Abstract
Background
Reduced auditory target P300 amplitude is a leading biomarker for psychotic disorders, although its relevance for differential diagnosis and link to specific clinical features (symptom profiles, functional impairment, and course) is unclear. This study aims to clarify the clinical significance of auditory target P300 using concurrent and retrospective clinical data from a longitudinal cohort with psychosis.
Methods
92 cases from an epidemiological study of first-admission psychosis were assessed using an auditory oddball paradigm at 15-year follow-up along with 44 never-psychotic adults. Subcomponents of auditory target P300 amplitude (i.e., a central positive P3a, a parietal positive P3b, and a frontal negative slow wave) were isolated using temporal-spatial principal components analysis.
Results
P3a amplitude was blunted across psychotic disorders relative to non-psychotic adults. P3b amplitude was reduced in schizophrenia specifically, including cases initially misclassified at baseline. The frontal negative slow wave did not distinguish among groups. P3b amplitude reduction was associated with several clinical features at the concurrent assessment, as well as previous time points, including recovery from psychosis even 5 years earlier and functioning even 15 years earlier.
Conclusions
Auditory target P300 amplitude yields both a schizophrenia-specific component (i.e., P3b) and a transdiagnostic psychosis component (i.e., P3a). The P3b component may also shed light on prognosis, real-world functioning, and course, as well as help to reduce misdiagnosis of psychotic disorders. Prospective studies are needed to test whether P3b tracks or predicts clinical status.
Keywords: Schizophrenia, Psychosis, P300 amplitude, Recovery, Remission
Introduction
Reduced auditory P300 amplitude has been linked to schizophrenia (Jeon & Polich, 2003; Bramon et al., 2005; Bramon et al., 2004), but specificity of this relationship is rarely studied. The few studies that compared schizophrenia with other psychotic disorders on auditory target P300 amplitude have yielded mixed results (Iwanami et al., 1994; Hermens et al., 2010; Kaur et al., 2012; Kaur et al., 2011; Salisbury et al., 1998; Salisbury et al., 1999; Ethridge et al., 2012; Mathalon et al., 2010). Consequently, it is unclear whether P300 amplitude reduction is best conceptualized as a unique characteristic of schizophrenia or a transdiagnostic indicator of psychosis.
There are several potential explanations for inconsistent findings. First, diagnostic misclassification is particularly pronounced early in illness course (Schwartz et al., 2000) and may have distorted results. For example, even using the best practice of consensus longitudinal diagnosis, half of the 470 cases with psychosis in our cohort changed diagnoses at least once during the first decade of illness (Bromet et al., 2011). In particular, many patients diagnosed initially with other psychoses were reclassified with schizophrenia at follow-up. Prior studies comparing P300 amplitude between schizophrenia and other psychotic disorders may have been subject to misclassification confounds due to a focus on recent-onset psychosis and/or lack of longer-term follow-up (Iwanami et al., 1994; Hermens et al., 2010; Kaur et al., 2012; Kaur et al., 2011; Salisbury et al., 1998; Salisbury et al., 1999; Ethridge et al., 2012; Mathalon et al., 2010). Thus, very little is known about auditory target P300 in psychotic disorders with diagnoses ascertained longitudinally to minimize misclassification.
Second, P300 amplitude is heterogeneous (Friedman et al., 1978; Sutton & Ruchkin, 1984) and reflects multiple overlapping neural processes (Dien et al., 2004; Rushby et al., 2005). The two most characterized processes are the P3a, a fronto-central index of attention to salient stimuli, and P3b, a parietal index of stimulus categorization and response (Nieuwenhuis et al., 2005). Past studies used different tasks that elicit these subcomponents to different degrees. For example, a mismatch negativity task (i.e., Kaur et al., 2012) elicits a P3a, whereas a two-stimulus oddball task (Salisbury et al., 1999) elicits both P3a and P3b. Thus, it is not clear to what extent auditory target P3a, P3b, or both distinguish schizophrenia from other psychotic disorders because prior studies did not attempt to differentiate these components.
Third, schizophrenia is heterogeneous in terms of symptoms, functioning, and course (Jablensky, 2006), and it is unclear what aspects of illness are related to P300 amplitude. P300 amplitude has been linked to greater psychotic (O’Donnell et al., 1993), disorganized (O’Donnell et al., 1993; Havermans et al., 1999), and negative symptoms (Pfefferbaum et al., 1989; Bruder et al., 2001), worse social and occupational functioning (Hermens et al., 2010; Light et al., 2014), and changes in symptoms (Mathalon et al., 2000). However, these literatures contain many null and inverse findings (Frodl-Bauch et al., 1999; Ford, 1999), including evidence that P300 amplitude reduction is present prior to onset and during periods of symptom remission (Ford et al., 1994). Very little is known about the association between P300 amplitude and recovery (i.e., sustained symptom remission with good functioning; Lieberman et al., 2008; New Freedom Commission on Mental Health, 2003), although it is an important target for research and treatment. It may be possible to link P300 amplitude reduction to recovery, especially among other psychotic disorders where recovery is more common.
Current Study
We sought to clarify the answers to these questions by assessing subcomponents of auditory target P300 amplitude in a sample of 92 cases with diverse psychotic disorders and 44 never-psychotic adults. To ensure maximal diagnostic accuracy, consensus diagnosis utilized information from multiple sources (i.e., semi-structured diagnostic interviews, review of medical records, and informant reports) collected at 5 time points spanning 10 years (first admission, 6-month, 2-year, 4-year, and 10-year). Information on symptoms (positive, negative, and disorganized), functioning (global and social), and course (remission and recovery) was available from these 5 assessments, plus a 6th assessment (15-year follow-up) at which we first assessed P300 amplitude. We tested if subcomponents of auditory target P300 amplitude derived from temporal-spatial principal components analysis (TSPCA) differentially relate to psychosis using concurrent and prospective data on diagnosis, symptoms, functioning, and course.
Methods
Participants
Participants for this study were drawn from the Suffolk County Mental Health Project (Bromet et al., 2011), an epidemiologic longitudinal study of first-admission psychosis. Participants were recruited from the 12 inpatient psychiatric facilities of Suffolk County, NY from 1989–1995; eligibility criteria were psychosis, age 15–60 year at admission, IQ > 70, and ability to provide informed consent. Study psychiatrists assigned DSM-IV diagnoses by consensus. This study was approved annually by the Institutional Review Board at Stony Brook University.
The EEG data examined in this study was collected 15 years after first admission (range: 12.4–19.1) on 41 participants with a schizophrenia spectrum diagnosis (SZ; schizophrenia [n = 28] or schizoaffective [n=13]) and 51 participants with other psychotic disorders (OP; mood disorder with psychosis [n = 38], substance-induced psychosis [n = 9], or psychosis not otherwise specified [n = 4]). We also assessed 44 never-psychotic adults (NA) who were also matched to cases on age, gender, and self-identified race. The full ERP battery included a flankers task (Foti et al., 2012), a semantic congruency task (Jackson et al 2015), and a task that involved viewing emotional faces; tasks were presented in counter-balanced order.
Clinical Assessment
At each assessment point, cases were evaluated using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID; First et al., 2001), the Scale for the Assessment of Positive Symptoms (SAPS; Andreasen, 1983a), and the Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1983b). The SANS was scored as a single index of negative symptoms, and the SAPS was subdivided into psychotic (hallucinations, delusions) and disorganized (bizarre behavior, thought disorder) subscales based on prior factor analysis (Kotov et al., 2010). Concurrent global assessment of functioning (GAF) was rated by consensus among study psychiatrists. Social functioning was calculated as the sum of social activity, social initiative, and sociosexual relations ratings of the Quality of Life Scale (Heinrichs et al., 1984). Remission from psychosis (Andreasen et al. 2005) and recovery from psychosis (Liberman et al., 2002), i.e., minimal symptoms and satisfactory functioning in the interval, were both defined according to consensus criteria.
Task
P300 Task
The P300 was elicited using a three-stimulus auditory oddball task. Participants wore headphones in a sound-attenuated dark room and were instructed to count the number of times a synthesized “you” sound was presented in each block. The frequent non-target (i.e., standard) stimulus was a synthesized “me” sound. Rare novel stimuli were chosen at random from a set of 25 non-repeating unique sounds (e.g., a flute noise) or synthesized noises (e.g., “beep”). Each stimulus was presented for 200ms at 75 dB and the interval between sound onsets was 1500ms. There were 250 trials divided into five blocks of 50 trials. Overall, 10% of stimuli were targets, 80% were frequent non-targets, and 10% were novels. Participants first were introduced to the “you” and “me” sounds, and then completed a practice block to provide an opportunity to ask questions. At end of each block, participants reported the number of targets that they heard. Accuracy was calculated as the sum of deviations from correct number of targets in each block, and expressed as a percent. Due to equipment malfunction, accuracy data was missing for two cases in the OP group.
EEG Recording, Processing, and Data Reduction
The EEG was recorded using the Active Two BioSemi system (BioSemi, Amsterdam, The Netherlands) using the 32 electrode cap based on the 10/20 system, plus the FCz and Iz electrodes. Data was sampled at 1024 Hz using a low-pass fifth-order sinc filter with −3 dB cutoff point at 208 Hz and digitized online at 24-bit resolution and a least significant bit value of 31.25 nV. Electrodes were measured with respect to a common mode sense active electrode that formed a monopolar channel. Offline analysis was performed using Brain Vision Analyzer software (Brain Products, Munich, Germany). Data were re-referenced to linked mastoid average and band-pass filtered from 0.1 Hz to 30 Hz. The EEG was segmented for each trial and corrected for blinks and eye movements recorded from four facial electrodes using a regression-based method (Gratton et al. 1983). Channels were rejected in each trial using a semiautomated procedure, with artifacts defined as a step of more than 50.0μV between samples, a difference of 300μV within a trial, or a maximum difference of less than 0.50μV within 100-msec intervals. Additional artifacts were identified using visual inspection. All subjects had at least 20 target trials for averaging. Stimulus-locked ERP averages were created and baseline corrected from the EEG activity from −200 msec to 0 msec.
Statistical Analysis
Temporal-Spatial Principal Analysis
The TSPCA followed published recommendations (Dien, 2012). We conducted a temporal PCA with promax rotation on the average waveforms for each electrode for target stimuli and standard stimuli, and then a spatial PCA on each of the temporal PCs using infomax rotation. A prominent temporal PC peaked at 327ms and was characterized as a broad central positivity, which we judged to be the P3a. A prominent temporal PC peaked at 497ms and was split by spatial rotation into a parietal positivity (first spatial component) and a frontal negativity (second spatial component). These two components were judged to be P3b and Negative Slow Wave, respectively (Dien, 2012), and both were retained for analysis since they occurred in the same time window. Further analyses examined the component scores for target stimuli for these three TSPCs: P3a, P3b, and Negative Slow Wave.
Group analyses were conducted using Univariate General Linear Model in SPSS (i.e., ANCOVA) separately for each TSPC. Diagnostic group (NA, SZ, OP) was entered as a between-subject factor and age and gender were entered as covariates. To be consistent with a previous report (Foti et al., 2012), we also tested two a priori contrasts pertaining to differential diagnosis: (1) NA vs all cases (i.e., general effect of psychotic illness), and (2) SZ vs OP (i.e., specific effect of schizophrenia). Schizophrenia and schizoaffective disorder did not differ on subcomponents (ps ranged from 0.10 for the Negative Slow Wave to 0.76 for P3b), supporting the decision to combine them into a single SZ group.
Individual difference analyses were conducted using partial correlations to control for the influence of age and gender. We examined full sample correlations of components with symptoms, functioning, and course to capture clinical utility of neural markers in a real-world scenario, namely when a patient presents with a history of psychosis but uncertain diagnosis. This is a common situation in many clinical and research contexts because long-term follow-up, detailed records, and informant reports are rarely available. For thoroughness, we also examined correlations separately by group when the whole-sample correlation was statistically significant.
Results
Sample Characteristics
The three groups were similar on demographic variables (see Table 1). Relative to OP, SZ was more likely to be taking antipsychotics (but not other medications), reported greater symptom levels and functioning deficits, and were less likely to be rated as meeting criteria for recovery or remission. The groups did not differ in number of target trials used in averaging. The SZ group were slightly less accurate in reporting number of targets, but overall accuracy was high (i.e. > 93%).
Table 1.
Sample characteristics
| Schizophrenia | Other Psychosis | Never Psychotic | Group Comparisons | |||||
|---|---|---|---|---|---|---|---|---|
| n = 41 | n = 51 | n = 44 | χ2 | p | ||||
|
|
|
|
|
|||||
| n | % | n | % | N | % | |||
| Gender | 3.00 | 0.22 | ||||||
| Male | 25 | 61 | 33 | 65 | 21 | 48 | ||
| Female | 16 | 39 | 18 | 35 | 23 | 52 | ||
| Race | 0.31 | 0.98 | ||||||
| Caucasian | 32 | 78 | 40 | 78 | 35 | 80 | ||
| Other | 9 | 22 | 11 | 22 | 9 | 20 | ||
| Medication in last month | ||||||||
| Antipsychotic | 33 | 83 | 11 | 22 | 61.09 | <0.01 | ||
| Antidepressant | 16 | 40 | 16 | 31 | 0.73 | 0.39 | ||
| Mood Stabilizer | 10 | 25 | 12 | 24 | 0.03 | 0.87 | ||
| Benzodiazapine | 6 | 15 | 7 | 14 | 0.03 | 0.86 | ||
| Remission | 22.97 | <0.01 | ||||||
| Remitted | 8 | 20 | 36 | 71 | ||||
| Not Remitted | 32 | 80 | 15 | 29 | ||||
| Recovery | 28.08 | <0.01 | ||||||
| Recovered | 3 | 8 | 31 | 62 | ||||
| Not Recovered | 37 | 93 | 19 | 38 | ||||
| Schizophrenia | Other Psychosis | Never Psychotic | ||||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | Test Statistic | p | |
|
|
|
|
|
|||||
| Age | 44.29 | 7.71 | 43.98 | 9.93 | 45.80 | 11.24 | F(2,133)=0.45 | 0.64 |
| Symptoms of Psychosis | ||||||||
| Negative | 19.08 | 11.77 | 6.41 | 9.52 | F(1,89)=32.22 | <0.01 | ||
| Psychotic | 2.98 | 5.66 | 0.86 | 3.38 | F(1,89)=4.89 | 0.03 | ||
| Disorganized | 3.13 | 5.07 | 1.51 | 2.85 | F(1,89)=3.69 | 0.06 | ||
| Global Assessment of Functioning | 47.18 | 11.90 | 64.96 | 13.08 | F(1,89)=44.85 | <0.01 | ||
| Social Functioning | 9.73 | 4.79 | 14.76 | 3.57 | F(1,89)=32.64 | <0.01 | ||
| Valid Target Trials (n=25) | 24.63 | 1.89 | 24.71 | 0.83 | 24.86 | 0.46 | F(2,133)=0.42 | 0.66 |
| Accuracy (%) | 93.4 | 0.14 | 98.1 | 0.04 | 99.0 | 0.03 | F(2,131)=5.56 | <0.01 |
Diagnostic Groups
Figure 1 displays the grand average waveforms by diagnostic group for several representative electrodes. The auditory target P300 amplitude, the prominent positive peak between 275ms and 350ms at midline central electrodes, appears blunted in SZ and OP relative to NA, observed most clearly at Cz. The relatively broad parietal positivity occurring after the peak of the P300 was reduced in SZ, observed across several electrodes, most notably T7 and PO3.
Figure 1.
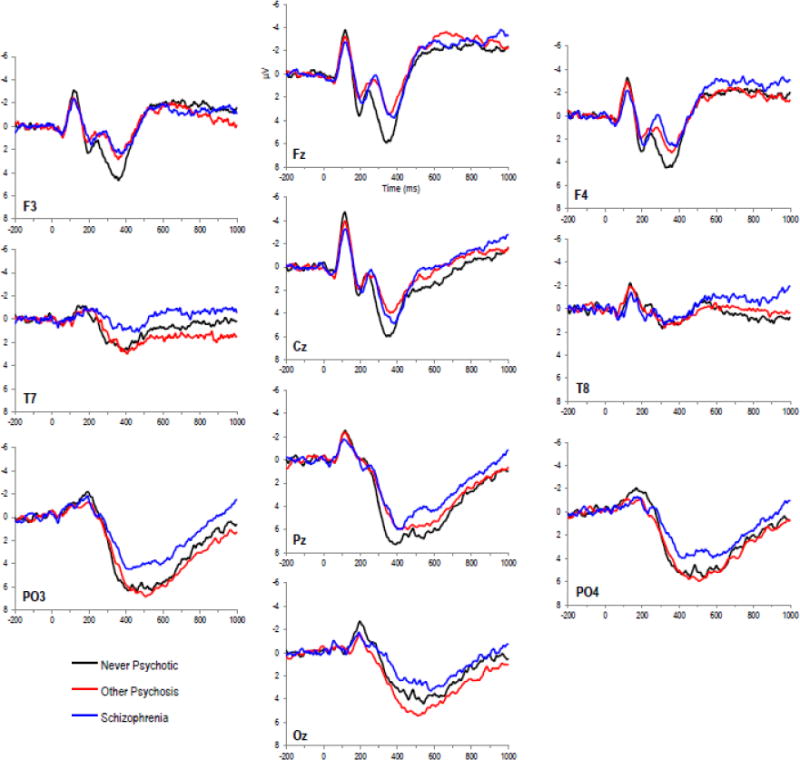
Average waveforms for target stimuli by group at representative electrodes. Waveforms presented with 200ms prestimulus baseline.
We next compared diagnostic groups on the TSPC scores (Table 2; Figure 2). Groups differed on the P3a, which was reduced in SZ and OP relative to NA (the former two groups did not differ). Groups also differed on the P3b, which was due to blunting in SZ relative to NA and OP (the latter two groups did not differ). No statistically significant group differences were observed for the Negative Slow Wave.
Table 2.
Diagnostic Group Differences
| Component | SZ (n=41) | OP (n=51) | NA (n=44) | Omnibus Anova | Apriori Contrast | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Mean | SD | Mean | SD | Mean | SD | Cases vs NA | SZ vs OP | ||
| P value | P value | ||||||||
| 327ms SF1 (P3a) | 3.45 | 4.44 | 3.76 | 4.35 | 6.58 | 4.77 | F(2,131) = 6.17, p < 0.01 | < 0.01 | ns |
| 497ms SF1 (P3b) | 3.49 | 3.49 | 5.85 | 3.89 | 5.71 | 3.50 | F(2,131) = 5.95, p < 0.01 | ns | < 0.01 |
| 497ms SF2 (Frontal Negative Slow Wave) | −1.76 | 4.11 | −1.95 | 4.34 | −1.50 | 2.77 | F(2,131) = 0.05, p = 0.95 | ns | ns |
Note: SZ= Schizophrenia spectrum disorders; OP = other psychotic disorders; NA = never-psychotic adults. Age and sex were entered as covariate in the analyses. In bivariate analyses, age correlated with P3b (r = −0.33, p < 0.05).
Figure 2.
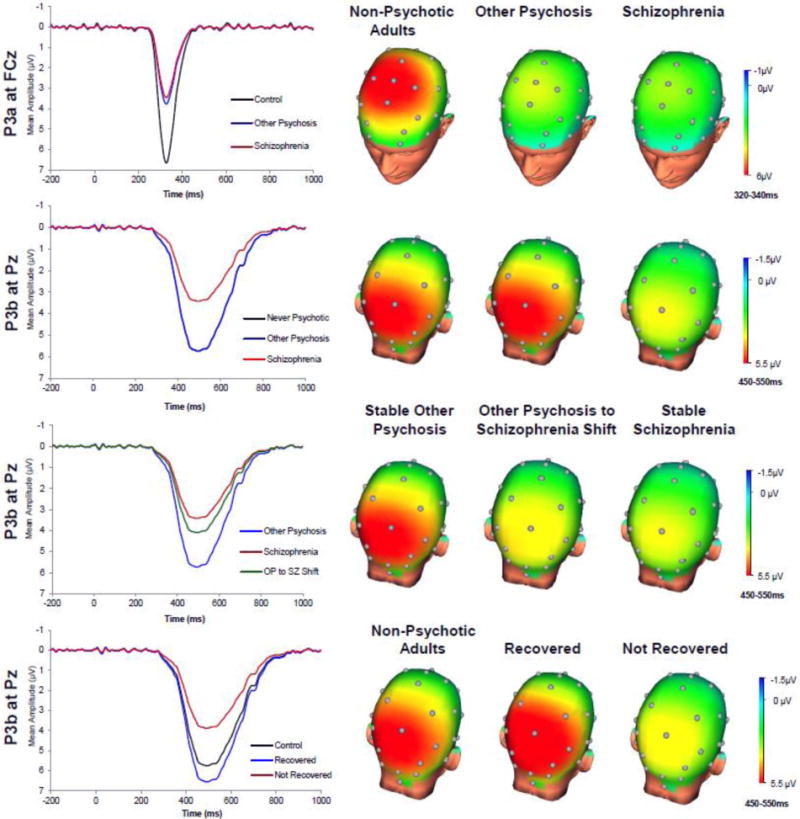
P3a and P3b temporal-spatial principal components and topographic head maps by group. In Figure c, the other psychosis group waveform and non-psychotic adults group waveform were similar and indistinguishable on the P3b component. For clarity, we present only the other psychosis waveform.
Of the 91 cases, 17 changed diagnostic classification since baseline assessment: 16 SZ cases were classified as OP at baseline, while 1 OP case was classified as SZ at baseline. The group effect predicting P3b amplitude in a 3-way ANCOVA (the single SZ to OP case was excluded) controlling for age and gender was statistically significant, F(2,86) = 5.15, p = 0.01; Means (SDs) for OP to SZ: 4.10 (3.66); for continuously SZ: 3.10 (3.40); and for continuously OP: 5.87 (3.92). As shown in Figure 2, the OP to SZ group exhibited reduced P3b relative to the continuously OP group (d = 0.46, one-tailed p = .04) but was not different from the continuously SZ group (Cohen’s d = 0.24, one-tailed p = 0.24). This suggests that early-course misclassification may increase the chance of spuriously reporting P300 amplitude reduction in both OP and SZ, rather than in SZ alone, due to inclusion of SZ cases into the OP group.
Clinical characteristics
We retained the P3a and P3b TSPCs in our individual differences analyses because they distinguished groups. Reduced P3b amplitude was associated with more negative symptoms, more disorganized symptoms, worse global and social functioning, lower likelihood of recovery, and lower likelihood of remission (Table 3). Figure 2 displays the P3b waveform for never psychotic adults, recovered cases, and non-recovered cases. Using the data collected at previous assessments, correlations with P3b were present for GAF by admission, for negative symptoms by 6-month follow-up, and for disorganized symptoms and social functioning by 2-year followup. This pattern suggests that reduced P3b amplitude is associated with enduring symptom and functioning profiles as early as 15 years prior to recording P300 amplitude. After segregating by group, reduced P3b amplitude was associated with worse global and social functioning in each group separately. In the OP sample, statistically significant correlations with P3b emerged by 10-year assessment for global functioning and recovery. Thus, there is evidence that P3b amplitude reduction reflects enduring chronic illness course within OP. No clinical features were related to P3a (all ps > 0.05).
Table 3.
Partial correlations between P3b amplitude assessed 15 year after admission and clinical features assessed at each time point controlling for age and gender
| Symptoms | Functioning | Course | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||||||||||
| Negative
|
Positive
|
Disorganized
|
Global
|
Social
|
Recovery
|
Remission
|
|||||||||||||||
| r | rSZ | rOP | r | rSZ | rOP | r | rSZ | rOP | r | rSZ | rOP | r | rSZ | rOP | d | dSZ | dOP | d | dSZ | dOP | |
| Concurrent | |||||||||||||||||||||
| 15 yr | −.32** | −.23 | −.19 | −.19 | – | – | −.21* | −.27 | −.15 | .40** | .32* | .30* | .38** | .36* | .29* | .74** | – | .47 | .60** | – | .26 |
| Retrospective | |||||||||||||||||||||
| 10 yr | −.28** | −.09 | −.18 | −.25* | −.25 | −.18 | −.23* | −.21 | −.19 | .41** | .24 | .35* | .36** | .31 | .27 | 1.05** | – | .82** | .45* | – | .55 |
| 4 yr | −.25* | .04 | −.23 | −.15 | – | – | −.28* | −.30 | −.20 | .30** | .21 | .10 | .25* | .04 | .20 | .44 | – | – | .71** | – | .74 |
| 2 yr | −.25* | .02 | −.22 | .16 | – | – | −.22 | – | – | .26* | −.04 | .20 | .24* | −.02 | .13 | .25 | – | – | .46 | – | – |
| 6 m | −.25* | −.09 | −.17 | −.08 | – | – | −.19 | – | – | .26* | .06 | .14 | .18 | – | – | .16 | – | – | .39 | – | – |
| Admission | −.16 | – | – | −.19 | – | – | −.16 | – | – | .26* | .24 | .13 | – | – | – | – | – | – | – | – | – |
Note:
= p < 0.05.
= p < 0.01. Social Functioning was not assessed at admission.
Discussion
The present investigation yielded three main findings. First, schizophrenia and other psychotic disorders were differently related to P300 subcomponents. The P3a component was reduced across the psychotic disorders and does not distinguish SZ from OP. In contrast, the P3b was reduced in schizophrenia spectrum disorders but not in other psychotic disorders. Second, P3b amplitude reduction showed a wide range of clinical correlates in the full sample, including greater negative and disorganized symptoms, worse global and social functioning, and less likelihood of recovery and remission. There is growing interest in psychiatry to identify alternative phenotypes grounded in neuroscience that link to meaningful outcomes (Insel et al., 2010), especially for psychosis (Tamminga et al., 2014). Based on our results, auditory target P3b amplitude, but not P3a amplitude, may represent a transdiagnostic marker of functioning and course in psychosis, including recovery, an outcome with virtually no known biological correlates. Third, P3b amplitude reduction correlated with clinical traits assessed several years earlier, especially negative symptoms and poor outcome. This suggests that P3b amplitude reduction reflects an enduring biological trait, rather than a state marker of illness, which has long been debated (Ford, 1999). Because biological factors influencing the course of psychosis are so poorly understood, longitudinal prospective investigations are needed to identify whether P3b amplitude predicts stable poor outcomes, or simply mirrors it.
There is a clear need to develop objective tools to aid in differential diagnosis in psychosis which is highly error-prone when based on cross-sectional information or short-term follow-up (Schwartz et al., 2000). Multi-wave follow-up can minimize misclassification, but is usually impractical in most clinical and research settings. That we found P3b amplitude reduction in cases of schizophrenia 15 years after initial misdiagnosis is consistent with the idea that P3b amplitude could be translated into a tool for helping distinguish schizophrenia from other psychotic disorders early in course.
Schizophrenia and other psychotic disorders may be characterized by somewhat distinct neural abnormalities. For example, reduced automatic attentional switching (i.e., reduced P3a) may represent a shared characteristic of psychotic disorders broadly, whereas reduced processing of saliency (i.e., reduced P3b) may be more characteristic of schizophrenia (Nieuwenhuis et al., 2005; Polich, 2007). In addition, P300 amplitude occurs during a period of cortical inhibition (Rockstroh et al., 1992), which may facilitate processing of a recent salient event at the cost of missing subsequent sensory information. Thus, it is possible that the neural mechanisms controlling cortical inhibition are somewhat faulty in schizophrenia but adequate in other psychotic disorders.
There are a number of limitations to the current study. First, larger samples are still needed to confirm our findings (e.g., all groups were comprised of 40–50 individuals). Second, our results may have been different had we examined an acutely ill population, rather than cases from a longitudinal cohort as their illness progressed. However, this sample is from a representative cohort, which allows us to generalize our findings to the general population of people who have been hospitalized for psychosis. Moreover, the longitudinal nature of the study provides a unique opportunity to improve diagnostic accuracy, as well as address long-standing questions regarding the link between P300 amplitude and recovery and remission. Third, although our retrospective correlations with clinical characteristics from early course point to long-standing neural deficits, we assessed event-related potentials in the second decade after psychosis onset and only once, and cannot thus distinguish whether P300 amplitude reduction is a preexisting risk factor with prognostic value or a consequence of chronic illness. However, converging lines of evidence suggest that P3b reduction may be a relatively stable trait-like biological marker of vulnerability to disease rather than a consequence of disease state. For example, P3b amplitude reduction presents in high-risk cases (Bramon et al, 2007) and unaffected biological relatives of cases (Ethridge et al., 2014). Fourth, we assessed P300 subcomponents using an auditory oddball paradigm, but other kinds of tasks or stimuli may yield different results. This particular auditory oddball task was chosen in part because meta-analysis has supported its use in schizophrenia research (Jeon & Polich, 2003).
Subcomponents of the target auditory oddball event-related potential may yield markers of psychosis (relative to never-psychotic), markers that distinguish between schizophrenia and other psychotic disorders, and markers for core clinical features of psychosis, such as functioning and recovery. Efforts to understand the link between P300 amplitude and psychosis (i.e., risk factors, consequences, and other models) may benefit from consideration of subcomponents underlying P300 amplitude, especially the P3b component. Present data suggest that P3b reduction may be a clinically useful biomarker of an enduring deficit in real-world functioning and illness course that may be apparent even before first hospitalization, and it may help to reduce misdiagnosis of new patients with psychotic disorders.
Acknowledgments
We want to thank Evelyn Bromet, cohort founder, and Al Hamdy, study interviewer.
Role of Funding Source
Financial Disclosures: National Institutes of Health (MH094398 to R.K.); Stony Brook University (Clinical Research Scholar Award to R.K.); Brain and Behavior Research Foundation (Young Investigator Grant to G.P.). These sponsors were not involved in design and conduct of the study, or collection, management, analysis, and interpretation of the data, or preparation, review, and approval of the manuscript. The first author takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors had full access to all the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
RK and GHP designed the study and wrote the protocol. GP undertook the analyses, literature review, and completed the first draft of the manuscript. GP, DF, RK, GHP, and EC contributed to revisions and have approved the final version.
Conflict of Interest
None
References
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) Iowa City: University of Iowa; 1983. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) Iowa City: University of Iowa; 1983. [Google Scholar]
- Andreasen NC, Carpenter WT, Jr, et al. Remission in schizophrenia: proposed criteria and rationale for consensus. The American journal of psychiatry. 2005;162(3):441–449. doi: 10.1176/appi.ajp.162.3.441. [DOI] [PubMed] [Google Scholar]
- Bramon E, McDonald C, et al. Is the P300 wave an endophenotype for schizophrenia? A meta- analysis and a family study. NeuroImage. 2005;27(4):960–968. doi: 10.1016/j.neuroimage.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Bramon E, Rabe-Hesketh S, et al. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophrenia research. 2004;70:2–3. 315–329. doi: 10.1016/j.schres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Bramon E, Shaikh M, Broome M, Lappin J, Bergé D, Day F, McGuire P. Abnormal P300 in people with high risk of developing psychosis. Neuroimage. 2008;41(2):553–560. doi: 10.1016/j.neuroimage.2007.12.038. [DOI] [PubMed] [Google Scholar]
- Bromet EJ, Kotov R, et al. Diagnostic shifts during the decade following first admission for psychosis. The American journal of psychiatry. 2011;168(11):1186–1194. doi: 10.1176/appi.ajp.2011.11010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder GE, Kayser J, et al. Event-related potentials in schizophrenia during tonal and phonetic oddball tasks: relations to diagnostic subtype, symptom features and verbal memory. Biological psychiatry. 2001;50(6):447–452. doi: 10.1016/s0006-3223(01)01168-4. [DOI] [PubMed] [Google Scholar]
- Dien J. Applying principal components analysis to event-related potentials: a tutorial. Developmental neuropsychology. 2012;37(6):497–517. doi: 10.1080/87565641.2012.697503. [DOI] [PubMed] [Google Scholar]
- Dien J, Spencer KM, et al. Parsing the late positive complex: mental chronometry and the ERP components that inhabit the neighborhood of the P300. Psychophysiology. 2004;41(5):665–678. doi: 10.1111/j.1469-8986.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- Ethridge LE, Hamm JP, Pearlson GD, Tamminga CA, Sweeney JA, Keshavan MS, Clementz BA. Event-Related Potential and Time-Frequency Endophenotypes for Schizophrenia and Psychotic Bipolar Disorder. Biological Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethridge LE, Hamm JP, Shapiro JR, Summerfelt AT, Keedy SK, Stevens MC, Clementz BA. Neural activations during auditory oddball processing discriminating schizophrenia and psychotic bipolar disorder. Biological psychiatry. 2012;72(9):766–774. doi: 10.1016/j.biopsych.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders—Patient Edition (SCID-I/P 2/2001 Revision) New York: Biometrics Research Department, New York State Psychiatric Institute; 2001. [Google Scholar]
- Ford JM, White PM, et al. ERPs in schizophrenia: effects of antipsychotic medication. Biological psychiatry. 1994;36(3):153–170. doi: 10.1016/0006-3223(94)91221-1. [DOI] [PubMed] [Google Scholar]
- Ford JM. Schizophrenia: the broken P300 and beyond. Psychophysiology. 1999;36(06):667–682. [PubMed] [Google Scholar]
- Foti D, Kotov R, et al. Beyond the broken error-related negativity: functional and diagnostic correlates of error processing in psychosis. Biological psychiatry. 2012;71(10):864–872. doi: 10.1016/j.biopsych.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D, Vaughan HG, Jr, et al. Stimulus and response related components of the late positive complex in visual discrimination tasks. Electroencephalography and clinical neurophysiology. 1978;45(3):319–330. doi: 10.1016/0013-4694(78)90184-0. [DOI] [PubMed] [Google Scholar]
- Frodl-Bauch T, Gallinat J, et al. P300 subcomponents reflect different aspects of psychopathology in schizophrenia. Biological psychiatry. 1999;45(1):116–126. doi: 10.1016/s0006-3223(98)00108-5. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and clinical neurophysiology. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Havermans R, Honig A, et al. A controlled study of temporal lobe structure volumes and P300 responses in schizophrenic patients with persistent auditory hallucinations. Schizophrenia research. 1999;38:2–3. 151–158. doi: 10.1016/s0920-9964(99)00006-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs DW, Hanlon TE, et al. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophrenia bulletin. 1984;10(3):388–398. doi: 10.1093/schbul/10.3.388. [DOI] [PubMed] [Google Scholar]
- Hermens DF, Ward PB, et al. Impaired MMN/P3a complex in first-episode psychosis: cognitive and psychosocial associations. Progress in neuro-psychopharmacology & biological psychiatry. 2010;34(6):822–829. doi: 10.1016/j.pnpbp.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Iwanami A, Suga I, et al. P300 component of event-related potentials in methamphetamine psychosis and schizophrenia. Progress in neuro-psychopharmacology & biological psychiatry. 1994;18(3):465–475. doi: 10.1016/0278-5846(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Jablensky A. Subtyping schizophrenia: implications for genetic research. Molecular psychiatry. 2006;11(9):815–836. doi: 10.1038/sj.mp.4001857. [DOI] [PubMed] [Google Scholar]
- Jeon YW, Polich J. Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophysiology. 2003;40(5):684–701. doi: 10.1111/1469-8986.00070. [DOI] [PubMed] [Google Scholar]
- Kaur M, Battisti RA, et al. MMN/P3a deficits in first episode psychosis: comparing schizophrenia-spectrum and affective-spectrum subgroups. Schizophrenia research. 2011;130:1–3. 203–209. doi: 10.1016/j.schres.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Kaur M, Battisti RA, et al. Neurophysiological biomarkers support bipolar-spectrum disorders within psychosis cluster. Journal of psychiatry & neuroscience: JPN. 2012;37(5):313–321. doi: 10.1503/jpn.110081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Guey LT, et al. Smoking in schizophrenia: diagnostic specificity, symptom correlates, and illness severity. Schizophrenia bulletin. 2010;36(1):173–181. doi: 10.1093/schbul/sbn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Drake RE, Sederer LI, et al. Science and Recovery in Schizophrenia. Psychiatr Serv. 2008:487–496. doi: 10.1176/ps.2008.59.5.487. [DOI] [PubMed] [Google Scholar]
- Liberman RP, Kopelowicz A, Ventura J, Gutkind D. Operational criteria and factors related to recovery from schizophrenia. International Review of Psychiatry. 2002;14(4):256–272. [Google Scholar]
- Light GA, Swerdlow NR, Thomas ML, Calkins ME, Green MF, Greenwood TA, Turetsky BI. Validation of mismatch negativity and P3a for use in multi-site studies of schizophrenia: characterization of demographic, clinical, cognitive, and functional correlates in COGS-2. Schizophrenia research. doi: 10.1016/j.schres.2014.09.042. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon DH, Ford JM, et al. Trait and state aspects of P300 amplitude reduction in schizophrenia: a retrospective longitudinal study. Biological psychiatry. 2000;47(5):434–449. doi: 10.1016/s0006-3223(99)00277-2. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Hoffman RE, Watson TD, Miller RM, Roach BJ, Ford JM. Neurophysiological distinction between schizophrenia and schizoaffective disorder. Frontiers in human neuroscience. 2009;3:70. doi: 10.3389/neuro.09.070.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell BF, Shenton ME, et al. The auditory N2 component in schizophrenia: relationship to MRI temporal lobe gray matter and to other ERP abnormalities. Biological psychiatry. 1993;34(1–2):26–40. doi: 10.1016/0006-3223(93)90253-a. [DOI] [PubMed] [Google Scholar]
- New Freedom Commission on Mental Health. Achieving the promise: Transforming mental health care in America, final report. Rockville, MD: U.S. Department of Health and Human Services; 2003. (DHHS Pub No SMA-03-3832). [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the P3, and the locus coeruleus–norepinephrine system. Psychological bulletin. 2005;131(4):510. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2007;118(10):2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, et al. P3 in schizophrenia is affected by stimulus modality, response requirements, medication status, and negative symptoms. Archives of general psychiatry. 1989;46(11):1035–1044. doi: 10.1001/archpsyc.1989.01810110077011. [DOI] [PubMed] [Google Scholar]
- Rockstroh B, Müller M, Cohen R, Elbert T. Probing the functional brain state during P300-evocation. Journal of Psychophysiology. 1992;6:175–175. [Google Scholar]
- Rushby JA, Barry RJ, et al. Separation of the components of the late positive complex in an ERP dishabituation paradigm. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2005;116(10):2363–2380. doi: 10.1016/j.clinph.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, Shenton ME, et al. First-episode schizophrenic psychosis differs from first-episode affective psychosis and controls in P300 amplitude over left temporal lobe. Archives of general psychiatry. 1998;55(2):173–180. doi: 10.1001/archpsyc.55.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Shenton ME, et al. P300 topography differs in schizophrenia and manic psychosis. Biological psychiatry. 1999;45(1):98–106. doi: 10.1016/s0006-3223(98)00208-x. [DOI] [PubMed] [Google Scholar]
- Schwartz JE, Fennig S, Tanenberg-Karant M, Carlson G, Craig T, Galambos N, Lavell J, Bromet EJ. Congruence of diagnoses 2 years after a first-admission diagnosis of psychosis. Archives of General Psychiatry. 2000;57(6):593–600. doi: 10.1001/archpsyc.57.6.593. [DOI] [PubMed] [Google Scholar]
- Sutton S, Ruchkin DS. The late positive complex. Advances and new problems. Annals of the New York Academy of Sciences. 1984;425:1–23. doi: 10.1111/j.1749-6632.1984.tb23520.x. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Pearlson G, Keshavan M, Sweeney J, Clementz B, Thaker G. Bipolar and Schizophrenia Network for Intermediate Phenotypes: Outcomes Across the Psychosis Continuum. Schizophrenia bulletin. 2014;40(Suppl 2):S131–S137. doi: 10.1093/schbul/sbt179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beijsterveldt CEM, Van Baal GCM. Twin and family studies of the human electroencephalogram: a review and a meta-analysis. Biological psychology. 2002;61(1):111–138. doi: 10.1016/s0301-0511(02)00055-8. [DOI] [PubMed] [Google Scholar]


