Abstract
Objective
To compare live birth rates, blastocyst to live birth efficiency, gestational age, and birth weight in a large cohort of patients undergoing single versus double thawed blastocyst transfer.
Design
Retrospective cohort study.
Setting
Large private ART practice.
Patient(s)
All autologous transfers (FBT) of one or two vitrified-warmed blastocysts from January 2009 through April 2012. Only supernumerary blastocysts with good morphology (BB or better) were vitrified.
Intervention(s)
Single or double FBT.
Main outcome measure(s)
Live birth, blastocyst to live birth efficiency, pre-term birth, low birth weight
Results
1696 FBTs were analyzed. No differences were observed in patient age, rate of embryo progression, or post-thaw blastomere survival. Double FBT yielded a higher live birth per transfer; however, 33% of births from double FBTs were twins versus only 0.6% of single FBTs. Double FBT was associated with significant increases in preterm birth and low birth weight, the latter of which was significant even when the analysis was limited to singletons. 38% of blastocysts transferred via single FBT resulted in a live born child versus only 34% with double FBT. This suggests that two single FBTs would result in more live born children with significantly fewer preterm births, when compared to double FBT.
Conclusions
Single FBT greatly decreased multiple and preterm birth risk while providing excellent live birth rates. Patients should be counseled that a greater overall number of live born children per couple can be expected when thawed blastocysts are transferred one at a time.
Support
Intramural Research Program and the Program in Reproductive and Adult Endocrinology, NICHD, NIH.
Keywords: vitrification, frozen embryo transfer, single embryo transfer, preterm birth, low birth weight
Introduction
The modern treatment goal for the infertile patient is the birth of one healthy child at a time. In the past, multiple embryos were transferred to increase pregnancy and live birth rates per fresh embryo transfer procedure. High rates of multiple gestation result from this practice. The increase in multiple births observed over the last 25 years is largely attributable to ART, with the proportion of twin births attributable to IVF conception rising steadily through 2011 (1). Many consider multiple births to be an adverse outcome in ART due to associated maternal and neonatal morbidity as well as the economic impact (2, 3). Risks associated with multiple pregnancy include prematurity, intrauterine growth restriction, low birth weight, cerebral palsy, learning disabilities, and developmental delay (4).
Strategies to curb the multiple birth epidemic aim to increase implantation via blastocyst transfer (5) and to improve cumulative live births per oocyte retrieval, via elective single embryo transfer coupled with future transfer of frozen-thawed embryos (6, 7). The practice of elective single embryo transfer is rising in the U.S., having increased from 1% in 2002 to 12% in 2011; however, the practice is underutilized in comparison to European countries such as Sweden (73.3%) (8,9). The number of frozen-thawed embryo transfers performed in the U.S. increased by 82.5% from 2006 to 2012 (10). It is well established that single embryo transfer reduces multiple birth and improves neonatal endpoints while providing acceptable live birth rates among single versus double fresh embryo transfer (6,11,12, 13, 14). However, there is a paucity of data comparing single versus double frozen-thawed blastocyst transfer, and study endpoints have been limited to pregnancy outcomes (clinical pregnancy, live birth, multiple birth, miscarriage, and ectopic) (15, 16). Recently, there has been a call for more substantial reporting of neonatal outcomes as opposed to ART cycle outcome alone (17, 18).
Our aim was to compare live birth rates, blastocyst-to-live birth efficiency, clinical pregnancy and multiple pregnancy rates, as well as preterm birth and birth weight in a large cohort of patients undergoing single versus double vitrified-thawed blastocyst transfer.
Materials and methods
Study Design
We performed a retrospective cohort study of all autologous single and double vitrified-thawed blastocyst transfers with known live-birth outcomes performed at our center from January 2009 through April 2012. The study was performed at the Shady Grove Fertility and Reproductive Science Center in Rockville, Maryland. Schulman Associates Institutional Review Board approved the retrospective review and analysis of data collected during routine clinical care.
Patients
All transfers of one or two autologous vitrified-warmed blastocysts from January 2009 through April 2012 were analyzed. Transfers of more than two embryos were excluded.
Vitrification/Warming
Modified Gardner and Schoolcraft grading was used to assess developing blastocysts (19). One of two senior embryologists reviewed all embryo grading, as is routine clinical practice at our center. Supernumerary blastocysts with an inner cell mass/trophectoderm grade of greater than or equal to BB by day 5 or 6 post oocyte retrieval underwent vitrification. Over the duration of the study, all embryo cryopreservation-thawing at our center was performed via a vitrification-warming method, performed as previously described (20).
Endometrial preparation protocol
Patients underwent ovarian and uterine suppression using combined hormonal oral contraceptive pills. After baseline hormonal assessment and transvaginal ultrasound documenting no ovarian cysts and a thin endometrium, patients were started on intramuscular estradiol valerate 4 mg every third day. When serum estradiol reached a level greater than 200 pg/mL and the endometrial double thickness was greater than or equal to 8mm on transvaginal ultrasound, patients were started on 50mg daily intramuscular progesterone in oil.
Embryo selection
Number of blastocysts transferred was determined by patients and their physicians as per routine clinical practice. Decisions regarding the number of cryopreserved blastocysts to transfer at our center are generally made based on a number of factors, including but not limited to, age of the patient at the time of cryopreservation, prior birth history; previous unsuccessful embryos transfers; the outcome of fresh embryo transfer cycles from which cryopreserved embryos were derived; the number of cryopreserved embryos available; medical and uterine factors; and infertility diagnosis. Though these are the primary factors generally considered in counseling, they did not all result in statistically confirmed differences in number of embryos transferred. Single embryo transfers were more likely to be performed in patients with a history of prior birth (both in general and specifically in the cycle from which cryopreserved embryos were derived), with fewer previous failed embryo transfers, and with uterine factor infertility. Single embryo transfers were also more common among patients with fewer cryopreserved embryos available, in part because in some cases, lack of multiple embryos precluded a choice.
Blastocyst quality grading at the time of vitrification did not play a role in decisions regarding how many embryos to transfer. All embryos achieved fully expanded blastocyst stage (expansion grade 4) prior to vitrification, and cryopreservation was limited to embryos with minimum grades of B for both the inner cell mass and trophectoderm. Thus, all cryopreserved blastocysts were considered good quality embryos with similarly high implantation potential. In addition, pre-vitrification grades were not linked to individual cryopreserved embryos, so this information was not available for use at the time of warming and transfer.
Embryo transfer
On the sixth day of progesterone replacement, ultrasound-guided blastocyst transfer was performed using the after-load technique, in which the outer sheath of the transfer catheter is left in place to maintain access to the uterine cavity.
Outcomes and Definitions
The primary outcome was live birth. Secondary outcomes were blastocyst-to-live birth efficiency, biochemical pregnancy (detectable serum hcg), clinical pregnancy, multiple gestation, gestational age at birth, and birth weight. Clinical pregnancy was defined as an intrauterine gestational sac on ultrasound. Live birth was defined as birth of a live infant greater than or equal to 24 weeks’ gestation. Blastocyst-to-live birth efficiency was calculated for each transfer as the number of live infants born greater than 24 weeks’ gestation divided by the number of blastocysts transferred. Blastocyst-to-live birth efficiency for each group was calculated as the mean of the per-transferred embryo efficiencies.
Preterm birth, very preterm birth, and extremely preterm birth were defined as birth prior to 37 weeks, 32 weeks, and 28 weeks, respectively (21). The following definitions were used for birth weight: low birth weight was defined as weight less than 2500 grams, very low birth weight was defined as less than 1500 grams, and extremely low birth weight was defined as less than 1000 grams (22).
Statistical analysis
Chi square analysis and Fisher’s exact test were used as appropriate to compare pregnancy, live-birth, and multiple gestation rates between patients who had single versus double thawed blastocyst transfer. Student’s t-test was used to evaluate continuous parameters. Sub-analyses of singleton pregnancies were used to control for multiple gestation when assessing intergroup differences in preterm birth and low birth weight. A p<0.05 was used for the definition of statistical significance.
Generalized estimating equation (GEE) modeling was used to compare the efficiency of conversion of transferred blastocysts to live born children. This analysis accounted for the potential correlation in outcomes among repeated cycles by the same subjects and estimated the effect of each independent factor as associated with embryo to live birth efficiency, after adjusting for the other variables in the model. Patient characteristics accounted for in the GEE modeling included age at the time of blastocyst vitrification and at the time of warming and transfer, infertility diagnoses, body mass index, parity, and number of prior unsuccessful transfers. Treatment-associated characteristics accounted for in the GEE modeling included freeze-all cycles, birth outcomes from fresh transfers of embryos from the same cohort, numbers of vitrified blastocysts, use of PGD/PGS, post-retrieval day of vitrification, percentage of blastocysts surviving vitrification and warming, and the percentage of cells that remained intact after warming.
Statistical analysis was performed using SPSS Statistics Version 22 (IBM Corporation, Armonk, NY).
Results
Cycle Characteristics
A total of 1696 transfers were analyzed. Characteristics of single and double transfer groups are presented in Table 1 on a per transfer basis. All associated data were updated for each transfer in instances where more than one transfer was performed in the same patient over the course of the study period. Single and double transfer groups were similar with regard to age at vitrification, day (post retrieval) of blastocyst vitrification, proportion of blastocysts surviving vitrification-warming, and mean proportion of intact cells after warming. The double transfer cohort had a younger age at transfer, a higher mean BMI, and were more likely to have PCOS as an infertility diagnosis. Single transfers were more likely to have male factor or uterine factor as an associated infertility diagnosis. However, when analyzed on a per patient basis, differences in BMI and uterine factor were no longer statistically significant, indicating the contributions of multiple transfers. Somewhat surprisingly, freeze-all in the antecedent cycle and pre-implantation genetic diagnosis/screening of transferred embryos were more common in the double tranfer group. This phenomenon may be related, in part, to financial considerations.
Table 1.
Cycle Characteristics Per Transfer
| Number of Vitrified-Warmed Blastocysts transferred | One | Two | p-value |
|---|---|---|---|
| Number of Transfers (Number of Patients) | 845 (711) | 851 (760) | -- |
| Patient Characteristics Per Transfer (± SD) | |||
| Age at Vitrification (years) | 33.7 ± 3.9 | 33.5 ± 4.0 | 0.34 |
| Age at Warming/Transfer (years) | 34.5 ± 3.9 | 34.0 ± 4.0 | 0.012 |
| Body Mass Index | 24.9 ± 5.0 | 25.4 ± 5.3 | 0.044 |
| Number Prior Transfers w/o Birth | 1.37 ± 1.26 | 1.40 ± 1.08 | 0.64 |
| Parity | 0.58 ± 0.65 | 0.36 ± 0.62 | <0.0001 |
| Prior Cycle Characteristics Per Transfer; N (%) | |||
| Freeze-all (No transfer) in Fresh Cycle | 81 (9.6) | 113 (13.3) | 0.017 |
| Live Birth from Fresh ET | 175 (22.9) | 60 (8.1) | <0.0001 |
| Infertility Diagnosis Per Transfer; N (%) transfers carrying diagnosis | |||
| Endometriosis | 61 (7.2) | 42 (4.9) | 0.053 |
| Male Factor | 315 (37.3) | 277 (32.6) | 0.041 |
| PCOS | 128 (15.2) | 176 (20.7) | 0.0030 |
| Tubal Factor | 108 (12.8) | 110 (12.9) | 0.93 |
| Uterine Factor | 47 (5.6) | 23 (2.7) | 0.0030 |
| Blastocyst Characteristics Per Transfer (± SD) | |||
| Day of Vitrification Post-Retrieval/Fertilization | 5.64 ± 0.57 | 5.60 ± 0.54 | 0.14 |
| Blastocysts Surviving Vitrification/Warming (%) | 95.6 ± 14.9 | 95.5 ± 12.6 | 0.84 |
| Intact Cells per Transferred Blastocyst (%) | 95.5 ± 5.4 | 95.2 ± 5.2 | 0.29 |
| Number of Vitrified Embryos Available for Transfer | 3.4 ± 3.0 | 4.6 ± 2.6 | <0.0001 |
| PGD/PGS; N (%) | 54 (6.4%) | 82 (9.6%) | 0.014 |
Cycle Outcomes, Single versus Double Vitrified-Warmed Blastocyst Transfer
Outcomes of vitrified-warmed embryo transfer cycles are depicted in Figure 1. Biochemical pregnancy, clinical pregnancy, and live birth were significantly higher in the double embryo transfer group. Multiple pregnancy was a frequent outcome in the double embryo group and occurred following fewer than 1% of pregnancies resulting from single embryo transfers (42.7% vs. 0.7%, p<0.0001).
Figure 1.
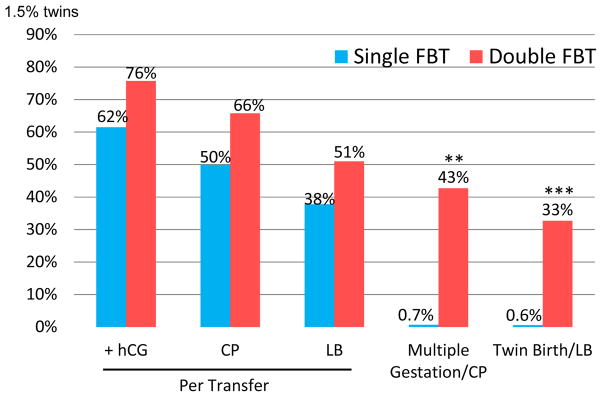
Pregnancy Outcomes Single vs. Double Frozen Blastocyst Transfer (FBT)*
* p<0.0001 for all comparisons
** 41.5% twins, 0.7% triplets, 0.4% quads in the DFBT group; No high order multiple pregnancies in the SFBT group
*** No high order multiple births in either group (Triplets and quads in DFBT group spontaneously reduced)
Blastocyst-to-Live Birth Efficiency, Single versus Double Vitrified-Warmed Blastocyst Transfer
When comparing live birth per embryo transferred, single embryo transfer had significantly more live births per embryo transferred (38.0% vs. 33.8%, p<0.04). The relative increase in live birth per embryo transferred was 12% among those transferred one at a time versus those transferred in pairs. The improved live birth efficiency of embryos in the single embryo group was the combined result of higher implantation (gestational sacs per embryo transferred) (50.3 vs. 47.4% p=0.17) and a lower rate of embryonic/fetal demise per implantation (proportion of gestational sacs not progressing to live birth) (24.5 vs. 28.6, p=0.12); though individually these were not statistically significant.
Among the 1696 transfers cycles evaluated, there were 1389 individual subjects (patients), which were included in the GEE model. Subjects underwent a range of 1 to 7 vitrified blastocyst transfer cycles. As shown in Supplemental Table 1, a positive coefficient indicates an association with higher embryo to live birth efficiency, and each coefficient represents the independent contribution of the associated variable. After adjusting for potentially confounding patient and treatment associated characteristics, the percentage of children born per transferred vitrified blastocyst was found to be significantly higher for transfers of one blastocyst compared to transfers of two blastocysts. The adjusted mean percentages of children per transferred blastocyst were 38.08% versus 33.52%. This 4.56% (95% CI 0.33–8.79%; p=0.034) absolute difference translates to a 13.6% relative advantage in embryo to birth efficiency for transfers of one versus two embryos. This difference in embryo to birth efficiency appeared to be primarily the result of a higher implantation rates (i.e. the percentages of confirmed gestational sacs observed through ultrasound observation per transferred embryo) for the blastocysts transferred singly rather than in pairs, although this trend did not reach the level of statistical significance and is thus inconclusive (GEE-adjusted mean implantation rates = 50.9% versus 46.8%, p=0.074). An unexpected finding of the model was a significant negative coefficient for parity. The contribution of prior cesarean section, which was not available in our dataset for analysis, may explain this in part. In addition, the inverse relationship between parity and embryo efficiency lost statistical significance when transfers to women with two or more prior births (N=106) were excluded.
Gestational Length, Single versus Double Vitrified-Warmed Blastocyst Transfer
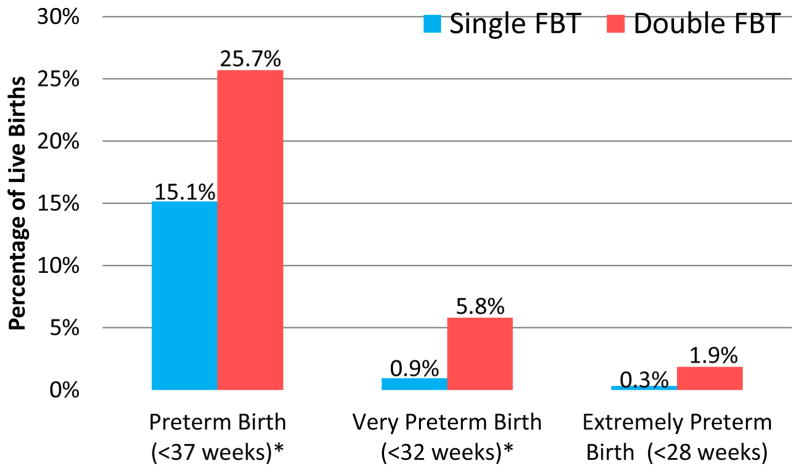
Mean gestational age was one week longer for the single embryo group (38 weeks and 5 days for the single embryo group compared to 37 weeks and 5 days in the double embryo group, p<0.0001). Figure 2 outlines the frequency and severity of prematurity in each group. Overall, rate of preterm birth was lower among the single embryo group (15.1% vs. 25.7%, p< 0.001). Very preterm birth was six times more likely to occur in the double embryo transfer group (p< 0.001). Differences in gestational age at birth were the result of early births in twin gestations. Singletons were born on average three weeks later than twins (38 weeks 5 days vs. 35 weeks 5 days, p <0.0001). There was no difference in preterm birth between the single and double transfer groups when the analysis was limited to singleton deliveries.
Figure 2.
Incidence and Severity of Prematurity, Single vs. Double Frozen Blastocyst Transfer (FBT)
Mean gestational age at birth was 38w5d ± 13d in the single embryo group vs. 37w5d ± 21d with double transfer (p<0.0001). The difference was attributable to increased twin delivery in the double embryo transfer group.
* P<0.001
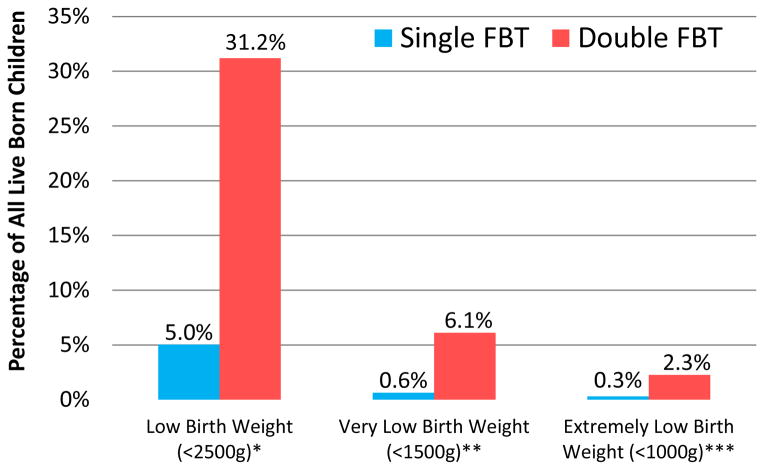
Birth Weight, Single versus Double Vitrified-Warmed Blastocyst Transfer
Low birth weight, very low birth weight, and extremely low birth weight were much more common in the double embryo transfer group (Figure 3). More than half of twin infants experienced low birth weight (51.8% vs. 7.4% singletons, p<0.0001). However, twin pregnancy did not fully account for lower birth weight among the double embryo transfer group. When limiting analysis of birth weight to singleton births, the single blastocyst transfer group maintained a higher average birth weight than double blastocyst transfer group (3,426 g vs. 3,245 g, p=0.001).
Figure 3.
Incidence and Severity of Low Birth Weight, Single vs. Double Frozen Blastocyst Transfer (FBT)
Mean birth weight was 3418 ± 605 grams in the single embryo group vs. 2837 ± 770 grams with double transfer (p<0.0001). A statistically significant decrease in birth weight persisted in the double transfer group when analysis was limited to singleton deliveries.
*P<0.0001; **P<0.001; ***P=0.024
Discussion
The shared goal of infertile couples and providers of fertility care should be the birth of a healthy infant, rather than a positive pregnancy test. In the current analysis of 1,696 vitrified-thawed blastocyst transfers, embryo-to-live birth efficiency, gestational length and birth weight were improved with single embryo transfer relative to transfer of two thawed embryos. To our knowledge, this represents the largest analysis to date comparing live birth outcomes between single and double frozen blastocyst transfer and the only such analysis to assess gestational length, birth weight, and embryo-to-live birth efficiency.
Extended culture enabling blastocyst transfer and vitrification are two advances in ART that have significantly increased the number of live births per stimulated IVF cycle. Embryo cryopreservation allows for transfer of a limited number of embryos while preserving embryos for future use; however, concerns have persisted regarding the implantation potential of frozen-thawed embryos (23, 24).
Two prior studies have evaluated the impact of single versus double frozen blastocyst transfer. A study by Yanaihara et al. in 2008 comparing single versus double frozen blastocyst transfer in 562 cycles found no statistically significant difference in clinical pregnancy or live birth rates between the two groups. However, ectopic and twin pregnancy rates were higher in the dual blastocyst transfer group, suggesting that single transfer should be the preferred method (15). In contrast, in a 2011 evaluation of 243 cycles, Berin et al. found significantly higher clinical pregnancy, live birth, and twin pregnancy rates with double frozen blastocyst transfer, consistent with the findings of the current study (16). Neither study analyzed embryo-to-live-birth efficiency or included data on preterm birth or low birth weight. Fauque et al. in 2010 evaluated fresh and frozen single versus double cleavage stage embryo transfer. In their subset of 97 frozen-thawed day 2 embryo transfers, no differences in clinical pregnancy, live birth, multiple birth rate, gestational age, or birth weight were found; however, there were only 7 live births for analysis per group (25). A recent study by Ishihara et al. evaluated frozen and fresh single embryo transfer in a large cohort and considered neonatal and maternal outcomes. The authors found that single frozen blastocyst transfer was associated with 66% singleton live birth per transfer, with 93% of these deliveries occurring at term, and that fewer than 4% were small for gestational age (26). However multiple embryo transfers were not included in the analysis for comparison.
The present study adds to this previous literature in that it considers a large, homogeneous cohort, including only transfers of high quality vitrified embryos at blastocyst stage and evaluates neonatal endpoints. Furthermore, this is the first study assessing embryo-to-birth efficiency for single versus double frozen-thawed embryo transfers. As expected, the transfer of two frozen-thawed blastocysts had a modest, but significant increase in biochemical pregnancy, clinical pregnancy, and live birth. Importantly, however, twin pregnancy was also significantly higher in the double blastocyst transfer group, whereas twin pregnancies accounted for less than 1% of those resulting from single blastocyst transfers.
Our analysis of transferred blastocyst to live born child efficiency provides compelling evidence that transferring embryos singly rather than in pairs would result in a significantly higher percentage of live born children per transferred embryo. While the inherent weaknesses of a retrospective study make this a less than ideal method of assessing this possibility, our analytical methods provided rigorous control over potentially confounding factors. The use of GEE modeling, in which the level of analysis was individual patients rather than treatment cycles, controlled for any correlation in outcomes within individual subjects while allowing for valid computation of probabilities without sacrificing power by limiting the analysis to one cycle per patient. The analysis adjusted for patient characteristics including age at treatment, BMI, prior parity and failed transfers, and infertility diagnoses including uterine factor which is well-known to adversely affect implantation. The analysis also adjusted for cycle-specific factors such as the outcome of fresh transfers from the same embryo cohort, cycles in which all viable embryos were cryopreserved, use of PGD/PGS, number of cryopreserved embryos, day of vitrification (indicative of the time needed to develop to the expanded blastocyst stage), percentage of warmed embryos to survive, and the post-warming percentage of intact cells in transferred blastocysts.
One factor that was not included in the analysis was blastocyst grade at the time of cryopreservation. However, our policy of cryopreserving only high quality blastocysts would have the effect of limiting differences in this variable. In addition, this information was not used, and was in fact not available, when embryos were warmed and decisions regarding how many embryos to transfer were made. Blastocyst grade at vitrification was thus effectively randomized with respect to the number of embryos subsequently transferred in cryopreserved embryo transfer cycles, and should not have had a confounding affect on treatment outcomes.
The adjusted estimate indicated an absolute difference of 4.56% (38.08% versus 33.52%) in favor of single embryo transfer in the percentage of children born per embryo transferred, amounting to a relative difference of 13.6% more children per embryo transferred for single compared to double embryo transfers. It should be noted that while statistically significant, the confidence interval for this estimate is wide. Thus, the true advantage of single embryo transfer may be considerably less than (or greater than) this estimate. Ideally, this benefit would be assessed through prospective randomized trials comparing single to double embryo transfer. However, in the absence of this gold standard level of evidence, the statistically significant 13.6% relative advantage derived from this well-controlled retrospective analysis represents our best estimate as to the increased efficiency of single embryo transfer.
This finding of greater embryo to birth efficiency with single embryo transfer is not unexpected. Assuming there exists some variation in cycle-to-cycle endometrial receptivity, as may be suggested by implantation defects associated with suboptimal endometrial thickness (27, 28) and elevated serum progesterone in stimulated cycles (29) and inter-cycle variability in histology (30), transferring embryos one at a time would minimize the potential for all embryos to be transferred to a non-receptive uterus, figuratively avoiding “putting ones eggs all in one basket”. In addition, single embryo transfers might avoid theoretical competition among embryos for implantation sites, nutrient supply, or other maternal resources, and would therefore maximize their opportunity for development.
Length of gestation and birth weight were also improved with the transfer of one blastocyst. The majority of observed adverse outcomes (preterm birth and low birth weight) occurred among twin gestations, which made up 49.3% of babies born from double blastocyst transfer versus 1.2% of those resulting from single transfer. However, when controlling for multiple pregnancy (i.e. limiting comparison of birth weight to live singleton births where a single gestational sac was seen on initial ultrasound), double blastocyst transfer was still associated with a lower birth weight. Though this phenomenon has not been previously reported among frozen blastocyst transfers, De Sutter et al. found that singletons born after fresh double embryo transfer had a lower birth weight than those born after fresh single embryo transfer. Furthermore, the 120 gram difference the authors reported is similar to the 181 gram difference found in the present study (31). Numerous studies have demonstrated lower birth weight among singletons born from ART (not limited to single embryo transfer) versus spontaneously conceived singletons (32–37). However, De Neubourg et al. observed no difference in the birth weight of spontaneously conceived singletons versus singletons born after fresh ART with single embryo transfer (38), suggesting transfer of multiple embryos as the causative factor of lower birth weight among ART singletons in general.
There exist several possibilities for our observed difference in singleton birth weight. Given the retrospective nature of the study, intrinsic differences in the single and double transfer groups may have played a role. Therefore, we conducted a stepwise multiple regression model of singleton birth weight. With age at vitrification, age at transfer, BMI, infertility diagnosis, day of vitrification (relative to oocyte retrieval), proportion of cells surviving vitrification-warming, infant sex, and number of blastocysts transferred included as potential covariates, only infant sex and number of embryos transferred remained significantly associated with singleton birth weight. The association of double transfer with lower birth weight among singleton pregnancies persisted. Given that our singleton birth weight analysis was limited to pregnancies noted to have one sac from first ultrasound, it is also unlikely that the higher birth weight observed among singletons following single transfer is accounted for by ‘vanishing twin syndrome,’ i.e. the spontaneous miscarriage of one twin in the first trimester (39, 40). In addition, an analysis of initial post-transfer serum hcg level (collected two weeks post transfer) among patients with singleton gestations via single versus double transfer revealed no difference. If early spontaneous reductions of twins to singletons in the double transfer group were the reason for lower observed singleton birth weights, one might expect a higher early hcg level in this group, which we did not find.
Strengths of our study include large, well-matched cohorts, inclusion of neonatal endpoints, and a focus on the clinically-relevant question of what differences in outcome can be expected when choosing between transferring one versus two high quality vitrified-thawed blastocysts. The results are generalizable and relevant to clinical decision-making regarding number of vitrified-warmed blastocysts to transfer. The main limitation is its retrospective design with inherent potential for bias. In general clinical practice, well-counseled patients opting for single embryo transfer may represent a better prognosis group. However, in the current study, the single embryo group was not limited to those with more than one embryo available. Had the study group included only those patients electing for transfer of a single embryo, our finding of increased embryo to live birth efficiency in this group would likely have been amplified.
Conclusions
Patient counseling is an important aspect of reproductive care. The current study demonstrates that in the modern era of ART, where the ultimate goal is a healthy newborn, patients should be counseled that sequential transfer of a single frozen-thawed embryos can be expected to result in a greater number of children born overall. Furthermore, these newborns will be less likely to experience low birth weight and preterm birth, due primarily to the reduction in twin pregnancy.
Supplementary Material
Acknowledgments
This work was supported by the Program in Reproductive and Adult Endocrinology and the Intramural Research Program, NICHD, NIH.
Footnotes
The authors have no financial disclosures.
The opinions and assertions contained in this article are the expressed views of the authors and are not considered official opinions of the Department of Health and Human Services, the Department of Defense, or of Walter Reed National Military Medical Center
This work was presented at American Society of Reproductive Medicine’s 69th Annual Meeting, in Boston, Massachusetts, on October 14, 2013.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kulkarni A, Jamieson D, Jones H, Kissin D, Gallo M, Macaluso M, Asashi E. Fertility treatments and multiple births in the United States. N Engl J Med. 2013;369(23):2218–25. doi: 10.1056/NEJMoa1301467. [DOI] [PubMed] [Google Scholar]
- 2.Conde-Agudelo A, Belizán JM, Lindmark G. Maternal morbidity and mortality associated with multiple gestations. Obstet Gynecol. 2000;95(6):899–904. [PubMed] [Google Scholar]
- 3.Lemos EV, Zhang D, Van Voorhis BJ, Hu XH. Healthcare expenses associated with multiple vs singleton pregnancies in the United States. Am J Obstet Gynecol. 2013;209(6):586.e1–586.e11. doi: 10.1016/j.ajog.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Reh A, Fino E, Krey L, Berkeley A, Noyes N, Grifo J. Optimizing Embryo Selection with Day 5 Transfer. Fertil Steril. 2010;93(2):609–15. doi: 10.1016/j.fertnstert.2009.02.070. [DOI] [PubMed] [Google Scholar]
- 5.Gardner DK, Vella P, Lane M, Wagley L, Schlenker T, Schoolcraft W. Culture and transfer of human blastocysts increases implantation rates and reduces the need for multiple embryo transfers. Fertil Steril. 1998;69(1):84–88. doi: 10.1016/s0015-0282(97)00438-x. [DOI] [PubMed] [Google Scholar]
- 6.Thurin A, Hausken J, Hillensjo T, Jablonowska B, Pinborg A, Strandell A, Bergh C. Elective single-embryo transfer versus double-embryo transfer in in vitro fertilitzation. N Engl J Med. 2004;351(23):2392–402. doi: 10.1056/NEJMoa041032. [DOI] [PubMed] [Google Scholar]
- 7.Veleva Z, Karinen P, Tomas C, Tapanainen JS, Martikainen H. Elective single embryo transfer with cryopreservation improves the outcome and diminishes the costs of IVF/ICSI. Hum Reprod. 2009;24(7):1632–9. doi: 10.1093/humrep/dep042. [DOI] [PubMed] [Google Scholar]
- 8.CDC: National Center for Chronic Disease Prevention and Health Promotion, Division of Reproductive Health. [Accessed August 19, 2014];2011 Assisted reproductive technology: National Summary Report. 2013 Dec; Available at http://www.cdc.gov/art/ART2011.
- 9.European Society of Human Reproduction and Embryology. [Accessed August 19, 2014];2010 ART Fact Sheet. 2013 Available at http://www.eshre.eu/guidelines-and-legal/art-fact-sheet.aspx.
- 10.Shapiro B, Daneshmand S, Garner F, Aguirre M, Hudson C. Clinical rationale for cryopreservation of entire embryo cohorts in lieu of fresh transfer. Fertil Steril. 2014;102(1):3–9. doi: 10.1016/j.fertnstert.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Pandian Z, Marjoribanks J, Ozturk O, Serour G, Bhattacharya S. Number of embryos for transfer following in vitro fertilisation or intra-cytoplasmic sperm injection. Cochrane Database Syst Rev. 2013 Jul 29;7:CD003416. doi: 10.1002/14651858.CD003416.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thurin Kjellberg A, Carlsson P, Bergh C. Randomized single versus double embryo transfer: obstetric and paediatric outcome and a cost-effectiveness analysis. Hum Reprod. 2006;21(1):210–6. doi: 10.1093/humrep/dei298. [DOI] [PubMed] [Google Scholar]
- 13.Stillman RJ, Richter KS, Banks NK, Graham JR. Elective single embryo transfer: a 6-year progressive implementation of 784 single blastocyst transfers and the influence of payment method on patient choice. Fertil Steril. 2009;92(6):1895–906. doi: 10.1016/j.fertnstert.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 14.Csokmay JM, Hill MJ, Chason RJ, Hennessy S, James AN, Cohen J, DeCherney AH, Payson MD. Experience with a patient-friendly, mandatory, single-blatocyst transfer policy: the power of one. Fertil Steril. 2011;96(3):580–4. doi: 10.1016/j.fertnstert.2011.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yanaihara A, Yorimitsu T, Motoyama H, Ohara M, Kawamura T. Clinical Outcome of Frozen Blastocyst Transfer; Single vs. Double Transfer. J Assist Reprod Genet. 2008;25:531–534. doi: 10.1007/s10815-008-9275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berin I, McLellan ST, Macklin EA, Toth TL, Wright DW. Frozeon-Thawed Embryo Transfer Cycles: Clinical Outcomes of Single and Double Blastocyst Transfers. J Assist Reprod Genet. 2011;28:575–81. doi: 10.1007/s10815-011-9551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Legro R, Wu X. Choosing the main outcome of an infertility trial is harder than you think. Fertil Steril. 2014;101(5):1201–2. doi: 10.1016/j.fertnstert.2014.03.051. [DOI] [PubMed] [Google Scholar]
- 18.Silver R. Infertility trial outcomes: healthy moms and babies. Fertil Steril. 2014;101(5):1209–16. doi: 10.1016/j.fertnstert.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 19.Gardner DKSW. in-vitro culture of human blastocyst. Carnforth: Parthenon; 1999. [Google Scholar]
- 20.Liebermann J, Tucker MJ. Vitrifying and warming of human oocytes, embryos, and blastocysts: vitrification procedures as an alternative to conventional cryopreservation. Methods Mol Biol. 2004;254:345–64. doi: 10.1385/1-59259-741-6:345. [DOI] [PubMed] [Google Scholar]
- 21.Blencowe H, Cousens S, Oestergaard M, Chous D, Moller AB, Narwal R, Adler A, Vera Garcia C, Rhode S, Say L, Lawn JE. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–72. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2012. Natl Vital Stat Rep. 2013;62(3):1–20. [PubMed] [Google Scholar]
- 23.Loutradi K, Kolibianakis E, Venetis C, Papanikolaou E, Pados F, Bontis I, Tarlatzis B. Cryopreservation of Human Embryos by Vitrification or Slow Freezing: A Systematic Review and Meta-analysis. Fertil Steril. 2008;90(1):186–93. doi: 10.1016/j.fertnstert.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Kolibianakis E, Zikopoulos K, Devroey P. Implantation Potential and Clinical Impact of Cryopreservation- A Review. Placenta. 2003;24:S27–33. doi: 10.1016/s0143-4004(03)00133-4. [DOI] [PubMed] [Google Scholar]
- 25.Fauque P, Jouannet P, Davy C, Guibert J, Viallon V, Epelbion S, Kunstmann JM, Patrat C. Cumulative results including obstetrical and neonatal outcome of fresh and frozen-thawed cycles in elective single versus double fresh embryo transfers. Fertil Steril. 2010;94(3):927–35. doi: 10.1016/j.fertnstert.2009.03.105. [DOI] [PubMed] [Google Scholar]
- 26.Ishihara O, Araki R, Akira K, Itakura A, Saito H, Damson GD. Impact of Frozen-Thawed Single-Blastocyst Transfer on Maternal and Neonatal Outcome: An Analysis of 277,042 Single-Embryo Transfer Cycles from 2008 to 2010 in Japan. Fertil Steril. 2014;101(1):128–133. doi: 10.1016/j.fertnstert.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 27.Richter KS, Bugge KR, Bromer JG, Levy MJ. Relationship between endometrial thickness and embryo implantation, based on 1,294 cycles of in vitro fertilization with transfer of two blastocyst-stage embryos. Fertil Steril. 2007;87(1):53–59. doi: 10.1016/j.fertnstert.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 28.Basir GS, OW-S, So WWK, Ng EHY, Ho PC. Evaluation of cycle-to-cycle variation of endometrial responsiveness using transvaginal sonography in women undergoing assisted reproduction. Ultrasound Obstet Gynecol. 2002;19:484–489. doi: 10.1046/j.1469-0705.2002.00685.x. [DOI] [PubMed] [Google Scholar]
- 29.Bosch E, Labarta E, Crespo J, Simon C, Remohi J, Jenkins J, et al. Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: analysis of over 4000 cycles. Hum Reprod. 2010;25:2092–100. doi: 10.1093/humrep/deq125. [DOI] [PubMed] [Google Scholar]
- 30.Murray MJ, Meyer WR, Zaino RJ, Lessey BA, Novotny DB, Ireland K, et al. A critical analysis of the accuracy, reproducibility, and clinical utility of histologic endometrial dating in fertile women. Fertil Steril. 2004;81(5):1333–1343. doi: 10.1016/j.fertnstert.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 31.De Sutter P, Delbaere I, Gerris J, Verstraelen H, Goetgeluk S, Van der Elst J, Temmerman M, Dhont M. Birthweight of singletons after assisted reproduction is higher after single-than after double- embryo transfer. Hum Reprod. 2006;21(10):2633–7. doi: 10.1093/humrep/del247. [DOI] [PubMed] [Google Scholar]
- 32.Helmerhorst F, Perquin D, Donker D, Keirse M. Perinatal outcome of singletons and twins after assisted conception: a systematic review of controlled studies. BMJ. 2004;328:261–5. doi: 10.1136/bmj.37957.560278.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans J, Hannan N, Edgell T, Vollenhoven B, Lutjen B, Osianlis T, Salamonsen L, Rombauts L. Fresh versus frozen embryo transfer: backing clinical decisions with scientific and clinical evidence. Hum Reprod Update. doi: 10.1093/humupd/dmu027. In press. [DOI] [PubMed] [Google Scholar]
- 34.Jackson R, Gibson K, Wu Y, Croughan M. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol. 2004;103:551–563. doi: 10.1097/01.AOG.0000114989.84822.51. [DOI] [PubMed] [Google Scholar]
- 35.Schieve L, Meikle S, Ferre C, Peterson H, Jeng G, Wilcox L. Low and very low birth weight in infants conceived with use of assisted reproductive technology. N Engl J Med. 2002;346:731–7. doi: 10.1056/NEJMoa010806. [DOI] [PubMed] [Google Scholar]
- 36.Schieve L, Ferre C, Peterson H, Macaluso M, Reynolds M, Wright V. Perinatal outcome among singleton infants conceived through assisted reproductive technology in the United States. Obstet Gynecol. 2004;103:1144–53. doi: 10.1097/01.AOG.0000127037.12652.76. [DOI] [PubMed] [Google Scholar]
- 37.DeGeyter C, De Geyter M, Steimann S, Zhang H, Holzgreve W. Comparative birth weights of singletons born after assisted reproduction and natural conception in previously infertile women. Hum Reprod. 2006 Mar;21(3):705–12. doi: 10.1093/humrep/dei378. [DOI] [PubMed] [Google Scholar]
- 38.De Neubourg D, Gerris J, Mangelschots K, Royen EV, Vercruyssen M, Steylemans A, Elseviers M. The obstetrical and neonatal outcome of babies born after single-embryo transfer in IVF/ICSI compares favourably to spontaneously conceived babies. Hum Reprod. 2006;21(4):1041–6. doi: 10.1093/humrep/dei424. [DOI] [PubMed] [Google Scholar]
- 39.Shebl O, Ebner T, Sommergruber M, Sir A, Tews G. Birth weight is lower for survivors of the vanishing twin syndrome: a case-control study. Fertil Steril. 2008;90(2):310–4. doi: 10.1016/j.fertnstert.2007.06.048. [DOI] [PubMed] [Google Scholar]
- 40.Almog B, Levin I, Wagman I, Kapustiansky R, Lessing JB, Amit A, Azem F. Adverse obstetric outcome for the vanishing twin syndrome. Reprod Biomed Online. 2010;20(2):256–60. doi: 10.1016/j.rbmo.2009.11.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.