Abstract
Patients with Alzheimer’s disease (AD) display amyloidopathy and tauopathy. In mouse models of AD, pharmacological inhibition using small molecule enzyme inhibitors, or genetic inactivation of Acyl-CoA: cholesterol acyltransferase 1 (ACAT1) diminished amyloidopathy and restored cognitive deficits. In microglia, ACAT1 blockage increases autophagosome formation and stimulates amyloid β peptide1–42 degradation. Here we hypothesize that in neurons ACAT1 blockage augments autophagy and increases autophagy-mediated degradation of P301L-tau protein. We tested this possibility in murine neuroblastoma cells ectopically expressing human tau, and in primary neurons isolated from triple transgenic AD (3XTg-AD) mice that express mutant forms of APP, PS1, and human tau. The results show that ACAT1 blockage increases autophagosome formation and decreases P301L-tau protein content without affecting endogenous mouse tau protein content. In vivo, lacking Acat1 decreases P301L-tau protein content in the brains of young 3XTg-AD mice but not in those of old mice, where extensive hyperphosphorylations and aggregation of P301L-tau take place. These results suggest that, in addition to ameliorating amyloidopathy in both young and old AD mice, ACAT1 blockage may benefit AD by reducing tauopathy at early stage.
Keywords: ACAT (SOAT), Alzheimer’s disease, Autophagy, Neuron, Tauopathy
1. Introduction
Alzheimer’s disease (AD) is the most common form of dementia in the aging population. Currently, there is no cure for AD. The hallmark of AD consists of extracellular amyloid plaques, mostly composed of amyloid beta (Aβ) peptides (especially Aβ1–42), and neurofibrillary tangles (NFTs), mostly composed of hyperphosphorylated tau. Aβ peptides are metabolites derived from the amyloid precursor protein (APP) by sequential proteolytic cleavages, as reviewed in (Masters and Selkoe, 2012). Tau is a soluble protein produced abundantly in neurons. Aβ and tau can interact with each other synergistically to trigger the disease (Eckert et al., 2010; Hurtado et al., 2010; Rhein et al., 2009; Vossel et al., 2010). Each toxic seed can also act on its own to accelerate the onset of the disease (Ittner and Götz, 2011). In addition to its involvement in AD, the accumulation of tau into aggregates without the amyloid plaque is associated with key pathological events in frontotemporal lobe degeneration and in other related tauopathies. It has been suggested that tauopathy is a collection of diseases caused by soluble but misfolded, or mislocalized, and/or hyperphosphorylated tau (Kopeikina et al., 2012). In addition to hyperphosphorylation, tau protein can also be modified by site-specific acetylations (Cohen et al., 2011; Cook et al., 2014). Human mutant P301L-tau is particularly prone to misfolding and to site-specific hyperphosphorylations (Terwel et al., 2005).
Acyl-CoA: cholesterol acyltransferase (ACAT) converts free cholesterol to cholesteryl esters and plays important roles in cellular cholesterol homeostasis. There are two ACAT isoforms in mammals, ACAT1 and ACAT2, with different tissue expression patterns. ACAT1 is a resident enzyme at the endoplasmic reticulum (ER) and is ubiquitously expressed in all tissues examined; ACAT2 is mainly expressed in the intestines and hepatocytes, and is also expressed in various other tissues at low levels. Both ACAT1 and ACAT2 are potential drug targets for treating cardiovascular disease, as reviewed in (Chang et al., 2009). Both enzymes are members of the membrane-bound O-acyltransferase (MBOAT) family, which share similar catalytic sites but participate in diverse biological processes (Chang et al., 2011). The roles of ACATs have been studied quite extensively in various systemic tissues; however, their roles in the CNS were unexplored until recently. Previously, it was believed that, although brain is a cholesterol-rich organ, it only contained unesterified cholesterol but no cholesteryl esters. Recent analyses showed that cholesteryl esters do exist in human and mouse brains. Importantly, in the brains of AD patients and in several mouse models for AD, the cholesteryl ester content is significantly elevated in the vulnerable regions affected by AD (Chan et al., 2012; Tajima et al., 2013). In different regions of the mouse brain, ACAT1 is the predominant enzyme for cellular cholesterol esterification (Bryleva et al., 2010). Early work showed that in cells expressing human APP (hAPP), inhibiting ACAT enzyme activity significantly reduced the amount of Aβ secreted into the growth medium (Puglielli et al., 2001). In studies using animal models, Kovacs and colleagues reported that treating a mouse model for AD with isotype-nonspecific ACAT inhibitors reduced Aβ and amyloid plaque levels, and rescued cognitive deficits (Hutter-Paier et al., 2004; Huttunen et al., 2010). Our laboratory showed that in the triple transgenic AD (3XTg-AD) mouse model that expresses hAPP, mutant presenilin-1 (PS1; the catalytic component of γ-secretase) and human mutant P301L-tau (Oddo et al., 2003b), gene knockout (KO) of Acat1 but not of Acat2, reduced human APP (hAPP) and Aβ1–42 levels, and rescued cognitive deficits (Bryleva et al., 2010). The 3XTg-AD mouse model displays memory dysfunction by 9 months of age (Oddo et al., 2003b). In symptomatic 3XTg-AD mice (at 10 months of age), we showed that adeno-associated virus-mediated Acat1 gene knock down (KD) caused reduction in hAPP and in Aβ1–42 levels in treated mice (Murphy et al., 2013). These results suggest that ACAT1 is a promising therapeutic target for AD.
To provide a mechanistic basis for the AD inhibitory actions of ACAT1 blockage, recently, we reported that in primary microglia isolated from neonatal mouse brains and in murine microglial cell line N9, blocking ACAT1 either by genetic inactivation or by using a potent ACAT1-specific inhibitor K604 (Ikenoya et al., 2007) increases autophagosome formation, stimulates lysosomal proteolysis, and facilitates Aβ1–42 peptide degradation in these cells (Shibuya et al., 2014). The enhancing effect of ACAT1 blockage on autophagy is independent of mammalian target of rapamycine (mTOR) signaling or ER stress response, but can be modulated by agents that block endogenous cholesterol biosynthesis. We also showed that the effect of ACAT1 blockage in increasing autophagy is additive with the effect of mTOR inhibitors (Shibuya et al., 2014). This work shows that blocking ACAT1 is a novel way to increase autophagy-mediated lysosomal proteolysis of Aβ1–42 in microglia.
Here we ask whether blocking ACAT1 can also increase autophagy in neurons. To test this possibility, we performed experiments in the mouse neuroblastoma cell line N2a, and in the primary cortical neurons isolated from the 3XTg-AD mouse. Human mutant P301L-tau expressed in the brains of 3XTg-AD mice is subject to autophagy-mediated degradation (Caccamo et al., 2010). Therefore, we also monitored the effect of ACAT1 blockage on human P301L-tau protein content in neurons. To test the in vivo significance of our results obtained in cell culture, we monitored the human P301L-tau protein content in the brains of 3XTg-AD mice with or without Acat1 gene KO.
2. Experimental procedures
2.1. Mice
Acat1 KO on C57BL/6 genetic background was as described (Meiner et al., 1996). The 3XTg-AD with and without Acat1 mouse lines on a mixed 129:C57BL/6 genetic background were produced and maintained as described previously (Bryleva et al., 2010; Murphy et al., 2013). All mouse procedures were approved by Dartmouth Institutional Animal Care and Use Committee.
2.2. Antibodies
Rabbit anti-ACAT1 (DM10) was as described previously (Chang et al., 1995). Mouse anti-human tau antibody (HT7) and mouse anti-human PHF-tau antibody (AT8) were from Thermo Fisher Scientific (Waltham, MA). Mouse anti-tau antibody (Tau-5) and rabbit anti-Atg5 antibody were from Millipore (Billerica, MA). Mouse anti-β tubulin was from GenScript (Piscataway, NJ). Rabbit anti-LC3 (for western blot) was from Novus (Littleton, CO). Rabbit anti-LC3 (for immunofluorescence), rabbit anti-p70S6K, rabbit anti-phosho-p70S6K (Thr389), rabbit anti-4E-BP1, and rabbit anti-phospho-4E-BP1 (Thr37/46) were from Cell Signaling Technology (Danvers, MA). Mouse anti-p62 was from Abcam (Cambridge, MA). Mouse anti-β actin was from Sigma (St. Louis, MO).
2.3. Cell culture
The mouse neuroblastoma cell line N2a (gift from Dr. Sam Sisodia at University of Chicago) was cultured in DMEM/Opti-MEM (50:50) with 10% fetal bovine serum (FBS) at 37 °C with 5% CO2 in a humidified incubator. Cells were incubated for 24 h with the ACAT1-specific inhibitor K604 (gift from Kowa Pharmaceuticals, Japan) or isotype-nonspecific ACAT inhibitor CI-1011(Selleckchem) at concentration as indicated. K604 and CI-1011 were dissolved in 100 % ethanol to make a 5 mM and 10 mM stock respectively, and stored at −20 °C till usage. Primary cortical neurons were isolated from mouse brains at postnatal day 0–3 using a procedure as described (Brewer, 1997; Bryleva et al., 2010). Cortical neurons were plated in 6 well plates at 350,000 cells/well and grown in 2 mL/well Neurobasal A (Life Technologies, Grand Island, NY) with 1xB27 (Life Technologies, Grand Island, NY), 0.5 mM L-glutamine, and 5 ng/mL fibroblast growth factor (Sigma, St. Louis, MO). Half of the media was replaced with fresh media once every 7 days. After 14–21 days in culture, cells were used for individual experiments as described in the legends of Figure 5 and Figure 6.
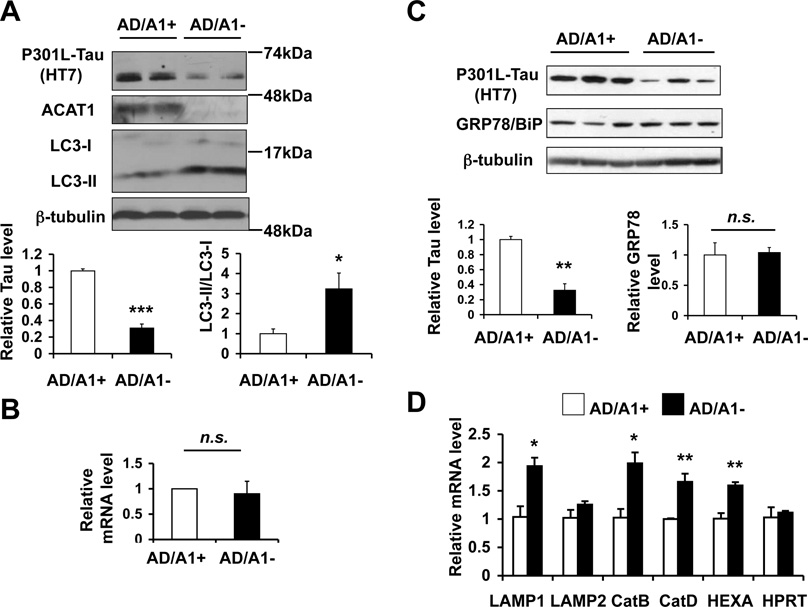
Fig. 5.
Acat1 knockout (KO) in AD neurons promotes P301L-Tau degradation by increasing autophagy A. Primary cortical neurons were isolated from neonatal 3XTg-AD mice with Acat1 (AD/A1+) or without Acat1 (AD/A1−) mice. P301L-Tau and LC3 levels in primary cortical neurons were analyzed by western blot. Representative blots are shown (n=3, *p<0.05, ***p<0.001). B. mRNA levels of human tau were examined by quantitative PCR (qPCR) in AD/A1+ and AD/A1− cortical neurons (n.s.; Not significant). C. GRP78/BiP levels were analyzed by Western blot in AD/A1+ or AD/A1− cortical neurons. The quantifications represent data from triplicates in one western blot (**p<0.01, n.s.; Not significant). D. Expression levels of TFEB-target genes were examined by qPCR in AD/A1+ or AD/A1− cortical neurons (n=2, *p<0.05, **p<0.01).
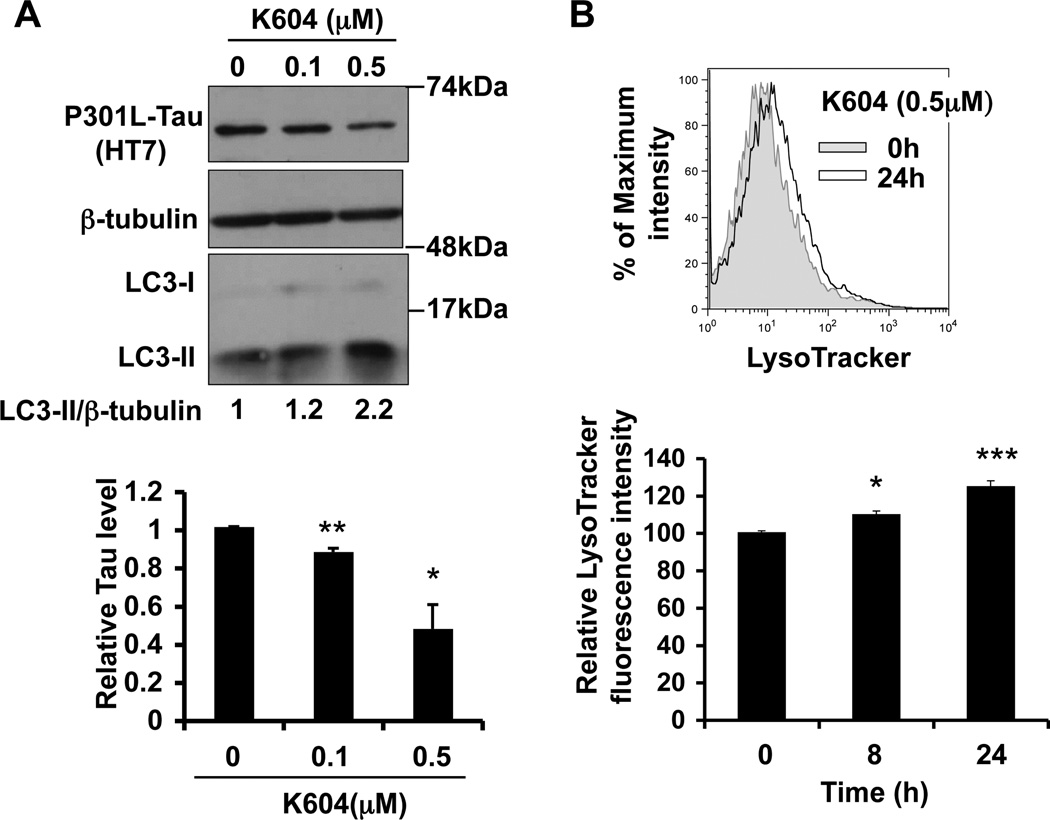
Fig. 6.
K604 stimulates autophagy-mediated proteolysis and increases P301L-Tau degradation in AD neurons A. AD/A1+ cortical neurons were treated with the indicated concentrations of K604 for 24 h. P301L-Tau and LC3 levels were analyzed by western blot. Representative blots were shown (n=2, *p<0.05, **p<0.01). B. AD/A1+ primary cortical neurons were treated with 0.5 µM K604 for time as indicated. Cellular acidic compartments were analyzed by LysoTracker staining (50 nM, 30 min) followed by flow cytometry. A representative histogram is shown (n=2, *p<0.05, **p<0.01)
2.4. Analysis of human tau levels in N2a cells
Plasmid DNA encoding WT-tau (pRK5-EGFP-Tau) and P301L-tau (pRK5-EGFPTau P301L) described previously (Hoover et al., 2010) were obtained from Addgene (Cambridge, MA). N2a cells were grown in 6 well plates to ~50% confluence and transfected with the plasmid as indicated by using Lipofectamine 3000 (Life Technologies, Grand Island, NY). Twenty-four hours after transfection, cells were incubated for 24 h with or without K604 at concentrations as indicated in fresh DMEM/Opti-MEM (50:50) containing 10% FBS, in the presence or absence of 5 mM 3-methyladenine (3MA) (Sigma, St. Louis, MO). Cells were then lysed in RIPA buffer (50 mM Tris-HCl, pH 7.6, 1% Triton X, 1% SDS, 0.5% sodium deoxycholate) with protease and phosphatase inhibitor cocktails (Sigma, St. Louis, MO), and analyzed for tau levels by western blot.
2.5. Gene KD by transfection of siRNAs
N2a cells were grown in 6 well plates to ~50% confluency and transfected with 10 nM of the siRNAs as indicated (Ambion, Silencer Select predesigned siRNA. Atg5: ID# s62452, Control: Negative control No.1) using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA). Twenty-four hours later, cells were trypsinized, reseeded on 6 well plates, and then incubated at 37 °C with 5% CO2 in a humidified incubator for 12 h. Cells were then transfected with pRK5-EGFP-Tau P301L and analyzed for tau levels as described above.
2.6. Immunofluorescence microscopy to visualize LC3 puncta
Cells were grown overnight on poly-D-lysine-coated glass coverslips in 6 well plates at a density of 150,000–300,000 cells/ well. Cells were washed with PBS and fixed with 4% paraformaldehyde for 15 min at room temp. Afterwards, cells were washed three times with PBS, incubated for 60 min at room temp in blocking buffer (3% BSA and 0.3% Triton in PBS) and incubated overnight at 4 °C with anti-LC3 antibody (Cell Signaling Technology, Danvers, MA) in blocking buffer. Cells were then washed three times with PBS and incubated with Alexa Fluor dye-conjugated secondary antibodies for 1h at room temp. Afterwards, cells were washed three times with PBS, rinsed with double-distilled water and mounted on glass slides with a drop of ProLong Gold antifade reagent (Invitrogen, Carlsbad, CA). Confocal images were obtained using a Zeiss LSM 510 confocal microscope with a 63× objective. Image analysis was performed using Image J software.
2.7. ACAT activity assay in intact cells
ACAT activity assay in intact cells was performed as previously described (Chang and Chang, 1986). The incubation time with [3H]-oleate-BSA was 2 h.
2.8. Cellular cholesterol/cholesterol ester analysis
Cellular free cholesterol and cholesteryl ester were separated by using pencilsize columns, and cellular cholesterol/cholesteryl ester levels were analyzed using Wako Free cholesterol E kit (Wako) as previously described (Shibuya et al., 2014). Total cholesterol values were obtained by adding the free cholesterol values and cholesteryl ester values.
2.9. Analysis of cellular acidic compartments by flow cytometry
The experiment was performed as described previously (Shibuya et al., 2014). Briefly, primary cortical neurons were pretreated for time as indicated with 0.5 µM K604. The control cells received solvent vehicle only. Cells were then incubated with 50 nM LysoTracker (Invitrogen, Carlsbad, CA) for 30 min and washed twice with PBS. LysoTracker positive (cellular acidic) compartments were analyzed by using flow cytometry using a BD FACSCanto (BD Biosciences, Franklin Lakes, NJ). Dead cells were excluded from analyses by propidium iodide staining. Data were analyzed by using FlowJo software (Tree Star Inc, Ashland, OR).
2.10. RNA Isolation and qPCR
Total RNAs were isolated by using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. One µg of total RNA was reverse-transcribed using SuperScript III reverse transriptase (Invitrogen, Carlsbad, CA) to prepare cDNA. qPCR was performed by using iTaq Universal SYBR Green Supermix (Bio-Rad, Hercules, CA). The following cycles were performed: An initial denaturation cycle of 94 °C for 5 min, followed by 40 amplification cycles of 94°C for 15 sec and 60°C for 1 min. Relative quantification was determined using ΔΔCT method. The mRNA expression values were normalized to β-actin mRNA level. The primers used in this study were previously reported (Shibuya et al., 2014)
2.11. Preparation of brain homogenates
Hemibrains from male AD/A1+ and AD/A1− mice at different ages as indicated were isolated and homogenized in 10% SDS solution that contained the protease inhibitor cocktail (Sigma, St. Louis, MO) and phosphatase inhibitor cocktail (Sigma, St. Louis, MO). Brain homogenates were snap-frozen in liquid nitrogen and stored at −80°C in individual aliquots until an alysis. For western blot analysis, the aliquots were thawed and protein concentration of each aliquot was determined by Lowry assay. Hemibrains from C57BL/6 mice or from Non-Transgenic mice (Non-Tg) derived from 3XTg-AD mice by breeding with Acat1 KO mice on C57BL/6 background were employed as negative controls.
2.12. Bielschowsky silver staining
Hemibrains were immersion fixed for 48 h in 4% paraformaldehyde at 4 °C and sectioned at 50 µm using a vibrotome. The sections were mounted on slides and silver stained according to Bielschowsky's method as described elsewhere (Lewis et al., 2000; Liu et al., 2012). The number of NFTs in the hippocampi was counted blindly. Two sections per mouse were used for analysis.
2.13. Statistical analysis
All quantification data are expressed as a mean with error bars representing the SEM. A two-tailed Student’s t test or MWU test was used when two values were compared. For multiple comparisons, a one-way ANOVA with a Turkey's post test was used. A p value of <0.05 was considered statistically significant.
3. Results
3.1. ACAT1 blockage promotes autophagy in the mouse neuroblastoma cell line N2a
To investigate whether blocking ACAT1 increases autophagy in neuronal cells, we began our study by treating N2a cells with a potent ACAT1-specific inhibitor K604 (Ikenoya et al., 2007). The result showed that at 0.1 to 1 µM, K604 inhibited ACAT activity by 60–80% (Fig. 1A). Next we added K604 to N2a cells at concentrations from 0.1 to 1 µM for 24 h, and examined LC3 levels by western blot. The result showed that K604 increased the LC3-II/LC3-I ratio, a reliable marker for autophagosome formation (Mizushima et al., 2010), in a dose-dependent manner (Fig. 1B). In mammalian cells, autophagy can be activated by inhibition of mTOR (Mizushima, 2007). To serve as a positive control, we treated N2a cells with rapamycin (RM), a potent mTOR signaling inhibitor, and showed that RM also increased the LC3-II/LC3-I ratio (Fig. 1B; RM). To validate the western blot result, we monitored the LC3 puncta in intact fixed cells by using immunofluorescence microscopy. The result revealed that the number of the fluorescent LC3 puncta was significantly increased in N2a cells after K604 or RM treatment (Fig. 1C). In addition to LC3, autophagy can be analyzed by monitoring p62 levels (Mizushima et al., 2010). p62 is a selective autophagic substrate, and p62 protein levels inversely correlate with autophagic activity (Bjørkøy et al., 2005). By western blot, we found that either K604 or RM significantly decreased the levels of p62 in N2a cells (Fig. 1D). These results demonstrate that blocking ACAT1 by K604 increases autophagy in N2a cells.
Fig. 1.
ACAT1 blockage promotes autophagy in the mouse neuroblastoma cell line N2a. A, E. N2a cells were treated with K604 or with CI-1011 at the indicated concentrations for 24 h and ACAT activity assay was performed in intact cells. Data are mean ± SEM of three experiments. **p < 0.01. ***p < 0.001. B. N2a cells were incubated with K604 at the indicated concentrations for 24 h or with 250 nM rapamycin(RM) for 24 h. Cellular LC3-II/LC3-I levels were analyzed by western blot. A representative blot of three experiments is shown. C. N2a cells were incubated with 1 µM K604 for 24 h or with 250 nM RM for 3 h, and immunostained for LC3. Representative pictures are shown. Numbers of LC3 puncta/cell (n>50) were counted using imageJ software. Data are mean ± SEM. **p<0.01, ***p<0.001. D. N2a cells were incubated with 1 µM K604 for 24 h or with 250 nM RM for 3 h. Cellular p62 levels and LC3-II levels were analyzed by western blot. A representative blot is shown. Data are mean ± SEM of three experiments. *p<0.05, **p < 0.01. ***p < 0.001. F. N2a cells were incubated with 5 µM CI-1011 for 24 h with or without 25 mM NH4Cl for the last 3 h of incubation. A representative blot of three experiments is shown.
CI-1011 is an isotype-nonspecific ACAT inhibitor that blocks both ACAT1 and ACAT2 with essentially the same efficacy (Ikenoya et al., 2007). Treating a mouse model for AD with CI-1011 reduced Aβ and amyloid plaque levels (Huttunen et al., 2010). To test if CI-1011 could also enhance autophagy in N2a cells, we first examined the efficacy of CI-1011 and found that it blocked ACAT activity in N2a cells in a dose-dependent manner (Fig. 1E). Consistent with a previous report (Ikenoya et al., 2007), when compared at several concentrations, CI-1011 is less efficient than K604 in inhibiting ACAT activity (Fig. 1E vs Fig. 1A). We next analyzed LC3-II levels in N2a cells after treating cells with CI-1011 at 5 µM. The result of a western blot showed that CI-1011 significantly increased the LC3-II level; the LC3-II level was further augmented by treating cells with NH4Cl (to block lysosome activity). This result suggests that similar to K604, CI-1011 also increases autophagy flux without interfering with the clearance of autophagosome. Because CI-1011 inhibits both ACAT1 and ACAT2, we decided to use K604 hereafter to specifically study the effect of ACAT1 blockage in neuronal cells.
3.2. Inhibiting ACAT1 in N2a cells enhances autophagy without altering mTOR signaling or total cellular cholesterol content
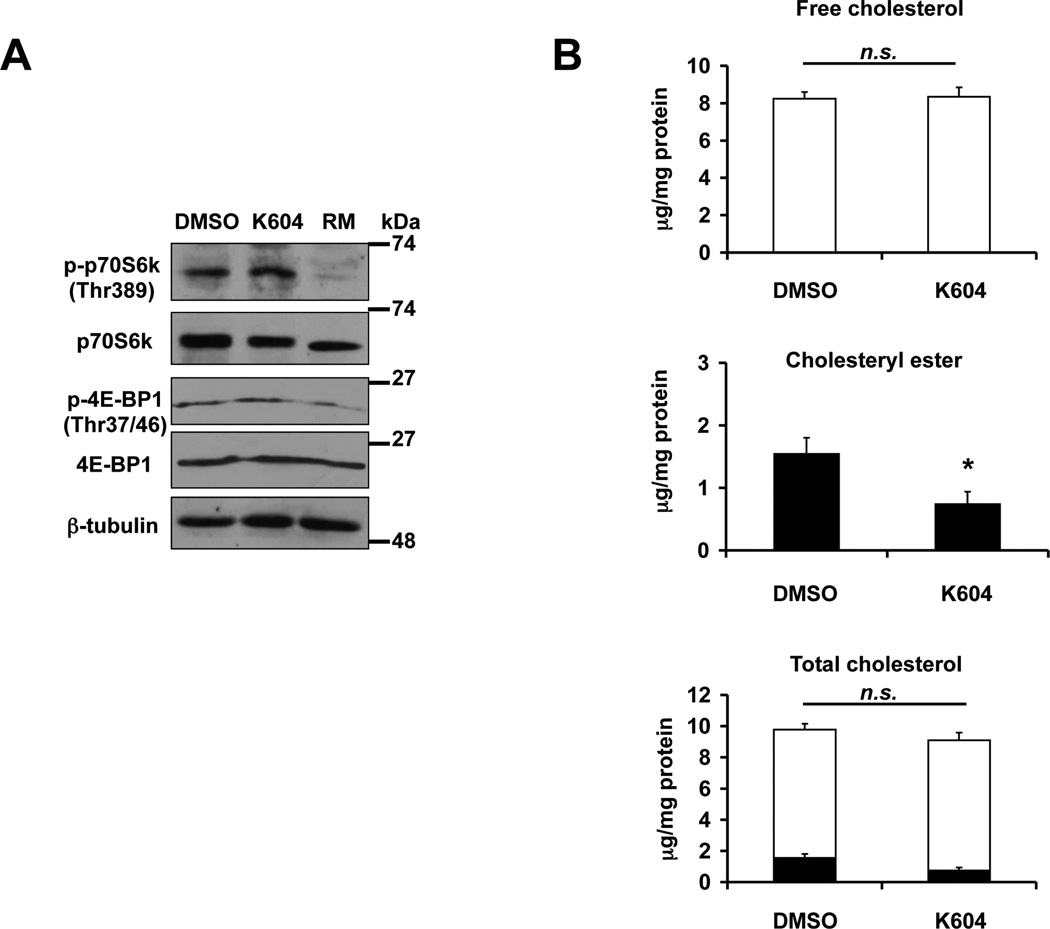
Previously we showed that ACAT1 blockage in microglia increased autophagy in an mTOR-independent manner (Shibuya et al., 2014). To determine whether K604 enhances autophagy in N2a cells by inhibiting mTOR signaling, we monitored mTOR kinase activity by analyzing the levels of phosphorylation of its substrates, p70S6 kinase and 4E-BP1 in cells treated with or without K604 (Fig. 2A). The result of the western blot analysis showed that K604 did not alter the phosphorylation levels of p70S6K and 4E-BP1. In contrast, as expected, RM significantly inhibited phosphorylation of these substrates. These results show that, similar to what we had reported in microglia (Shibuya et al., 2014), blocking ACAT1 by K604 increases autophagy in N2a cells by an mTOR-independent mechanism.
Fig. 2.
Inhibiting ACAT1 by K604 in N2a cells does not alter mTOR signaling or cellular total cholesterol content. A. N2a cells were treated with 1 µM K604 or with 250 nM RM for 24 h. mTOR activity was analyzed by western blot for phospho- and total levels of p70S6K and 4E-BP1. The blot is representative of three experiments. B. N2a cells were treated with 1 µM K604 for 24 h. Cellular lipids were extracted, and cellular cholesterol and cholesteryl ester levels were determined as described in Experimental procedures. Total cholesterol values were obtained by adding the free cholesterol values and cholesteryl ester values. Data are mean ± SEM of three experiments. *p < 0.5. n.s., Not significant.
In various cell culture systems examined, cellular cholesterol levels are reported to affect autophagy in various manners (Cheng et al., 2006; Xu et al., 2010; Ouimet et al., 2011). In our previous study, we showed in microglial cells, inhibiting ACAT1 by K604 enhanced autophagy without altering total cellular cholesterol (free cholesterol and cholesteryl ester) content (Shibuya et al., 2014). To test whether K604 may affect total cholesterol content in the N2a cells, we examined free cholesterol and cholesteryl ester contents with or without K604 treatment. The result showed that treating N2a cells with K604 for 24 h decreased the cholesteryl ester content by approximately 50% (Fig. 2B; middle panel), without affecting either the free cholesterol or the total cholesterol contents (Fig. 2B; top and bottom panels). This result suggests that similar to the result observed in microglial cells, in N2a cells, the mechanism by which ACAT1 blockage promotes autophagy is not by altering total cellular cholesterol contents in cells.
3.3. K604 reduces human tau protein content ectopically expressed in N2a cells
It was recently shown that in N2a cells and in rat primary neurons, enhancing autophagy by using trehalose, an mTOR-independent enhancer of autophagy, promotes degradation of human tau ectopically expressed in these cells (Krüger et al., 2012). Since K604 promotes autophagy in N2a cells, we asked if K604 could promote human tau degradation in N2a cells. To test this possibility, we first expressed human wild type-tau (WT-tau) or human mutant tau (P301L-tau) in N2a cells by transient transfection, and then examined the effects of K604 on tau levels. The result of the western blot analysis showed that K604 decreased the levels of both WT-tau and P301L-tau in a dose-dependent manner (Fig. 3A). In western blot analysis, the human tau protein was detected as multiple bands, presumably because of phosphorylations at multiple sites within tau (Matsumura et al., 1999; Sontag et al., 1996). The result of a control experiment showed that, as expected, RM also reduced both the WT-tau and P301L-tau levels in these cells (Fig. 3A; RM). These data implied that augmenting autophagy either by K604 or by RM stimulated human tau degradation. N2a cells express endogenous mouse tau. We monitored the mouse endogenous tau by using the antibody Tau-5 and showed that in N2a cells, the endogenous tau protein content was not affected by K604 treatment (Fig. 3B).
Fig. 3.
K604 reduces human Tau levels in N2a cells. A. Wild-type tau (WT-Tau) or the P301L-mutant tau (P301L-Tau) was transiently overexpressed in N2a cells. Then cells were treated with K604 at the indicated concentrations or 250 nM RM for 24 h. Tau levels were analyzed by western blot using the HT7 antibody for total human tau proteins. A representative blot is shown. Non-transfected cells (Non-TF) were used as a negative control. Data are mean ± SEM of three experiments *p<0.05, **p<0.01, ***p<0.001. B. N2a cells were treated with K604 at the indicated concentrations for 24 h. Endogenous mouse tau levels in N2a cells were analyzed by western blot using the Tau-5 antibody for mouse tau (mTau). A representative blot is shown. Data are mean ± SEM of three experiments. n.s., Not significant.
3.4. K604 reduces human tau protein content in N2a cells via augmented autophagy
The results described above strongly suggest that K604 decreases human tau protein content by increasing autophagy. Atg5 is an essential protein for autophagosome formation (Mizushima et al., 2001). We performed Atg5 KD in N2a cells that transiently express P301L-tau. A negative control experiment was carried out by using the control siRNA (Ctrl KD). Afterwards, we compared the effect of K604 on P301L-tau levels in Atg5 KD cells vs Ctrl KD cells (Fig. 4A). We also included RM treatment as a positive control to promote the mTOR-dependent autophagy. The results showed that Atg5 KD diminished the Atg5 protein levels by approximately 90% (Fig. 4B; Atg5), and dramatically reduced the LC3-II levels (Fig. 4B; LC3-II). In contrast to the result obtained in Ctrl cells, in Atg5 KD cells, neither RM nor K604 was able to increase the residual LC3-II levels (Fig. 4B; quantitation shown at bottom right panel). We also found that when compared to Ctrl KD, Atg5 KD significantly increased the P301L-tau levels (Fig. 4B; Tau Ctrl KD vs Tau Atg5 KD; quantitation shown at the bottom left panel); after Atg5 KD, neither K604 nor RM was able to reduce the P301L-tau levels (Fig. 4B; quantitation shown at the bottom left panel). These data demonstrate that after autophagy is attenuated by Atg5 KD, similar to RM, K604 is no longer able to reduce P301L-tau content in N2a cells. 3-methyladenine (3MA) is a class III PI3-kinase inhibitor; treating cells with 3MA effectively inhibits autophagy (Mizushima et al., 2010). We treated N2a cells with 3MA and found that similar to the effect of Atg5 KD, 3MA significantly increased the mutant tau level and abolished the effect of K604 on P301L-tau (Fig. 4C). Together, these results demonstrate that in N2a cells, K604 decreases P301L-tau via increased autophagy.
Fig. 4.
K604 promotes human tau degradation in N2a cells via augmented autophagy A. Experimental design for Atg5 knockdown (KD). Briefly, N2a cells were transfected with control siRNA (Ctrl KD) or Atg5 siRNA (Atg5 KD) for 36 h, followed by transfection with P301L-Tau construct for 24 h. Cells were then treated with K604 at 1 µM for 24 h or with RM at 250 nM for 3h. Cells were lysed and then subjected to western blot analysis. B. Experiments were performed in a manner described in (A) and cellular levels of P301L-Tau, Atg5, and LC3 in Ctrl KD cells or Atg5 KD cells were analyzed by western blot. Representative blots are shown. Data are mean ± SEM of three experiments. *p<0.05, ***p<0.001, n.s., Not significant. C. N2a cells were transfected with P301L-Tau construct for 24 h. Cells were then treated with 1 µM K604 in the presence or absence of 5 mM 3-methyladenine (3MA). Representative blots are shown. Data are mean ± SEM of three experiments. ***p<0.001.
3.5. Acat1 KO decreases P301L-tau protein content by increasing autophagosome formation in AD neurons
N2a is a fast-growing mouse neuroblastoma cell line. We sought to determine whether genetic inactivation of ACAT1 in primary neurons could elicit the same effects as what the ACAT1 inhibitor K604 did in N2a cells. The 3XTg-AD mouse expresses human mutant P301L-tau (Oddo et al., 2003b). We isolated primary cortical neurons from neonatal 3XTg-AD mice with Acat1 (AD/A1+) or without Acat1 (AD/A1−) and compared the P301L-tau levels and autophagosome formation. The results showed that when compared to AD/A1+ neurons, in AD/A1− neurons, the P301L-tau levels were decreased by approximately 70 %, whereas the LC3-II/LC3-I ratio was increased by about 3 fold (Fig. 5A). The result of a quantitative PCR (qPCR) experiment showed that the mRNA levels of human mutant tau were comparable between AD/A1+ and AD/A1− neurons (Fig. 5B), demonstrating that in AD neurons, Acat1 KO reduces the protein level without affecting the mRNA level of P301L-tau. Autophagy can be activated as a consequence of ER stress (Ogata et al., 2006). We had previously shown that in microglia, ACAT1 blockage increased autophagy without inducing ER stress (Shibuya et al., 2014). The protein content of GRP78/BiP has been used as a protein marker to monitor ER stress; its level is increased upon ER stress (Harding et al., 2002). We monitored GRP78/BiP content by western blot, and showed that its level is the same between AD/A1+ and AD/A1− neurons (Fig. 5C). These results show that similar to what was shown in microglia, in cortical neurons, Acat1 KO enhances autophagy without altering ER stress response.
The transcription factor EB (TFEB) regulates lysosome biogenesis by increasing transcription of multiple target genes (Sardiello et al., 2009). We had previously shown that in cultured microglia and in adult microglia freshly isolated from 3XTg-AD mice, ACAT1 blockage increased the expressions of various TFEB-target genes (Shibuya et al., 2014). To investigate whether Acat1 KO increases lysosome biogenesis in AD neurons, we isolated mRNAs from AD/A1+ or AD/A1− cortical neurons and performed qPCR analysis. The results showed that Acat1 KO increased the expression levels of four different TFEB-target genes (Fig. 5D); the result of a control experiment showed that Acat1 KO did not alter the expression of a house keeping enzyme hypoxanthine-guanine phosphoribosyltransferase (HPRT) (Fig. 5D; HPRT). These data demonstrate that in AD neurons, Acat1 KO stimulates TFEB-mediated lysosome biogenesis.
3.6. K604 stimulates autophagy, increases cellular acidic compartments, and decreases P301L-tau content in AD neurons
In Figure 3, we showed that K604 reduces P301L-tau levels in N2a cells. To examine whether K604 can reduce P301L-tau levels in primary neurons, we treated AD/A1+ cortical neurons with K604 at the indicated concentrations for 24 h and analyzed the levels of P301L-tau and LC3 by western blot. The results showed that similar to what was shown in N2a cells, K604 reduced P301L-tau levels and increased LC3-II levels; the dose dependency of the K604 effects in primary neurons and in N2a cells were similar (Fig. 6A). In microglia, ACAT1 blockage stimulated lysosomal proteolysis and increased cellular acidic compartments (i.e. late endosomes/lysosomes); this increase was demonstrated by using flow cytometry after cells were stained with the dye LysoTracker (Shibuya et al., 2014). To examine whether K604 causes the same effect in neurons, we treated AD/A1+ cortical neurons with K604 at 0.5 µM for various amount of time as indicated, and estimated cellular acidic compartments. The result revealed that treating the AD/A1+ cortical neurons with K604 for 8 to 24 h significantly increased the LysoTracker-positive compartments (Fig. 6B). Together, these results demonstrate that similar to the effects of Acat1 KO in AD neurons described in Figure. 5, the ACAT1-specific inhibitor K604 stimulates autophagy-mediated lysosomal proteolysis and decreases P301L-tau content in AD neurons.
3.7. Acat1 KO decreases P301L-tau levels in the brains of young AD mice but not in old AD mice
We sought to test the physiological significance of our findings in N2a cells and in cortical neurons by monitoring the P301L-tau levels in the brains of AD mice with or without Acat1. We prepared brain samples in 10% sodium dodecyl sulfate (SDS) from age-matched male AD/A1+ and AD/A1− mice, and analyzed tau protein content in the brain homogenates by western blot. We first monitored P301L-tau levels by using the human tau specific antibody HT7. The results showed that, when compared with the AD/A1+ mice, at 2 and 4 months of age, the AD/A1− mice contained significantly less P301L-tau (Fig. 7A and B). We next monitored the mouse endogenous tau in the brains of WT and Acat1 KO mice. The result showed that at 4 months of age, Acat1 KO did not alter the endogenous tau levels (Fig. 7C).
Fig. 7.
Acat1 KO decreases P301L-Tau levels in brains of young AD mice but not in old AD mice. (A, B) Hemibrains from male AD/A1+ and AD/A1− mice at different ages were homogenized and analyzed by western blot. Total P301L-Tau levels were detected by using the HT7 antibody that recognizes total human tau. Hemibrains from C57BL/6 mice (WT) or from Non-Transgenic mice (Non-Tg) were employed as negative controls. A. 2 months (n=4 per genotype, data quantification was performed by using the short exposure film), B. 4 months (n=8 for AD/A1+, n=7 for AD/A1−, data quantification was performed by using the long exposure film). C. Mouse endogenous tau levels were analyzed in WT and Acat1 KO male mice on C57BL/6 background at 4 months. The Tau-5 antibody that recognizes human and mouse tau was used to detect mouse tau (mTau). A representative blot of results from two experiments is shown (3 WT vs 3 Acat1 KO/experiment; total 6 WT vs 6 Acat1 KO). n.s., Not significant.
We next used the AT8 antibody, which specifically recognizes the phosphorylated tau at Ser202/Thr205, to monitor phosphorylation of P301L-tau. In the 3XTg-AD mouse model, Ser202/Thr205-specific hyperphosphorylation of P301L-tau becomes apparent between 12 to 15 months of ages (Oddo et al., 2003a, 2003b). We compared the phosphorylated P301L-tau levels in the AD/A1+ and the AD/A1− mice at 2 or 21 months of age, by using the AT8 antibody. The result showed that at 2 months of age, no AT8 specific phosphorylation was detectable in these mice, while at 21 months of age, highly significant levels of AT8 specific phosphorylation occurred in both types of these mice (Fig. 8A). We next compared the levels of total P301L-tau and phosphorylated P301L-tau in the old AD/A1+ and AD/A1− mice. The result showed that at 17 and 21 months of age, for both total P301L-tau and hyperphosphorylated P301L-tau, comparable levels were present in the brains of AD/A1+ and AD/A1− mice (Fig. 8B and C). We also examined the number of NFTs in the hippocampi of AD mice at 17 months of age, and found that no significant difference in NFTs existed between AD/A1+ and AD/A1− mice (Fig. 8D).
Fig. 8.
Acat1 KO does not decrease phosphorylated P301L-Tau levels in AD mouse brains (A–C). Hemibrain homogenates from AD/A1+ and AD/A1− male mice at different ages were analyzed by western blot for total and phosphorylated P301L-Tau. The HT7 antibody and Phospho-PHF-tau pSer202+Thr205 antibody (AT8) were used to detect total and phosphorylated tau (p-Tau), respectively. A. 2 and 21 months of age. Two-month old AD mice contained undetectable levels of p-Tau. B. 17 months old (n=5 per genotype, n.s., Not significant). C. 21 months old (n=7 for AD/A1+, n=6 for AD/A1−, n.s., Not significant). D. Neurofibrillary tangles (NFTs) in 17-months old mice hippocampi were analyzed by Bielschowsky silver stain. The tangles were quantified blindly. The insets in the top left corners of the panels are magnified views of the neurons in the hippocampus. Arrows indicate neurons with NFTs. Scale bars: 300 µm, 50 µm (inset). Horizontal bars represent median values (n=7 per genotype, n.s.; Not significant).
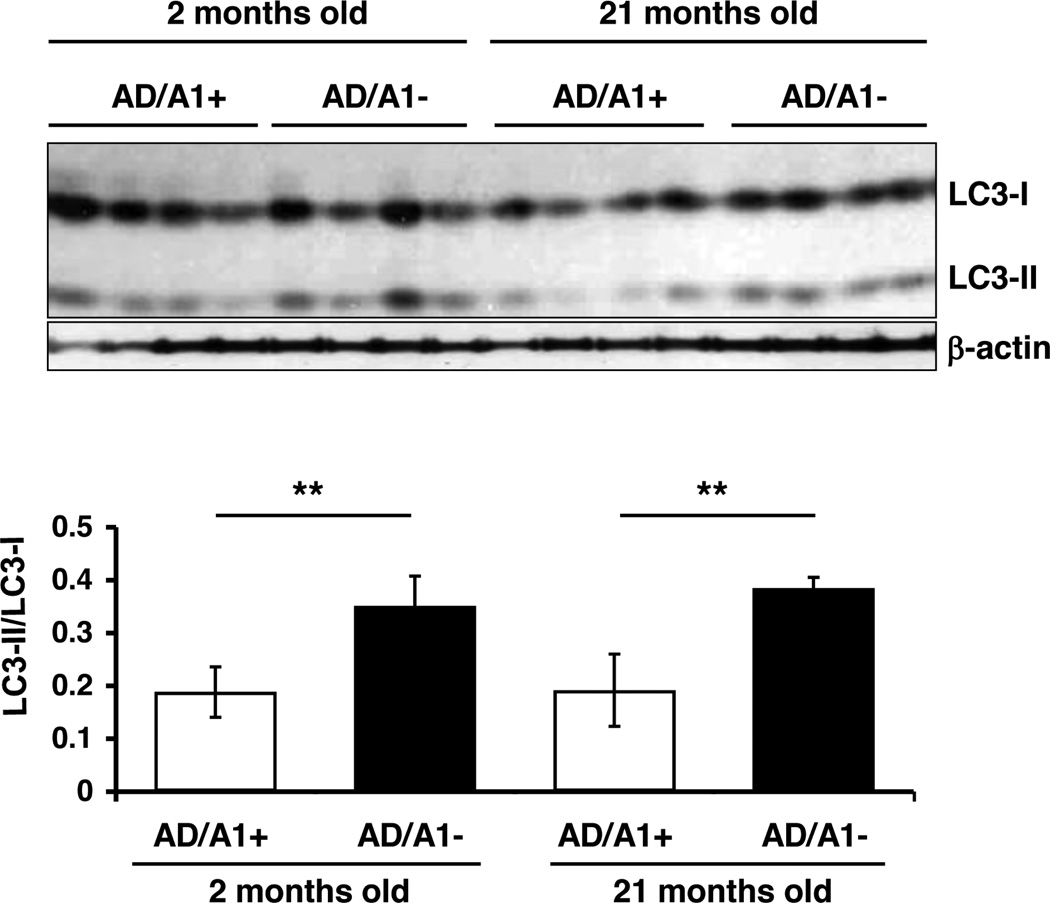
The results presented in Figures 7 and 8 showed that Acat1 KO was effective in reducing total P301L-tau in young AD mice but not in old AD mice. These results raised the possibility that Acat1 KO in AD mice might increase autophagy in young AD mice only. To test this possibility, we monitored the LC3II/LC3I ratio in the brain homogenates of AD/A1+ and AD/A1− mice at 2 months and at 21 months of age. The result showed that at both ages, the AD/A1− mice exhibited significantly increased LC3II/LC3I ratio in their brains than the AD/A1+ mice (Fig. 9). This result demonstrates that the inability of Acat1 KO in reducing P301L-tau in old AD mice is not because Acat1 KO is unable to increase autophagosome in these mice.
Fig. 9.
Acat1 KO increases autophagy in brains of young and old AD mice. LC3 levels were analyzed by western blot in hemibrains from AD/A1+ or AD/A1− male mice at 4 and 21 months of age (n=4 per genotype, **p<0.01).
4. Discussion
Autophagy is the major pathway for organelle and long-lived protein turnover (Mizushima et al., 2008). We have previously reported that ACAT1 blockage increases autophagosome formation and augments lysosomal proteolysis to stimulate Aβ1–42 degradation in cultured microglia and in vivo (Shibuya et al., 2014). In the CNS, microglia are phagocytes and play key roles in scavenging undesirable materials, as reviewed in (Daniel Lee and Landreth, 2010). On the other hand, unlike microglia, neurons are the basic building blocks for information transfer; impaired autophagy has been reported in neurons of AD patients, and in neurons of mouse models related to AD (Boland et al., 2008; Lee et al., 2010; Neely et al., 2011). It is therefore important to find out whether the AD inhibitory actions of ACAT1 blockage demonstrated in microglia also occur in neurons. Here we show that in cell culture, blocking ACAT1 by using the small molecule ACAT1 inhibitor K604 or by Acat1 gene KO increases autophagy in an mTOR signaling-independent manner, and reduces P301L-tau in a neuronal cell line, and in primary cortical neurons isolated from the 3XTg-AD mouse. In vivo, Acat1 KO significantly increases autophagosome formation in the brains of AD mice at both young and old ages. Thus, similar to its action in microglia, ACAT1 blockage stimulates autophagosome formation in AD neurons.
Modulating cellular cholesterol levels affects autophagy in various cell types. The effect of cholesterol on autophagy appears to depend on cell types. For instance, in macrophages, cholesterol loading by acetylated LDL enhances autophagy to promote cholesterol efflux (Ouimet et al., 2011). Similarly, in smooth muscle cells, providing excess free cholesterol stimulates autophagy to protect from cell death (Xu et al., 2010). On the contrary in fibroblast cell lines, reducing cellular cholesterol enhances autophagy (Cheng et al., 2006). In the current study, we found that in N2a cells inhibiting ACAT1 by K604 for 24 h didn’t reduce the total cellular cholesterol content. Similarly, in microglia, K604 treatment for 24 h promotes autophagy without affecting the total cellular cholesterol content (Shibuya et al., 2014). Thus, at least in cultured microglial cells and neuronal cells, ACAT1 blockage stimulates autophagy not by altering total cellular cholesterol content. Lipid rafts are cholesterol/sphingolipid-rich microdomain(s) present in various cellular membranes. The mitochondria-associated ER membrane (MAM) is the junctional membrane between the mitochondria and the ER, and can be isolated biochemically; reviewed in (Vance, 2014). MAM is shown to be rich in cholesterol and rich in sphingolipid (ceramide) contents (Hayashi and Fujimoto, 2010); it is also enriched in ACAT1 protein content (Area-Gomez et al., 2012). It is possible that blocking ACAT1 may increase the local cholesterol and/or other lipid content in MAM (and possibly in other membranes); such alteration may signal an increase in autophagic function. We are currently designing experiments to test this possibility.
Our biochemical analysis in brain homogenates showed that, at 2–4 months of age, Acat1 KO led to a significant decrease in the total human tau content in the brains of AD mice. However, at 17–21 months of age, it did not reduce the total tau protein or the hyperphosphoryated tau, even though Acat1 KO still increased autophagy in these mice. At present it is not clear why Acat1 KO was not effective in reducing the total or hyperphosphorylated P301L-tau in old AD mice. Similar to our results, Oddo and colleagues reported that in the 3XTg-AD mouse model, enhancing autophagy by RM administration before the disease onset, but not after the disease onset, reduces hyperphosphorylated tau levels (Majumder et al., 2011). Results of the western blot analyses reported here showed that in the 3XTg-AD mice, the AD prone-phosphorylation of P301L-tau was not detectable in the brains at 2 months of age, but became easily detectable at 17–21 months of age. Certain mutations in tau, including P301L-tau, cause tau to become hyperhposphorylated and more prone to form insoluble aggregates (Goedert and Spillantini, 2011). We suspect that in the old 3XTg-AD mouse brain, the hyperphosphorylated/aggregated P301L-tau may become much less susceptible to autophagy-mediated degradation in vivo. Other explanations are also possible.
The insoluble, aggregated tau eventually produces NFTs, which constitute one of the hallmarks for AD. However, recently, it has been shown that in a mouse model of tauopathy, NFTs may not be the major toxic tau species (SantaCruz et al., 2005). Instead, the misfolded, non-aggregated tau can cause trans-synaptic spread of tau pathology in cultured cells (Frost et al., 2009; Guo and Lee, 2011; Michel et al., 2013) and in vivo (Clavaguera et al., 2009; de Calignon et al., 2012; Liu et al., 2012). It has also been shown that the tau protein plays important roles in mediating the toxicity generated by the metabolites produced from hAPP (Jin et al., 2011; Roberson et al., 2007). Agents that reduce misfolding-prone tau could be therapeutic towards treating tauopathies (Brunden et al., 2010). We have previously shown that ACAT1 blockage by gene KO or by gene KD decreases amyloid pathology in both the young and the old 3XTg-AD mice (Bryleva et al., 2010; Murphy et al., 2013). Our results described in this manuscript suggest that, at least in the 3XTg-AD mouse model, in addition to its beneficial effect on amyloidopathy, ACAT1 blockage could also benefit AD by reducing tauopathy at early stage. Whether our findings are applicable to other mouse models for tauopathies and/or human AD needs to be validated in the future.
Our current and previous work (Shibuya et al., 2014) suggest the use of ACAT1-specific inhibitor to treat AD and other related neurodegenerative diseases. Over the last few decades, various pharmaceutical companies have produced more than 20 different “ACAT inhibitors”, originally intended for treating cardiovascular diseases. Most of these inhibitors were discovered before the first ACAT gene (ACAT1) was identified (Chang et al., 1993). ACAT1 was later shown to be the first member of the MBOAT family (Hofmann, 2000). In humans, there are 11 MBOATs (Chang et al., 2011). MBOATs contain multiple membrane spanning domains, share similar catalytic sites, but play different roles in various biological processes. More recent studies revealed that many “ACAT inhibitors” are not mono-specific; they inhibit other MBOAT family members. For examples, the “ACAT inhibitor” CI976 inhibits multiple membrane trafficking steps, in part by inhibiting the enzyme activity of lysophospholipid acyltransferase 3 (Schmidt and Brown, 2009), which is an MBOAT member. The “ACAT inhibitor” CI-1011 fed to animals or fed to humens decreased the plasma concentrations of total triglyceride content (Llaverías et al., 2003), presumably because CI 1011 also inhibits the enzyme activity of diacyl glycerol acyltransferase 1 (DGAT1), a different MBOAT member. At present K604 is the only small molecule ACAT1 inhibitor available. In cell free biochemical assays, K604 at 0.5 µM inhibited ACAT1 enzymatic activity by 70% without significantly inhibiting the ACAT2 enzyme activity (Ikenoya et al., 2007). In cultured neurons and cultured microglial cells, our results show that the effects of K604 at 0.5 µM corroborate the effects of Acat1 KO. However, these results do not exclude the possibility that at higher concentration, K604 may inhibit other member(s) of the MBOAT family. A second concern for using the currently available ACAT inhibitors to treat neurodegenerative diseases is that many ACAT inhibitors are very hydrophobic compounds and possess membrane active property (Homan and Hamelehle, 2001). The membrane active compounds can be sequestered within the lipid bilayer and reach high local concentration to affect membrane properties non-specifically. The short term and long term effects of these compounds in the CNS are unknown. To treat AD and other related neurodegenerative diseases, we encourage the development of new small molecule ACAT1-specific inhibitors that are blood brain barrier permeable while devoid of various off-target side effect(s) described above.
Supplementary Material
Highlights.
Blocking ACAT1 increases autophagy in neurons.
Acat1 KO increases autophagosome in the brain of 3xTg-AD mouse.
Blocking ACAT1 decreases human tau in neurons.
Acat1 KO decreases human tau in the brain of pre-symptomatic 3xTg-AD mouse.
Acknowledgements
We thank members of the Chang lab at Dartmouth for stimulating discussions. This work was supported by National Institutes of Health Grant AG37609 to T.Y.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests.
References
- Area-Gomez E, Del Carmen Lara Castillo M, Tambini MD, Guardia-Laguarta C, de Groof AJC, Madra M, Ikenouchi J, Umeda M, Bird TD, Sturley SL, Schon EA. Upregulated function of mitochondria-associated ER membranes in Alzheimer disease. EMBO J. 2012;31:4106–4123. doi: 10.1038/emboj.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørkøy G, Lamark T, Brech A, Outzen H, Perander M, Øvervatn A, Stenmark H, Johansen T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J. Cell Biol. 2005;171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, Nixon RA. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease. J. Neurosci. 2008;28:6926–6937. doi: 10.1523/JNEUROSCI.0800-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer GJ. Isolation and culture of adult rat hippocampal neurons. J. Neurosci. Methods. 1997;71:143–155. doi: 10.1016/s0165-0270(96)00136-7. [DOI] [PubMed] [Google Scholar]
- Brunden KR, Ballatore C, Crowe A, Smith I, Amos B, Lee VM-Y, Trojanowski JQ. Tau-directed drug discovery for Alzheimer’s disease and related tauopathies: A focus on tau assembly inhibitors. Exp. Neurol., Beta-amyloid and tau protein abnormalities in Alzheimer’s disease. 2010;223:304–310. doi: 10.1016/j.expneurol.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryleva EY, Rogers MA, Chang CCY, Buen F, Harris BT, Rousselet E, Seidah NG, Oddo S, LaFerla FM, Spencer TA, Hickey WF, Chang T-Y. ACAT1 gene ablation increases 24(S)-hydroxycholesterol content in the brain and ameliorates amyloid pathology in mice with AD. Proc. Natl. Acad. Sci. 2010;107:3081–3086. doi: 10.1073/pnas.0913828107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mTOR, Aβ and tau: Effects on cognitive impairments. J. Biol. Chem. 2010 doi: 10.1074/jbc.M110.100420. jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CCY, Chang TY. Cycloheximide sensitivity in regulation of acyl coenzyme A:cholesterol acyltransferase activity in Chinese hamster ovary cells. 2. Effect of sterol endogenously synthesized. Biochemistry (Mosc.) 1986;25:1700–1706. doi: 10.1021/bi00355a039. [DOI] [PubMed] [Google Scholar]
- Chang CCY, Chen J, Thomas MA, Cheng D, A DPV, Newton RS, Pape ME, Chang TY. Regulation and Immunolocalization of Acyl-Coenzyme A:Cholesterol Acyltransferase in Mammalian Cells as Studied with Specific Antibodies. J. Biol. Chem. 1995;270:29532–29540. doi: 10.1074/jbc.270.49.29532. [DOI] [PubMed] [Google Scholar]
- Chang CCY, Huh HY, Cadigan KM, Chang TY. Molecular cloning and functional expression of human acyl-coenzyme A:cholesterol acyltransferase cDNA in mutant Chinese hamster ovary cells. J. Biol. Chem. 1993;268:20747–20755. [PubMed] [Google Scholar]
- Chang CCY, Sun J, Chang TY. Membrane-bound O-acyltransferases (MBOATs) Front. Biol. 2011;6:177–182. [Google Scholar]
- Chang TY, Li BL, Chang CCY, Urano Y. Acyl-coenzyme A:cholesterol acyltransferases. Am. J. Physiol. - Endocrinol. Metab. 2009;297:E1–E9. doi: 10.1152/ajpendo.90926.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RB, Oliveira TG, Cortes EP, Honig LS, Duff KE, Small SA, Wenk MR, Shui G, Paolo GD. Comparative Lipidomic Analysis of Mouse and Human Brain with Alzheimer Disease. J. Biol. Chem. 2012;287:2678–2688. doi: 10.1074/jbc.M111.274142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Ohsaki Y, Tauchi-Sato K, Fujita A, Fujimoto T. Cholesterol depletion induces autophagy. Biochem. Biophys. Res. Commun. 2006;351:246–252. doi: 10.1016/j.bbrc.2006.10.042. [DOI] [PubMed] [Google Scholar]
- Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M, Jucker M, Goedert M, Tolnay M. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen TJ, Guo JL, Hurtado DE, Kwong LK, Mills IP, Trojanowski JQ, Lee VMY. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat. Commun. 2011;2:252. doi: 10.1038/ncomms1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook C, Carlomagno Y, Gendron TF, Dunmore J, Scheffel K, Stetler C, Davis M, Dickson D, Jarpe M, DeTure M, Petrucelli L. Acetylation of the KXGS motifs in tau is a critical determinant in modulation of tau aggregation and clearance. Hum. Mol. Genet. 2014;23:104–116. doi: 10.1093/hmg/ddt402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Calignon A, Polydoro M, Suárez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, Pitstick R, Sahara N, Ashe KH, Carlson GA, Spires-Jones TL, Hyman BT. Propagation of Tau Pathology in a Model of Early Alzheimer’s Disease. Neuron. 2012;73:685–697. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert A, Schulz KL, Rhein V, Götz J. Convergence of Amyloid-β and Tau Pathologies on Mitochondria In Vivo. Mol. Neurobiol. 2010;41:107–114. doi: 10.1007/s12035-010-8109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost B, Jacks RL, Diamond MI. Propagation of Tau Misfolding from the Outside to the Inside of a Cell. J. Biol. Chem. 2009;284:12845–12852. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG. Pathogenesis of the Tauopathies. J. Mol. Neurosci. 2011;45:425–431. doi: 10.1007/s12031-011-9593-4. [DOI] [PubMed] [Google Scholar]
- Guo JL, Lee VMY. Seeding of Normal Tau by Pathological Tau Conformers Drives Pathogenesis of Alzheimer-like Tangles. J. Biol. Chem. 2011;286:15317–15331. doi: 10.1074/jbc.M110.209296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Calfon M, Urano F, Novoa I, Ron D. Transcriptional and Translational Control in the Mammalian Unfolded Protein Response. Annu. Rev. Cell Dev. Biol. 2002;18:575–599. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Fujimoto M. Detergent-resistant microdomains determine the localization of sigma-1 receptors to the endoplasmic reticulum-mitochondria junction. Mol. Pharmacol. 2010;77:517–528. doi: 10.1124/mol.109.062539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann K. A superfamily of membrane-bound O-acyltransferases with implications for wnt signaling. Trends Biochem. Sci. 2000;25:111–112. doi: 10.1016/s0968-0004(99)01539-x. [DOI] [PubMed] [Google Scholar]
- Homan R, Hamelehle KL. Influence of membrane partitioning on inhibitors of membrane-bound enzymes. J. Pharm. Sci. 2001;90:1859–1867. doi: 10.1002/jps.1135. [DOI] [PubMed] [Google Scholar]
- Hoover BR, Reed MN, Su J, Penrod RD, Kotilinek LA, Grant MK, Pitstick R, Carlson GA, Lanier LM, Yuan L-L, Ashe KH, Liao D. Tau Mislocalization to Dendritic Spines Mediates Synaptic Dysfunction Independently of Neurodegeneration. Neuron. 2010;68:1067–1081. doi: 10.1016/j.neuron.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado DE, Molina-Porcel L, Iba M, Aboagye AK, Paul SM, Trojanowski JQ, Lee VMY. Aβ Accelerates the Spatiotemporal Progression of Tau Pathology and Augments Tau Amyloidosis in an Alzheimer Mouse Model. Am. J. Pathol. 2010;177:1977–1988. doi: 10.2353/ajpath.2010.100346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter-Paier B, Huttunen HJ, Puglielli L, Eckman CB, Kim DY, Hofmeister A, Moir RD, Domnitz SB, Frosch MP, Windisch M, Kovacs DM. The ACAT Inhibitor CP-113,818 Markedly Reduces Amyloid Pathology in a Mouse Model of Alzheimer’s Disease. Neuron. 2004;44:227–238. doi: 10.1016/j.neuron.2004.08.043. [DOI] [PubMed] [Google Scholar]
- Huttunen HJ, Havas D, Peach C, Barren C, Duller S, Xia W, Frosch MP, Hutter-Paier B, Windisch M, Kovacs DM. The Acyl-Coenzyme A:Cholesterol Acyltransferase Inhibitor CI-1011 Reverses Diffuse Brain Amyloid Pathology in Aged Amyloid Precursor Protein Transgenic Mice. J. Neuropathol. Exp. Neurol. 2010;69:777–788. doi: 10.1097/NEN.0b013e3181e77ed9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenoya M, Yoshinaka Y, Kobayashi H, Kawamine K, Shibuya K, Sato F, Sawanobori K, Watanabe T, Miyazaki A. A selective ACAT-1 inhibitor, K-604, suppresses fatty streak lesions in fat-fed hamsters without affecting plasma cholesterol levels. Atherosclerosis. 2007;191:290–297. doi: 10.1016/j.atherosclerosis.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Ittner LM, Götz J. Amyloid-β and tau — a toxic pas de deux in Alzheimer’s disease. Nat. Rev. Neurosci. 2011;12:67–72. doi: 10.1038/nrn2967. [DOI] [PubMed] [Google Scholar]
- Jin M, Shepardson N, Yang T, Chen G, Walsh D, Selkoe DJ. Soluble amyloid β-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proc. Natl. Acad. Sci. 2011;108:5819–5824. doi: 10.1073/pnas.1017033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopeikina KJ, Hyman BT, Spires-Jones TL. Soluble forms of tau are toxic in Alzheimer’s disease. Transl. Neurosci. 2012;3:223–233. doi: 10.2478/s13380-012-0032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger U, Wang Y, Kumar S, Mandelkow E-M. Autophagic degradation of tau in primary neurons and its enhancement by trehalose. Neurobiol. Aging. 2012;33:2291–2305. doi: 10.1016/j.neurobiolaging.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Llaverías G, Laguna JC, Alegret M. Pharmacology of the ACAT inhibitor avasimibe (CI-1011) Cardiovasc Drug Rev. 2003;21:33–50. [PubMed] [Google Scholar]
- Lee CYD, Landreth GE. The role of microglia in amyloid clearance from the AD brain. J. Neural Transm. Vienna Austria 1996. 2010;117:949–960. doi: 10.1007/s00702-010-0433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM, Wolfe DM, Martinez-Vicente M, Massey AC, Sovak G, Uchiyama Y, Westaway D, Cuervo AM, Nixon RA. Lysosomal Proteolysis and Autophagy Require Presenilin 1 and Are Disrupted by Alzheimer-Related PS1 Mutations. Cell. 2010;141:1146–1158. doi: 10.1016/j.cell.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Murphy MP, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin W-L, Yen S-H, Dickson DW, Davies P, Hutton M. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat. Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, Duff K. Trans-synaptic spread of tau pathology in vivo. PloS One. 2012;7:e31302. doi: 10.1371/journal.pone.0031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder S, Richardson A, Strong R, Oddo S. Inducing Autophagy by Rapamycin Before, but Not After, the Formation of Plaques and Tangles Ameliorates Cognitive Deficits. PLoS ONE. 2011;6:e25416. doi: 10.1371/journal.pone.0025416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters CL, Selkoe DJ. Biochemistry of Amyloid β-Protein and Amyloid Deposits in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2012 doi: 10.1101/cshperspect.a006262. a006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura N, Yamazaki T, Ihara Y. Stable Expression in Chinese Hamster Ovary Cells of Mutated Tau Genes Causing Frontotemporal Dementia and Parkinsonism Linked to Chromosome 17 (FTDP-17) Am. J. Pathol. 1999;154:1649–1656. doi: 10.1016/S0002-9440(10)65420-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiner VL, Cases S, Myers HM, Sande ER, Bellosta S, Schambelan M, Pitas RE, McGuire J, Herz J, Farese RV. Disruption of the acyl-CoA:cholesterol acyltransferase gene in mice: Evidence suggesting multiple cholesterol esterification enzymes in mammals. Proc. Natl. Acad. Sci. 1996;93:14041–14046. doi: 10.1073/pnas.93.24.14041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel CH, Kumar S, Pinotsi D, Tunnacliffe A, George-Hyslop PS, Mandelkow E, Mandelkow E-M, Kaminski CF, Schierle GSK. Extracellular Monomeric Tau is Sufficient to Initiate the Spread of Tau Pathology. J. Biol. Chem. 2013 doi: 10.1074/jbc.M113.515445. jbc.M113.515445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Yamamoto A, Hatano M, Kobayashi Y, Kabeya Y, Suzuki K, Tokuhisa T, Ohsumi Y, Yoshimori T. Dissection of Autophagosome Formation Using Apg5-Deficient Mouse Embryonic Stem Cells. J. Cell Biol. 2001;152:657–668. doi: 10.1083/jcb.152.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Yoshimorim T, Levine B. Methods in Mammalian Autophagy Research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SR, Chang CC, Dogbevia G, Bryleva EY, Bowen Z, Hasan MT, Chang T-Y. Acat1 Knockdown Gene Therapy Decreases Amyloid-β in a Mouse Model of Alzheimer’s Disease. Mol. Ther. 2013;21:1497–1506. doi: 10.1038/mt.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely KM, Green KN, LaFerla FM. Presenilin Is Necessary for Efficient Proteolysis through the Autophagy–Lysosome System in a γ-Secretase-Independent Manner. J. Neurosci. 2011;31:2781–2791. doi: 10.1523/JNEUROSCI.5156-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol. Aging, Molecular and Cellular Basis of Synaptic Loss and Dysfunction in Alzheimer’s Disease. 2003a;24:1063–1070. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-Transgenic Model of Alzheimer’s Disease with Plaques and Tangles: Intracellular Aβ and Synaptic Dysfunction. Neuron. 2003b;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback JA, Urano F, Imaizumi K. Autophagy Is Activated for Cell Survival after Endoplasmic Reticulum Stress. Mol. Cell. Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimet M, Franklin V, Mak E, Liao X, Tabas I, Marcel YL. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab. 2011;13:655–667. doi: 10.1016/j.cmet.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglielli L, Konopka G, Pack-Chung E, Ingano LAM, Berezovska O, Hyman BT, Chang TY, Tanzi RE, Kovacs DM. Acyl-coenzyme A: cholesterol acyltransferase modulates the generation of the amyloid β-peptide. Nat. Cell Biol. 2001;3:905–912. doi: 10.1038/ncb1001-905. [DOI] [PubMed] [Google Scholar]
- Rhein V, Song X, Wiesner A, Ittner LM, Baysang G, Meier F, Ozmen L, Bluethmann H, Dröse S, Brandt U, Savaskan E, Czech C, Götz J, Eckert A. Amyloid-β and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proc. Natl. Acad. Sci. 2009;106:20057–20062. doi: 10.1073/pnas.0905529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu G-Q, Mucke L. Reducing Endogenous Tau Ameliorates Amyloid β-Induced Deficits in an Alzheimer’s Disease Mouse Model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- SantaCruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH. Tau Suppression in a Neurodegenerative Mouse Model Improves Memory Function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardiello M, Palmieri M, Ronza A, di, Medina DL, Valenza M, Gennarino VA, Malta CD, Donaudy F, Embrione V, Polishchuk RS, Banfi S, Parenti G, Cattaneo E, Ballabio A. A Gene Network Regulating Lysosomal Biogenesis and Function. Science. 2009;325:473–477. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- Schmidt JA, Brown WJ. Lysophosphatidic acid acyltransferase 3 regulates Golgi complex structure and function. J Cell Biol. 2009;186:211–218. doi: 10.1083/jcb.200904147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya Y, Chang CCY, Huang LH, Bryleva EY, Chang TY. Inhibiting ACAT1/SOAT1 in Microglia Stimulates Autophagy-Mediated Lysosomal Proteolysis and Increases Aβ1–42 Clearance. J. Neurosci. 2014;34:14484–14501. doi: 10.1523/JNEUROSCI.2567-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag E, Nunbhakdi-Craig V, Lee G, Bloom GS, Mumby MC. Regulation of the Phosphorylation State and Microtubule-Binding Activity of Tau by Protein Phosphatase 2A. Neuron. 1996;17:1201–1207. doi: 10.1016/s0896-6273(00)80250-0. [DOI] [PubMed] [Google Scholar]
- Tajima Y, Ishikawa M, Maekawa K, Murayama M, Senoo Y, Nishimaki-Mogami T, Nakanishi H, Ikeda K, Arita M, Taguchi R, Okuno A, Mikawa R, Niida S, Takikawa O, Saito Y. Lipidomic analysis of brain tissues and plasma in a mouse model expressing mutated human amyloid precursor protein/tau for Alzheimer’s disease. Lipids Health Dis. 2013;12:68. doi: 10.1186/1476-511X-12-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwel D, Lasrado R, Snauwaert J, Vandeweert E, Haesendonck CV, Borghgraef P, Leuven FV. Changed Conformation of Mutant Tau-P301L Underlies the Moribund Tauopathy, Absent in Progressive, Nonlethal Axonopathy of Tau-4R/2N Transgenic Mice. J. Biol. Chem. 2005;280:3963–3973. doi: 10.1074/jbc.M409876200. [DOI] [PubMed] [Google Scholar]
- Vossel KA, Zhang K, Brodbeck J, Daub AC, Sharma P, Finkbeiner S, Cui B, Mucke L. Tau Reduction Prevents Aβ-Induced Defects in Axonal Transport. Science. 2010;330:198–198. doi: 10.1126/science.1194653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance JE. MAM (mitochondria-associated membranes) in mammalian cells: lipids and beyond. Biochimica et biophysica acta. 2014;1841:595–609. doi: 10.1016/j.bbalip.2013.11.014. [DOI] [PubMed] [Google Scholar]
- Xu K, Yang Y, Yan M, Zhan J, Fu X, Zheng X. Autophagy plays a protective role in free cholesterol overload-induced death of smooth muscle cells. J. Lipid Res. 2010;51:2581–2590. doi: 10.1194/jlr.M005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.