Abstract
Auditory thalamus (medial geniculate body [MGB]) receives ascending inhibitory GABAergic inputs from inferior colliculus (IC) and descending GABAergic projections from thalamic reticular nucleus (TRN) with both inputs postulated to play a role in shaping temporal responses. Previous studies suggested that enhanced processing of temporally rich stimuli occurs at the level of MGB, with our recent study demonstrating enhanced GABA sensitivity in MGB compared to IC. The present study used sinusoidal amplitude modulated (SAM) stimuli to generate modulation transfer functions (MTFs), to examine the role of GABAergic inhibition in shaping the response properties of MGB single units in anesthetized rats. Rate MTFs (rMTFs) were parsed into “bandpass (BP)”, “mixed (Mixed)”, “highpass (HP)” or “atypical” response types, with most units showing the Mixed response type. GABAA receptor blockade with iontophoretic application of the GABAA receptor (GABAAR) antagonist gabazine (GBZ) selectively altered the response properties of most MGB neurons examined. Mixed and HP units showed significant GABAAR mediated SAM evoked rate response changes at higher modulation frequencies (fms), which were also altered by NMDA receptor blockade (2R)-amino-5-phosphonopentanoate (AP5). Bandpass units, and the lower arm of Mixed units responded to GABAAR blockade with increased responses to SAM stimuli at or near the rate best modulation frequency (rBMF). The ability of GABA circuits to shape responses at higher modulation frequencies is an emergent property of MGB units, not observed at lower levels of the auditory pathway and may reflect activation of MGB NMDA receptors (Rabang and Bartlett, 2011, Rabang et al., 2012). Together, GABAARs exert selective rate control over selected fms, generally without changing the units’ response type. These results showed that coding of modulated stimuli at the level of auditory thalamus is at least, in part, strongly controlled by GABA neurotransmission, in delicate balance with glutamatergic neurotransmission.
Keywords: Auditory thalamus, SAM response, Inhibition-excitation balance
The temporally complex features of acoustic stimuli are used by most species as specific communication calls, including speech for humans. Sinusoidal amplitude modulated (SAM) stimuli are frequently used as a reliable proxy to examine how different substations of the auditory system process temporally changing stimuli as one ascends the auditory neuraxis. Neurons at higher levels of the auditory system respond to SAM stimuli with large and complex variations in spike rate. The response properties of auditory neurons to amplitude modulated stimuli were well described for auditory cortex (AC) and inferior colliculus (IC) (Krishna and Semple, 2000, Wallace et al., 2000, Wallace et al., 2002, Lu and Wang, 2004). The medial geniculate body (MGB), the auditory thalamic “relay station”, receives excitatory and inhibitory ascending information from the IC and direct and indirect descending excitatory and inhibitory input from AC (Winer et al., 1996, Bartlett et al., 2000, Malmierca, 2003). Studies have examined the temporal processing features of MGB units, comparing them to other auditory structures, including studies in anesthetized cat (Rouiller et al., 1979), guinea pig (Wallace et al., 2007), and recent series of in-depth studies in unanesthetized marmoset (Bartlett and Wang, 2007, 2011). Collectively, these studies suggested that MGB neurons display unique and more complex responses to modulated and click train stimuli than do neurons in the IC (Bartlett and Wang, 2007, 2011). This conclusion is based, in part, on finding large number of MGB neurons exhibiting both synchronized and nonsynchronized discharge patterns (Bartlett and Wang, 2007, 2011). These MGB findings contrast, quantitatively, to response properties described at lower levels of the auditory neuraxis using similar stimuli (Krishna and Semple, 2000, Joris et al., 2004). A number of studies at lower levels of the auditory neuraxis suggested that glycinergic and/or GABAergic inhibition play a role in shaping responses to modulated stimuli (Burger and Pollak, 1998, Koch and Grothe, 1998, Caspary et al., 2002). Studies in chinchilla cochlear nucleus and IC suggested that glycine or GABA selectively alters response-rate at or below best modulation frequency (BMF), frequently changing band-pass responses to more low-pass responses (Burger and Pollak, 1998, Caspary et al., 2002). Other rat IC studies found that AMPA or NMDA receptor blockade resulted in decreased discharge rates while GABAA receptor blockade produced an increase in firing rate (Kelly and Zhang, 2002). Koch and Grothe (1998) found that GABAergic inhibition sharpened tuning of frequency modulated signals for a majority of IC neurons in the big brown bat. Three separate IC iontophoretic studies found that GABAA receptor blockade increased near-CF, tone-evoked discharge rates, suggesting that inhibitory inputs onto IC neurons had co-tuned frequency response areas, with some inhibitory neurons likely tuned more broadly than the IC neurons onto which they projected (Yang et al., 1992, Le Beau et al., 1996, Palombi and Caspary, 1996). These experiments suggested that glutamatergic and GABAergic inputs selectively regulate IC response properties to SAM stimuli.
Based on the identification of high affinity GABAARs mediating tonic inhibitory current and enhanced GABA sensitivity in auditory thalamus (Richardson et al., 2011, Cai et al., 2013), and the findings and models by Bartlett, Wang and colleagues (Bartlett and Wang, 2007, 2011, Rabang and Bartlett, 2011, Rebang et al., 2012), the present study sought to characterize the role of GABAergic inhibition in shaping responses to SAM stimuli for commonly observed SAM-response-types seen for MGB neurons.
Experimental Procedures
All experiments were carried out in accordance to protocols approved by the Laboratory Animal Care and Use Committee of Southern Illinois University School of Medicine.
1. Surgery procedure
Thirty-seven adult male FBN (Fisher Brown Norway) rats (4–6 mos) were initially anesthetized with I.M. injection (1.4 ml/kg) of a 3:1 mixture of ketamine-HCl (100 mg/ml) and xylazine (20 mg/ml). Anesthesia was maintained by ip injections of urethane (initially 1.3 ml/kg, then one-third initial amount for maintenance doses; 750 mg/kg, Sigma, St. Louis, MO). Urethane was chosen as the anesthetic agent because its actions are on multiple neurotransmitter systems rather than simply potentiating the effects of inhibitory systems, thus it may have less net effect on GABAergic neurotransmission than other anesthetic agents (Hara and Harris, 2002). Rats were placed in a modified stereotaxic frame in an IAC sound-attenuating booth (Industrial Acoustic Co., Inc., New York, NY) with body temperature maintained at 37 ± 0.5 °C by a thermostatically controlled heating blanket. Similar to Caspary et al. (2005), prior to surgery, auditory brainstem responses (ABRs) to click and 4 kHz, 8 kHz, 12 kHz, 16 kHz, 24 kHz and 32 kHz tones (3 ms duration, 1 ms ramp) were obtained. None of the animals used in the present study showed any signs of hearing loss. To access the left MGB, a 2X2 mm craniotomy was drilled, exposing the dorsal surface of cortex (−5.5 mm from bregma; 3.5 mm lateral from midline). MGB unit recording depth was between 4800 um to 6800 um from the surface (Paxinos and Watson, 2007, Cai et al., 2013).
2. Acoustic stimuli and electrophysiological recording
Similar to Cai et al. (2013), a carbon fiber electrode attached to a five-barrel iontophoretic electrode, Carbostar-6 (Kation Scientific, Minneapolis, MN), was coupled to a headstage preamplifier, Multichannel Acquisition Processor (MAP) system and PC running MAP software and Sort Client (Plexon Inc., Dallas, TX) for real-time spike sorting. A piezoelectric advancer (David Kopf Ins., Tujunga, CA) advanced the electrode into the left MGB using 70–80 dB broadband noise pips as search signal. Single units (3:1 SNR) were discriminated based on waveform morphology and principle component analysis. Stimulus presentation real-time data display and analysis used ANECS software (Blue Hills Scientific, Dr. K. Hancock, Boston, MA) coupled to TDT System III hardware. Acoustic signals were amplified (TDT-ED1), transduced (TDT-EC1) and presented to the right ear canal using polypropylene tubing. The sound system was calibrated off-line using a 0.25 inch Bruel & Kjaer model 4938 microphone (Naerum, Denmark) into a simulated rat ear (2–48 kHz ± 2dB) (Palombi and Caspary, 1996). SAM carrier frequency (fc) was set at the unit's characteristic frequency (CF) or broadband noise (BBN); rMTFs and tMTFs were determined for each unit at 30 dB above CF threshold in response to 2/sec, 450 msec long SAM stimuli (4 ms raise-fall time, 100% depth) with fms stepped between 2 and 512 Hz. Spikes were collected by the carbon fiber over a 500 ms period, following stimulus onset for 10 stimulus repetitions at each envelope frequency.
3. Iontophoresis and histology
A multi-barrel electrode was coupled to a constant current system (BH-2 NeuroPhore System). The balancing barrel was filled with potassium acetate (KAc, 2 M), with remaining barrels filled with γ-Aminobutyric acid (GABA, 500 mM, pH = 4.0, Sigma-Aldrich, St. Louis, MO), gabazine (SR-95531) (GBZ, 10 mM, Sigma-Aldrich, St. Louis, MO) and AP5 (100 mM, pH = 7.4). Retaining currents were set at −15 nA as previous studies (Cai et al., 2013, Duque et al., 2013). The KAc filled balancing barrel neutralizes all currents by passing current equal and opposite currents being used. Additional control runs using the same current used to eject the test drug, but unbalance through the balancing barrel, were routinely utilized to rule out possible current effects. Once an MGB unit was located, drug delivering was performed with ejection currents kept between 0 to 100 nA to avoid excessive diffusion (Foeller et al., 2001). Neurons reported here showed full recovery following cessation of drug application.
Rat brains were removed following a saline and 4% paraformaldehyde perfusion, placed in 20% sucrose overnight, sectioned at 50 μm and stained with fast thionin for localization (Palombi and Caspary, 1996, Duque et al., 2013). Localizations of the recording sites were carefully mapped according to the rat brain atlas (Paxinos and Watson, 2007) at the end of the experiments.
4. Data analysis
Responses were analyzed offline, and MTFs were determined using both spike rate (rMTF) and temporal synchronization (tMTF) measurement at each fm tested. Phase locking ability was evaluated by standard vector strength (VS)(Goldberg and Brown, 1969, Yin et al., 2011), not including the first 25ms onset response.
n was the number of spikes over all trials, and θi was the phase of each spike in radians. VS may vary from 0 to 1 (perfect synchronization). Control runs were obtained before and after drug application. Z-score tests were used to calculate the point-to-point differences between pre- and post-drug application within each frequency and determined which part of the MTFs was most affected by drug application. A reversible response rate change from control of greater than 15 percent was considered a positive drug effect.
For Mixed type responders, collapsed group data was normalized to the minimum fm (normalized fm = 0) which divides the rMTF into lower (normalized fm = −1, −2, −3…) and higher fms (normalized fm = 1, 2, 3…) sections and plotted in fms steps above and below this minima with average response rate at each normalized fm. For high-pass (HP) responders, collapsed group data was plotted with average response rate as fm steps. Two-Factor repeated measures ANOVAs were used to test differences before and after drug delivery and post hoc t-tests with Bonferroni correction were used to test individual fm significance. Analyses and figures were executed using Sigmaplot 10 (Systat Software, Inc., Chicago, IL), SPSS and Excel, with p value less than 0.05 considered statistically significant.
Results
The role of GABAergic inhibition, acting through GABAARs onto 106 well isolated MGB units, was examined in efforts to characterize GABA’s role in shaping responses to SAM stimuli. Previous MGB single unit recording studies using temporally rich or SAM stimuli, classified rMTFs into four main response types: bandpass (BP), Mixed, highpass (HP), and atypical. BP units were those units with strong selectivity for a narrow subset of fms, therefore showing a single peaked rMTF. In contrast to BP type, units classified as “atypical” types responded equally to different fms with no fm selectivity seen in their rMTF. HP response types included units showing increasing rate responses as fm increased. The most prevalent response type in the present study was the Mixed type, which included units having two rate response peaks separated by a fixed minimum dividing the rMTF into lower and higher fms sections. Similar to the distribution of units responding to SAM stimuli described for the guinea pig MGB (Wallace et al., 2007) and marmoset (Bartlett, 2013), most BP, Mixed and HP units were localized in ventral division of the MGB (vMGB, 43/106, 41%) and near the boundary (34/106, 32%) between dorsal MGB (dMGB) and vMGB. Relatively fewer BP, Mixed and HP units (25/106, 24%) were found in dMGB (Table 1).
Table 1.
SAM Unit Response Type Based on rMTF and Location
| rMTF Category |
Location | Total | Percentage | |||
|---|---|---|---|---|---|---|
| dMGB | boundary | vMGB | unknown | |||
| BP | 7 | 10 | 13 | 1 | 31 | 29.25 |
| Mixed | 8 | 14 | 12 | 3 | 37 | 34.91 |
| HP | 6 | 6 | 14 | / | 26 | 24.53 |
| Atypical | 4 | 4 | 4 | / | 12 | 11.32 |
| Total | 25 | 34 | 43 | 4 | 106 | 100 |
Mixed units and GABA Neurotransmission
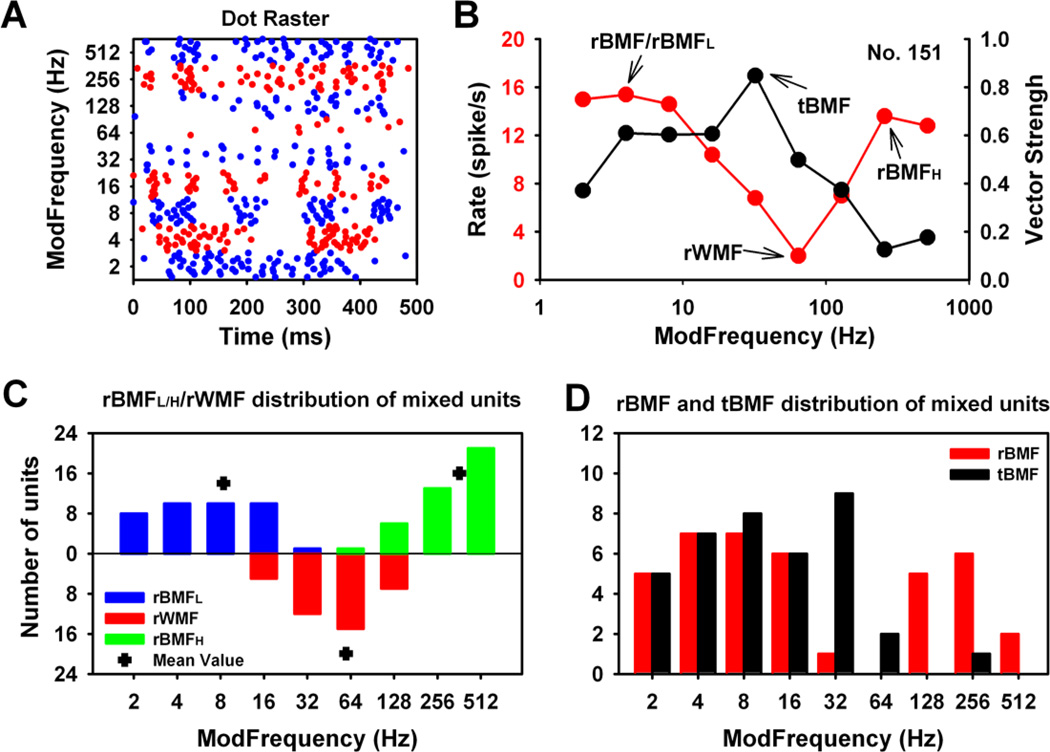
“Mixed” responders constituted the major (35%, 37/106) response type, a percentage similar to that seen in the thalamus of unanesthetized marmoset (Bartlett and Wang, 2007). Mixed responders showed rMTF peaks at both high and low fms. The shape of rMTFs of Mixed units resembled the shapes of letters “V”, “N” or “M”, with a clear transition minimum embedded between two peaks (rBMFL and rBMFH) (Fig. 1B, red trace). Mixed responders typically showed rMTFs formed at low fm by synchronous/time-locked responses and at high fm by asynchronous or onset responses with clear minima, showing few or no spikes between the two rMTF segments (Figs. 1A, 1B). The raster display for this representative Mixed SAM responder shows time-locked responses at low fms, the clear cessation of responses to SAM stimuli near 64 Hz, followed by a resumption of phasic, responses (Fig. 1A). This transfer point was defined as the rate worst modulation frequency (rWMF), embedded between two rBMF peaks (Fig. 1B). The group data for Mixed units showed minima (rWMFs) between the two arms of the rMTF, were ranged between 16 Hz to 128 Hz (Average rWMF = 59.50 Hz) (Fig. 1C). Mixed units mostly showed two rate best modulation frequencies (rBMFs; either rBMFL=Low side; or rBMFH=high side): rBMFL and rBMFH (Fig. 1B). Average rBMFL and rBMFH values were 8.40 Hz and 363.70 Hz, respectively (Fig. 1C). A single absolute rBMF value for Mixed units was chosen by comparing the maximum response rate at rBMFL and rBMFH, with group data showing that rBMFL was more likely to represent unit’s absolute rBMF (Fig. 1D). The rBMFs distribution of the Mixed units ranged between lower fms (2 to 16 Hz), with the exception of two with BMFs at higher fms, 128 Hz and 256 Hz (Fig. 1D). The tBMF for the Mixed unit was designated as the fms reflecting the highest level of synchrony (highest VS value) based on the tMTF (Fig. 1B, black trace). The distribution of tBMFs showed that most Mixed units followed modulations/synchronized up to 64 Hz (Fig. 1D).
Figure 1. Basic response properties of the Mixed units in the MGB.
(A). Dot raster of a representative Mixed MGB unit. Time-locked responses were seen for lower fms while non-synchronized responses were shown for higher fms. Typical for the Mixed MGB units, a clear transition minima was seen in the area around 64 Hz. (B) Modulation transfer function curves for this representative unit showed clear transitions between lower and higher fms in both rMTF and tMTF. (C) Group data showed that the distribution of the rBMFs and rWMFs followed the shape of the Mixed units’ rMTF. Lower rBMFs range between 2 Hz and 32 Hz (mean = 8.4 Hz), higher rBMFs range between 64 Hz and 512 Hz (mean = 363.7 Hz). Minimum transition frequencies were between 16 Hz and 128 Hz, with few or no responses at rWMFs. (D) The distribution of the absolute rBMFs (the highest rMTF peak). As expected, the distribution of tBMFs was focused on the lower fms.
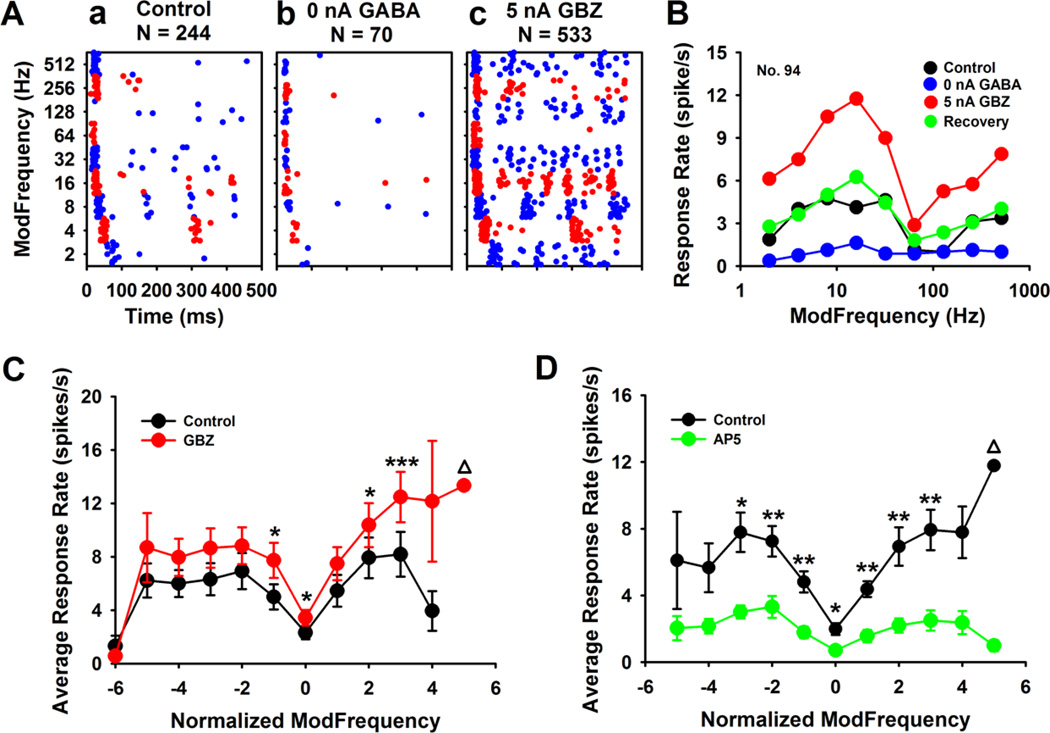
Eighty-four percent (31/37) of Mixed units showing SAM responses were suppressed by GABA application; with 59% (22/37) responding to GABAAR blockade with GBZ. For this Mixed response exemplar (Figs. 2A, 2B), GABAAR blockade, with GBZ, significantly increased responses near the rBMFL and at higher fms in the upper arm of the rMTF (Fig. 2Ac). Leaking GABA (holding current = 0 nA) onto this unit inhibited virtually all sustained responses elicited by SAM stimuli, leaving only onset responses across fms (Fig. 2Ab). Mixed units could be sub-classified based on their responses to GABA and GBZ application. Thirty-six percent (8/22) of Mixed units responded to GABAAR blockade with response rate selectively increased at higher fms or near rBMF (6/22, 27%).
Figure 2. GABA neurotransmission in the Mixed units in the MGB.
Most Mixed units were very sensitive to GABA application. (Ab&B) A representative Mixed unit showed dramatically decreased responses when tuning off the holding current (0 nA, leaking GABA) and a notable increase with 5 nA GBZ application (Ac&B) compared with the control recording (Aa). (B) The rMTF curves showed that responses to GABA/GBZ application were focused at or near rBMF. (C) When collapsed according to the minima, group data (n = 16) indicated a selective significant increase in response rate at rBMF and higher fms of the Mixed units. (D). Similar selectivity were seen in 10 Mixed units with AP5 application, which indicated the rBMF and higher fm tail could be shaped by GABAergic and glutamatergic neurotransmission. ANOVA indicated significant differences before and after GBZ (F1,15 = 40.93, p = 0.00001) or AP5 delivery (F1,11 = 23.05, p = 0.00055). N: number of spikes, Δ The edge points of the rMTF curves were not included into ANOVA calculation, due to the small number of data samples after lining up the minimum. Post-hoc t-test with bonferroni correction, * p < 0.05, ** p < 0.01.
Non-selective parallel shifts to GABAAR blockade were observed for 18% (4/22) of MGB units. GABA effects on Mixed units were also focused on higher fms (13/31, 42%) and fms near rBMF (6/31, 19%). GABAAR agonist/blockade generally resulted in only slight changes in the onset discharge rate with most notable increases in sustained responses (Figs. 2A).
When Mixed unit data was collapsed by lining up the minimum transfer points, rWMF (normalized fm = 0) of the rMTFs (Fig 2C), GABAAR blockade resulted in significant (F1,15 = 40.93, p = 0.00001) and selective changes in response rate at the highest fms (normalized fm 2 and 3) and near rBMFs (normalized fm 0 and −1). In their model of MGB SAM response types, Rabang et al., (2012) postulated that the emergence in MGB of Mixed and HP SAM response types was due to activation of NMDA receptors at higher fms. In support of their hypothesis, NMDA receptor blockade with AP5 showed similar rMTF changes to that seen with GABAergic inhibition (Fig. 2D, F1,11 = 23.05, p = 0.00055).
As will be delineated below, HP SAM responders also showed a similar selective increase to GABAAR blockade at the highest fms (Figs. 3, 4). When one considers the impact of GABAAR blockade on only the upper arm of the rMTF from Mixed responders, together with the impact of GABAAR blockade on high-pass (HP) responders, more than 60% of changes due to GABAAR blockade selectively occur at the higher fms in response to SAM stimuli. Selective increases in response rate at higher rMTFs with GABAAR blockade has not previously been described in similar studies at lower levels of the auditory neuroaxis (see Discussion).
Figure 3. GABA inhibition in the HP units in the MGB.
Highpass (HP) units showed increased responses to SAM stimuli at higher fm (Aa, Ca). When compared with the control condition, GABA or GBZ application showed selective effects at higher fms (Ab, Cb), with slight or no GABAAR actions at lower fms (B, D).
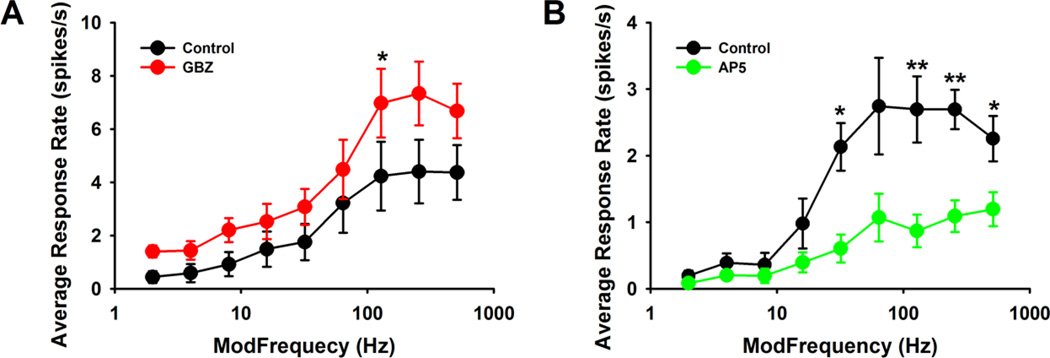
Figure 4. Effects by GABAergic and glutamatergic neurotransmission onto the HP units.
Collapsed group data were plotted with average response rate as the fm stepped (A). Group data (n = 10) showed GBZ manipulation onto HP units. Significant increases were seen across all the fms, with the more notable increases at the higher fms, 128 Hz, 256 Hz and 512 Hz. (B). Group data (n = 8) showed AP5 manipulation onto HP units. Significant decreases of response rate showed up at all the fms higher than 64 Hz, with no difference seen at lower fms. Two-Factor ANOVA indicated significant differences before and after GBZ (F1,9 = 20.02, p = 0.00155) or AP5 delivery (F1,7 = 62.40, p = 0.00010). Post-hoc t-test with bonferroni correction, * p < 0.05, ** p < 0.01.
Similar to our previous report (Cai et al., 2013), Mixed units were extremely sensitive to GABA manipulation (see exemplar in Fig. 2A). Changes in SAM responses of >15%, compared to control condition, occurred with application of low dose GABA and reduced Mixed unit spike rates by an average of 48.15 ± 3.71% (Mean ± SE) at a mean dose of 10.97 ± 2.02 nA (Mean ± SE). Spike rates were enhanced by an average of 63.78 ± 8.33% (Mean ± SE) with GBZ application.
HP units and GABA Neurotransmission
Twenty-five percent (26/106) of units categorized as HP showed increased responses to SAM stimuli as fm increased. Among the 26 HP units, 16 were suppressed by GABA application (Figs. 3A, 3B) with 10 showing increased rate responses at higher fm with GABAAR blockade (Figs. 3C, 3D). As noted above, the HP unit group data revealed that GABAAR blockade significantly increased SAM evoked response rates (F1,9 = 20.02, p = 0.00155), especially at 128 Hz (p = 0.01). Increasing tendencies were shown at 256 Hz (p = 0.06) and 512 Hz (p = 0.07) (Fig. 4A). As was the case with Mixed units, the impact of GABAAR blockade at the highest fms suggested the involvement of NMDA receptors in shaping HP responses at higher fms. NMDA receptor blockade with AP5 significantly depressed SAM evoked responses (F1,7 = 62.40, p = 0.00010), especially at higher fms in a manner similar to what was seen with GABA application (Fig. 4B. This dramatic inhibition of high side fms responses by GABA or AP5 strongly suggested that a delicate balance between excitation and inhibition in MGB exerts a significant control on responses to modulated stimuli at higher modulation frequencies.
BP units and GABA Neurotransmission
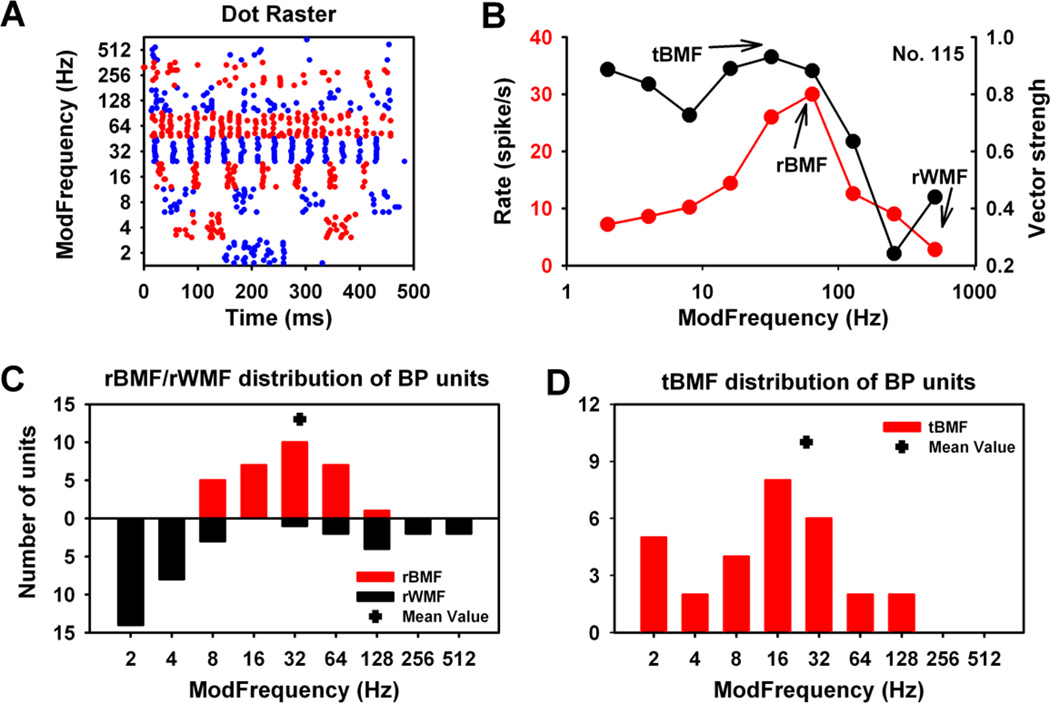
BP units showed high fm selectivity, with a single peak in rMTF defined as the rBMF (Fig. 5B, red trace). The rWMF within BP category was defined as the fm with the lowest response rate across the rMTF, which is either above or below the rBMF. Thirty-one BP units (31/106, 29%) were the second most prevalent MGB SAM response type (Table 1), and are the most prevalent response type at lower brainstem auditory structures. The dot raster display from an MGB BP unit showed SAM responses (Fig. 5A) with dramatically increasing discharge rate over a mid-range fms (16 Hz to 128 Hz), plotted for this exemplar in the companion rMTF (Fig. 5B). The ability of this unit to temporally follow/synchronize to SAM stimuli is shown in the tMTF (Fig 5B, black trace) with vector strength plotted against fm. MGB BP units typically showed clearly discernible tBMFs as well as rBMFs. The distribution of rBMFs for the 31 BP units ranged between 8 Hz and 128 Hz with a mean rBMF at 34.93 Hz (Fig. 5C). BP unit rWMFs were primarily at the lowest fms (Fig. 5C), which likely reflects the smaller number of modulation envelopes at lowest fms. The distribution of tBMFs ranged between 2 Hz to 128 Hz, with no BP unit showing tBMF higher than 128 Hz. The largest number of BP units had tBMFs at 16 Hz, with the mean tBMF for BP units at 26.00 Hz (Fig. 5D).
Figure 5. Basic response properties of the BP units in the MGB.
(A). Dot raster of a representative bandpass (BP) MGB unit. Time-locked responses were shown for lower and middle range fms with responses decreasing as fms increased. (B) rMTF showing a peak response at 64 Hz, marked as rBMF. rMTF and tMTF were consistent for fms higher than rBMF, but deviate significantly at lower fms, reflecting the high levels of timelocked/ synchronous responses below 64 Hz. (C) Group distribution of rBMFs and rWMFs for BP units. rBMF for BP units were at fms between 8 Hz to 128 Hz. More BP units showed rWMF at low fms (2 Hz to 8 Hz). (D) The distribution of tBMFs was between 2 Hz and 128 Hz with the average tBMF value of 26 Hz.
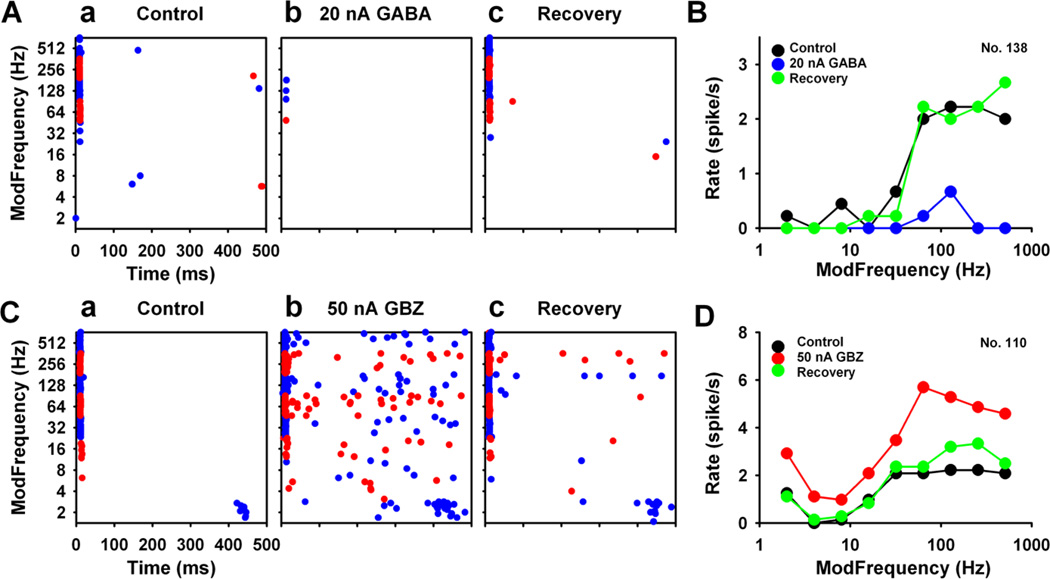
GABA and/or the nonselective GABAAR antagonist, GBZ, were applied onto 31 BP responders. GABA rapidly and profoundly suppressed SAM evoked response at or near rBMF (Figs. 6Ab, B). In contrast to GABA’s suppression of SAM evoked activity, GABAAR blockade increased responses to SAM stimuli at or near rBMF (Figs. 6Ac, B). MGB units were exquisitely sensitive to low-dose GABA application, resulting in an eighty-five percent dramatic reduction in sustain activity, and remaining only onset responses to the SAM stimuli (Fig. 6Ab). GABAAR blockade resulted in notable increases in SAM evoked responses (Fig. 6Ac), suggesting a delicate GABAergic modulation impacting the response properties of MGB neurons.
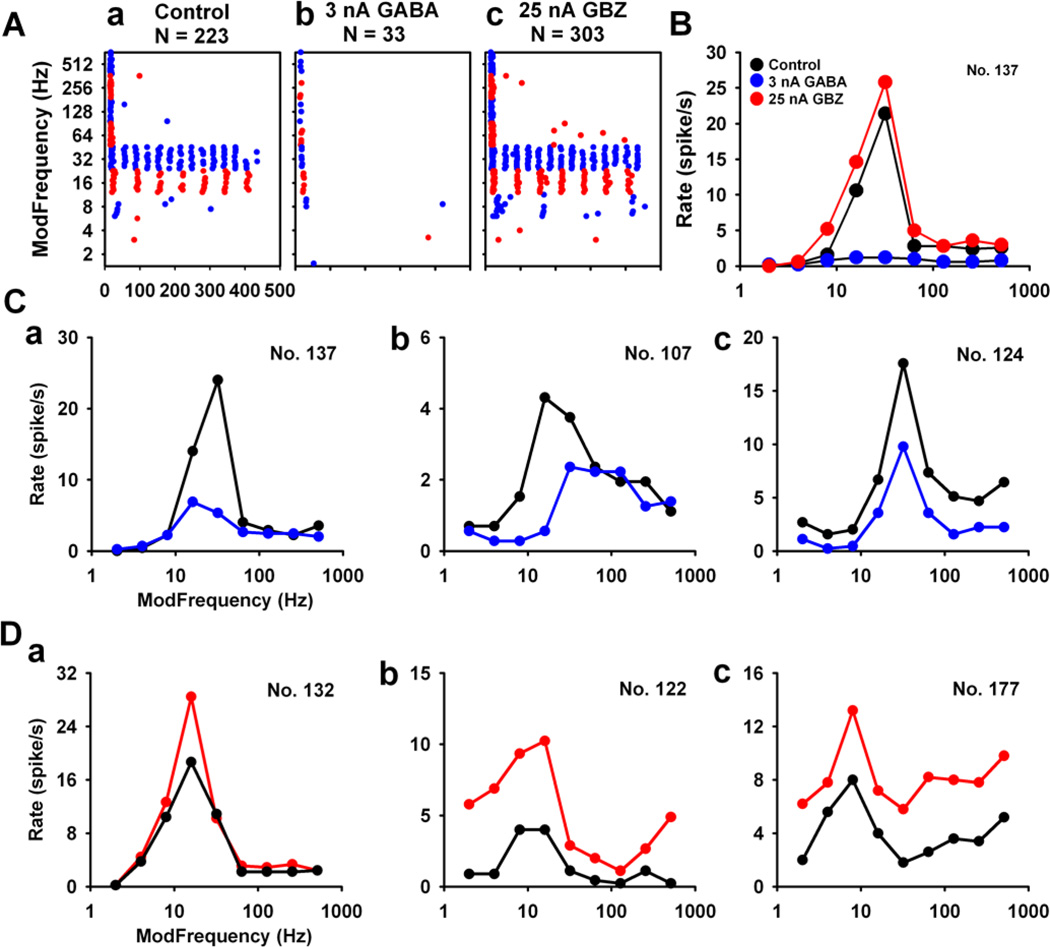
Figure 6. GABA neurotransmission in the BP units in the MGB.
(A) Dot raster of a representative BP unit showed dramatically reduced responses with application of 3 nA GABA (Ab). GABAAR blockade with GBZ application (25 nA) had a less notable effect (Ac) compared to the control response to SAM stimuli (Aa). (B) rMTF curves showed selective action to GABAAR manipulation were mainly focused at or near rBMFs (B, Ca, Da). A subset of BP neurons showed GABAAR actions at rBMF and at lower fms (Cb, Db). A few BP MGB neurons responded to GABAAR manipulation non-selectively reflected in a parallel shift from the control rMTF (Cc, Dc).
Of 31 BP units, 74% showed significant response changes (> 15%) to applied GABA (23/31 units) with almost half of BP units showing significant GBZ responses (14/31, 45%). Responsive units were sub-classified by MGB location and selectivity of GABAAR action. Over half (52%, 12/23) of the drug-affected BP units showed selective changes in response rate to GABAAR manipulation at or near rBMF. About 50% of the total spikes were inhibited in these units (Fig. 6Ca,). GABAAR blockade with GBZ resulted in a 46.76 ± 9.35% (Mean ± SE) selective increase in discharge rate to SAM stimuli. Fifty percent of MGB units showing GBZ mediated increased discharge rate showed maximal changes at or near-rBMF (Fig. 6Da). GABA application and GBZ blockade significantly altered rBMF amplitude for half of the BP response type, while not significantly altering the rMTF shape in response to SAM stimuli (Figs. 6Ca, Da).
Discussion
SAM stimuli have been used to examine the ability of the central auditory processor to selectively respond to rapidly changing temporal features, modeling the acoustic signal properties of speech and species specific vocalizations (Joris et al., 2004, Yin et al., 2011). The MGB receives both ascending and descending inhibitory GABAergic inputs from the IC and the TRN respectively, which activate both synaptic and extrasynaptic GABAARs (Richardson et al., 2011). Although auditory thalamus (MGB) has frequently been regarded as a simple “relay” between IC and AC, the present and recent studies indicated that important hierarchical acoustic processing, sensory, and emotional gating occurs in the auditory thalamus (Eggermont and Roberts, 2004, Weinberger, 2011).
Studies in unanesthetized marmoset show that MGB neurons display increasingly complex response properties to modulated sounds and click stimuli (Bartlett and Wang, 2007, 2011, Rabang and Bartlett, 2011). Wallace et al. (2000, 2002, 2007) examined responses to modulated pure-tone and click stimuli in these three auditory structures and found increased complexity in coding as one ascended the auditory neuraxis. As described in extensive reviews of temporal coding, the upper fm limit of phase-locking decreased, and there was an increased tendency of steady-state delay as one moved from IC through MGB to AC (Joris et al., 2004, Nelken, 2004). These studies found that MGB neurons were involved in the further computations/refinement of the neural representation of temporal modulations relative to what is observed in the IC, which continued as one moved to auditory cortex.
In agreement with the findings reviewed above, the present study describes a cohort of well-isolated single MGB units in anesthetized rat MGB showing similar complex responses to SAM stimuli, supporting qualitatively shared coding properties across species and anesthetic state. Similar to the marmoset, a majority of rat MGB units showed “Mixed” responses to SAM stimuli. SAM elicited responses from Mixed units showed highly synchronized responses at lower fms, and little or/no tonic synchronization at higher fms. The relatively stable minima transfer point (Fig. 1B) is a characteristic feature of Mixed units described here and by Bartlett and Wang (2007). This minimum and thus the overall shape of the Mixed rMTF curves were relatively stable even with GABAAR or NMDA receptor blockade. This suggests that this minimum was not shaped by GABAergic/glutamatergic circuits and likely represents intrinsic MGB neuronal properties or reflects ascending or descending inputs to MGB.
Most commonly, rMTFs generated in response to modulated stimuli in the IC show low- or band-pass responses, with the upper portion of the rMTF fixed near zero spikes for non-suppressive response types (Shaddock Palombi et al., 2001). Unlike neurons in lower brainstem nuclei (IC and CN), large numbers of MGB units respond to modulated stimuli with Mixed and HP shaped rMTFs. Besides the dominant Mixed units, the present study also found 25% HP units in the MGB, which did not respond to low fms, a response type rarely seen at lower levels of the central auditory system (Krishna and Semple, 2000, Caspary et al., 2002, Joris et al., 2004). In the IC, GABAAR blockade generally does not alter the rMTF at higher fms (Burger and Pollak, 1998, Caspary et al., 2002). In the present study, MGB HP unit responses were readily altered by GABAAR at the increased discharge rates seen with increasing fms. These findings strongly suggest a role for GABAergic neurotransmission in shaping modulated responses at higher fms for HP units and also for the upper limb of Mixed units. Careful comparison of rMTF shapes of the combined rMTFs of HP and Mixed units data and common effects of GABAAR and NMDA receptor manipulation on these two response types suggest that the Mixed-type unit is an emergent property in MGB, as reflecting a melding of BP and HP response types.
Possible role of GABAergic inhibition within MGB
In auditory thalamus, GABA can act through either synaptic or extrasynaptic GABAARs. A rate code transformation for modulated signals has been extensively described for the MGB (Bartlett and Wang, 2007, 2011, Rabang and Bartlett, 2011). We previously reported that the MGB units showed a dose-dependent, enhanced sensitivity to GABA application relative to units in the IC (Cai et al., 2013). A number of earlier studies described a role for GABA or glycine in controlling response magnitude at or near rBMF, while shaping selective properties of SAM and SFM responses in the IC and the DCN (Palombi and Caspary, 1996, Koch and Grothe, 1998, Shaddock Palombi et al., 2001, Caspary et al., 2002, 2005). GABA has multiple roles in the MGB. Similar to GABA’s action in IC, it can modulate discharge rate near BMF at low fms, but unique to MGB, GABA inhibition appears critically involved in shaping rate responses at higher fms, likely acting at extrasynaptic GABAARs mediating a tonic inhibitory current. In the present study, when NMDA receptors were blocked, rMTF changes were selectively decreased at higher fms and resembling what was seen with GABA application. Collectively, GABAAR and NMDA changes are consistent with the model presented by Rabang and Bartlett (Rabang and Bartlett, 2011). We therefore advanced the possibility that enhanced GABA sensitivity via tonic GABAAR and slow NMDAR signaling underpins a delicate control of the resting membrane potential, which selectively controls discharge rate and filtering at higher fms in MGB. Rabang and Bartlett (2011) suggested that at high fms of rMTFs reflect repetitive glutamatergic excitation resulting and delayed activation of NMDA receptors. The present findings support this hypothesis finding that NMDA receptor blockade AP5 selectively lowered MGB unit SAM responses at higher fms. GABAAR blockade can interact with NMDA receptors by tonically depolarizing MGB neurons (Richardson et al., 2011) releasing the Mg++-block enhancing NMDA activation at higher modulation rates.
These findings provide additional support for a complex and delicate balance between inhibition and excitation that exists for MGB neurons previously observed in whole animal and slice studies (Nelson and Erulkar, 1963, Aitkin and Dunlop, 1968, Hu et al., 1994, Hu, 1995, Peruzzi et al., 1997, Bartlett and Smith, 1999).
Conclusion
The present study described the properties of MGB units in response to SAM stimuli and the role of inhibition. These findings suggest that: 1. GABAAR blockade selectively enhance modulation discharge rate at higher fms for Mixed and HP response types. This action can be partially blocked by NMDA receptor blockade, which selectively reduces rate responses at higher fms; 2. Effects of GABAAR activation or blockade were centered on/around rBMF for BP response types and at the lower portion of the rMTF for Mixed units; 3. The most common, Mixed type response, may be a combination of BP and HP response types separated by a likely non-synaptically mediated minimal transfer point; 4. Collectively, GABAergic inhibition shapes fm selectivity and likely plays an important role, together with glutamatergic excitation, in selective control of temporal response properties at the level of the MGB.
Medial geniculate body shows emergent properties in its coding of modulated stimuli.
A delicate balance of tonic GABAAR and NMDA signaling regulates coding of modulated signals at higher modulation frequencies.
GABA’s role in shaping responses to higher modulation frequencies in MGB has not been seen in lower auditory structures.
Acknowledgments
The authors would like to thank Dr. Edward Bartlett for valuable comments on an earlier version of this manuscript and Dr. Brozoski for help with the statistical analysis. The study was supported by NIH grant DC000151 to Donald M. Caspary.
Abbreviations
- ABR
auditory brainstem response
- AC
primary auditory cortex
- AMPA
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- ANOVA
analysis of variance
- AP5
(2R)-amino-5-phosphonovaleric acid
- BBN
broadband noise
- BMF
best modulation frequency
- BP
bandpass
- CF
characteristic frequency
- d/vMGB
dorsal/ventral medial geniculate body
- FBN
Fisher Brown Norway
- fc
carrier frequency
- fm
modulation frequency
- GABA
γ-Aminobutyric acid
- GABAAR
GABAA receptor
- GBZ
gabazine
- HP
highpass
- IC
inferior colliculus
- KAc
potassium acetate
- MAP
multichannel acquisition processor
- MGB
medial geniculate body
- MTF
modulation transfer function
- NMDA
N-Methyl-D-aspartic acid
- r/tBMF
rate/temporal best modulation frequency
- r/tMTF
rate/temporal modulation transfer function
- rWMF
rate worst modulation frequency
- TRN
thalamic reticular nucleus
- SAM
sinusoidal amplitude modulation
- SNR
signal-to-noise ratio
- VS
vector strength
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Aitkin LM, Dunlop CW. Interplay of excitation and inhibition in the cat medial geniculate body. Journal of neurophysiology. 1968;31:44–61. doi: 10.1152/jn.1968.31.1.44. [DOI] [PubMed] [Google Scholar]
- Bartlett EL. The organization and physiology of the auditory thalamus and its role in processing acoustic features important for speech perception. Brain and language. 2013;126:29–48. doi: 10.1016/j.bandl.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett EL, Smith PH. Anatomic, intrinsic, and synaptic properties of dorsal and ventral division neurons in rat medial geniculate body. Journal of neurophysiology. 1999;81:1999–2016. doi: 10.1152/jn.1999.81.5.1999. [DOI] [PubMed] [Google Scholar]
- Bartlett EL, Stark JM, Guillery RW, Smith PH. Comparison of the fine structure of cortical and collicular terminals in the rat medial geniculate body. Neuroscience. 2000;100:811–828. doi: 10.1016/s0306-4522(00)00340-7. [DOI] [PubMed] [Google Scholar]
- Bartlett EL, Wang X. Neural representations of temporally modulated signals in the auditory thalamus of awake primates. Journal of neurophysiology. 2007;97:1005–1017. doi: 10.1152/jn.00593.2006. [DOI] [PubMed] [Google Scholar]
- Bartlett EL, Wang X. Correlation of neural response properties with auditory thalamus subdivisions in the awake marmoset. Journal of neurophysiology. 2011;105:2647–2667. doi: 10.1152/jn.00238.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger RM, Pollak GD. Analysis of the role of inhibition in shaping responses to sinusoidally amplitude-modulated signals in the inferior colliculus. Journal of neurophysiology. 1998;80:1686–1701. doi: 10.1152/jn.1998.80.4.1686. [DOI] [PubMed] [Google Scholar]
- Cai R, Kalappa BI, Brozoski TJ, Ling LL, Caspary DM. Is GABA neurotransmission enhanced in auditory thalamus relative to inferior colliculus? Journal of neurophysiology. 2013;111:229–238. doi: 10.1152/jn.00556.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Palombi PS, Hughes LF. GABAergic inputs shape responses to amplitude modulated stimuli in the inferior colliculus. Hearing research. 2002;168:163–173. doi: 10.1016/s0378-5955(02)00363-5. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Schatteman TA, Hughes LF. Age-related changes in the inhibitory response properties of dorsal cochlear nucleus output neurons: role of inhibitory inputs. The Journal of neuroscience. 2005;25:10952–10959. doi: 10.1523/JNEUROSCI.2451-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque D, Malmierca MS, Caspary DM. Modulation of stimulus-specific adaptation by GABAA receptor activation or blockade in the medial geniculate body of the anaesthetized rat. The Journal of physiology. 2013;592:729–743. doi: 10.1113/jphysiol.2013.261941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends in neurosciences. 2004;27:676–682. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Foeller E, Vater M, Kossl M. Laminar analysis of inhibition in the gerbil primary auditory cortex. Journal of the Association for Research in Otolaryngology : JARO. 2001;2:279–296. doi: 10.1007/s101620010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM, Brown PB. Response of binaural neurons of dog superior olivary complex to dichotic tonal stimuli: some physiological mechanisms of sould localization. Journal of neurophysiology. 1969;32:613–636. doi: 10.1152/jn.1969.32.4.613. [DOI] [PubMed] [Google Scholar]
- Hara K, Harris RA. The anesthetic mechanism of urethane: the effects on neurotransmitter-gated ion channels. Anesthesia and analgesia. 2002;94:313–318. doi: 10.1097/00000539-200202000-00015. [DOI] [PubMed] [Google Scholar]
- Hu B. Cellular basis of temporal synaptic signalling: an in vitro electrophysiological study in rat auditory thalamus. The Journal of physiology. 1995;483(Pt 1):167–182. doi: 10.1113/jphysiol.1995.sp020576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Senatorov V, Mooney D. Lemniscal and non-lemniscal synaptic transmission in rat auditory thalamus. The Journal of physiology. 1994;479(Pt 2):217–231. doi: 10.1113/jphysiol.1994.sp020290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris PX, Schreiner CE, Rees A. Neural processing of amplitude-modulated sounds. Physiological reviews. 2004;84:541–577. doi: 10.1152/physrev.00029.2003. [DOI] [PubMed] [Google Scholar]
- Kelly JB, Zhang H. Contribution of AMPA and NMDA receptors to excitatory responses in the inferior colliculus. Hearing research. 2002;168:35–42. doi: 10.1016/s0378-5955(02)00372-6. [DOI] [PubMed] [Google Scholar]
- Koch U, Grothe B. GABAergic and glycinergic inhibition sharpens tuning for frequency modulations in the inferior colliculus of the big brown bat. Journal of neurophysiology. 1998;80:71–82. doi: 10.1152/jn.1998.80.1.71. [DOI] [PubMed] [Google Scholar]
- Krishna BS, Semple MN. Auditory temporal processing: responses to sinusoidally amplitude-modulated tones in the inferior colliculus. Journal of neurophysiology. 2000;84:255–273. doi: 10.1152/jn.2000.84.1.255. [DOI] [PubMed] [Google Scholar]
- Le Beau FE, Rees A, Malmierca MS. Contribution of GABA- and glycine-mediated inhibition to the monaural temporal response properties of neurons in the inferior colliculus. Journal of neurophysiology. 1996;75:902–919. doi: 10.1152/jn.1996.75.2.902. [DOI] [PubMed] [Google Scholar]
- Lu T, Wang X. Information content of auditory cortical responses to time-varying acoustic stimuli. Journal of neurophysiology. 2004;91:301–313. doi: 10.1152/jn.00022.2003. [DOI] [PubMed] [Google Scholar]
- Malmierca MS. The structure and physiology of the rat auditory system: an overview. International review of neurobiology. 2003;56:147–211. doi: 10.1016/s0074-7742(03)56005-6. [DOI] [PubMed] [Google Scholar]
- Nelken I. Processing of complex stimuli and natural scenes in the auditory cortex. Current opinion in neurobiology. 2004;14:474–480. doi: 10.1016/j.conb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Nelson PG, Erulkar SD. Synaptic mechanisms of excitation and inhibition in the central auditory pathway. Journal of neurophysiology. 1963;26:908–923. doi: 10.1152/jn.1963.26.6.908. [DOI] [PubMed] [Google Scholar]
- Palombi PS, Caspary DM. GABA inputs control discharge rate primarily within frequency receptive fields of inferior colliculus neurons. Journal of neurophysiology. 1996;75:2211–2219. doi: 10.1152/jn.1996.75.6.2211. [DOI] [PubMed] [Google Scholar]
- Paxinos W, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 2007. [Google Scholar]
- Peruzzi D, Bartlett E, Smith PH, Oliver DL. A monosynaptic GABAergic input from the inferior colliculus to the medial geniculate body in rat. The Journal of neuroscience. 1997;17:3766–3777. doi: 10.1523/JNEUROSCI.17-10-03766.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabang CF, Bartlett EL. A computational model of cellular mechanisms of temporal coding in the medial geniculate body (MGB) PLoS One. 2011;6:e29375. doi: 10.1371/journal.pone.0029375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabang CF, Parthasarathy A, Venkataraman Y, Fisher ZL, Gardner SM, Bartlett EL. A computational model of inferior colliculus responses to amplitude modulated sounds in young and aged rats. Frontiers in neural circuits. 2012;6:77. doi: 10.3389/fncir.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson BD, Ling LL, Uteshev VV, Caspary DM. Extrasynaptic GABA(A) receptors and tonic inhibition in rat auditory thalamus. PLoS One. 2011;6:e16508. doi: 10.1371/journal.pone.0016508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouiller EM, de Ribaupierre Y, de Ribaupierre F. Phase-locked responses to low frequency tones in the medial geniculate body. Hearing research. 1979;1:213–226. doi: 10.1016/0378-5955(80)90008-8. [DOI] [PubMed] [Google Scholar]
- Shaddock Palombi P, Backoff PM, Caspary DM. Responses of young and aged rat inferior colliculus neurons to sinusoidally amplitude modulated stimuli. Hearing research. 2001;153:174–180. doi: 10.1016/s0378-5955(00)00264-1. [DOI] [PubMed] [Google Scholar]
- Wallace MN, Anderson LA, Palmer AR. Phase-locked responses to pure tones in the auditory thalamus. Journal of neurophysiology. 2007;98:1941–1952. doi: 10.1152/jn.00697.2007. [DOI] [PubMed] [Google Scholar]
- Wallace MN, Rutkowski RG, Shackleton TM, Palmer AR. Phase-locked responses to pure tones in guinea pig auditory cortex. Neuroreport. 2000;11:3989–3993. doi: 10.1097/00001756-200012180-00017. [DOI] [PubMed] [Google Scholar]
- Wallace MN, Shackleton TM, Palmer AR. Phase-locked responses to pure tones in the primary auditory cortex. Hearing research. 2002;172:160–171. doi: 10.1016/s0378-5955(02)00580-4. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. The medial geniculate, not the amygdala, as the root of auditory fear conditioning. Hearing research. 2011;274:61–74. doi: 10.1016/j.heares.2010.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA, Saint Marie RL, Larue DT, Oliver DL. GABAergic feedforward projections from the inferior colliculus to the medial geniculate body. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:8005–8010. doi: 10.1073/pnas.93.15.8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Pollak GD, Resler C. GABAergic circuits sharpen tuning curves and modify response properties in the mustache bat inferior colliculus. Journal of neurophysiology. 1992;68:1760–1774. doi: 10.1152/jn.1992.68.5.1760. [DOI] [PubMed] [Google Scholar]
- Yin P, Johnson JS, O'Connor KN, Sutter ML. Coding of amplitude modulation in primary auditory cortex. Journal of neurophysiology. 2011;105:582–600. doi: 10.1152/jn.00621.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]