Abstract
BACKGROUND
The spectrum, prevalence, and prognostic implications of abnormal left ventricular geometry (LVG) in patients with lacunar stroke are unknown. We examined the spectrum of LVG and its relationship with vascular risk factors and outcomes after lacunar stroke.
METHODS
LVG was determined with transthoracic echocardiography for 1961 patients with MRI-verified recent lacunar stroke participating in the Secondary Prevention of Small Subcortical Strokes trial. Multivariable logistic regression and Cox proportional hazards models were used to identify characteristics independently associated with LVG and to estimate risk from abnormal LVG for recurrent stroke and death.
RESULTS
Abnormal LVG was present in 77%. Hispanic (OR 1.4, 95% CI 1.1–1.8) or black (OR 2.0, 1.3–2.9) race-ethnicity, diabetes (OR 1.3, 1.0–1.7), hypertension, impaired renal function (OR 1.8, 1.2–2.5), intracranial stenosis (OR 1.5,1.1–2.1), and abnormal left ventricular function (OR 2.0,1.4–3.0) were independently associated with abnormal LVG. Subjects with abnormal LVG also more frequently had advanced manifestations of small vessel disease specifically previous subcortical infarcts and white matter hyperintensities. After adjusting for assigned treatments, clinical risk factors, and advanced manifestations of small vessel disease, subjects with abnormal LVG remained at increased risk of stroke recurrence (HR 1.5, CI 1.0–2.4). There was no interaction between LVG and assigned antiplatelet or blood pressure target. Abnormal LVG was not associated with mortality.
CONCLUSIONS
LVG consistent with chronic hypertensive changes was highly prevalent and correlated with neuroradiological manifestations of small vessel disease in lacunar stroke patients. These results support the constructs that both cerebral small vessel disease and LVG represent end-organ consequences of chronic hypertension.
REGISTRATION
Keywords: lacunar stroke, transthoracic echocardiogram, left ventricular geometry, hypertension, secondary prevention, small vessel disease
BACKGROUND
Transthoracic echocardiography is routinely used to help ascertain stroke mechanism after an ischemic cerebrovascular event.[1,2] However its use, particularly in the context of lacunar (subcortical) infarction, is controversial,[3] with some investigators concluding that transthoracic echocardiography is of little to no utility in the workup of lacunar stroke.
Left ventricular geometry refers to the relationship between left ventricular wall thickness and left ventricular mass, indexed to body surface area. It is a conceptualization of cardiac remodeling in response to physiologic (e.g. athletic strength or endurance training) or pathologic (e.g., response to the pressure overload of hypertension or volume overload) changes.
Though abnormal left ventricular geometry in keeping with chronic hypertension (i.e. concentric remodeling or concentric hypertrophy) is the most common echocardiographic finding reported in previous small series of patients with lacunar stroke, it is unknown whether transthoracic echocardiography yields additional clinical or prognostic information in this cohort of patients.
Our aim was to define the prevalence, spectrum, and prognostic implications of abnormal left ventricular geometry in patients with symptomatic lacunar infarcts. Data were from the Secondary Prevention of Small Subcortical Strokes (SPS3) Trial involving patients with recent MRI-proven symptomatic lacunar infarcts. All participants, 86% of whom had hypertension at baseline,[4] had a transthoracic echocardiogram prior to randomization.
METHODS
All participants were part of the SPS3 trial, the design, rationale, participant characteristics, and results of which have been previously published.[4–7] Briefly, participants were enrolled within 180 days of a symptomatic lacunar infarct with a corresponding lesion on magnetic resonance imaging (MRI). Patients with a major-risk cardioembolic source (supplemental table 1), ipsilateral carotid stenosis >50%, or previous cortical infarcts were ineligible for the trial. Eligible patients were randomized in 2×2 factorial design to (1) a double-blinded antiplatelet intervention (1:1): 325 mg daily aspirin and either placebo or 75 mg daily clopidogrel, and (2) a prospective, open-label, systolic blood pressure target (1:1) of 130–139 mm Hg vs. <130 mm Hg. The study was approved at each site by the local institutional review committee. All participants gave informed consent.
Prior to enrolment, participants were required to have a transthoracic echocardiogram to exclude any participant with a major-risk cardiogenic embolic source. Echocardiography was not performed according to a pre-specified protocol or interpreted centrally, and site personnel reported available information from echocardiography reports on a standardized data form. All participants who had measurements reported for left ventricular end-diastolic dimension (LVEDD), interventricular septal thickness at end-diastole (IVST), and posterior wall thickness at end-diastole (LVPWT) were included in these analyses. (Supplementary Figure I) LVEDD measurements were obtained from the two dimensional echocardiographic parasternal long-axis views of the left ventricle at the tips of the mitral valve for the internal dimension, interventricular septal thickness and posterior wall thickness. The left ventricular end-systolic measurement was also obtained from this view and a fractional shortening calculated. A qualitative assessment of the left ventricular systolic performance was obtained per site protocol and recorded as normal or abnormal (mild or moderate-severe). An estimate of left ventricular ejection fraction was also made qualitatively or semi-quantitatively by Simpson’s biplane technique. Ejection fractions > 50% were considered normal, 40–50% mildly reduced left ventricular function, and moderate-severely reduced left ventricular dysfunction if below 40%.
Left ventricular geometry was indexed to body surface area and classified per Devereaux as normal, concentric remodeling, concentric hypertrophy, or eccentric hypertrophy.[8] Body surface area was estimated according to the Mosteller formula.[9]
Previous subcortical stroke or transient ischemic attack was defined as a clinical episode occurring prior to the qualifying event associated with a classical lacunar stroke syndrome. Diabetes was defined as (1) a clinical history of diabetes at the time of the qualifying event (91%) or (2) initiation of anti-diabetic medications over the first three months of follow-up (9%). Ischemic heart disease was defined as any clinical history of myocardial infarction, angina, or coronary artery bypass surgery, angioplasty or stenting. Glomerular filtration rate was estimated using the Chronic Kidney Disease Epidemiology Collaboration equation.[10] Blood pressure at entry was defined as the average of two screening systolic blood pressure determinations done at least one week apart.[6] To determine severity of hypertension, an adjustment was made by adding 5 mmHg to the systolic blood pressure for each antihypertensive medication the participant was taking at that time, to a maximum of 4 medications. Normotensive was defined as <120 mmHg, pre-hypertensive as 120–<140 mmHg, stage I hypertension as 140–<160 mmHg, and stage II hypertension as ≥160 mmHg.
De-identified magnetic resonance images (MRI) were submitted to the SPS3 Coordinating Center for interpretation by a neuroradiologist blinded to clinical information and assigned treatment.[11] Old subcortical infarcts were defined as hypointense areas on fluid-attenuated inversion recovery (FLAIR) and/or T1 measuring ≥3 mm, but no more than 15 mm in maximum dimension. Hypointense lesions at the level of the anterior commissure, convexity, or midbrain, were considered enlarged peri-vascular spaces (EPVS) and not classified as infarcts, unless the lesion was surrounded by a hyperintense halo on FLAIR.[11,13] Burden of white matter hyperintensities was evaluated visually on FLAIR images using the Age-Related White Matter Changes (ARWMC) scale. A priori, ARWMC scores of 0–4 were defined as none-mild disease, 5–8 moderate, and 9+ severe.[11,13]
Participant characteristics were compared between groups using Student’s t-test or ANOVA for continuous variables and a chi-square test (or Fisher’s exact test if expected cell count < 5) for categorical data. Multivariable binomial logistic regression was used to identify participant characteristics independently associated with left ventricular geometry group at study entry. To estimate risk associated with abnormal left ventricular geometry at study entry for each of recurrent stroke and death, we fit multivariable Cox proportional hazards models adjusted for assigned treatments and clinical variables previously reported as independently predictive of recurrent stroke[14] and death[15] in this cohort. All analyses were performed using SPSS for Windows, Version 20 (Armonk, NY). All statistical tests were two-sided, no adjustments were made for multiple comparisons, and significance was accepted at the 0.05 level.
RESULTS
The 65% of the 3020 SPS3 participants with the necessary echocardiographic measurements (LVEDD, IVST, LVPWT) recorded to determine left ventricular geometry are included in these analyses. Included participants did not differ strikingly from excluded participants. (Supplementary Table II) The average age of the 1961 included participants was 63 years, 64% were male, and histories of hypertension, diabetes, and prior symptomatic lacunar infarct were present in 75%, 36%, and 14% of patients respectively. Qualitative assessment of left ventricular function was normal for 86% of patients. Participants were followed for a mean of 3.7 (SD 1.9, median 3.6) years.
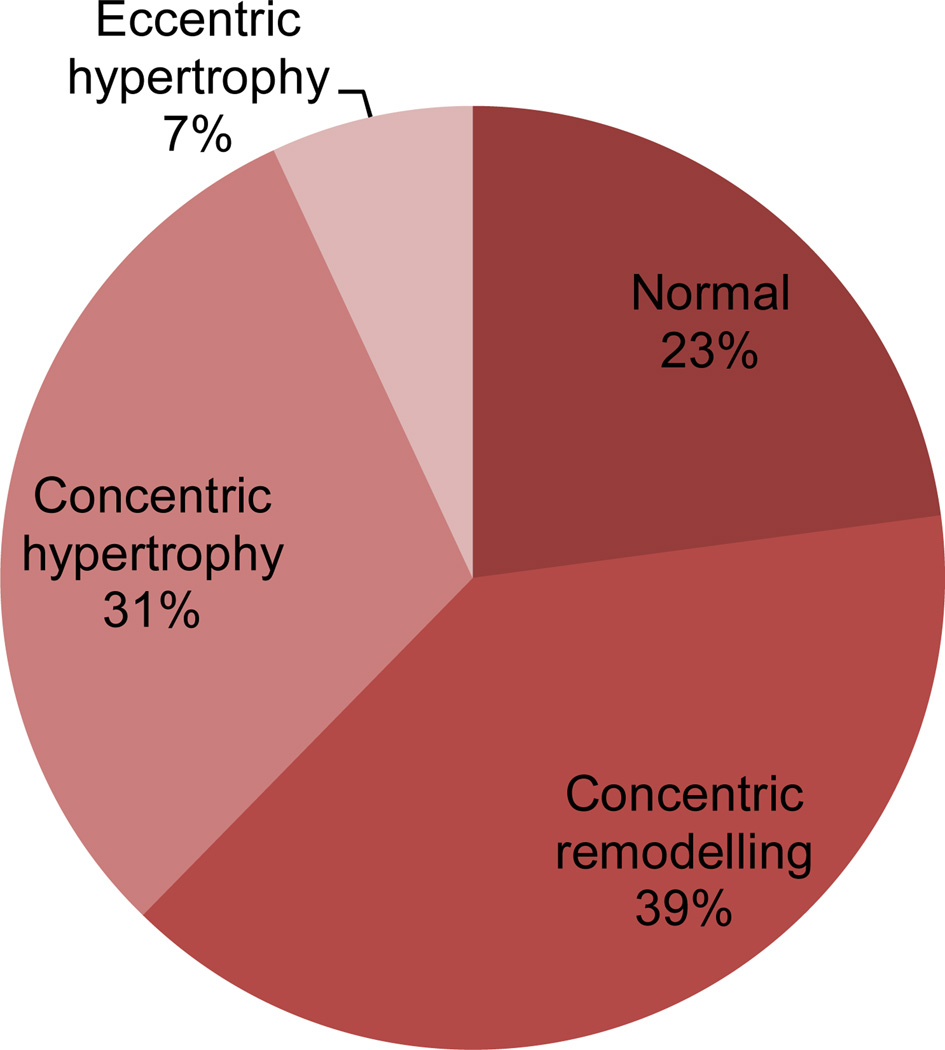
Over 75% of the participants had abnormal left ventricular geometry. (Figure 1) As compared to those with normal geometry (n = 449), participants with abnormal geometry (n = 1512) were slightly older, more often of Hispanic or black race-ethnicity, and had a higher prevalence of diabetes, hypertension, more severe hypertension, impaired renal function, intracranial stenosis, and abnormal left ventricular function. (Tables 1 and 2) Participants with abnormal vs. normal geometry more frequently had old small subcortical infarcts on MRI (43% vs. 34%, p < 0.001). White matter hyperintensities were also more prevalent among those with abnormal (ARWMC score 0–4 46%, 5–8 29%, >8 24%) vs. normal (ARWMC score 0–4 60%, 5–8 23%, >8 18%) geometry (p < 0.001).
Figure 1.
Left ventricular geometry of participants
Table 1.
Characteristics of participants by left ventricular geometry
| Normal geometry (n = 449) |
Abnormal geometry (n = 1512) |
p-value | |
|---|---|---|---|
| Age, mean (SD) | 62 (11) | 64 (11) | 0.002 |
| Male, % | 67 | 63 | 0.1 |
| Race-ethnicity, %* | < 0.001 | ||
| Non-Hispanic white | 58 | 45 | |
| Hispanic | 31 | 37 | |
| Black | 9 | 16 | |
| Other/multiple | 2 | 3 | |
| History of hypertension, % | 68 | 77 | < 0.001 |
| Severity of hypertension, %* | < 0.001 | ||
| Normal | 2 | 2 | |
| Pre-hypertensive | 25 | 16 | |
| Stage I | 45 | 41 | |
| Stage II | 28 | 41 | |
| Diabetes, % | 30 | 38 | 0.002 |
| Ischemic heart disease, % | 10 | 9 | 0.7 |
| Estimated GFR (mL/min/1.73m2) | |||
| mean (SD) | 85 (19) | 81 (20) | < 0.001 |
| < 60, % | 10 | 17 | 0.001 |
| Prior symptomatic lacunar infarct, % | 15 | 14 | 0.8 |
| Fractional shortening, mean (SD) | 38 (11) | 36 (15) | 0.009 |
| Fractional shortening ≤ 25%, % | 6 | 9 | 0.1 |
| Left ventricular function*,**, % | < 0.001 | ||
| normal | 91 | 84 | |
| mild dysfunction | 8 | 15 | |
| moderate-severe dysfunction | 1 | 1 | |
| Intracranial stenosis ≥50%, % (n = 1850) | 11 | 19 | < 0.001 |
Abbreviations: SD = standard deviation, GFR = glomerular filtration rate. ARWMC = age-related white matter changes
For subsequent analyses, and due to small numbers, severity of hypertension categories normal and pre-hypertensive were combined into non-hypertensive, and left ventricular function categories of mild and moderate-severe dysfunction were combined into abnormal dysfunction
As per qualitative assessment
Table 2.
Participant characteristics from Table 1 independently associated with abnormal left ventricular geometry
| Odds ratio (95% CI) | |
|---|---|
| Race-ethnicity | |
| Hispanic | 1.4 (1.1, 1.8) |
| Black | 2.0 (1.3, 2.9) |
| All others | reference group |
| Diabetes | 1.3 (1.0, 1.7) |
| Severity of hypertension | |
| Non-hypertensive | reference group |
| Stage I | 1.4 (1.0, 1.8) |
| Stage II | 1.9 (1.4, 2.5) |
| Estimated glomerular filtration rate < 60 mL/min/1.73m2 | 1.8 (1.2, 2.5) |
| Abnormal left ventricular function* | 2.0 (1.4, 3.0) |
| Intracranial stenosis ≥ 50% | 1.5 (1.1, 2.1) |
As per qualitative assessment
Amongst patients with abnormal geometry, concentric remodeling and concentric hypertrophy were more common than eccentric hypertrophy. (Figure 1) Those with concentric hypertrophy were more often female and had a history of hypertension and more severe hypertension than those with concentric remodeling. Concentric hypertrophy was also associated with more neuroradiological manifestations of small vessel disease, i.e. higher ARWMC score and previous subcortical infarct on MRI. (Table 3)
Table 3.
Characteristics of participants by type of abnormal left ventricular geometry
| Concentric remodeling (n = 773) |
Concentric hypertrophy (n = 603) |
Eccentric hypertrophy (n = 136) |
p-value | |
|---|---|---|---|---|
| Age, mean (SD) | 63 (11) | 64 (11) | 65 (11) | 0.1 |
| Male, % | 70 | 56 | 53 | < 0.001 |
| Race-ethnicity, %* | < 0.001 | |||
| Non-Hispanic white | 50 | 38 | 46 | |
| Hispanic | 30 | 46 | 38 | |
| Black | 17 | 14 | 15 | |
| Other/multiple | 3 | 2 | 1 | |
| History of hypertension, % | 71 | 83 | 79 | < 0.001 |
| Severity of hypertension, % | < 0.001 | |||
| Non-hypertensive | 22 | 13 | 16 | |
| Stage I | 43 | 38 | 40 | |
| Stage II | 35 | 49 | 44 | |
| Diabetes, % | 40 | 35 | 36 | 0.2 |
| Ischemic heart disease, % | 9 | 9 | 14 | 0.2 |
| Estimated GFR (mL/min/1.73m2), | ||||
| mean (SD) | 82 (20) | 79 (20) | 79 (21) | 0.01 |
| < 60, % | 15 | 18 | 18 | 0.2 |
| Prior symptomatic lacunar infarct, % | 15 | 13 | 16 | 0.6 |
| Fractional shortening | ||||
| mean (SD) | 35 (17) | 37 (13) | 36 (10) | 0.2 |
| ≤ 25%, % | 10 | 6 | 12 | 0.04 |
| Abnormal left ventricular function*, % | 14 | 17 | 27 | < 0.001 |
| Intracranial stenosis ≥50%, % | 18 | 21 | 15 | 0.2 |
| Small subcortical infarcts on FLAIR/T1 (hypointense) on MRI, % | 39 | 50 | 41 | < 0.001 |
| White matter hyperintensities | ||||
| ARWMC score, mean (SD) | 5.6 (4) | 6.3 (4) | 5.7 (4) | 0.005 |
| ARWMC score | 0.04 | |||
| none-mild (0–4) | 49 | 41 | 49 | |
| moderate (5–8) | 29 | 31 | 28 | |
| severe (>8) | 22 | 28 | 23 | |
GFR = glomerular filtration rate; ARWMC = Age Related White Matter Changes
As per qualitative assessment
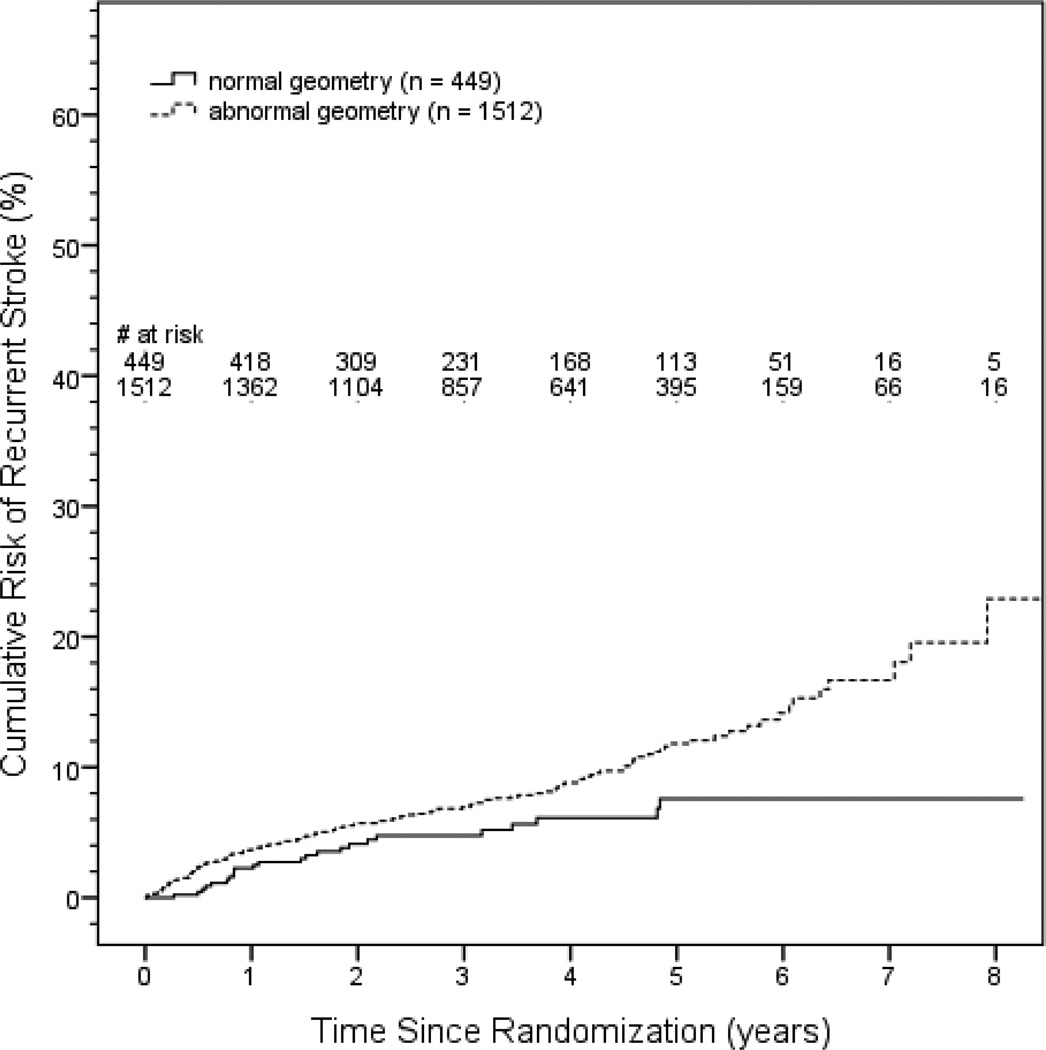
Recurrent stroke occurred in 163 patients (136 ischemic, 24 hemorrhagic, 3 uncertain/unknown) and death in 122 patients. Participants with abnormal geometry were at mildly increased risk of recurrent stroke (adjusted HR 1.6, 95% CI 1.0, 2.2, p = 0.04) after adjusting for assigned treatment groups and published clinical risk factors predictive of recurrent stroke in SPS3 participants13, i.e. male sex, black race-ethnicity, diabetes, and previous symptomatic subcortical infarct. (Table 4, Figure 2) None of severity of hypertension, intracranial stenosis, or left ventricular dysfunction was additionally predictive of recurrent stroke nor appreciably changed the estimated HR for abnormal left ventricular geometry in this model. There was no significant interaction between left ventricular geometry and either assigned antiplatelet (p=0.9) or blood pressure target (p = 0.9). None of the individual components of the left ventricular geometry formula (i.e. left ventricular end diastolic dimension, mass index, relative wall thickness), either as continuous measures or categorized as normal versus abnormal, was singularly predictive of recurrent stroke in the adjusted model (data not shown). We also investigated if abnormal left ventricular geometry remained predictive after additionally adjusting for cerebral manifestations of small vessel disease, i.e. we added terms for subcortical infarcts and ARWMC score group (0–4, 5–8, 9+) to the adjusted model described above. The estimated risk for recurrent stroke with abnormal geometry remained relatively unchanged (adjusted HR 1.5, 95% CI 1.0, 2.4, p = 0.05).
Table 4.
Hazard ratios (HR) for recurrent stroke and death by left ventricular geometry.
| # of events (n) | Unadjusted HR (95% CI) |
Adjusted* HR (95% CI) |
|
|---|---|---|---|
| Recurrent stroke | |||
| Normal geometry | 24 (449) | reference group | reference group |
| Abnormal geometry | 139 (1512) | 1.7 (1.1, 2.6) | 1.6 (1.0, 2.4) |
| Death | |||
| Normal geometry | 18 (449) | reference group | reference group |
| Abnormal geometry | 104 (1512) | 1.6 (0.97, 2.6) | 1.3 (0.76, 2.1) |
Recurrent stroke: Multivariable model adjusted for assigned treatment groups, male sex, Black race-ethnicity, diabetes, and previous symptomatic subcortical infarct13
Death: Multivariable model adjusted for age, body mass index, history of hypertension, systolic blood pressure, diabetes, low hemoglobin (< 13 g/dL), estimated glomerular filtration rate, and interactions of assigned antiplatelet with non-hypertension and ischemic heart disease14
Figure 2.
Kaplan-Meier curve for recurrent stroke by left ventricular geometry
Annualized hemorrhagic stroke rates were similar across the groups of normal geometry (0.39%, 6 of 24 strokes), concentric remodeling (0.41%, 11 of 80), and concentric hypertrophy (0.32%, 7 of 51), with no hemorrhagic strokes in the eccentric hypertrophy group (0 of 8 strokes). Abnormal left ventricular geometry was not predictive of death (adjusted HR 1.3, 95% CI 0.76, 2.1, p = 0.4). (Table 4)
DISCUSSION
In this cohort of patients with recent symptomatic lacunar stroke, abnormal left ventricular geometry, especially those changes consistent with chronic hypertension, was highly prevalent. Further, left ventricular geometry consistent with chronic hypertension had distinct risk factors and was associated with neuroradiological correlates of cerebral small vessel disease. We also found abnormal left ventricular geometry to be predictive of stroke recurrence.
Both concentric remodeling and concentric hypertrophy subtypes may develop in the context of chronic hypertension. [16] Pathophysiologically, geometric changes that occur in the transition from normal to concentric hypertrophy in the setting of systemic hypertension are postulated to be a response of the left ventricle to increased pressure overload, operating through left ventricular wall tension and stress. As left ventricular systolic pressure rises, wall tension increases and left ventricular radius remains normal or may even decrease. This increases wall stress which is then ‘normalized’ by increasing wall thickness resulting in concentric remodeling.[17] Recovery of left ventricular volume then results in concentric hypertrophy, the most advanced hypertrophic change with hypertension.[17]
About 75% of SPS3 participants have hypertension, yet in previous analyses we did not find that history of hypertension, severity of hypertension, or blood pressure at study entry were predictive of recurrent stroke.[14] The association between abnormal geometry and stroke recurrence, independent of hypertension, suggests that abnormal geometry may be a marker for suboptimal duration or degree of blood pressure control from the perspective of secondary stroke prevention. Abnormal left ventricular geometry is known to reverse over years in the context of adequately controlled hypertension.[18]
In population-based studies, abnormal left ventricular geometry is associated with cerebrovascular events and mortality.[19–21] In our cohort, abnormal left ventricular geometry carried an increased risk of stroke, but not for death. While our results may be due to play of chance, the lack of association between abnormal geometry and death may be explained by a comparatively short period of follow-up. (Other large studies examining the relationship between geometry and prognosis had mean/median follow-up periods ranging from 4–8 years.) It is also possible that in this cohort with symptomatic cerebral small vessel disease and a relatively young age that geometry portends different prognostic implications than in the general population. The association between concentric hypertrophy and increased vascular risk, for example, is postulated to be partly due to the association of increased large-vessel atherosclerosis with this geometry subtype.[16,22] In our cohort, however, the overall prevalence of clinically manifest large vessel disease was quite low with only 3% of patients having a history of ischemic heart disease, fewer still having a history of peripheral vascular disease, and patients with symptomatic carotid disease and major-risk cardioembolic conditions being excluded from the study. Thus, it is possible in this comparatively etiologically “pure” cohort, hypertensive end-organ damage in the context of microvascular brain disease is not associated with an excess risk of death.
Limitations to our analysis include its post-hoc nature. Interpretation of baseline echocardiograms in SPS3 was not centralized, which while increasing the generalizability of our findings, may also introduce inter-rater variability that blunted relationships. Furthermore, adequate quantitative echocardiography measurements were only available for two-thirds of participants and were limited to a single study at study entry, and we cannot exclude that an unidentified selection bias related to left ventricular geometry may have occurred. Finally, as SPS3 participants were subsequently treated to systolic blood pressure targets that have been shown to modify left ventricular geometry and vascular prognosis over time, having a single study limited our ability to interpret the relationship between left ventricular geometry and stroke recurrence during the median follow-up of 3.6 years.
The relationship between left ventricular geometry, its evolution alongside cerebral microvascular changes in the context of small vessel disease, and its prognostic implications in this context requires further study. Our findings should be viewed as hypothesis-generating and suggest that routine transthoracic echocardiography may be of value in patients with lacunar infarction given our finding that abnormal left ventricular geometry provides independent additional prognostic information about increased risk of recurrent infarction.
CONCLUSIONS
Abnormal left ventricular geometry is common in lacunar stroke, and abnormal geometry subtypes are associated with distinct patient characteristics and correlate with neuroradiological findings. Abnormal geometry was independently associated with stroke recurrence in this population. Our findings suggest that abnormal left ventricular geometry on transthoracic echocardiography may provide additional prognostic information about risk of stroke recurrence in lacunar stroke patients.
Supplementary Material
ACKNOWLEDGEMENTS
SOURCES OF FUNDING
The study was supported by a grant (U01NS038529) from the US National Institutes of Health-National Institute of Neurological Disorders and Stroke (NIH-NINDS)
The authors thank Mr. Franco Cermeno for his able assistance with graphics
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST/DISCLOSURES
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, Douglas PS, Faxon DP, Gillam LD, Kimball TR, Kussmaul WG, Pearlman AS, Philbrick JT, Rakowski H, Thys DM, Antman EM, Smith SC, Jr, Alpert JS, Gregoratos G, Anderson JL, Hiratzka LF, Hunt SA, Fuster V, Jacobs AK, Gibbons RJ, Russell RO American College of C, American Heart A, American Society of E. Acc/aha/ase 2003 guideline update for the clinical application of echocardiography: Summary article: A report of the american college of cardiology/american heart association task force on practice guidelines (acc/aha/ase committee to update the 1997 guidelines for the clinical application of echocardiography) Circulation. 2003;108:1146–1162. doi: 10.1161/01.CIR.0000073597.57414.A9. [DOI] [PubMed] [Google Scholar]
- 2.European Stroke Organisation Executive C, Committee ESOW. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovascular diseases. 2008;25:457–507. doi: 10.1159/000131083. [DOI] [PubMed] [Google Scholar]
- 3.Rabinstein AA, Chirinos JA, Fernandez FR, Zambrano JP. Is tee useful in patients with small subcortical strokes? European journal of neurology : the official journal of the European Federation of Neurological Societies. 2006;13:522–527. doi: 10.1111/j.1468-1331.2006.01283.x. [DOI] [PubMed] [Google Scholar]
- 4.The SPS3 Group. Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, Pearce LA, Pergola PE, Szychowski JM. Blood-pressure targets in patients with recent lacunar stroke: The sps3 randomised trial. Lancet. 2013;382:507–515. doi: 10.1016/S0140-6736(13)60852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The SPS3 Investigators. Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, Pearce LA. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. The New England journal of medicine. 2012;367:817–825. doi: 10.1056/NEJMoa1204133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benavente OR, White CL, Pearce L, Pergola P, Roldan A, Benavente MF, Coffey C, McClure LA, Szychowski JM, Conwit R, Heberling PA, Howard G, Bazan C, Vidal-Pergola G, Talbert R, Hart RG. The secondary prevention of small subcortical strokes (sps3) study. International journal of stroke : official journal of the International Stroke Society. 2011;6:164–175. doi: 10.1111/j.1747-4949.2010.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White CL, Szychowski JM, Roldan A, Benavente MF, Pretell EJ, Del Brutto OH, Kase CS, Arauz A, Meyer BC, Meissner I, Demaerschalk BM, McClure LA, Coffey CS, Pearce LA, Conwit R, Irby LH, Peri K, Pergola PE, Hart RG, Benavente OR Investigators SPS. Clinical features and racial/ethnic differences among the 3020 participants in the secondary prevention of small subcortical strokes (sps3) trial. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2013;22:764–774. doi: 10.1016/j.jstrokecerebrovasdis.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. The American journal of cardiology. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 9.Mosteller RD. Simplified calculation of body-surface area. The New England journal of medicine. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J Ckd EPI. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benavente OR, Pearce LA, Bazan C, Roldan AM, Catanese L, Bhat Livezey VM, Vidal-Pergola G, McClure LA, Hart RG Investigators SPS. Clinical-mri correlations in a multiethnic cohort with recent lacunar stroke: The sps3 trial. Int J Stroke. doi: 10.1111/ijs.12282. epub ahead of print 27 May 2014 101111/ijs12282 2014. [DOI] [PubMed] [Google Scholar]
- 12.Benavente OR, Pearce LA, Bazan C, Roldan AM, Catanese L, Bhat Livezey VM, Vidal-Pergola G, McClure LA, Hart RG Investigators SPS. Clinical-mri correlations in a multiethnic cohort with recent lacunar stroke: The sps3 trial. International journal of stroke : official journal of the International Stroke Society. 2014;9:1057–1064. doi: 10.1111/ijs.12282. [DOI] [PubMed] [Google Scholar]
- 13.Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjogren M, Wallin A, Ader H, Leys D, Pantoni L, Pasquier F, Erkinjuntti T, Scheltens P. A new rating scale for age-related white matter changes applicable to mri and ct. Stroke. 2001;32:1318–1322. doi: 10.1161/01.str.32.6.1318. [DOI] [PubMed] [Google Scholar]
- 14.Hart RG, Pearce LA, Bakheet MF, Benavente OR, Conwit RA, McClure LA, et al. Predictors of stroke recurrence in patients with recent lacunar stroke and response to interventions according to risk status: Secondary prevention of small subcortical strokes trial. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2014;2014:618–624. doi: 10.1016/j.jstrokecerebrovasdis.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma M, Pearce LA, Benavente OR, Anderson DC, Connolly SJ, Palacio S, Coffey CS, Hart RG. Predictors of mortality in patients with lacunar stroke in the secondary prevention of small subcortical strokes trial. Stroke. 2014;45:2989–2994. doi: 10.1161/STROKEAHA.114.005789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devereux RB, Roman MJ. Left ventricular hypertrophy in hypertension: Stimuli, patterns, and consequences. Hypertension research : official journal of the Japanese Society of Hypertension. 1999;22:1–9. doi: 10.1291/hypres.22.1. [DOI] [PubMed] [Google Scholar]
- 17.Shah AM, Pfeffer MA. Left ventricular size, mass, and shape: Is the sum greater than the parts? JACC Heart failure. 2014;2:523–525. doi: 10.1016/j.jchf.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devereux RB, Palmieri V, Liu JE, Wachtell K, Bella JN, Boman K, Gerdts E, Nieminen MS, Papademetriou V, Dahlof B. Progressive hypertrophy regression with sustained pressure reduction in hypertension: The losartan intervention for endpoint reduction study. Journal of hypertension. 2002;20:1445–1450. doi: 10.1097/00004872-200207000-00033. [DOI] [PubMed] [Google Scholar]
- 19.Krumholz HM, Larson M, Levy D. Prognosis of left ventricular geometric patterns in the framingham heart study. Journal of the American College of Cardiology. 1995;25:879–884. doi: 10.1016/0735-1097(94)00473-4. [DOI] [PubMed] [Google Scholar]
- 20.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: The mesa (multi-ethnic study of atherosclerosis) study. Journal of the American College of Cardiology. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerdts E, Cramariuc D, de Simone G, Wachtell K, Dahlof B, Devereux RB. Impact of left ventricular geometry on prognosis in hypertensive patients with left ventricular hypertrophy (the life study) European journal of echocardiography : the journal of the Working Group on Echocardiography of the European Society of Cardiology. 2008;9:809–815. doi: 10.1093/ejechocard/jen155. [DOI] [PubMed] [Google Scholar]
- 22.Roman MJ, Pickering TG, Schwartz JE, Pini R, Devereux RB. Association of carotid atherosclerosis and left ventricular hypertrophy. Journal of the American College of Cardiology. 1995;25:83–90. doi: 10.1016/0735-1097(94)00316-i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.