Abstract
Idiopathic pulmonary fibrosis is a severe chronic lung disease with a high mortality rate. Excessive TGF-β signaling is recognized as a central player in lung fibrosis. However, the related mechanisms remain unclear. Herein we used a novel Tbx4 lung enhancer-driven Tet-On transgenic system to inhibit TGF-β signaling in mouse lung resident mesenchymal cells at different stages of bleomycin-induced fibrosis by conditionally knocking out TGF-β receptor II or expressing a dominant-negative TGF-β receptor II. Abrogation of mesenchymal TGF-β signaling markedly attenuated bleomycin-induced fibrotic pathology, which was independent of altered early inflammation. Furthermore, a novel TGF-β downstream target gene P4HA3 (an α-subunit of collagen prolyl hydroxylase) was identified, and its expression was significantly increased in fibroblastic foci of both bleomycin-induced fibrotic mouse lungs and idiopathic pulmonary fibrosis patients’ lungs. The relationship between activated TGF-β signaling, upregulation of P4HA3, as well as increased hydroxyproline/collagen production was further verified in cultured lung fibroblasts. Moreover, inhibition of collagen prolyl hydroxylase by pyridine-2,5-dicarboxylate attenuated both TGF-β-stimulated collagen production in cultured fibroblasts and bleomycin-induced mouse lung fibrosis. These data indicate that increased expression and activity of collagen prolyl hydroxylase is one of the important mechanisms underlying TGF-β-mediated profibrotic effects. Inhibition of collagen prolyl hydroxylase may be a new promising approach for preventing and treating pulmonary fibrosis.
Keywords: TGF-beta signaling, Lung mesenchymal cells, pulmonary fibrosis, collagen prolyl hydroxylase
Introduction
Idiopathic pulmonary fibrosis (IPF) is one of the most severe chronic lung diseases [1]. While two drugs that are able to reduce the progression of mild to moderate IPF are becoming available [2, 3], the need for further targeted and specific therapies for this devastating disease is imperative. Although IPF has been extensively studied, the molecular pathogenic mechanisms remain unclear. Aberrant response and crosstalk between lung epithelial and mesenchymal cells upon injury are two critical processes to initiate uncontrollable myofibroblast accumulation with the formation of the fibroblastic foci and excessive collagen deposition [4, 5].
TGF-β, a well-recognized pro-fibrotic growth factor, is one of the important molecules involved in the aberrant crosstalk between different cell compartments. Increased expression and activation of TGF-β1 has been identified in both IPF patients and experimental models of lung fibrosis [6]. TGF-β ligands are synthesized and secreted in inactive “latent” forms in a complex with latent TGF-β binding protein and bound to the matrix in the tissue. Extracellular matrix stretch and interaction with cell surface integrins are required for TGF-β activation and release. The active TGF-β can then bind to the cognate TGF-β receptor II and I (TβRII and TβRI) complex, initiating Ser/Thr phosphorylation of intracellular downstream Smad2/3 and other non-Smad molecules, subsequently targeting gene transcriptional activation and expression. Studies by us and other groups have shown that global blockade of TGF-β activation or downstream Smad3 activation is able to prevent bleomycin (BLM)-induced lung fibrosis in mice [7–9]. However, in addition to its adverse pro-fibrotic effect, TGF-β signaling is also essential in maintaining tissue homeostasis and appropriate immune reactivity by regulating a variety of cells and cross-talking to many other signaling cascades. Therefore, therapeutic blockade of entire TGF-β signaling in treating lung fibrosis may not be ideal. Direct and specific abrogation of TGF-β-mediated excessive collagen production and deposition will have many therapeutic advantages.
Although it has been reported that abrogation of TβRII in lung alveolar epithelial cells partially prevents BLM-induced lung fibrosis [10], the role of TGF-β signaling in lung mesenchymal cells in mediating collagen production and lung fibrosis is not clear. One major obstacle to perform such a study is the lack of lung mesenchyme-specific genetic targeting tools and the heterogeneous properties of adult lung mesenchymal cells. Recently, we have developed a lung mesenchyme-specific Tet-On transgenic mouse line, in which only lung mesenchymal cells are selectively targeted during lung development [11]. By taking advantage of this unique genetic tool, we generated adult lung mesenchyme-specific TβRII conditional knockout mice as well as dominant-negative TβRII transgenic mice, in which lung mesenchymal TGF-β signaling can be inhibited in certain types of lung resident mesenchymal cells at selected stages of BLM-induced lung fibrosis. We found that blockade of TGF-β signaling in resident lung mesenchymal cells inhibited BLM-induced lung fibrosis in mice. The anti-fibrotic effect of mesenchymal TGF-β signal abrogation was independent of its early anti-inflammatory activity. Furthermore, P4HA3 (an α-subunit of prolyl hydroxylase that is involved in triple helical procollagen synthesis) was identified as a novel TGF-β target gene, and its increased expression was detected in lung fibroblastic foci of BLM-treated mice. Markedly increased P4HA3 expression was also detected in human IPF lungs. The relationship between activated TGF-β signaling, upregulation of P4HA3, as well as increased collagen production was further verified in cultured lung fibroblasts. Moreover, inhibition of collagen prolyl hydroxylase by pyridine-2,5-dicarboxylate significantly reduced procollagen production in cultured fibroblasts, and fibrotic lesion in BLM-damaged mouse lung in vivo. Therefore, prolyl hydroxylase may turn out to be a novel potential therapeutic target to interfere with excessive collagen production and deposition in IPF patients.
Materials and Methods
Mouse strains and breeding
Tbx4-rtTA mouse line was generated in our lab [11]. Floxed-TβRII and TetO-dnTβRII mice were obtained from Mouse Repository at NCI-Frederick and Jackson Laboratory, respectively [12, 13]. TetO-Cre mouse line was originally provided by Dr. Jeffrey Whitsett at Cincinnati Children’s Hospital [14]. Lung mesenchymal-specific TβRII conditional knockout mice (TβRIIfx/fx/Tbx4-rtTA/TetO-Cre) were generated by crossing TβRIIfx/fx and TβRIIfx/+/Tbx4-rtTA/TetO-Cre mice with Dox induction. Lung mesenchymal-specific expression of dominant-negative TβRII transgenic mice (Tbx4-rtTA/TetO-dnTβRII) were generated by crossing TetO-dnTβRII and Tbx4-rtTA mice with doxycycline (Dox) induction as described above. All mice were bred in the C57BL/6 strain background, and the studies were approved by Institutional Animal Care and Use Committee at the Saban Research Institute of Children’s Hospital Los Angeles.
Lung tissue biopsy specimens from de-identified IPF patients and donor
Three IPF patients (males, 63±2 years old) were evaluated for expression of P4HA3 by immunohistochemistry. Diagnosis of IPF was established according to the ATS/ERS/JRS/ALAT statement based on the pattern of usual interstitial pneumonia determined by HRCT and open lung biopsy [15]. Slides from two morphologically normal lungs (two males, 55 and 63 years old) were used as controls. The study was approved by the Bioethics Committee (IRB) at the National Institute of Respiratory Diseases, Mexico.
Bleomycin-induced pulmonary fibrosis model and PDC treatment
Anesthetized 8-week-old mice as described above were administered with 2.5 or 4 u/kg BLM (Sigma, St. Louis, MO) diluted in 120μL of saline, or saline alone by intratracheal instillation using an intratracheal aerosolizer (MicroSprayer® Aerosolizer - Model IA, Penn-Century, Wyndmoor, PA [16]) at day 0. The mouse lungs were then harvested at different time points (7, 14, and 28 Days post BLM treatment). Lung mesenchymal-specific transgenic expression (Cre or dnTβRII) was induced by Dox administration (625mg/kg food, TestDiet, and 0.5 mg/ml drinking water, Sigma-Aldrich). Different starting time points relatively to BLM challenge were chosen for Dox induction. 10 mM PDC (Sigma) was prepared in PBS, and delivered to the lungs by the intratracheal aerosolizer.
Lung histopathology and semi-quantitative evaluation
Lung tissues were fixed in 4% paraformaldehyde and 5 µm paraffin tissue sections were stained with hematoxylin and eosin (H&E) or Sirius Red [17]. Lung inflammation on Day 7 was evaluated using a semi-quantitative method, as previously described [18, 19]. Scores of 0–5 were generated based on the numbers of inflammatory foci in peri-vascular, peri-bronchiolar, as well as alveolar regions under high power field (200X magnification, 0= no inflammatory foci, 1=≤5, 2=6–15, 3=16–25, 4=26–35, 5=≥35). Thus, the total inflammation score is the sum of these three measurements in a range of 0–15, presented by mean ± SEM. Lung fibrosis on Day 14 and Day 28 was also evaluated using Ashcroft semi-quantitative scoring system [7, 20].
Hydroxyproline assay and immunohistochemisty
Collagen contents of the lung tissues or the cultured cells were evaluated by measuring hydroxyproline according to the manufacturer’s instructions of a commercial assay kit (Biovision, Mountview, CA).
Immunostaining of paraffin lung sections was performed following our previously published protocol [7]. Rabbit anti-P4HA3 was purchased from EnoGene (E305231).
Cell culture, inhibitor treatment, shRNA Lentiviral particle transduction, and analysis
Primary lung fibroblast cell was isolated from 3 month-old wild type mice. Briefly, aseptically minced lung tissues were digested with 0.3 mg/ml of type IV collagenase and 0.5 mg/ml of trypsin for 1 hour at 37°C with intermittent agitation. The cell suspension was filtered through a 70 um cell strainer to remove undigested tissue mass, then centrifuged and resuspended in F-12K medium containing 10%FBS, 1% penicillin/streptomycin, and 200 mM L-glutamine. Cells were incubated in plastic flask at 37°C for 1 hour, non-adherent cells were removed. Viable fibroblasts attached to the flask to grow and began to exhibit the spindle-shape in 2 to 3 days. Human lung fibroblast cell line HLF1 was obtained from ATCC (CCL-153).
5×105 of the above cells were seeded to 100mm dish and cultured for 48 hours, followed by 24-hour starvation before stimulation with 5 ng/ml TGF-β1 (Cell Signaling). To inhibit TGF-β pathway or P4H activity, cultured cells were pre-treated with media containing 1 µM of SB-505124 (Sigma) or 7.2 mM PDC for 1h prior to TGF-β1 stimulation. The cells were then incubated with TGF-β1 for 24 hours, and harvested for analyses of RNA, protein, and hydroxyproline. To determine whether TGF-β1-induced P4HA3 gene expression is dependent on protein or RNA synthesis, cultured cells were pretreated with 30 μg/ml of cycloheximide (Sigma) or 5 μg/ml of actinomycin D (Sigma) for 1 hour prior to TGF-β1 stimulation, and P4HA3 gene expression was analyzed after 4 hours.
To knock down P4HA3 expression in cultured HLF1 cells, human P4HA3 shRNA Lentiviral particles (sc-97000-V) and scramble control shRNA Lentiviral particles (sc-108080) were obtained from Santa Cruz Biotechnology. The cells were transduced with shRNA Lentiviral particles according to the manufacturer’s protocol, and followed by two-week selection with 4 µg/ml puromycin (Santa Cruz Biotechnology). TGF-β treatment and analyses of these cells were then conducted as described above.
Measurement of gene expression by real-time PCR and western blot
Total RNA was isolated from snap-frozen lung tissues using the RNeasy kit (Qiagen). cDNAs were synthesized and quantified using iScript kit and SsoFast EvaGreen Supermix (Bio-Rad), as described previously [21]. The primers for P4HA3 are 5’-GAG TAC CGC ATC AGC AAA AG -3’ and 5’-CCC TCC GAT TCC ATA GTT CAC-3’.
Detection of lung tissue proteins has been previously described [22]. Proteins of interest were detected using the following antibodies: pSmad3 (#600-401-919, Rockland), Smad3 (#ab28379, Abcam), P4HA3 (#E305231, EnoGene), HIF-1α (#610958, BD Bioscience), Collagen Type I (#600-401-103, Rockland), and GAPDH (# 10R-G109A, Fitzgerald).
Data presentation and statistical analysis
At least 6 mice were analyzed in each experimental subgroup. Genetically manipulated mice and wild type mice were littermates. All quantitative data were expressed as mean ± SEM. Statistical difference between two independent groups was assessed by an independent samples t-test; p-values ≤0.05 were considered statistically significant.
Results
Specific inhibition of lung mesenchymal TGF-β signaling significantly attenuated bleomycin-induced early inflammation as well as later fibrosis in mouse lung
TGF-β signaling has cell-type specific functions, and plays complex roles under both physiological and pathological conditions. The pathogenic mechanisms of altered TGF-β signaling in lung mesenchymal compartment during lung fibrosis are not carefully studied due to absence of genetic approaches to specifically target lung mesenchymal cells. Using a Tbx4 lung enhancer driven Tet-On system recently developed in our lab [11], we were able to manipulate TGF-β signaling in adult lung mesenchymal cells by two different genetic approaches. One was Dox-induced conditional knockout (CKO) of TβRII in mice with genotypes of Tbx4-rtTA/TetO-Cre/ TβRIIfx/fx [12], and the other was Dox-induced transient expression of a dominant-negative TβRII (dnTβRII) transgene in mice with genotypes of Tbx4-rtTA/TetO-dnTβRII [13]. Unlike TβRII CKO, dnTβRII-mediated inhibition of TGF-β signaling is reversible upon withdrawal of Dox induction.
In order to determine the role of TGF-β signaling in Tbx4-rtTA-targeted mesenchymal cells during lung injury-induced fibrotic repair, endogenous TGF-β signaling in these lung cells was inhibited by Dox induction in either TβRII CKO or dnTβRII transgenic mice starting 7 days before intra-tracheal instillation of BLM. Lung specimens from the mice treated with BLM or saline control were obtained at specific time points after BLM injury, such as day 7 to capture the inflammatory stage, and day 14 and 28 for the fibrotic stage. Reduced expression of endogenous TβRII or induction of transgenic dnTβRII-c-Myc protein in entire lung tissue lysates was verified (Fig.1A), and the related changes in TGF-β signaling activity were compared by measuring Smad3 phosphorylation among BLM-treated mouse lungs with different genotypes (Fig.1A). Compared to BLM-injured wild type (WT) lungs, Smad3 phosphorylation was significantly attenuated both in TβRII CKO as well as in dnTβRII lungs, particularly from day 7 to day 14 after BLM challenge. Overall lung fibrosis at day 28, shown by collagen staining of the left lung (Fig.1B), was significantly inhibited in both TβRII CKO and dnTβRII transgenic lungs. No phenotypic difference of lung fibrosis was observed between the BLM-injured WT mice with Dox treatment and the mice without Dox treatment (Supplemental Fig.1), suggesting that reduced fibrosis in both BLM-challenged TβRII CKO and dnTβRII mouse lungs is due to specific inhibition of mesenchymal TβRII activity, but not caused by Dox treatment [23].
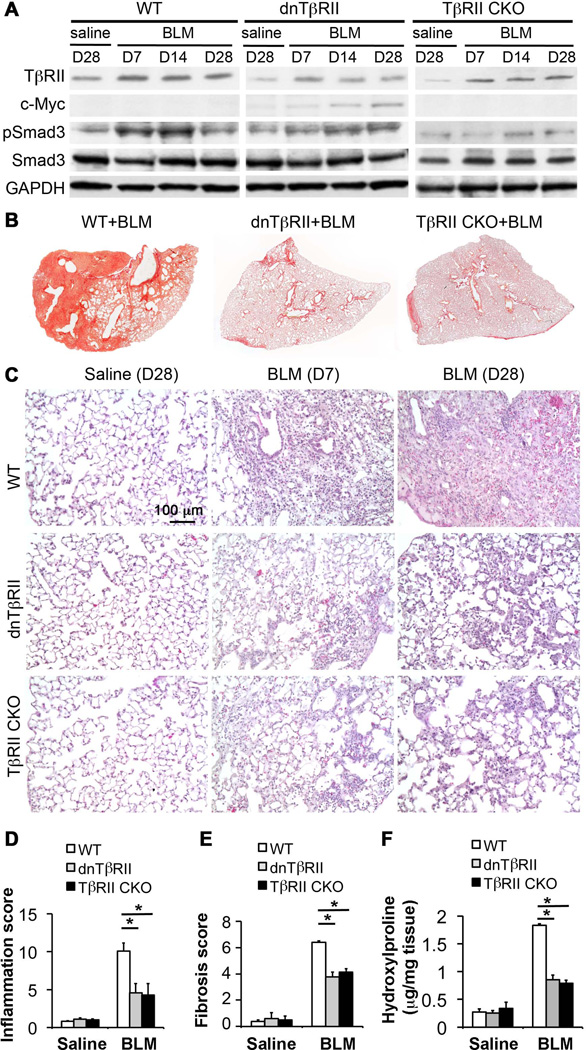
Fig.1. Reduced TGF-β signaling activity in lung mesenchyme resulted in attenuation of pulmonary inflammation and fibrosis upon bleomycin injury.
(A) Dynamic changes of TβRII, Smad3 protein and activation (phosphorylation) in mouse lung tissues with indicated genotypes after BLM-mediated lung injury. C-Myc is the epitope tag for dnTβRII. GAPDH is a loading control. (B) Sirius Red collagen staining of the mouse lungs with indicated genotypes, which were harvested 28 days after BLM treatment. (C) Histopathology, shown by H&E staining, of the mouse lungs with indicated genotypes at early inflammation stage versus late fibrotic stage. (D) Inflammation was evaluated based on histopathology with scores from 0 (no inflammation) to 15 (worst inflammation). (E) Lung fibrosis was quantified using Ashcroft method. (F) The overall level of lung tissue collagen was measured based on the content of hydroxyproline, presented as hydroxyproline (µg) per mg lung tissue. *P<0.05.
Short-term blockade of lung mesenchymal TβRII-mediated signaling did not result in any changes in lung structure and the basal level of collagen in the saline-treated control groups of both TβRII CKO and dnTβRII mice (Fig.1C–1F). Interestingly, constant inhibition of lung mesenchymal TGF-β signaling starting before BLM challenge significantly inhibited, but did not fully block early lung inflammation, shown by lung histology on day 7 (Fig.1C), and the related inflammatory scoring (Fig.1D). Likewise, continuous blockade of lung mesenchymal TβRII activity resulted in significant attenuation of fibrotic pathology at day 28 (Fig.1C). Semi-quantitative Ashcroft fibrosis score in WT+BLM group was significantly higher than both dnTβRII+BLM group and TβRII CKO+BLM group (Fig.1E). Consistently, increase of collagen in lung tissues, as measured by hydroxyproline content, was remarkably attenuated in dnTβRII+BLM and TβRII CKO+BLM groups compared to WT+BLM group (Fig.1F).
Lung mesenchymal TGF-β signaling in the fibrotic stage, but not the inflammatory stage, is critical to the development of bleomycin-induced pulmonary fibrosis
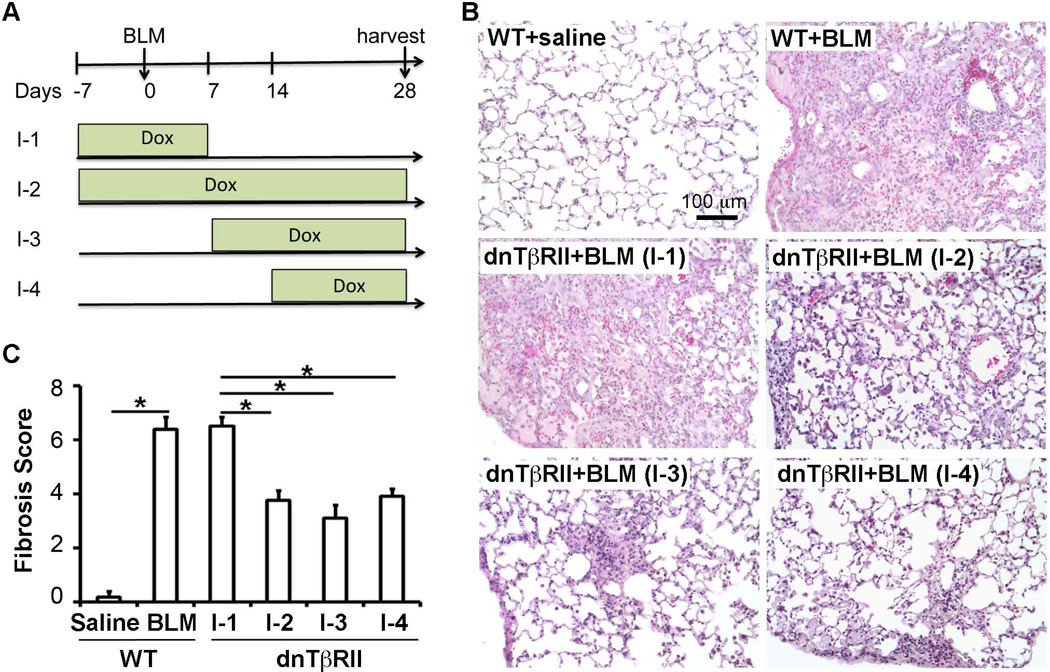
As shown in Fig.1, inhibition of lung mesenchymal TGF-β signaling prior to BLM challenge significantly attenuated both BLM-induced early lung inflammation and later lung fibrosis. In this context, it is important to determine whether the reduction in lung fibrosis by blocking lung mesenchymal TGF-β signaling is due to inhibition of lung inflammation at the early stage or direct inhibition of the later fibrotic process, or both. By taking advantage of lung mesenchyme-specific reversible inhibition of TβRII activity in this inducible dnTβRII transgenic mouse model, dnTβRII expression was induced at different stages of BLM-induced lung fibrosis, as illustrated in Fig.2A. Compared to full course induction of dnTβRII from 7 days before BLM-challenge to day 28, dnTβRII expression was selectively restricted to (1) 7 days before BLM-challenge to day 7; (2) day 7 to day 28; and (3) day 14 to day 28. Interestingly, inhibition of lung mesenchymal TGF-β signaling at the initiation phase of BLM-induced injury (7 days before BLM to day 7), a period characterized by acute lung inflammation, had no effect on the severity of fibrosis (I-1 in Fig.2). In contrast, mesenchymal expression of dnTβRII starting from day 7 or day 14 to day 28 resulted in significant reduction of lung fibrosis (I-3 or I-4 in Fig.2), a phenotypic effect similar to that seen in the lungs with dnTβRII induction from 7 days before BLM injury to day 28 (I-2 in Fig.2). This suggests that mesenchymal TGF-β signaling plays critical role in later fibrotic collagen production and deposition, independent of its effect on early inflammatory response.
Fig.2. Lung mesenchymal TGF-β signal at late fibrotic stage, but not early inflammatory stage, contributed to lung fibrosis.
(A) Lung mesenchymal TGF-β signaling was inhibited at different stages in BLM-induced lung fibrosis by controlling Dox-induced dnTβRII expression as indicated; I-1: 7 days before BLM to day 28 after BLM, I-2: 7 days before BLM to day 7 after BLM, I-3: Day 7 to day 28 after BLM, I-4: Day 14 to day 28 after BLM. (B) Fibrotic histopathology was shown by H&E-stained lung tissue sections, with indicated genotypes and Dox induction scheme. (C) Fibrotic changes were quantitatively compared using Ashcroft scores among the lungs with dnTβRII-mediated inhibition at different stages after BLM injury. *P<0.05.
Mesenchymal TGF-β signaling upregulates P4HA3, a subunit of collagen prolyl hydroxylase, during bleomycin-induced lung fibrosis
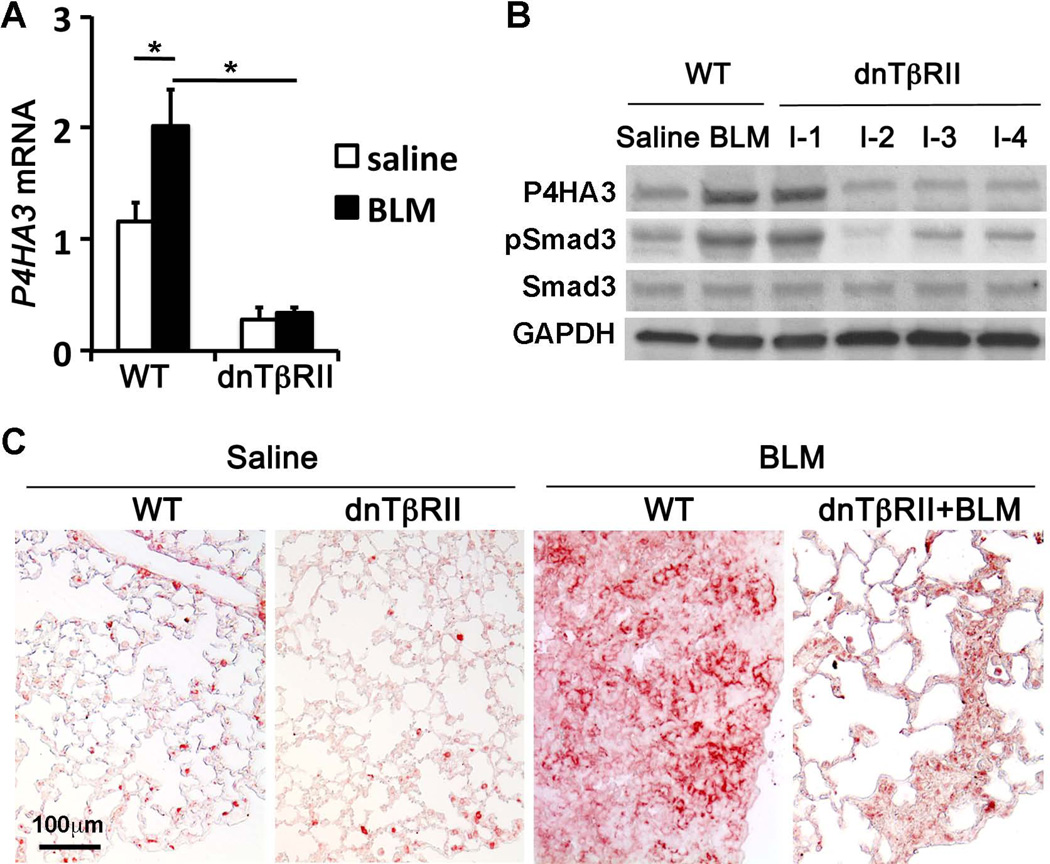
In order to understand the molecular mechanisms by which elevated mesenchymal TGF-β signaling causes excessive collagen production and deposition in BLM-induced lung fibrosis, we compared gene expression for many potential TGF-β target molecules that are directly involved in collagen production between BLM-treated WT lungs with severe fibrosis and dnTβRII transgenic lungs with attenuated fibrosis. Interestingly, expression of prolyl 4-hydroxylase, α polypeptide III (P4HA3) was significantly and constantly increased in WT+BLM lungs at both mRNA and protein levels, compared to saline controls even at day 28 after BLM injury (Fig.3A–3B), while inhibition of TGF-β signaling in dnTβRII+BLM lungs resulted in significant reduction of P4HA3 expression to the level similar to that in non-fibrotic lungs of saline controls (Fig.3A–3B). Furthermore, by immunohistochemistry we verified that increased P4HA3 was mainly localized in the fibroblastic foci of day 28 WT+BLM lungs (Fig.3C).
Fig.3. Altered expression of P4HA3 was associated with changed TGF-β pathway activity and lung fibrosis induced by BLM treatment.
Lung tissues were harvested at day 28 after BLM challenge from the WT or dnTβRII transgenic mice with Dox induction from 7 days before BLM treatment to the end. (A) Expression of P4HA3 at the mRNA level was measured by real-time PCR. (B) Expression of P4HA3 at the protein level was detected by western blot. Smad3 phosphorylation was used to evaluate TGF-β pathway activity. GAPDH was used as a loading control. (C) P4HA3 was upregulated in fibrotic foci of BLM-treated WT mouse lung at day 28, which was significantly reduced in dnTβRII lungs where fibrosis was reduced.
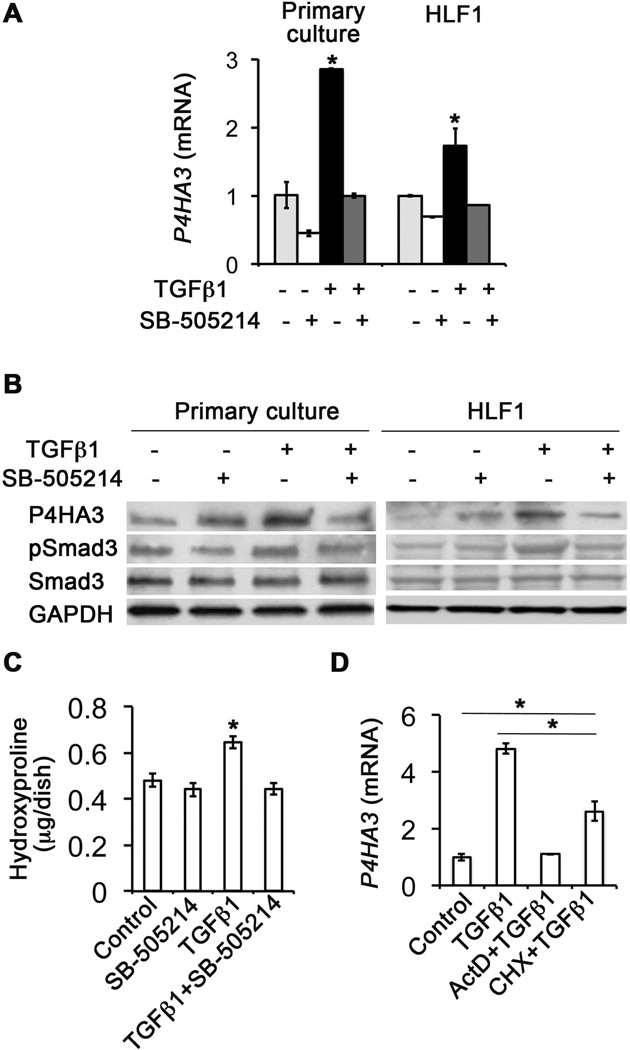
P4HA3 is an α subunit of prolyl 4-hydroxylases (P4Hs) [24, 25]. P4Hs catalyze formation of 4-hydroxyproline that is essential for triple helix procollagen synthesis in endoplasmic reticulum [26]. The procollagen is then exported, and further processed to form tropocollagen and collagen fibril in extracellular space. Therefore, proline hydroxylation of collagen propeptide is one of the key steps for final collagen fibril formation and deposition. Thus, the relationship between TGF-β signaling, P4HA3 expression and collagen production in mouse lung primary fibroblasts as well as in a human lung fibroblast cell line HLF1 (Fig.4) was investigated. Not surprisingly, addition of TGF-β1 to the cell culture medium significantly upregulated expression of P4HA3 at both mRNA and protein levels (Fig.4A–4B). Blockade of intracellular TGF-β signaling activation by adding TGF-β receptor I inhibitor SB-505124 simultaneously inhibited TGF-β-induced phosphorylation of Smad3 and expression of P4HA3, as well as proline hydroxylation of the cell lysate protein (Fig.4C), supporting that TGF-β pathway specifically stimulates P4HA3 expression and protein proline hydroxylation. Interestingly, P4HA3 gene expression induced by exogenous TGF-β1 in cultured lung fibroblast was reduced, but not blocked, by pretreating cells with protein synthesis inhibitor cycloheximide (Fig.4D). In contrast, inhibition of gene transcription by pretreating cells with actinomycin D fully blocked TGF-β1-induced P4HA3 expression. Therefore, TGF-β-induced P4HA3 expression may be mediated by both direct and indirect mechanisms.
Fig.4. TGF-β1 specifically stimulated P4HA3 expression and collagen production in cultured mouse lung primary fibroblasts and human lung fibroblast cell line HLF1.
(A) P4HA3 expression at the mRNA level was quantified by real-time PCR. Additions of TGF-β (5 ng/ml) and its TβRI inhibitor SB-505214 (1 µM) were indicated. (B) P4HA3 protein was increased upon TGF-β1 stimulation for 24 hours, which could be blocked by SB-505124. (C) Increased collagen production, detected by the content of hydroxyproline, was also associated with elevated expression of P4HA3 upon TGF-β stimulation, which was also inhibited by SB-505124 in mouse lung primary fibroblasts. (D) P4HA3 gene expression was quantified by real-time PCR. TGF-β-induced P4HA3 expression was partially, but not fully, inhibited by pretreating cells with a protein synthesis inhibitor cycloheximide (30 μg/ml). In contrast, TGF-β-stimulated P4HA3 expression was fully abrogated by pretreating cells with a RNA synthesis inhibitor actinomycin D (5 μg/ml). *P<0.05.
The relationship between TGF-β-induced P4HA3 expression and TGF-β pro-fibrotic effects was also evaluated in cultured human lung fibroblast, where P4HA3 expression was knocked down by transducing the cells with P4HA3 shRNA Lentiviral particles (Supplemental Fig.2). Compared to scramble shRNA control, knock down of P4HA3 expression by P4HA3 shRNA specifically attenuated TGF-β1-induced secretion of collagen precursor (Supplemental Fig.2), suggesting that P4HA3 is one of the downstream targets that mediate TGF-β1 profibrotic effects.
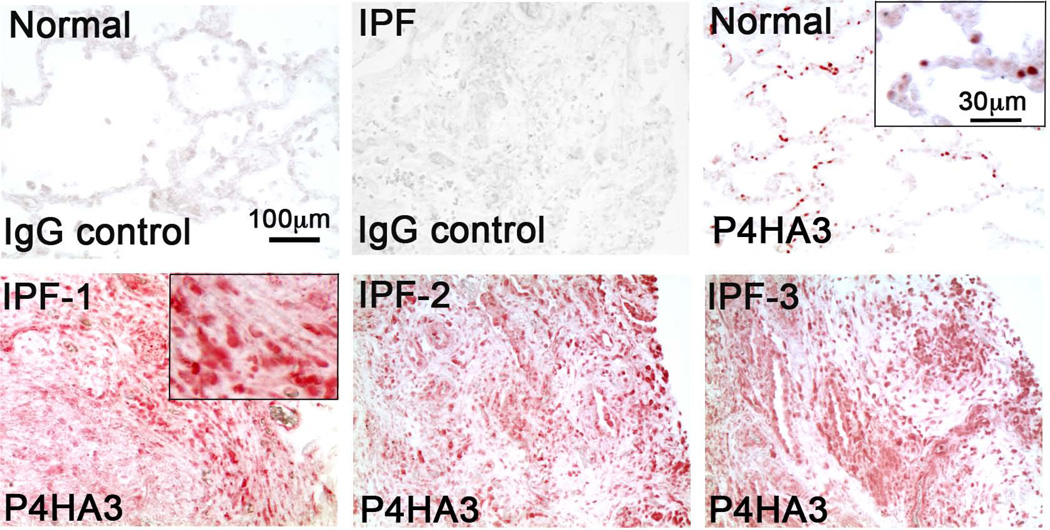
Increased P4HA3 expression was also detected in fibroblastic foci of human IPF lungs
In order to evaluate the role of P4HA3 in lung fibrosis of IPF patients, de-identified human IPF lung biopsies were therefore stained with a specific P4HA3 antibody to compare its expression and localization in IPF lungs with non-fibrotic lungs. Interestingly, increased P4HA3 staining was detected in IPF fibrotic lung, particularly close to the edge of fibroblastic foci (Fig.5). This suggests that P4HA3 may play an important role in mediating excessive collagen synthesis and deposition in human IPF, similar to its role in BLM-induced lung fibrosis in mice.
Fig.5. Increased P4HA3 protein was detected in human IPF lung biopsy.
Lung tissue sections from normal donors and IPF patients were stained by rabbit anti-P4HA3 antibody (red). Increased P4HA3 staining was seen in IPF lungs. Normal rabbit IgG was used as a negative control for immunostaining of both normal and IPF lungs. Higher magnification pictures are included as inserts.
Inhibition of prolyl 4-hydroxylase attenuated TGF-β induced collagen production in vitro and bleomycin-induced lung fibrosis in vivo
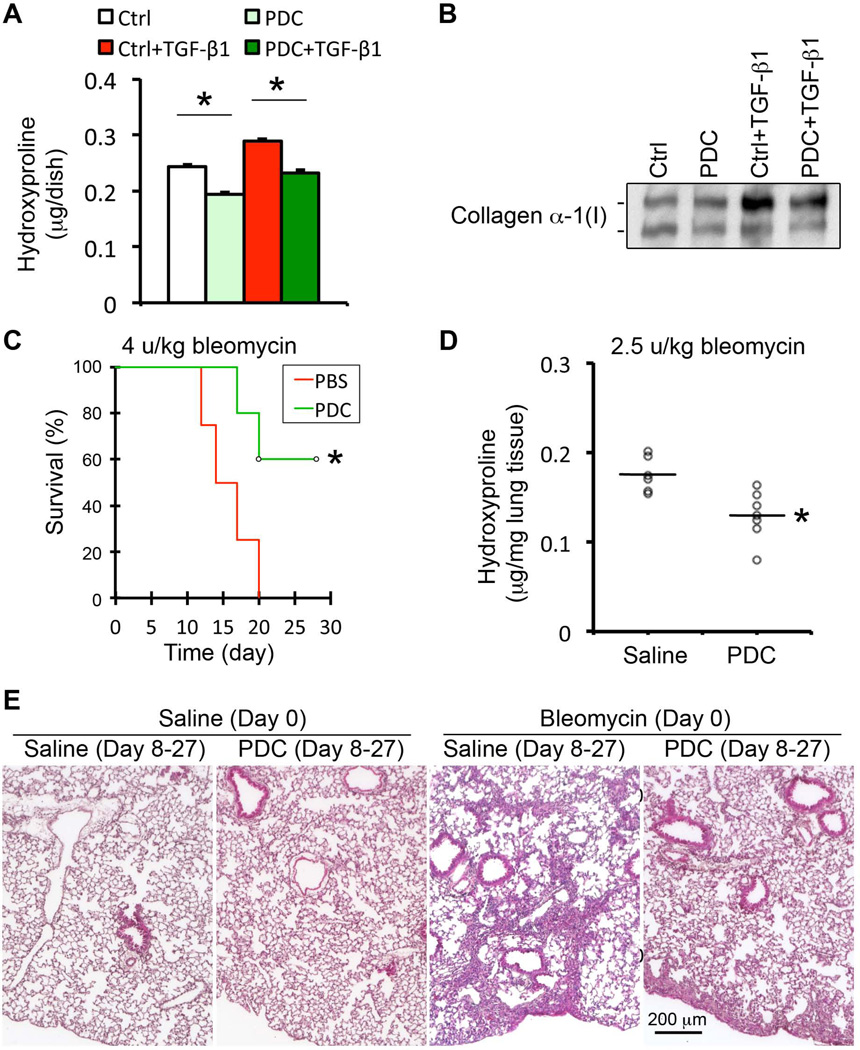
The collagen proline hydroxylation catalyzed by the P4H in endoplasmic reticulum requires Fe2+, 2-oxoglutarate, O2, and ascorbate. Thus, a number of analogues of 2-oxoglutarate are able to inhibit P4H competitively. Pyridine 2,5-dicarboxylate (PDC) is known as a specific inhibitor of the collagen P4H with 0.8 µM of Ki (the concentration required to produce half maximum inhibition), but does not inhibit HIF-P4Hs (>300 µM of Ki) in a simple enzymatic reaction system [27]. We then tested whether addition of PDC to the cultured human lung fibroblasts was able to inhibit TGF-β-induced collagen production. By measuring cellular hydroxyproline and secreted collagen in the conditioned medium of cultured HLF1, we found that 7.2 mM of PDC was able to inhibit both cellular endogenous proline hydroxylation and TGF-β-induced increase in proline hydroxylation (Fig.6). Consistently, increased secretion of collagen precursor to the conditioned medium by TGF-β stimulation was also significantly inhibited by PDC treatment (Fig.6B). Since inhibition of HIF-P4H activity could reduce HIF-1α proline hydroxylation and subsequently decrease HIF-1α ubiquitination and protein degradation, we then examined the protein level of intracellular HIF-1α protein to determine whether the PDC used here also inhibited HIF-P4H in cultured lung fibroblasts. As shown in Supplemental Fig.3, HIF-1α protein level was not increased upon PDC treatment, indicating that the cellular HIF-P4H activity was not inhibited. These data suggested that PDC was able to penetrate into cell and specifically inhibit collagen hydroxylation and secretion to outside cells.
Fig.6. Inhibition of collagen prolyl hydroxylase by PDC significantly reduced collagen production in vitro and attenuated BLM-induced lung fibrosis in vivo.
(A–B) Inhibition of collagen prolyl hydroxylase by PDC (7.2 mM) in cultured human lung fibroblast cell line HLF1 significantly reduced TGF-β-stimulated proline hydroxylation (A) in cell lysate, and collagen production (B) detected in the conditioned medium. (C) Intratracheal delivery of PDC significantly reduced the mortality rate in high dose BLM-challenged mice during fibrotic stage. (D–E) Local delivery of PDC also significantly attenuated BLM-induced mouse lung fibrosis, shown by lung tissue hydroxyproline measurement (D) and fibrotic histopathology in H&E stained lung tissue sections (E).
We then evaluated PDC as a potential anti-fibrotic agent in BLM-induced mouse lung fibrosis. In order to avoid systemic effects of PDC-mediated inhibition of collagen hydroxylation, we chose local delivery of PDC by intra-tracheal spray starting from day 8 after BLM-induced injury. 100 µl of 10 mM PDC was given to the mouse every other day until day 18, and then every other 2 days to the end of the study (day 28). Our results showed that administration of PDC significantly reduced high dose BLM-induced mortality rates (Fig.6C), and low dose BLM-induced lung fibrosis, shown by the reduced content of hydroxyproline and fibrotic histopathology (Fig.6D–6E). Moreover, PDC treatment did not cause any detectable adverse effect in the normal lung (Fig.6E), suggesting that the dose (100 µl of 10 mM PDC per local delivery) used in this experiment was acceptably safe.
Discussion
TGF-β signaling has profound physiologic and pathologic effects. Although hyperactive TGF-β signaling mediates excessive collagen production and deposition during lung fibrosis [28], full blockade of TGF-β pathways results in disruption of tissue function and homeostasis, such as excessive inflammation and an emphysema-like phenotype in the lung [22, 29]. Therefore, effective and safe drugs targeting TGF-β signaling have not been successfully developed [30]. Answers to questions regarding where, when and how excessive TGF-β signaling mediates lung fibrosis are urgently needed.
Lung mesenchyme in adult is quite heterogeneous, including airway and vascular smooth muscle cells, lipofibroblasts, myofibroblasts, pericytes, etc. Hoyles et al have used a Col1A2-CreERT driver line to knockout TβRII in all fibroblasts in the entire body from postnatal day 7 to 14, and studied BLM-induced lung fibrosis in these knockout mice at ages 6–8 weeks [31]. Although significant reduction of lung fibrosis was observed at day 14, there was no clue as to whether TGF-β signaling in fibroblasts per se was involved in initiation versus progression of pulmonary fibrosis. Furthermore, mesenchymal cells from other organs/systems including circulating fibrocytes were also targeted by Col1A2-CreERT driver line. In order to specifically manipulate TGF-β signaling in lung resident mesenchymal cells in vivo at selected stages of experimental lung fibrosis, we used a unique Tbx4-lung enhancer driven Tet-On genetic tool to target lung resident mesenchymal cells [11]. We found that the majority of cells targeted by the Tbx4-lung enhancer in the adult lung were positive for NG2 (Supplemental Fig.4), a marker for pericytes, which are known to be expanded in fibrotic lungs of mouse BLM models as well as in IPF patients [32, 33]. The cellular pattern of Tbx4 lung enhancer activation remained the same in BLM-induced fibrotic mouse lung (Supplemental Fig.4).
As shown in our study, inhibition of TGF-β signaling in this lung mesenchymal cell subpopulation during the early inflammatory stage of BLM injury had no significant impact on later lung fibrosis, although significant attenuation of pulmonary inflammation was observed. Blockade of lung-specific mesenchymal TGF-β signaling even 14 days after BLM injury was still able to attenuate lung fibrosis examined at day 28, although the fibrotic process is thought to be initiated around day 10 in this BLM-induced lung fibrosis model. This suggests that lung mesenchymal TGF-β-mediated fibrotic progression is independent of the early inflammatory processes and even fibrotic initiation. This may be very important in helping design new therapeutic strategies to stop progression of lung fibrosis in IPF patients.
In order to understand the molecular mechanisms by which excessive TGF-β signaling promotes aberrant collagen production and deposition during injury repair, we found a novel TGF-β target gene P4HA3 that is directly involved in the key step of collagen synthesis. More importantly, significant increase of P4HA3 expression was also detected in IPF patients’ lungs, particularly within fibroblastic foci. P4HA3 is one isoform of the prolyl hydroxylase α-subunits [25], and is able to catalyze the formation of 4-hydroxyproline in Xxx-Pro-Gly triplets of collagen pro-peptides in the endoplasmic reticulum. Hydroxyproline is essential for forming triple helix pro-collagen before it is secreted into the extracellular matrix and converted to tropocollagen and then collagen fibrils. The relationship between activated TGF-β signaling and induction of P4HA3 expression was further verified in mouse and human lung fibroblast cultures. Moreover, for the first time, we have shown that PDC is able to inhibit intracellular collagen P4H and reduce collagen production and deposition, and in particular, local lung delivery of PDC can attenuate BLM-induced pulmonary fibrosis in mice. This suggests that local inhibition of collagen P4H may turn out to be a promising new therapeutic target to intervene in the vicious cycle of aberrant TGF-β signal activation and excessive collagen production during the progression of pulmonary fibrosis. However, the optimal regimen of PDC usage in anti-fibrotic treatment and its toxicological effects in vivo remain to be further investigated as higher doses of PDC may affect both collagen P4H and HIF-P4H activity.
Supplementary Material
Acknowledgements
This work was supported by NIH Grants HL109932 and HL068597 (WS), California Institute of Regenerative Medicine Training Grant to DW supporting YL and WX, a pilot research grant of the Saban Research Institute of Children’s Hospital Los Angeles (WS), and the Canadian Institute for Health Research (MK).
Footnotes
No conflict of interest were declared
Author Contribution Statement
Concept and design, Y.L., W.S., Acquisition of data, Y.L., W.X., R.D., H.C., B.Q., M.S., W.S. Analysis and interpretation, D.W., M.S., J.G., M.K., W.S., Drafting of the manuscript: Y.L., W.X., W.S.
References
- 1.Ley B, Collard HR, King TE. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:431–440. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 2.King TE, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 3.Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 4.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 5.King TE, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–1961. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez IE, Eickelberg O. New cellular and molecular mechanisms of lung injury and fibrosis in idiopathic pulmonary fibrosis. Lancet. 2012;380:680–688. doi: 10.1016/S0140-6736(12)61144-1. [DOI] [PubMed] [Google Scholar]
- 7.Zhao J, Shi W, Wang YL, et al. Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. AmJPhysiol Lung Cell MolPhysiol. 2002;282:L585–L593. doi: 10.1152/ajplung.00151.2001. [DOI] [PubMed] [Google Scholar]
- 8.Bonniaud P, Kolb M, Galt T, et al. Smad3 null mice develop airspace enlargement and are resistant to TGF-beta-mediated pulmonary fibrosis. J Immunol. 2004;173:2099–2108. doi: 10.4049/jimmunol.173.3.2099. [DOI] [PubMed] [Google Scholar]
- 9.Sheppard D. Integrin-mediated activation of latent transforming growth factor beta. Cancer Metastasis Rev. 2005;24:395–402. doi: 10.1007/s10555-005-5131-6. [DOI] [PubMed] [Google Scholar]
- 10.Li M, Krishnaveni MS, Li C, et al. Epithelium-specific deletion of TGF-β receptor type II protects mice from bleomycin-induced pulmonary fibrosis. J Clin Invest. 2011;121:277–287. doi: 10.1172/JCI42090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang W, Menke DB, Jiang M, et al. Spatial-temporal targeting of lung-specific mesenchyme by a Tbx4 enhancer. BMC Biol. 2013;11:111. doi: 10.1186/1741-7007-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chytil A, Magnuson MA, Wright CV, et al. Conditional inactivation of the TGF-beta type II receptor using Cre:Lox. Genesis. 2002;32:73–75. doi: 10.1002/gene.10046. [DOI] [PubMed] [Google Scholar]
- 13.Frugier T, Koishi K, Matthaei KI, et al. Transgenic mice carrying a tetracycline-inducible, truncated transforming growth factor beta receptor (TbetaRII) Genesis. 2005;42:1–5. doi: 10.1002/gene.20115. [DOI] [PubMed] [Google Scholar]
- 14.Perl AK, Wert SE, Nagy A, et al. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci U S A. 2002;99:10482–10487. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maytal Bivas-Benita RZ, Hans E. Junginger, Gerrit borchard. non-invasive pulmonary aerosol delivery in mice by the endotracheal route. european Journal of Pharmaceutics and Biopharmaceutics. 2005:214–218. doi: 10.1016/j.ejpb.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Tarantal AF, Chen H, Shi TT, et al. Overexpression of transforming growth factor-beta1 in fetal monkey lung results in prenatal pulmonary fibrosis. Eur Respir J. 2010;36:907–914. doi: 10.1183/09031936.00011810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu W, Lan Q, Chen M, et al. Adoptive transfer of induced-Treg cells effectively attenuates murine airway allergic inflammation. PloS one. 2012;7:e40314. doi: 10.1371/journal.pone.0040314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richards IM, Kolbasa KP, Winterrowd GE, et al. Role of intercellular adhesion molecule-1 in antigen-induced lung inflammation in brown Norway rats. The American journal of physiology. 1996;271:L267–L276. doi: 10.1152/ajplung.1996.271.2.L267. [DOI] [PubMed] [Google Scholar]
- 20.Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988;41:467–470. doi: 10.1136/jcp.41.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun J, Chen H, Chen C, et al. Prenatal lung epithelial cell-specific abrogation of Alk3-bone morphogenetic protein signaling causes neonatal respiratory distress by disrupting distal airway formation. The American journal of pathology. 2008;172:571–582. doi: 10.2353/ajpath.2008.070286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H, Sun J, Buckley S, et al. Abnormal mouse lung alveolarization caused by Smad3 deficiency is a developmental antecedent of centrilobular emphysema. Am J Physiol Lung Cell Mol Physiol. 2005;288:L683–L691. doi: 10.1152/ajplung.00298.2004. [DOI] [PubMed] [Google Scholar]
- 23.Fujita M, Ye Q, Ouchi H, et al. Doxycycline attenuated pulmonary fibrosis induced by bleomycin in mice. Antimicrobial agents and chemotherapy. 2006;50:739–743. doi: 10.1128/AAC.50.2.739-743.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Den Diepstraten C, Papay K, Bolender Z, et al. Cloning of a novel prolyl 4-hydroxylase subunit expressed in the fibrous cap of human atherosclerotic plaque. Circulation. 2003;108:508–511. doi: 10.1161/01.CIR.0000080883.53863.5C. [DOI] [PubMed] [Google Scholar]
- 25.Kukkola L, Hieta R, Kivirikko KI, et al. Identification and characterization of a third human, rat, and mouse collagen prolyl 4-hydroxylase isoenzyme. J Biol Chem. 2003;278:47685–47693. doi: 10.1074/jbc.M306806200. [DOI] [PubMed] [Google Scholar]
- 26.Myllyharju J. Prolyl 4-hydroxylases, the key enzymes of collagen biosynthesis. Matrix Biol. 2003;22:15–24. doi: 10.1016/s0945-053x(03)00006-4. [DOI] [PubMed] [Google Scholar]
- 27.Myllyharju J. Prolyl 4-hydroxylases, key enzymes in the synthesis of collagens and regulation of the response to hypoxia, and their roles as treatment targets. Ann Med. 2008;40:402–417. doi: 10.1080/07853890801986594. [DOI] [PubMed] [Google Scholar]
- 28.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 29.Kulkarni AB, Huh CG, Becker D, et al. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kisseleva T, Brenner DA. Mechanisms of fibrogenesis. Exp Biol Med (Maywood) 2008;233:109–122. doi: 10.3181/0707-MR-190. [DOI] [PubMed] [Google Scholar]
- 31.Hoyles RK, Derrett-Smith EC, Khan K, et al. An essential role for resident fibroblasts in experimental lung fibrosis is defined by lineage-specific deletion of high-affinity type II transforming growth factor β receptor. Am J Respir Crit Care Med. 2011;183:249–261. doi: 10.1164/rccm.201002-0279OC. [DOI] [PubMed] [Google Scholar]
- 32.Rock JR, Barkauskas CE, Cronce MJ, et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A. 2011;108:E1475–E1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hung C, Linn G, Chow YH, et al. Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med. 2013;188:820–830. doi: 10.1164/rccm.201212-2297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.