Abstract
Meprins are oligomeric metalloproteinases that are abundantly expressed in the brush-border membranes of renal proximal tubules. During acute kidney injury (AKI) induced by cisplatin or ischemia-reperfusion, membrane-bound meprins are shed and their localization is altered from the apical membranes toward the basolateral surface of the proximal tubules. Meprins are capable of cleaving basement membrane proteins in vitro, however, it is not known whether meprins are able of degrade extracellular matrix proteins under pathophysiological conditions in vivo. The present study demonstrates that a basement membrane protein, nidogen-1, is cleaved and excreted in the urine of mice subjected to cisplatin-induced nephrotoxicity, a model of AKI. Cleaved nidogen-1 was not detected in the urine of untreated mice, but during the progression of cisplatin nephrotoxicity, the excretion of cleaved nidogen-1 increased in a time-dependent manner. The meprin inhibitor actinonin markedly prevented urinary excretion of the cleaved nidogen-1. In addition, meprin β-deficient mice, but not meprin α-deficient mice, subjected to cisplatin nephrotoxicity significantly suppressed excretion of cleaved nidogen-1, further suggesting that meprin β is involved in the cleavage of nidogen-1. These studies provide strong evidence for a pathophysiological link between meprin β and urinary excretion of cleaved nidogen-1 during cisplatin-induced AKI.
Keywords: Meprin A, meprin β, metalloproteinase, nidogen, acute kidney injury, cisplatin, actinonin, meprin β-KO mice
1. Introduction
Meprins are oligomer metalloproteinases of the ‘astacin’ family that were initially isolated and characterized from brush-border membranes of kidneys (Beynon et al., 1981; Kenny and Ingram, 1987) and intestines (Sterchi et al., 1982). Meprins are expressed as oligomeric forms composed of α and/or β subunits. The heteroligomeric form comprised both of α and β subunits (meprin αβ) is called heteromeric meprin A and the homodimeric form comprised only of meprin β subunits is called meprin B (Bertenshaw et al., 2003); (Bond et al., 2005). Heteromeric meprin A as well as meprin B are expressed as membrane-bound forms in the brush-border membranes of the renal proximal tubules (Bond et al., 2005; Sterchi et al., 2008). Heteromeric meprin A is anchored to the apical membranes of proximal tubules via the meprin β-subunit (Marchand et al., 1994). Homomeric meprin A is a homo-oligomer of meprin α subunits that can form high molecular weight complexes and is a secreted form of meprin A (Ishmael et al., 2001). During acute kidney injury (AKI) induced either by cisplatin or ischemia-reperfusion, localization of membrane-bound meprins in the proximal tubules was shown to be altered resulting in the redistribution of meprins toward the basolateral surface of the proximal tubule. (Bylander et al., 2008; Herzog et al., 2007; Walker et al., 1998). Since meprin β and heteromeric meprin A are membrane-bound meprins, their redistribution during AKI suggests ectodomain shedding and a recent report has demonstrated that a disintegrin and metalloproteinase domain-containing protein 10 (ADAM10) is involved in the shedding of meprin β and heteromeric meprin A from the membranes (Herzog et al., 2014). Although, actinonin, a potent pharmacological inhibitor of meprins, significantly reduced AKI due to cisplatin nephrotoxicity and IR (Kaushal et al., 2013), there is very little information available on the pathophysiological substrates targeted by meprins in vivo (Jefferson et al., 2013; Kaushal et al., 2013; Ongeri et al., 2011).
Current evidence for proteolytic degradation of target substrates by meprins has mostly come from in vitro studies. These studies have demonstrated that meprins are capable of hydrolyzing and processing a large number of substrates including basement membrane proteins, cytokines, adherens junction proteins, hormones, bioactive peptides, and cell-surface proteins in vitro (Jefferson et al., 2013; Kaushal et al., 2013). We previously demonstrated that heteromeric meprin A purified from rat kidney cortices was able to degrade extracellular matrix (ECM) proteins including collagen IV, laminin, nidogen, fibronectin, and gelatin in vitro (Kaushal et al., 1994; Walker et al., 1998). Consistent with the purified meprin from rat kidney, recombinant human meprin β was shown to degrade ECM proteins in vitro (Kruse et al., 2004). The ECM proteins are known to play an important role in epithelial cell attachment mediated by cellular receptors and specific matrix components. Cell-matrix adhesions regulate important cellular functions and have profound effects in cell proliferation, migration, and differentiation (Yurchenco, 2011). However, very little is known about the alterations in ECM proteins and matrix remodeling of the renal tubular basement membrane in vivo during AKI.
Cisplatin, a chemotherapeutic agent commonly used to treat a wide variety of solid tumors, induces nephrotoxicity as one of the side effects (Miller et al., 2010) and limits its use in higher doses to increase antitumor efficacy. Although the primary target of cisplatin in the kidney is proximal tubular epithelial cells, the molecular mechanisms of cisplatin-induced nephrotoxicity are not completely understood. Currently, it is virtually unknown whether ECM proteins are cleaved in vivo during cisplatin nephrotoxicity. When the laminin/nidogen complex was incubated with heteromeric meprin A purified from kidney cortex, there was a preferential cleavage of nidogen-1 and a cleaved fragment of nidogen-1 was produced (Walker et al., 1998). However, under pathophysiological conditions in cisplatin nephrotoxicity in vivo, it is not known whether meprins are involved in degradation of nidogen-1. In the present study, using a potent meprin inhibitor, actinonin, as well as meprin β-deficient and meprin α-deficient mice we provide evidence that meprin β is capable of cleaving the basement membrane protein nidogen-1 during cisplatin-induced AKI.
2. Materials and Methods
2.1. Reagents
Cisplatin was purchased from Novaplus (Bedford, OH). All other chemicals were from Sigma-Aldrich unless specified otherwise. The antibodies against meprin α (AF3220 and AF4007), meprin β (AF3300), and pro-meprin β (MAB 28592) were from R&D Systems (Minneapolis, MN). The nidogen-1 antibody (MAB 1886) was from Millipore (Temecula, CA). Antibodies against GAPDH (sc-32233) and Na+/K+-ATPase (sc-28800) as well as all HRP-conjugated secondary antibodies were from Santa Cruz Biotechnology (Dallas, TX). Fluorescent-labeled secondary antibodies (Alexa Fluor 488 donkey anti-rabbit, Alexa Fluor 488 donkey anti-goat, Alexa Fluor 594 donkey anti-goat, and Alexa Fluor 594 donkey anti-rat) were from Molecular Probes (Eugene, OR).
2.2. Animals
Male mice (C57Bl/6n), 10-12 weeks old were purchased from Charles Rivers Labs (Wilmington, MA) and housed with free access to food and water in accordance with standard procedures. The animal protocol for these studies was approved by the Institutional Animal Care and Use Committee (IACUC) of the Central Arkansas Veterans Healthcare System. Meprin α-KO and meprin β-KO mice were a generous gift from Dr. Judith Bond (Pennsylvania State University, Hershey, PA) and bred at our facility.
2.3. Cisplatin nephrotoxicity model of acute kidney injury and urine collection
Male mice (10-12 weeks old) were injected intraperitoneally (i.p.) with a single dose of cisplatin (20 mg/kg body weight). To examine the effect of the meprin inhibitor actinonin, mice received a single injection i.p. of actinonin (in saline/EtOH) in a dose of 20 mg/kg b.w., one hour before the injection with cisplatin. Control mice were injected with vehicle. Animals were kept in metabolic cages (4 mice per cage) and urine samples were collected on ice at 4, 8, 12, 24, 48 and 72 hours after injection with cisplatin. Urine samples were supplemented with EDTA to final concentration of 5 mM, pH 8.0 and centrifuged at 1000 × g for 5 minutes at 4°C. The cleared supernatants were stored at −20°C until used for western blot. Control urine samples were obtained from the same mice a week before starting the cisplatin treatment. Kidneys were harvested and fixed in formalin for histology and immunohistochemistry or snap-frozen in liquid nitrogen and stored at −80°C until used for western blotting. BUN and creatinine were determined from serum collected at the time of sacrifice using the diagnostic kits from Pointe Scientific (Canton, MI).
2.4. Immunohistochemistry
Deparaffinized kidney sections (5 μm) were immunostained with polyclonal goat anti-meprin β antibody or anti-meprin α antibody and polyclonal rabbit anti-Na+/K+-ATPase antibody at 4°C overnight. After washing with phosphate-buffered saline (PBS), slices were incubated with secondary antibodies donkey anti-goat Alexa Fluor 594 or donkey anti-rabbit Alexa Fluor 488 and nuclei counterstained with mounting medium containing DAPI (Vectashield, Burlingame, CA). Epi-immunofluorescence was recorded on an Olympus BX51 microscope. For immunostaining of nidogen-1, frozen sections of perfused mouse kidneys were incubated with polyclonal goat anti-meprin β antibody and monoclonal rat anti-nidogen-1 antibody.
2.5. Genotyping of meprin α-KO and meprin β-KO mice
Mouse tail biopsies were digested overnight with proteinase K to prepare genomic DNA. For detection of meprin β by PCR the protocol described (Norman et al., 2003) was used with LA Taq DNA polymerase ( TakaRa, Clontech). The expected product size of the meprin β amplicon of the meprin β-KO mice is 3.7 kb and the meprin β amplicon of wild-type mice is 2.2 kb. For the detection of meprin α by PCR the protocol described (Yura et al., 2009) was used with the following modification for the reverse primer: 5’-GAAAAGGCAGGTAAGACAATGTGCCTG-3’. The expected product size for the meprin α amplicon of meprin α-KO mice is 4.5 kb, the meprin α amplicon in wild-type mice is 3.3 kb.
2.6. Meprin α and meprin β expression in kidney tissue
Kidney lysates were prepared in Cell Lysis buffer (Cell Signaling, Danvers, MA) supplemented with 1 mM PMSF, 1 μg/ml pepstatin A, 1 μg/ml leupeptin using a Dounce homogenizer. The lysate was spun at 16000 × g for 10 min at 4°C and the protein concentration of the supernatant was determined by bicinchoninic acid (BCA) protein assay. Equal protein amounts (40 μg) were separated by 7.5% PAGE-SDS and western blots probed with antibodies to meprin α and meprin β. Blots were stripped and reprobed for GAPDH as loading control. Signals were detected by chemiluminescence (SuperSignal WestPico, Pierce, Rockford, IL).
2.7. Urinary excretion of cleaved nidogen-1
Urine samples were collected as described above under “Cisplatin nephrotoxicity model of acute kidney injury and urine collection”. Aliquots of equal protein content were separated by 10% PAGE-SDS and western blots probed with antibodies to nidogen-1. Signals were detected by chemiluminescence (SuperSignal WestPico). Bands of the excreted nidogen-1 fragment were analyzed by densitometry using QuantityOne software (BioRad Laboratories, Hercules, CA).
2.8. Statistical Analyses
Data are presented as the mean ± SEM. p< 0.05 was considered significant. Statistical analyses were performed by pairwise comparison of samples using Student's T-test.
3. Results
3.1. Meprin β distribution and expression in kidney sections in response to cisplatin
Double-immunofluorescence staining of kidney sections with meprin β (red) and Na+/K+-ATPase (green) confirmed that meprin β localization is altered in the injured kidney during cisplatin nephrotoxicity (Herzog et al., 2014; Herzog et al., 2007; Kaushal et al., 2013) (Fig 1A). No meprin staining was observed in the luminal surface of distal tubules, glomeruli, and collecting ducts. Kidney sections obtained from the cisplatin nephrotoxicity model were also immunostained with meprin β (green) and basement membrane protein nidogen-1 (red) antibodies (Fig 1B). Nidogen-1 showed clear staining (red) of the basement membranes of kidney sections from both control and 3d cisplatin-treated mice. Meprin staining in 3d cisplatin sections was redistributed throughout the proximal tubule and toward the basolateral plasma membrane. Thus, meprin β with its enormous degradative potential may be detrimental in renal injury due to altered localization and processing during AKI. meprin β protein levels weremarkedly decreased in 3d cisplatin-treated mice whereas there was no change in the meprin α expression (Fig 1C). The decrease in meprin β is attributed to the increased excretion of meprin β in response to cisplatin.
Figure 1. Meprin β distribution and its protein levels in kidney are altered in cisplatin treated mice.
Double-immunofluorescence staining of kidney sections using antibodies against meprin β and Na+/K+-ATPase. Kidney sections were co-stained with primary antibodies against meprin β and Na+/K+-ATPase followed by Alexa Fluor 594 (red) and Alexa Fluor 488 secondary antibodies (green),respectively, as described in Materials and Methods. Nuclei were stained with DAPI (blue). Epifluorescence pictures were recorded on an Olympus BX51 microscope. B. Double-immunofluorescence staining of kidney sections using antibodies against meprin β and nidogen-1. Kidney sections prepared from perfused frozen kidneys were stained with primary antibodies against meprin β and nidogen-1 followed by Alexa Fluor 488 donkey anti-goat secondary antibody (green) and Alexa Fluor 594 donkey anti rat secondary antibody (red), respectively. Nuclei were stained with DAPI (blue). C. Meprin α and meprin β protein expression after cisplatin administration. Wild-type mice (3 mice each) were treated with cisplatin for 1 day and 3 day or left untreated (controls). Aliquots of kidney homogenates of mice were separated by 7.5% PAGE-SDS, transferred to PVDF membranes, and immunoblots probed for meprin α, meprin β, and GAPDH as loading control.
3.2. Effect of actinonin on altered distribution of meprin α and meprin β in response to cisplatin
Double-immunofluorescence staining of kidney sections with meprin α (red) and Na+/K+-ATPase (green) or meprin β (red) and Na+/K+-ATPase (green) demonstrated that redistribution of meprin α and meprin β was partially reversed on treatment with the meprin inhibitor actinonin (Fig 2). The reversal in distribution is due to the renoprotective role of actinonin during cisplatin injury.
Figure 2.
Effect of meprin inhibitor actinonin on altered distribution of meprin α and meprin β during cisplatin injury. Kidney sections of mice treated with cisplatin with or without actinonin as described in Material and Methods were co-stained with antibodies against meprin α (red) or meprin β (red) respectively and Na+/K+-ATPase (green). Nuclei were stained with DAPI (blue). Epifluorescence pictures were recorded on an Olympus BX51 microscope.
3.3. Cleavage of nidogen during cisplatin-induced nephrotoxicity and effect of meprin inhibitor actinonin
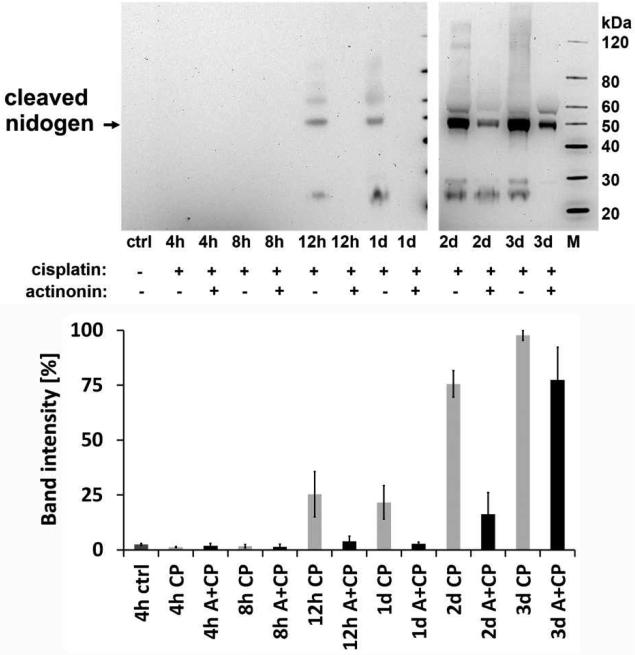
We next examined whether meprins are able to degrade the basement membrane protein nidogen-1 in vivo during cisplatin nephrotoxicity. We observed that a nidogen-1 fragment (~50 kDa) that was undetectable in the urine of normal mice increased significantly during cisplatin-induced AKI (Fig 3). This urinary nidogen-1 fragment was first detected 12 h after cisplatin administration and the excretion of this fragment subsequently increased during the progression of the injury. Administration of the meprin inhibitor actinonin markedly prevented the urinary excretion of the cleaved nidogen product at all of the time points assayed (Fig 3).
Figure 3. Time-course of urinary excretion of cleaved nidogen in mice after cisplatin injection and effect of meprin inhibitor actinonin.
The urinary excretion of cleaved nidogen-1 was monitored in mice injected with either actinonin at 20 mg/kg b.w. or vehicle 1 h before injecting cisplatin at 20 mg/kg b.w. as described in Materials and Methods. Aliquots of equal protein content were analyzed by western blot using monoclonal antibody to nidogen-1 (upper panel). Western blot band intensities were analyzed by densitometry. Bands were normalized to the strongest cleaved nidogen band obtained on the western blot (lower panel). A in the figure is actinonin and CP is cisplatin. Error bars represent the S.E.M, n=3.
These results suggest that meprins may mediate the cleavage of nidogen from the basement membrane. In this model, serum creatinine and blood urea nitrogen (BUN) start rising 2 d after cisplatin treatment (Herzog et al., 2007), suggesting that excretion of the nidogen-1 fragment in this model occurs prior to the rise in serum creatinine. Although actinonin, a naturally occurring hydroxamate, has proved to be a most effective inhibitor of meprins and inhibits meprin α and meprin A at a nanomolar range (Ki = 20 nM), it can also inhibit other metalloproteinases and aminopeptidases at higher concentrations (Kruse et al., 2004).
3.4. Genotyping and protein expression of meprin subunits in meprin α-KO and meprin β-KO mice
To confirm whether meprin α and/or meprin β are involved in vivo in the degradation of nidogen-1 we next examined whether meprin α-knockout (KO) and/or meprin β-KO mice lack the ability to cleave nidogen-1 and excrete the cleaved nidogen-1 product in the urine in response to cisplatin nephrotoxicity. Meprin α-KO and meprin β-KO mice were generated in Dr. Judith Bond's laboratory (Norman et al., 2003; Yura et al., 2009). These mice develop normally, the renal function of the null mice is normal, and no differences were observed in serum creatinine and BUN values between the null and wild-type mice. There was no meprin α detected in meprin β-null mice suggesting that heteromeric meprin A is absent in meprin β-KO mice (Norman et al., 2003).
This observation is in accordance with the previous findings that meprin α is present in the brush border membrane by virtue of its association with its membrane-bound binding partner meprin β. We established breeding colonies of meprin α-KO and meprin β-KO mice in our laboratory and genotyping confirmed the deficiency of meprin α and meprin β genes in the knockout mice (Fig 4A). Both meprin β and meprin α proteins were absent in kidneys of meprin β-KO mice confirming that meprin β-KO mice are deficient in heteromeric meprin A at the brush-border membranes (Fig 4B).
Figure 4. Genotyping and protein expression of meprin subunits in meprin α-KO and meprin β-KO mice.
A. Genomic DNA was prepared from tail biopsies of meprin α-KO and meprin β-KO mice as well as wild-type mice. PCR with meprin α- and meprin β-specific primers was performed using LATaQ (TaKaRa, Clontech) and products separated by agarose gel electrophoresis and visualized with ethidium bromide. B. Aliquots of kidney homogenates from meprin α-KO and meprin β-KO as well as wild-type mice were separated by 7.5% PAGE-SDS and western blots probed with antibodies specific to meprin α (top panels), meprin β (lower panel, left) and the pro-form of meprin β (lower panel, right).
3.5. Meprin β- KO mice subjected to cisplatin nephrotoxicity do not cleave nidogen-1
Meprin β-KO mice when subjected to cisplatin nephrotoxicity were unable to excrete a 50-kDa nidogen-1 product in the urine (Fig 5A), suggesting that meprin β is involved in the proteolytic cleavage of nidogen-1 protein.
Figure 5.
Time-course of urinary excretion of cleaved nidogen-1 in meprin β-KO and meprin α-KO mice after cisplatin injection. A. Wild-type (wt) and meprin β-KO (bko) mice were administered with cisplatin or vehicle as described in Materials and Methods. Aliquots of equal protein content were analyzed by western blot using a monoclonal antibody to nidogen-1 (upper panel). Western blot band intensities were analyzed by densitometry. Bands were normalized to the strongest cleaved nidogen band obtained on the western blot (lower panel). Error bars represent the S.E.M, n=3. *P< 0.05, **p<0.01, ***p<0.001. B. Wild-type (wt) and meprin α-KO (ako) mice were administered cisplatin or vehicle as described in Materials and Methods. Aliquots of equal protein content were analyzed by western blot using a monoclonal antibody to nidogen-1 (upper panel). Western blot band intensities were analyzed by densitometry. Bands were normalized to the strongest cleaved nidogen band obtained on the western blot (lower panel).
In contrast, meprin α-KO mice escreted a 50-kDa nidogen-1 fragment in the urine (Fig 5B), indicating that meprin α is not involved in the cleavage of nidogen-1 during cisplatin injury.
Taken together, these studies demonstrate that meprin β is involved in the cleavage of nidogen-1 during cisplatin nephrotoxicity and provides the first evidence of degradation of an extracellular matrix component by meprin β in vivo.
4. Discussion
Basement membrane proteins play important roles in cell-matrix interactions that regulate subsequent cell proliferation, migration, differentiation, tissue development, and repair (LeBleu et al., 2007; Timpl, 1996). Degradation of basement proteins in response to injury may not only impair these cellular processes and related signaling pathways but also influence remodeling of the ECM. In previous in vitro studies, we have shown that heteromeric meprin A purified from the kidney is capable of degrading ECM proteins. In the present study we have provided evidence that meprin β is able to cleave the basement membrane protein nidogen-1 in vivo during cisplatin-induced AKI. We have demonstrated that a 50-kDa nidogen-1 cleavage product is excreted as early as 12 h after cisplatin administration and the excretion is further increased during the progression of cisplatin nephrotoxicity. We further showed that excretion of the 50-kDa nidogen-1 fragment into urine during AKI is markedly suppressed by the meprin inhibitor actinonin. Meprin β-KO mice, but not meprin α-KO mice, subjected to cisplatin nephrotoxicity significantly prevented excretion of cleaved nidogen-1, further confirming that meprin β is involved in the nidogen-1 cleavage. Taken together, these studies provided evidence for a pathophysiological link between meprin β and nidogen-1 cleavage in vivo during cisplatin-induced AKI.
Nidogen-1, a 150-kDa sulfated glycoprotein, is an important component of renal kidney basement membranes (LeBleu et al., 2007; Ries et al., 2001; Timpl, 1996). Nidogen-1 is composed of distinct G1, G2, and G3 globular domains (Fox et al., 1991), with the G1 and G2 domains separated by a link region and the G2 and G3 domains separated by a longer rod-like region. Nidogen-1 acts as a connecting element between collagen IV and laminin and integrates other basement membrane components, in particular perlecan, into the ECM (Fox et al., 1991; Ho et al., 2008; Ries et al., 2001). In the basement membranes, the G2 domain of nidogen-1 interacts with collagen IV and perlecan (Fox et al., 1991; Ho et al., 2008; Reinhardt et al., 1993; Ries et al., 2001) and the G3 domain binds to laminin particularly with high affinity (Poschl et al., 1996). Nidogen-1 is susceptible to degradation by numerous serine proteases and metalloproteinases in vitro (Kruse et al., 2004; Sage et al., 2012; Sires et al., 1993; Walker et al., 1998) but is resistant to degradation when bound with laminin (Mayer et al., 1995). Our studies demonstrate that meprin β is involved in the preferential cleavage of nidogen in the basement membrane during cisplatin-induced AKI and its cleavage product was identified in the urine. Our previous in vitro study also showed that nidogen is cleaved by meprin purified from rat kidney (Walker et al., 1998). Thus, degradation of nidogen-1 by meprin β will perturb the capacity of nidogen to bind to other basement membrane partners and affect the supramolecular organization of basement membranes. Proteolytic degradation of nidogen-1 by cathepsin S impairs its ability to bind to other basement membrane proteins (Sage et al., 2012). Basement membrane assembly in skin is prevented in the absence of nidogen-1 and nidogen-2 (Nischt et al., 2007). Since nidogen-1 crosslinks laminin and type IV collagen networks, the loss of intact nidogen-1 due to degradation by meprin β may result in a defective basement membrane at the nidogen-1 linking region. This may also potentiate proteolytic cleavage of laminin or collagen IV and excretion of their cleaved products.
Nidogen-1 protein in the basement membranes also plays a critical role in promoting attachment of cells (Dong et al., 1995; Salmivirta et al., 2002). The link region between the G1 and G2 domains contains an EGF repeat that was previously shown to participate in cell adhesion through integrin α3β1 (Dedhar et al., 1992). The first EGF repeat between the G2 and G3 domains contains an RGD sequence involved in cell attachment through interaction with integrin αvβ3 (Dedhar et al., 1992; Dong et al., 1995). Degradation of nidogen-1 will impair the ability of cells to adhere through the nidogen-1 receptors, α3β1 and αvβ3 integrins. In addition, nidogen-1 may also play a critical role in transducing intracellular signaling via integrin-mediated cell adhesion in addition to its role in assembly of the basement membrane. Upon binding to extracellular ECM ligands, including nidogen, integrins are activated and interact with signaling molecules inside the cell (Harburger and Calderwood, 2009; Kaushal et al., 2014). Once cells are attached to the basement membrane ligands, activated integrins transduce intracellular signaling by recruitment and tyrosine phosphorylation of signaling proteins including focal adhesion kinase, Src, phosphatidylinositol-3 kinase (PI3-K)/Akt-1, and cytoskeletal adaptor proteins including talin, p130Cas, paxillin, and vinculin (Mitra et al., 2005; Vachon, 2011). Thus, signaling originating from β1 integrins promotes alterations in cell survival, proliferation, morphology, and function. Degradation of nidogen-1 will impair the signaling pathways mediated by tyrosine phosphorylation of signaling molecules including FAK, Src, and PI3-kinase and their corresponding signaling pathways in adherent renal cells. Thus, nidogen-1 may play a critical role in transducing intracellular signaling via integrin-mediated adhesion in addition to its role in assembly of the basement membrane.
In conclusion, our studies demonstrate that a basement membrane protein, nidogen-1, is cleaved during the progression of cisplatin-induced nephrotoxicity. The cleavage of nidogen-1 during nephrotoxicity was markedly prevented by a meprin inhibitor, actinonin. In addition, meprin β-KO mice subjected to cisplatin nephrotoxicity significantly abolished nidogen-1 cleavage. Taken together, these studies provide the first evidence for a pathophysiological link between meprin β and nidogen-1 cleavage in vivo during cisplatin-induced nephrotoxicity.
Highlights.
Basement membrane protein nidogen-1 is cleaved in cisplatin-induced nephrotoxicity.
Meprin inhibitor actinonin abolished nidogen-1 cleavage during cisplatin nephrotoxicity.
Meprin β-KO mice subjected to cisplatin nephrotoxicity prevented nidogen-1 cleavage.
This study provides the first in vivo evidence of nidogen-1 cleavage by meprin β and a pathophysiological link between meprin β and nidogen-1 in cisplatin nephrotoxicity.
Acknowledgements
This work was supported by NIH grant R01DK081690 and VA Merit Award BX000444 to GPK and VA Merit Award BX000828 to RSH. We thank Judith Bond, Ph.D., for generously providing meprin α-KO and meprin β-KO mice for these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bertenshaw GP, Norcum MT, Bond JS. Structure of homo- and hetero-oligomeric meprin metalloproteases. Dimers, tetramers, and high molecular mass multimers. J Biol Chem. 2003;278:2522–2532. doi: 10.1074/jbc.M208808200. [DOI] [PubMed] [Google Scholar]
- Beynon RJ, Shannon JD, Bond JS. Purification and characterization of a metalloendoproteinase from mouse kidney. Biochem J. 1981;199:591–598. doi: 10.1042/bj1990591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond JS, Matters GL, Banerjee S, Dusheck RE. Meprin metalloprotease expression and regulation in kidney, intestine, urinary tract infections and cancer. FEBS Lett. 2005;579:3317–3322. doi: 10.1016/j.febslet.2005.03.045. [DOI] [PubMed] [Google Scholar]
- Bylander J, Li Q, Ramesh G, Zhang B, Reeves WB, Bond JS. Targeted disruption of the meprin metalloproteinase beta gene protects against renal ischemiareperfusion injury in mice. Am J Physiol Renal Physiol. 2008;294:F480–490. doi: 10.1152/ajprenal.00214.2007. [DOI] [PubMed] [Google Scholar]
- Dedhar S, Jewell K, Rojiani M, Gray V. The receptor for the basement membrane glycoprotein entactin is the integrin alpha 3/beta 1. J Biol Chem. 1992;267:18908–18914. [PubMed] [Google Scholar]
- Dong LJ, Hsieh JC, Chung AE. Two distinct cell attachment sites in entactin are revealed by amino acid substitutions and deletion of the RGD sequence in the cysteine-rich epidermal growth factor repeat 2. J Biol Chem. 1995;270:15838–15843. doi: 10.1074/jbc.270.26.15838. [DOI] [PubMed] [Google Scholar]
- Fox JW, Mayer U, Nischt R, Aumailley M, Reinhardt D, Wiedemann H, Mann K, Timpl R, Krieg T, Engel J, et al. Recombinant nidogen consists of three globular domains and mediates binding of laminin to collagen type IV. The EMBO journal. 1991;10:3137–3146. doi: 10.1002/j.1460-2075.1991.tb04875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harburger DS, Calderwood DA. Integrin signalling at a glance. Journal of cell science. 2009;122:159–163. doi: 10.1242/jcs.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog C, Haun RS, Ludwig A, Shah SV, Kaushal GP. ADAM10 is the major sheddase responsible for the release of membrane-associated meprin A. J Biol Chem. 2014;289:13308–13322. doi: 10.1074/jbc.M114.559088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog C, Seth R, Shah SV, Kaushal GP. Role of meprin A in renal tubular epithelial cell injury. Kidney Int. 2007;71:1009–1018. doi: 10.1038/sj.ki.5002189. [DOI] [PubMed] [Google Scholar]
- Ho MS, Bose K, Mokkapati S, Nischt R, Smyth N. Nidogens-Extracellular matrix linker molecules. Microscopy research and technique. 2008;71:387–395. doi: 10.1002/jemt.20567. [DOI] [PubMed] [Google Scholar]
- Ishmael FT, Norcum MT, Benkovic SJ, Bond JS. Multimeric structure of the secreted meprin A metalloproteinase and characterization of the functional protomer. J Biol Chem. 2001;276:23207–23211. doi: 10.1074/jbc.M102654200. [DOI] [PubMed] [Google Scholar]
- Jefferson T, Auf dem Keller U, Bellac C, Metz VV, Broder C, Hedrich J, Ohler A, Maier W, Magdolen V, Sterchi E, Bond JS, Jayakumar A, Traupe H, Chalaris A, Rose-John S, Pietrzik CU, Postina R, Overall CM, Becker-Pauly C. The substrate degradome of meprin metalloproteases reveals an unexpected proteolytic link between meprin beta and ADAM10. Cellular and molecular life sciences : CMLS. 2013;70:309–333. doi: 10.1007/s00018-012-1106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal GP, Elbein A, Carver WE. The Extracellular Matrix. In: Baynes J,DM, editor. Medical Biochemistry. Elsevier Mosby Inc; 2014. pp. 380–392. [Google Scholar]
- Kaushal GP, Haun RS, Herzog C, Shah SV. Meprin A metalloproteinase and its role in acute kidney injury. Am J Physiol Renal Physiol. 2013;304:F1150–1158. doi: 10.1152/ajprenal.00014.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal GP, Walker PD, Shah SV. An old enzyme with a new function: purification and characterization of a distinct matrix-degrading metalloproteinase in rat kidney cortex and its identification as meprin. J Cell Biol. 1994;126:1319–1327. doi: 10.1083/jcb.126.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny AJ, Ingram J. Proteins of the kidney microvillar membrane. Purification and properties of the phosphoramidon-insensitive endopeptidase ('endopeptidase-2′) from rat kidney. Biochem J. 1987;245:515–524. doi: 10.1042/bj2450515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse MN, Becker C, Lottaz D, Kohler D, Yiallouros I, Krell HW, Sterchi EE, Stocker W. Human meprin alpha and beta homo-oligomers: cleavage of basement membrane proteins and sensitivity to metalloprotease inhibitors. Biochem J. 2004;378:383–389. doi: 10.1042/BJ20031163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBleu VS, Macdonald B, Kalluri R. Structure and function of basement membranes. Experimental biology and medicine. 2007;232:1121–1129. doi: 10.3181/0703-MR-72. [DOI] [PubMed] [Google Scholar]
- Marchand P, Tang J, Bond JS. Membrane association and oligomeric organization of the alpha and beta subunits of mouse meprin A. J Biol Chem. 1994;269:15388–15393. [PubMed] [Google Scholar]
- Mayer U, Zimmermann K, Mann K, Reinhardt D, Timpl R, Nischt R. Binding properties and protease stability of recombinant human nidogen. Eur J Biochem. 1995;227:681–686. doi: 10.1111/j.1432-1033.1995.tb20188.x. [DOI] [PubMed] [Google Scholar]
- Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of Cisplatin nephrotoxicity. Toxins. 2010;2:2490–2518. doi: 10.3390/toxins2112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nature reviews Molecular cell biology. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- Nischt R, Schmidt C, Mirancea N, Baranowsky A, Mokkapati S, Smyth N, Woenne EC, Stark HJ, Boukamp P, Breitkreutz D. Lack of nidogen-1 and -2 prevents basement membrane assembly in skin-organotypic coculture. J Invest Dermatol. 2007;127:545–554. doi: 10.1038/sj.jid.5700562. [DOI] [PubMed] [Google Scholar]
- Norman LP, Jiang W, Han X, Saunders TL, Bond JS. Targeted disruption of the meprin beta gene in mice leads to underrepresentation of knockout mice and changes in renal gene expression profiles. Mol Cell Biol. 2003;23:1221–1230. doi: 10.1128/MCB.23.4.1221-1230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongeri EM, Anyanwu O, Reeves WB, Bond JS. Villin and actin in the mouse kidney brush-border membrane bind to and are degraded by meprins, an interaction that contributes to injury in ischemia-reperfusion. Am J Physiol Renal Physiol. 2011;301:F871–882. doi: 10.1152/ajprenal.00703.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poschl E, Mayer U, Stetefeld J, Baumgartner R, Holak TA, Huber R, Timpl R. Site-directed mutagenesis and structural interpretation of the nidogen binding site of the laminin gamma1 chain. The EMBO journal. 1996;15:5154–5159. [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D, Mann K, Nischt R, Fox JW, Chu ML, Krieg T, Timpl R. Mapping of nidogen binding sites for collagen type IV, heparan sulfate proteoglycan, and zinc. J Biol Chem. 1993;268:10881–10887. [PubMed] [Google Scholar]
- Ries A, Gohring W, Fox JW, Timpl R, Sasaki T. Recombinant domains of mouse nidogen-1 and their binding to basement membrane proteins and monoclonal antibodies. Eur J Biochem. 2001;268:5119–5128. doi: 10.1046/j.0014-2956.2001.02437.x. [DOI] [PubMed] [Google Scholar]
- Sage J, Leblanc-Noblesse E, Nizard C, Sasaki T, Schnebert S, Perrier E, Kurfurst R, Bromme D, Lalmanach G, Lecaille F. Cleavage of nidogen-1 by cathepsin S impairs its binding to basement membrane partners. PLoS One. 2012;7:e43494. doi: 10.1371/journal.pone.0043494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmivirta K, Talts JF, Olsson M, Sasaki T, Timpl R, Ekblom P. Binding of mouse nidogen-2 to basement membrane components and cells and its expression in embryonic and adult tissues suggest complementary functions of the two nidogens. Experimental cell research. 2002;279:188–201. doi: 10.1006/excr.2002.5611. [DOI] [PubMed] [Google Scholar]
- Sires UI, Griffin GL, Broekelmann TJ, Mecham RP, Murphy G, Chung AE, Welgus HG, Senior RM. Degradation of entactin by matrix metalloproteinases. Susceptibility to matrilysin and identification of cleavage sites. J Biol Chem. 1993;268:2069–2074. [PubMed] [Google Scholar]
- Sterchi EE, Green JR, Lentze MJ. Non-pancreatic hydrolysis of N-benzoyl-l-tyrosylp-aminobenzoic acid (PABA-peptide) in the human small intestine. Clin Sci (Lond) 1982;62:557–560. doi: 10.1042/cs0620557. [DOI] [PubMed] [Google Scholar]
- Sterchi EE, Stocker W, Bond JS. Meprins, membrane-bound and secreted astacin metalloproteinases. Mol Aspects Med. 2008;29:309–328. doi: 10.1016/j.mam.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R. Macromolecular organization of basement membranes. Current opinion in cell biology. 1996;8:618–624. doi: 10.1016/s0955-0674(96)80102-5. [DOI] [PubMed] [Google Scholar]
- Vachon PH. Integrin signaling, cell survival, and anoikis: distinctions, differences, and differentiation. Journal of signal transduction. 2011;2011:738137. doi: 10.1155/2011/738137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker PD, Kaushal GP, Shah SV. Meprin A, the major matrix degrading enzyme in renal tubules, produces a novel nidogen fragment in vitro and in vivo. Kidney Int. 1998;53:1673–1680. doi: 10.1046/j.1523-1755.1998.00949.x. [DOI] [PubMed] [Google Scholar]
- Yura RE, Bradley SG, Ramesh G, Reeves WB, Bond JS. Meprin A metalloproteases enhance renal damage and bladder inflammation after LPS challenge. Am J Physiol Renal Physiol. 2009;296:F135–144. doi: 10.1152/ajprenal.90524.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harbor perspectives in biology. 2011;3 doi: 10.1101/cshperspect.a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]