Abstract
Fibroblast growth factor (FGF)-21 is secreted from the liver, pancreas, and adipose in response to prolonged fasting/starvation to facilitate lipid and glucose metabolism. Northern elephant seals naturally fast for several months, maintaining a relatively elevated metabolic rate to satisfy their energetic requirements. Thus, to better understand the impact of prolonged food deprivation on FGF21-associated changes, we analyzed the expression of FGF21, FGF receptor-1 (FGFR1), β-klotho (KLB; a co-activator of FGFR) in adipose, and plasma FGF21, glucose and 3-hydroxybutyrate in fasted elephant seal pups. Expression of FGFR1 and KLB mRNA decreased 98% and 43%, respectively, with fasting duration. While the 80% decrease in mean adipose FGF21 mRNA expression with fasting did not reach statistical significance, it paralleled the 39% decrease in plasma FGF21 concentrations suggesting that FGF21 is suppressed with fasting in elephant seals. Data demonstrate an atypical response of FGF21 to prolonged fasting in a mammal suggesting that FGF21-mediated mechanisms have evolved differentially in elephant seals. Furthermore, the typical fasting-induced, FGF21-mediated actions such as the inhibition of lipolysis in adipose may not be required in elephant seals as part of a naturally adapted mechanism to support their unique metabolic demands during prolonged fasting.
Keywords: fibroblast growth factor 21, fasting, food deprivation, elephant seal
1. Introduction
Northern elephant seal pups endure a post-weaning fast for 2–3 months, after which they set out to sea to forage, before returning to the rookery and fasting again for 1–2 months for molting (Riedman, 1990). Throughout fasting, the maintenance of their metabolic rate and energetic requirements are primarily satisfied by fat oxidation in the subcutaneous blubber that is the main site of fat storage (Iverson, 2002; Champagne et al., 2005; Kelso et al. 2012; Crocker et al., 2014).
During prolonged food deprivation in terrestrial mammals, fibroblast growth factor (FGF)-21 is secreted mainly from liver and adipose (Kharitonenkov and Shanafelt, 2009). FGF21 belongs to the group of vertebrate endocrine FGFs, which include FGF15/19, 21, and 23 (Itoh and Ornitz, 2011). FGF21 exerts its metabolic actions in adipose via FGF receptor-1 (FGFR1) in the presence of the co-activator, β-klotho (KLB), which is required for the FGF21-induced activation of target cells (Adams et al., 2013).
Food deprivation shifts fuel utilization from carbohydrates (glucose and glycogen) to lipids. White adipose tissue (WAT) is the main energy storage site in the body, and after hepatic glycogen is depleted, the triacylglycerides (TG) stored in WAT are oxidized to fatty acids and used for energy production. Derived free fatty acids (FFA) stimulate FGF21 secretion mainly from the liver and the WAT (Muise et al., 2008; Chen et al., 2011). FGF21 may initiate adaptive physiological responses during food-deprived condition such as hepatic ketogenesis (Badman et al., 2007), somatic growth suppression, and hibernation-like behavior (Inagaki et al., 2008; Potthoff et al., 2012). Additionally, FGF21 attenuates lipolysis in WAT via a negative feedback loop (Hotta et al., 2009). Collectively, this suite of cellular events contributes to survival during starvation or protracted periods of food deprivation; however, data on the effects of prolonged fasting on FGF21 in a naturally adapted mammal are lacking.
If FGF21 were to contribute to metabolism during prolonged fasting in elephant seals, FGF21 and its receptor would be enhanced with fasting duration; however, the conditions in fasting seals may contradict those required for typical FGF21-mediated cellular events. FGF21 inhibits lipolysis in WAT during fasting (Hotta et al., 2009) and FGF21 pharmacologically stimulates glucose uptake and ameliorates glucose tolerance and insulin sensitivity (Xu et al., 2009; Lin et al., 2013). However, fasting elephant seals rely on lipid oxidation in the subcutaneous blubber and hepatic gluconeogenesis in tandem with the Cori cycle and an insulin resistance (IR)-like condition via reduction of glucose uptake (Champagne et al., 2005; Viscarra et al., 2011; Houser et al., 2012). In addition, FGF21 promotes torpor to conserve energy (Inagaki et al., 2008); however, fasting elephant seal pups remain metabolically and physically active, normo-thermic, and maintain high levels of energy expenditure without increasing ketoacids despite high rates of beta-oxidation of fatty acids (Champagne et al., 2005; Crocker et al. 2014). FGF21 also regulates energy homeostasis by activating mitochondrial oxidative function that enhances the oxidative capacity of adipose via an AMPK-SIRT1-PGC-1α-dependent pathway (Chau et al., 2010). Conversely, fasting duration is associated with increased AMPK activation in adipose in the elephant seals (Viscarra et al. 2011) suggesting that FGF21 may be up-regulated, at least locally, with fasting in seals. Nonetheless, these contradictory conditions that may be potentially imparted by FGF21 provide an intriguing situation, which may extend our understanding of the cellular events mediated by FGF21. Therefore, we hypothesized that the expression of FGF21, FGFR1, and KLB decrease with fasting in northern elephant seal pups.
2. Material and Methods
All procedures were reviewed and approved by the Institutional Animal Care and Use Committees of both the University of California Merced and Sonoma State University, USA. All work was performed under National Marine Fisheries Service marine mammal permit no. 87-1743.
2.1 Sample Collection
Adipose (subcutaneous blubber) biopsies, and plasma samples were collected from northern elephant seal pups at Año Nuevo State Park, CA during the autumn haul-out that follows the first trip to sea in 2011 and 2012. Different cohort of animals were sampled within the first week (early; N = 8) and after 3–4 weeks of fasting (late; N = 9). Sampling procedures were performed as described previously (Vázquez-Medina et al., 2011). Briefly, animals were sedated with tiletamine hydrochloride and zolazepam hydrochloride, which were administered intramuscularly. Immobilization was maintained by injecting ketamine as required. After immobilization, a spinal needle was inserted into the extradural spinal vein, and blood samples were collected, placed on ice, and centrifuged on site to separate the plasma. Tissue samples were rinsed with ice-cold sterile saline solution and placed in cryogenic vials. Plasma and tissue samples were frozen by immersion in liquid nitrogen and stored at −80°C until analyzed.
2.2 Quantitative RT-PCR
RNA extraction, cDNA synthesis and qPCR were performed according to a previously reported procedure (Suzuki et al., 2013). Briefly, RNA was extracted from adipose with TRIzol (Life Technologies, Grand Island, NY). cDNA was synthesized with 1 μg of RNA and oligo (dT) primer using a QuantiTect® Reverse Transcription Kit (Qiagen, Valencia, CA). Quantitative PCR reactions were performed for KLB (158 bp, accession no.: AB986562), FGFR1 (110 bp, AB986561), and FGF21 (184 bp, AB986560) using gene-specific primers, which were designed to amplify a region that covered several exons (Table 1). GAPDH expression was also quantified as an internal standard. Each of the PCR fragments was sub-cloned and sequenced, as described previously (Suzuki et al., 2010). Real time PCR was performed using 1 μL of the cDNA for each gene with the plasmid as a standard.
Table 1.
Primer sequences used for qPCR.
| Primers | |
|---|---|
| FGF21 FW | 5’ -cgacagcggtacctctacac-3’? |
| FGF21 RV | 5’ -ggccctggcacaggaacc-S’ |
| β-klotho FW | 5’ -gagaatggctggttcacagacagtc-3’ |
| β-klotho RV | 5’ -gccattcaaagccatccaggagaga-3’ |
| FGFR1 FW | 5’ -ccttctagtgggactcctaacc-3’ |
| FGFR1 RV | ?’ -atgctccaggtggcgtaacg-3’ |
2.3 Measurement of plasma FGF21, glucose, and 3-hydroxybutyrate
Plasma FGF21 concentration was measured using a commercially available ELISA kit (Abnova, Taipei, Taiwan) according to the manufacturer’s instructions. Plasma concentrations of glucose and 3-hydroxybutyrate were measured with an Analox GM7 analyser (Analox Instruments, London, UK). Due to the limited plasma volume that remained after the FGF21 analyses, the glucose and 3-hydroxybutyrate (3-HBA) measurements were performed on 5 samples per fasting period.
2.4 Statistical analysis
The differences in mean values (± SEM) between the early and late fasting periods were compared using the Student’s t-test. Differences were considered significant at p < 0.05. Two aberrant values were rejected from the plasma FGF21 concentration data as detected by Smirnov–Grubbs test.
3. Results
3.1 Expression of Adipose FGFR1, KLB, and FGF21
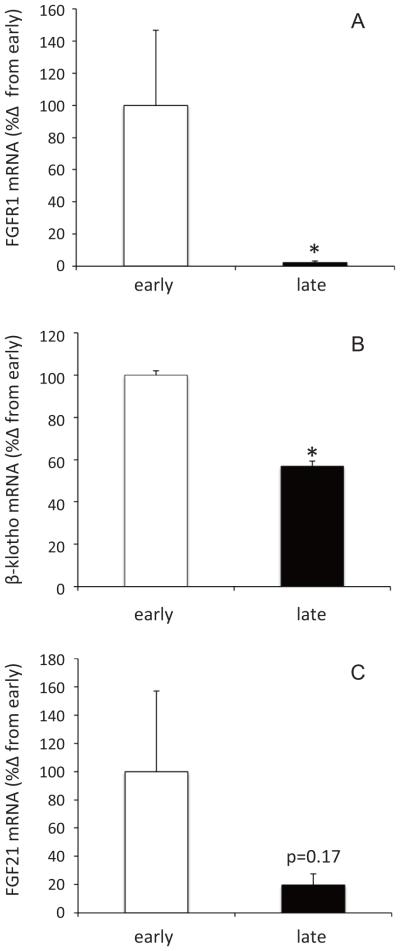
To evaluate changes in FGF21-mediated signaling in adipose, the mRNA expressions of FGFR1, KLB, and FGF21 were measured by qRT-PCR. The expressions of FGFR1 and KLB mRNA decreased 98% and 43%, respectively, with fasting (Figure 1A and 1B). While not statistically significant (p = 0.17), mean adipose FGF21 mRNA expression decreased 80% with fasting (Figure 1C).
Figure 1.
Changes in mRNA expressions of fibroblast growth factor receptor-1 (A), β-Klotho (B), and fibroblast growth factor 21 (C) in adipose of early- and late-fasted northern elephant seal pups. * denotes significant difference at p<0.05.
3.2 Changes in the plasma FGF21 concentration
The plasma FGF21 concentrations were measured to better assess the relevance of the cellular events. The average and individual data of plasma FGF21 levels are shown in Figure 2. Similar to the adipose FGF21 expression levels, plasma FGF21 concentrations decreased 39% (early fasting: 0.33 ± 0.05 ng/mL vs. late fasting: 0.20 ± 0.02 ng/mL) with fasting duration, corroborating the decreasing tendency in adipose mRNA levels.
Figure 2.
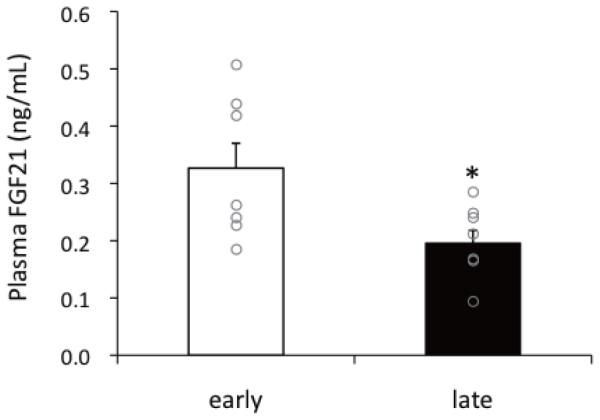
Change in mean (± SEM) plasma fibroblast growth factor 21 concentration between early- and late-fasted northern elephant seal pups. Circles are shown individual data. * denotes significant difference at p<0.05.
3.3 Changes in plasma glucose and ketone concentrations
Measurements of plasma glucose and 3-hydroxybutyrate concentrations were performed to further assess the potential shifts in substrate metabolism. Neither plasma glucose (early: 148 ± 6 mg/mL vs. late: 142 ± 6 ng/mL) nor 3-HBA (early: 223 ± 24 μM vs. late: 226 ± 7 μM) were significantly altered with fasting duration.
4. Discussion
While prolonged food deprivation/starvation typically stimulates FGF21-mediated signaling to suppress glucose and lipid metabolism to manage substrate utilization during a period of energetic burden, this study demonstrates a unique and atypical response in a mammal naturally adapted to protracted fasting. Unlike other mammals, FGF21 is suppressed with fasting duration in northern elephant seals. These data suggest that FGF21 may not contribute to the regulation of lipid and glucose metabolism during prolonged food deprivation in seals and/or that FGF21 may only contribute to cellular metabolism during the very early stage of their long-term fasting.
The co-activator, KLB, is essential for FGF21 activation, thus cells without KLB cannot respond to FGF21 (Suzuki et al., 2008). Therefore, the down-regulation of adipose KLB in the presence of decreasing plasma FGF21 levels during late fasting strongly suggests that FGF21-mediated signaling may not contribute to lipid and/or glucose metabolism in adipose during prolonged food deprivation in seals. In rat and human adipocytes, TNFα can suppress KLB expression and impair FGF21 signaling as a consequence of insulin resistance (Díaz-Delfín et al. 2012). Previously, we reported an increase in muscle TNFα (Suzuki et al., 2013) and IR-like condition in adipose (Viscarra et al., 2011) during late fasting in elephant seal pups suggesting that a local increase in TNFα and/or induction of an IR-like condition may contribute to the down-regulation of KLB. Consistent with the decrease in KLB, adipose FGFR1 expression was also suppressed with fasting duration. FGFR1 expression is stimulated by FGF21 in the mouse (Adams et al., 2013). The parallel decreases in adipose FGFR1 and plasma FGF21 levels during late fasting suggest that the FGF21 receptor content is mediated by circulating levels. The suppression of adipose FGFR1 and KLB in the presence of decreased plasma FGF21 is indicative of down-regulation of cellular FGF21-mediated signaling. Furthermore, while physical activity may increase FGF21 (Cuevas-Ramos et al., 2011), during this time of fasting northern elephant seals maintain a relatively high metabolic rate (hypermetabolism) and are physically active (Kelso et al. 2013) suggesting that the suppression of FGF21 and its associated cellular cascade is more a function of fasting dynamics in seals than a change in physical activity and/or metabolic rate.
Circulating FGF21 is primarily secreted from the liver and adipose (Kharitonenkov and Shanafelt, 2009). Because an examination of hepatic FGF21 expression in elephant seals is not be possible, we cannot assess the contribution of each organ to plasma FGF21; however, given that adipose constitutes approximately 35–38% of a juvenile elephant seal’s body mass and is metabolically active (Kelso et al. 2012), the parallel decreases in adipose FGF21 expression and plasma FGF21 suggest that adipose is a significant source of FGF21 in elephant seals. The levels measured here are similar to those in humans, although levels in humans vary greatly (Gälman et al., 2008).
In other mammals, PPARγ activation induces FGF21 expression pharmacologically (Muise et al., 2008; Wang et al., 2008). In elephant seals, adipose PPARγ expression decreases with prolonged fasting (Viscarra et al., 2011) suggesting that the decreased adipose FGF21 expression may have resulted from reduced PPARγ expression. Paradoxically, fasting in seals is associated with suppressed FGF21, and this suppression is not likely to contribute to substrate metabolism as evident by the lack of changes in plasma glucose and 3-HBA. These data are coincident with previous studies demonstrating that the maintenance of blood glucose and inconsequential accumulation of ketoacids via the Cori cycle and an IR-like condition in the seals (Champagne et al., 2005; Viscarra and Ortiz, 2013; Crocker et al., 2014) may facilitate the conservation of circulating glucose by relying on lipid oxidation for energy.
Collectively, these data suggest that, unlike other fasted/starved mammals, prolonged fasting attenuates FGF21-mediated signaling in elephant seals, a mammal naturally adapted to such a behavior. FGF21 is a potent activator of glucose uptake via GLUT1 through PPARγ activation in adipocytes (Moyers et al., 2007; Muise et al., 2008). In fasting elephant seal pups, an IR-like condition develops in adipose with prolonged food deprivation with PPARγ downregulation (Viscarra et al., 2011). The decreases in plasma FGF21 and the expressions of adipose FGFR1 and KLB with fasting duration may contribute to IR via down-regulation of PPARγ expression. This atypical FGF21 response to food deprivation may be an important adapted mechanism that contributes to the maintenance of elevated circulating glucose to support the fasting metabolism of elephant seals. Thus, the typical fasting responses mediated by FGF21 activation, including the inhibition of lipolysis in adipose and the induction of torpor, are not evident in elephant seals.
Highlights.
We tested effects of fast on FGF21-associated changes in elephant seal.
Expression of FGF receptor-1 and its co-activator in adipose was decreased by fast.
Plasma FGF21 levels were also decreased with a trend of decrease in adipose FGF21.
Their atypical metabolism responses during fast do not require FGF21 functions.
Acknowledgments
We sincerely thank JT Sharick (SSU) for his great help with collecting samples. M Suzuki was supported by a grant for Overseas Researcher from Nihon University and Grant-in-Aid for Scientific Research (C, 23580265) from the Japan Society for the Promotion of Science. JP Vazquez-Medina was supported by fellowships from The University of California Institute for Mexico and The United States (UC MEXUS). J Viscarra was supported by a supplement to NIH NHLBI (R01HL91767). RM Ortiz was partially supported by NIH NHLBI (K02HL103787). The research was partially funded by NIH NHLBI (R01HL91767).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams AC, Yang C, Coskun T, Cheng CC, Gimeno RE, Luo Y, Kharitonenkov A. The breadth of FGF21's metabolic actions are governed by FGFR1 in adipose tissue. Mol Metab. 2013;2:31–37. doi: 10.1016/j.molmet.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gälman C, Lundåsen T, Kharitonenkov A, Bina HA, Eriksson M, Hafström I, Dahlin M. Åmark P., Angelin B. Rudling, M. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARα activation in man. Cell Metab. 2008:169–174. doi: 10.1016/j.cmet.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Cuevas-Ramos D, Almeda-Valdés P, Meza-Arana CE, Brito-Córdova G, Gómez-Pérez FJ, Mehta R, Oseguera-Moguel J, Aguilar-Salinas CA. Exercise increases serum fibroblast growth factor 21 (FGF21) levels. PLoS One. 2012;7:e38022. doi: 10.1371/journal.pone.0038022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne CD, Houser DS, Crocker DE. Glucose production and substrate cycle activity in a fasting adapted animal, the northern elephant seal. J Exp Biol. 2005;208:859–868. doi: 10.1242/jeb.01476. [DOI] [PubMed] [Google Scholar]
- Chau MD, Gao J, Yang Q, Wu Z, Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK–SIRT1–PGC-1α pathway. Proc Nat Acad Sci. 2010;107:12553–12558. doi: 10.1073/pnas.1006962107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Hoo RLC, Konishi M, Itoh N, Lee PC, Ye HY, Lam KSI, Xu A. Growth hormone induces hepatic production of fibroblast growth factor 21 through a mechanism dependent on lipolysis in adipocytes. J Biol Chem. 2011;286:34559–34566. doi: 10.1074/jbc.M111.285965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker DE, Champagne CD, Fowler MA, Houser DS. Adiposity and fat metabolism in lactating and fasting northern elephant seals. Adv Nutr. 2014;5:57–64. doi: 10.3945/an.113.004663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Delfín J, Hondares E, Iglesias R, Giralt M, Caelles C, Villarroya F. TNF-α represses β-Klotho expression and impairs FGF21 action in adipose cells: involvement of JNK1 in the FGF21 pathway. Endocrinology. 2012;153:4238–4245. doi: 10.1210/en.2012-1193. [DOI] [PubMed] [Google Scholar]
- Ho KY, Veldhuis JD, Johnson ML, Furlanetto R, Evans WS, Alberti KG, Thorner MO. Fasting enhances growth hormone secretion and amplifies the complex rhythms of growth hormone secretion in man. J Clin Invest. 1988;81:968–975. doi: 10.1172/JCI113450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser DS, Crocker DE, Tift MS, Champagne CD. Glucose oxidation and nonoxidative glucose disposal during prolonged fasts of the northern elephant seal pup (Mirounga angustirostris) Am J Physiol Regul Integr Comp Physiol. 2012;303:R562–R570. doi: 10.1152/ajpregu.00101.2012. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Lin VY, Goetz R, Mohammadi M, Mangelsdorf DJ, Kliewer SA. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. 2008;8:77–83. doi: 10.1016/j.cmet.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta Y, Nakamura H, Konishi M, Murata Y, Takagi H, Matsumura S, Inoue K, Fushiki T, Itoh N. Fibroblast growth factor 21 regulates lipolysis in white adipose tissue but is not required for ketogenesis and triglyceride clearance in liver. Endocrinology. 2009;150:4625–4633. doi: 10.1210/en.2009-0119. [DOI] [PubMed] [Google Scholar]
- Itoh N, Ornitz DM. Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J Biochem. 2011;149:121–130. doi: 10.1093/jb/mvq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyers JS, Shiyanova TL, Mehrbod F, Dunbar JD, Noblitt TW, Otto KA, Reifel-Miller A, Kharitonenkov A. Molecular determinants of FGF-21 activity-synergy and cross-talk with PPARγ signaling. J Cell Physiol. 2007;210:1–6. doi: 10.1002/jcp.20847. [DOI] [PubMed] [Google Scholar]
- Muise ES, Azzolina B, Kuo DW, El-Sherbeini M, Tan Y, Yuan X, Mu J, Thompson JR, Berger JP, Wong KK. Adipose fibroblast growth factor 21 is up-regulated by peroxisome proliferator-activated receptor gamma and altered metabolic states. Mol Pharmacol. 2008;74:403–412. doi: 10.1124/mol.108.044826. [DOI] [PubMed] [Google Scholar]
- Lin Z, Tian H, Lam KS, Lin S, Hoo RC, Konishi M, Itoh N, Wang Y, Bornstein SR, Xu A, Li X. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013;17:779–789. doi: 10.1016/j.cmet.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Olivares-Reyes JA, Arellano-Plancarte A, Castillo-Hernandez JR. Angiotensin II and the development of insulin resistance: implications for diabetes. Mol Cell Endocrinol. 2009;302:128–139. doi: 10.1016/j.mce.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK. Tumor necrosis factor-α induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes. 2005;54:2939–2945. doi: 10.2337/diabetes.54.10.2939. [DOI] [PubMed] [Google Scholar]
- Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/19 and 21: From feast to famine. Genes Dev. 2012;26:312–324. doi: 10.1101/gad.184788.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidman M. The Pinnipeds: Seals, sea lions, and walruses. University of California Press; Berekeley, CA: 1990. [Google Scholar]
- Suzuki M. Expression and localization of aquaporin-1 on the apical membrane of enterocyte in small intestine of bottlenose dolphin. J Comp Physiol B. 2010;180:229–238. doi: 10.1007/s00360-009-0397-6. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Uehara Y, Motomura-Matsuzaka K, Oki J, Koyama Y, Kimura M, Asada M, Komi-Kuramochi A, Oka S.x, Imamura T. Klotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c. Mol Endocrinol. 2008;22:1006–1014. doi: 10.1210/me.2007-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Vázquez-Medina JP, Viscarra JA, Soñanez-Organis JG, Crocker DE, Ortiz RM. Activation of systemic, but not local, renin–angiotensin system is associated with upregulation of TNF-α during prolonged fasting in northern elephant seal pups. J Exp Biol. 2013;216:3215–3221. doi: 10.1242/jeb.085225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Medina JP, Crocker DE, Forman HJ, Ortiz RM. Prolonged fasting does not increase oxidative damage or inflammation in postweaned northern elephant seal pups. J Exp Biol. 2010;213:2524–2530. doi: 10.1242/jeb.041335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscarra JA, Champagne CD, Vazquez-Medina JP, Crocker DE, Ortiz RM. Increased AMPK activity compensates for reduced insulin sensitivity during prolonged fasting in northern elephant seal pups. FASEB J. 2011;25:858–6. [Google Scholar]
- Viscarra JA, Ortiz RM. Cellular mechanisms regulating fuel metabolism in mammals: role of adipose tissue and lipids during prolonged food deprivation. Metabolism. 2013;62:889–97. doi: 10.1016/j.metabol.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Qiang L, Farmer SR. Identification of a domain within peroxisome proliferator-activated receptor regulating expression of a group of genes containing fibroblast growth factor 21 that are selectively repressed by SIRT1 in adipocytes. Mol Cell Biol. 2008;28:188–200. doi: 10.1128/MCB.00992-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, Chen JL, Jung DY, Zhang A, Ko HJ, Kim JK, Véniant MM. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]