Abstract
Imidazole, 1-methylimidazole and 4-nitroimidazole bind to yeast cytochrome c peroxidase (yCcP) with apparent equilibrium dissociation constants () of 3.3 ± 0.4, 0.85 ± 0.11, and ~0.2 M, respectively, at pH 7. This is the weakest imidazole binding to a heme protein reported to date and it is about 120 times weaker than imidazole binding to metmyoglobin. Spectroscopic changes associated with imidazole and 1-methylimidazole binding to yCcP suggest partial ionization of bound imidazole to imidazolate. The pKa for ionization of bound imidazole is estimated to be 7.4 ± 0.2, about 7 units lower than that of free imidazole and about 3 units lower than imidazole bound to metmyoglobin. Equilibrium binding of imidazole to CcP(H52L) is biphasic with low- and high-affinity phases having values of 9.5 ± 4.5 and 0.13 ± 0.04 M, respectively. CcP(H52L) binding of 1-methylimidazole is monophasic with an affinity similar to those of yCcP and rCcP. Binding of 1-methylimidazole to rCcP is associated with two kinetic phases, the initial binding complete within 10 s, followed by a process that is consistent with 1-methylimidazole binding to a cavity created by movement of Trp-191 from the interior of the protein to the surface. Both the equilibrium binding and kinetics of 1-methylimidazole binding to yCcP are pH dependent. yCcP has a four-fold increase in 1-methylimidazole binding affinity on decreasing the pH from 7.5 to 4.0, an observation that is unique among the many studies on binding of imidazole and imidazole derivatives to heme proteins.
Keywords: Cytochrome c Peroxidase, CcP(H52L), Imidazole, 1-Methylimidazole, 4-Nitroimidazole
1. Introduction
The reactivity of the heme group within diverse heme proteins can be explored via ligand binding reactions. Heme proteins in their Fe(III) redox state bind ligands that are weakly basic such as azide, cyanide, fluoride and imidazole [1]. We have a long-standing interest in elucidating the differences in ligand binding to metmyoglobin (metMb) and cytochrome c peroxidase (CcP), representatives of two different heme protein classes [2-8]. The pH dependence of ligand binding to metMb and CcP are substantially different, reflecting differences in the role of acidic and basic groups within the two proteins in the binding process. For ligands such as azide, cyanide, and fluoride, the binding mechanism for both metMb and CcP is preferential diffusion of the uncharged protonated form of the ligand into the distal heme pocket followed by deprotonation and anion binding to the heme iron. The distal histidine in CcP, His-52, serves as a base-catalyst in the ligand binding reactions, facilitating deprotonation of HN3, HCN, and HF within the heme pocket. The active site of CcP is shown in Fig. 1.
Figure 1.
CcP Active Site
Active site structure of yCcP showing the distal (Arg-48, Trp-51, and His-52) and proximal (His-175, Trp-191, Asp-235) heme pockets. Coordinates from the Protein Data Bank entry 2CYP.
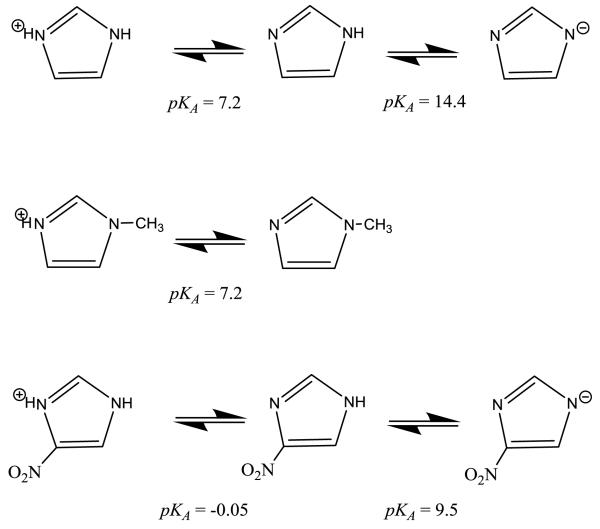
The binding of imidazole to heme proteins is potentially more complex than that of azide, cyanide and fluoride since imidazole can exist in three different forms: the imidazolium cation, neutral imidazole, and the imidazolate anion, Scheme 1. The most extensive studies of imidazole binding to heme proteins have been carried out using metMb [6,7,9-18]. The binding of imidazole is pH dependent with maximum affinity near pH 8. At this pH sperm whale metMb and horse metMb imidazole complexes have equilibrium dissociation constants of 14 and 27 mM, respectively [6,9]. Two ionizable groups in horse metMb influence imidazole binding. The first, with an apparent pKa of 6.2, is attributed to His-97 and the second, with a pKa of 9.0, is attributed to ionization of the heme-bound water [6].
Scheme 1.
Protonation of imidazole has a relatively small effect on the rate of binding to metMb, with the Alberty group concluding that imidazole and the imidazolium ion bind to sperm whale metMb with rate constants of 360 and 127 M−1s−1, respectively [10]. We have found similar results with horse metMb [6]. The imidazolate anion does not contribute to the observed rate of binding in metMb but bound imidazole ionizes to the imidazolate form with an apparent pKa of 10.34 [18].
Previous studies on the interaction of imidazole with CcP have found small spectral perturbations in the Soret region [19-22]. There is a small red-shift in the Soret band with the maximum increase in absorbance occurring between 414 and 418 nm [19,22]. The apparent KD value for the CcP/imidazole complex was 20 mM [22]. In the reported studies, imidazole does not bind to the heme iron but rather binds within a cavity formed in the proximal heme pocket by movement of Trp-191 from the interior of the protein to the surface [19,20]. The movement of Trp-191 creates two conformations of the enzyme, with native CcP existing in a “closed” conformation, with Trp-191 in van der Waals contact of the heme, 96% of the time and in an “open” conformation, with Trp-191 exposed to the solvent 4% of the time [20]. The conformational equilibrium is due to the large scale movement of residues 190 to 195 that comprise a loop on the surface of CcP. Mutating Trp-191 to a glycine residue has little effect on the overall structure of CcP but does create a stable cavity within the proximal heme pocket. CcP(W191G) binds a wide range of ligands including imidazole. The apparent KD for the CcP(W191G)/imidazole complex is 0.7 mM [19]. The apparent KD values for the imidazole complexes of CcP and CcP(W191G) are consistent with one another when taking the conformational equilibrium into account.
We have found that by using much higher concentrations of imidazole than previously used [19-22] we can induce imidazole binding to the heme iron in CcP. The apparent KD value for imidazole binding to the heme is 3.3 ± 0.4 M, over two orders of magnitude larger than binding of imidazole within the Trp-191 cavity. In order to explore the relative importance of protonation and deprotonation phenomena in the mechanism of imidazole binding to the heme in CcP, we have investigated the binding of imidazole, 1-methylimidazole (MIM) and 4-nitroimidazole (4NI) to yeast CcP (yCcP), to a recombinant form of CcP (rCcP), and a CcP mutant that has the distal histidine replaced by a leucine residue, CcP(H52L). The two substituted imidazoles have very different propensities to form the imidazolium cation and imidazolate anion in comparison to imidazole itself (Scheme 1), and CcP(H52L) lacks the distal histidine, which has been shown to be important in the binding of anionic ligands to CcP. During the course of our studies we have determined the kinetics of imidazole binding into the Trp-191 cavity for both yCcP and rCcP at pH 7 and the binding of 1-methylimidazole into the Trp-191 cavity for yCcP between pH 4.1 and 7.5.
In a companion paper [23], we test the idea that the polarity of the distal heme pocket has a significant influence on binding of neutral ligands such as imidazole. In that paper we show that making the distal heme pocket more apolar can enhance imidazole binding to CcP by more than 4.5 orders of magnitude, essentially converting the heme in CcP from a very weak imidazole binder to variants that have heme/imidazole affinities among the highest reported.
2. Materials and Methods
2.1. yCcP, rCcP and CcP(H52L)
Both yCcP and rCcP were used in this study. James Satterlee, Washington State University, kindly provided the expression system for rCcP [24]. The gene for rCcP includes an N-terminal methionine for bacterial expression and the exact amino acid sequence of mature baker’s yeast CcP [25]. The rCcP gene is inserted into the multiple cloning site of the Novagene vector pET24a(+) under control of the T7 promoter. The N-terminal methionine is removed by the expression system and isolated rCcP has the exact amino acid sequence as yCcP. The CcP(H52L) gene was constructed using the Stratagene QuikChange mutagenesis kit and sequenced in both directions to assure that only the intended mutation was introduced. Expression of the recombinant proteins has been described previously [24]. Purification of yCcP, rCcP, and the CcP mutants is carried out using the same protocol [24, 26-28]. Briefly, the soluble proteins are extracted from disrupted cells (either yeast or E. coli) using 50 mM potassium phosphate buffer, pH 6.0, centrifuged to remove insoluble material, and passed through a Sephadex G-75 column. Fractions containing apoCcP were pooled, the pH adjusted to 7.5, and a 5-fold molar excess of hemin added. After incubation in the dark for 1 to 2 hours, the pH was adjusted to pH 6.0, centrifuged, and applied to a DEAE-Sepharose FastFlow column equilibrated in 50 mM potassium phosphate buffer, pH 6.0. HoloCcP was eluted using a linear gradient consisting of 200 mL 50 mM potassium phosphate and 200 mL of 1.0 M potassium phosphate, both at pH 6.0. Fractions were monitored by UV-vis spectroscopy and fractions with absorbance ratios (408nm/280nm) >1 were pooled. The sample was concentrated on a small DEAE-Sepharose FastFlow column and dialyzed against several changes of deionized distilled water to induce crystallization. On occasion, CcP samples were lyophilized after dialysis against deionized distilled water.
2.2. Other Materials
Potassium acetate and potassium phosphate salts were obtained from Fisher Scientific. Imidazole, 1-methylimidazole, and 4-nitroimidazole were obtained from Aldrich Chemical Co. Buffers between pH 4.0 and 5.5 were 0.010 M acetate with added KH2PO4 to adjust the ionic strength. Between pH 5.5 and 8.0, the buffers were mixtures of KH2PO4 and K2HPO4, controlling ionic strength to the desired values.
2.3. Spectroscopic Measurements and Protein Concentration Determination
Protein spectra were determined using one of several instruments including a Cary Model 219 and a Varian/Cary Model 3E double-beam spectrophotometers and a Hewlett Packard Model 8452A diode-array spectrophotometer. Concentration of stock protein solutions were determined spectrophotometrically at pH 6.0 using extinction coefficients in the Soret band of 98 mM−1 cm−1 at 408 nm for yCcP, 101 mM−1 cm−1 at 408 nm for rCcP, and 97 mM−1 cm−1 at 404 nm for CcP(H52L) [27-29].
2.4. Equilibrium Binding
Spectroscopic changes associated with ligand binding to the heme enabled monitoring of complex formation. Determination of the equilibrium constants was done by titrating about 10 μM protein solutions with increasing concentrations of buffered ligand. Mixtures were incubated for up to 25 hours while monitoring changes in the spectroscopic properties. Equilibrium constants were determined by fitting the changes in absorbance as a function of ligand concentration to appropriate models using non-linear least squares regression analysis. Spectra of the complexes were calculated from the spectroscopic and titration data.
2.5. Kinetic Measurements
The rates of complex formation were determined using manual mixing of the reactants followed by spectrophotometric observation. Reactions were carried out under pseudo-first order conditions with excess ligand. Protein concentrations were generally about 10 μM. Observed rate constants were determined at a minimum of five different ligand concentrations, varying by at least a factor of five for each experiment.
3. Results
3.1. Equilibrium Binding of Imidazole, 1-Methylimidazole and 4-Nitroimidazole to CcP
Initial studies indicated that yCcP bound imidazole more weakly than did metmyoglobin and that very high concentrations of imidazole would be needed to show significant complex formation. Addition of up to 5 M imidazole to yCcP causes a red-shift of the Soret band from 408 nm in the free enzyme to 412 nm in the presence of imidazole accompanied by a decrease in the amplitude of the Soret band, Fig. 2A. The data in Fig. 2A are for samples incubated between 4 and 22 hours. The largest absorbance changes occur at 436 and 382 nm as seen in the difference spectra, Fig. 2B. Absorbance changes in the visible region of the spectrum are consistent with the conversion of a five-coordinate, high-spin heme in the free enzyme to a six-coordinate, low-spin heme in the complex.
Figure 2.
yCcP/Imidazole titration at pH 7.0
Titration of yCcP with imidazole at pH 7.0. Panel A. Spectrum of yCcP (thin solid line), spectrum of 4.9 M imidazole (dashed-double dotted line), spectra of yCcP in the presence of increasing concentrations of imidazole after correcting for the absorbance of imidazole (dashed lines), calculated spectrum for the yCcP/imidazole complex (thick solid line). The very high absorbance of imidazole in the UV region leads to inaccurate corrections to the spectra and data for yCcP/imidazole mixtures are not shown below 350 nm. The α, β, and Soret bands of the yCcP/imidazole complex occur at 570, 542, and 412 nm, respectively. Panel B. Difference spectra in the presence and absence of increasing concentrations of imidazole (dashed lines). The thick solid line represents the difference spectrum between the calculated spectrum for 100% formation of the yCcP/imidazole complex and yCcP. The maximum increase and decrease in absorbance in the Soret region occurs at 436 and 382 nm, respectively. Panel C. The difference in Δ(A436 - A382) for mixtures of yCcP and imidazole equilibrated for 4 hours (solid circles) and 22 hours (solid triangles) plotted as a function of the imidazole concentration. The data were fit to a simple hyperbolic titration equation, Eq. 1 of the text, using non-linear least squares regression. The solid line is the calculated best-fit line with . Experimental conditions: [yCcP] = 7.91 μM, [imidazole] = 0.98 to 4.90 M, 1.0 M ionic strength potassium phosphate buffer, pH 7.0, 25 °C.
A plot of the difference in absorbance at 436 and 382 nm, Δ(A436- A382), as a function of the imidazole concentration is hyperbolic from which an apparent equilibrium dissociation constant () can be determined, Fig. 2C. The absorbance changes were fit to Eq. 1 using non-linear
| (1) |
least-squares regression. At pH 7.0, the best-fit value for of the yCcP/imidazole complex is 3.3 ± 0.4 M, Table 1. Only about 60% of yCcP is bound to imidazole at the highest ligand concentrations used in these studies. However, the spectrum of the imidazole/yCcP complex can be calculated from the data and is shown in Fig. 2A. Selected spectral parameters are collected in Table 2.
Table 1.
Equilibrium dissociation constants for ligand complexes of CcP, CcP(H52L) and metMb at pH 7 a
| Enzyme | phase | (M) Imidazole | (M) 1-Methylimidazole | (M) 4-Nitroimidazole |
|---|---|---|---|---|
| yCcP | 3.3 ± 0.4 | 0.85 ± 0.11 | ~0.2 | |
| rCcP | 4.6 ± 0.8 | 0.63 ± 0.04 | ~0.1 | |
| CcP(H52L) | high | 0.13 ± 0.04 (<46%) | 1.1 ± 0.2 | ~0.03 |
| low | 9.5 ± 4.5 (>54%) | |||
| metMb b | (2.8 ± 0.7) × 10−2 | (8.7 ± 1.0) × 10−2 | (2.5 ± 0.7) × 10−3 |
Table 2.
Peak positions and extinction coefficients for CcP, CcP(H52L), metMb, and their imidazole complexes at pH 7.0.
| Proteina | protein band λ (ε) b |
δ band λ (ε) b |
Soret band λ (ε) b |
visible bands λ (ε) b |
|
|---|---|---|---|---|---|
| yCcP | 282 (77) | 374sh (57) | 408 (98) | 508 (11.3) | 646 (3.3) |
| rCcP | 281 (76) | 374sh (60) | 408 (101) | 504 (11.2) | 641 (3.5) |
| H52L | 282 (86) | 379sh (81) | 404 (97) | 511 (12.7) | 651 (4.0) |
| metMb | 280 (39) | 354sh (37) | 409 (188) | 503 (11.3) | 581 (4.6) |
| yCcP·imidazole | - | - | 412 (85) | 542 (14.7) | 570 (12.9) |
| rCcP·imidazole | - | 360sh (31) | 412 (109) | 540 (10.7) | 574sh (7.9) |
| metMb-imidazole | 280sh (36) | 361 (32) | 415 (146) | 535 (12.9) | 365sh (9.1) |
| yCcP·MIM | - | 350 (28) | 416 (131) | 542 (13.3) | 572 (11.9) |
| rCcP·MIM | - | 346 (20) | 420 (121) | 544 (12.4) | 574sh (10.1) |
| H52L-MIM | - | 420 (133) | 544 (13.7) | 572sh (12.8) | |
| metMb·MIM | 280sh (35) | 357 (28) | 417 (141) | 534 (13.2) | 562sh (9.8) |
Abbreviations: MIM, 1-methylimidazole.
λ, wavelength in nm. ε, extinction coefficient in mM−1 cm−1.
A titration of rCcP with imidazole at pH 7.0 is shown in Fig. S1 of Appendix A, Supplementary Material provided with this article. The best-fit value for of the rCcP/imidazole complex is 4.6 ± 0.8 M, about 40% larger than for the yCcP/imidazole complex, Table 1. In addition, there are minor differences in the spectroscopic changes that occur upon addition of imidazole to rCcP compared to the changes upon addition of imidazole to yCcP. The most obvious is that the maximum absorbance change occurs at 420 nm during the rCcP-imidazole titration compared to 436 nm in the yCcP-imidazole titration, Figs. 2B and S1B. In addition, the intensity of the Soret band increases upon addition imidazole to rCcP in contrast to the decrease in Soret intensity as imidazole is added to yCcP, Figs. 2A and S1A. The calculated spectra of the imidazole complexes of yCcP and rCcP are somewhat different, with α, β, and γ (Soret) bands occurring at 570, 542, and 412 for the yCcP-imidazole complex and 574, 540, and 412 nm for the rCcP-imidazole complex, Table 2. Most notable is that the extinction coefficient at the Soret maximum is 85 mM−1 cm−1 for the yCcP-imidazole complex and 109 mM−1 cm−1 for the rCcP-imidazole complex. As will be discussed later, these differences are attributed to differences in ionization of the bound imidazole to imidazolate in the two complexes.
The binding of 1-methylimidazole (MIM) to yCcP causes an increase in the absorptivity in the Soret band with a red-shift in peak position to 416 nm Fig. 3A. Prominent α and β bands appear at 572 and 542 nm, respectively. The difference spectra, Fig. 3B, has the largest increase in absorptivity at 420 nm for the yCcP/MIM complex. The equilibrium titration curve is shown in Fig. 3C. Non-linear regression gives a best-fit value for of the yCcP/MIM complex of 0.85 ± 0.11 M. The calculated spectrum of the yCcP/MIM complex is shown in Fig. 3A and selected spectral parameters collected in Table 2.
Figure 3.
yCcP/MIM titration at pH 7.0
Titration of yCcP with 1-methylimidazole (MIM) at pH 7.0. Panel A. Spectrum of yCcP (thin solid line) and 1.0 M MIM (dashed double-dotted line). Spectrum of yCcP in the presence of increasing concentrations of MIM (dashed lines). The spectra have been corrected for the absorbance of MIM and are not shown below 300 nm due to distortion in the corrected spectra. Calculated spectrum of the yCcP/MIM complex (thick solid line). The α, β, and Soret bands occur at 572, 542, and 416 nm, respectively, for the yCcP/MIM complex. Panel B. Difference spectra in the presence and absence of increasing concentrations of imidazole. The maximum increase and decrease in absorbance in the Soret region occurs at 420 and 380 nm, respectively. The thick solid line represents the difference spectrum between the yCcP/MIM complex and yCcP. Panel C. The difference in Δ(A420 – A380) for mixtures of yCcP and MIM equilibrated for 24 hours plotted as a function of the MIM concentration. The data were fit to a simple hyperbolic titration equation, Eq. 1 of the text, using non-linear least squares regression. The solid line is the calculated best-fit line with . Experimental conditions: [yCcP] = 4.45 μM, [MIM] = 0.099 to 0.977 M, 1.0 M ionic strength potassium phosphate buffer, pH 7.0, 25 °C.
The binding of 1-methylimidazole (MIM) to rCcP causes an increase in the absorptivity in the Soret band with a red-shift in peak position to 420 nm Fig. S2A (Supplementary Material). Prominent α and β bands appear at 574 and 544 nm, respectively. The difference spectra, Fig. S2B, show maximum and minimum changes in absorptivity at 424 and 384 nm, respectively. The equilibrium titration curve is shown in Fig. S2C. Non-linear regression gives a best-fit value for of the rCcP/MIM complex of 0.63 ± 0.04 M, Table 1. The calculated spectrum of the yCcP/MIM complex is shown in Fig. S2A and selected spectral parameters collected in Table 2.
4-Nitroimidazole (4NI) has limited solubility in aqueous buffer with saturated solutions having a concentration of less than 6 mM at neutral pH [7]. Adding ~5 mM 4NI to both yCcP and rCcP causes a small perturbation to the spectrum of yCcP, Fig. S3 in the Supplementary Material. The absorbance increase at 416 nm is consistent with complex formation. Assuming that the spectrum of the 4NI complexes of yCcP and rCcP are similar to those of other heme protein/4NI complexes, we estimate that about 2% of yCcP and 5% of rCcP is bound to 4NI at the highest ligand concentrations used. This estimate of complex formed gives values of approximately 0.2 M and 0.1 M for the yCcP and rCcP 4NI complexes, respectively, Table 1. The Supplementary Material provides more information about estimating the value for the CcP/4NI complexes. The amount of CcP/4NI complex formed at the highest concentration of ligand used is too small to calculate the spectrum of the CcP/4NI complex with accuracy.
3.2. Equilibrium Binding of Imidazole, 1-Methylimidazole and 4-Nitroimidazole to CcP(H52L)
To determine the involvement of the distal histidine, His-52, in CcP in binding imidazole to CcP, the binding of imidazole, 1-methlyimidazole, and 4-nitroimidazole to CcP(H52L) was investigated. Addition of up to 5 M imidazole to CcP(H52L) causes spectral changes similar to those for imidazole binding to yCcP, Fig. 4A. The Soret band decreases in intensity and shifts to the red, in this case from 406 to 410 nm. There does not appear to be an isosbestic point during the titration and the difference spectrum between CcP(H52L) in the presence and absence of imidazole has different shapes at low and high imidazole concentrations (Fig. 4B). At low imidazole concentrations the difference spectrum in the Soret region has minimum and maximum values near 382 and 418 nm, respectively, while the difference spectrum at high imidazole concentration has minimum and maximum values near 382 and 442 nm, respectively. The increase in absorbance at 418 nm in the first phase of the reaction indicates bound imidazole while the increase at 442 nm in the second phase of the reaction indicates bound imidazolate.
Figure 4.
CcP(H52L)/Imidazole titration at pH 7.0
Titration of CcP(H52L) with imidazole. Panel A. Spectrum of CcP(H52L) with Soret maximum at 406 nm (solid line). Spectra of CcP(H52L) in the presence of increasing concentrations of imidazole (dashed lines). The spectra have been corrected by subtracting the absorbance due to imidazole. The high absorbance values associated with imidazole distort the corrected spectra and data below 350 nm is not shown. Panel B. Difference spectra between CcP(H52L) in the presence increasing concentrations of imidazole (dashed lines). At the lowest concentration of imidazole (0.155 M) the minimum and maximum in the Soret region occur at 382 and 418 nm, respectively. At the highest concentration of imidazole, 4.77 M, the difference spectrum has minimum and maximum at 382 and 442 nm, respectively. Panel C. Plot of the difference in Δ(A442 –A382) as a function of imidazole concentration. Samples incubated between 24 and 27 hours. The data were fit to a biphasic titration equation, Eq. 2 of the text, using non-linear least squares regression. The solid line is the calculated using the best-fit parameters (see text). Experimental conditions: [CcP(H52L)] = 8.66 μM, [Imidazole] = 0.155 to 4.77 M, pH 7.0, 1.0 M ionic strength potassium phosphate buffer, 25 °C.
A plot of the difference in absorbance at 442 and 382 nm as a function of the imidazole concentration is shown in Fig. 4C. The equilibrium titration curve is biphasic. The plot of Δ(A442 – A382) versus imidazole concentration was fit to Eq. 2 using non- linear least-squares
| (2) |
regression. The best-fit value of ΔA0 is −0.496 ± 0.003. The parameters for the high-affinity phase are designated ΔAh and and have best-fit values of 0.150 ± 0.012 and 0.13 ± 0.04 M, respectively. The parameters for the low-affinity phase are designated ΔA1 and . The last term does not saturate and only the ratio of can be determined. The best-fit value of is (3.6 ± 0.3) × 10−2 M−1. Since the low-affinity phase shows no sign of saturation at 5 M imidazole, must be greater than 5 M. An upper limit of 14 M for can be determined, assuming that the CcP(H52L)/imidazolate complex has a spectrum similar to that of the metMb/imidazolate complex [6]. The value of is reported as 9.5 ± 4.5 M in Table 1. The very large value of prevents saturation of the H52L mutant with imidazole and the spectrum of fully-formed imidazole/CcP(H52L) complex cannot be determined.
Addition of 1-methylimidazole to CcP(H52L) causes a red-shift of the Soret band, Fig. 5A. In the visible region of the spectrum, the absorbance in the charge-transfer bands at 508 and 650 nm decreases, accompanied by increases in absorbance in the α and β bands at 574 and 544 nm, respectively. The difference spectrum between the CcP(H52L) in the presence and absence of MIM is shown in Fig. 5B. The largest change in absorbance occurs at 424 nm.
Figure 5.
CcP(H52L)/MIM titration at pH 7.0
Titration of CcP(H52L) with 1-methylimidazole (MIM) at pH 7.0. Panel A. Spectrum of CcP(H52L) (thin solid line) and 1.0 M MIM (dashed double-dotted line). Spectra of CcP(H52L) in the presence of increasing concentrations of MIM (dashed lines). The spectra have been corrected for the absorbance of MIM and are not shown below ~370 nm due to distortion in the corrected spectra. Calculated spectrum of the CcP(H52L)/MIM complex (thick solid line). Panel B. Difference spectra of CcP(H52L) in the presence of increasing concentrations of MIM. (dashed lines). Difference spectrum between the CcP(H52L)/MIM complex and CcP(H52L) (thick solid line). Panel C. Change in Δ(A424 – A508) as a function of the MIM concentration The data were fit to a simple hyperbolic titration equation, Eq. 1 of the text, using non-linear least squares regression. The solid line is the calculated best-fit line with . Experimental conditions: [CcP(H52L)] = 7.94 μM, [MIM] = 0.00 to 0.969 M, 1.0 M ionic strength potassium phosphate buffer, pH 7.0, 25 °C.
The binding of MIM to CcP(H52L) was followed by monitoring the changes in absorbance at 424 and 508 nm as a function of the 1-methylimidazole concentration, Fig. 5C. The change in absorbance fits a simple hyperbolic binding curve, Eq. 1, with a value of 1.1 ± 0.2 M, Table 1. The calculated spectrum for 100% complex formation is shown in Fig. 5A and selected spectral parameters are given in Table 2.
Addition of a near-saturated solution of 4-nitroimidazole to CcP(H52L) causes small perturbations to the spectrum of the mutant (Fig. S4 in the Supplementary Material). The spectral perturbations are larger than those observed for the yCcP/4NI interaction and we estimate that about 14% of the CcP(H52L)/4NI complex is formed at 4.80 mM 4NI. The estimated fraction of complex formation leads to a value of ~0.03 M for the CcP(H52L)/4NI complex at pH 7.0, Table 1. The Supplementary Material provides additional information on estimating for the CcP(H52L)/4NI complex.
3.3. pH Dependence of 1-Methylmidazole Binding to CcP
The pH dependencies of ligand binding are of interest since information about enzyme discrimination between protonated and unprotonated forms of the ligand as well as the involvement of any acidic or basic groups within the protein can be obtained. Unfortunately, the binding of imidazole and 4NI to yCcP is too weak to be able to determine the pH dependence of binding with sufficient accuracy to be useful. However, yCcP binds MIM strongly enough that the pH dependence of for the yCcP/MIM complex can be determined between pH 4.0 and 7.5. At each pH, the absorbance changes upon MIM addition were hyperbolic and the data fit to Eq. 1. Best-fit values for as a function of pH are shown in Fig. 6. To obtain the limiting values of at high and low pH, the values were fit to an equation representing a single acid/base ionization, Eq. 3. In Eq. 3, KDa and KDb are the acidic and basic limits of ,
| (3) |
respectively, and Ka is the acid dissociation constant of the ionizable group responsible for the pH dependence of MIM binding. The values of KDa and KDb for the yCcP/MIM complex are 0.20 ± 0.03 M and 0.82 ± 0.09 M, respectively and the pKa value for the ionizable group is 5.2 ± 0.3. Note that the pKa of the ionizable group affecting MIM binding is not that of MIM itself, which is 7.2, Scheme 1 [30].
Figure 6.
pH Dependence of for the yCcP/MIM Reaction
pH dependence of the equilibrium dissociation constant, , for the CcP/MIM complex. The data are plotted as . The data were fit to an equation representing a single acid-base ionization, Eq. 3 of the text, using non-linear least-squares regression. The apparent pKa for the acidic group affecting MIM binding affinity is 5.2 ± 0.3.
3.4. Kinetics of 1-Methyimidazole Binding to CcP
The kinetics of both imidazole and 1-methylimidazole binding to CcP are complex with evidence of at least two kinetic phases for each system. We will present the results of 1-methylimidazole binding first since it is in some respects simpler than the kinetics of imidazole binding. The first phase of the MIM/CcP reaction is complete within the time it takes to mix the two reactants manually, Fig. S5 Supplementary Material. The initial reaction is followed by a slower reaction that occurs over the period of several hours. Fig. 7 shows the reaction between 9.07 μM rCcP and 0.992 M MIM over 4 hours at pH 7.0. The first spectrum was determined within 30 s of mixing the two reactants and a substantial change in the spectrum is already apparent, Fig. 7A. This change is attributed to initial binding of MIM to the heme iron in CcP. Over the course of four hours the spectrum continues to change consistent with an apparent first order reaction. The difference spectra, Fig. 7B, provide evidence that the spectrum of the initial reaction product is different than the spectrum of the final reaction product. The maximum change in absorbance in the 30 s spectrum occurs at 428 nm while the maximum change in the spectrum after 4 h occurs at 424 nm. Fitting the slow absorbance change to an exponential equation gives an observed rate constant of (1.13 ± 0.06) × 10−4 s−1. The rate constant for the slow absorbance change is denoted and the rate constant for the fast initial absorbance change is denoted by . Although we cannot determine for the CcP/MIM reaction we can determine for the CcP/imidazole reaction (see below).
Figure 7.
Reaction of rCcP with MIM at pH 7.0
Time dependence of the spectrum of rCcP upon addition of 1-methylimidazole (MIM) at pH 7.0. Panel A. rCcP (dashed line). Spectra of the reaction mixture (solid lines) at 10 minute intervals, beginning ~30 seconds after mixing the reactants and continuing for 4 hours. Dashed-dotted line – spectrum of 1.0 M MIM. All spectra have been corrected by subtracting the absorbance due to MIM. Panel B. Difference spectra between the corrected absorbance of reaction mixture and rCcP. The first difference spectrum is determined withi ~30 seconds of mixing and the following at 10 minute intervals for four hours. Panel C. Time dependence of the difference in absorbance at 424 and 388 nm. The data were fit to a single exponential function using non-linear least squares regression, with the best-fit shown by the solid line. The dashed line shows the expected value of Δ(A424 – A388) if no reaction were to occur. Experimental conditions: [rCcP] = 9.07 μM, [MIM] = 0.992 M, pH 7.0, 1.0 M ionic strength potassium phosphate buffer, 25 °C.
The value of for the yCcP/MIM reaction was determined as a function of both the MIM concentration and the pH, Fig. 7. Between pH 5.5 and 6.5, Fig. 8A, decreases with increasing concentration of MIM similar to the concentration dependence of 1,2-dimethylimidazole binding within the Trp-191 cavity of CcP [20]. Between pH 4.1 and 5.0 and at pH 7.0 and 7.5, the value of attains a minimum value at intermediate values of MIM and then increases with increasing ligand concentration, Figs. 8B and 8C. A detailed analysis of the concentration dependence of will be presented in the Discussion section. The kinetics for the rCcP/MIM reaction is similar to that of the yCcP/MIM reaction and is shown as a function of the MIM concentration at pH 7.0, Fig. S6, Supplementary Material.
Figure 8.
Concentration and pH dependence of for yCcP/MIM reaction
Dependence of the observed rate constant, , for the reaction of rCcP and 1-methylimidazole (MIM) on the concentration of MIM at various pH values. Panel A. Data at pH 5.5 (solid circles), pH 6.0 (solid triangles) and pH 6.5 (open circles). Panel B. Data at pH 4.1 (solid circles), pH 4.5 (open circles), and pH 5.0 (solid triangle). Panel C. Data at pH 7.0 (solid circles) –left-hand axis. Data at pH 7.5 (open circles) – right-hand axis. The data were fit to an extended cavity binding model discussed in the text and the solid lines are calculated from the best-fit parameters of the model. Experimental conditions: 1.0 M ionic strength potassium phosphate buffers with 10 mM potassium acetate between pH 4.1 and 5.0, 25 °C.
3.5. Kinetics of Imidazole Binding to CcP
Fig. 9 shows the spectroscopic changes in the Soret region that occur upon manually mixing rCcP and imidazole and following the reaction over a period of four hours at pH 7.0. The initial spectrum was acquired within 30 s of mixing the two reactants and subsequent spectra were obtained at 60 s intervals. Every tenth spectrum is displayed in Fig. 9A and 9B. After mixing, the reactant concentrations were 8.76 μM rCcP and 3.15 M imidazole. All spectra have been corrected for the imidazole absorbance and the distortion in the corrected spectra below about 350 nm is evident. Fig. 9B shows the difference spectrum between the reaction mixture and the free enzyme. The 30 s difference spectrum has a maximum in the Soret region at 416 nm while later spectra have maximum differences at either 418 or 420 nm on the diode-array spectrophotometer. Fig. 9C shows a plot of Δ(A418 – A378) over the course of 4 hours. The biphasic nature of the reaction is evident. Fitting Δ(A418 – A378) to a double exponential equation give best-fit values of (2.13 ± 0.08) × 10−2 s−1 and (2.91 ± 0.01) × 10−4 s−1 for and , respectively.
Figure 9.
Reaction of rCcP with imidazole at pH 7.0
Time dependence of the spectrum of rCcP upon addition of 3.15 M imidazole at pH 7.0. Panel A. rCcP (dashed line). Spectra of the reaction mixture (solid lines) at 10 minute intervals, beginning ~30 seconds after mixing the reactants and continuing for 4 hours. All spectra have been corrected by subtracting the absorbance due to imidazole. Panel B. Difference spectra between the corrected absorbance of reaction mixture and rCcP. The first difference spectrum is determined within ~30 seconds of mixing and the following at 10 minute intervals for four hours. Panel C. Time dependence of the difference in Δ(A418 – A378). The data were fit to a two exponential function using non-linear least squares regression, with the best-fit shown by the solid line. Experimental conditions: [rCcP] = 9.07 μM, [imidazole] = 3.15 M, pH 7.0, 1.0 M ionic strength potassium phosphate buffer, 25 °C.
The dependence of and on the imidazole concentration at pH 7.0 for both rCcP and yCcP is shown in Fig. 10. There is considerable scatter in the data for but it appears as if the rate constant is independent of the ligand concentration. The average value of for the yCcP and rCcP reactions are (4.1 ± 0.6) × 10−2 s−1 and (3.3 ± 1.1) × 10−2 s−1, respectively. The rate constant for the slower reaction, , decreases with increasing imidazole concentration and is consistent with the data for binding imidazole and imidazole derivatives in the Trp-191 cavity of CcP [20].
Figure 10.
and for yCcP and rCcP – Imidazole reactions
Dependence of the observed rate constants for the reaction between CcP and imidazole on the imidazole concentration. Panel A. for the yCcP/imidazole reaction (solid circles) and the rCcP/imidazole reaction (open circles). The solid lines are the average values for for the two proteins. Panel B. for the yCcP/imidazole reaction (solid circles) and the rCcP/imidazole reaction (open circles). The data were fit to an extended cavity binding model discussed in the test and the solid lines are calculated form the best-fit parameters of the model. Experimental conditions: 1.0 M ionic strength potassium phosphate buffer, pH 7.0, 25 °C.
4. Discussion
4.1. Mechanism of Imidazole Binding to CcP
Ligand binding to CcP has long been known to be complex [2,3], even for simple ligands such as cyanide. Three kinetic processes are observed for cyanide binding, the initial bimolecular binding of cyanide to the heme iron followed by two isomerizations observed above pH 7. The fastest isomerization has rates that vary between 0.18 and 1.9 s−1 while the slower has rates that vary between 3.5 × 10−5 s−1 and 4.0 × 10−2 s−1 depending upon the pH.
We observed two kinetic processes during the reaction between imidazole and CcP, Figs. 9 and 10. The rate constant for the faster reaction, is independent of imidazole concentration and , the rate constant for the slower reaction, decreases with imidazole concentration at pH 7.0, Fig. 10. The imidazole concentration dependence of is consistent with ligand binding within the Trp-191 cavity of CcP [20]. This leads us to postulate a mechanism in which the ligand initially binds to the heme iron in CcP followed by ligand binding in the Trp-191 cavity, Eq. 4.
| (4) |
In Eq. 4, ligand binding to the heme is indicated by LH and ligand binding to the Trp-191 cavity is indicated by LC. There are several things to note about the mechanism shown in Eq. 4. The first is that ligand binding to the heme is much faster () than binding into the Trp-191 cavity () but that ligand binding to the heme is much weaker ( values in the molar range, Table 1) than binding in the cavity (apparent KD values in the millimolar range [20,22]). The second is that the mechanism is schematic and does not describe the concentration dependence of the two observed rate constants.
We have little information about the initial binding of the ligand to the heme. For imidazole, is independent of the ligand concentration at pH 7 and has average values of 0.041 ± 0.006 s−1 and 0.033 ± 0.011 s−1 for the yCcP and rCcP, respectively, Fig. 10. We also note that the two reactions illustrated in Eq. 4 account for the total absorbance change in the imidazole/CcP reaction, Fig. 9C, leading to the conclusion that the rate of imidazole binding to the heme is limited by conformational equilibria within CcP. For MIM, the initial binding is too fast to measure using our techniques, Fig. S5, but a lower limit of 0.35 s−1 can be obtained assuming that 97% of the reaction is complete within the 10 s required to mix the reactants. This is about 10 times faster than for the imidazole/CcP reaction and within the range of the fast isomerization reaction observed in the kinetics of cyanide binding to CcP [3].
The concentration dependence of for imidazole binding to both yCcP and rCcP at pH 7.0, Fig. 10, and for MIM binding to rCcP between pH 5.5 and 6.5, Fig 8A, is consistent with ligand binding within the Trp-191 cavity of CcP [20]. However, the absorbance changes we observe in this second phase of the reaction at the high concentrations of ligand used in this study are much larger than those associated with either imidazole or MIM binding within the Trp-191 cavity at the lower concentrations used in previous studies [19,20,22]. We propose that the large absorbance changes are the effect of ligand binding in the Trp-191 cavity on the properties of the heme-bound ligand, i.e., ligand binding in the Trp-191 cavity stabilizes ligand binding at the heme. Support for this idea can be obtained from the present data.
The rate of the first kinetic process is two to three orders of magnitude faster than the rate of the second so that the first reaction can equilibrate before the second has proceeded to any significant extent. We can use the absorbance changes associated with the first phase of the reaction to estimate the equilibrium dissociation constant, designated KD1, for ligand binding to the heme iron in the absence of a ligand occupying the Trp-191 cavity. In addition we can use the total absorbance change from the kinetic studies to determine a kinetically-derived value for the observed equilibrium dissociation constant, where . The value of should be equal to obtained from the overnight incubations and included in Table 1. It should be noted that , represents imidazole binding to the heme iron in the presence of imidazole occupying the Trp-191 cavity. Figs. S7 to S9 (Supplementary Material) shows the determination of both KD1 and for the yCcP/imidazole, the rCcP/imidazole, and the rCcP/MIM complexes. The binding of imidazole and MIM to the heme iron in the absence of a ligand occupying the Trp-191 cavity is so weak that the derived values of KD1 can only be considered estimates. The derived values of KD1 are 47 ± 19, 13 ± 13, and 20 ± 5 M for the yCcP/imidazole, the rCcP/imidazole, and the rCcP/MIM complexes, respectively. The kinetically-derived values of are 3.7 ± 0.5, 5.5 ± 0.9, and 0.71 ± 0.07 M for these complexes, respectively, within experimental error of the values given in Table 1.
Probably the most interesting aspect of the kinetics of imidazole and MIM binding to CcP is the concentration dependence of , Figs. 8 and 10. As mentioned above, the concentration dependence of for imidazole binding to both yCcP and rCcP at pH 7 and for MIM binding to yCcP between pH 5.5 and 6.5, Fig. 8A, are similar to ligand binding in the Trp-191 cavity as described by Cao et al. [20]. However, MIM binding outside of this pH range shows a more complex behavior with an initial decrease in followed by an increase at higher MIM concentrations. The effect is most pronounce at the pH extremes, pH 4.1, 7.0 and 7.5, Fig. 8B and 8C. We propose an extended cavity binding model, shown in Scheme 2, in which the dynamics of the Trp-191 cavity depend upon heme ligation, i.e., whether the heme in CcP is 5-coordinate as in wild-type CcP or whether a ligand such as MIM is bound to the heme. In Scheme 2 we use equilibrium association constants for ease in writing the mechanism and kinetic equations derived from Scheme 2. In the following discussion we will refer to both KA and KD as appropriate.
Scheme 2.
In the absence of added ligand, CcP is about 96% in the closed-cavity form (EC) and 4% in the open-cavity form (EO) [20]. Upon mixing enzyme and ligand (L), an equilibrium is established between EC and the enzyme complex with ligand bound to the heme (LHEC) within a time frame determined by , less than 10 s for MIM. This initial heme binding is very weak, with an estimated value for KD1 = 1/KA1 of 20 M at pH 7, Fig. S9 (Supplementary Material). The fraction of enzyme converted to LHEC will be less than 9% after the first phase of the reaction is complete at the highest MIM concentrations used in the kinetic studies. For the second phase of the reaction, , there are two pathways for ligand to bind within the Trp-191 cavity. In the upper pathway of Scheme 2, ligand binds to the free enzyme in a rate controlled by opening to the Trp-191 crevice as described by Cao et al. [20]. The upper pathway is sufficient to describe the MIM binding data between pH 5.5 and 6.5.
To explain the increase in at high MIM concentrations at the pH values between 4.1 and 5.0 and between 7.0 and 7.5 we need a pathway that is not limited by the rate of crevice opening. The lower pathway in Scheme 2 provides such a mechanism. We propose that the rates of cavity opening and closing are faster when a ligand is bound to the heme group in CcP than in the free enzyme and that the rate-limiting step for ligand binding into the Trp-191 cavity when a ligand is bound to the heme group in CcP, LHEO, is the ligand association rate characterized by the rate constant k4 in Scheme 2.
In Scheme 2, the vertical steps represent ligand binding to the heme in CcP, which are proposed to be in rapid equilibrium compared to the cavity binding steps. Those steps can be characterized by the experimentally determined equilibrium association constant, , which depends upon the pH (Fig. 5) and KA1 = 1/KD1. The horizontal steps in Scheme 2 represent ligand binding into the Trp-191 cavity. In the upper pathway, binding into the cavity of free CcP is limited by the rate of cavity opening, k1, followed by rapid equilibration of ligand with the open form of the enzyme, EO, a step characterized by the equilibrium association constant KA2. In the lower pathway, ligand binds into the Trp-191 cavity of the enzyme species with MIM bound to the heme of CcP, represented by LHEC in Scheme 2. In this pathway, we assume that the open and closed forms of the cavity equilibrate rapidly, K3, compared to the rate of ligand binding within the open cavity, k4.
Using the rate and equilibrium constants defined in Scheme 2, we can derive an expression for over the range of experimental conditions used in this study, Eq. 5. Scheme 2 defines four
| (5) |
rate constants and four equilibrium constants to describe the binding of MIM within the Trp-191 cavity in the CcP/MIM system. The shape of the binding curves shown in Fig. 8 can be described empirically by 3 parameters, two defining the limits of at high and low ligand concentration and one essentially describing the curvature of the plots. In order to use Eq. 5 to fit the data constraints will need to be placed on the parameters. The thermodynamic cycle in Scheme 2 requires that from which one parameter can be eliminated by expressing it in terms of the other variables. We choose to eliminate k−4. is independently determined from the equilibrium experiments, Fig. 6 and Table 1. The values of k1 and k−1 can be estimated to be approximately 1 × 10−4 and 2.4 × 10−3s−1, respectively, from the data of Cao et al. [20] at pH 6. We have found best-fit values for k1 and k−1 of 9.4 × 10−5 and 2.3 × 10−3 s−1, respectively. We assume that these values are independent of pH. We can make a reasonable assumption to eliminate K3. We assume that binding of ligand to the heme in free CcP does not depend on whether the Trp-191 crevice is open or closed. This assumption leads to the relationship that K3 = k1/k−1 = 0.041.
Using the considerations made above, the three undetermined parameters in Scheme 2 are KA1, KA2, and k4. Preliminary fitting showed that three independent parameters, along with the imposed constraints, produced very large variations in the parameters indicating an ill-defined system not amenable to non-linear least squares regression. We made one further assumption to constrain the system. We assume that KA1 is related to in a constant ratio independent of pH. At pH 7, we estimate that KA1 is about 4% of , Fig. S9. In the final fitting procedure we have set leaving only two adjustable parameters to fit the data, KA2 and k4. Best-fit values for KA2 and k4 as a function of pH are provided in Table 3 and are used in Eq. 5 to calculate the solid lines in Fig. 7. The values of KA2 are reasonably constant between pH 4.1 and 6.5 with an average value of 200 ± 70 M−1 (KD2 = 5 ± 2 mM), while k4 averages (4.1 ± 0.6) × 10−2 M−1s−1.
Table 3.
Kinetic parameters for 1-methylimidazole binding into the Trp-191 cavity of the yCcP/MIM complex. a
| pH |
b (M−1) |
KA2 (M−1) |
k4 (M−1 s−1) |
|---|---|---|---|
| 4.1 | 4.72 | (2.94 ± 0.28) × 102 | (1.55 ± 0.08) × 10−1 |
| 4.5 | 4.37 | (9.19 ± 0.52) × 101 | (2.49 ± 0.16) × 10−2 |
| 5.0 | 3.53 | (1.74 ± 0.17) × 102 | (2.72 ± 0.35) × 10−2 |
| 5.5 | 2.48 | (1.73 ± 0.35) × 102 | (1.14 ± 0.03) × 10−2 |
| 6.0 | 1.74 | (2.64 ± 0.18) × 102 | (1.60 ± 0.03) × 10−2 |
| 6.5 | 1.40 | (2.13 ± 0.27) × 102 | (1.26 ± 0.03) × 10−2 |
| 7.0 | 1.30 | (2.13 ± 0.81) × 103 | (9.21 ± 3.17) × 10−3 |
| 7.5 | 1.24 | (4.52 ± 5.10) × 103 | (5.48 ± 1.2) × 100 |
The data in Fig. 7 were fit to Eq. 5 for the Extended Cavity Model – Scheme 2 in the text. Four of the parameters defined in Scheme 2 have fixed values, independent of pH: k1 = 9.4 × 10−5 s−1; k−1 = 2.3 × 10−3 s−1; K3 = 0.041. KA1 varies with pH according to the relationship KA1 = 0.04.
was calculated from the best-fit line shown in Fig. 5.
Scheme 2 is consistent with the general features of the pH and concentration dependence of over a broad range of experimental conditions and the kinetic parameters are reasonable when compared to independently determined values where possible. The assumptions made, primarily that ligand binding to the heme changes the crevice dynamics, that the crevice dynamics are independent of pH, and that ratio of KA1 to is independent of pH await further investigation.
4.2. Heme Protein/Imidazole Binding Affinity
Imidazole is a very important ligand for heme proteins. The imidazole side-chain of histidine occupies the fifth coordination site of the heme iron in many heme proteins including myoglobin [31], hemoglobin [32], cytochromes a, b, and c [33-35], the peroxidases [36], the heme sensor proteins such as FixL [37] and the direct oxygen sensor of E. coli [38]. The b-type cytochromes have bis-imidazole coordination, with imidazole side-chains bound to both axial coordination-sites on the heme [34]. In general, exogenous imidazole will bind to five-coordinate heme proteins or to heme proteins that have weakly-bound ligands at the sixth coordination site [1].
A brief review of the literature indicates that most studies of imidazole binding to heme proteins have been done with two classes of heme proteins, the globins and the c-type cytochromes. Interestingly, the reported KD values for the imidazole complexes of these two classes span about the same range of affinities. The low end of the affinity range is exemplified by sperm whale metmyoglobin and horse cytochrome c with KD values of 26 and 34 mM for their imidazole complexes, respectively [18,39], and the high end exemplified by Chironomus plumosus metHb and Rhodobacter sphaeroides cytochrome c1, with KD values of 29 and 33 μM for their imidazole complexes, respectively [40,41]. The binding affinity for each class spans about three orders of magnitude. The range of ligand affinities is due to differences in the heme environment within each protein. The effect of the heme environment can be investigated using site-directed mutagenesis. The highest reported imidazole affinity that we could find in the literature is for a mutant of Rhodobacter capsulatus cytochrome c2 in which the methionine that coordinates to the heme iron in the wild-type cytochrome is replaced with an asparagine residue [42]. The imidazole complex of R. capsulatus cytochrome c2(M96N) has a KD value of 1.8 μM about 400-fold smaller than that of the wild-type cytochrome.
There have been a few studies of imidazole binding to heme enzymes. Imidazole complexes of nitric oxide synthase and cytochrome P450CAM have KD values of 63 and 400 μM, respectively [43, 44], within the range of imidazole affinities shown by the globins and the c-type cytochromes. Only partial formation of a horseradish peroxidase (HRP)/imidazole complex was observed at pH 9.3 [45], while the chloroperoxidase/imidazole complex has a reported KD value of 0.25 M at pH 6 [46], the largest reported KD value for any heme protein/imidazole complex prior to the current study.
The investigation of imidazole binding to CcP is complicated by binding of imidazole within a cavity created by movement of Trp-191 from the interior of CcP to the surface. Binding of imidazole within the Trp-191 cavity is about two orders of magnitude stronger than binding to the heme iron in CcP. This means that our equilibrium studies of imidazole binding to the heme iron in CcP are actually studies of binding to a CcP molecule with imidazole bound in the Trp-191 cavity. The weak imidazole binding shown by HRP, CPO, and CcP suggest that the peroxidases, as a class, have very low affinity for imidazole. Importantly, this study extends the span of observed imidazole affinities by heme proteins to over six orders of magnitude, with KD values ranging from 1.8 μM for R. capsulatus cytochrome c2(M96N) to 4.6 M for rCcP.
4.3. Ionization of CcP-Bound Imidazole
Bound imidazole can exist in two forms, either as neutral imidazole or the imidazolate anion. Model studies have shown that the electronic absorption spectra of imidazole/heme and imidazolate/heme complexes can be distinguished. The Soret bands of model heme/bis-imidazole complexes shift about 9 nm to the red in highly alkaline solutions, consistent with formation of the bis-imidazolate complexes [47-49]. The Soret bands of the horse metMb/imidazole and metHb/imidazole complexes shift from 414 and 412 at neutral pH to 438 and 436 nm above pH 11 [6, 50] while the Soret band of the C. plumosus metHb/imidazole complex shifts from 413 to 417 nm upon alkalization [40]. In addition, the Soret bands of the imidazole complexes decrease between 13% and 44% upon ionization to the imidazolate complexes.
The binding of imidazole to yCcP causes a 4 nm red-shift in the position of the Soret band and a decrease absorptivity at the Soret maximum, Fig. 2A. The Soret band is broad with a significant increase in absorbance near 436 nm, Fig. 2B. The decrease in the overall intensity of the Soret band along with increased absorbance at 436 nm suggest that the yCcP/imidazole complex at pH 7 is a mixture of at least two species, including both imidazole and imidazolate forms [6, 50]. This suggestion is strengthened by comparison of the spectral properties of the yCcP/imidazole complex with those of the yCcP/MIM complex, which cannot form a bound imidazolate species, Fig. 2. Upon addition of MIM to yCcP, the Soret band shifts from 408 nm to 416 nm with an increase in absorptivity. The difference spectrum shows a maximum difference at 420 nm, consistent with the 416 nm maximum for the Soret band.
We can estimate the fraction of bound imidazole and imidazolate in the yCcP/imidazole complex at pH 7 from the absorbance increase at 436 nm if we can establish reasonable values for the extinction coefficients of the imidazolate complex and the neutral imidazole complex at this wavelength. The alkaline imidazolate complexes of metMb and metHb provide suitable models for the imidazolate form of the complex and these species have extinction coefficients of 86 and 94 mM−1 cm−1 at 436 and 438 nm, respectively [6,50]. As models for the neutral imidazole/yCcP complex we use the spectra of the metMb/imidazole complex, Table 2, and of the imidazole complexes of CcP(triVal) and CcP(triLeu) [23]. These species have extinction coefficients of between 124 and 146 at their Soret maxima and extinction coefficients of between 25 and 31 mM−1 cm−1 at 436 nm. Using the range of extinction coefficients at 436 nm, we calculate that between 22 and 32% of the bound imidazole in the yCcP complex is ionized at pH 7.0, Fig. 2. Using these same extinction coefficients we calculate that only 1 to 11% of the bound imidazole is ionized in the rCcP/imidazole complex at pH 7.0, Fig. S1 (Supplementary Material). The difference in ionization gives rise to the difference in spectroscopic properties for the yCcP and rCcP imidazole complexes.
The estimates for the fraction of bound imidazolate anion in the yCcP and rCcP imidazole complexes at pH 7 allows us to calculate estimated pKa values for ionization of bound imidazole. The estimated pKa values are of 7.4 ± 0.2 and 8.2 ± 0.5 for the yCcP and rCcP imidazole complexes, respectively. These pKa values are about 7 and 6 units lower than that of free imidazole, Scheme 1, and about 3 and 2 units lower than the equivalent pKa values for metMb and metHb [18, 50]. The most acidic of all heme-bound imidazoles is that of soybean legHb, with a reported pKa of between 6.5 and 7.0 [51].
4.4. pH Dependence 1-Methylimidazole Binding to CcP
The binding of 1-methylimidazole is very similar to binding of imidazole in both model Fe(III) porphyrins and heme proteins, with binding affinities for the two ligands generally within a factor of 5 of one another [6,39,40] although exceptions have been reported [41]. CcP fits within this pattern, binding MIM is about four times stronger than imidazole at pH 7. The increase in affinity has allowed us to determine the pH dependence of MIM binding to CcP over the pH range 4.0 to 7.5, Fig. 6. Interestingly, the binding is relatively independent of pH, increasing by only a factor of 4 as the pH decreases from pH 7.5 to 4.0. Although small, the increase in affinity for MIM by the heme iron in CcP with decreasing pH is unique among heme proteins. All heme proteins studied to date show decreasing affinity for imidazole with decreasing pH below pH 7 due to formation of the imidazolium ion [6,12,39,40,52]. The unique pH dependence of MIM binding as well as the multiphasic kinetics emphasize the complexity of imidazole and MIM binding to CcP. The apparent KD values shown in Fig. 6 depend upon the initial ligand binding equilibria as well as any reactions that may stabilize the initially formed complex. Because of the complexity of the binding reaction we cannot identify the ionizable group that affects MIM binding, i.e., the group responsible for the apparent pKa of 5.2, Fig. 6.
Metmyoglobin shows very little discrimination in the rates of imidazole and imidazolium ion binding [6,7,9,10]. When imidazolium ion binds to the heme iron, it must release the imidazolium proton. The fate of the released proton affects the pH dependence of binding. MetMb releases the acidic proton from ligands such as the imidazolium ion, HF, HCN, and HN3 into solution [6-8] while the proton is retained within the peroxidase complexes, binding to the distal histidine [8,53]. For MIM binding to CcP, we suggest that there is little discrimination between the neutral base and the imidazolium cation in terms of entry into the distal heme pocket and binding to the heme iron, similar to the findings for metMb. Upon imidazolium ion binding to the heme, the imidazolium proton is transferred to the distal histidine. With little discrimination between binding of imidazole and the imidazolium ion and with retention of the imidazolium proton within the complex, the formation of the initial CcP/MIM complex would be relatively independent of pH. Stabilization of the initial complex by binding MIM in the Trp-191 cavity could increase the overall binding affinity at low pH, Fig. 6.
4.5. Binding Imidazole and Imidazole Derivatives to CcP(H52L)
From the work of Alberty and colleagues [9,10], imidazole and the imidazolium ion can enter the heme pocket and bind to the heme with similar rates. Base catalysis of imidazole binding to the heme iron in CcP by the distal histidine should not be required and we anticipated that removal of the His-52 would have little effect upon the binding of imidazole and its derivatives. As anticipated, replacing the distal histidine with an apolar residue has little effect on the binding of MIM, Table 1. Binding of 4NI to both CcP and CcP(H52L) is weak but CcP(H52L) has an estimated 7-fold higher affinity for 4NI than does CcP, Table 1.
However, binding of imidazole to CcP(H52L) is more complex than binding to CcP. A major difference in the properties of CcP and CcP(H52L) is that binding of imidazole to CcP(H52L) is biphasic. The two phases are assumed to be due to two different conformations of CcP(H52L), which are is slow exchange and have different ligand binding affinities. (It should be noted that multiple forms of the CcP(H52L)/cyanide complex have been detected by NMR studies [54].) The major phase of imidazole binding is the low-affinity phase where an estimated KD of 9.5 ± 4.5 M could be determined from fitting the absorbance data to a biphasic titration curve. This is about 3 times weaker binding of imidazole by yCcP but of the same magnitude. The minor binding phase is the high-affinity phase, which has a 25-fold greater affinity for imidazole than yCcP. As we will see in the accompanying paper [23], making the distal heme pocket more apolar by replacing Arg-48, Trp-51, and His-52 with apolar residues increases the binding affinity for imidazole and the imidazole derivatives by up to 4.5 orders of magnitude. The high-affinity binding of imidazole to CcP(H52L) could be a reflection of decreasing distal pocket polarity in the mutant.
The spectral features of imidazole binding to CcP(H52L), Fig. 4, are also quite interesting. We were not able to go to high enough imidazole concentrations to saturate the mutant with ligand and were unable to calculate the spectrum for 100% complex formation. However, there are aspects of the binding that are similar to binding of imidazole to yCcP, namely that the Soret band shifts to the red by about 4 nm with a decrease in absorptivity. At the higher concentrations of imidazole, the largest increase in absorbance occurs at 442 nm, characteristic of imidazolate binding and similar to the absorbance changes seen with imidazole binding to yCcP, Fig. 2C. However, in contrast to yCcP/imidazole binding, the difference spectrum at low imidazole concentrations has the largest increase in absorbance at 418 nm rather than at 442 nm and is characteristic of neutral imidazole binding. The biphasic nature of imidazole binding to CcP(H52L) suggests that the high-affinity conformation favors neutral imidazole binding while the low-affinity conformation favors imidazolate binding.
4.6. Conclusions
The binding of imidazole to the heme group in CcP is very weak with an apparent equilibrium dissociation constant of 3.3 ± 0.4 at pH 7. This is the weakest imidazole binding to a heme protein reported to date and it is about 120 times weaker than imidazole binding to metmyoglobin. Likewise, the binding of 1-methylimidazole and 4-nitroimidazole to CcP are weak with apparent equilibrium dissociation constants of 0.85 ± 0.11 and ~0.2 M, respectively. Spectroscopic changes associated with imidazole and 1-methylimidazole binding to yCcP suggest partial ionization of bound imidazole to imidazolate. The pKa for ionization of bound imidazole is estimated to be 7.4 ± 0.2, about 7 units lower than that of free imidazole and about 3 units lower than imidazole bound to metmyoglobin.
Replacing the distal histidine residue with leucine has relatively little effect on the binding of imidazole and 1-methylimidazole. Equilibrium binding of imidazole to CcP(H52L) is biphasic with low- and high-affinity phases having values of 9.5 ± 4.5 and 0.13 ± 0.04 M, respectively. CcP(H52L) binding of 1-methylimidazole is monophasic with an affinity similar to those of yCcP and rCcP.
Binding of 1-methylimidazole to rCcP is associated with two kinetic phases, the initial binding complete within 10 s, followed by a process that is consistent with 1-methylimidazole binding to a cavity created by movement of Trp-191 from the interior of the protein to the surface. Both the equilibrium binding and kinetics of 1-methylimidazole binding to yCcP are pH dependent. yCcP has a four-fold increase in 1-methylimidazole binding affinity on decreasing the pH from 7.5 to 4.0, an observation that is unique among the many studies on binding of imidazole and imidazole derivatives to heme proteins.
Supplementary Material
Highlights.
CcP binds two molecules of imidazole, one to the heme and one in the Trp-191 cavity
The heme in CcP has the lowest reported affinity for imidazole of any heme protein
The pKA for heme-bound imidazole in CcP is 7.4 compared to 14.4 for free imidazole
CcPs binding affinity for 1-methylimidazole increases 4-fold between pH 7.5 and 4.0
Abbreviations
- CcP
generic abbreviation for cytochrome c peroxidase
- yCcP
authentic yeast cytochrome c peroxidase isolated from S. cervisiae
- rCcP
recombinant cytochrome c peroxidase expressed in E. coli with an amino acid sequence identical to that of yCcP
- metMb
metmyoglobin
- metHb
methemoglobin
- HRP
horseradish peroxidase
- CPO
chloroperoxidase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors attest that they have no potential conflict of interest including financial, personal, or other relationships that could inappropriately influence, or be perceived to influence, this work.
Appendix A. Supplementary Material
Supplementary material associated with this article can be found in the online version at doi:
References
- [1].Antonini E, Brunori M. Hemoglobin and myoglobin in their reaction with ligands. North-Holland Publishing Company; Amsterdam - London: 1971. [Google Scholar]
- [2].Erman JE. Kinetic studies of fluoride binding by cytochrome c peroxidase. Biochemistry. 1974;13:34–39. doi: 10.1021/bi00698a006. [DOI] [PubMed] [Google Scholar]
- [3].Erman JE. Kinetic and equilibrium studies of cyanide binding by cytochrome c peroxidase. Biochemistry. 1974;13:39–44. doi: 10.1021/bi00698a007. [DOI] [PubMed] [Google Scholar]
- [4].Lent B, Conroy CW, Erman JE. The effect of ionic strength on the kinetics of fluoride biding to cytochrome c peroxidase. Arch. Biochem. Biophys. 1976;177:56–61. doi: 10.1016/0003-9861(76)90415-x. [DOI] [PubMed] [Google Scholar]
- [5].Merryweather J, Summers F, Vitello LB, Erman JE. Metmyoglobin/fluoride: effect of distal histidine protonation on the association and dissociation rate constants. Arch. Biochem. Biophys. 1998;358:359–368. doi: 10.1006/abbi.1998.0872. [DOI] [PubMed] [Google Scholar]
- [6].Lin J, Vitello LB, Erman JE. Imidazole binding to horse metmyoglobin: dependence upon pH and ionic strength. Arch. Biochem. Biophys. 1998;352:214–228. doi: 10.1006/abbi.1998.0619. [DOI] [PubMed] [Google Scholar]
- [7].Taylor KC, Vitello LB, Erman JE. 4-Nitroimidazole binding to horse metmyoglobin: evidence for preferential anion binding. Arch. Biochem. Biophys. 2000;382:284–295. doi: 10.1006/abbi.2000.2039. [DOI] [PubMed] [Google Scholar]
- [8].Jacobson T, Williamson J, Wasilewski A, Felesik J, Vitello LB, Erman JE. Azide binding to yeast cytochrome c peroxidase and horse metmyoglobin: comparative thermodynamic investigation using isothermal titration calorimetry. Arch. Biochem. Biophys. 2004;382:25–136. doi: 10.1016/j.abb.2003.12.016. [DOI] [PubMed] [Google Scholar]
- [9].Divan WF, Goldsack DE, Alberty RA. Temperature jump kinetic studies of the binding of imidazole by sperm whale metmyoglobin. J. Biol. Chem. 1965;240:2437–2441. [PubMed] [Google Scholar]
- [10].Goldsack DE, Eberlein WS, Alberty RA. Temperature jump studies of sperm whale metmyoglobin. III. Effect of heme-linked groups on ligand binding. J. Biol. Chem. 1966;241:2653–2660. [PubMed] [Google Scholar]
- [11].Morishima I, Neya S, Yonezawa T. Proton NMR study of hemproteins. Ionization and orientation of iron-bound imidazole in methemoglobin and metmyoglobin. Biochim. Biophys. Acta, Protein Structure. 1980;621:218–226. doi: 10.1016/0005-2795(80)90173-7. [DOI] [PubMed] [Google Scholar]
- [12].Bolognese M, Cannillo E, Ascenzi P, Giacometti GM, Merli A, Brunori M. Reactivity of ferric Aplysia and sperm whale myoglobins towards imidazole. X-ray and binding study. J. Mol. Biol. 1982;158:305–315. doi: 10.1016/0022-2836(82)90435-1. [DOI] [PubMed] [Google Scholar]
- [13].Lionetti C, Guanziroli MG, Frigerio F, Ascenzi P, Bolognesi M. X-ray crystal structure of the ferric sperm whale myoglobin: imidazole complex at 2.0 Å resolution. J. Mol. Biol. 1991;217:409–412. doi: 10.1016/0022-2836(91)90744-q. [DOI] [PubMed] [Google Scholar]
- [14].Chen Y, Liu G, Tang W. 1H NMR saturation transfer studies of exogenous ligands binding to metmyoglobin. Spectroscopy Letts. 1996;29:1381–1396. [Google Scholar]
- [15].Yamamoto Y. 1H NMR study of the heme molecular structure in sperm whale met-aquo and met-imidazole myoglobins. Bull. Chem. Soc. Japan. 1996;69:2947–2953. [Google Scholar]
- [16].Qian C, Yai Y, Wu Y, Tang W. Kinetic studies of imidazole and its methyl derivative binding to metmyoglobin: effects of substitute methyl on the binding affinity. BioMetals. 2000;13:267–272. doi: 10.1023/a:1009274731507. [DOI] [PubMed] [Google Scholar]
- [17].Zakariassen H, Sorlie M. Heat capacity changes in heme protein-ligand interactions. Thermochimica Acta. 2007;464:24–28. [Google Scholar]
- [18].George P, Hanania GIH, Irvine DH, Abu-Issa I. The effect of co-ordination on ionization. Part IV. Imidazole and its ferrimyoglobin complex. J. Chem. Soc. 1964:5689–5694. [Google Scholar]
- [19].Fitzgerald MM, Churchill MJ, McRee DE, Goodin DB. Small molecule binding to an artificially created cavity at the active site of cytochrome c peroxidase. Biochemistry. 1994;33:3807–3818. [PubMed] [Google Scholar]
- [20].Cao Y, Musah RA, Wilcox SK, Goodin DB, McRee DE. Protein conformer selection by ligand binding observed with crystallography. Protein Science. 1998;7:72–78. doi: 10.1002/pro.5560070107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bonagura CA, Sundaramoorthy M, Bhaskar B, Poulos TL. The effects of an engineered cation site on the structure, activity, and EPR properties of cytochrome c peroxidase. Biochemistry. 1999;38:5538–5545. doi: 10.1021/bi982996k. [DOI] [PubMed] [Google Scholar]
- [22].Sigman JA, Pond AE, Dawson JH, Lu Y. Engineering cytochrome c peroxidase into cytochrome P450: a proximal effect on heme-thiolate ligation. Biochemistry. 1999;38:11122–11129. doi: 10.1021/bi990815o. [DOI] [PubMed] [Google Scholar]
- [23].Bidwai A, Ayala C, Vitello LB, Erman JE. Apolar distal pocket mutants of yeast cytochrome c peroxidase: binding of imidazole, 1-methylimidazole, and 4-nitroimidazole to the triAla, triVal, and triLeu variants. Biochim. Biophys. Acta. doi: 10.1016/j.bbapap.2015.04.014. submitted for publication - accompanying article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Takio K, Titani K, Ericsson LH, Yonetani T. Primary structure of yeast cytochrome c peroxidase II. The complete amino acid sequence. Arch. Biochem. Biophys. 1980;203:615–6299. doi: 10.1016/0003-9861(80)90219-2. [DOI] [PubMed] [Google Scholar]
- [25].Teske JG, Savenkova MI, Mauro JM, Erman JE, Satterlee JD. Yeast cytochrome c peroxidase expression in Escherichia coli and rapid isolation of various highly purified holoenzymes. Protein Expression Purif. 2000;19:139–147. doi: 10.1006/prep.2000.1220. [DOI] [PubMed] [Google Scholar]
- [26].Yonetani T, Ray GS. Studies on cytochrome c peroxidase. I. Purification and some properties. J. Biol. Chem. 1965;240:4503–4508. [PubMed] [Google Scholar]
- [27].Vitello LB, Huang M, Erman JE. pH-dependent spectral and kinetic properties of cytochrome c peroxidase: comparison of freshly isolated and stored enzyme. Biochemistry. 1990;29:4283–4288. doi: 10.1021/bi00470a004. [DOI] [PubMed] [Google Scholar]
- [28].Erman JE, Vitello LB, Miller MA, Shaw A, Browne KA, Kraut J. Histidine 52 is a critical residue for rapid formation of cytochrome c peroxidase compound I. Biochemistry. 1993;32:9798–9806. doi: 10.1021/bi00088a035. [DOI] [PubMed] [Google Scholar]
- [29].Pearl NM, Jacobson T, Arisa M, Vitello LB, Erman JE. Effect of single-site charge-reversal mutations on the catalytic properties of yeast cytochrome c peroxidase: Mutations near the high-affinity cytochrome c binding site. Biochemistry. 2007;46:8263–8272. doi: 10.1021/bi700623u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Eilbeck WJ, West MS. Thermochemical studies of vitamin B12. Part II. Thermodynamic data for the interaction of imidazole and methylimidazoles with aquocobalamin (vitamin B12) J. Chem. Soc. Dalton Trans. 1976:274–278. [Google Scholar]
- [31].Kendrew JC, Dickerson RE, Strandberg BE, Hart RG, Davies DR. Structure of myoglobin. A three-dimensional fourier synthesis at 2 Å resolution. Nature (London) 1960;185:422–427. doi: 10.1038/185422a0. [DOI] [PubMed] [Google Scholar]
- [32].Bolton W, Perutz MF. Three dimensional fourier synthesis of horse deoxyhaemoglobin at 2.8 angstrom units resolution. Nature. 1964;228:551–552. doi: 10.1038/228551a0. [DOI] [PubMed] [Google Scholar]
- [33].Martin CT, Scholes CP, Chan SI. The identification of histidine ligands to cytochrome a in cytochrome c oxidase. J. Biol. Chem. 1985;260:2857–2861. [PubMed] [Google Scholar]
- [34].Durley RCE, Mathews FS. Refinement and structural analysis of bovine cytochrome b5 at 1.5 Å resolution. Acta Cryst. D. 1996;52:65–76. doi: 10.1107/S0907444995007827. [DOI] [PubMed] [Google Scholar]
- [35].Dickerson RE, Takano T, Eisenberg D, Kallai OB, Samson L, Cooper A, Margoliash E. Ferricytochrome c. I. General features of the horse and bonito proteins at 2.8 Å resolution. J. Biol. Chem. 1971;246:1511–1535. [PubMed] [Google Scholar]
- [36].Finzel BC, Poulos TL, Kraut J. Crystal structure of yeast cytochrome c peroxidase refined at 1.7-Å resolution. J. Biol. Chem. 1984;259:13027–13036. [PubMed] [Google Scholar]
- [37].Gong W, Hao B, Mansy SS, Gonzalez G, Gilles-Gonzalez MA, Chan MK. Structure of a biological oxygen sensor: A new mechanism for heme-driven signal transduction. Proc. Natl. Acad. Sci. USA. 1998;95:15177–15182. doi: 10.1073/pnas.95.26.15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Park H, Suquet C, Satterlee JD, Kang CH. Insights into signal transduction involving PAS domain oxygen-sensing heme proteins from the x-ray crystal structure of Escherichia Coli Dos heme domain (Ec DosH) Biochemistry. 2004;43:2738–2746. doi: 10.1021/bi035980p. [DOI] [PubMed] [Google Scholar]
- [39].Schejter A, Aviram I. The reaction of cytochrome c with imidazole. Biochemistry. 1969;8:149–153. doi: 10.1021/bi00829a021. [DOI] [PubMed] [Google Scholar]
- [40].Mohr P, Scheler W, Schumann H, Müller K. Ligand-protein interactions in imidazole and 1,2,4-triazole complexes of methaemoglobin from Chironomus plumosus. Eur. J. Biochem. 1967;3:158–163. doi: 10.1111/j.1432-1033.1967.tb19511.x. [DOI] [PubMed] [Google Scholar]
- [41].Kokhan O, Shinkarev VP, Wright CA. Binding of imidazole to the heme of cytochrome c1 and inhibition of the bc1 complex from Rhodobacter sphaeroides. I. Equilibrium and modeling studies. J. Biol. Chem. 2010;285:22513–22521. doi: 10.1074/jbc.M110.128058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Dumortier C, Fitch J, Van Petegem F, Vermeulen W, Meyer TE, Van Beeumen JJ, Cusanovich MA. Protein dynamics in the region of the sixth ligand methionine revealed by studies of imidazole binding to Rhodobacter capsulatus cytochrome c2 hinge mutants. Biochemistry. 2004;43:7717–7724. doi: 10.1021/bi0362370. [DOI] [PubMed] [Google Scholar]
- [43].Chabin RM, McCauley E, Calaycay JR, Kelly TM, MacNaul KL, Wolfe GC, Hutchinson NI, Madhusudanaraju S, Schmidt JA, Kozarich JW, Wong KK. Active-site structure analysis of recombinant human inducible nitric oxide synthase using imidazole. Biochemistry. 1996;35:9567–9575. doi: 10.1021/bi960476o. [DOI] [PubMed] [Google Scholar]
- [44].Lipscomb JD. Electron paramagnetic resonance detectable states of P-450CAM. Biochemistry. 1980;19:3590–3599. doi: 10.1021/bi00556a027. [DOI] [PubMed] [Google Scholar]
- [45].Howes BD, Feis A, Indiani C, Marzocchi MP, Smulevich G. Formation of two types of low-spin heme in horseradish peroxidase isoenzyme A2 at low temperature. J. Biol. Inorg. Chem. 2000;5:227–235. doi: 10.1007/s007750050367. [DOI] [PubMed] [Google Scholar]
- [46].Sono M, Dawson JH, Hall K, Hager LP. Ligand and halide binding properties of chloroperoxidase: peroxidase-type active site heme environment with cytochrome P-450 type endogenous axial ligand and spectroscopic properties. Biochemistry. 1986;25:347–356. doi: 10.1021/bi00350a011. [DOI] [PubMed] [Google Scholar]
- [47].Peisach J, Mims WB. Linear electric field effect in electron paramagnetic resonance for two bisimidazole-heme complexes, model compounds for B and H hemichromes of hemoglobin and for cytochrome b5. Biochemistry. 1977;16:2795–2799. doi: 10.1021/bi00631a033. [DOI] [PubMed] [Google Scholar]
- [48].Nappa M, Valentine JS, Snyder PA. Imidazolate complexes of ferric porphyrins. J. Am. Chem. Soc. 1977;99:5799–5798. doi: 10.1021/ja00459a045. [DOI] [PubMed] [Google Scholar]
- [49].Quinn R, Nappa M, Valentine JS. New five-and six-coordinate imidazole and imidazolate complexes of ferric tetraphenylporphyrin. J. Amer. Chem. Soc. 1982;103:2588–2595. [Google Scholar]
- [50].Scheler W. Zur physikalischen chemie des methämoglobin-imidazol-komplexes. Acta biol. med. germ. 1959;2:468–480. [PubMed] [Google Scholar]
- [51].Sievers G, Gadsby PMA, Peterson J, Thomson AJ. Magnetic circular dichroism spectra of soybean leghaemoglobin a at room temperature and 4.2 K. Biochim. Biophys. Acta. 1983;742:637–647. [Google Scholar]
- [52].Baldwin DA, Marques HM, Pratt JM. Hemes and hemoproteins, 2: The pH-dependent equilibria of microperoxidase-8 and characterization of the coordination sphere of Fe(III) J. Inorg. Biochem. 1986;27:245–254. doi: 10.1016/0162-0134(86)80065-4. [DOI] [PubMed] [Google Scholar]
- [53].Thanabal V, de Ropp JS, La Mar GN. Proton NMR characterization of the catalytically relevant proximal and distal hydrogen-bonding networks in ligated resting state horseradish peroxidase. J. Amer. Chem. Soc. 1988;110:3027–3035. [Google Scholar]
- [54].Satterlee JD, Savenkova MI, Foshay M, Erman JE. Temperature, pH, and solvent isotope dependent properties of the active sites of resting-state and cyanide-ligated recombinant cytochrome c peroxidase(H52L) revealed by proton hyperfine resonance spectra. Biochemistry. 2003;42:10772–10782. doi: 10.1021/bi034633c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.