Abstract
Addiction is a widespread public health issue with social and economic ramifications. Substance abuse disorders are often accompanied by disruptions in circadian rhythms including sleep/wake cycles, which can exacerbate symptoms of addiction and dependence. Additionally, genetic disturbance of circadian molecular mechanisms can predispose some individuals to substance abuse disorders. In this review, we will discuss how circadian genes can regulate midbrain dopaminergic activity and subsequently, drug intake and reward. We will also suggest future directions for research on circadian genes and drugs of abuse.
Introduction
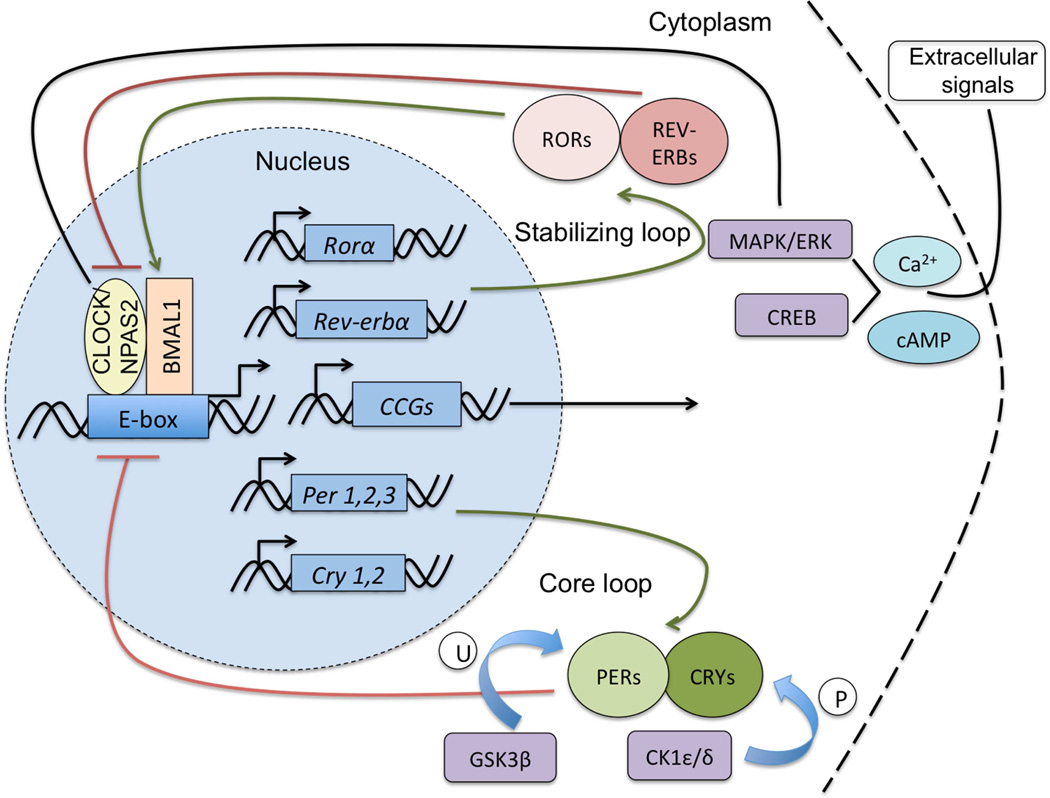
The majority of living organisms display daily cycles in behavior and physiology that enable them to adapt to their environment and react to a variety of stimuli known as zeitgebers or “time-givers” (e.g. light, food, etc.). Circadian rhythms enable organisms to adaptively entrain to their environmental conditions to optimize behavioral responses for survival. The central rhythm-generating nucleus in the mammalian brain is the suprachiasmatic nucleus (SCN) of the hypothalamus. Additional subsidiary oscillators have been identified in extra-SCN brain regions and peripheral tissues and these can be coordinated by the SCN or independently controlled (1). Circadian molecular clock machinery is present in all cell types throughout the body. This mechanism consists of an interconnected series of core and accessory transcriptional-translational feedback loops modulated by regulatory kinases (see fig. 1). The activity of the clock components is regulated over a diurnal timescale. Integral to the mammalian circadian clock are the transcription factors, Circadian Locomotor Output Cycles Kaput (CLOCK), or Neuronal PAS Domain Protein 2 (NPAS2) and Brain and Muscle Arnt-like Protein 1 (BMAL1). These proteins heterodimerize and promote transcription of the Period (Per1, Per2, Per3) and Cryptochrome (Cry1, Cry2) genes. Throughout the 24-hour day, PER and CRY proteins in turn are phosphorylated and feed back into the nucleus to inhibit the transcriptional activity of the CLOCK/NPAS2-BMAL1 complex, and hence their own expression. CLOCK/NPAS2 and BMAL1 additionally regulate the transcription of many other genes by binding to E-box elements in their promoter regions. Among these clock-controlled genes are those that underlie aspects of neuronal signaling in mesolimbic systems involved in reward processing and the development of addictive behaviors (2–6).
Figure 1.
A series of transcriptional and translational feedback loops comprise the core molecular clock in mammals. At the heart of the clock are the transcription factors CLOCK (or NPAS2) and BMAL1 which heterodimerize in the nucleus and bind to Enhancer Box (E-box) sequences in many genes to regulate their transcription. Targets include the Per and Cry genes. Over the course of 24 hours, PER and CRY proteins dimerize and shuttle back into the nucleus where CRY directly inhibits the CLOCK/NPAS2-BMAL1 complex forming a negative feedback mechanism. Additionally, CLOCK/NPAS2 and BMAL1 also regulate the expression of the nuclear hormone receptors Rev-erbα and Rorα, which can repress or activate Bmal1 transcription. Other regulatory proteins act on the molecular clock through phosphorylation including Casein kinase 1 (CK1) proteins and ubiquitination by Glycogen synthase kinase beta (GSK3β). Intracellular calcium signaling cascades can also act to regulate the activity of core circadian proteins through kinase-dependent pathways. CCGs, Clock controlled genes; P, phosphorylation; U, ubiquitination.
In addition to the SCN, midbrain and forebrain regions express molecular clock elements at the cellular level and are also indirectly connected with the SCN through anatomical projections (see fig. 2). Mesocoritcolimbic brain circuitry has been shown to be important for the processing of rewarding stimuli, including drugs of abuse, which can remodel the system to cause addiction in vulnerable individuals. Major components of this circuitry that are important for alcohol responses include the ventral tegmental area (VTA), nucleus accumbens (NAc), amygdala, hippocampus, and medial prefrontal cortical regions. Koob and Volkow (2010) review decades of clinical and pre-clinical studies showing that discrete aspects of mesocorticolimbic circuitry are engaged during binge drug use, withdrawal/negative affect, and relapse, encompassing all stages of the addiction cycle (7). Much progress has been made in the identification of molecular and physiological adaptations that underlie substance use disorders. The neurotransmitter dopamine (DA) features prominently in the behavioral response to drugs of abuse as well as natural rewards. Activation of the midbrain DA system can confer incentive salience to environmental stimuli and promote motivational or goal-directed behavior (8, 9). The time course of this signaling has been shown to correspond to reward value and predicted outcomes (10–12). This role of dopamine goes beyond serving a hedonic purpose to one that motivates behavior in the direction of obtaining a pleasurable substance, as dopamine depletion does not abolish unconditioned affective reaction patterns to sucrose and quinine (8). These principles also support a reinforcement learning model of dopamine action, which contributes to goal-oriented behavior (13). Reinforcement learning models help explain the unique advantage of addictive drugs over natural reinforcers in that rapid pharmacokinetic and prolonged effects of drugs on dopamine release may promote overlearning on drug-related stimuli including cues (13, 14). Elements of the dopaminergic system and reward have been shown to be under circadian regulation. Diurnal variations observed in the rewarding value of natural and drug reinforcers suggest that within distinct regions of the mesocorticolimbic system, rhythms in expression of circadian and dopamine-related proteins may coincide with rhythms in reward behavior to promote dependence (15, 16). A conceptual model of the interaction between circadian misalignment, mesocorticolimbic circuitry and the development of alcohol use disorders (AUDs) in adolescents has been proposed by Hasler & Clark (2013)(17). In this review we will highlight a number of recent studies providing strong evidence that circadian genes regulate several aspects of dopaminergic transmission.
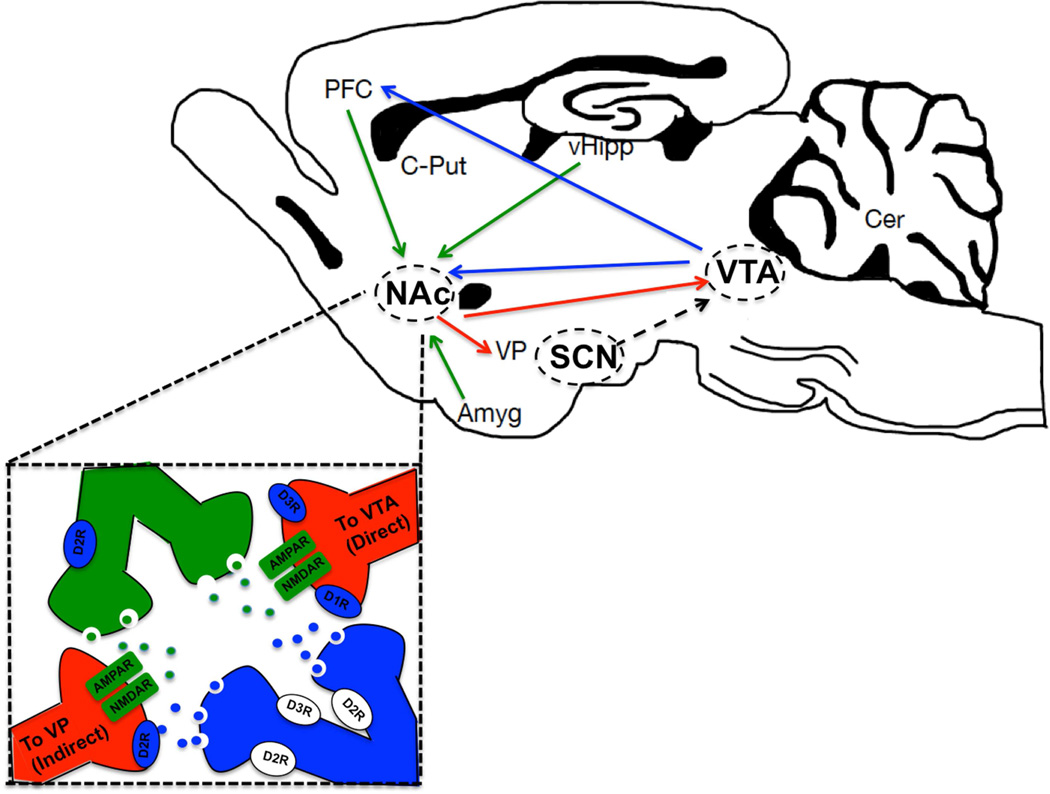
Figure 2.
The master clock, the supraciasmatic nucleus (SCN), projects indirectly to mesolimbic brain regions through three distinct hypothalamic/epithalamic nuclei including the medial pre-optic area (mPOA), the dorsomedial hypothalamus (DMH) and the lateral habenula (LHb). These in turn project to the ventral tegmental area (VTA) to mediate reward and motivational behavior. The VTA sends a dense dopaminergic projection (blue) to the nucleus accumbens (NAc) within the ventral striatum as well as the prefrontal cortex (PFC) in theforebrain. Additional inputs to the NAc include glutamatergic afferents (green) from the PFC, vental hippocampus (vHipp) and Amygdala (Amyg). This GABAergic nucleus sends a reciprocal projection (red) to the VTA through the “direct” pathway and projects to the ventral pallidum (VP) through the “indirect pathway.” NAc medium spiny neurons (MSNs) differentially express dopamine receptors and are critical in mediating responses to drugs of abuse and rewarding stimuli. C-put, caudate putamen; Cer, cerebellum.
Although the master pacemaker is located in the SCN, circadian genes and proteins are widely expressed throughout the brain and periphery, thereby forming SCN-independent pacemakers that entrain to other non-photic stimuli including food and drugs (18, 19). Many studies have established that addictive drugs are able to serve as zeitgebers and can reliably entrain anticipatory activity rhythms in animals when given regularly. This locomotor activity has been likened to the seeking behavior characteristic of drug addiction. Additionally, the circadian regulation of dopamine transmission and signaling plays a role in reward (20). For example, daily methamphetamine injections have been shown to entrain animals and induce anticipatory locomotor activity to the time of injection (21). Ethanol, cocaine, and nicotine have also been shown to induce this anticipatory behavior and alter behavioral rhythms (20, 22, 23). In rodents with SCN lesions methamphetamine in the drinking water restores activity rhythms in a robust manner (24). In addition, methamphetamine treatment shifts the expression of the Per genes in striatal regions in a manner that matches shifts in activity rhythms, independent of the SCN rhythms (18). Rewarding stimuli such as food or chocolate can entrain both behavioral and Per1 expression rhythms, which persist for several days in several brain regions (including dorsal medial hypothalamus, nucleus accumbens, prefrontal cortex, and the central amygdala) (25). Therefore, both behavioral and molecular rhythms appear to be affected by rewarding stimuli, including drugs of abuse.
Alcohol can disrupt circadian rhythms and circadian disruption can promote alcohol intake (26). Decades of research support the notion that there exists a bidirectional relationship between alcohol abuse and circadian rhythm disruptions. However, how alcohol abuse and circadian rhythm disruptions interact as risk factors for one another remains unclear. Alcoholism in human populations is associated with disruptions in circadian rhythms, which can persist during abstinence and increase risk for relapse (27–31). Moreover, circadian gene disruptions may be a risk factor for addiction. Genetic variations (single nucleotide polymorphisms or mutations) in Clock, Per2 and Per3 genes have been associated with alcoholism in humans (32, 33). Additionally, mice and rats selectively bred for high alcohol consumption have altered circadian phenotypes (34–36). Taken together, these studies suggest shared genetic linkage between alcohol-related behaviors and the circadian genes. These studies are well reviewed and conceptually integrated by Logan et al. (2014) (37).
Here, we will discuss how neurotransmitter systems and reward circuitry are under circadian control and modulated by drug and alcohol experience, as well as how specific circadian genes regulate drug and alcohol responses. We take note that studies on the role of circadian genes in the response to drugs of abuse, such as cocaine, far outnumber alcohol-focused studies. Hence, we encourage further study of the interactions between alcohol, circadian rhythms, and circadian genes in a systematic manner.
Alcohol disrupts circadian rhythms
Studies have shown that chronic alcohol can disrupt the circadian pattern in a variety of hormonal and behavioral rhythms (20, 26, 38–41). High levels of alcohol can affect biological rhythms such as disruption of circadian sleep rhythms (42, 43) and spontaneous locomotor activity (44). The SCN is responsive to ethanol in that acute ethanol can prevent photic resetting and chronic ethanol disrupts photic entrainment (45–48). Additionally, studies have illustrated that the SCN develops rapid tolerance to the effects of EtOH (49, 50).
Kakihana et al. (1976) (38) demonstrated that chronic consumption of alcohol dampens the circadian rhythm of ACTH release, as measured by plasma corticosterone levels in mice. Other studies have shown that chronic ethanol treatment reduces the circadian variations in plasma corticosterone concentration (51) and gonadotrophin production in females (52). Furthermore, it reduces the number of vasopressin (AVP), vasoactive intestinal polypeptide (VIP), gastrin-releasing peptide (GRP), and somatostatin (SST) - containing neurons in the SCN of Wistar rats (39). Chronic ethanol can also alter the characteristics of biochemical circadian rhythms in Wistar rats. Chronic alcohol delayed peak times of glucose, potassium and lactic acid rhythms (by 18 h, 3 h, and 3 h respectively), and advanced peak times of cholesterol rhythms by 3 h and 9 h respectively during ethanol treatment(40). Significant changes in the range and mean of all the biochemical circadian rhythms studied were also observed during ethanol treatment.
Alcohol disrupts circadian gene expression
Relatively few human studies have examined the effects of alcohol on circadian gene expression. Huang et al. (2010) (53) assessed the mRNA levels (from peripheral blood mononuclear cells) of circadian genes in patients with alcohol dependence and undergoing alcohol-withdrawal treatment. mRNA levels of Clock were markedly lower in alcohol dependent subjects upon admission to treatment and lasting at least one week into alcohol withdrawal. McCarthy et al. (2013) (54) preformed a study to determine if alcohol dependent subjects have altered Per2 expression rhythms in peripheral tissues. In this study, skin fibroblasts were collected from alcohol dependent (and control) subjects for culture and use in a bioluminescent reporter gene (Per2::luciferase) assay to measure circadian rhythms in gene expression for 5 days. They found that the Per2::Luc period was inversely correlated with illness severity (defined as the number of alcohol dependence criteria met). More studies are needed to determine whether changes in molecular rhythms are consistent across studies and/or if they vary depending on cell type or physiological state. It would be of great interest to the field if future studies included a treatment group (i.e. subjects receiving an FDA approved treatment for alcohol dependence) to determine whether alterations in molecular rhythms can be rescued as individual symptoms improve.
There are also relatively few studies reporting the effect of alcohol intake on circadian gene expression in animal models. Observed changes in molecular rhythms depend on the alcohol paradigm, length and dose of alcohol exposure, and tissue type. Melendez et al. (2012) identified gene expression changes (using microarrays) in several brain regions in response to chronic intermittent ethanol (CIE), a paradigm known to induce dependence-like behaviors in mice. Results of this study indicate that the most differentially expressed genes in the NAc were involved in circadian rhythms (55). Recently, we found that chronic alcohol consumption (in a two bottle choice paradigm) and abstinence resulted in decreased Clock expression in the NAc and VTA (56). Moreover, Chen et al. (2004) (57) found that chronic ethanol intake via liquid diet shifted Per1 and Per2 gene expression rhythms in the arcuate nucleus (an area that sends projections to the NAc). Reports on the effects of chronic alcohol intake on circadian gene expression in the SCN are quite variable and discussed in Logan et al. (2014) (37). To date, there are no studies examining the diurnal expression of circadian genes in both the SCN and the mesocorticolimbic brain regions known to be important for alcohol intake. Thus, it is important to acknowledge that changes in peripheral gene expression may have little or no connection to potential changes in the SCN circadian clock. It will be important for future studies to characterize the effects of alcohol on diurnal expression of circadian genes in the SCN, as well as in mesocorticolimbic regions.
Given these limited human and animal model studies, it will be important to perform studies to measure circadian gene expression from post-mortem brain tissue of alcohol dependent individuals (and controls), ideally collected at several circadian time points to determine rhythmicity and duration parameters. The circadian field is moving forward with the identification of novel protein-protein and protein-DNA interactions, illuminating interactions between circadian, neurotransmission, metabolic, and immune signaling (58–61). These types of studies will guide our interpretation of the extra-SCN role of circadian genes in the context of the neuronal adaptations seen in reward-related brain circuitry with alcohol dependence.
Circadian genes regulate behavioral responses to drugs of abuse
Genetic animal models have also revealed that circadian genes are important regulators of behavioral responses to drugs of abuse. The first studies to reveal this relationship were performed in Drosophila melanogaster and showed that flies bearing mutations in the circadian genes Clock, Per, Cycle, or Doubletime all fail to sensitize to cocaine (62). Later, Pohl and colleagues (2013) tested several circadian mutations in Drosophila melanogaster, and found that mutations in Per, Tim, and Cyc completely block the development of ethanol tolerance. Importantly, Pohl et al. (2013) revealed that the ability of these genes to abolish ethanol tolerance was independent of a nonfunctional circadian clock (63). Abarca et al.(2002) (64) found that mPer1 null mutant mice also fail to sensitize to cocaine and exhibit increased cocaine conditioned place preference (CPP), a measure of cocaine reward. In contrast, mPer2 null mutants exhibit hypersensitization to cocaine and normal levels of cocaine CPP. In addition, studies show that mPer1 might partially regulate morphine dependence, as mutants for this gene show a reduction in morphine CPP (65). Furthermore, Per2Brdm mutants are hypersensitive to ethanol, exhibiting increased ethanol preference and consumption, increased sedation, and decreased hypothermia (33, 66). Intriguingly, reports are inconsistent for ethanol intake measures in Per1 mutant mice, where ethanol selfadministration measures (operant and voluntary) and sedation have been reported as increased or not significantly different than WT mice (6, 67). Incongruent findings such as this can be due to the use of different strain backgrounds, as well as possible gender differences and testing conditions across laboratories (68).
Several studies from our lab and others have shown that Clock can act as a negative regulator of drug reward. McClung et al. (2005) and Ozburn et al. (2012, 2013) identified a key role for the Clock gene in drugs of abuse. Mice bearing a dominant negative mutation in Clock (ClockΔ19 mice) exhibit increased cocaine CPP compared with wildtype (WT) littermates (3, 69). Ozburn et al. (2012) examined whether results from the conditioned reward study were relevant to cocaine intake using a clinically relevant operant intravenous cocaine self-administration paradigm (IVSA). We found that WT mice exhibited a diurnal variation in acquisition and maintenance of drug intake that is absent in ClockΔ19 mice. A greater percentage of Clock mutant mice acquired cocaine self-administration, regardless of time of day tested. Furthermore, mutant mice self-administered more cocaine than WT mice. Using fixed ratio (to assess sensitivity to reinforcing properties of cocaine) and progressive ratio (to assess motivation for cocaine) schedules of reinforcement dose-response paradigms, Ozburn et al. (2012) (69) also found that cocaine is a more efficacious reinforcer in ClockΔ19 mice than in WT mice. Importantly, this study found that ClockΔ19 mice exhibited similar learning and readily acquired food self-administration, indicating the mutation does not have an effect on learning.
In addition to this cocaine phenotype, Clock mutants exhibit increased locomotor activity, reduced anxiety-like and depression-like behavior, and increased intracranial self-stimulation (ICSS) at a lower threshold (70, 71). ClockΔ19 mutants also have an increase in dopaminergic activity in the ventral tegmental area (VTA) and a general increase in glutamatergic tone that might underlie these behaviors (3, 72). Many of these behavioral and physiological phenotypes are rescued by expressing functional CLOCK in the VTA of ClockΔ19 mutants or are recapitulated by reducing Clock expression in the VTA of wild type mice via RNA interference (71, 73). Ozburn et al. (2013) found that the hyperhedonic phenotype of ClockΔ19 mutants extended to a very different class of drug (ethanol) (56). ClockΔ19 mutants exhibited significantly increased ethanol intake in a continuous access two-bottle choice paradigm. This effect was more robust in female mice. Moreover, chronic ethanol experience resulted in a long-lasting decrease in VTA Clock expression. Furthermore, they found that reducing Clock expression in the VTA (via RNAi using viral mediated gene transfer) resulted in significantly increased ethanol intake similar to the ClockΔ19 mice (56). They also found that ClockΔ19 mice exhibit significantly augmented responses to the sedative effects of ethanol and ketamine, but not pentobarbital. However, their drinking behavior was not affected by acamprosate, an FDA-approved drug for the treatment of alcoholism, suggesting that their increased glutamatergic tone might underlie the increased sensitivity to the sedative/hypnotic properties of ethanol but not its rewarding properties. Taken together, these studies identified a significant role for Clock in the VTA as a negative regulator of drug and alcohol reinforcement, where decreased CLOCK function increases vulnerability for drug and alcohol use. Furthermore, we implicate the VTA dopamine system in this response.
The dopaminergic reward circuit
Drugs of abuse including alcohol, cocaine, methamphetamine, and opioids act directly on the dopamine system as well as other signaling pathways to promote seeking behavior. Generally, these substances elicit their effects by increasing dopamine release from the VTA to target regions including the NAc, which is critically positioned to integrate limbic information for the generation of motivational action (9, 74–76). Genetic studies in rodent models highlight the importance of dopamine signaling in the development of addictive behavior. Mice with a complete knock out of D1-type dopamine receptors exhibit reduced alcohol intake and preference and do not self-administer cocaine (77). Human imaging studies also support a key role of DA suggesting that fast DA changes are associated with the subjective perception of reward (78, 79). The DA system is activated by acute administration of all addictive drugs but the system does not necessarily account for all reinforcing effects of drugs.
While it has been established that ethanol affects neural function by modulating various neurotransmitter systems including GABA, glutamate and norepinephrine (80–82), attention over the past few decades has focused on the DA system, which has been shown to mediate positive reinforcing effects of ethanol. Behavioral pharmacology of ethanol reinforcement has been elucidated through the use of operant procedures. Systemic administration of dopaminergic agents including receptor subtype-specific agonists and antagonists alter responding for ethanol reinforcement suggesting a role for the signaling system in modulating voluntary ethanol drinking behavior (83). Additionally, electrophysiological studies point to a stimulatory action of ethanol on VTA DA neuronal firing rate through an array of mechanisms, including ionic and synaptic effects, and a marked decrease in dopaminergic activity in ethanol-withdrawn animals (84–87). Sophisticated experiments in vitro have revealed that ethanol-induced excitation of VTA DA neurons is modulated by GABAergic transmission in the nucleus and produces phases of excitation and inhibition (88). Recently, the specific relationship between VTA DA firing patterns leading to differential accumbal dopamine release in the NAc terminal field and ethanol drinking behaviors has been investigated with the use of optogenetic techniques. Bass et al. (2013) found that tonic but not phasic dopamine transmission reduces ethanol self-administration in rats presumably through a dopamine D2 autoreceptor-mediated feedback mechanism (89). Within the NAc, drugs of abuse elicit striking changes in plasticity of excitatory and inhibitory synaptic signaling onto GABAergic medium spiny neurons (MSNs), the principal projection neurons of this region. Persistent psychostimulant-induced adaptations in the NAc are thought to underlie drug dependence and the functional differences between D1R and D2R expressing MSNs in reward behavior have been investigated using genetic strategies (90, 91). With regard to ethanol-induced synaptic alterations in MSNs, Jeanes et al. (2014) recently described cell-type specific effects of chronic intermittent ethanol (CIE) exposure on NMDAR-LTD in the NAc shell (92). These and many other studies point to long-lasting cellular effects of psychostimulants and ethanol on excitatory neurotransmission in the VTA and NAc as a result of changes in firing of mesolimbic dopaminergic neurons (93–96). Transition to addiction involves neuroplasticity in all limbic regions and is thought to develop through cascades of dysfunction beginning with dopamine signaling in the VTA and affecting target regions including the ventral striatum, dorsal striatum, OFC, PFC, and amygdala and facilitating the transition from use to abuse to dependence (7).
Circadian genes and dopaminergic reward circuitry
Several studies have highlighted the role of circadian genes in the direct regulation of dopaminergic reward circuitry. Within mesolimbic nuclei, virtually all aspects of dopaminergic activity including neuronal firing patterns, neurotransmitter synthesis, release, degradation and postsynaptic actions are subject to circadian transcriptional influence and display diurnal variation (97). This regulation of signaling plays a role in reward-related behavior as all drugs of abuse exert their actions by impinging on dopaminergic circuitry and any disruption of this system may increase vulnerability to the rewarding properties of drugs. Additionally, diurnal variation in dopaminergic neuronal activity may underlie the diurnal variation in behavioral responses to drugs as previously described. Indeed, within the VTA, rhythms have been observed in the expression of DA receptors as well as tyrosine hydroxylase (TH) and monoamine oxidase (MAOA), the enzymes responsible for the synthesis and degradation of DA respectively (3, 97–101). There is also evidence to support the idea that these regulatory genes may be clock-controlled genes (CCGs), as they contain canonical E-box sites in their promoter regions and are bound by CLOCK and BMAL1 (Fig.3) (99, 100).
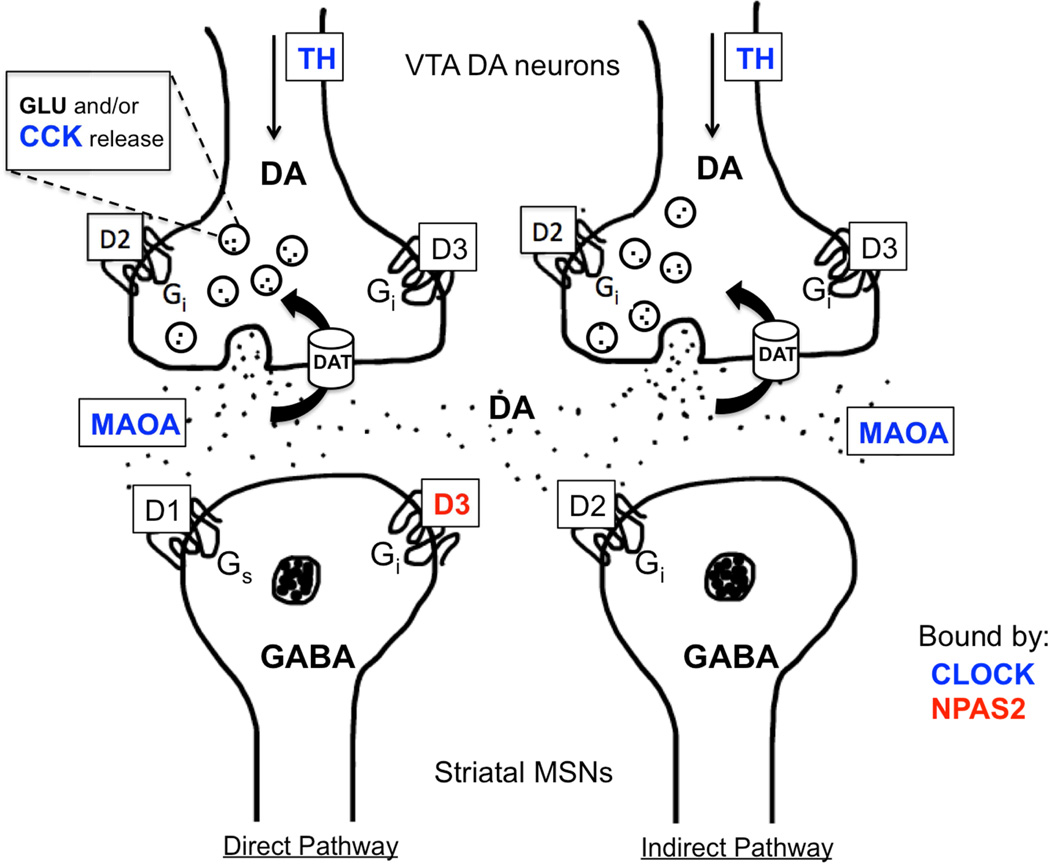
Figure 3.
Transcriptional targets of CLOCK and NPAS2 include a variety of genes that regulate dopamine (DA) synthesis, release, uptake and transmission within key reward circuitry. At the site of presynaptic DA release from VTA neurons onto postsynaptic GABAergic medium spiny neurons (MSNs) of the NAc, a variety of mechanisms are under circadian transcriptional control by direct binding of CLOCK to DA-related genes. Presynaptically, CLOCK negatively regulates the transcription of tyrosine hydroxylase (TH) to affect DA synthesis. CLOCK also positively regulates the activity of the neuropeptide, CCK, which negatively influences DA output and MAOA, the enzyme responsible for the breakdown of DA in the synaptic space. CLOCK and NPAS2 differentially regulate DA transmission as NPAS2 has been found to specifically regulate expression of Drd3. Functional studies support the role of VTA Clock and striatal Npas2 as negative and positive regulators or drug reward respectively.
A critical role of core circadian proteins in the regulation of CCGs in the midbrain has been elucidated through studies of clock gene mutations. Clock is expressed in the VTA and NAc and is implicated in the modulation of reward processing and moodrelated behavior (3, 102). Abnormal mesolimbic signaling has been well characterized in ClockΔ19 mutant mice. ClockΔ19 mice exhibit heightened sensitivity to rewarding substances compared with WT mice suggesting aberrant reward processing (3, 69). We performed a microarray analysis several years ago and found differential regulation of several key dopamine-related genes within the VTA of Clock mutants (3). Moreover, there is an increase in the mean firing rate and bursting of DA neurons, which can be normalized by chronic lithium treatment, as well as an increase in TH mRNA expression (3, 103). The notion that CLOCK directly regulates the activity of key mechanisms in DA transmission within the VTA is further supported by the finding that CLOCK short-hairpin RNA delivered specifically to this nucleus recapitulates the abnormal signaling in WT mice including increased DA release into the NAc (73). These findings provide mechanistic insight into the circadian transcriptional control of reward processing and suggest a unique function of CLOCK as an inhibitor of TH transcription in contrast to its widespread activational role throughout the brain and periphery. Recently, a role for the circadian nuclear receptor, REV-ERBα, in the repression of TH within the ventral midbrain has been elucidated, highlighting the importance of regulatory circadian proteins in transcriptional control of DA-related genes (105). Mutant studies have also shed light on various other dopamine-related CCGs including the neuropeptide cholecystokinin (Cck), which serves as a negative regulator of DA transmission in vivo (106, 107). ClockΔ19 mice have reduced levels of Cck mRNA. Furthermore, knockdown of Cck specifically in the VTA is sufficient to recapitulate a manic-like behavioral phenotype in WT mice (58). Results from this study support the role of CLOCK as a direct positive regulator of Cck, which critically modulates DA output and reward-related responses. Other neurotransmitter signaling systems are also thought to be altered following clock gene disruptions including the glutamatergic system, which appears to regulate aspects of increased sensitivity to ethanol and ketamine in ClockΔ19 mice (56). Other studies have implicated altered glutamatergic tone in the Clock mutants in the increased drug preference phenotype and have shown that functional CLOCK and PER2 are essential to maintain normal glutamate levels and uptake by transporters (72, 108, 109). Results of microarray studies in Clock mutants and following Clock knock down also demonstrate a downregulation of GABAergic genes in the VTA suggesting a potential dampening of inhibitory control and disinhibition of DA neuronal activity however this relationship could be investigated directly in the future through electrophysiological studies of VTA DA neurons (3).
In addition to circadian gene regulation of processes involved in DA production and release from VTA cell bodies, several studies highlight the circadian control of other aspects of dopamine signaling in afferent target regions of the VTA. Chief among these and relevant to reward processing and motivated behavior is the striatum and predominantly the NAc. NAc MSNs are a critical site for drug-induced plastic changes as DA reliably modulates glutamatergic transmission at excitatory synapses in the region through DA receptor signaling pathways (110). DA receptor expression has been shown to be rhythmic and canonical E-box sites are present in the Drd1 and Drd2 genes, which are differentially expressed by NAc MSNs, suggesting that these are also direct CCGs and may be involved in mediating drug responses (111). Interestingly, within the NAc, NPAS2 is highly enriched (112) and NPAS2 has recently been shown to be critical for mediating cocaine CPP (Ozburn et al., in press). Npas2 mutant mice have a decrease in cocaine CPP and this can be recapitulated via selective knock-down of Npas2 only in the NAc (Ozburn et al., in press). In contrast, knock-down of Clock in the NAc has no effect. Additionally, Npas2 knock down in the NAc disrupts the diurnal expression of Drd1, Drd2 and Drd3 with greatest effect on the latter (Ozburn et al., in press). The prominent increase in Drd3 gene expression in Npas2 knock-down mice may have implications for DA signaling through postsynaptic mechanisms that are relevant for reward processing (113). Although alcohol related behaviors have not been reported for Npas2 mutants, specific inhibitors of the D3 receptor reduce operant alcohol self-administration and reinstatement of alcohol seeking behaviors (114). While CLOCK and NPAS2 are generally considered homologous in structure and function, we have uncovered differential cell-type specific expression of the two transcription factors in the striatum with NPAS2 being highly enriched in D1-containing MSNs while CLOCK expression is more ubiquitous (Ozburn et al., in press). As activation of the D1-direct pathway is associated with learning positive reinforcers, this points to a functional distinction between circadian transcriptional mechanisms in reward relevant circuitry (115). With regard to behavioral discrepancies observed between Clock and Npas2 manipulation in the NAc, we propose that CLOCK regulates drug reward primarily through actions on DA transmission in the VTA while NPAS2 acts primarily within the NAc. However, since the VTA releases dopamine into the NAc, mutations of Clock in the VTA affect the function of the NAc over time. Since Clock gene disruption in the ClockΔ19 model leads to changes in DA transmission in the striatum, a potential consequence of increased dopaminergic tone in Clock mutants is a decrease in D1:D2 receptor ratio in the striatum due to a strong upregulation of D2 receptor expression, which could reflect a compensatory mechanism to counteract dysregulated DA transcription and release (116). Additionally, Dzirasa et al (2010) have characterized specific abnormalities in the NAc of Clock mutants including reduced levels of the AMPA-type glutamate receptor subunit GluA1 and phospho-GluA1 as well as altered signaling between the NAc and prefrontal regions correlated with exploratory drive behavior. These changes among others suggest a potential shifting of excitatory synaptic weight in the NAc, which could be a result of the mutant hyperdopaminergic state (109). A similar homeostatic mechanism may be at play in Per2 mutant mice, which exhibit lower Maoa expression in the NAc accompanied by higher DA levels. In these mice, the ratio of D1 to D2 receptors is also significantly decreased (100). These findings suggest that clock gene disruptions impact DA signaling at multiple levels and through a variety of mechanisms likely contributing to abnormal behavior. Further studies will be necessary to determine the extent to which these disruptions directly affect CCGs and may indirectly impact plasticity in afferent signaling pathways thus altering responses to drugs of abuse and mediating addictive behavior.
Conclusions
Circadian rhythm disruptions and addiction vulnerability go hand in hand. Moreover, chronic exposure to alcohol and other substances leads to lasting changes in rhythms that contribute to the cycle of addiction and relapse. Recent studies have determined that genes that control circadian rhythms are keenly involved in regulating the dopaminergic reward circuitry and this regulation may be the cause of this increase in vulnerability and the plasticity that contributes to addiction. Results of these investigations may uncover new therapeutic targets aimed at the treatment of substance abuse disorders and also inform optimal administration times for existing therapeutic agents. This is important given the strong circadian rhythms in dopamine receptors for example if these receptors are to be targeted for drug development. It would also be important to identify compounds which increase rhythm stability but do not reinforce the entrainment of the circadian system to drugs of abuse within reward-related pathways. There is much we still need to learn about how these diurnal rhythms are involved in drug craving, seeking and sensitization or tolerance.
Acknowledgements
Studies from our group were supported by NIDA (DA023988 to CAM), NIMH (MH082876 to CAM), NIAAA (AA020452 to ARO), and NARSAD Young Investigator Award (to ARO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
References
- 1.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 2.Vitaterna MH, Ko CH, Chang AM, Buhr ED, Fruechte EM, Schook A, et al. The mouse Clock mutation reduces circadian pacemaker amplitude and enhances efficacy of resetting stimuli and phase-response curve amplitude. Proc Natl Acad Sci U S A. 2006;103:9327–9332. doi: 10.1073/pnas.0603601103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, et al. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci U S A. 2005;102:9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci U S A. 2002;99:9026–9030. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Logan RW, McCulley WD, 3rd, Seggio JA, Rosenwasser AM. Effects of withdrawal from chronic intermittent ethanol vapor on the level and circadian periodicity of running-wheel activity in C57BL/6J and C3H/HeJ mice. Alcohol Clin Exp Res. 2012;36:467–476. doi: 10.1111/j.1530-0277.2011.01634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gamsby JJ, Templeton EL, Bonvini LA, Wang W, Loros JJ, Dunlap JC, et al. The circadian Per1 and Per2 genes influence alcohol intake, reinforcement, and blood alcohol levels. Behav Brain Res. 2013;249:15–21. doi: 10.1016/j.bbr.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 9.Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- 10.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 11.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 12.Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- 13.Montague PR, Hyman SE, Cohen JD. Computational roles for dopamine in behavioural control. Nature. 2004;431:760–767. doi: 10.1038/nature03015. [DOI] [PubMed] [Google Scholar]
- 14.Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- 15.Webb IC, Baltazar RM, Wang X, Pitchers KK, Coolen LM, Lehman MN. Diurnal variations in natural and drug reward, mesolimbic tyrosine hydroxylase, and clock gene expression in the male rat. J Biol Rhythms. 2009;24:465–476. doi: 10.1177/0748730409346657. [DOI] [PubMed] [Google Scholar]
- 16.Baltazar RM, Coolen LM, Webb IC. Diurnal rhythms in neural activation in the mesolimbic reward system: critical role of the medial prefrontal cortex. Eur J Neurosci. 2013;38:2319–2327. doi: 10.1111/ejn.12224. [DOI] [PubMed] [Google Scholar]
- 17.Hasler BP, Clark DB. Circadian misalignment, reward-related brain function, and adolescent alcohol involvement. Alcohol Clin Exp Res. 2013;37:558–565. doi: 10.1111/acer.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iijima M, Nikaido T, Akiyama M, Moriya T, Shibata S. Methamphetamine-induced, suprachiasmatic nucleus-independent circadian rhythms of activity and mPer gene expression in the striatum of the mouse. Eur J Neurosci. 2002;16:921–929. doi: 10.1046/j.1460-9568.2002.02140.x. [DOI] [PubMed] [Google Scholar]
- 19.Stephan FK. Phase-Shifts of Circadian-Rhythms in Activity Entrained to Food Access. Physiology & Behavior. 1984;32:663–671. doi: 10.1016/0031-9384(84)90323-8. [DOI] [PubMed] [Google Scholar]
- 20.Kosobud AEK, Gillman AG, Leffel JK, Pecoraro NC, Rebec GV, Timberlake W. Drugs of abuse can entrain circadian rhythms. The Scientific World Journal. 2007;7:203–212. doi: 10.1100/tsw.2007.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kosobud AEK, Pecoraro NC, Rebec GV, Timberlake W. Circadian activity precedes daily methamphetamine injections in the rat. Neuroscience Letters. 1998;250:99–102. doi: 10.1016/s0304-3940(98)00439-x. [DOI] [PubMed] [Google Scholar]
- 22.Gillman AG, Kosobud AEK, Timberlake W. Pre- and post-nicotine circadian activity rhythms can be differentiated by a paired environmental cue. Physiology and Behavior. 2008;93:337–350. doi: 10.1016/j.physbeh.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White W, Feldon J, Heidbreder CA, White IM. Effects of administering cocaine at the same versus varying times of day on circadian activity patterns and sensitization in rats. Behavioral Neuroscience. 2000;114:972–982. doi: 10.1037//0735-7044.114.5.972. [DOI] [PubMed] [Google Scholar]
- 24.Masubuchi S, Honma S, Abe H, Ishizaki K, Namihira M, Ikeda M, et al. Clock genes outside the suprachiasmatic nucleus involved in manifestation of locomotor activity rhythm in rats. European Journal of Neuroscience. 2000;12:4206–4214. [PubMed] [Google Scholar]
- 25.Ángeles-Castellanos M, Salgado-Delgado R, Rodríguez K, Buijs RM, Escobar C. Expectancy for food or expectancy for chocolate reveals timing systems for metabolism and reward. Neuroscience. 2008;155:297–307. doi: 10.1016/j.neuroscience.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Spanagel R, Rosenwasser AM, Schumann G, Sarkar DK. Alcohol consumption and the body's biological clock. Alcohol Clin Exp Res. 2005;29:1550–1557. doi: 10.1097/01.alc.0000175074.70807.fd. [DOI] [PubMed] [Google Scholar]
- 27.Brower KKJ. Alcohol's effects on sleep in alcoholics. Alcohol research & health. 2001;25:110–125. [PMC free article] [PubMed] [Google Scholar]
- 28.Fonzi S, Solinas GP, Costelli P, Parodi C, Murialdo G, Bo P, et al. Melatonin and cortisol circadian secretion during ethanol withdrawal in chronic alcoholics. Chronobiologia. 1994;21:109–112. [PubMed] [Google Scholar]
- 29.Kuhlwein E, Hauger RL, Irwin MR. Abnormal nocturnal melatonin secretion and disordered sleep in abstinent alcoholics. Biol Psychiatry. 2003;54:1437–1443. doi: 10.1016/s0006-3223(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 30.Landolt HP, Gillin JC. Sleep abnormalities during abstinence in alcohol-dependent patients. Aetiology and management. CNS Drugs. 2001;15:413–425. doi: 10.2165/00023210-200115050-00006. [DOI] [PubMed] [Google Scholar]
- 31.Sano H, Suzuki Y, Yazaki R, Tamefusa K, Ohara K, Yokoyama T, et al. Circadian variation in plasma 5-hydroxyindoleacetic acid level during and after alcohol withdrawal: phase advances in alcoholic patients compared with normal subjects. Acta Psychiatr Scand. 1993;87:291–296. doi: 10.1111/j.1600-0447.1993.tb03374.x. [DOI] [PubMed] [Google Scholar]
- 32.Sjoholm L, Kovanen L, Saarikoski S, Schalling M, Lavebratt C, Partonen T. CLOCK is suggested to associate with comorbid alcohol use and depressive disorders. Journal of Circadian Rhythms. 2010;8:1. doi: 10.1186/1740-3391-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, et al. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nature Medicine. 2005;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- 34.Hofstetter JR, Grahame NJ, Mayeda AR. Circadian activity rhythms in highalcohol- preferring and low-alcohol-preferring mice. Alcohol. 2003;30:81–85. doi: 10.1016/s0741-8329(03)00095-8. [DOI] [PubMed] [Google Scholar]
- 35.McCulley WD, 3rd, Ascheid S, Crabbe JC, Rosenwasser AM. Selective breeding for ethanol-related traits alters circadian phenotype. Alcohol. 2013;47:187–194. doi: 10.1016/j.alcohol.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenwasser AM, Fecteau ME, Logan RW, Reed JD, Cotter SJ, Seggio JA. Circadian activity rhythms in selectively bred ethanol-preferring and nonpreferring rats. Alcohol. 2005;36:69–81. doi: 10.1016/j.alcohol.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Logan RW, Williams WP, McClung CA. Circadian rhythms and addiction: Mechanistic insights and future directions. Behav Neurosci. 2014 doi: 10.1037/a0036268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kakihana R, Moore JA. Circadian rhythm of corticosterone in mice: the effect of chronic consumption of alcohol. Psychopharmacologia. 1976;46:301–305. doi: 10.1007/BF00421118. [DOI] [PubMed] [Google Scholar]
- 39.Madeira MD, Andrade JP, Lieberman AR, Sousa N, Almeida OF, Paula-Barbosa MM. Chronic alcohol consumption and withdrawal do not induce cell death in the suprachiasmatic nucleus, but lead to irreversible depression of peptide immunoreactivity and mRNA levels. J Neurosci. 1997;17:1302–1319. doi: 10.1523/JNEUROSCI.17-04-01302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajakrishnan V, Subramanian P, Viswanathan P, Menon VP. Effect of chronic ethanol ingestion on biochemical circadian rhythms in Wistar rats. Alcohol. 1999;18:147–152. doi: 10.1016/s0741-8329(98)00077-9. [DOI] [PubMed] [Google Scholar]
- 41.Rosenwasser AM, Fecteau ME, Logan RW. Effects of ethanol intake and ethanol withdrawal on free-running circadian activity rhythms in rats. Physiol Behav. 2005;84:537–542. doi: 10.1016/j.physbeh.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 42.Gilliam DM, Collins AC. Circadian and genetic influences on tissue sensitivity and sleep time to ethanol in LS and SS mice. Pharmacol Biochem Behav. 1983;18:803–808. doi: 10.1016/0091-3057(83)90026-6. [DOI] [PubMed] [Google Scholar]
- 43.Hilakivi L, Tuomisto L, Hilakivi I, Kiianmaa K, Hellevuo K, Hyytia P. Effect of prenatal alcohol exposure on neonatal sleep-wake behaviour and adult alcohol consumption in the AA and ANA rat lines. Alcohol Alcohol. 1987;22:231–240. [PubMed] [Google Scholar]
- 44.Deimling MJ, Schnell RC. Circadian rhythms in the biological response and disposition of ethanol in the mouse. J Pharmacol Exp Ther. 1980;213:1–8. [PubMed] [Google Scholar]
- 45.Ruby CL, Brager AJ, DePaul MA, Prosser RA, Glass JD. Chronic ethanol attenuates circadian photic phase resetting and alters nocturnal activity patterns in the hamster. Am J Physiol Regul Integr Comp Physiol. 2009;297:R729–R737. doi: 10.1152/ajpregu.00268.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruby CL, Prosser RA, DePaul MA, Roberts RJ, Glass JD. Acute ethanol impairs photic and nonphotic circadian phase resetting in the Syrian hamster. Am J Physiol Regul Integr Comp Physiol. 2009;296:R411–R418. doi: 10.1152/ajpregu.90782.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brager AJ, Ruby CL, Prosser RA, Glass JD. Chronic ethanol disrupts circadian photic entrainment and daily locomotor activity in the mouse. Alcohol Clin Exp Res. 2010;34:1266–1273. doi: 10.1111/j.1530-0277.2010.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brager AJ, Ruby CL, Prosser RA, Glass JD. Acute ethanol disrupts photic and serotonergic circadian clock phase-resetting in the mouse. Alcohol Clin Exp Res. 2011;35:1467–1474. doi: 10.1111/j.1530-0277.2011.01483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lindsay JH, Glass JD, Amicarelli M, Prosser RA. The mammalian circadian clock in the suprachiasmatic nucleus exhibits rapid tolerance to ethanol in vivo and in vitro. Alcohol Clin Exp Res. 2014;38:760–769. doi: 10.1111/acer.12303. [DOI] [PubMed] [Google Scholar]
- 50.Prosser RA, Glass JD. The mammalian circadian clock exhibits acute tolerance to ethanol. Alcohol Clin Exp Res. 2009;33:2088–2093. doi: 10.1111/j.1530-0277.2009.01048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tabakoff B, Jafee RC, Ritzmann RF. Corticosterone concentrations in mice during ethanol drinking and withdrawal. J Pharm Pharmacol. 1978;30:371–374. doi: 10.1111/j.2042-7158.1978.tb13259.x. [DOI] [PubMed] [Google Scholar]
- 52.Alfonso M, Duran R, Marco J. Ethanol-induced alterations in gonadotrophins secretion during the estrous cycle of rats. Alcohol Alcohol. 1993;28:667–674. [PubMed] [Google Scholar]
- 53.Huang MC, Ho CW, Chen CH, Liu SC, Chen CC, Leu SJ. Reduced expression of circadian clock genes in male alcoholic patients. Alcohol Clin Exp Res. 2010;34:1899–1904. doi: 10.1111/j.1530-0277.2010.01278.x. [DOI] [PubMed] [Google Scholar]
- 54.McCarthy MJ, Fernandes M, Kranzler HR, Covault JM, Welsh DK. Circadian clock period inversely correlates with illness severity in cells from patients with alcohol use disorders. Alcohol Clin Exp Res. 2013;37:1304–1310. doi: 10.1111/acer.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melendez RI, McGinty JF, Kalivas PW, Becker HC. Brain region-specific gene expression changes after chronic intermittent ethanol exposure and early withdrawal in C57BL/6J mice. Addict Biol. 2012;17:351–364. doi: 10.1111/j.1369-1600.2011.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ozburn AR, Falcon E, Mukherjee S, Gillman A, Arey R, Spencer S, et al. The role of clock in ethanol-related behaviors. Neuropsychopharmacology. 2013;38:2393–2400. doi: 10.1038/npp.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen CP, Kuhn P, Advis JP, Sarkar DK. Chronic ethanol consumption impairs the circadian rhythm of pro-opiomelanocortin and period genes mRNA expression in the hypothalamus of the male rat. J Neurochem. 2004;88:1547–1554. doi: 10.1046/j.1471-4159.2003.02300.x. [DOI] [PubMed] [Google Scholar]
- 58.Arey RN, Enwright JF, 3rd, Spencer SM, Falcon E, Ozburn AR, Ghose S, et al. An important role for Cholecystokinin, a CLOCK target gene, in the development and treatment of manic-like behaviors. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koike N, Yoo SH, Huang HC, Kumar V, Lee C, Kim TK, et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338:349–354. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Logan RW, Sarkar DK. Circadian nature of immune function. Mol Cell Endocrinol. 2012;349:82–90. doi: 10.1016/j.mce.2011.06.039. [DOI] [PubMed] [Google Scholar]
- 62.Andretic R, Chaney S, Hirsh J. Requirement of circadian genes for cocaine sensitization in Drosophila. Science. 1999;285:1066–1068. doi: 10.1126/science.285.5430.1066. [DOI] [PubMed] [Google Scholar]
- 63.Pohl JB, Ghezzi A, Lew LK, Robles RB, Cormack L, Atkinson NS. Circadian genes differentially affect tolerance to ethanol in Drosophila. Alcohol Clin Exp Res. 2013;37:1862–1871. doi: 10.1111/acer.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:9026–9030. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Y, Wang Y, Wan C, Zhou W, Peng T, Wang Z, et al. The role of mPer1 in morphine dependence in mice. Neuroscience. 2005;130:383–388. doi: 10.1016/j.neuroscience.2004.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perreau-Lenz S, Zghoul T, de Fonseca FR, Spanagel R, Bilbao A. Circadian regulation of central ethanol sensitivity by the mPer2 gene. Addict Biol. 2009;14:253–259. doi: 10.1111/j.1369-1600.2009.00165.x. [DOI] [PubMed] [Google Scholar]
- 67.Zghoul T, Abarca C, Sanchis-Segura C, Albrecht U, Schumann G, Spanagel R. Ethanol self-administration and reinstatement of ethanol-seeking behavior in Per1(Brdm1) mutant mice. Psychopharmacology (Berl) 2007;190:13–19. doi: 10.1007/s00213-006-0592-z. [DOI] [PubMed] [Google Scholar]
- 68.Wahlsten D, Bachmanov A, Finn DA, Crabbe JC. Stability of inbred mouse strain differences in behavior and brain size between laboratories and across decades. Proc Natl Acad Sci U S A. 2006;103:16364–16369. doi: 10.1073/pnas.0605342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ozburn AR, Larson EB, Self DW, McClung CA. Cocaine self-administration behaviors in ClockDelta19 mice. Psychopharmacology (Berl) 2012;223:169–177. doi: 10.1007/s00213-012-2704-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, et al. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, et al. Manialike behavior induced by disruption of CLOCK. Proceedings of the National Academy of Sciences. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beaule C, Swanstrom A, Leone MJ, Herzog ED. Circadian modulation of gene expression, but not glutamate uptake, in mouse and rat cortical astrocytes. PLoS One. 2009;4:e7476. doi: 10.1371/journal.pone.0007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mukherjee S, Coque L, Cao JL, Kumar J, Chakravarty S, Asaithamby A, et al. Knockdown of Clock in the Ventral Tegmental Area Through RNA Interference Results in a Mixed State of Mania and Depression-Like Behavior. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wise RA. Drug-activation of brain reward pathways. Drug Alcohol Depend. 1998;51:13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]
- 75.Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–228. [PubMed] [Google Scholar]
- 76.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 77.El-Ghundi M, George SR, Drago J, Fletcher PJ, Fan T, Nguyen T, et al. Disruption of dopamine D1 receptor gene expression attenuates alcohol-seeking behavior. Eur J Pharmacol. 1998;353:149–158. doi: 10.1016/s0014-2999(98)00414-2. [DOI] [PubMed] [Google Scholar]
- 78.Grace AA. The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction. 2000;95(Suppl 2):S119–S128. doi: 10.1080/09652140050111690. [DOI] [PubMed] [Google Scholar]
- 79.Volkow ND, Wang GJ, Ma Y, Fowler JS, Zhu W, Maynard L, et al. Expectation enhances the regional brain metabolic and the reinforcing effects of stimulants in cocaine abusers. J Neurosci. 2003;23:11461–11468. doi: 10.1523/JNEUROSCI.23-36-11461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 81.Woodward JJ. Ethanol and NMDA receptor signaling. Crit Rev Neurobiol. 2000;14:69–89. doi: 10.1080/08913810008443548. [DOI] [PubMed] [Google Scholar]
- 82.Weinshenker D, Rust NC, Miller NS, Palmiter RD. Ethanol-associated behaviors of mice lacking norepinephrine. J Neurosci. 2000;20:3157–3164. doi: 10.1523/JNEUROSCI.20-09-03157.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103:121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 84.Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- 85.Brodie MS, Appel SB. Dopaminergic neurons in the ventral tegmental area of C57BL/6J and DBA/2J mice differ in sensitivity to ethanol excitation. Alcohol Clin Exp Res. 2000;24:1120–1124. [PubMed] [Google Scholar]
- 86.Okamoto H, Miki T, Lee KY, Yokoyama T, Kuma H, Gu H, et al. Effects of chronic ethanol administration on the expression levels of neurotrophic factors in the rat hippocampus. Okajimas Folia Anat Jpn. 2006;83:1–6. doi: 10.2535/ofaj.83.1. [DOI] [PubMed] [Google Scholar]
- 87.Diana M, Pistis M, Muntoni A, Gessa G. Mesolimbic dopaminergic reduction outlasts ethanol withdrawal syndrome: evidence of protracted abstinence. Neuroscience. 1996;71:411–415. doi: 10.1016/0306-4522(95)00482-3. [DOI] [PubMed] [Google Scholar]
- 88.Theile JW, Morikawa H, Gonzales RA, Morrisett RA. GABAergic transmission modulates ethanol excitation of ventral tegmental area dopamine neurons. Neuroscience. 2011;172:94–103. doi: 10.1016/j.neuroscience.2010.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bass CE, Grinevich VP, Gioia D, Day-Brown JD, Bonin KD, Stuber GD, et al. Optogenetic stimulation of VTA dopamine neurons reveals that tonic but not phasic patterns of dopamine transmission reduce ethanol self-administration. Front Behav Neurosci. 2013;7:173. doi: 10.3389/fnbeh.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 91.Lobo MK, Covington HE, 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jeanes ZM, Buske TR, Morrisett RA. Cell type-specific synaptic encoding of ethanol exposure in the nucleus accumbens shell. Neuroscience. 2014;277C:184–195. doi: 10.1016/j.neuroscience.2014.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- 94.Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, et al. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McClung CA. Circadian rhythms, the mesolimbic dopaminergic circuit, and drug addiction. Scientific World Journal. 2007;7:194–202. doi: 10.1100/tsw.2007.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sleipness EP, Jansen HT, Schenk JO, Sorg BA. Time-of-day differences in dopamine clearance in the rat medial prefrontal cortex and nucleus accumbens. Synapse. 2008;62:877–885. doi: 10.1002/syn.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sleipness EP, Sorg BA, Jansen HT. Diurnal differences in dopamine transporter and tyrosine hydroxylase levels in rat brain: dependence on the suprachiasmatic nucleus. Brain research. 2007;1129:34–42. doi: 10.1016/j.brainres.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 100.Hampp G, Ripperger JA, Houben T, Schmutz I, Blex C, Perreau-Lenz S, et al. Regulation of monoamine oxidase A by circadian-clock components implies clock influence on mood. Curr Biol. 2008;18:678–683. doi: 10.1016/j.cub.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 101.Weber M, Lauterburg T, Tobler I, Burgunder JM. Circadian patterns of neurotransmitter related gene expression in motor regions of the rat brain. Neuroscience letters. 2004;358:17–20. doi: 10.1016/j.neulet.2003.12.053. [DOI] [PubMed] [Google Scholar]
- 102.Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, et al. Manialike behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Coque L, Mukherjee S, Cao JL, Spencer S, Marvin M, Falcon E, et al. Specific role of VTA dopamine neuronal firing rates and morphology in the reversal of anxiety-related, but not depression-related behavior in the ClockDelta19 mouse model of mania. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:1478–1488. doi: 10.1038/npp.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mukherjee S, Coque L, Cao JL, Kumar J, Chakravarty S, Asaithamby A, et al. Knockdown of Clock in the ventral tegmental area through RNA interference results in a mixed state of mania and depression-like behavior. Biological psychiatry. 2010;68:503–511. doi: 10.1016/j.biopsych.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chung S, Lee EJ, Yun S, Choe HK, Park SB, Son HJ, et al. Impact of Circadian Nuclear Receptor REV-ERBalpha on Midbrain Dopamine Production and Mood Regulation. Cell. 2014;157:858–868. doi: 10.1016/j.cell.2014.03.039. [DOI] [PubMed] [Google Scholar]
- 106.Schade R, Vick K, Ott T, Sohr R, Pfister C, Bellach J, et al. Circadian rhythms of dopamine and cholecystokinin in nucleus accumbens and striatum of rats--influence on dopaminergic stimulation. Chronobiol Int. 1995;12:87–99. doi: 10.3109/07420529509064504. [DOI] [PubMed] [Google Scholar]
- 107.Lanca AJ, De Cabo C, Arifuzzaman AI, Vaccarino FJ. Cholecystokinergic innervation of nucleus accumbens subregions. Peptides. 1998;19:859–868. doi: 10.1016/s0196-9781(98)00032-1. [DOI] [PubMed] [Google Scholar]
- 108.Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, et al. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med. 2005;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- 109.Dzirasa K, Coque L, Sidor MM, Kumar S, Dancy EA, Takahashi JS, et al. Lithium ameliorates nucleus accumbens phase-signaling dysfunction in a genetic mouse model of mania. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:16314–16323. doi: 10.1523/JNEUROSCI.4289-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12:623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Akhisaroglu M, Kurtuncu M, Manev H, Uz T. Diurnal rhythms in quinpiroleinduced locomotor behaviors and striatal D2/D3 receptor levels in mice. Pharmacol Biochem Behav. 2005;80:371–377. doi: 10.1016/j.pbb.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 112.Garcia JA, Zhang D, Estill SJ, Michnoff C, Rutter J, Reick M, et al. Impaired cued and contextual memory in NPAS2-deficient mice. Science. 2000;288:2226–2230. doi: 10.1126/science.288.5474.2226. [DOI] [PubMed] [Google Scholar]
- 113.Le Foll B, Goldberg SR, Sokoloff P. The dopamine D3 receptor and drug dependence: effects on reward or beyond? Neuropharmacology. 2005;49:525–541. doi: 10.1016/j.neuropharm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 114.Heidbreder CA, Andreoli M, Marcon C, Hutcheson DM, Gardner EL, Ashby CR., Jr Evidence for the role of dopamine D3 receptors in oral operant alcohol self-administration and reinstatement of alcohol-seeking behavior in mice. Addict Biol. 2007;12:35–50. doi: 10.1111/j.1369-1600.2007.00051.x. [DOI] [PubMed] [Google Scholar]
- 115.Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Spencer S, Falcon E, Kumar J, Krishnan V, Mukherjee S, Birnbaum SG, et al. Circadian genes Period 1 and Period 2 in the nucleus accumbens regulate anxiety-related behavior. The European journal of neuroscience. 2012 doi: 10.1111/ejn.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]