Abstract
Objective
To determine whether oocyte cryopreservation (OC) for deferred reproduction is cost-effective per live birth using a model constructed from observed clinical practice.
Design
Decision-tree mathematical model with sensitivity analyses.
Setting
Not applicable.
Patients
A simulated cohort of women wishing to delay childbearing until age 40 years.
Interventions
Not applicable.
Main Outcome Measure
Cost per live birth.
Results
Our primary model predicted that OC at age 35 years by women planning to defer pregnancy attempts until age 40 would decrease cost per live birth to $39,946 (and increase odds of live birth to 62% by the end of the model),indicating OC to be a cost-effective strategy relative to forgoing OC, which was associated with a predicted cost per live birth of $55,060 (and 42% chance of live birth). If fresh autologous ART was added at age 40 prior to thawing oocytes, 74% obtained a live birth, though at an increased cost of $61,887. Separate sensitivity analyses demonstrated that OC remained cost-effective so long as patients underwent OC prior to age 38, more than 49% of those not obtaining a spontaneously conceived live birth returned to thaw oocytes, and likelihood of obtaining a spontaneously conceived live birth after six months’ attempts at age 40 was less than 35%.
Conclusions
In women who plan to delay childbearing until age 40, oocyte cryopreservation before 38 years of age reduces the cost to obtain a live birth.
Keywords: oocyte cryopreservation, vitrification, fertility preservation, cost analysis, ART
Introduction
In recent years, there has been an increase in the number of women delaying childbirth for educational, professional, and personal pursuits (1). The Organization for Economic Co-operation and Development recently reported that among member countries, 27.8 years was the average age of first birth. This statistic has risen steadily since the 1970s (2). The US fertility rate is now the lowest ever reported (3).
Well established is that female fertility precipitously declines with advancing age (4-10), as is the limited ability of conventional assisted reproductive technology (ART) to surmount age-related infertility (11, 12). In fact, the live birth rate per treatment cycle drops by nearly 50% for women initiating IVF after the age of 40 (13). Thus, involuntary childlessness is a relatively frequent consequence of delaying conception attempts.
In order to maintain the possibility of creating their families at a later date, some women are beginning to embrace the use of oocyte cryopreservation (OC) as a technologic bridge from reproductive prime to preferred conception age. OC has the potential to extend fertility beyond a woman’s natural reproductive lifespan as well as preserve a woman’s option to parent genetically-linked children with a future partner. Nearly 90% of surveyed women cited lack of partner as their primary reason for pursuing OC, and the majority found ‘the egg freezing process’ to be ‘empowering’ (14). Recently, OC has become more accepted as accumulating data has demonstrated pregnancy rates comparable to those from IVF cycles using fresh female gametes (15-20). The American Society for Reproductive Medicine recently lifted the technology’s “experimental” designation, corroborating these data (21).
Recent acceptance of OC as a conventional therapy has spurred interest in the question of whether it is cost-effective relative to more established ART options. Two recently published studies that endeavored to answer this question had markedly different results. A US-based model by Hirschfeld-Cytron et al. found that OC was not cost-effective (22), while a Netherlands-based analysis by Van Loendersloot et al. found that OC provided an overall cost savings of $24,600 per live birth (23). A subsequent co-authored letter by these two groups explained that the differences in their results were likely due to variations in model inputs and design, including age, cost estimates of ART, and probabilities of success with cryopreserved oocytes (24).
Given that OC for deferred childbearing is evolving into a mainstream therapy, that outcomes from studies evaluating cost-effectiveness were conflicting, and that OC cycle data from our center is available to inform model design, we sought to conduct a cost-effectiveness analysis of OC (as performed in the US) using a real-data driven approach. Our main study objective was to determine whether cryopreserving oocytes at age 35 with the intention to thaw, fertilize, and implant at age 40 is more cost-effective than attempting pregnancy and, if needed, undergoing conventional IVF at age 40.
Materials and Methods
Model Design
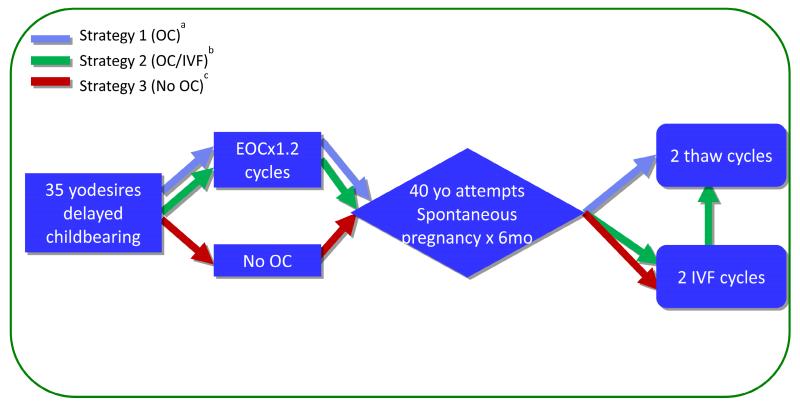
We constructed a decision-tree mathematical model involving theoretical 35 year-old women with personal reasons (e.g. career, lack of partner) for deferring childbearing until 40 years of age. Our model examined three treatment strategies available to such women, as depicted in Figure 1 and described below. We based the described strategies on observed patient treatment choices and clinical outcomes, and on professional guidelines (14, 25).
Figure 1.
Schematic Representation of Treatment Strategies Analyzed in Cost-effectiveness Analysis
aStrategy 1 (OC): OC × 1.2 cycles (mean number required to obtain 16 MII oocytes at age 35 years), attempt spontaneous conception at age 40 years for 6 months, 2 oocyte thaw cycles using stored oocytes if no live birth
bStrategy 2 (OC/IVF): OC × 1.2 cycles, attempt spontaneous conception at age 40 years for 6 months, 2 fresh autologous ART cycles if no live birth, 2 oocyte thaw cycles using stored oocytes if still no live birth
cStrategy 3 (No OC): No OC at age 35 years, attempt spontaneous conception at age 40 years for 6 months, 2 fresh autologous ART cycles if no live birth
Approval was obtained from the NYU School of Medicine Institutional Review Board to retrospectively analyze the OC cycles completed at the New York University Langone Medical Center Fertility Center from June of 2007 to April of 2014. These real data furnished the inputs for our cost model. We found that with 8 mature, meiosis II (MIIs) oocytes available per thaw cycle, outcomes were comparable to fresh IVF (20). The average 35 year-old patient needed to complete 1.2 OC treatment cycles in order to bank 16 MII for two future potential thaw cycles.
Fertility treatment strategies modeled were as follows and were based on the strategies most often employed in clinical practice (Figure 1):
Strategy 1: “OC” (notated in blue in all figures). In this strategy, a patients elect to undergo OC at age 35 to obtain at least 16 MII oocytes for potential use after age 40. In Strategy 1 (as in all three strategies), women attempt spontaneous conception via timed intercourse for a period of 6 months upon reaching 40 years of age. If no spontaneous live birth is obtained, the women then proceed with two IVF cycles using previously banked oocytes.
Strategy 2: “OC/IVF”(notated in green) is similar to Strategy 1 in that women also undergo OC at age 35 and attempt spontaneous conception at age 40. However, in Strategy 2, if no live birth is obtained spontaneously, the women undergo two fresh autologous IVF cycles at age 40 prior to thawing banked cryopreserved oocytes. We have observed this strategy employed by patients in an attempt to maximize chances at autologous live birth (14).
Strategy 3: “No OC” (notated in red). In this strategy, women wishing to defer childbearing decline the option to undergo OC at age 35, and instead attempt spontaneous pregnancy for 6 months upon reaching the age of 40 (25). Then, if no live birth is achieved, they undergo two cycles of fresh IVF.
Model Inputs
Model inputs for natural fecundity at age 40 were derived from the published literature. In all three strategies, a 16% total live birth rate was used as the result of six months of attempting spontaneous pregnancy by a 40 year-old woman. This number was calculated by taking the likelihood of a pregnancy over 6 months’ attempts (6) and subtracting the expected proportion of biochemical and clinical miscarriages (26).
For ART success, a dataset containing all fresh, autologous ART cycle starts reported in 2011 was obtained from the SART Research Committee. SART CORS includes data from 90% of all ART clinics in the US. Mean live birth per cycle start by age was calculated from the SART CORS database for 2011 (the most recent available reporting year). These data provided ART live birth model inputs for all three treatment strategies. The mean live birth per cycle start from fresh, autologous, ART at age 40 years was 16.82%. Live birth rates from oocytes cryopreserved, thawed, and fertilized at experienced centers are now known to be comparable to those of fresh IVF completed at the age of freeze (15-20). Therefore we used 33.04%, the mean live birth per fresh, autologous cycle start at age 35 years calculated from SART CORS 2011, as the model input for live birth rate per oocyte thaw cycle in Strategies 1 and 2 (Supplemental Table 1).
OC and oocyte storage for five years, thaw cycle, and fresh IVF charges (each including medication costs) were randomly obtained for17 regionally diverse fertility clinics via published pricing on internet websites (27-30) or by phone or email inquiries made in July 2014. Median charges obtained were used as primary model inputs (OC and storage: $15,048; OC thaw cycle: $5,094; fresh autologous ART cycle: $14,987) (Supplemental Table 2). Charges obtained by this sampling method were consistent with those published by the Livestrong Foundation, Attain Fertility, Cost Helper Health, and RESOLVE (31-34).
Sensitivity Analyses
Cost per live birth was calculated for each strategy using a data-driven mathematical decision-tree probability model based on the inputs described. Sensitivity analyses were performed to compare the cost-effectiveness of each strategy while varying different model inputs, including the age at cryopreservation, probability of spontaneously conceived live birth at 40 years of age, cost of OC, and the cost of an IVF cycle at 40 years of age. First, in order to determine up to what age the cost benefit per live birth persisted, we varied age at OC over the range of 25 to 40 years of age. In this analysis, we utilized the mean number of cycles required to obtain 16 MII oocytes and the mean live birth per cycle start for each specific age group. All remaining model inputs were consistent with those used in the primary model. Second, natural fecundity has proven difficult to study, and estimates in older women may be prone to underestimation, due in part to confounding by partner age and coital frequency; therefore, precise estimates are limited (34). To determine how OC cost-effectiveness was impacted by the magnitude of age-related fertility decline, we varied the proportion of women obtaining a live birth from 6 months’ attempts at spontaneous conception from 0-50%. Third, given the wide reported range of OC cycle cost ($10,804-$17,000, Supplemental Table 2), we varied OC cycle cost to determine if there were price point(s) that altered the cost-effectiveness of the models’ treatment strategies. Fourth, given the complex pricing structures used in IVF cycles (which mean that published per cycle pricing may overestimate actual costs) as well as the uncertainty of future IVF costs, we also varied IVF cycle cost to determine potential price points that may alter the cost-effectiveness of the three strategies. Finally, since the proportion of women undergoing OC for social reasons who ultimately return to use their eggs is not known but is highly determinative of the therapy’s cost-benefit, we varied this proportion from 0 to 80%.
Results
In our primary model, which used OC at age 35, pregnancy attempts at age 40, and median US ART charges, Strategy 1 (OC) was most cost-effective, with a mean cost per live birth of $39,946 with 62% of patients predicted to achieve live birth by the end of the model. Strategy 2 (OC/IVF), in which patients underwent fresh IVF at age 40 years prior to thawing oocytes, resulted in the highest likelihood of live birth by the end of the model at 74%; however, cost per live birth was also greatest with this strategy at $61,887. Strategy 3 (No OC), in which patients did not cryopreserve oocytes, resulted in only 42% of women obtaining a live birth at a cost per live birth of $55,060. In short, in our primary model, Strategy 1 (OC) was more cost-effective than Strategy 3 (No OC), which was more cost-effective than Strategy 2 (OC/IVF).
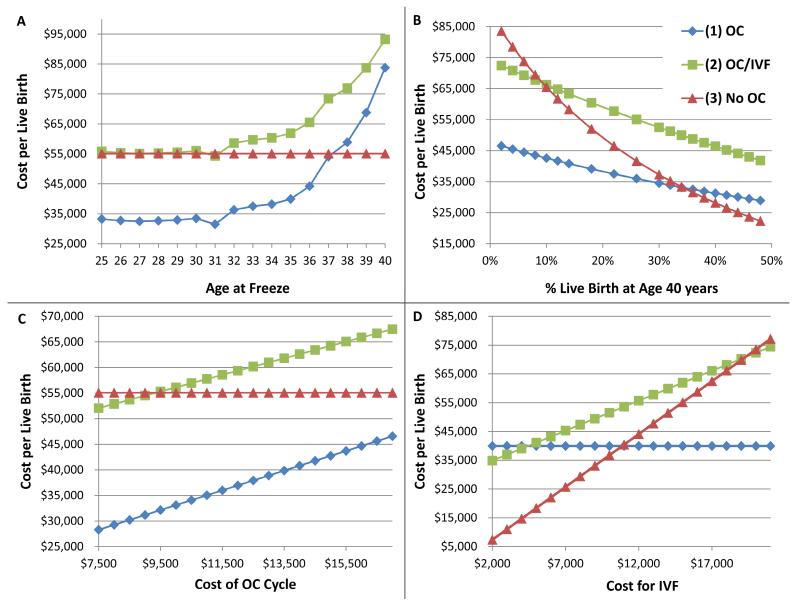
Given that OC for deferred reproduction was cost-effective when patients underwent OC at age 35, we endeavored to determine up to what age the cost benefit per live birth persisted. As expected in this analysis, in Strategy 3, where no OC had been performed, likelihood and cost per live birth remained constant across ages at 42% and $55,060 respectively (Red line, Figure 2, Panel A). Importantly, Strategy 1 (OC) was always more cost-effective than Strategy 2 (OC/IVF) and was more cost-effective than Strategy 3 (No OC) through the age of 37 years. This is depicted graphically at the point of intersection of blue and red lines in Figure 2, Panel A, where cost-per live birth for Strategy 1 (OC) surpasses that of Strategy 2 (No OC) after the age of 37 years. Cost-effectiveness of Strategy 2 (OC/IVF) was similar to Strategy 3 (No OC) only up to the age of 31years, after which Strategy 3 was more cost-effective. In our model, women who underwent OC prior to the age of 35 using Strategy 2 (OC/IVF) had at least a 74% chance of achieving live birth.
Figure 2.
Sensitivity Analyses Comparing Cost-Effectiveness Between Treatment Strategies While Varying Individual Model Inputs.
Each panel represents a separate sensitivity analysis, in which the model input named on the X-axis was varied, while all other model inputs were held constant. Cost per live birth is represented on the Y-axis, such that lower points on each graph represent the more cost effective strategy for the given model input. Model inputs varied in the sensitivity analyses included: A) age at oocyte cryopreservation (OC), B) probability of live birth at age 40 years from 6 months’ attempts at spontaneous conception, C) cost of OC, and D) cost of an IVF cycle. In all panels, the blue line represents Strategy 1 (OC), the green line represents Strategy 2 (OC/IVF), and the red line represents Strategy 3 (No OC). See Figure 1 and methods for a detailed description of each strategy.
We further evaluated the impact of age-related fertility decline by varying the likelihood that a woman at age 40 years will succeed in having a live born child after attempting natural conception of 6 months. As expected, the cost per live birth with Strategy 3 (No OC) decreased rapidly with improved natural fecundity at age 40 (Figure 2, Panel B). However, cost-effectiveness of Strategy 3 (No OC) did not surpass that of Strategy 1 (OC) until fecundity over 6 months reached 35%, more than twice that indicated by published literature.
Given the wide range of reported OC cycle costs, we performed sensitivity analysis varying the cost for an OC cycle (including medications) (Figure 2, Panel C). Age at OC was fixed at 35 years of age. In this analysis, Strategy 1 (OC) was always the most cost-effective option; Strategy 2 (OC/IVF) only became more cost-effective than Strategy 3 (No OC) when the cost of an OC cycle was less than $9,341, which was lower than the lower limit of the reported range of costs. Further, we also varied the cost of an IVF cycle (Figure 2, Panel D), and observed that Strategy 3 (No OC) was more cost-effective than the other strategies only when the cost of IVF was less than $11,000, which was again lower than the lower limit of the reported range of costs for IVF cycles.
Finally, we attempted to answer the question of what percentage of women would need to use their frozen oocytes in order to warrant pursing OC treatment from a cost perspective. Since the primary model indicated that Strategy 2 (OC/IVF) was not cost-effective even if 100% of those still not pregnant after 2 fresh IVF cycles at age 40 years returned to thaw, this strategy was omitted from this sensitivity analysis. OC was determined to be cost-effective when more than 49% of those women, who did not achieve a live birth after 6 months of attempting spontaneous pregnancy at age 40 years, returned to thaw their oocytes for fertilization and embryo transfer (not depicted graphically).
Discussion
Our data-driven analyses established several clinically useful cut-points for patients and clinicians considering whether OC for deferred childbearing is warranted from a cost perspective. Specifically, among women planning to defer pregnancy attempts until age 40, our model predicted a lower overall cost per live birth among those electing for OC prior to age 38. Our model further indicated that among women with cryopreserved oocytes, fresh IVF prior to oocyte thaw cycle(s) would increase the chances of live birth, though at an increased cost. Women cryopreserving oocytes by age 35 and undergoing fresh IVF at age of desired childbearing (40 years) had at least a 74% chance of live birth according to model outputs.
A major strength of our model derives from its data-driven approach and reliance on observed patient practices. The number of OC cycles needed and costs incurred were based on 7 years of oocyte yield data among patients undergoing OC treatments for personal (non-medical) indications at our clinic. In addition, our model adds to existing literature on this question, in that the treatment strategies assessed by the model are those we have seen employed most commonly by women of advancing reproductive age considering OC for personal indications (14). In their 2011 model, Van Loendersloot et al. did not allow for any spontaneous pregnancy attempts at age 40 among women with oocytes cryopreserved, and those without cryopreserved oocytes attempted for 12 months prior to moving to IVF. The ASRM Practice Committee has stated that ‘women older than 35 years should receive an expedited evaluation and undergo treatment after 6 months of failed attempts to conceive or earlier’(10). Hirschfeld-Cytron et al. allowed for the possibility of spontaneous pregnancy at age 40 years with 6 months’ attempts in all groups; however, their model involved OC at the young age of 25 years, which as stated above, does not accord with patient patterns in our experience. While the former study reported a significant cost savings per live birth with OC, the latter did not find OC to be cost-effective unless the cost of an IVF cycle was greater than $22,000.
Though it builds on existing models, our study has several limitations. Decision-tree models are inherently limited by the accuracy and precision of their data inputs. Since OC remains in its relative infancy, sufficient thaw cycle outcome data by age at OC for women undergoing OC for personal indications are not yet available. Therefore, by necessity, our model assumed that women receiving blastocysts resulting from OC would have live birth rates equivalent to infertile couples undergoing fresh, autologous ART at the same age. Though numerous studies have demonstrated the general non-inferiority of oocyte thaw cycles (15-20), these equivalent success rates likely rely to some extent on the center’s experience level with OC as well as practices regarding the number of oocytes thawed and number of embryos transferred. Our model compensates for this to some extent by indicating 8 MII oocytes per thaw cycle, which equated with success rates comparable to fresh IVF at our center (20). The live birth rates per thaw cycle by age at OC used in our model are somewhat higher than those reported in a recent meta-analysis by Cil et al.; however, the highest number of vitrified-thawed oocytes fertilized in their model was 6, and their meta-analysis of vitrified oocytes included patients undergoing OC for medical fertility preservation and studies published as early as 2003, since which time, success rates have likely improved (36).
In addition, costs associated with OC and oocyte thaw cycles vary widely, and the proportion of women who will ultimately utilize their cryopreserved oocytes remains unknown. Sensitivity analyses compare values within a range of reasonable uncertainty and represent an accepted method to evaluate and account for inputs with wide ranges (37). Here, they provide useful insight into the question of whether OC completed for non-medical indications is cost-effective. Indeed, estimating costs has proved to be particularly challenging for this work. By varying age at cryopreservation, cost of treatment, cost of an IVF cycle, and natural fecundity at age 40 in separate analyses, we were able to obtain more specific information that clinicians and patients considering OC can use to determine whether OC will be cost-effective for a particular woman’s situation. In this way, we are able to show the cost points at which our conclusions change and improve the applicability of these findings across many clinical scenarios as well as a range of possible future costs.
Varying age at cryopreservation, we determined the age-cost-effectiveness threshold for undergoing OC to be 38 years. This information is encouraging for patients and providers of OC, in that it is consistent with the mean age of women seeking OC observed in our clinical practice. Among 1,439 OC cycles performed at our center for personal, non-medical indications, we observed a decrease in mean patient age from 40.0 to 37.9 years (p<.0001) from 2005 to 2013. Though cost effective based on this sensitivity analysis, it is important to note that it may be impractical for both financial and societal reasons for women to seek OC in their 20s and early 30s.
Our model indicated that at least 49% of those women who do not obtain a live birth after6 months’ attempts at spontaneous conception beginning at age 40 must utilize their cryopreserved oocytes, in order for OC to be cost-effective per live birth. Approximately 12% of all women who have undergone OC at our center have returned to attempt pregnancy using cryopreserved oocytes (14); however, this percentage will likely increase given that many of these women cryopreserved their oocytes recently and have not had sufficient time to return to attempt pregnancy.
In addition to reliance on inputs, it is important to recognize the necessary simplicity of mathematical models relative to individual clinical situations. For example, in the present study, costs of ectopic pregnancies and spontaneous abortions were not explicitly considered in the decision tree. However, since miscarriage occurs more frequently among pregnancies resulting from older oocytes (e.g. the spontaneous and fresh ART pregnancies at age 40 occurring in our model), inclusion of these costs would likely have increased the cost-effectiveness of OC predicted by the model. Probabilities of canceled cycles and of no transfer (due to lack of oocytes surviving thaw, no oocytes obtained at retrieval in fresh cycles, or failed fertilization of all oocytes) were not explicitly modeled. In our experience, cycles resulting in no transfer are quite unlikely among patients with 8 MII oocytes available to thaw (20) and represent a more common occurrence among patients undergoing fresh ART at age 40. In addition, frequencies of all these outcomes (miscarriage, ectopic, cycle cancellation, and no transfer) were largely accounted for by using inputs for live birth per-cycle start rather than per-embryo transfer. Therefore, cost per live birth in each strategy takes into account the cost of cycles that do not proceed to transfer and that do not progress from implantation to live birth.
Variation in age at pregnancy attempts and oocyte thaw was not explicitly modeled; however, it is unusual for women to return earlier than 5 years after OC, and if patients wait longer to attempt pregnancy (e.g. after age 40 years), OC only becomes more cost effective, since spontaneous pregnancy and autologous live birth rates decrease with age. We did not model ovulation induction and/or intrauterine insemination in women who did not conceive spontaneously at the age of 40 years. A recent randomized controlled trial compared IVF versus clomiphene or gonadotropin ovulation induction with IUI in women ages 38-42 years with unexplained infertility of ≥ 6 months’ duration. The authors found that IVF decreased time to pregnancy by 3-4 months, with 5% live birth per ovulation induction cycle versus 15% per IVF cycle. The study did not include a cost analysis of these therapies (38). An additional simplification was that, as with prior published models, ours did not consider the possibility of the patient who changes her decision to defer child bearing and obtains a spontaneously conceived live birth prior to the age of 40.
Loss of individual productivity and/or absence from work was not included in our analysis, which focused on clinical rather than societal costs. Our analysis did not directly account for potential changes in the cost of IVF over the five year period between OC and pregnancy attempts at age 40 years due to potential inflation/deflation or changes in technology or other factors, however sensitivity analyses varied the cost of OC and IVF cycles to evaluate potential effects on our findings were the cost of IVF to change. Using similar methods to sample regionally diverse clinics for IVF pricing, we found that the median cost of an ART cycle including medications was relatively stable from 2011 to 2014 and were consistent with those reported by ASRM and RESOLVE (39). Of note, the majority of clinics include the cost of assisted hatching and ICSI in their IVF pricing. As there are likely higher rates of assisted hatching and ICSI in older IVF patients, our sensitivity analyses also take into account potential differences in cost due to differences in these procedures by age group. Furthermore, if the cost of fresh autologous ART were to increase, so would the cost-effectiveness of OC, by comparison. From the insurer’s perspective, it is important to recognize that our model is based on financial charges -- which may overestimate actual costs. From the individual patient and her health care provider/counselor’s perspective, it is important to recognize that costs presented here represent average cost per live birth, which does not necessarily equal the cost to an individual woman.
Finally, and perhaps most importantly, many women pursuing this technology may seek it out as an insurance policy of sorts, which our model cannot address. It is not possible to estimate the value of increased likelihood of having a genetic child or the “peace of mind” cryopreserved oocytes may provide to an individual woman.
Conclusion
Importantly, for women younger than age 38, OC proved to be a cost-effective means to increase the likelihood of conceiving and delivering a genetically-related child. Analysis indicated that fresh ART prior to an oocyte thaw cycle(s) increased the chances of live birth among women with cryopreserved oocytes, though at an increased cost. Women cryopreserving by age 35 who then undergo fresh ART attempts prior to oocyte thaw had at least a 74% chance of live birth according to model outputs. These findings support, from a cost-perspective, an integral role for OC technology in reproductive planning for women who are not ready to complete childbearing before reaching the upper end of reproductive prime.
Supplementary Material
Table 1.
Number of MII Oocytes Obtained by Age at Cryopreservation at NYU Fertility Center from 2007-2014
| Age (years) |
No. OC cycles (N=1545) |
No. MII oocytes (mean±SD) |
Minimum- Maximum No. MII oocytes |
Mean No. Cycles required to obtain 16 MII oocytes |
|---|---|---|---|---|
| ≤ 30 | 12 | 14.75 ± 6.8 | 6-26 | 1.1 |
| 31 | 6 | 16.2±13.1 | 5-41 | 1.0 |
| 32 | 9 | 13.7±9.5 | 4-27 | 1.2 |
| 33 | 37 | 13.2±7.5 | 2-32 | 1.2 |
| 34 | 96 | 13.1±9.4 | 0-50 | 1.2 |
| 35 | 126 | 13±8 | 1-39 | 1.2 |
| 36 | 150 | 12.3±8.6 | 1-47 | 1.3 |
| 37 | 234 | 10.3±7.4 | 0-44 | 1.6 |
| 38 | 239 | 10.1±6.8 | 0-37 | 1.6 |
| 39 | 232 | 9.6±6.7 | 0-37 | 1.7 |
| 40 | 176 | 8.5±6.1 | 0-35 | 1.9 |
| 41 | 108 | 8.6±6.3 | 1-35 | 1.9 |
| 42 | 72 | 7.9±6 | 1-33 | 2.0 |
| 43 | 27 | 9.3±5 | 0-20 | 1.7 |
| 44 | 13 | 6.3±4 | 1-14 | 2.5 |
| 45 | 8 | 3.9±2.5 | 1-9 | 4.1 |
Acknowledgements
The authors would like to acknowledgements Valerie Baker, MD and the SART Research Committee for their assistance in the formulation and execution of this study.
SART wishes to thank all of its members for providing clinical information to the SART CORS database for use by patients and researchers. Without the efforts of our members, this research would not have been possible.
This work was supported by the Program in Reproductive and Adult Endocrinology and the Intramural Research Program, NICHD, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no financial disclosures.
The opinions and assertions contained in this article are the expressed views of the authors and are not considered official opinions of the Department of Health and Human Services, Texas Fertility Center, or New York University Langone Medical Center.
This work was presented at American Society of Reproductive Medicine’s 68th Annual Meeting, San Diego, California, on October 22, 2012.
References
- 1.Mills M, Rindfuss RR, McDonald P, teVelde E, ESHRE Reproduction and Society Task Force Why do people postpone parenthood? Reasons and social policy incentives. Hum Reprod Update. 2011;17:848–60. doi: 10.1093/humupd/dmr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. [Last Accessed September 7, 2014];OECD Family Database, SF2.3: Mean age of mothers at First Childbirth. 2012 Feb 24; Last updated: Available at: http://www.oecd.org/els/soc/SF2.3%20Mean%20age%20of%20mother%20at%20first%20childbirth%20-%20updated%20240212.pdf.
- 3.Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. [Last Accessed: September 7, 2014];Births: Final data for 2012. Natl Vital Stat Rep. 2013 62(9):1–87. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr62/nvsr62_09.pdf#table01. [PubMed] [Google Scholar]
- 4.Tietze C. Reproductive span and rate of reproduction among Hutterite women. Fertil Steril. 1957;8:89–97. doi: 10.1016/s0015-0282(16)32587-0. [DOI] [PubMed] [Google Scholar]
- 5.Guttmacher AF. Factors affecting normal expectancy of conception. J Am Med Assoc. 1956;161:855–60. doi: 10.1001/jama.1956.02970090081016. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz D, Mayaux MJ. Female fecundity as a function of age: results of artificial insemination in 2193 nulliparous women with azoospermic husbands. Federation CECOS. N Engl J Med. 1982;306:404–6. doi: 10.1056/NEJM198202183060706. [DOI] [PubMed] [Google Scholar]
- 7.Leridon H. Human Fertility: The Basic Component. Chicago University Press; Chicago: 1977. [Google Scholar]
- 8.Wood J. Fecundity and natural fertility in humans. In: Milligen S, editor. Reviews of Reproductive Biology. Oxford University Press; Oxford: 1989. pp. 61–109. [PubMed] [Google Scholar]
- 9.Leridon H. A new estimate of permanent sterility by age: sterility defined as the inability to conceive. Popul Stud. 2008;62:15–24. doi: 10.1080/00324720701804207. [DOI] [PubMed] [Google Scholar]
- 10.American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee Female age-related fertility decline. Fertil Steril. 2014;101:633–4. doi: 10.1016/j.fertnstert.2013.12.032. Committee Opinion No. 589. [DOI] [PubMed] [Google Scholar]
- 11.Leridon H. Can assisted reproduction technology compensate for the natural decline in fertility with age? A model assessment. Hum Reprod. 2004;19:1548–53. doi: 10.1093/humrep/deh304. [DOI] [PubMed] [Google Scholar]
- 12.Hourvitz A, Machinger R, Maman E, Baum M, Dor J, Levron J. Assisted reproduction in women over 40 years of age: how old is too old? Reprod Biomed Online. 2009;19:599–603. doi: 10.1016/j.rbmo.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 13. [Last accessed: August 26, 2014];Society for Assisted Reproductive Technologies National Data Summary. 2012 Available at: www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?ClinicPKID=0.
- 14.Hodes-Wertz B, Druckenmiller S, Smith M, Noyes N. What do reproductive-age women who undergo oocyte cryopreservation think about the process as a means to preserve fertility? Fertil Steril. 2013;100:1343–9. doi: 10.1016/j.fertnstert.2013.07.201. [DOI] [PubMed] [Google Scholar]
- 15.Rienzi L, Cobo A, Paffoni A, Scarduelli C, Capalbo A, Vajta G, et al. Consistent and predictable delivery rates after oocyte vitrification: an observational longitudinal cohort multicentric study. Hum Reprod. 2012;27:1606–12. doi: 10.1093/humrep/des088. Hum Reprod. 2012; 27:1606-12. [DOI] [PubMed] [Google Scholar]
- 16.Boldt J. Current results with slow freezing and vitrification of the human oocyte. Reprod Biomed Online. 2011;23:314–22. doi: 10.1016/j.rbmo.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Grifo JA, Noyes N. Delivery rate using cryopreserved oocytes is comparable to conventional in vitro fertilization using fresh oocytes: potential fertility preservation for female cancer patients. Fertil Steril. 2010;93:391–6. doi: 10.1016/j.fertnstert.2009.02.067. [DOI] [PubMed] [Google Scholar]
- 18.Cobo A, Kuwayama M, Pérez S, Ruiz A, Pellicer A, Remohí J. Comparison of concomitant outcome achieved with fresh and cryopreserved donor oocytes vitrified by the Cryotop method. Fertil Steril. 2008;89:1657–64. doi: 10.1016/j.fertnstert.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 19.Nagy ZP, Chang CC, Shapiro DB, Bernal DP, Elsner CW, Mitchell-Leef D, Toledo AA, Kort HI. Clinical evaluation of the efficiency of an oocyte donation program using egg cryo-banking. Fertil Steril. 2009;92:520–6. doi: 10.1016/j.fertnstert.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Goldman KN, Noyes NL, Knopman JM, McCaffrey C, Grifo JA. Oocyte efficiency: does live birth rate differ when analyzing cryopreserved and fresh oocytes on a per-oocyte basis? Fertil Steril. 2013;100:712–7. doi: 10.1016/j.fertnstert.2013.04.040. [DOI] [PubMed] [Google Scholar]
- 21.Practice Committees of American Society for Reproductive Medicine. Society for Assisted Reproductive Technology Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99:37–43. doi: 10.1016/j.fertnstert.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 22.Hirshfeld-Cytron J, Grobman WA, Milad MP. Fertility preservation for social indications: a cost-based decision analysis. Fertil Steril. 2012;97:665–70. doi: 10.1016/j.fertnstert.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 23.Van Loendersloot LL, Moolenaar LM, Mol BW, Repping S, van der Veen F, Goddijn M. Expanding reproductive lifespan: a cost-effectiveness study on oocyte freezing. Hum Reprod. 2011;26:3054–60. doi: 10.1093/humrep/der284. [DOI] [PubMed] [Google Scholar]
- 24.Hirshfeld-Cytron J, van Loendersloot LL, Mol BW, Goddijn M, Grobman WA, Moolenaar LM, Milad MP. Cost-effective analysis of oocyte cryopreservation: stunning similarities but differences remain. Hum Reprod. 2012;27:3639. doi: 10.1093/humrep/des339. [DOI] [PubMed] [Google Scholar]
- 25.Practice Committee of the American Society of Reproductive Medicine Diagnostic Evaluation of the Infertile Female: A Committee Opinion. Fertil Steril. 2012;98:302–7. doi: 10.1016/j.fertnstert.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 26.Miller JF, Williamson E, Glue J, Gordon YB, Grudzinskas JG, Sykes A. Fetal loss after implantation. A prospective study. Lancet. 1980;2:554–6. doi: 10.1016/s0140-6736(80)91991-1. [DOI] [PubMed] [Google Scholar]
- 27.Florida Institute for Reproductive Medicine [Last accessed: September 7, 2014]; Available at: www.fertilityjacksonville.com.
- 28.NYU Fertility Center [Last accessed September 8, 2014]; Available at: http://www.nyufertilitycenter.org/financial_information.
- 29.Extend Fertility [Last accessed: September 7, 2014]; Available at: www.extendfertility.com/experts/faq.php#cost.
- 30.Egg Freezing Center [Last accessed September 8, 2014]; Available at: http://www.eggfreezingcenter.com/oocyte-cryopreservation.html.
- 31.Cost Helper: Health [Last accessed September 8, 2014]; Available at: http://health.costhelper.com/freezing-eggs.html.
- 32.Livestrong Foundation [Last accessed September 8, 2014]; Available at: http://www.livestrong.org/we-can-help/fertility-services/fertility-preservation-options-women.
- 33.Attain Fertility [Last accessed July 11, 2014]; Available at: www.attainfertility.com/article/ivf-costs.
- 34.RESOLVE: The National Infertility Association [Last accessed September 7, 2014]; Available at: www.resolve.org/family-building-options/insurance_coverage/the-costs-of-infertility-treatment.html.
- 35.Klein J, Sauer MJ. Assessing fertility in women of advanced reproductive age. Am J Obstet Gynecol. 2001;185:758–70. doi: 10.1067/mob.2001.114689. [DOI] [PubMed] [Google Scholar]
- 36.Cil AP, Bang H, Oktay K. Age-specific probability of live birth with oocyte cryopreservation: an individual patient data meta-analysis. Fertil Steril. 2013;100:492–9. doi: 10.1016/j.fertnstert.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richardson WS, Detsky AS. Users’ Guide to the Medical Literature: VII. How to use a clinical decision analysis: A. Are the results of the study valid? JAMA. 1995;273:1292–1295. doi: 10.1001/jama.273.16.1292. [DOI] [PubMed] [Google Scholar]
- 38.Goldman MB, Thornton KL, Ryley D, Alper MM, Fung JL, Hornstein MD, Reindollar RH. A randomized clinical trial to determine optimal infertility treatment in older couples: the Fortyand Over Treatment Trial (FORT-T) Fertil Steril. 2014;101:1574–81. doi: 10.1016/j.fertnstert.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu B, Mumford S, Segars JH, Armstrong AY. Cost-effectiveness analysis comparing continuation of art with conversion to iui in patients with low follicle numbers. Fertil Steril. 2011;96:S2. doi: 10.1016/j.fertnstert.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.