SUMMARY
Objective
This review summarizes the therapeutic potential of midazolam as an anticonvulsant antidote for organophosphate (OP) intoxication.
Methods
Benzodiazepines are widely used for acute seizures and status epilepticus (SE), a neurological emergency of persistent seizures that can lead to severe neuronal damage or death. Midazolam is a benzodiazepine hypnotic with a rapid onset and short duration of action.
Results
Midazolam is considered the new drug of choice for persistent acute seizures and SE, including those caused by neurotoxic OPs and nerve agents. Midazolam is a positive allosteric modulator of synaptic GABA-A receptors in the brain. It potentiates GABAergic inhibition and thereby controls hyperexcitability and seizures. Midazolam is administered intravenously or intramuscularly to control acute seizures and SE. Due to its favorable pharmacokinetic features, midazolam is being considered as a replacement anticonvulsant for diazepam in the antidote kit for nerve agents. Clinical studies such as the recent RAMPART trial have confirmed the anticonvulsant efficacy of midazolam in SE in prehospital settings.
Significance
In experimental models, midazolam is effective when given at the onset of seizures caused by nerve agents. However, benzodiazepines are less effective at terminating seizures when given 30 min or later after OP exposure or seizure onset likely because of internalization or down-regulation of synaptic, but not extrasynaptic, GABA-A receptors, which can lead to diminished potency and seizure recurrence.
Keywords: Benzodiazepines, diazepam, midazolam, organophosphates, nerve agents, status epilepticus
INTRODUCTION
Benzodiazepines are a first-line therapy for acute seizures and status epilepticus (SE), a life-threatening emergency characterized by persistent seizures lasting 30 min or more without regaining consciousness. Three benzodiazepines (diazepam, lorazepam and midazolam) are frequently recommended for SE because they suppress a variety of seizures.1 Midazolam is a safe benzodiazepine with a short half-life and rapid onset of action. It has powerful sedative, anticonvulsant, anxiolytic, and amnestic properties.2 It is used as premedication to inhibit anxiety and unpleasant memories during surgical procedures.2,3 Midazolam was first synthesized in 1975 by Armin Walser and Rodney Fryer at Hoffmann-LaRoche in the United States. Since then, it has become a frequently used benzodiazepine and is also included in the World Health Organization’s list of essential medicines. Midazolam is considered as the new drug of choice for acute seizures and SE, including nerve agent-induced seizures.4 It is administered intravenously (IV) or intramuscularly (IM) to control acute repetitive seizures and SE.5,6,7 Midazolam differs from other benzodiazepines in that it is water-soluble, providing increased product stability, better injection-site tolerance, and faster absorption.8 Midazolam is being considered as a replacement anticonvulsant for diazepam in the military antidote kit for nerve agents. This review describes recent advances in midazolam pharmacology with an emphasis on its mechanism of action, clinical uses, and therapeutic potential in treating acute seizures and SE caused by chemical agents.
CLINICAL PHARMACOLOGY OF MIDAZOLAM
The most prominent effects of midazolam are sedation, hypnosis, decreased anxiety, muscle relaxation, anterograde amnesia, and anticonvulsant activity. Midazolam is a rapid acting benzodiazepine with pronounced intensity. The antiseizure activity of midazolam is thought to result from its allosteric potentiation of synaptic GABA-A receptors. Like other benzodiazepines, midazolam does not activate GABA-A receptors directly; rather, it allosterically modulates the effects of GABA, the primary inhibitory neurotransmitter in the brain. GABA-A receptors are pentameric in structure, with five subunits that form a central chloride ion channel. 9,10,11 The extracellular domains form the primary recognition sites for GABA and the allosteric recognition sites for benzodiazepines and neurosteroids.12 The major isoforms of the GABA-A receptor consist of 2α,2β, and 1γ or δ-subunits. The GABA-A receptor mediates two types of inhibition, characterized as synaptic (phasic) and extrasynaptic (tonic) inhibition. Synaptic release of GABA results in the activation of low affinity γ-containing synaptic receptors, while ambient GABA levels persistently activate high-affinity δ-containing extrasynaptic receptors. Benzodiazepines bind to γ-containing synaptic receptors, leading to allosteric potentiation of GABA-gated hyperpolarization of the neuron, and thereby inhibit neuronal excitability. 9,11,13,14
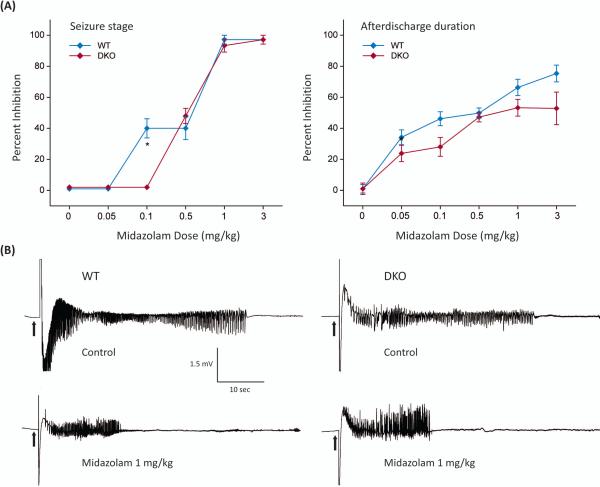
Benzodiazepines also bind to other targets including TSPO, a 19kDa cholesterol transporter protein that plays a key role in the biosynthesis of neurosteroids.15,16,17 Therefore, midazolam likely may interact with TSPO. There is evidence suggesting that midazolam can augment neurosteroidogenesis and, as a result, increase the levels of endogenous neurosteroids.18,19 Neurosteroids such as allopregnanolone rapidly alter neuronal excitability through direct interaction with GABA-A receptors, thereby producing robust protection against seizures.12,20,21 Unlike benzodiazepines, neurosteroids increase inhibition by acting at two distinct GABA-A receptors that mediate synaptic (phasic) and extrasynaptic (tonic) inhibition. Neurosteroids promote extrasynaptic receptor-mediated tonic inhibition that plays a critical role in controlling seizures. However, midazolam treatment, at anticonvulsant dosage, was not associated with significant increase in brain levels of the neurosteroid allopregnanolone.22 Therefore, the extent to which this mechanism contributes to midazolam actions remains unclear. Midazolam has been tested in seizure models utilizing δ-subunit knockout (DKO) mice which lack δ-subunit extrasynaptic GABA-A receptors.22 The anticonvulsant potency of midazolam was undiminished in DKO mice compared to the control (Fig.1), indicating that extrasynaptic GABA-A receptors are not involved in the protective activity of the drug.
Fig. 1. Anticonvulsant activity of midazolam in mice lacking extrasynaptic GABA-A receptors.
(A) Percent inhibition of fully kindled seizures and afterdischarge seizure duration in wild-type (WT) and δ-subunit extrasynaptic GABA-A receptor knockout (DKO) mice by midazolam. (B) Depth EEG recordings illustrating inhibition of afterdischarge seizure activity in a fully kindled WT and DKO mouse by midazolam. The anticonvulsant efficacy of midazolam was tested in the kindling paradigm, which is a model of human complex partial seizures. Kindling is one of the most widely used model of epilepsy in which repeated stimulation, subthreshold for motor seizures, of limbic structures triggers progressive intensification of behavioral and electrographic seizure activity and, therefore, shares many features with human complex partial seizures. Once an animal has been kindled, the heightened response to the stimulus is permanent and seizures occur upon stimulation even after several months. In this study, mice were surgically implanted with a bipolar stainless-steel wire electrode in the right hippocampus. After recovery from surgery, they received once-daily kindling stimulations (60 Hz 1-ms bipolar pulses for 1 s) at 125% of their individual afterdischarge threshold until stage 5 seizures were elicited on 3 consecutive days (considered as the “fully kindled” state). Midazolam (0.05 to 3 mg/kg, SC) was given 15-min prior to stimulation and the seizure activity was rated based on the severity of behavioral seizures and afterdischarge duration in fully-kindled mice. *p <0.05 vs. WT group.
Midazolam is used for the treatment of acute seizures and moderate to severe insomnia, and for inducing sedation and amnesia before medical procedures.3 It is used frequently to suppress a variety of acute seizures or SE, most notably seizures triggered by ethanol-withdrawal and organophosphate (OP) poisonings. Midazolam hydrochloride is available as 1 mg/ml and 5 mg/ml injections for IM or IV administration. These products are very stable with a shelf-life of 36 months. Midazolam is absorbed quickly following IM administration, and maximum plasma concentration is reached in about 30 minutes with over 90% bioavailability. The elimination half-life is 1.5 to 2.5 h.
Currently, three benzodiazepines are used in the management of acute seizures and SE: diazepam, lorazepam, and midazolam (Table 1). Although diazepam is absorbed through an IM route, such injections have been reported as providing a slow, erratic and incomplete absorption.23, 24, 25 A rectal gel of diazepam (Diastat) is available for the outpatient management of acute seizures or acute repetitive seizures. Unlike diazepam, midazolam is water soluble and less likely to cause thrombophlebitis. Midazolam has been tested clinically for the treatment of SE.5,7,26,27 A meta-analysis has confirmed that midazolam by any route is superior to diazepam.28 Midazolam offers several unique advantages over other benzodiazepines, including its rapid onset of action, short duration, water solubility, and extended shelf life.6 It has the longest shelf life of the benzodiazepines, 2-3 years at room temperature. It is available in a water-soluble form for formulation of aqueous injectable products. Midazolam is chemically a 8-chloro-6-(2-fluorophenyl)-1-methyl-4H-imidazo[1,5-a][1,4]benzodiazepine. The free base is a lipophilic substance with low solubility in water. The basic nitrogen in position 2 of the imidazobenzodiazepine ring system enables midazolam to form water-soluble salts with acids. At pH <5, midazolam is deprotonated and converts to an open ring configuration due to acid-catalyzed hydrolysis of the 4,5-double bond of the diazepine ring.29 These physicochemical features are attractive in formulating midazolam into aqueous injectable products with longer shelf-lives.
Table 1.
General pharmacotherapeutic profile of benzodiazepines for SE.
| Lorazepam | Diazepam | Midazolam | References | |
|---|---|---|---|---|
| Routes of administration | IM, IV | IM, IV, rectal | IM, IV | 1,2,23 |
| Half-life | 14 hours | 43 hours | 1.9 hours | 1,2 |
| Solubility | Lipophilic | Lipophilic | Hydrophilic | 1,8,29 |
| Shelf-life of an injectable product | 1 year if refrigerated, 30-60 days room temperature | 3 years if refrigerated, 30-60 days room temperature | 2-3 years at room temperature | 7,29 |
| Clinical indications | Early SE | Early SE | Prehospital SE | 6,7,26,30,34 |
| Pharmacokinetics by IM injection | Slow, erratic or incomplete absorption | Slow, erratic or incomplete absorption | Efficient absorption | 7,23,24,25 |
| Drug distribution kinetics | Biphasic kinetics after IV administration | Biphasic kinetics after IV administration | Monophasic kinetics | 1,2 |
| Mean SE termination rate | 60% by IV route | 43% by IV route | 73% by IM route | 5,7,26-28, 30-34 |
| Major adverse effect | Respiratory depression (10% by IV route) | Respiratory depression (10% by IV route) | Respiratory depression (6.4% by IM route) | 5,7,26-28, 30-34 |
| Meta-analysis for SE efficacy | Superior to diazepam | Inferior to lorazepam and midazolam | Non-inferior to lorazepam; Superior to diazepam | 7,26,27,28 |
| Efficacy against OP intoxication-induced SE | Effective if given early; less effective for delayed treatment | Effective if given early; less effective for delayed treatment | Effective if given early; less effective for delayed treatment | 4,42,43,49,55,66,69,70,71 |
Midazolam is evaluated extensively as a treatment option for SE, in both the pediatric and adult populations.26,30-34 The Rapid Anticonvulsant Medication Prior to Arrival Trial (RAMPART), which made a phase 3 evaluation of IM midazolam versus IV lorazepam, found similar protection profiles and recurrent seizure rates for children and adults with SE treated by paramedics.6,7 RAMPART involved 4314 paramedics, 33 EMS agencies, and 79 receiving hospitals across the United States. In this double-blind, randomized, noninferiority trial in 448 subjects, it was clearly demonstrated that IM midazolam is as effective as IV lorazepam, with a similar degree of safety, in terminating SE seizures before arrival at the hospital. Moreover, the IM midazolam group had a significant improvement in seizure-cessation rates upon emergency department arrival. 7 This study provides strong evidence that IM midazolam is safe and effective for the management of prehospital SE. Midazolam autoinjectors provided a faster and more reliable administration of midazolam for treating persistent seizures in the prehospital setting.
Midazolam causes side effects including sedation, cognitive impairment, dependency, and subsequent withdrawal syndrome.1 Paradoxical reactions such as agitation and involuntary movements have been reported in children and the elderly. There are also limitations common with diazepam that include partial seizure resistance, development of tolerance, and withdrawal syndrome, as well as adverse cognitive impairment and prolonged sedation.1 Cardiorespiratory adverse events have been reported in a few cases. At high doses, midazolam is known to cause respiratory depression. Midazolam, when given in combination with CNS drugs, like analgesic opiates or hypnotic barbiturates, may increase the likelihood of respiratory depression, causing respiratory arrest or even death.
EXPERIMENTAL PHARMACOLOGY OF MIDAZOLAM IN OP MODELS
Organophosphate (OP) pesticides such as parathion, chlorpyriphos, and diisopropylfluorophosphate (DFP) are neurotoxic chemicals. Nerve agents (nerve gases) such as sarin and soman are chemical threat agents with potential use in weapons of mass destruction. Nerve agents are the most toxic of the known chemical agents. They are classified into “G-series,” such as GA (tabun), GB (sarin), GD (soman) and GF (cyclosarin), and “V-series,” such as VX and VR. They are volatile liquids and pose both a vapor and liquid hazard when dispersed by missiles, shells, and sprays. They act by irreversibly inhibiting acetylcholinesterase (AChE), the enzyme that hydrolyzes acetylcholine. Toxic manifestations following nerve agents and OP exposure include hypersecretion, respiratory distress, tremor and convulsions leading to SE. Such devastating neurotoxicity was witnessed in sarin attacks in Japan and Syria.35,36,37 The RBC AChE enzyme activity is used as a biomarker of nerve gas exposure. Nerve agent induced SE can last several hours, causing profound brain damage, resulting in death or long-term neuronal dysfunction.38,39 The development of brain damage following seizures is a time-dependent process, and with increased time spent in seizures, the chances of brain damage increases. Therefore, rapid control of nerve agent-induced seizures is critical for neuroprotection and survival.38 Presently there are very few effective antidotes for OP intoxication, especially for rapid and effective termination of neurotoxic manifestations, seizures, and brain damage. Pyridostigmine bromide is FDA-approved for use as a pretreatment, but it has limited usefulness in post-exposure emergencies.
Current treatment for nerve agent and OP intoxication includes a specialized three drug combination regimen: (i) atropine sulfate--a muscarinic receptor antagonist; (ii) 2-PAM (pralidoxime chloride)--a drug to regenerate acetylcholinesterase activity; and (iii) diazepam--a benzodiazepine anticonvulsant.40,41 This regimen is distributed in CHEMPACKs with autoinjectors for use in case of chemical attacks or accidents. The federal CHEMPACK is a unique program of the strategic national stockpile, which stocks atropine, 2-PAM and diazepam in autoinjectors and multi-dose vials for distribution to state and local authorities in preparation for chemical threat agents. A diazepam injection (5 mg/ml) is packaged in 2 ml disposable autoinjectors that deliver their entire 2 ml contents automatically upon activation. Prefilled DuoDote® autoinjectors are available and provide a single intramuscular dose of atropine (2.1 mg/0.7 ml) and 2-PAM (600 mg/2 ml). These antidote products are designated for administration by emergency medical services personnel. Atropine sulfate (AtropenR®) autoinjectors are available in a variety of doses (0.25, 0.5, 1 and 2 mg), specifically designed for self or caregiver administration.
There are limitations with the current medical regimen for OP intoxication.42 Atropine is a critical antidote for survival from cardio-respiratory distress. It competitively blocks the effects of excess acetylcholine at muscarinic receptors in the peripheral and central nervous system. Atropine would lose its effectiveness if not given within minutes after the onset of seizures.43,44 2-PAM can partly rescue the cholinergic system by reactivating AChE, an important element in post-exposure treatment. 2-PAM and related oximes cannot enter into the brain, and thus offer little or limited neuroprotection. Therefore, benzodiazepines are the first-line drugs for the treatment of convulsions and SE caused by nerve agents. Diazepam is the current drug of choice for nerve agent seizures. The drug was provided to the military in the 1990s in an autoinjector form for IM delivery of 10 mg of diazepam. Diazepam is still considered to be part of the CHEMPACK as an anticonvulsant antidote for nerve agent intoxication. However, benzodiazepines have significant limitations. Diazepam has been shown in experimental studies to provide only partial protection against the neurotoxic manifestations of nerve agent exposure.39,43-47 There is evidence for partial or complete resistance of seizures or SE, a condition known as refractory SE that occurs later after a seizure following OP poisoning. Diazepam must be administered within 10 to 30 minutes, after which there is no protection against seizures and progressive neurological damage occurs.43,48,49 This timeline is often not practical in many incidents including emergencies and mass casualties. Moreover, diazepam is erratically absorbed via IM autoinjectors. A progressive, time-dependent loss in the anticonvulsant efficacy of diazepam occurs with delayed administration after seizure onset.42 Moreover, repeated high doses of diazepam are needed to control recurrent seizures, resulting in sedation, respiratory depression and tolerance. Seizures induced by OP intoxication can become self-sustaining and develop time-dependent refractory SE with over 25% mortality.50 The diazepam auto-injector for administration by caregivers in an outpatient setting for acute seizures is reported to cause injection site pain and hemorrhage.51 Avizafone, a water-soluble prodrug of diazepam developed elsewhere, has better formulation features for an autoinjection delivery.52,53,54 It must, however, be injected at a higher molar dose than diazepam for seizure protection. These limitations have prompted the search for alternative anticonvulsants to diazepam.
Midazolam has been suggested as a better anticonvulsant than diazepam for nerve agent seizures. Midazolam has been tested extensively in a variety of animal models of OP exposure.4,43,55-58 Most experiments are conducted in a guinea-pig model that is designed to closely simulate the military antidote regimen (pyridostigmine pretreatment and atropine sulfate+2-PAM). Rat and primate models were also utilized for testing the anticonvulsant therapies for OP intoxication. A comparison of seizure onset following nerve agents tabun, sarin, soman, cycloserin and VR showed similar patterns of seizure onset and progression with an average onset time of 6 to 10 min in guinea pigs.59 The average time to onset for seizures in rats following soman is similar to that of guinea pigs.49
Midazolam was tested against seizures induced by sarin, tabun, soman and VX in guinea pigs and rats. It was given IM at doses 0.5 to 10 mg/kg. Midazolam was quite effective when given 5 min after seizure onset, but lost its efficacy when given 40 min after onset in soman models in rats.43,55 Midazolam terminated seizures much faster than lorazepam and other benzodiazepines, with an average latency of 15 min. The ED50 values range from 0.7 to 2.2 mg/kg depending on the type of nerve agent and time of midazolam administration. There is a good correlation between pharmacokinetics for rapid absorption and anticonvulsant action of IM midazolam in soman-exposed animals.57
Among six benzodiazepines (avizafone, clonazepam, diazepam, loprazolam, lorazepam, and midazolam) tested in a soman model in guinea pigs, midazolam was found to be the most potent and rapidly acting drug when given 5 or 40 min after seizure onset.55 Midazolam in combination with an anticholinergic drug was reportedly very effective in terminating soman-induced seizures in guinea pigs when given 5 or 40 min after seizure onset.56
The anticonvulsant effectiveness of midazolam in terminating seizures elicited by soman was evaluated by different routes (intramuscular, intranasal or sublingual) of administration in guinea pigs.4 Midazolam appears effective in treating soman-induced seizures when given by all three routes, but with differences in potency and speed of action. Nasal application of midazolam is reportedly more effective with additional advantages such as rapid and convenient route of application.60,61 However, the speed of anticonvulsant activity of midazolam is found to be similar for the IM and intranasal routes of administration.4 Nasal midazolam delivery has been suggested as a rapid route for seizure suppression, 33 but intranasal administration would not be practical in treating a casualty exposed to nerve agents, which produces copious nasal secretions. Overall, experimental studies show that midazolam is a fast-acting anticonvulsant capable of terminating seizures elicited by nerve agents when administered early after exposure. There are still strong indications of seizure resistance or recurrence following an initial anticonvulsant effect, however, especially with delayed treatment with therapeutic doses.
Nerve agent seizures are initiated by cholinergic hyperactivation and quickly become self-sustained SE by progressive recruitment of glutamate receptors. The limbic forebrain plays a critical role in the initiation, propagation, and maintenance of seizures following nerve agent exposures. Midazolam provided anticonvulsant effects when injected directly into the anterior piriform cortex, amygdala, and area tempestas in a pretreatment protocol 30-min prior to sarin exposure in rats.62 Further studies confirmed midazolam effectiveness following microinjection into the perirhinal and entorhinal cortices but the drug was ineffective in the mediodorsal thalamus.63 These studies indicate neuroanatomical specificity for midazolam control of nerve agent-induced seizures.
Midazolam has been evaluated in pediatric and aged rodent models to mimic the likely age-related differences in susceptibility to nerve agents and varied anticonvulsant responses to benzodiazepines. Very young and older animals are more susceptible to the lethal effects of nerve agents than the young adults. The extent of neuronal injury due to nerve agent-induced seizures increases with increasing age. In pediatric rat pups, the median effective anticonvulsant dose for protection against sarin or VX-induced seizures increases with age. Younger animals may need a much lower dose of midazolam to terminate seizures than young adult animals.64
Midazolam has been evaluated for its anticonvulsant effects in primate models. Midazolam was tested at various doses following IM injection or auto-injector delivery in monkeys exposed to nerve agents.65,66 In comparison to diazepam (0.43 mg/kg), which showed 25% success rate for seizure cessation, midazolam (0.28 – 0.3 mg/kg) was faster and more successful (90%) in terminating soman-induced seizures in rhesus monkeys.65,66 Midazolam was also tested in advanced phase trials in monkeys using atropine and 2-PAM as a standard antidote regimen. Midazolam was reportedly effective in terminating or suppressing seizures when given at the onset of seizures. Additional details of outcomes are expected to be available in the near future.
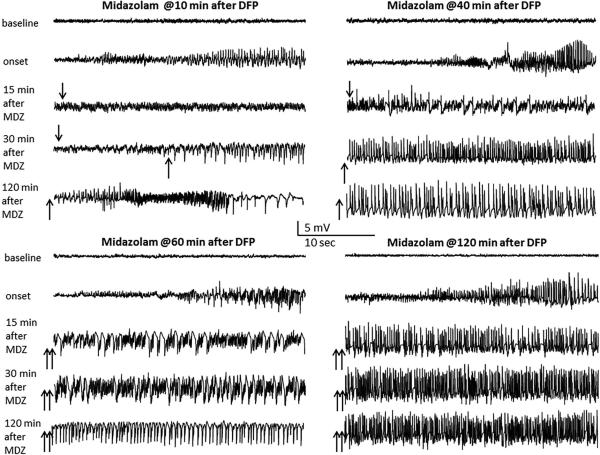
Many experimental studies in rodents suggest that seizures often recur after termination of the initial SE by benzodiazepines. This can ultimately result in little or no protection against neuropathology.49,67 The phenomenon of seizure recurrence is widely recognized as problematic with benzodiazepine treatment of nerve agent seizures. 24,49,55,68,69 Recently, the efficacy of the benzodiazepines diazepam and midazolam has been tested in the DFP model of OP intoxication in rats using a post-exposure treatment.70,71 Although diazepam controlled seizures initially when given at 10 or 40 min, it was partly or completely ineffective when given at 60 or 120 min after DFP --a profile indicative of refractoriness to benzodiazepines (Fig.2). There was a high frequency of seizure recurrence after initial seizure cessation even with early treatment. Midazolam appear to be less than optimal to control seizure activity with delayed (>60-min) therapy. Overall, OP intoxication-induced SE that responds initially to diazepam or midazolam becomes progressively resistant to delayed treatment, confirming the benzodiazepine insensitivity of nerve agent seizures, SE and neuronal injury.
Fig. 2. Time-dependent diminished anticonvulsant effect of midazolam against diisopropylfluoro-phosphate (DFP) intoxication-induced status epilepticus in rats.
Traces represent 25-sec epochs from a depth electrode in the hippocampus at various time points after midazolam in DFP-intoxicated rats. Organophosphate insecticides such as DFP are neurotoxic chemicals that cause lethal convulsions and injury. Like nerve agents, DFP is an irreversible cholinesterase inhibitor and thereby cause persistent seizures, status epilepticus (SE), and brain injury. In this study, we investigated the efficacy of midazolam in DFP model of OP intoxication in rats. Rats were exposed to DFP (2-4 mg/kg, SC) and were given standard antidotes, atropine and pralidoxime to increase the survival rate. Midazolam was administered at 10, 40, 60 or 120-min after DFP exposure. The progression of seizures was monitored by video-EEG recordings for 24-hours. DFP caused seizures within ~10 min and progressed into SE lasting for several hours. Midazolam (2 mg/kg, IM) effectively controlled seizures when given at 10 min, but it was partially or completely ineffective at 40, 60 or 120 min after DFP--a profile indicative of resistance to benzodiazepines. Seizure suppression (↓), seizures recurrence (↑), and seizure resistance (↑↑) at various intervals after midazolam treatment were indicated with arrows.
The mechanisms underlying the development of resistance to benzodiazepines remain unclear. A variety of mechanistic premises have been proposed for the development of pharmacoresistance of SE.50, 72-75 Molecular and electrophysiological studies in animal models of SE indicate that such phenomenon may involve internalization and down-regulation of synaptic GABA-A receptors from neuron membrane sites or other dysfunctions in GABAergic synaptic transmission that manifest as the seizures progress such as desensitization of synaptic GABA-A receptors.73-78 It is clearly demonstrated that postsynaptic GABAergic inhibition displays a high degree of plasticity in the hippocampus in that during prolonged epileptiform bursting, the rate of synaptic GABA-A receptor internalization increases rapidly, the subunit composition of these receptors swiftly changes (down-regulation of benzodiazepine-sensitive γ2-subunits), and the benzodiazepines ultimately lose efficacy due to limited network inhibition to shunt excessive seizure discharges.75 A significant decrease in the surface expression of γ2-containing synaptic GABA-A receptors (targets for benzodiazepines) are observed during persistent SE, while no such change is evident in δ-containing extrasynaptic GABA-A receptors (targets for neurosteroids). 73, 75 Consequently, these complex changes in subunit plasticity can transduce a reduction in synaptic or phasic inhibition but not extrasynaptic tonic inhibition. Overall, a combination of multifaceted, dynamic plasticity mechanisms may likely contribute to time-related loss of anticonvulsant efficacy of benzodiazepines.
FUTURE DIRECTIONS
Benzodiazepines are a safe treatment option for acute seizures and SE even in outpatient settings.79 Persistent convulsive seizures and SE are devastating central manifestations of exposure to OP agents that can lead to severe brain damage or death. Effective control of such seizures is critical for survival and prevention of neuronal injury, a major goal of anticonvulsant antidote treatments for OP intoxication. Benzodiazepines are the current drugs of choice for terminating seizures and SE caused by nerve agents. The currently fielded benzodiazepine diazepam has several limitations such as limited efficacy and inconsistent absorption from IM administration. Midazolam is likely to replace diazepam in the nerve agent treatment regimen because of its superior pharmacokinetic features. It is effective for controlling persistent acute seizures and SE, especially those caused by nerve agents and OP pesticides. The anticonvulsant activity of midazolam is a result of the drug binding to benzodiazepine sites on synaptic GABA-A receptors. Such protective effects are not mediated via extrasynaptic GABA-A receptors, which are the putative targets of endogenous anticonvulsant neurosteroids.
Midazolam has been tested in experimental models of OP poisoning and is effective for treatment of seizures caused by sarin and other chemical nerve agents. Like other benzodiazepines, midazolam is very effective when given early after seizure onset but loses its protective efficacy over time or when given in a delayed setting. Midazolam is less than optimal for effective control of nerve agent-induced recurrent seizures, which occur later after initial seizure cessation. Overall, benzodiazepines, although helpful in many cases, have often failed to be protective against persistent SE and neurodegeneration that occur at later times after nerve agent exposure in experimental settings. Such benzodiazepine refractory SE often observed later after OP exposure or seizure onset has been attributed to seizure activity-induced internalization or down-regulation of synaptic, but not extrasynaptic, GABA-A receptors and consequently a reduction in synaptic inhibition. Therefore, newer anticonvulsants superior to benzodiazepines are required to effectively terminate nerve agent-induced seizures and SE, and suppress seizure recurrence even when given late after chemical exposures or seizure onset.
ACKNOWLEDGMENTS
This work was partly supported by the CounterACT Program, National Institutes of Health, Office of the Director and the National Institute of Neurologic Disorders and Stroke [Grant U01 NS083460]. The authors thank Xin Wu, Ramkumar Kuruba, Bryan Clossen and Iyan Younus for technical assistance.
Footnotes
DISCLOSURE
The authors have no competing financial interests. We confirm that we have read the journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclaimer. The views expressed in this article are those of the authors and do not reflect the official policy of the Texas A&M University, National Institutes of Health, Department of Defense, or any other United States Government agencies.
REFERENCES
- 1.Riss J, Cloyd J, Gates J, et al. Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand. 2008;118:69–86. doi: 10.1111/j.1600-0404.2008.01004.x. [DOI] [PubMed] [Google Scholar]
- 2.Mandrioli R, Mercolini L, Raggi MA. Benzodiazepine metabolism: an analytical perspective. Curr Drug Metab. 2008;9:827–844. doi: 10.2174/138920008786049258. [DOI] [PubMed] [Google Scholar]
- 3.Luyk NH, Boyle MA, Ward-Booth RP. Evaluation of the anxiolytic and amnestic effects of diazepam and midazolam for minor oral surgery. Anesth Prog. 1987;34:37–42. [PMC free article] [PubMed] [Google Scholar]
- 4.McDonough JH, Van Shura KE, LaMont JC, et al. Comparison of the intramuscular, intranasal or sublingual routes of midazolam administration for the control of soman-induced seizures. Basic Clin Pharmacol Toxicol. 2009;104:27–34. doi: 10.1111/j.1742-7843.2008.00326.x. [DOI] [PubMed] [Google Scholar]
- 5.Galvin GM, Jelinek GA. Midazolam: an effective intravenous agent for seizure control. Arch Emerg Med. 1987;4:169–172. doi: 10.1136/emj.4.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silbergleit R, Lowenstein D, Durkalski V, et al. RAMPART (Rapid Anticonvulsant Medication Prior to Arrival Trial): a double-blind randomized clinical trial of the efficacy of intramuscular midazolam versus intravenous lorazepam in the prehospital treatment of status epilepticus by paramedics. Epilepsia. 2011;52(Suppl 8):S45–S47. doi: 10.1111/j.1528-1167.2011.03235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silbergleit R, Durkalski V, Lowenstein D, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 2012;366:591–600. doi: 10.1056/NEJMoa1107494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanto JH. Midazolam: The first water-soluble benzodiazepine; pharmacology, pharmacokinetics and efficacy in insomnia and anesthesia. Pharmacotherapy. 1985;5:138–155. doi: 10.1002/j.1875-9114.1985.tb03411.x. [DOI] [PubMed] [Google Scholar]
- 9.Mihic SJ, Whiting PJ, Klein RL, et al. A single amino acid of the human GABA-A receptor γ2-subunit determines benzodiazepine efficacy. J Biologic Chem. 1994;269:32768–32773. [PubMed] [Google Scholar]
- 10.Mohler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Therap. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- 11.Kucken AM, Teissére JA, Seffinga-Clark J, et al. Structural requirements for imidazobenzodiazepine binding to GABA-A receptors. Mol Pharmacol. 2003;63:289–296. doi: 10.1124/mol.63.2.289. 1195. [DOI] [PubMed] [Google Scholar]
- 12.Carver CM, Reddy DS. Neurosteroid interactions with synaptic and extrasynaptic GABA-A receptors: Regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology. 2013;230:151–188. doi: 10.1007/s00213-013-3276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rovira C, Ben-Ari Y. Developmental study of benzodiazepine effects on monosynaptic GABA-A-mediated IPSPs of rat hippocampal neurons. J Neurophysiol. 1993;70:1076–1085. doi: 10.1152/jn.1993.70.3.1076. [DOI] [PubMed] [Google Scholar]
- 14.Sarto-Jackson I, Sieghart W. Assembly of GABA-A receptors. Mol Membr Biol. 2008;25:302–310. doi: 10.1080/09687680801914516. [DOI] [PubMed] [Google Scholar]
- 15.Reddy DS, Kulkarni SK. Role of GABA-A and mitochondrial diazepam binding inhibitor receptors in the antistress activity of neurosteroids in mice. Psychopharmacology. 1996;128:280–292. doi: 10.1007/s002130050136. [DOI] [PubMed] [Google Scholar]
- 16.Gavish M, Bachman I, Shoukrun R, et al. Enigma of the peripheral benzodiazepine receptor. Pharmacol Rev. 1999;51:629–650. [PubMed] [Google Scholar]
- 17.Papadopoulos V, Baraldi M, Guilarte TR, et al. Translocator protein (18 kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Tokuda K, O'Dell KA, Izumi Y, et al. Midazolam inhibits hippocampal long-term potentiation and learning through dual central and peripheral benzodiazepine receptor activation and neurosteroidogenesis. J Neurosci. 2010;30:16788–16795. doi: 10.1523/JNEUROSCI.4101-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhir A, Rogawski MA. Role of neurosteroids in the anticonvulsant activity of midazolam. Br J Pharmacol. 2012;165:2684–2691. doi: 10.1111/j.1476-5381.2011.01733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reddy DS. Role of anticonvulsant and antiepileptogenic neurosteroids in the pathophysiology and treatment of epilepsy. Front Endocrinol. 2011;2(article 38):1–11. doi: 10.3389/fendo.2011.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carver CM, Wu X, Reddy DS. Perimenstrual-like hormonal regulation of extrasynaptic δ-containing GABA-A receptors mediated tonic inhibition and neurosteroid sensitivity. J Neurosci. 2014;34:14181–14197. doi: 10.1523/JNEUROSCI.0596-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reddy S, Younus I, Reddy DS. Antiseizure activity of midazolam in mice lacking δ-subunit extrasynaptic GABA-A receptors. J Pharmacol Exp Therap. 2015 doi: 10.1124/jpet.114.222075. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garnett WR1, Barr WH, Edinboro LE, et al. Diazepam autoinjector intramuscular delivery system versus diazepam rectal gel: A pharmacokinetic comparison. Epilepsy Res. 2011;93:11–6. doi: 10.1016/j.eplepsyres.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Divoll M, Greenblatt DJ, Ochs HR, et al. Absolute bioavailability of oral and intramuscular diazepam: effects of age and sex. Anesth Analg. 1983;62:1–8. [PubMed] [Google Scholar]
- 25.Lehmann C, Wannarka GL. Bioavailability and dose proportionality of intramuscular diazepam administered by autoinjector. J Clin Pharmacol. 2008;48:436–444. doi: 10.1177/0091270007311572. [DOI] [PubMed] [Google Scholar]
- 26.Alldredge BK, Gelb AM, Isaacs SM, et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med. 2001;345:631–637. doi: 10.1056/NEJMoa002141. [DOI] [PubMed] [Google Scholar]
- 27.Shorvon S, Ferlisi M. The treatment of super-refractory status epilepticus: a critical review of available therapies and a clinical treatment protocol. Brain. 2011;134(Pt 10):2802–2818. doi: 10.1093/brain/awr215. [DOI] [PubMed] [Google Scholar]
- 28.McMullan J, Sasson C, Pancioli A, et al. Midazolam versus diazepam for the treatment of status epilepticus in children and young adults: a meta-analysis. Acad Emerg Med. 2010;17:575–582. doi: 10.1111/j.1553-2712.2010.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orive MM, Gallo B, Alonso RM, et al. Spectrophotometric study of the acid-base equilibria of an imidazobenzodiazepine, midazolam, and its determination in pharmaceutical formulations. Mikrochim Acta. 1989;I:181–190. [Google Scholar]
- 30.Pellock JM. Use of midazolam for refractory status epilepticus in pediatric patients. J Child Neurol. 1998;13:581–587. doi: 10.1177/088307389801301201. [DOI] [PubMed] [Google Scholar]
- 31.Rivera R, Segnini M, Baltodano A, et al. Midazolam in the treatment of status epilepticus in children. Crit Care Med. 1993;21:991–994. doi: 10.1097/00003246-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Parent JM, Lowenstein DH. Treatment of refractory generalized status epilepticus with continuous infusion of midazolam. Neurology. 1994;44:1837–40. doi: 10.1212/wnl.44.10.1837. [DOI] [PubMed] [Google Scholar]
- 33.Thakker A, Shanbag P. A randomized controlled trial of intranasal-midazolam versus intravenous-diazepam for acute childhood seizures. J Neurol. 2013;260:470–474. doi: 10.1007/s00415-012-6659-3. [DOI] [PubMed] [Google Scholar]
- 34.Lahat E, Aladjem M, Eshel G, et al. Midazolam in treatment of epileptic seizures. Pediatr Neurol. 1992;8:215–216. doi: 10.1016/0887-8994(92)90071-6. [DOI] [PubMed] [Google Scholar]
- 35.Yanagisawa N, Morita H, Nakajima T. Sarin experiences in Japan: acute toxicity and long-term effects. J Neurol Sci. 2006;249:76–85. doi: 10.1016/j.jns.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Dolgin E. Syrian gas attack reinforces need for better anti-sarin drugs. Nat Med. 2013;19:1194–1195. doi: 10.1038/nm1013-1194. [DOI] [PubMed] [Google Scholar]
- 37.Rosman Y, Eisenkraft A, Milk N, et al. Lessons learned from the Syrian sarin attack: evaluation of a clinical syndrome through social media. Ann Intern Med. 2014;160:644–648. doi: 10.7326/M13-2799. [DOI] [PubMed] [Google Scholar]
- 38.Shih TM, Duniho SM, McDonough JH. Control of nerve agent-induced seizures is critical for neuroprotection and survival. Toxicol Appl Pharmacol. 2003;188:69–80. doi: 10.1016/s0041-008x(03)00019-x. [DOI] [PubMed] [Google Scholar]
- 39.McDonough JH, Jr, Shih TM. Neuropharmacological mechanisms of nerve agent-induced seizure and neuropathology. Neurosci Biobehav Rev. 1997;21:559–579. doi: 10.1016/s0149-7634(96)00050-4. [DOI] [PubMed] [Google Scholar]
- 40.Bajgar J. Organophosphates/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis, and treatment. Adv Clin Chem. 2004;38:151–216. doi: 10.1016/s0065-2423(04)38006-6. [DOI] [PubMed] [Google Scholar]
- 41.Eddleston M, Buckley NA, Eyer P, et al. Management of acute organophosphorus pesticide poisoning. Lancet. 2008;371(9612):597–607. doi: 10.1016/S0140-6736(07)61202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDonough JH, McMonagle JD, Shih TM. Time-dependent reduction in the anticonvulsant effectiveness of diazepam against soman-induced seizures in guinea pigs. Drug Chem Toxicol. 2010;33:279–283. doi: 10.3109/01480540903483417. [DOI] [PubMed] [Google Scholar]
- 43.Shih T, McDonough JH, Jr, Koplovitz I. Anticonvulsants for soman-induced seizure activity. J Biomed Sci. 1999;6:86–96. doi: 10.1007/BF02256439. [DOI] [PubMed] [Google Scholar]
- 44.Skovira JW, McDonough JH, Jr, Shih TM. Protection against sarin-induced seizures in rats by direct brain microinjection of scopolamine, midazolam or MK-801. J Mol Neurosci. 2010;40:56–62. doi: 10.1007/s12031-009-9253-0. [DOI] [PubMed] [Google Scholar]
- 45.Hayward IJ, Wall HG, Jaax NK, et al. Decreased brain pathology in organophosphate-exposed rhesus monkeys following benzodiazepine therapy. J Neurol Sci. 1990;98:99–106. doi: 10.1016/0022-510x(90)90185-p. [DOI] [PubMed] [Google Scholar]
- 46.Lallement G, Clarencon D, Brochier G, et al. Efficacy of atropine/pralidoxime/diazepam or atropine/HI-6/prodiazepam in primates intoxicated by soman. Pharmacol Biochem Behav. 1997;56:325–332. doi: 10.1016/s0091-3057(96)00292-4. [DOI] [PubMed] [Google Scholar]
- 47.Capacio BR, Whalley CE, Byers CE, et al. Intramuscular diazepam pharmacokinetics in soman-exposed guinea pigs. J Appl Toxicol. 2001;21(Suppl 1):S67–S74. doi: 10.1002/jat.813. [DOI] [PubMed] [Google Scholar]
- 48.Goodkin HP, Liu X, Holmes GL. Diazepam terminates brief but not prolonged seizures in young, naïve rats. Epilepsia. 2003;44:1109–1112. doi: 10.1046/j.1528-1157.2003.62402.x. [DOI] [PubMed] [Google Scholar]
- 49.Apland JP, Aroniadou-Anderjaska V, Figueiredo TH, et al. The limitations of diazepam as a treatment for nerve agent-induced seizures and neuropathology in rats; Comparison with UBP302. J Pharmacol Exp Ther. 2014;351:359–372. doi: 10.1124/jpet.114.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wasterlain CG, Chen JW. Mechanistic and pharmacologic aspects of status epilepticus and its treatment with new antiepileptic drugs. Epilepsia. 2008;49(Suppl 9):63–73. doi: 10.1111/j.1528-1167.2008.01928.x. [DOI] [PubMed] [Google Scholar]
- 51.Rogin J, Wheless J, Abou-Khalil B, et al. Safety and effectiveness of long-term treatment with diazepam auto-injector administered by caregivers in an outpatient setting for the treatment of acute repetitive seizures. Epilepsia. 2014;55:1444–1451. doi: 10.1111/epi.12685. [DOI] [PubMed] [Google Scholar]
- 52.Clement JG, Broxup B. Efficacy of diazepam and avizafone against soman-induced neuropathology in brain of rats. Neurotoxicology. 1993;14:485–504. [PubMed] [Google Scholar]
- 53.Lallement G, Renault F, Baubichon D, et al. Compared efficacy of diazepam or avizafone to prevent soman-induced electroencephalographic disturbances and neuropathology in primates: relationship to plasmatic benzodiazepine pharmacokinetics. Arch Toxicol. 2000;74:480–486. doi: 10.1007/s002040000146. [DOI] [PubMed] [Google Scholar]
- 54.Abbara C, Rousseau JM, Turcant A, et al. Bioavailability of diazepam after intramuscular injection of its water-soluble prodrug alone or with atropine-pralidoxime in healthy volunteers. Br J Pharmacol. 2009;157:1390–1397. doi: 10.1111/j.1476-5381.2009.00330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McDonough JH, Jr, McMonagle J, Copeland T, et al. Comparative evaluation of benzodiazepines for control of soman-induced seizures. Arch Toxicol. 1999;73:473–478. doi: 10.1007/s002040050637. [DOI] [PubMed] [Google Scholar]
- 56.Koplovitz I, Schulz S, Shutz M, et al. Combination anticonvulsant treatment of soman-induced seizures. J Appl Toxicol. 2001;21(Suppl 1):S53–55. doi: 10.1002/jat.811. [DOI] [PubMed] [Google Scholar]
- 57.Capacio BR, Byers CE, Merk KA, et al. Pharmacokinetic studies of intramuscular midazolam in guinea pigs challenged with soman. Drug Chem Toxicol. 2004;27:95–110. doi: 10.1081/dct-120030727. [DOI] [PubMed] [Google Scholar]
- 58.Ramarao G, Afley P, Acharya J, et al. Efficacy of antidotes (midazolam, atropine and HI-6) on nerve agent induced molecular and neuropathological changes. MC Neurosci. 2014;15:47. doi: 10.1186/1471-2202-15-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shih TM, McDonough JH., Jr Organophosphorus nerve agents-induced seizures and efficacy of atropine sulfate as anticonvulsant treatment. Pharmacol Biochem Behav. 1999;64:147–153. doi: 10.1016/s0091-3057(99)00114-8. [DOI] [PubMed] [Google Scholar]
- 60.Gilat E, Goldman M, Lahat E, et al. Nasal midazolam as a novel anticonvulsive treatment against organophosphate-induced seizure activity in the guinea pig. Arch Toxicol. 2003;77:167–172. doi: 10.1007/s00204-002-0425-8. [DOI] [PubMed] [Google Scholar]
- 61.Gilat E, Kadar T, Levy A, Rabinovitz I, et al. Anticonvulsant treatment of sarin-induced seizures with nasal midazolam: an electrographic, behavioral, and histological study in freely moving rats. Toxicol Appl Pharmacol. 2005;209:74–85. doi: 10.1016/j.taap.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 62.Skovira JW, McDonough JH, Shih TM. Protection against sarin-induced seizures in rats by direct brain microinjection of scopolamine, midazolam or MK-801. J Mol Neurosci. 2010;40:56–62. doi: 10.1007/s12031-009-9253-0. [DOI] [PubMed] [Google Scholar]
- 63.Skovira JW, Shih TM, McDonough JH. Neuropharmacological specificity of brain structures involved in soman-induced seizures. Neurotoxicology. 2012;33:463–468. doi: 10.1016/j.neuro.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 64.Miller S. Evaluation of anticonvulsants to treat nerve agent-induced seizures in pediatric rats. US Army Med Def Biosci Rev. 2014:49–49. [Google Scholar]
- 65.McDonough JH, Capacio BR, Shih T-M. Benzodiazepine dosage necessary to terminate soman-induced seizures in a rhesus monkey model. US Army Med Def Biosci Rev. 2000;1:297–297. [Google Scholar]
- 66.McDonough JH, Capacio BR, Shih T-M. Treatment of nerve agent-induced status epilepticus in the non-human primates. US Army Med Def Biosci Rev. 2002:7–7. [Google Scholar]
- 67.Singhi S, Murthy A, Singhi P, et al. Continuous midazolam versus diazepam infusion for refractory convulsive status epilepticus. J Child Neurol. 2002;17:106–110. doi: 10.1177/088307380201700203. [DOI] [PubMed] [Google Scholar]
- 68.McDonough JH, Jr, Zoeffel LD, McMonagle J, et al. Anticonvulsant treatment of nerve agent seizures: anticholinergics versus diazepam in soman-intoxicated guinea pigs. Epilepsy Res. 2000;38:1–14. doi: 10.1016/s0920-1211(99)00060-1. [DOI] [PubMed] [Google Scholar]
- 69.Eisenkraft A, Rosman Y, Statlender L, et al. Early seizure recurrence after organophosphate intoxication. US Army Med Def Biosci Rev. 2014:31–31. [Google Scholar]
- 70.Wu X, Kuruba R, Reddy DS. Midazolam refractory seizures and brain injury following acute organophosphate intoxication. American Epilespy Society Abst. 2014 #1.031. [Google Scholar]
- 71.Kuruba R, Wu X, Reddy DS. Benzodiazepine resistant status epilepticus and brain injury in DFP model of organophosphate intoxication. American Epilespy Society Abst. 2014 #1.029. [Google Scholar]
- 72.Goodkin HP, Kapur J. The impact of diazepam's discovery on the treatment and understanding of status epilepticus. Epilepsia. 2009;50:2011–2018. doi: 10.1111/j.1528-1167.2009.02257.x. [DOI] [PubMed] [Google Scholar]
- 73.Goodkin HP, Joshi S, Mtchedlishvili Z, et al. Subunit-specific trafficking of GABA-A receptors during status epilepticus. J Neurosci. 2008;28:2527–2538. doi: 10.1523/JNEUROSCI.3426-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goodkin HP, Sun C, Yeh JL, et al. GABA-A receptor internalization during seizures. Epilepsia. 2007;48(Suppl 5):109–113. doi: 10.1111/j.1528-1167.2007.01297.x. [DOI] [PubMed] [Google Scholar]
- 75.Goodkin HP, Yeh JL, Kapur J. Status epilepticus increases the intracellular accumulation of GABA A receptors. J Neurosci. 2005;25:5511–5520. doi: 10.1523/JNEUROSCI.0900-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Naylor DE, Liu H, Wasterlain CG. Trafficking of GABA-A receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci. 2005;25:7724–7733. doi: 10.1523/JNEUROSCI.4944-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Deeb TZ, Maguire J, Moss SJ. Possible alterations in GABA-A receptor signaling that underlie benzodiazepine-resistant seizures. Epilepsia. 2012;53:79–88. doi: 10.1111/epi.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vinkers CH, Olivier B. Mechanisms underlying tolerance after long-term benzodiazepine use: A future for subtype-selective GABA-A receptor modulators? Adv Pharmacol Sci. 2012;2012:416864. doi: 10.1155/2012/416864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reddy DS. Clinical pharmacology of current antiepileptic drugs. Int J Pharm Sci Nanotech. 2014;7:2305–2319. [Google Scholar]