Abstract
We describe an atypical man with diffuse large B cell lymphoma localized to the sphenoid wing and adjacent cavernous sinus, initially presenting with isolated ipsilateral facial pain mimicking trigeminal neuralgia due to invasion of Meckel’s cave but subsequently progressing to intra-axial extension and having synchronous features of systemic lymphoma. Primary central nervous system lymphoma is uncommon, accounting for approximately 2% of all primary intra-cranial tumors, but its incidence has been steadily increasing in some groups [1]. It usually arises in periventricular cerebral white matter, reports of lymphoma in extra-axial regions are rare [2]. This man highlights the importance of maintaining lymphoma in the differential diagnosis of tumors of the skull base presenting with trigeminal neuralgia-like symptoms.
Keywords: Lymphoma, Skull base, Trigeminal neuralgia
1. Introduction
Primary central nervous system (CNS) lymphoma is uncommon, accounting for approximately 2% of all primary intra-cranial tumors, but its incidence has been steadily increasing in some groups [1]. It usually arises in periventricular cerebral white matter, reports of lymphoma in extra-axial regions are rare [2].
2. Case report
A 55-year-old man presented with a 6 month history of severe, paroxysmal, electric-like facial pain in the distribution of the right V3 nerve that initially involved the upper lip and cheek but gradually progressed to include numbness over the chin. Physical examination revealed no evidence of neurological deficit other than diminished sensation over the chin. Other medical conditions included coronary artery disease managed with aspirin, clopidogrel, lisinopril and metoprolol, hypercholesterolemia managed with rosuvastatin and ezetimbe, and depression managed with sertraline. Family and social history were non-contributory.
The man was presumptively diagnosed with trigeminal neuralgia. He did not tolerate carbamazepine (discontinued following development of rash) and did not respond to pregabalin or gabapentin. Based on this lack of response to pharmacotherapy, the patient elected to pursue microvascular decompression. An MRI study conducted as part of the work-up for this procedure demonstrated an avidly enhancing extra-axial right sphenoid wing mass impinging on the cisternal segment of the right trigeminal nerve, measuring approximately 40 mm anterior-posteriorly × 20 mm left to right × 25 mm superior-inferiorly in greatest dimensions and with a prominent dural tail extending approximately 10 mm laterally across the middle cranial fossa (Fig. 1). The mass extended into the infratemporal fossa through a markedly expanded right foramen ovale (measuring approximately 7 mm), extended slightly into the right inferior orbital fissure, and appeared to extend into the foramen rotundum. There was marked effacement of the right Meckel’s cave (Fig. 2), partial effacement of the right cavernous sinus, and a moderate mass effect on the anteromedial aspect of the right temporal lobe. The lesion was adjacent to the paraclinoid right internal carotid artery but notably did not cause stenosis or compression. Based on the anatomic location and radiographical characteristics of this lesion, the mass was thought to be most consistent with either trigeminal schwannoma or meningioma and was treated conservatively. However, the man returned earlier than expected with progressive pain symptoms within 8 weeks.
Fig. 1.
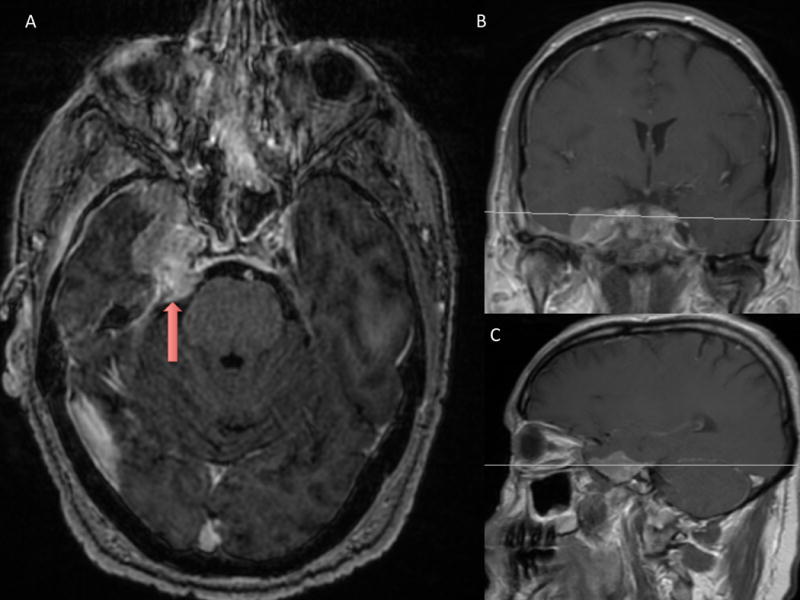
An enhancing mass (red arrow) is seen on axial (A), coronal (B), and (C) sagittal MRI. The mass measured approximately 40 mm anterior to posterior × 20 mm right to left × 25 mm superior to inferior in greatest dimensions. Medially, it encroached upon the right cavernous sinus which appeared mildly compressed or slightly invaded. The mass extended into the prepontine cistern in the area of the right trigeminal nerve and extended into Meckel’s cave to the level of the foramen ovale.
Fig. 2.
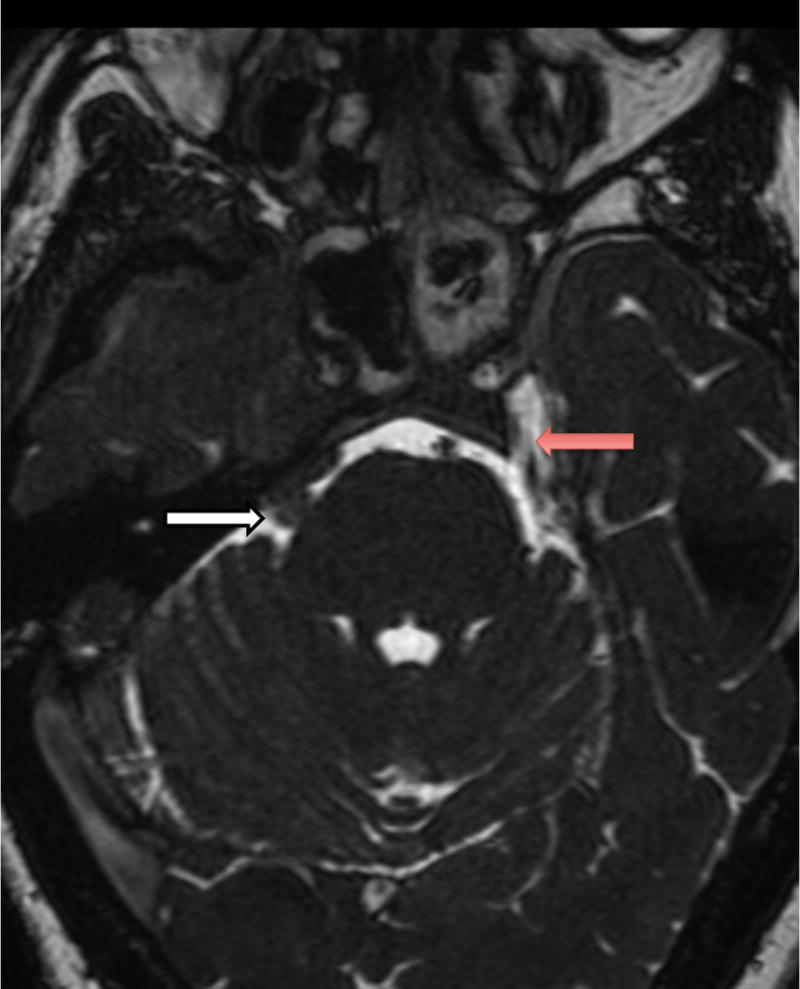
Axial fast imaging employing steady state acquisition (FIESTA) sequence MRI demonstrates the anatomy of the cranial nerves in relation to the mass lesion. On the patient’s left side, the trigeminal nerve can be seen taking its usual course through Meckel’s cave (red arrow). On the patient’s right side, the trigmenal nerve (white arrow) is obscured by a tumor mass that obliterates Meckel’s cave.
A repeat MRI study showed that the mass had increased in size and also demonstrated increased subcortical white matter T2-weighted hyperintensity suggesting pial invasion. He proceeded to undergo right V3 rhizotomy and right temporal craniotomy. An intra-operative frozen pathological specimen of the tumor was indeterminate and partial resection of the right sphenoid wing mass (sparing the cavernous sinus segment) was achieved in view of the indeterminate pathology result and risk of injury to structures in the cavernous sinus. Permanent histopathology revealed a tumor consisting of medium to large sized lymphoid cells with polymorphic and multi-lobulated nuclei of B cell phenotype (CD20 positive). The cells were Pax5 positive, Bcl2 negative, Bcl6 positive, MUM1 negative, negative for EBER in situ hybridization and had a high Ki-67 labeling index of 50%. These findings were indicative of diffuse large B cell lymphoma (DLBCL).
The patient’s facial pain was dramatically alleviated following the partial resection. Given the histopathological identity of the tumor, he was referred for staging investigations. Lactate dehydrogenase measurement was within normal limits and human immunodeficiency virus serology was negative. MRI of the spine found a T1-weighted hypointense focus in the right anterior C4 vertebral body corresponding to slightly increased metabolic activity on positron emission tomography (PET) scanning. Scrotal ultrasound and whole body CT scans revealed no evidence of metastatic disease. Cerebrospinal fluid cytology and bone marrow biopsy revealed no abnormal cells or leptomeningeal involvement. The man was diagnosed as having DLBCL with synchronous primary CNS and systemic pathological features and was started on high dose methotrexate and rituximab for CNS disease followed by focal radiation to the tumor area for local control and additional pain relief. Consolidation systemic multidrug chemotherapy (R-CHOP: rituximab, cyclophosphamide, hydroxydaunomycin, oncovin, prednisone) followed.
The man reported a significant improvement in facial pain following surgery, with only mild intermittent headaches. There are no residual neurological deficits. There have been no signs of relapse over 4 months of close follow-up.
3. Discussion
This extremely rare presentation with lancinating facial pain from CNS lymphoma involving Meckel’s cave near the sphenoid wing at the skull base illustrates the challenge in diagnosis of DLBCL presenting in an atypical location that clinically and radiographically mimicked other more common disorders. Clinically, our patient’s paroxysmal pain in a unilateral V3 distribution appeared to be trigeminal neuralgia which in the vast majority of patients is caused by focal compression and demyelination of the trigeminal nerve root at its entry into the pons by an aberrant loop of vasculature [3], and is associated with tumors in only about 10% of patients [4]. Radiographically, the lesion appeared consistent with schwannoma or meningioma which are the most common tumors associated with trigeminal neuralgia-like neuropathy [4].
To our knowledge, malignant lymphoma arising from the sphenoid wing has previously been reported in only one patient [5]. Two reports described lymphoma arising from Meckel’s cave [6, 7], and some others reported it arising orbitally or in the cavernous sinus [8]. These previously reported skull base lesions have frequently been misdiagnosed as meningiomas or schwannomas, as in the present patient. To our knowledge also, only one prior report of primary malignant lymphoma of Meckel’s cave has described presenting symptoms resembling trigeminal neuralgia, but it included involvement of all three distributions of the trigeminal nerve and was accompanied by the emergence of other progressive neurological defects [7]. Our patient is unique in the highly specific involvement of a single branch of the facial nerve in its clinical presentation and the absence of other appreciable neurological deficits.
Like these prior reports, we considered the radiographic studies of our patient to be consistent with meningioma or schwannoma rather than atypical alternatives including lymphoma. The MRI findings of extranodal non-Hodgkin lymphoma of the skull base are highly similar to those associated with meningioma and other more common neoplasms of this region. In particular, tumor infiltration along dural surfaces of the planum sphenoidale, cavernous sinus and other structures can appear almost identical to the dural tail that is characteristic of meningioma. One small series of five patients with non-Hodgkin lymphoma of the skull base confirmed the difficulty in differentiating these neoplasms but suggested that bone enhancement without hyperostotic reaction and cavernous sinus invasion without narrowing of the carotid artery lumen could be helpful in distinguishing lymphoma from meningioma [9]. The latter is typically characterized by hyperostotic bone involvement which actually represents invasion of the bone by the tumor [10] and does not generally preserve the carotid artery lumen. These features appear to apply to our patient as radiography did not show a hyperostotic reaction to bone involvement and demonstrated delicate sparing of the carotid artery lumen in both MRI studies taken 8 weeks apart, despite growth of the tumor over this period. Recognition of these radiographic features could help inform surgeons of the possibility of lymphoma, enabling earlier diagnosis and better management.
Primary CNS lymphoma is a rare form of extranodal non-Hodgkin lymphoma that accounts for approximately 2% of all primary CNS tumors in the United States, although its incidence appears to be rising in the elderly [1, 11]. Prognosis of primary CNS lymphoma remains guarded. The preferred treatment involves induction chemotherapy including high dose methotrexate with consolidation radiation therapy which has been shown to achieve up to 45% overall survival at 2 years [12] (although the value of consolidation radiotherapy in patients who experience a complete radiographic response to induction chemotherapy has been challenged [13]). Rituximab, a monoclonal anti-CD20 antibody, also improves outcomes [14]. Early diagnosis and intervention for these patients likely improves outcomes and enables a more rational therapeutic approach. Our patient emphasizes the importance of considering the possibility of primary lymphoma in unusual locations to avoid misdiagnosis and delay of prompt oncologic management.
Neuroimaging is important in the diagnostic workup of trigeminal neuralgia to identify potential neoplastic lesions resulting in trigeminal neuralgia-like symptoms.
Primary CNS lymphoma is a rare form of extranodal non-Hodgkin lymphoma that accounts for approximately 2% of all primary CNS tumors in the United States.
While the majority of trigeminal neuralgia results from neurovascular compression at the root entry zone, approximately 10% of cases result from other etiologies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest/Disclosures
The authors declare that they have no financial or other conflicts of interest in relation to this research and its publication.
References
- 1.Dolecek TA, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14(Suppl 5):v1–49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hochberg FH, Miller DC. Primary central nervous system lymphoma. J Neurosurg. 1988;68(6):835–53. doi: 10.3171/jns.1988.68.6.0835. [DOI] [PubMed] [Google Scholar]
- 3.Love S, Coakham HB. Trigeminal neuralgia: pathology and pathogenesis. Brain. 2001;124(Pt 12):2347–60. doi: 10.1093/brain/124.12.2347. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin MR, et al. Microvascular decompression of cranial nerves: lessons learned after 4400 operations. J Neurosurg. 1999;90(1):1–8. doi: 10.3171/jns.1999.90.1.0001. [DOI] [PubMed] [Google Scholar]
- 5.Hodgson DJ, et al. Intracranial extracerebral follicular lymphoma mimicking a sphenoid wing meningioma. Journal of Neurology, Neurosurgery & Psychiatry. 1999;67(2):251–252. doi: 10.1136/jnnp.67.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdel Aziz KM, van Loveren HR. Primary Lymphoma of Meckel’s Cave Mimicking Trigeminal Schwannoma: Case Report. Neurosurgery. 1999;44(4):859–862. doi: 10.1097/00006123-199904000-00096. [DOI] [PubMed] [Google Scholar]
- 7.Kinoshita M, et al. Primary malignant lymphoma of the trigeminal region treated with rapid infusion of high-dose MTX and radiation: case report and review of the literature. Surg Neurol. 2003;60(4):343–8. doi: 10.1016/s0090-3019(02)01046-7. discussion 348. [DOI] [PubMed] [Google Scholar]
- 8.Roman-Goldstein SM, et al. Atypical central nervous system lymphoma at the cranial base: report of four cases. Neurosurgery. 1998;43(3):613–5. doi: 10.1097/00006123-199809000-00119. discussion 615–6. [DOI] [PubMed] [Google Scholar]
- 9.Han MH, et al. Non-Hodgkin lymphoma of the central skull base: MR manifestations. J Comput Assist Tomogr. 1993;17(4):567–71. doi: 10.1097/00004728-199307000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Bikmaz K, Mrak R, Al-Mefty O. Management of bone-invasive, hyperostotic sphenoid wing meningiomas. J Neurosurg. 2007;107(5):905–12. doi: 10.3171/JNS-07/11/0905. [DOI] [PubMed] [Google Scholar]
- 11.Kadan-Lottick NS, Skluzacek MC, Gurney JG. Decreasing incidence rates of primary central nervous system lymphoma. Cancer. 2002;95(1):193–202. doi: 10.1002/cncr.10643. [DOI] [PubMed] [Google Scholar]
- 12.Ferreri AJM, et al. A multicenter study of treatment of primary CNS lymphoma. Neurology. 2002;58(10):1513–1520. doi: 10.1212/wnl.58.10.1513. [DOI] [PubMed] [Google Scholar]
- 13.Thiel E, et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol. 2010;11(11):1036–47. doi: 10.1016/S1470-2045(10)70229-1. [DOI] [PubMed] [Google Scholar]
- 14.Batchelor TT, et al. Rituximab monotherapy for patients with recurrent primary CNS lymphoma. Neurology. 2011;76(10):929–930. doi: 10.1212/WNL.0b013e31820f2d94. [DOI] [PMC free article] [PubMed] [Google Scholar]


