Abstract
Objective
To test whether more physiologically assessed hot flashes were associated with greater connectivity in the default mode network (DMN), the network of brain regions active during rest. We particularly focus on DMN networks supporting the hippocampus as this region is rich in estrogen receptors and has previously been linked to hot flashes.
Design
Women underwent 24 hours of physiologic and diary hot flash monitoring, functional magnetic resonance imaging, 72 hours of sleep actigraphy monitoring, a blood draw, questionnaires, and physical measures.
Setting
Community
Participants
Twenty midlife women ages 40–60 with their uterus and both ovaries and not taking hormone therapy
Interventions
None
Main outcome measures
DMN functional connectivity
Results
Controlling for age, race, education, a greater number of physiologically-monitored hot flashes were associated with greater DMN connectivity [beta, B (standard error, SE)=.004 (.002), p<.05], particularly hippocampal DMN connectivity [B(SE)=.005 (.002), p<.05]. Findings were most pronounced for sleep physiologic hot flashes [with hippocampal DMN, B(SE)= .02 (.007), p<0.01]. Associations persisted additionally controlling for sleep, depressive symptoms, and serum estradiol concentrations.
Conclusions
More physiologically-monitored hot flashes were associated with greater DMN connectivity, particularly networks supporting the hippocampus. Findings were most pronounced for sleep hot flashes. Findings underscore the importance of continued investigation of the central nervous system in efforts to understand this classic menopausal phenomenon.
Keywords: Hot flashes, vasomotor symptoms, brain, hippocampus, default mode network
Introduction
Hot flashes are the classic symptom of the menopausal transition, experienced by over 70% of women at some point during the menopausal transition (1). Hot flashes are associated with impairments in quality of life (2), depressed mood (3), reported sleep disturbance (4), and possibly even poorer memory function(5). Hot flashes are a leading driver of medical treatment seeking at midlife for women (6, 7).
Despite their prevalence and impact on women’s lives, the understanding of the physiology of hot flashes remains incompletely understood. Leading models conceptualize hot flashes as originating in the central nervous system (8), yet there has been limited data investigating relations between the brain and hot flashes. Some data supports acute changes in brain regions associated with awareness of bodily sensation, such as the insula and prefrontal cortex, acutely during hot flashes and the involvement of brain stem areas in the triggering of the hot flash (9, 10). However, little research has investigated brain network differences associated with hot flashes.
Hot flashes occur in the context of estrogen withdrawal, and the effects of estrogen on brain structure and function in humans remains controversial (11, 12). However, some prior studies have suggested decrements in verbal memory during the peri-menopause, a time when hot flashes are common (13–15). Although subjective hot flashes have not been associated with cognitive function, physiologically-measured hot flashes, particularly those occurring during sleep, have been linked to poorer verbal memory performance (5). Furthermore, brain regions involved in verbal memory including the hippocampus and prefrontal cortex are rich in estrogen receptors (16, 17). Acute doses of estradiol are associated with greater functional connectivity between the hippocampus and prefrontal cortex (18).
The default mode network (DMN) is a recently-discovered network of brain regions that are active during rest in the absence of external stimuli (19, 20). Activity of this neural network is distributed across brain regions and occurs spontaneously. Suppression of the DMN is associated with better memory formation, and less suppression of the DMN predicts poorer attention to later tasks (21, 22). The DMN appears to be involved in a range of psychiatric and medical conditions, and DMN hyperactivity appears characteristic of major depressive disorder (23).
We tested whether both self-reported and physiologically assessed hot flashes were associated with altered functional connectivity in the DMN, particularly networks supporting the hippocampus. We hypothesized that a higher frequency of physiologically-assessed (but not self-reported) hot flashes would be associated with greater functional connectivity in the DMN, particularly networks supporting the hippocampus. We tested these hypotheses in a sample of midlife women who underwent both brain imaging and detailed ambulatory physiologic hot flash monitoring. Physiologic assessment of hot flashes is particularly important to testing relations between hot flashes and the brain, as prior work has indicated that it is physiologically-detected hot flashes rather than self-reported hot flashes that are most related to cognition (5) and peripheral nervous system physiology (24, 25).
Materials and Methods
Study Sample
Twenty women who were a subsample of a larger parent study of 300 women focused on menopause and cardiovascular function underwent brain imaging. At study entry, nine women reported having daily hot flashes and 11 reported having no hot flashes in the past six months. Parent study inclusion criteria included being age 40–60; having a uterus and at least one ovary; not pregnant; being late perimenopausal (amenorrhea >2–<12 months) or postmenopausal status (amenorrhea ≥ 12 months) in accordance with STRAW+10 criteria (26)); without a history of heart disease, stroke, arrhythmia, or breast cancer; and not taking hormone therapy, SSRI/SNRI antidepressants, clonidine, beta blockers, calcium channel blockers, gabapentin, or insulin within three months. Additional criteria for inclusion in the brain imaging sub-study included no metal in the body and no history of chronic migraines, concussion, stroke, brain injury, dementia, or Parkinson’s disease. Of the twenty women who underwent the brain imaging protocol, one woman completed only part of the imaging protocol due to time limitations and an additional subject experienced hot flash monitor failure; thereby 18 women are included in these analyses.
Design and Procedures
The parent study protocol included anthropometric measures, questionnaires, blood specimens, and a 3-day ambulatory hot flash and actigraphy sleep assessment protocol. Women in the brain imaging substudy additionally underwent magnetic resonance imaging (MRI) on a separate day. Procedures were approved by the University of Pittsburgh Institutional Review Board. All participants provided written informed consent.
Hot flashes
Hot flash monitoring was conducted with an ambulatory sternal skin conductance monitor and electronic diary. Sternal skin conductance was recorded via the VU-AMS monitor (Amsterdam, the Netherlands), a portable device worn in a pouch around the waist. This device measures sternal skin conductance sampled at 1 Hz from the sternum via a 0.5 Volt constant voltage circuit passed between two Ag/AgCl electrodes (UFI) filled with 0.05M KCL Velvachol/glycol paste (27). Participants were instructed to avoid exercising and showering during monitoring. Physiologic hot flashes were classified via standard methods, with skin conductance rise of 2 μmho in 30 seconds (28) flagged automatically by UFI software (DPSv3.6; Morro Bay, CA) and edited for artifact (29). Given that some women show submaximal hot flashes failing to reach the 2 μmho criterion (30, 31), all potential hot flash events were also visually inspected, and events showing the characteristic hot flash pattern yet <2 micro mho/30 sec rise were coded as hot flashes. This coding has been shown to be reliable (κ=.86) (30, 31). A 20-minute lockout period was implemented after the start of the flash during which no hot flashes were coded.
To report hot flashes, participants were instructed to 1) complete a portable electronic diary (Palm Z22, Palm, Inc.) during waking hours and 2) press event mark buttons on their wrist actigraph and hot flash monitor (waking and sleeping hours) when experiencing a hot flash.
MRI Acquisition
Imaging data were collected at the University of Pittsburgh Magnetic Resonance Research Center (MRRC) using a 3T Siemens Trio machine, and 12-channel Siemens head coil. A standard high-resolution T1-weighted volumetric magnetization prepared rapid gradient echo scans (MPRAGE) sequence was acquired in axial orientation (160 slices, 256×240, 1mm isotropic). For the resting-state scan, T2*-weighted BOLD acquisition was done using a gradient-echo echo planar imaging sequence: TR=2,000 msec, TE=34 msec, matrix=128×128×29, voxel size=2×2×3 mm3, oblique axial acquisition, integrated parallel acquisition techniques=2. Images were acquired over 5 minutes (150 volumes). Subjects were instructed to lie still with their eyes open, look at a fixation cross, think of nothing in particular, and not to fall asleep.
MRI Processing
A seed based ROI (Region of Interest) analysis method was carried out in SPM8 (Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm). Resting-state functional images were slice time corrected, realigned, normalized to MNI space (using the MPRAGE), spatially smoothed with a 6-mm kernel, and temporally band-pass filtered (0.009 of 0.08 Hz). Functional connectivity analysis was performed using a seed-driven approach with the Conn toolbox (32). A component-based noise correction method (aCompCor) was used to remove physiological and other spurious sources of noise (33). In addition, significant principle components of the signals from white matter and CSF regions were removed together with movement related covariates. The REX (http://web.mit.edu/swg/software.htm) was used to extract the primary eigenvariate time-series of each ROI and then the CONN toolbox was used to extract network connectivity strength for the DMN, using the ROIs defined by Shirer et al (34). These ROIs include the medial prefrontal cortex, anterior cingulate cortex, orbitofrontal cortex, superior frontal gyrus, midcingulate cortex, posterior cingulate cortex, precuneus, angular gyrus, thalamus, and hippocampus. A correlation map was produced for each subject by extracting the residual BOLD time course from the ROIs and computing Pearson’s correlation coefficients between that time course and the time course of all other voxels. Correlation coefficients were converted to normally distributed z-scores using Fisher’s transform. The mean DMN strength was defined as the mean z score across all nodes of the DMN. The mean hippocampal DMN score was the mean z score for connectivity between the hippocampus and all the other nodes of the DMN.
Sleep
Participants wore a wrist actigraph and completed a sleep diary each day during the monitoring period. Actigraphy data were collected with a Minimitter Actiwatch-2 (Respironics, Inc., Murrysville, PA) in 1-min epochs and analyzed with the Actiware (Version 6.0.1) software program. Sleep diary data for bedtime and rise time were entered for calculation of sleep-wake variables. Actigraphy outcome variables included total sleep time (within the bedtime and rise time interval), sleep latency (bedtime to first sleep period), wakefulness after sleep onset (WASO; total minutes of wakefulness between sleep onset and final wake time) and sleep efficiency (100%*total sleep time⁄time in bed). Participants also completed the Pittsburgh Sleep Quality Index (35), a well-validated questionnaire measure of sleep quality.
Covariates
Demographics, menstrual history, and health behaviors were assessed by questionnaires and interview. Menopausal status was obtained from reported bleeding patterns, categorized as perimenopausal (>2–<12months amenorrhea), or postmenopausal (≥12 months amenorrhea). Race/ethnicity was self-reported by the participant. Education was assessed as years of completed education and due to small cell sizes classified as less than or greater than a college for analysis. Depressive symptoms were assessed via the Center for Epidemiologic Studies Depression Scale (36) and trait anxiety via the Spielberger State-Trait Anxiety Inventory (37). Concentrations of E2 were obtained from a morning fasting blood sample and assessed using liquid chromatography-tandem mass spectrometry, with inter- and intra-assay coefficients of variation of 5.0 and 8.1%, respectively and a lower limit of detection of 0.5 pg/ml.
Statistical analysis
Variables were examined for distributions, outliers, and cell sizes. Average DMN was log and square transformed due to skew. To account for variations in monitoring times, hot flash rates were calculated, with the number of hot flashes (either physiologically detected or self-reported) divided by monitoring time and standardized to a 24-hour period. Hot flash rates were set to 0 for women without hot flashes. Associations between hot flashes and DMN variables were tested in linear regression models, controlling for a priori selected covariates age, race/ethnicity, and education. Additional covariates depressive symptoms, trait anxiety, and estradiol concentrations were separately added into multivariable models in secondary models. All tests were two tailed with an alpha set to 0.05. Analyses were conducted using SAS v9.4 (SAS Institute, Cary, NC).
Results
Participants were on average 55 years old and approximately a quarter of the women were African American, with the remainder non-Hispanic Caucasian (Table 1). Most of the women (85%) were postmenopausal. Nine women reported having hot flashes, yet on physiologic monitoring 15 women showed physiologic hot flashes, a finding that is consistent with prior findings that women tend to under-report hot flashes relative to physiologic monitoring (38). For the sample as a whole, women reported an average of 2 hot flashes/24 hours, and showed 7 hot flashes/24 hours on physiologic monitoring.
Table 1.
Sample characteristics
| N | 18 |
| Age, M (SD) | 54.9 (3.5) |
| Race, N (%) | |
| White | 13 (72.2) |
| Nonwhite | 5 (27.8) |
| Education, N (%) | |
| <college | 6 (33.3) |
| ≥college | 12 (66.7) |
| Menopause status, N (%) | |
| Perimenopausal | 3 (16.7) |
| Postmenopausal | 17 (83.3) |
| BMI, M (SD) | 29.0 (4.9) |
| CESD score, M (SD) | 7.6 (7.7) |
| Trait anxiety, M (SD) | 32.1 (8.6) |
| Serum estradiol, pg/mL, M (SD) | 10.9 (16.9) |
| Actigraphy-assessed sleep time, hours, M (SD) | 6.3 (1.3) |
| Physiologic hot flash rate, number, M (SD) | |
| 24-hr | 7.2 (6.6) |
| Wakinga | 5.3 (5.5) |
| Sleepa | 1.9 (1.9) |
| Self-report hot flash rate, number, M (SD) | |
| 24-hr | 2.3 (2.9) |
| Wakinga | 1.5 (2.2) |
| Sleepa | 0.7 (0.8) |
Standardized to sleep and wake periods of 17 and 7 hours respectively for ease of interpretation
We first tested the relation between hot flashes and DMN connectivity. More physiologic hot flashes were associated with both greater total DMN connectivity, as well as greater hippocampal DMN connectivity (Table 2; Figure 1; Supplemental Figure 1). Associations were most pronounced for the left hippocampus and for physiologic hot flashes occurring during sleep, and associations persisted after covarying for age, race, and education. We next examined relations between hot flashes and a priori-selected frontal-hippocampal connectivity, finding that physiologically-monitored sleep hot flashes were positively associated with connectivity in five of the six regions in multivariable models (Table 3). No significant associations were observed between self-reported hot flashes and DMN connectivity.
Table 2.
Relation between hot flashes and DMN activity
| Average DMN§ | Average hippocampal DMN | Average left hippocampal DMN | Average right hippocampal DMN | |
|---|---|---|---|---|
|
| ||||
| B(SE) | B(SE) | B(SE) | B(SE) | |
| Physiologic hot flash rate | ||||
| 24-hr | .004 (.002)b | .005 (.002)b | .006 (.003)b | .004 (.003) |
| Sleep | .01 (.006)b | .02 (.007)c | .03 (.008)c | .02 (.01) |
| Wake | .004 (.002)b | .005 (.003) | .006 (.003)a | .005 (.003) |
| Self-report hot flash rate | ||||
| 24-hr | .00006 (.005) | .0007 (.006) | .002 (.007) | −.0007 (.008) |
| Sleep | .002 (.02) | .004 (.02) | .01 (.02) | −.005 (.03) |
| Waking | −.0003 (.006) | .0005 (.008) | .002 (.010) | −.0006 (.01) |
p<0.10
p<0.05
p<0.01
DMN: Default Mode Network
log transformed
Covariates: age, race, education
Figure 1.
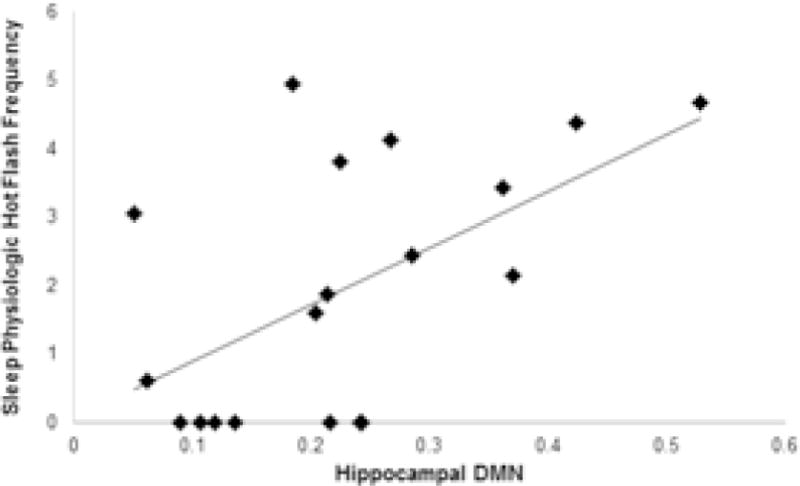
Scatterplot of relation between sleep physiologic hot flashes and hippocampal DMN
Note: Values of horizontal axis represent mean of the normalized correlations between the hippocampus and all the other nodes of the DMN
Table 3.
Relation between physiologically-monitored hot flashes and specific Dorsal DMN regions
| MPC-ACC-OC–LH | MPC-ACC-OC–RH | RSFG–LH | RSFG–RH | MCC–LH | MCC–RH | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| B(SE) | B(SE) | B(SE) | P | B(SE) | B(SE) | B(SE) | |
| 24-hr | .008 (.009) | .01 (.006)b | .008 (.008) | .003 (.006) | .02 (.006)b | .009 (.006) | |
| Sleep | .06 (.03)b | .07 (.02)c | .06 (.02)b | .03 (.02) | .08 (.02)c | .05 (.02)b | |
| Wake | .004 (.01) | .01 (.008) | .006 (.009) | .0009 (.007) | .02 (.008)a | .008 (.007) | |
p<0.10
p<0.05
p<0.01
Covariates: age, race, education
DMN: Default Mode Network; MPP-ACC-OC: Medial Prefrontal Cortex, Anterior Cingulate Cortex, Orbitofrontal Cortex; LH: Left Hippocampus; RH: Right Hippocampus; RSFG: Right Superior Frontal Gyrus; MCC: Midcingulate Cortex
Given that relations between hot flashes and DMN connectivity were observed most strongly for sleep hot flashes, we examined whether sleep characteristics accounted for observed relations. None of the relations between hot flashes and DMN connectivity were accounted for by any of the sleep variables assessed, such as sleep time, sleep efficiency, wake after sleep onset, or questionnaire-sleep quality [e.g., relations between physiologic sleep flashes and DMN outcome controlling for actigraphy-assessed sleep time, for total DMN: b(SE)=.01(.006), p=.048; hippocampal DMN: b(SE)=.02(.007), p=.01; left hippocampal DMN: b(SE)=.03(.009), p=.007]. Notably, the sleep indices were weakly or largely unrelated to the DMN outcomes (data not shown)].
We considered several additional analyses. In models of relations between physiologic hot flashes and DMN outcomes, we additionally adjusted for covariates such as depressive symptoms, trait anxiety, and serum estradiol concentrations. Results were unchanged (data not shown).
Discussion
This study was the first to investigate the relation between menopausal hot flashes and resting state connectivity, showing that more physiologically-monitored hot flashes (but not reported hot flashes) were associated with greater activity in the DMN. Findings were most striking for DMN connectivity to the hippocampus. Moreover, relations were most pronounced for physiologically monitored hot flashes occurring during sleep as compared to daytime.
The DMN is an organized network in the brain that is active during rest. Data indicates that hyperconnectivity and hyperactivity of the DMN, as well failure to deactivate components of the DMN, is associated with maladaptive emotional states such as major depressive disorder (23, 39). Elevated DMN activity has also been linked to rumination (23), and alterations in DMN activity have been linked to chronic pain (40) and possibly insomnia (41). It is notable that the findings here were for physiologically-assessed hot flashes and not subjectively-experienced hot flashes, arguing against a purely symptom-perception or somatic focus explanation of links between hot flashes and the DMN. Moreover, depressive and anxious symptoms were measured here and did not account for observed associations. Notably, suppression of the DMN is associated with better memory formation (21, 22). However, why the DMN is linked to hot flashes is not entirely clear and requires further investigation.
The current study pointed to DMN connectivity to the hippocampus in particular as related to hot flashes. A large literature indicates that the hippocampus may be sensitive to changing estrogen levels in the menopause transition and possibly to hot flashes (42). The abrupt declines in endogenous estradiol that mark the menopause transition may negatively impact the hippocampus, which is a region rich in estrogen receptors (16). Memory problems are a leading cognitive change women report during the menopause transition; in one study 60% of women transitioning through the menopause reporting unfavorable memory changes (43). Reproductive hormones may be important to these relations. In a longitudinal study of women transitioning through the menopause indicated, perimenopausal women who used hormone therapy had improved verbal memory and increased activation in the left hippocampus during this memory task relatively to their counterparts who had never used hormone therapy (11). Further, experimental suppression of the reproductive axis in young women results in changes in verbal memory encoding which can be restored with restoration of ovarian function (44, 45). Finally, prior work has shown more overnight physiologic hot flashes related to poorer verbal memory function (5). This body of literature points to adverse changes in the hippocampus and the functions it serves during the menopause transition and with its characteristic symptoms. Our findings for hippocampal DMN connectivity and hot flashes are consistent with this work. However, it is notable that endogenous estradiol concentrations, measured with state of the art methods, did not account for these relations, suggesting that other physiologic processes, such as HPA axis activity linked to both hot flashes and hippocampal function, may be important to investigate in future research on hot flashes and DMN activity.
Findings were most pronounced for physiologically monitored hot flashes, particularly hot flashes during sleep compared to during waking hours. These findings are consistent with other research showing that physiologically monitored hot flashes, rather than self-reported hot flashes, were associated with verbal memory (5). Physiologically monitored hot flashes differ from self-reported hot flashes in several important ways. Like the results observed here, most studies that use physiologic hot flash measures in the ambulatory setting detect more hot flashes than are reported (5, 25, 38). Although these measures merit continued refinement for use in large studies (46), physiologic hot flash measures have the advantage of not relying upon attention, perception of hot flashes, emotional influences on hot flash perceptions, or adherence to reporting (47, 48). These issues are particular prominent in the ambulatory setting when distracting factors, emotional experiences, and competing activities are common. Physiologic hot flashes show a circadian rhythm (49) and may be more related to other physiologic indices such as cardiac vagal control than are self-reported hot flashes (24, 25). Physiologic hot flash measures may be particularly useful for measuring hot flashes during sleep, when hot flash reporting may be particularly difficult and influenced by the quality of sleep itself (50, 51).
Notably, the strongest associations here were for hot flashes detected during sleep. Why associations between hot flashes and DMN connectivity were most pronounced for sleep rather than waking hot flashes is not entirely clear, but these findings are consistent with prior work showing overnight hot flashes most related to verbal memory (5). The associations between sleep physiologic hot flashes and DMN connectivity were not accounted for by sleep characterizes such as sleep time, waking during the night, or subjective sleep quality, broadly consistent with prior work (5). In fact, sleep indices were weakly or unrelated to DMN connectivity in this study. Whether sleep hot flashes (aka “night sweats”) and waking hot flashes have different underlying physiologies is not known, although it is notable that other work has found that reductions in cardiac vagal control with hot flashes were particularly large for sleep physiologic hot flashes (25). Further, investigation of the unique role of sleeping hot flashes in relation to brain function is warranted, with more intensive measures (e.g., polysomnography) of sleep.
This study has several limitations. The main limitation of the study was its small size which may have limited power to detect associations, although it is notable that such consistent findings were noted between DMN activity and hot flashes despite this small sample. However, these findings should be replicated in future work. Primarily postmenopausal women were included here and thereby any differences by menopausal stage could not be investigated. Although the sleep measures used here were extensive, future work should further expand the sleep battery given the particular importance of nocturnal hot flashes.
This study had several strengths. This is the first study to investigate resting state connectivity in relation to menopausal hot flashes. Hot flashes were investigated via rigorous methods: physiologic monitoring and prospective diary report and during wake and sleep as a woman went about her daily activity. These methods allowed investigation of any similarities or differences in the patterns of associations across indices and states. Sleep was measured via actigraphy, an improvement over prior work that has largely used self-reported sleep. Moreover, other related important indices, including depression, anxiety, and rigorously-measured endogenous estradiol concentrations were assessed and their roles considered.
Conclusions
More menopausal hot flashes were associated with greater DMN connectivity, particularly to the hippocampus. These associations were most pronounced for physiologically monitored hot flashes occurring during sleeping hours. As opposed to prior work showing changes in brain function acutely during hot flashes, the current data support differences in DMN connectivity that distinguish women with varying degrees of hot flash burden. Most investigations have focused on peripheral physiology in attempting to understand the physiology of hot flashes. These findings underscore the importance of continued investigation of central nervous system function in investigating the propensity towards and underlying physiology of hot flashes.
Supplementary Material
Supplementary Figure. DMN connectivity by physiologic hot flash frequency (median split), A: none-low hot flashes; B: medium-high hot flashes.
Capsule.
Among midlife women, more physiologically-monitored hot flashes, particularly during sleep, were associated with greater default mode network connectivity, particularly for networks supporting the hippocampus.
Acknowledgments
This research was supported by the National Institutes of Health, National Heart Lung and Blood Institute (R01HL105647 and K24HL123565 to Thurston) and a seed grant from the Department of Psychiatry at University of Pittsburgh.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Thurston: none; Maki: Abbot, Pfizer, Noven (consulting); Derby: none; Sejdić: none; Aizenstein: none.
References
- 1.Gold E, Colvin A, Avis N, Bromberger J, Greendale G, Powell L, et al. Longitudinal analysis of vasomotor symptoms and race/ethnicity across the menopausal transition: Study of Women’s Health Across the Nation (SWAN) Am J Public Health. 2006;96:1226–35. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avis NE, Colvin A, Bromberger JT, Hess R, Matthews KA, Ory M, et al. Change in health-related quality of life over the menopausal transition in a multiethnic cohort of middle-aged women: Study of Women’s Health Across the Nation. Menopause. 2009;16:860–9. doi: 10.1097/gme.0b013e3181a3cdaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bromberger JT, Matthews KA, Schott LL, Brockwell S, Avis NE, Kravitz HM, et al. Depressive symptoms during the menopausal transition: the Study of Women’s Health Across the Nation (SWAN) J Affect Disord. 2007;103:267–72. doi: 10.1097/gme.0b013e3181a3cdaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kravitz HM, Zhao X, Bromberger JT, Gold EB, Hall MH, Matthews KA, et al. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep. 2008;31:979–90. [PMC free article] [PubMed] [Google Scholar]
- 5.Maki PM, Drogos LL, Rubin LH, Banuvar S, Shulman LP, Geller SE. Objective hot flashes are negatively related to verbal memory performance in midlife women. Menopause. 2008;15:848–56. doi: 10.1097/gme.0b013e31816d815e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholson WK, Ellison SA, Grason H, Powe NR. Patterns of ambulatory care use for gynecologic conditions: A national study. Am J Obstet Gynecol. 2001;184:523–30. doi: 10.1067/mob.2001.111795. [DOI] [PubMed] [Google Scholar]
- 7.Williams RE, Kalilani L, DiBenedetti DB, Zhou X, Fehnel SE, Clark RV. Healthcare seeking and treatment for menopausal symptoms in the United States. Maturitas. 2007;58:348–58. doi: 10.1016/j.maturitas.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Freedman RR. Menopausal hot flashes: mechanisms, endocrinology, treatment. J Steroid Biochem. 2014;142:115–20. doi: 10.1016/j.jsbmb.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diwadkar VA, Murphy ER, Freedman RR. Temporal sequencing of brain activations during naturally occurring thermoregulatory events. Cereb cortex. 2014;24:3006–13. doi: 10.1093/cercor/bht155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedman RR, Benton MD, Genik RJ, 2nd, Graydon FX. Cortical activation during menopausal hot flashes. Fertil Steril. 2006;85:674–8. doi: 10.1016/j.fertnstert.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 11.Maki PM, Resnick SM. Longitudinal effects of estrogen replacement therapy on PET cerebral blood flow and cognition. Neurobiol Aging. 2000;21:373–83. doi: 10.1016/s0197-4580(00)00123-8. [DOI] [PubMed] [Google Scholar]
- 12.Wnuk A, Korol DL, Erickson KI. Estrogens, hormone therapy, and hippocampal volume in postmenopausal women. Maturitas. 2012;73:186–90. doi: 10.1016/j.maturitas.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epperson CN, Sammel MD, Freeman EW. Menopause effects on verbal memory: findings from a longitudinal community cohort. J Clin Endocr Metab. 2013;98:3829–38. doi: 10.1210/jc.2013-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greendale GA, Huang MH, Wight RG, Seeman T, Luetters C, Avis NE, et al. Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology. 2009;72:1850–7. doi: 10.1212/WNL.0b013e3181a71193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber MT, Maki PM, McDermott MP. Cognition and mood in perimenopause: a systematic review and meta-analysis. J Steroid Biochem. 2014;142:90–8. doi: 10.1016/j.jsbmb.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishunina TA, Fischer DF, Swaab DF. Estrogen receptor alpha and its splice variants in the hippocampus in aging and Alzheimer’s disease. Neurobiol Aging. 2007;28:1670–81. doi: 10.1016/j.neurobiolaging.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 17.Osterlund MK, Gustafsson JA, Keller E, Hurd YL. Estrogen receptor beta (ERbeta) messenger ribonucleic acid (mRNA) expression within the human forebrain: distinct distribution pattern to ERalpha mRNA. J Clin Endocr Metab. 2000;85:3840–6. doi: 10.1210/jcem.85.10.6913. [DOI] [PubMed] [Google Scholar]
- 18.Ottowitz WE, Siedlecki KL, Lindquist MA, Dougherty DD, Fischman AJ, Hall JE. Evaluation of prefrontal-hippocampal effective connectivity following 24 hours of estrogen infusion: an FDG-PET study. Psychoneuroendocrino. 2008;33:1419–25. doi: 10.1016/j.psyneuen.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freedman RR. Menopause and sleep. Menopause. 2014;21:534–5. doi: 10.1097/GME.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 20.Raichle ME. A paradigm shift in functional brain imaging. J Neurosci. 2009;29:12729–34. doi: 10.1523/JNEUROSCI.4366-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daselaar S, Prince S, Cabeza R. When less means more: deactivations during encoding that predict subsequent memory. Neuroimage. 2004;23:921–27. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 22.Daselaar S, Prince S, Dennis N, Hayes S, Kim H, Cabeza R. Posterior midline and ventral parietal activity is associated with retrieval success and encoding failure. Front Hum Neurosci. 2009;3:1–10. doi: 10.3389/neuro.09.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crandall CJ, Tseng CH, Crawford SL, Thurston RC, Gold EB, Johnston JM, et al. Association of menopausal vasomotor symptoms with increased bone turnover during the menopausal transition. J Bone Miner Res. 2011;26:840–9. doi: 10.1002/jbmr.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thurston RC, Christie IC, Matthews KA. Hot flashes and cardiac vagal control: a link to cardiovascular risk? Menopause. 2010;17:456–61. doi: 10.1097/gme.0b013e3181c7dea7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thurston R, Christie I, Matthews K. Hot flashes and cardiac vagal control during women’s daily lives. Menopause. 2012;19:406–12. doi: 10.1097/gme.0b013e3182337166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, et al. Executive Summary of the Stages of Reproductive Aging Workshop + 10: Addressing the Unfinished Agenda of Staging Reproductive Aging. J Clin Endocrinol Metab. 2012 doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dormire SL, Carpenter JS. An alternative to Unibase/glycol as an effective nonhydrating electrolyte medium for the measurement of electrodermal activity. Psychophysiology. 2002;39:423–6. doi: 10.1017.S0048577201393149. [DOI] [PubMed] [Google Scholar]
- 28.Freedman RR. Laboratory and ambulatory monitoring of menopausal hot flashes. Psychophysiology. 1989;26:573–9. doi: 10.1111/j.1469-8986.1989.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 29.Carpenter JS, Andrykowski MA, Freedman RR, Munn R. Feasibility and psychometrics of an ambulatory hot flash monitoring device. Menopause. 1999;6:209–15. doi: 10.1097/00042192-199906030-00006. [DOI] [PubMed] [Google Scholar]
- 30.Thurston R, Matthews K, Hernandez J, De La Torre F. Improving the performance of physiologic hot flash measures with support vector machines. Psychophysiology. 2009;46:285–92. doi: 10.1111/j.1469-8986.2008.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thurston R, Hernandez J, Del Rio J, De la Torre F. Support vector machines to improve physiologic hot flash measures: Application to the ambulatory setting. Psychophysiology. 2011;48:1015–21. doi: 10.1111/j.1469-8986.2010.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–41. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 33.Thurston R, Chang Y, Derby C, Bromberger J, Harlow S, Janssen I, et al. Abuse and subclinical cardiovascular disease among midlife women: Findings from The Study of Women’s Health Across the Nation. Stroke. 2014;45 doi: 10.1161/STROKEAHA.114.005928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warren MP. Are researchers studying the wrong kind of hot flashes? Menopause. 2014;21:113. doi: 10.1097/GME.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 35.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 36.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:385–401. [Google Scholar]
- 37.Spielberger CD. Manual for the State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press; 1983. [Google Scholar]
- 38.Mann E, Hunter MS. Concordance between self-reported and sternal skin conductance measures of hot flushes in symptomatic perimenopausal and postmenopausal women: a systematic review. Menopause. 2011;18:709–22. doi: 10.1097/gme.0b013e318204a1fb. [DOI] [PubMed] [Google Scholar]
- 39.Crandall CJ, Zheng Y, Crawford SL, Thurston RC, Gold EB, Johnston JM, et al. Presence of vasomotor symptoms is associated with lower bone mineral density: a longitudinal analysis. Menopause. 2009;16:239–46. doi: 10.1097/gme.0b013e3181857964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balabanovic J, Ayers B, Hunter MS. Women’s experiences of Group Cognitive Behaviour Therapy for hot flushes and night sweats following breast cancer treatment: an interpretative phenomenological analysis. Maturitas. 2012;72:236–42. doi: 10.1016/j.maturitas.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 41.Picchioni D, Duyn JH, Horovitz SG. Sleep and the functional connectome. Neuroimage. 2013;80:387–96. doi: 10.1016/j.neuroimage.2013.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greendale GA, Derby CA, Maki PM. Perimenopause and cognition. Obstet Gynecol Clin North Am. 2011;38:519–35. doi: 10.1016/j.ogc.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woods NF, Mitchell ES, Adams C. Memory functioning among midlife women: observations from the Seattle Midlife Women’s Health Study. Menopause. 2000;7:257–65. [PubMed] [Google Scholar]
- 44.Craig MC, Fletcher PC, Daly EM, Rymer J, Brammer M, Giampietro V, et al. Reversibility of the effects of acute ovarian hormone suppression on verbal memory and prefrontal function in pre-menopausal women. Psychoneuroendocrino. 2008;33:1426–31. doi: 10.1016/j.psyneuen.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 45.Craig MC, Fletcher PC, Daly EM, Rymer J, Cutter WJ, Brammer M, et al. Gonadotropin hormone releasing hormone agonists alter prefrontal function during verbal encoding in young women. Psychoneuroendocrino. 2007;32:1116–27. doi: 10.1016/j.psyneuen.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 46.Miller HG, Li RM. Measuring hot flashes: summary of a National Institutes of Health workshop. Mayo Clin Proc. 2004;79:777–81. doi: 10.4065/79.6.777. [DOI] [PubMed] [Google Scholar]
- 47.Hunter MS, Mann E. A cognitive model of menopausal hot flushes and night sweats. J Psychomsom Res. 2010;69:491–501. doi: 10.1016/j.jpsychores.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Thurston RC, Blumenthal JA, Babyak MA, Sherwood A. Emotional antecedents of hot flashes during daily life. Psychosom Med. 2005;67:137–46. doi: 10.1097/01.psy.0000149255.04806.07. [DOI] [PubMed] [Google Scholar]
- 49.Freedman RR, Norton D, Woodward S, Cornelissen G. Core body temperature and circadian rhythm of hot flashes in menopausal women. J Clin Endocrinol Metab. 1995;80:2354–8. doi: 10.1210/jcem.80.8.7629229. [DOI] [PubMed] [Google Scholar]
- 50.Thurston RC, Santoro N, Matthews KA. Are vasomotor symptoms associated with sleep characteristics among symptomatic midlife women? Comparisons of self-report and objective measures. Menopause. 2012 doi: 10.1097/gme.0b013e3182422973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thurston RC, Blumenthal JA, Babyak MA, Sherwood A. Association between hot flashes, sleep complaints, and psychological functioning among healthy menopausal women. Int J Behav Med. 2006;13:163–72. doi: 10.1207/s15327558ijbm1302_8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure. DMN connectivity by physiologic hot flash frequency (median split), A: none-low hot flashes; B: medium-high hot flashes.


