Abstract
Heavy drinking smokers constitute a distinct sub-population of smokers for whom traditional smoking cessation therapies may not be effective. Recent evidence suggested that combined varenicline (VAR) and naltrexone (NTX) therapy may be more efficacious than either monotherapy alone in reducing smoking and drinking-related behavior in this population. The manner in which individuals smoke a cigarette (i.e., smoking topography) may be predictive of smoking cessation outcomes, yet the effects of smoking pharmacotherapies on puffing behavior have not been thoroughly examined. Therefore, the current double-blind medication study examined the effects of VAR alone (1mg BID), low dose NTX alone (25mg QD), the combination of VAR+NTX, and placebo on smoking topography measures in heavy drinking, non-treatment seeking daily smokers (n=120). After a 9-day titration period, participants completed a laboratory session in which they smoked their first cigarette of the day using a smoking topography device following 12-hrs of nicotine abstinence and consumption of an alcoholic beverage (BrAC = 0.06 g/dl). The primary measures were puff count, volume, duration, and velocity and inter-puff interval (IPI). Independent of medication group, puff velocity and IPI increased, while puff volume and duration decreased, over the course of the cigarette. The active medication groups, vs. the placebo group, had significantly blunted puff duration and velocity slopes over the course of the cigarette, and this effect was particularly evident in the VAR+NTX group. Additionally, the VAR+NTX group demonstrated lower average IPI than the monotherapy groups and lower average puff volume than all other groups. These results suggest that smoking pharmacotherapies, particularly the combination of VAR+NTX, alter smoking topography in heavy drinking smokers, producing a pattern of less intense puffing behavior. As smoking topography has been predictive of the ability to quit smoking, future studies should examine how smoking pharmacotherapies’ effects on puffing behavior relate to smoking cessation outcomes.
Keywords: Naltrexone, varenicline, heavy drinking smokers, smoking topography
1. Introduction
Heavy drinking smokers represent a prominent and distinct subgroup of substance users who often present unique treatment challenges (Dani and Harris, 2005, Littleton et al., 2007). Levels of alcohol use are higher in smokers than non-smokers and the prevalence of smoking is higher in heavy drinkers compared with non-drinkers (Dawson, 2000). Because of this, heavy drinking smokers experience more health consequences, including impaired brain morphology and function (Durazzo et al., 2007) and greater risk for various cancers (Ebbert et al., 2005), than those who only drink or smoke. The co-use of these substances also has clinical importance, as greater alcohol use is associated with decreased odds of quitting smoking and smokers are four times more likely to have a smoking lapse during drinking episodes (Hymowitz et al., 1997, Kahler et al., 2010, Kahler et al., 2008). Thus, while there are currently no pharmacological treatments tailored to heavy drinking smokers, recent work in this population has focused on developing medications that can reduce both alcohol and cigarette consumption (Fridberg et al., 2014, Ray et al., 2014a).
There is evidence that varenicline (VAR) and naltrexone (NTX), both alone and in combination, may reduce smoking behavior and alcohol consumption and, therefore, hold promise as a treatment for heavy drinking smokers. Varenicline is a front-line treatment for smoking cessation and in heavy drinking smokers has been shown to reduce the number of cigarettes smoked and alcoholic beverages consumed per day, while also attenuating alcohol craving (Fucito et al., 2011, McKee et al., 2009, Mitchell et al., 2012). Naltrexone (50 mg) is FDA-approved for the treatment of alcohol dependence, but has also shown some promise as an adjunct treatment for smoking cessation (King et al., 2006, King et al., 2012). Of note, NTX may be primarily effective among heavy drinking smokers by preferentially reducing alcohol consumption and smoking urge while also improving smoking quit rates in comparison with non-heavy drinking smokers (Fridberg et al., 2014, King et al., 2009a, O’Malley et al., 2009). Finally, recent evidence from our group suggests that the combination of VAR and low dose NTX (25 mg) may be more effective in reducing cigarette craving, smoking behavior, and alcohol consumption than either medication alone (Ray et al., 2014a). Although the early evidence on combined VAR+NTX therapy as a targeted treatment for heavy drinking smokers is promising, additional studies are needed to replicate and extend these preliminary results by identifying biobehavioral mechanisms by which combined therapy may provide advantages over traditional monotherapies.
The manner in which an individual smokes a single cigarette, i.e., smoking topography, is an objective and reliable index of smoking intensity and reinforcement (Perkins et al., 2012). Importantly, preliminary evidence suggests that smoking topography measures may be more predictive of smoking cessation outcomes than other traditional measures of individual differences in smoking behavior, including severity of nicotine dependence and cigarettes per day (Strasser et al., 2004; Franken et al., 2006). For example, in a clinical trial comparing nicotine replacement therapies (NRTs) in heavy adult smokers, several pre-treatment smoking topography measures, including lower puff volume (capacity of each puff), lower puff velocity (flow rate of each puff), and higher interpuff interval (IPI; time between each puff), were predictive of greater abstinence rates independent of treatment group (Strasser et al., 2004). Similarly, in a NRT trial in adolescent smokers, lower puff volume at baseline was associated with better treatment outcomes (Franken et al., 2006). Finally, greater puff volume and longer puff duration at pretreatment baselines were related to poorer cessation outcomes in female smokers treated with NRT, but not those receiving VAR (McClure et al., 2013). Therefore, it appears that individuals with a less “intense” pattern of smoking/puffing behavior during a single cigarette, as indexed by a lower average puff volume, velocity, and duration and higher IPI, may have greater odds of maintaining abstinence during a quit attempt (McClure et al., 2013).
Despite the potentially meaningful association between smoking topography and smoking cessation outcomes, few studies have examined the effects of pharmacotherapies on smoking topography. In non-treatment seeking daily smokers, NTX, but not buproprion, significantly reduced puff count compared with placebo (Rukstalis et al., 2005). Conversely, two other studies of non-treatment seeking smokers reported that neither VAR nor bupropion treatment directly affected any individual smoking topography measure (McKee et al., 2012; Ashare et al., 2012); although, VAR was found to reduce a measure of daily smoking behavior that was comprised from an individual’s cigarettes per day and total puff volume (Ashare et al., 2012). While smoking topography is a reliable index of an individual’s smoking intensity and may be related to cessation outcomes, additional research is needed to determine whether topography measures are sensitive to the effects of smoking pharmacotherapies.
In sum, smoking topography measures, particularly puff volume and duration, may be predictive of smoking cessation outcomes. However, the effects of smoking pharmacotherapies on smoking topography remain unclear, particularly among hard-to-treat subgroups such as heavy drinking smokers. While there is early, but mixed, evidence suggesting that particular measures of smoking topography may be sensitive to VAR and NTX monotherapy (Rukstalis et al., 2005; McKee et al., 2012; Ashare et al., 2012), no studies have examined the combined effects of these medications on puffing characteristics. Therefore, the goal of this study was to examine whether VAR (1 mg/twice daily), low dose NTX (25 mg), and their combination affect smoking topography (vs. placebo) in heavy drinking smokers. Based on a prior study in this sample that found VAR + NTX combined therapy was more effective than VAR or NTX monotherapy and placebo in reducing cigarette craving, as well as daily smoking and drinking behavior (Ray et al., 2014a), we hypothesized that VAR and NTX treatment, both alone and in combination, will produce a less intense pattern of puffing behavior over the course of a single cigarette compared with placebo (i.e., a lower puff volume, velocity, and duration and higher IPI) and also that the combination of VAR and NTX will be more effective than either monotherapy alone in producing these changes.
2. Methods
2.1. Participants & Screening Procedures
The study was approved by the Institutional Review Board of the University of California, Los Angeles and was in accordance with the Declaration of Helsinki. Detailed methodology of the general experimental and screening procedures has been previously published elsewhere (Ray et al., 2014a, Ray et al., 2014b). A community-based sample of non-treatment seeking, daily smokers was recruited via online and print advertisements in the Los Angeles area. Participants were reminded at multiple points throughout the recruitment and screening processes that this was not a treatment study. Interested individuals called the laboratory and completed a telephone-screening interview to determine initial eligibility. Potential participants were eligible if they: (1) were between 21 and 55 years of age; (2) reported smoking 10 or more cigarettes per day and did not report more than 3 months of smoking abstinence in the past year; (3) fit the criteria for heavy drinking according to the National Institute on Alcohol Abuse and Alcoholism (NIAAA) guidelines (Health and Services, 1995): for men, >14 drinks per week or ≥5 drinks per occasion at least once per month over the past 12 months; for women, >7 drinks per week or ≥4 drinks per occasion at least once per month; (4) were of good general health; (5) were not currently pregnant or planning to become pregnant during the course of the study; (6); did not report use of cocaine, methamphetamine, heroin or other illicit drugs (other than marijuana) in the previous 60 days; and (7) reported no history of psychotic disorders, bipolar disorders, or major depression with suicidal ideation in their lifetime.
Individuals who met the initial eligibility requirements were invited to the laboratory for in-person screening, in which they provided informed consent. The in-person screening also consisted of a general physical examination by the study physician and the completion of several questionnaires, which included the Beck Depression Inventory (BDI-II; Beck, 1996), demographic and lifetime substance use history questionnaires, the Fagerstrom Test of Nicotine Dependence (FTND; Heatherton et al., 1991), the Wisconsin Smoking Withdrawal Scale (Welsch et al., 1999), and the Time Line Follow Back to assess cigarette and alcohol use over the past 30 days (Sobell et al., 1986). Participants were asked to abstain from drinking alcohol for 24 h prior to the in-person screening visit, which was confirmed by breathalyzer. Urine drug screens and pregnancy tests were also performed. Individuals who passed the physical exam, had a BDI score < 20 (no current symptoms of moderate depression of higher), had a breath alcohol concentration (BrAC) of 0.000 g/dl, and tested negative for drug use and pregnancy were randomized to a medication condition. Finally, expired carbon monoxide (CO) levels were collected at the screen in order to later verify overnight abstinence prior to the experimental session, as described below.
A total of 427 individuals (79% male) were screened in person, and 130 individuals (67% male) were randomized in a double-blind fashion to one of the following medication conditions: (a) VAR alone (n=34), (b) NTX alone (n=35), VAR + NTX (n=31), and placebo (n=30). A total of 120 individuals completed the study (n = 30 in each group), however 11 individuals had smoking topography data that could not be analyzed due to instrumentation error, leaving the final group sizes as follows: VAR = 29, NTX = 28, VAR + NTX = 25, and placebo = 27.
2.2. Experimental Procedures & Smoking Topography Measures
Participants took the study medication on a daily basis for 9 days and subsequently completed an experimental session on day 9. The participants were titrated on VAR as follows: days 1–2, 0.5mg per day, days 3–5, 0.5mg twice per day, and days 6–9, 1mg twice per day. Naltrexone was administered at 25mg per day for a period of 9 days. Placebo pills were matched to the active medications in number of pills and packaging. Study medications were packed into opaque capsules with 50mg of riboflavin. Medication compliance was monitored by testing a urine sample for riboflavin content at each testing session by examining it under an ultraviolet light (Del Boca et al., 1996).
Participants were asked to abstain from cigarettes and alcohol for 12- and 24-hours prior to the experimental session, respectively. Upon arrival to the laboratory, participants were required to provide expired CO levels of less than 10ppm (or below 50% of initial screening value) and a BrAC of 0.000 g/dl in order to proceed with the session. After completing baseline measures, participants received a loading dose of alcohol designed to reach a target BrAC of 0.060g/dl, calculated using published guidelines (Brick, 2006), in order to examine the effects of the medication conditions on acute response to alcohol (reported elsewhere: Ray et al., 2014a). The alcohol administration was not blinded, such that both participants and experimenters were aware that alcohol was being consumed. Upon reaching the target dose (30 min post-alcohol administration), participants smoked their first cigarette of the day in the laboratory using a CReSS Pocket smoking topography device (Borgwaldt). Participants smoked their own cigarette and no smoking instructions were provided. The primary topography measures were puff count (number of puffs), puff volume (mean capacity of each puff in ml), puff duration (mean length of each puff in seconds), puff velocity (mean flow rate of each puff in ml/s), and inter-puff interval (IPI; mean time between each puff in seconds).
2.3. Statistical Analyses
Medication group differences on demographics and cigarette puff count were analyzed using a series of ANOVA’s with Tukey’s HSD tests implemented as pairwise post hoc tests of medication groups. Smoking topography variables with data at the level of a single puff (i.e. puff duration, velocity, volume, and IPI) were analyzed using a series of multilevel models in SAS version 9.4 using proc mixed. Puff duration and IPI data were positively skewed and, therefore, log transformed data for these variables were used in the multilevel models. For each multilevel model, the proportion of the cigarette smoked (Cig%, computed as current puff number / total puff count) was a level 1 predictor, which was treated as random at the subject level (level 2). Medication variables were treated as level 2 predictors, as both main effects (i.e., the average of the topography measure by medication group) and moderators of Cig% (i.e. moderators of slope over the course of the cigarette). An unstructured covariance matrix was specified as well as Satterthwaite approximated degrees of freedom. Medication group was coded using an orthogonal contrasting scheme to avoid collinearity of tests and to provide tests of a priori differences of interest. With the four medication groups (Placebo, NTX alone, VAR alone, and VAR+NTX), we defined 3 medication contrasts. The first contrast tested whether active medications in aggregate differed from placebo (Active Contrast: All active medication groups vs. Placebo). The second contrast compared the two monotherapy groups to each other (Monotherapy Contrast: NTX alone vs. VAR alone). The third and final contrast tested whether the combined medication group was superior to the monotherapy groups (Combined Contrast: VAR+NTX vs. NTX alone and VAR alone). As the contrasts being examined were orthogonal and a priori defined, the α for these contrast effects was set to 0.05. Lastly, if a significant medication contrast was observed, post hoc tests were conducted to compare each active medication group individually to placebo using a dummy-coding scheme.
3. Results
3.1. Sample Characteristics
Full sample characteristics are presented in Table 1. Medication groups did not differ on most demographic characteristics, with the exception of the placebo group being significantly older than both the NTX and VAR+NTX groups (F (3, 104) = 4.57, p < 0.01; Tukey’s HSD p’s ≤ 0.01). Medication groups did not differ in terms of smoking or drinking variables, including FTND score, cigarettes per day, drinking days in the past month, drinks per drinking day (p’s ≥ 0.47).
Table 1.
Sample characteristics
| VAR (N = 29) | NTX (N = 28) | VAR+NTX (N = 25) | Placebo (N = 27) | |
|---|---|---|---|---|
| Age | 34.24 (10.92) | 30.50 (8.60) | 30.40 (8.71) | 38.88 (9.81)* |
| Education (years) | 13.86 (3.31) | 13.21 (3.98) | 13.76 (3.26) | 13.96 (4.00) |
| Sex (% Male) | 65.52% | 75.00% | 52% | 66.67% |
| Ethnicity (% Caucasian) | 33.33% | 39.29% | 29.17% | 53.85% |
| FTND Score | 3.55 (1.86) | 3.50 (1.95) | 3.76 (1.59) | 4.04 (1.99) |
| Cigarettes per Day | 14.08 (4.76) | 14.01 (5.25) | 14.46 (7.31) | 14.13 (5.17) |
| Drinking Days per Month | 22.03 (8.15) | 21.71 (7.66) | 18.84 (7.98) | 20.56 (8.25) |
| Drinks Per Drinking Day | 6.49 (4.48) | 6.43 (3.29) | 7.20 (3.52) | 6.08 (3.32) |
| Screen CO level (ppm) | 0.015 (0.012) | 0.014 (0.010) | 0.013 (0.007) | 0.016 (0.012) |
| Session CO level (ppm) | 0.006 (0.004) | 0.007 (0.007) | 0.005 (0.003) | 0.008 (0.008) |
Data are presented as mean (standard deviation) or percentage. The CO level assessed at the screening session (Screen CO) did not have an abstinence requirement, while the CO level assessed at the experimental session (Session CO) was after a 12 hour abstinence requirement.
The placebo group was significantly older than both the NTX and NTX + VAR groups (F (3, 104) = 4.57, p < 0.01; Tukey’s HSD p’s ≤ 0.01)
3.2. Smoking Topography Results
Group means for each smoking topography measure are reported in Table 2.
Table 2.
Smoking topography averages for each medication group
| VAR (n = 29) | NTX (n = 28) | VAR+NTX (n = 25) | Placebo (n = 27) | |
|---|---|---|---|---|
| Puff Count | 15.69 (6.15) | 15.55 (6.15) | 17.84 (6.62) | 15.39 (6.49) |
| Puff Duration (ms) | 1712.99 (614.75) | 1616.31 (625.64) | 1546.17 (662.11) | 1573.06 (637.12) |
| Puff Velocity (ml/s) | 36.66 (10.20) | 38.38 (10.38) | 33.78 (10.98) | 40.29 (10.57) |
| Puff Volume (ml) | 58.93 (20.46) | 57.74 (20.82) | 50.19 (22.04) | 60.85 (21.21) |
| IPI (ms) | 19860.47 (9895.95) | 23824.24 (10071.11) | 16156.87 (10071.11) | 19936.82 (9729.62) |
Data are presented as mean (standard deviation).
3.2.1. Puff Count
On average, participants took 16.00 (SD = 5.91) puffs through the smoking topography device. Medication groups did not differ in puff count (p’s ≥0.11).
3.2.2. Puff Duration
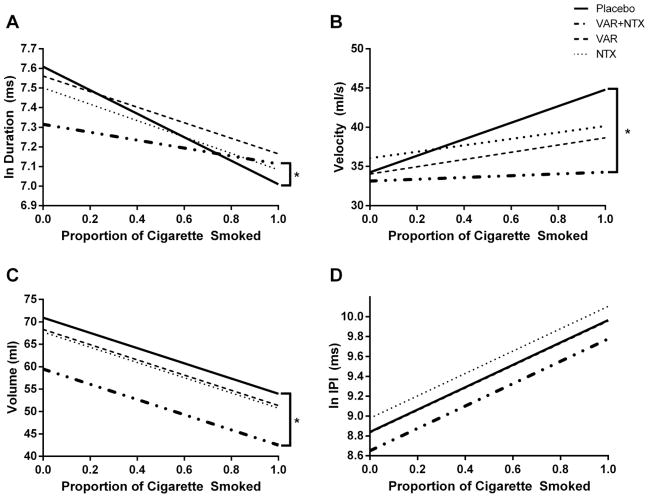
The medication group contrast results for the smoking topography variables that were analyzed at the level of a single puff (i.e. puff duration, velocity, volume, and IPI) are provided in Table 3. Independent of medication group, puff duration decreased over the course of the cigarette (Cig%: β = −0.41, t = −7.67, p < 0.001). The active medication groups (i.e., the aggregate of VAR+NTX combined, VAR alone, and NTX alone) had a significantly blunted puff duration slope compared with the placebo group (Figure 1A; Cig% × Active Contrast: β = 0.20, t = 2.16, p < 0.05), but this rate of change did not differ between the active medication groups (p’s > 0.11). Post hoc analyses indicated that the combined VAR+NTX group, but not the monotherapy groups, displayed a significantly flatter puff duration slope than the placebo group (β = 0.38, t = 2.61, p = 0.01).
Table 3.
Medication group contrast results
| Active Contrast | Monotherapy Contrast | Combined Contrast | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Main Effect | × Cig% | Main Effect | × Cig% | Main Effect | × Cig% | |
| Puff Duration | −0.023 | .196* | 0.035 | 0.011 | −0.082 | 0.137 |
| Puff Velocity | −1.89 | −5.44* | −0.91 | 0.26 | −2.05 | −2.15 |
| Puff Volume | −4.32 | 4.88 | 0.29 | 3.64 | −5.70† | 1.36 |
| IPI | −0.015 | −0.178 | −0.073 | −0.075 | −0.170* | −0.154 |
Results are presented as beta coefficients (β). Active Contrast tested whether active medications in aggregate differed from placebo (i.e., all active medication groups vs. the placebo group). Monotherapy Contrast compared the two monotherapy groups to each other (i.e., NTX alone vs. VAR alone). Combined Contrast tested whether the combined medication group was different than the monotherapy groups (i.e., VAR+NTX vs. NTX alone and VAR alone). Cig% refers to the slope of the respective topography measure over the course of the cigarette, while x Cig% refers to the statistical interaction of the medication contrast and Cig%.
p < 0.05,
p = 0.08
Figure 1.
Puff duration (A), velocity (B), volume (C), and IPI (D) as predicted by medication group and proportion of the cigarette smoked. Brackets with an asterisk refer to a significant post hoc difference (p < 0.05) between an active medication group and the placebo group. Raw data is presented for puff velocity and volume, while log transformed data is presented for duration and IPI. A) Puff Duration. Active medication groups together had blunted slopes as compared with the placebo group (Cig% × Active Contrast, p < 0.05). Post hoc comparisons indicated that only the combined VAR+NTX group had a significantly flatter slope than the placebo group (p < 0.05). B) Puff velocity. Active medication groups together had blunted slopes compared with the placebo group (Cig% × Active Contrast, p < 0.05). Post hoc analyses indicated that only the combined VAR + NTX group had a significantly flatter slope than placebo (p < 0.05). C) Puff volume. The combined VAR+NTX group had marginally lower average volume than monotherapy groups (Combined Contrast main effect, p = 0.08). Post hoc comparisons indicated that only the combined VAR+NTX group had significantly lower average puff volume as compared with placebo (p < 0.05). D) IPI. The combined VAR+NTX group was found to have lower average IPI than the monotherapy groups (Combined contrast main effect, p < 0.05). However, post hoc comparisons did not reveal differences between the individual active medication groups and the placebo group.
3.2.3. Puff Velocity
Puff velocity significantly increased over the course of the cigarette (β = 5.12, t = 3.89, p < 0.001). Similar to puff duration, the active medication groups in aggregate had a significantly flattened puff velocity slope compared with the placebo group (Figure 1B; Cig% × Active Contrast: β = −5.44, t = −2.41, p < 0.05), but not compared with each other (p’s > 0.32). Post hoc analyses showed a significant difference only between the combined VAR+NTX therapy and placebo groups in the slope of puff velocity (β = −8.62, t = −2.35, p < 0.05), with the combined group displaying a blunted increase in velocity over the course of the cigarette.
3.2.4. Puff Volume
Puff volume decreased over the course of the cigarette (β = −16.98, t = −7.09, p < 0.001), but the slope of the decrease did not differ between medication groups (p’s ≥0.25). However, the combined medication group displayed a marginally lower average puff volume than the monotherapy groups (Figure 1C; Combined Contrast main effect: β = −5.70, t = −1.77, p = 0.08). A post hoc examination revealed that only the combined VAR+NTX group demonstrated a significantly lower average volume than the placebo group (β = −12.00, t = −2.20, p < 0.05).
3.2.5. IPI
Interpuff interval increased over the course of the cigarette (β = 1.13, t = 7.12, p < 0.001), but this rate of change did not differ between medication groups (p’s ≥ 0.52). While the combined VAR+NTX group had a significantly lower average IPI than the monotherapy groups (Figure 1D; Combined Contrast main effect: β = −0.170, t = −2.14, p < 0.05), post hoc analyses did not reveal a significant difference in average IPI between an active medication group and the placebo group (p’s ≥ 0.17).
3.2.6. Covariates
Because the placebo group was significantly older than both the NTX and VAR+NTX groups and CO levels may be a proxy measure of recent smoking heaviness, both age and CO levels were examined as potential covariates. Age was a significant covariate for puff volume (p < 0.05), but not duration, velocity, or IPI. Screening session CO levels were significantly associated with puff duration (p < 0.05), volume (p < 0.01), and IPI (p = 0.07), while CO levels obtained at baseline during the experimental session were only associated with puff volume (p < 0.05). Despite age and CO levels being significant covariates for select variables, all results reported above remained significant (or for the main effect of puff volume, remained a trend) after adding these covariates to the multilevel models.
4. Discussion
The present study tested whether VAR, low dose NTX, and their combination alter smoking topography in heavy drinking smokers. In the overall sample of smokers, each topography variable displayed a distinct trajectory over the course of smoking a single cigarette, with puff duration and volume decreasing and puff velocity and IPI increasing during this time. Importantly, the slopes and averages of these topography trajectories were significantly affected by smoking pharmacotherapies, particularly the combination of VAR+NTX. The active medication groups demonstrated both a significantly blunted increase in puff velocity and an attenuated decrease in puff duration compared with placebo, an effect which was predominantly driven by the combined VAR+NTX group. Additionally, the combined VAR+NTX group had lower average puff volume than the monotherapy and placebo groups and reduced IPI compared with the monotherapy groups. These results suggest that smoking topography measures are sensitive to the effects of smoking pharmacotherapies and that the combination of VAR+NTX may be producing changes in smoking behavior that are distinguishable than either monotherapy alone.
As reported by others (Collins et al., 2009, Guyatt et al., 1989, Veilleux et al., 2011), we found that individual smoking topography variables possess unique trajectories during a smoking event, with puff duration and volume decreasing and velocity and IPI increasing over the course of a single cigarette. Although it is currently unclear as to why smoking topography variables have these distinct patterns, it has been speculated that these trajectories may be related to an individual’s titration of the amount of nicotine received per puff (Guyatt et al., 1989, Kolonen et al., 1992). As a cigarette is smoked, the amount of nicotine increases on a per puff basis while the volume and duration of each puff correspondingly decrease to regulate the amount of nicotine being consumed (Guyatt et al., 1989). The finding that smoking pharmacotherapies, particularly the combination of VAR+NTX, blunt the slopes of puff duration and velocity and reduce the average puff volume during a cigarette may suggest that these medications are changing the stereotypical pattern by which smokers self-regulate their nicotine intake, potentially by decreasing the positively reinforcing value of nicotine itself (Oncken et al., 2006). An additional implication of these findings is that a medication’s ability to affect the slope of individual puff characteristics over the course of a cigarette should be reported in addition to the average of such measures. All prior laboratory studies examining the effects of monotherapies on smoking topography have only compared the average of individual puff characteristics without analyzing how these measures dynamically change throughout a cigarette, which may have contributed to the mixed findings between these studies (Rukstalis et al., 2005; McKee et al., 2012; Ashare et al., 2012). In sum, smoking pharmacotherapies appear to alter the manner in which an individual self-regulates nicotine intake during a smoking event, which may in turn suggest that these individuals are smoking with an overall lower intensity reflective of experiencing diminished positively reinforcing effects.
Prior smoking topography studies have indicated that a less intense pattern of puffing behavior, as characterized by lower average puff volume, velocity, and duration and greater IPI, is associated with better treatment outcomes in smoking cessation trials (Strasser et al., 2004; Franken et al., 2006; MccClure et al., 2013). In support of our original hypotheses, active smoking pharmacotherapies, compared with placebo, produced an overall pattern of smoking topography (i.e., reduced puff volume, velocity, and duration) that is typically associated with lower puffing intensity (Strasser et al., 2004; Mcclure et al., 2013). Additionally, the combined treatment of VAR+NTX appeared to be more effective at reducing puffing intensity than either monotherapy alone by blunting the slopes of puff velocity and duration while also decreasing overall average puff volume. Contrary to our original hypothesis, combined VAR+NTX treatment was associated with a decreased IPI compared to the monotherapies (but not placebo). This finding is difficult to explain, particularly because no other laboratory studies have reported a pharmacological effect on IPI in either direction (McKee et al., 2012; Ashare et al., 2012). Yet, the combined therapy group smoked their cigarette with shorter, slower, and shallower puffs than the placebo group over an equivalent number of total puffs. Therefore, we speculate that, even after accounting for the reduced interval between puffs, this overall pattern of puffing behavior in the combined therapy group would still result in a decrease in nicotine exposure and may be reflective of reduced reinforcing effects of nicotine. However, given IPI’s positive predictive association with smoking abstinence (Strasser et al., 2004), it still needs to be determined whether a pharmacotherapy’s ability to blunt puff duration, velocity, volume, and IPI would ultimately be associated with beneficial cessation outcomes. As most studies have examined the association between baseline, pretreatment smoking topography measures and smoking cessation outcomes, future studies are needed to elucidate how pharmacotherapy-induced changes in components of puff intensity are related to long-term changes in smoking behavior within the context of a clinical trial.
The present study had a number of strengths, such as employing a randomized placebo-controlled design and being the first study to examine the effects of combined VAR+NTX therapy on smoking topography. However, there were also several study limitations that must be noted. First, alcohol was administered prior to smoking in all sessions and there was no study condition in which a placebo beverage was administered. While heavy drinking smokers frequently smoke after alcohol consumption, which accordingly increases the real-world validity of the current study, comparable doses of alcohol have been shown to influence average puff volume, count, and duration (King et al., 2009b, Mintz et al., 1985, Nil et al., 1984). Thus, without a placebo beverage condition, it is impossible to determine whether the pharmacotherapies are mitigating alcohol’s influence on smoking topography or directly affecting puffing behavior independently of alcohol consumption. Future studies should employ a placebo condition in order to clarify the relationship between alcohol consumption, combined VAR+NTX administration, and smoking topography in this population. Secondly, several studies have indicated that men and women display different puffing characteristics (Melikian et al., 2007; King et al., 2009b; Perkins et al., 2012). Although the present study did enroll both male and female smokers, the sample sizes of each medication group were neither large nor evenly distributed enough to examine sex as an additional factor. Finally, participants were allowed to provide their own cigarettes for the smoking portion of the study, but the brand of cigarette was not recorded and, therefore, could not be examined as a potential covariate for the smoking topography outcomes. As nicotine yield can vary by cigarette brand and topography measures may reflect individual differences in nicotine titration, we cannot rule out that between-subject differences in cigarette type could have contributed to the current findings.
In conclusion, the current study is the first laboratory study to show that smoking pharmacotherapies may affect puff duration, velocity, volume, and IPI. The results presented in this manuscript provide evidence that VAR and NTX treatment, both alone and in combination, may change the fashion in which heavy drinking daily smokers smoke a single cigarette by producing a less intense overall pattern of puffing behavior. In particular, the combination of VAR and NTX appears to be more effective than either monotherapy alone at reducing puffing intensity by blunting the slopes of puff velocity and duration while also decreasing overall average puff volume. Future studies should determine how a smoking pharmacotherapy’s ability to produce changes in puffing behavior relates both to the immediate reinforcing effects of each puff and to smoking cessation outcomes in a clinical trial. Furthermore, additional studies are needed to clarify how treatment seeking status may relate to a pharmacotherapy’s effects on smoking topography. Previous studies have shown that the motivation to quit smoking, or lack thereof, may affect a pharmacotherapies’ ability to promote abstinence and reduce craving (Perkins et al., 2008). However, all prior studies examining the effects of pharmacotherapies on smoking topography, including the current study, have only enrolled non-treatment seeking smokers (Rukstalis et al., 2005; McKee et al., 2012; Ashare et al., 2012). Thus, it remains to be determined whether varenicline, naltrexone, and their combination would produce comparable changes in puffing behavior in treatment and non-treatment seeking populations.
Highlights.
Smoking topography was measured in heavy drinking smokers after pharmacotherapy
Participants received varenicline, naltrexone, varenicline + naltrexone, or placebo
Varenicline + naltrexone blunted puff duration and velocity trajectories vs. placebo
Varenicline + naltrexone decreased mean puff volume vs. all other groups
Varenicline + naltrexone may reduce puffing intensity in heavy drinking smokers
Acknowledgments
This research was supported grants from the California Tobacco Related Disease Research Program (TRDRP; 18KT-0020 LAR), the National Institute on Drug Abuse (DA030898 LAR), and the UCLA Clinical and Translational Science Institute (UL1RR033176 and UL1TR000124). DJOR is supported by a grant from TRDRP (23FT-0102) and LAR is a paid consultant for GSK.
Footnotes
Contributors
LAR designed the study and details of the protocol. SB and DJOR were responsible for the data analysis and interpretation. All authors contributed to writing the article and approve of its content.
None of the authors have any other conflicts of interest to disclose.
Neither the funding sources nor GSK had a role in the analysis and interpretation of the data or writing of the manuscript. The content is solely the view of the authors and does not necessarily represent the official views of the National Institutes of Health or the TRDRP.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashare RL, Tang KZ, Mesaros AC, Blair IA, Leone F, Strasser AA. Effects of 21 days of varenicline versus placebo on smoking behaviors and urges among non-treatment seeking smokers. J Psychopharmacol. 2012;26:1383–90. doi: 10.1177/0269881112449397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown G. Manual for the Beck Depression Inventory-II. San Antonio, TX: 1996. [Google Scholar]
- Brick J. Standardization of alcohol calculations in research. Alcohol Clin Exp Res. 2006;30:1276–87. doi: 10.1111/j.1530-0277.2006.00155.x. [DOI] [PubMed] [Google Scholar]
- Collins CC, Epstein DH, Parzynski CS, Zimmerman D, Moolchan ET, Heishman SJ. Puffing behavior during the smoking of a single cigarette in tobacco-dependent adolescents. Nicotine & Tobacco Research. 2009:ntp176. doi: 10.1093/ntr/ntp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nature neuroscience. 2005;8:1465–70. doi: 10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- Dawson DA. Drinking as a risk factor for sustained smoking. Drug and alcohol dependence. 2000;59:235–49. doi: 10.1016/s0376-8716(99)00130-1. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Kranzler HR, Brown J, Korner PF. Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcohol Clin Exp Res. 1996;20:1412–7. doi: 10.1111/j.1530-0277.1996.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Meyerhoff DJ. The neurobiological and neurocognitive consequences of chronic cigarette smoking in alcohol use disorders. Alcohol and alcoholism. 2007;42:174–85. doi: 10.1093/alcalc/agm020. [DOI] [PubMed] [Google Scholar]
- Ebbert JO, Janney CA, Sellers TA, Folsom AR, Cerhan JR. The association of alcohol consumption with coronary heart disease mortality and cancer incidence varies by smoking history. Journal of general internal medicine. 2005;20:14–20. doi: 10.1111/j.1525-1497.2005.40129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken FH, Pickworth WB, Epstein DH, Moolchan ET. Smoking rates and topography predict adolescent smoking cessation following treatment with nicotine replacement therapy. Cancer Epidemiology Biomarkers & Prevention. 2006;15:154–7. doi: 10.1158/1055-9965.EPI-05-0167. [DOI] [PubMed] [Google Scholar]
- Fridberg DJ, Cao D, Grant JE, King AC. Naltrexone Improves Quit Rates, Attenuates Smoking Urge, and Reduces Alcohol Use in Heavy Drinking Smokers Attempting to Quit Smoking. Alcoholism: Clinical and Experimental Research. 2014;38:2622–9. doi: 10.1111/acer.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O’Malley SS. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology (Berl) 2011;215:655–63. doi: 10.1007/s00213-010-2160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt AR, Kirkham AJ, Baldry AG, Dixon M, Cumming G. How does puffing behavior alter during the smoking of a single cigarette? Pharmacology Biochemistry and Behavior. 1989;33:189–95. doi: 10.1016/0091-3057(89)90449-8. [DOI] [PubMed] [Google Scholar]
- Health UDo, Services H. The physicians’ guide to helping patients with alcohol problems. Rockville, MD: US Department of Health and Human Services, Public Heath Service, National Institute of Health (NIH), National Institute on Alcohol Abuse and Alcoholism (NIAAA); 1995. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hymowitz N, Cummings KM, Hyland A, Lynn WR, Pechacek TF, Hartwell TD. Predictors of smoking cessation in a cohort of adult smokers followed for five years. Tobacco control. 1997;6:S57. doi: 10.1136/tc.6.suppl_2.s57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Spillane NS, Metrik J. Alcohol use and initial smoking lapses among heavy drinkers in smoking cessation treatment. Nicotine & Tobacco Research. 2010;12:781–5. doi: 10.1093/ntr/ntq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Strong DR, Papandonatos GD, Colby SM, Clark MA, Boergers J, et al. Cigarette smoking and the lifetime alcohol involvement continuum. Drug and alcohol dependence. 2008;93:111–20. doi: 10.1016/j.drugalcdep.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, Cao D, Vanier C, Wilcox T. Naltrexone decreases heavy drinking rates in smoking cessation treatment: an exploratory study. Alcoholism, clinical and experimental research. 2009a;33:1044–50. doi: 10.1111/j.1530-0277.2009.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, de Wit H, Riley RC, Cao D, Niaura R, Hatsukami D. Efficacy of naltrexone in smoking cessation: a preliminary study and an examination of sex differences. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2006;8:671–82. doi: 10.1080/14622200600789767. [DOI] [PubMed] [Google Scholar]
- King A, McNamara P, Conrad M, Cao D. Alcohol-induced increases in smoking behavior for nicotinized and denicotinized cigarettes in men and women. Psychopharmacology (Berl) 2009b;207:107–17. doi: 10.1007/s00213-009-1638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Cao D, O’Malley SS, Kranzler HR, Cai X, deWit H, et al. Effects of naltrexone on smoking cessation outcomes and weight gain in nicotine-dependent men and women. J Clin Psychopharmacol. 2012;32:630–6. doi: 10.1097/JCP.0b013e3182676956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolonen S, Tuomisto J, Puustinen P, Airaksinen MM. Puffing behavior during the smoking of a single cigarette in a naturalistic environment. Pharmacology Biochemistry and Behavior. 1992;41:701–6. doi: 10.1016/0091-3057(92)90215-2. [DOI] [PubMed] [Google Scholar]
- Littleton J, Barron S, Prendergast M, Nixon SJ. Smoking kills (alcoholics)! shouldn’t we do something about it? Alcohol and alcoholism. 2007;42:167–73. doi: 10.1093/alcalc/agm019. [DOI] [PubMed] [Google Scholar]
- McClure EA, Saladin ME, Baker NL, Carpenter MJ, Gray KM. Smoking topography and abstinence in adult female smokers. Addict Behav. 2013;38:2833–6. doi: 10.1016/j.addbeh.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, et al. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry. 2009;66:185–90. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Weinberger AH, Shi J, Tetrault J, Coppola S. Developing and validating a human laboratory model to screen medications for smoking cessation. Nicotine Tob Res. 2012;14:1362–71. doi: 10.1093/ntr/nts090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melikian AA, Djordjevic MV, Hosey J, Zhang J, Chen S, Zang E, Muscat J, Stellman SD. Gender differences relative to smoking behavior and emissions of toxins from mainstream cigarette smoke. Nicotine Tob Res. 2007;9:377–87. doi: 10.1080/14622200701188836. [DOI] [PubMed] [Google Scholar]
- Mintz J, Boyd G, Rose JE, Charuvastra V, Jarvik ME. Alcohol increases cigarette smoking: a laboratory demonstration. Addict Behav. 1985;10:203–7. doi: 10.1016/0306-4603(85)90001-2. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Teague CH, Kayser AS, Bartlett SE, Fields HL. Varenicline decreases alcohol consumption in heavy-drinking smokers. Psychopharmacology (Berl) 2012;223:299–306. doi: 10.1007/s00213-012-2717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nil R, Buzzi R, Bättig K. Effects of single doses of alcohol and caffeine on cigarette smoke puffing behavior. Pharmacology Biochemistry and Behavior. 1984;20:583–90. doi: 10.1016/0091-3057(84)90308-3. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, McKee SA, Leeman RF, Cooney NL, Meandzija B, et al. Dose-dependent reduction of hazardous alcohol use in a placebo-controlled trial of naltrexone for smoking cessation. Int J Neuropsychopharmacol. 2009;12:589–97. doi: 10.1017/S146114570800936X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oncken C, Gonzales D, Nides M, Rennard S, Watsky E, Billing CB, et al. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med. 2006;166:1571–7. doi: 10.1001/archinte.166.15.1571. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA. The reliability of puff topography and subjective responses during ad lib smoking of a single cigarette. Nicotine & Tobacco Research. 2012;14:490–4. doi: 10.1093/ntr/ntr150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Courtney KE, Ghahremani DG, Miotto K, Brody A, London ED. Varenicline, low dose naltrexone, and their combination for heavy-drinking smokers: human laboratory findings. Psychopharmacology (Berl) 2014a doi: 10.1007/s00213-014-3519-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Courtney KE, Ghahremani DG, Miotto K, Brody A, London ED. Varenicline, naltrexone, and their combination for heavy-drinking smokers: preliminary neuroimaging findings. Am J Drug Alcohol Abuse. 2014b:1–10. doi: 10.3109/00952990.2014.927881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukstalis M, Jepson C, Strasser A, Lynch KG, Perkins K, Patterson F, et al. Naltrexone reduces the relative reinforcing value of nicotine in a cigarette smoking choice paradigm. Psychopharmacology (Berl) 2005;180:41–8. doi: 10.1007/s00213-004-2136-8. [DOI] [PubMed] [Google Scholar]
- Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E. The reliability of a timeline method for assessing normal drinker college students’ recent drinking history: utility for alcohol research. Addict Behav. 1986;11:149–61. doi: 10.1016/0306-4603(86)90040-7. [DOI] [PubMed] [Google Scholar]
- Strasser AA, Pickworth WB, Patterson F, Lerman C. Smoking topography predicts abstinence following treatment with nicotine replacement therapy. Cancer Epidemiology Biomarkers & Prevention. 2004;13:1800–4. [PubMed] [Google Scholar]
- Veilleux JC, Kassel JD, Heinz AJ, Braun A, Wardle MC, Greenstein J, et al. Predictors and sequelae of smoking topography over the course of a single cigarette in adolescent light smokers. Journal of adolescent health. 2011;48:176–81. doi: 10.1016/j.jadohealth.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Exp Clin Psychopharmacol. 1999;7:354–61. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]