Abstract
Objective
The efficacy of epilepsy surgery depends critically upon successful localization of the epileptogenic zone. Magnetoencephalography (MEG) enables non-invasive detection of interictal spike activity in epilepsy, which can then be localized in three dimensions using magnetic source imaging (MSI) techniques. However, the clinical value of MEG in the pre-surgical epilepsy evaluation is not fully understood, as studies to date are limited by either a lack of long-term seizure outcomes or small sample size.
Methods
We performed a retrospective cohort study of focal epilepsy patients who received MEG for interictal spike mapping followed by surgical resection at our institution.
Results
We studied 132 surgical patients, with mean post-operative follow-up of 3.6 years (minimum 1 year). Dipole source modelling was successful in 103 (78%) patients, while no interictal spikes were seen in others. Among patients with successful dipole modelling, MEG findings were concordant with and specific to: i) the region of resection in 66% of patients, ii) invasive electrocorticography (ECoG) findings in 67% of individuals, and iii) the MRI abnormality in 74% of cases. MEG showed discordant lateralization in ~5% of cases. After surgery, 70% of all patients achieved seizure-freedom (Engel class I outcome). Whereas 85% of patients with concordant and specific MEG findings became seizure-free, this outcome was achieved by only 37% of individuals with MEG findings that were non-specific or discordant with the region of resection (χ2 = 26.4, p < 0.001). MEG reliability was comparable in patients with or without localized scalp EEG, and overall, localizing MEG findings predicted seizure freedom with an odds ratio of 5.11 (2.23–11.8, 95% CI).
Significance
MEG is a valuable tool for non-invasive interictal spike mapping in epilepsy surgery, including patients with non-localized findings on long-term EEG monitoring, and localization of the epileptogenic zone using MEG is associated with improved seizure outcomes.
Keywords: epilepsy surgery, epileptogenic zone, MEG, magnetic source imaging, interictal spike
Introduction
Seizures are resistant to anti-epileptic drugs in one third of patients with focal epilepsy, leading to significant morbidity.1 Resective epilepsy surgery leads to seizure-freedom in approximately two-thirds of patients with temporal lobe epilepsy (TLE), and in more than one-half of individuals with an extra-temporal epileptogenic zone.2–5 Despite these successes, there is substantial room for improvement in the localization and surgical treatment of drug-resistant epilepsy. While invasive electro-diagnostic techniques such as electrocorticography (ECoG) are the gold standard for seizure focus localization, recordings require additional surgical intervention and are limited to the area of electrode coverage.6 Improved methods for non-invasive mapping of epileptogenic networks across the whole brain remain critically important to the pre-surgical evaluation, as adequate localization of the seizure focus is the most important predictor of seizure freedom in epilepsy surgery.7; 8
Magnetoencephalography (MEG) is a non-invasive tool that can help delineate the epileptogenic zone, in part by localizing interictal epileptic spikes.9; 10 MEG possesses high spatio-temporal resolution without signal deterioration from the skull and scalp that may limit signal propagation with EEG.11 Brain source imaging with MEG can be achieved using equivalent current dipole modeling of interictal spikes, and the position and orientation of the estimated dipole can be overlaid onto the patient’s own co-registered magnetic resonance imaging (MRI) to aid surgical planning.9; 12 This process of interictal spike modeling of MEG data and dipole map overlay is often referred to as magnetic source imaging (MSI). However, despite advances in source localization techniques, MEG is performed in only a minority of pre-surgical epilepsy evaluations, and the clinical value of MEG in surgical epilepsy treatment has been less well established compared to diagnostic modalities such as EEG, ECoG, and MRI.13
Previous studies have compared interictal MEG to other diagnostic modalities for epileptogenic zone localization.14; 15 Stefan and colleagues report the largest series of MEG in epilepsy to date, describing a 70% sensitivity of MEG for detecting epileptic activity in 455 patients, of which 131 had epilepsy surgery.16 However, without detailed analysis of post-operative seizure outcome, only limited conclusions can be drawn regarding the predictive value of MEG in achieving long-term seizure-freedom. While other studies have directly related MEG findings to surgical outcome, their statistical conclusions have been limited by small sample size.14; 17–19 Furthermore, given the additional technical challenges related to source localization of deeper brain structures such as the mesial temporal lobe, some have argued that MEG has diminished clinical utility in TLE compared to neocortical epilepsy.20; 21 Thus, the predictive value of MEG spike mapping in epilepsy surgery remains incompletely understood.
Here we report a retrospective cohort study including 132 patients with focal epilepsy who underwent MEG followed by resective epilepsy surgery at our institution, with seizure outcomes determined at mean post-operative follow-up of 3.6 years (minimum 1 year). We examine the concordance of spike activity mapped by MSI with the area of resection and with the results of other diagnostic modalities, and relate MEG findings to seizure outcome. To our knowledge, this represents the largest reported series of MEG in surgical epilepsy patients with comprehensive long-term seizure outcome data.
Methods
Patients
We retrospectively examined 348 MEG recordings performed in 310 epilepsy patients referred for studies at the University of California, San Francisco (UCSF) Biomagnetic Imaging Laboratory (BIL) between June 1, 2004 and June 30, 2013. Among these, we identified 144 patients who underwent resective surgery for focal epilepsy at our institution following MEG recordings. Twelve patients without at least one year of post-operative follow-up were excluded, and 132 patients were analyzed. Overall, 43% of patients who underwent extra-temporal resection and 36% of those who received temporal lobectomy for intractable epilepsy at our institution during the study period received MEG. While referral criteria in our practice are not strict, MEG is typically performed on patients in whom surgical intervention is being considered, but there is uncertainty regarding seizure focus localization. Prior to MEG, patients have had interictal scalp EEG, high-resolution 3T MRI with epilepsy specific protocols, formal comprehensive neuropsychological evaluation, and long-term inpatient video monitoring with scalp EEG (with the exception of two patients in the present study who did not have long-term EEG). If after these studies, the extent of the epileptogenic zone is not yet clearly delineated for potential surgical resection, or if a lack of concordance between neuroimaging, electrophysiology, and clinical symptomatology is suspected, MEG is pursued. For patients who underwent multiple MEG recording sessions, onlythe last session prior to surgery was considered inthis study. All procedures were in full compliance with UCSF clinical research policies, with research protocol approval by the UCSF Committee on Human Research.
MEG data acquisition and analysis
Simultaneous EEG and MEG recordings were performed inside a magnetically shielded room, with a 275 channel whole-head axial gradiometer system (VSM MedTech, Port Coquitlam, British Columbia). Data were recorded from each patient in a passband of 0–75 Hz (300 Hz sample rate) using a CTF 275 channel whole cortex MEG helmet. Twenty-one channel scalp EEG was recorded simultaneously using a modified international 10–20 system that includes subtemporal electrodes. Thirty to forty minutes of spontaneous data were obtained in 10–15 min intervals with the patient asleep and awake. The position of the head in the MEG dewar relative to the MEG sensors was determined via indicator coils before and after each interval to ensure adequate sampling of the entire magnetic field. The data were bandpass filtered offline at 1–70 Hz.
Spikes were visually identified by a certified EEG technologist (MM) and were confirmed by a board-certified clinical neurophysiologist and epileptologist (HEK). EEG spikes were identified based on the criteria defined by the International Federation of Clinical Neurophysiology (IFCN) for EEG epileptiform discharges.22 MEG spikes were chosen for analysis based on duration (< 80 ms), morphology, field map, and lack of associated artifact. The onset of each spike, defined as the rising deflection of the first sharp negativity from the baseline, was marked and equivalent current dipoles were fit using commercial software provided by CTF Systems (VSM MedTech, Port Coquitlam, British Columbia). Only sources with a goodness of fit higher than 90% were accepted. Co-registration of dipoles to MRI scans was performed using fiducials (nasion and preauricular points) to produce magnetic source images (MSI) of dipoles superimposed on anatomic images. The authors then inspected these results and classified the spike dipoles according to their location.. Of note, simultaneous EEG during MEG was used to define and confirm spikes on MEG, in order to ascertain that a signal was not an artifact or another physiologic feature. EEG was also used as an aid to identify MEG spikes in cases where there is MEG artifact (i.e. by averaging EEG spikes), but EEG was not used to constrain source modeling.
We estimated the concordance between the lobe or lobar regions(s) indicated by spike dipoles localization and three separate reference regions: i) the lobe (or anterior/posterior sublobar region of the frontal lobe) of resection determined by examination of post-operative MRI and operative reports, ii) the epileptogenic zone as estimated by epileptologist review of invasive intra-operative and extra-operative ECoG recordings, including both ictal activity and interictal spikes when available, and iii) the region of abnormality, if present, seen on MRI. Dipoles were considered concordant with the reference region if the dipole location indicated spike source in the same lobe or lobar region. If the region indicated by spike dipoles encompassed more than one lobar region of epileptogenic tissue extent, but the reference region also overlapped these multiple regions (e.g., left lateral parieto-occipital spikes preceding a parieto-occipital cortical resection), findings were considered concordant. Spike activity was considered concordant and specific if no appreciable (>10%) dipole source estimates were observed in other lobes, but considered non-specific in the setting of another distal region with >10% dipoles (the 10% limit was to allow occasional spurious spikes that do not co-localize with the vast majority of dipoles during a recording session). Other scenarios included cases in which dipoles were ipsilateral but in a different region compared to the reference area (concordant lateralization only), and cases of discordant lateralization in which >50% of dipoles were contralateral. Spike localization and the determination of concordance between regions were performed while blinded to the patient’s seizure outcome.
Evaluation of clinical data and seizure outcomes
For all patients, we retrospectively reviewed outpatient and inpatient provider notes, diagnostic and laboratory reports, operative records, and pathology reports. Clinical and demographic data including patient gender, age, handedness, epilepsy duration, surgical history, medication history, MRI results, EEG findings, positron emission tomography (PET) results, use of implanted electrodes for long-term recording, side and region of surgery, as well as the use of intra-operative ECoG were recorded. Details regarding patients’ epilepsy history and seizure semiology, including seizure type and frequency, were obtained from pre-operative and post-operative assessments by epileptologists. Epilepsy risk factors were recorded as listed previously.8 Seizure outcome was determined by the latest patient follow-up with the epileptologist using a modified Engel classification system.23
Surgical decisions were made by a comprehensive team of epileptologists, neurosurgeons, neuropsychologists, neuroradiologists, and other practitioners. Standard pre-operative evaluation included MEG, scalp EEG, and structural MRI, and often included neuropsychological evaluation, positron emission tomography (PET), and long-term video-EEG monitoring with or without extra-operative ECoG using surgically implanted subdural and/or depth electrodes. Intra-operative interictal ECoG was also performed in the majority of cases. Resections were customized to incorporate epileptogenic regions and cerebral lesions, and to preserve eloquent cortex, where applicable. For cases of mesial TLE, anterior temporal lobectomy was performed, including tailored resection of the lateral temporal cortex, amygdala, and hippocampus. Multi-lobe resections were often performed in cases with presumed multi-focal or hemispheric epilepsy syndromes in 18 (14%) patients. Surgical specimens were analyzed by neuropathologists. Cases of malformation of cortical development (MCD) included both type I and II focal cortical dysplasia (FCD) on the modified Palmini classification system.24
Statistical analysis
Individual chi-square (χ2) tests were used to evaluate potential associations between MEG findings defined above (concordant/specific versus non-specific, lateralized only, or discordant) and: i) long-term scalp EEG findings (localized, lateralized only, or not lateralized) ii) location of resection (temporal lobe versus extra-temporal/multi-lobe), and iii) epilepsy pathology (lesional versus non-lesional). To identify associations between various factors of interest and post-operative seizure outcome (Engel I versus Engel II-IV), univariate analysis was performed using a χ2 test for categorical variables (e.g. gender) or an unpaired Student’s t-test for continuous variables (eg., age). An odds ratio (OR) was calculated to examine the association between concordant and specific vs. non-specific or discordant MEG in predicting seizure freedom after resection, with a 95% confidence interval (CI). Statistical significance was assessed at p < 0.05. Statistical analyses were performed using SPSS version 22 (IBM, Somers, NY).
Results
We identified 132 patients with drug-resistant focal epilepsy who were referred for MEG for localization of the epileptogenic zone, followed by resective epilepsy surgery at our institution, and with at least 1 year post-operative follow-up (mean, 3.6 years). Mean age (± SEM) at the time of surgery was 27.3 years (range, 3–68 years), and 73 (55%) individuals were male. Other patient characteristics are provided in Table 1.
Table 1.
Patient characteristics
| Age at surgery | years | 27.3 ± 1.3 |
| Gender | Male | 73 (55) |
| Female | 59 (45) | |
| Handedness | Right | 97 (74) |
| Left | 18 (14) | |
| Ambidextrous | 4 (3) | |
| Not yet lateralized or unknown | 13 (10) | |
| Duration epilepsy | years | 14.0 ± 1.0 |
| Lobe involved | Temporal | 75 (57) |
| Frontal | 27 (21) | |
| Parietal | 7 (5) | |
| Occipital | 5 (4) | |
| Multiple | 18 (14) | |
| Seizure frequency | no. per week | 11.0 ± 1.9 |
| History of generalized seizures | Yes | 78 (59) |
| No | 54 (41) | |
| Side of surgery | Right | 55 (42) |
| Left | 77 (58) | |
| Previous resection | No | 112 (85) |
| Yes | 20 (15) |
Data are N (%) for categorical variables or mean ± SEM for continuous variables. N = 132 patients.
MCD: malformation of cortical development; MTS: mesial temporal sclerosis.
Interictal spike dipole modelling
Among 132 patients, 103 (78%) had successful modelling of MEG dipole activity corresponding to interictal spikes. Spike activity was not observed during recordings in 25 (19%) patients, and metallic artifact (e.g., dental implants) limited MEG interpretation in 4 (3%) cases. No differences in patient age, seizure frequency, or duration of epilepsy were observed between patients with or without successful dipole modelling (p > 0.05, unpaired t-tests).
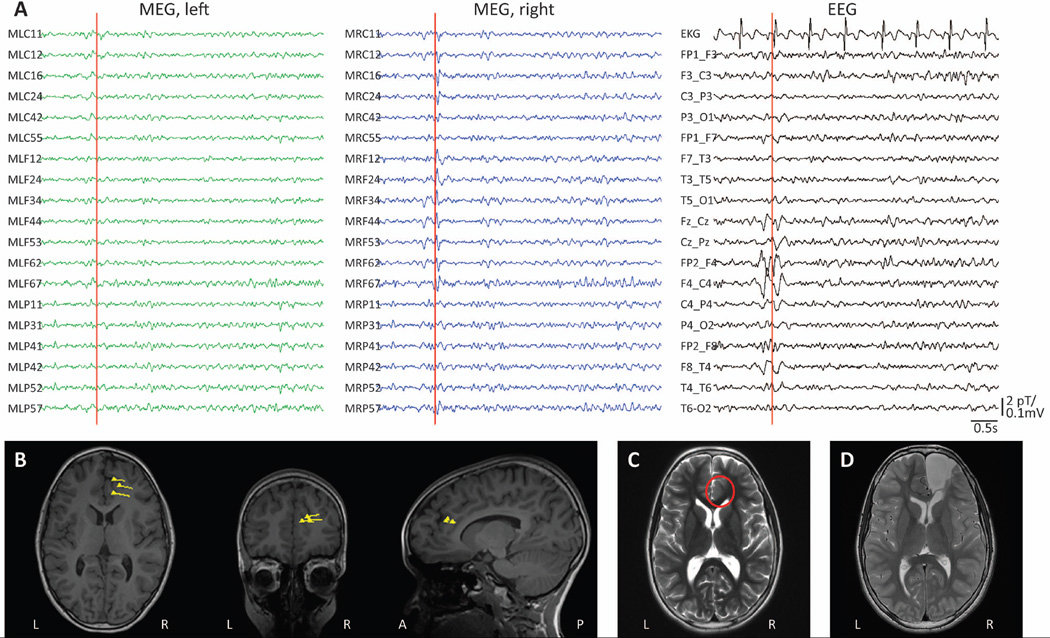
An example of successful dipole localization is displayed in Figure 1. In this 12-year-old female with drug-resistant focal epilepsy, simultaneous MEG/EEG recordings demonstrated interictal spikes in the right frontal/central region (Fig. 1A), localized by equivalent current dipole modelling to the right anterior cingulate cortex (Fig. 1B). MRI revealed a subtle blurring of gray-white matter differentiation at this location (Fig. 1C), which was resected under the guidance of intra-operative ECoG (Fig. 1D). Post-operatively, neuropathological examination suggested FCD type IIA, and the patient remains seizure-free (Engel I outcome) two years after surgery.
Figure 1. Example of MSI dipole modelling with simultaneous MEG/EEG recordings.
A) Recordings from selected MEG channels and simultaneous EEG in a 12-year-old female with drug-resistant focal epilepsy. A representative interictal spike is seen in both MEG and EEG recordings localizing to the right frontal/central region. B) Localization of single dipole sources corresponding to the spike in A, and similar spikes during the recording, shown as triangles with vector tails superimposed on T1-weighted anatomical MRI. C) Pre-operative T2-weighted axial MRI showing a subtle abnormal blurring of gray-white matter differentiation in the right anterior cingulate region (circle), proximal to the location of MEG dipoles. D) Post-operative T2-weighted axial MRI demonstrating the resection cavity. Neuropathological examination revealed FCD type IIA, and the patient remains seizure-free two years after surgery. EEG: electroencephalography; FCD: focal cortical dysplasia; MEG: magnetoencephalography; MRI: magnetic resonance imaging; MSI: magnetic source imaging.
Concordance between MEG findings and the epileptogenic zone
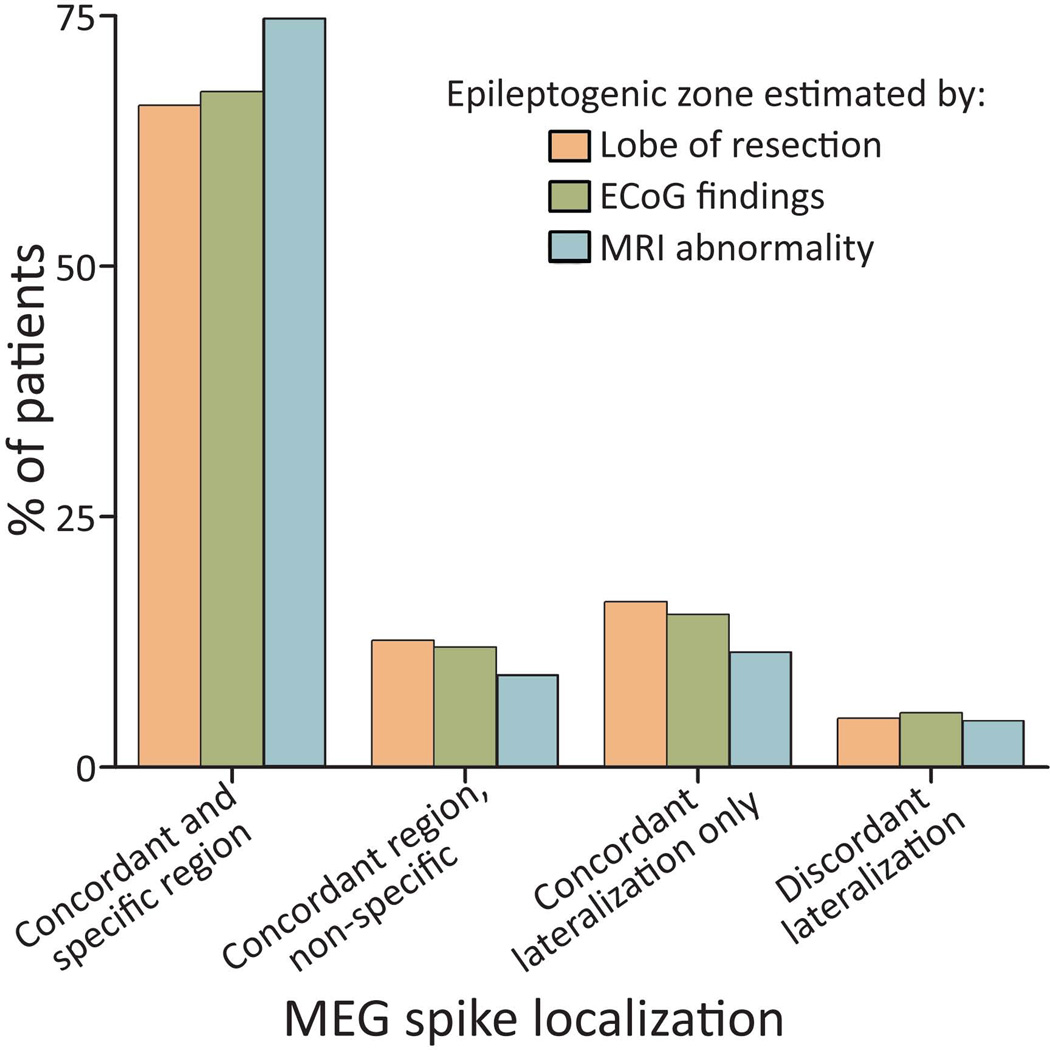
To estimate the accuracy and reliability of MEG, we examined the concordance between MEG spike localization with three reference regions: the region of resection, the epileptogenic zone delineated by intra-operative or extra-operative ECoG, and the area of abnormal MRI findings (Fig. 2). These reference regions coincided with each other in most, but not all, cases. MEG findings were concordant with and specific to: i) the lobe of resection in 68 (66%) of 103 patients with successful MEG dipole modelling, ii) ECoG findings in 62 (67%) of 92 patients who had invasive recordings, and iii) an MRI abnormality in 65 (74%) of 87 cases with a radiological lesion. Across all reference categories, MEG dipoles were concordant with the reference region but non-specific (i.e. spikes also seen in another lobe) in 9–13% of comparisons, dipoles localized to the same side but not region as the reference area in 11–17% of cases, and MEG was discordant, with most dipoles localizing to the contralateral hemisphere, in 5% of cases. These results suggest that among patients in whom interictal spikes are mapped, MEG provides accurate and specific localization of the involved lobe in about two-thirds of cases, with discordant lateralization approximately 5% of the time.
Figure 2. MEG concordance with the area of resection, ECoG, and MRI.
Shown is the number of patients with concordance between the region of MEG spike activity to three reference regions: i) the region of resection, ii) the epileptogenic zone delineated by ECoG, and iii) MRI abnormality. Cases are classified as concordant and specific, concordant but non-specific (same region, but >10% spikes also noted elsewhere), concordant lateralization only (same side, different region), and discordant lateralization (>50% spikes contralateral). Only 103 (78%) of 132 patients are included in this graph, as no spikes were modelled with MEG in 29 patients. ECoG: electrocorticography; MEG: magnetoencephalography; MRI: magnetic resonance imaging.
We also asked whether MEG reliability might differ across patients with different long-term EEG results, pathological findings, or surgery location (Table 2). The rate of concordant/specific MEG results (i.e. first group in Fig. 2) was comparable in patients with localized, lateralized only, or non-lateralized long-term findings on long-term scalp EEG (Table 2A), and no significant difference in MEG concordance was observed in patients with lesional vs. non-lesional epilepsy (Table 2B), or in individuals with temporal lobe vs. extra-temporal/multi-lobe epilepsy (Table 3C). Overall, MEG reliability appears similar across these various patient groups.
Table 2.
MEG findings by EEG results, pathology, and location
| MEG findings | ||
|---|---|---|
| Concordant and specific |
Non-specific, lateralized-only or discordant |
|
| A) Long-term scalp EEG findings | ||
| Localized | 42 (69) | 19 (31) |
| Lateralized only | 15 (58) | 11 (42) |
| Not lateralized | 9 (64) | 5 (36) |
| χ2 = 2.0, p = 0.56 | ||
| B) Lesional vs. non-lesional pathology* | ||
| Lesional | 47 (67) | 23 (33) |
| Non-lesional | 21 (64) | 12 (36) |
| χ2 = 0.1, p = 0.73 | ||
| B)Temporal lobe vs. extra-temporal/multi-lobe | ||
| Temporal | 37 (64) | 21 (36) |
| Extra-temporal/multi-lobe | 31 (69) | 14 (31) |
| χ2 = 0.3, p = 0.59 | ||
| Total | 68 (66) | 35 (34) |
Data are N (%). N = 103 patients with MEG spikes modelled (except 2 patients who did not undergo long-term EEG are excluded from A).
Lesional epilepsy is defined by a distinct lesion (e.g. tumor, mesial temporal sclerosis), while cases with gliosis only or no pathological findings are considered non-lesional.
Table 3.
Seizure outcomes and associated factors
| Engel I | Engel II–IV | p value | ||
|---|---|---|---|---|
| A) Patient demographics | ||||
| Age at surgery | years | 26.3 ± 1.5 | 29.5 ± 2.4 | 0.26 |
| Gender | Male | 54 (74) | 19 (26) | 0.26 |
| Female | 38 (64) | 21 (36) | ||
| Handedness | Right | 66 (68) | 31 (32) | 0.91 |
| Left | 13 (72) | 5 (28) | ||
| Ambidextrous | 3 (75) | 1 (25) | ||
| Not yet lateralized or unknown | 10 (77) | 3 (23) | ||
| B) Epilepsy characteristics | ||||
| Duration epilepsy | years | 13.4 ± 1.2 | 15.5 ± 1.8 | 0.34 |
| Seizure frequency | no. per week | 10.2 ± 2.3 | 13.0 ± 3.5 | 0.50 |
| AED regimen tried | no. | 4.5 ± 0.3 | 5.7 ± 0.5 | 0.03* |
| Epilepsy risk factors | no. | 0.78 ± 0.10 | 0.85 ± 0.12 | 0.69 |
| Lobe involved | Temporal | 55 (73) | 20 (27) | 0.43 |
| Frontal | 16 (59) | 11 (41) | ||
| Parietal | 5 (71) | 2 (29) | ||
| Occipital | 4 (80) | 1 (20) | ||
| Multi-lobe | 8 (57) | 6 (43) | ||
| Hemispherectomy | 4 (100) | 0 | ||
| Primary Pathology | Gliosis only | 23 (55) | 19 (45) | 0.05 |
| FCD | 26 (79) | 7 (21) | ||
| MTS | 21 (84) | 4 (16) | ||
| Tumor | 17 (77) | 5 (23) | ||
| Ischemia/infarct | 3 (60) | 2 (40) | ||
| Other | 2 (40) | 3 (60) | ||
| History of generalized seizures | Yes | 48 (62) | 30 (38) | 0.02* |
| No | 44 (82) | 10 (18) | ||
| C) Pre-operative diagnostics | ||||
| MEG | Concordant and specific | 58 (85) | 10 (15) | < 0.001* |
| Non-specific or discordant | 13 (37) | 22 (63) | ||
| No spikes modelled | 21 (72) | 8 (28) | ||
| MRI | Abnormal | 79 (73) | 30 (27) | 0.14 |
| Normal | 13 (57) | 10 (43) | ||
| Ictal scalp EEG | Localized | 63 (80) | 16 (20) | < 0.01* |
| Not localized | 26 (52) | 24 (48) | ||
| Implanted ECoG | Performed | 35 (60) | 23 (40) | 0.06 |
| Not performed | 57 (77) | 17 (23) | ||
| PET (when performed) | Abnormal | 17 (65) | 9 (35) | 0.12 |
| Normal | 12 (92) | 1 (8) | ||
| D) Operative factors | ||||
| Side of surgery | Right | 39 (71) | 16 (29) | 0.85 |
| Left | 53 (69) | 24 (31) | ||
| Intra-operative ECoG | Performed | 76 (70) | 32 (30) | 0.81 |
| Not performed | 16 (67) | 8 (33) | ||
| Extent of resection (Tumor, vascular malformation, tuber, cyst only) | Gross-total | 16 (80) | 4 (20) | 0.05 |
| Subtotal | 2 (33) | 4 (67) | ||
| Previous resection | No | 80 (71) | 32 (29) | 0.30 |
| Yes | 12 (60) | 8 (40) | ||
| TOTAL | 92 (70) | 40 (30) | ||
Data are N (%) for categorical variables or mean ± SEM for continuous variables.
Statistically significant value (p < 0.05) from χ2 test (categorical) or t-test (continuous) comparing patients with Engel I versus II–IV seizure outcomes.
AED: anti-epileptic drug; ECoG: electrocorticography, EEG: electroencephalography; FCD: focal cortical dysplasia; MEG: magnetoencephalography; MRI: magnetic resonance imaging; MTS: mesial temporal sclerosis; PET: positron emission tomography.
MEG concordance in predicting seizure outcome
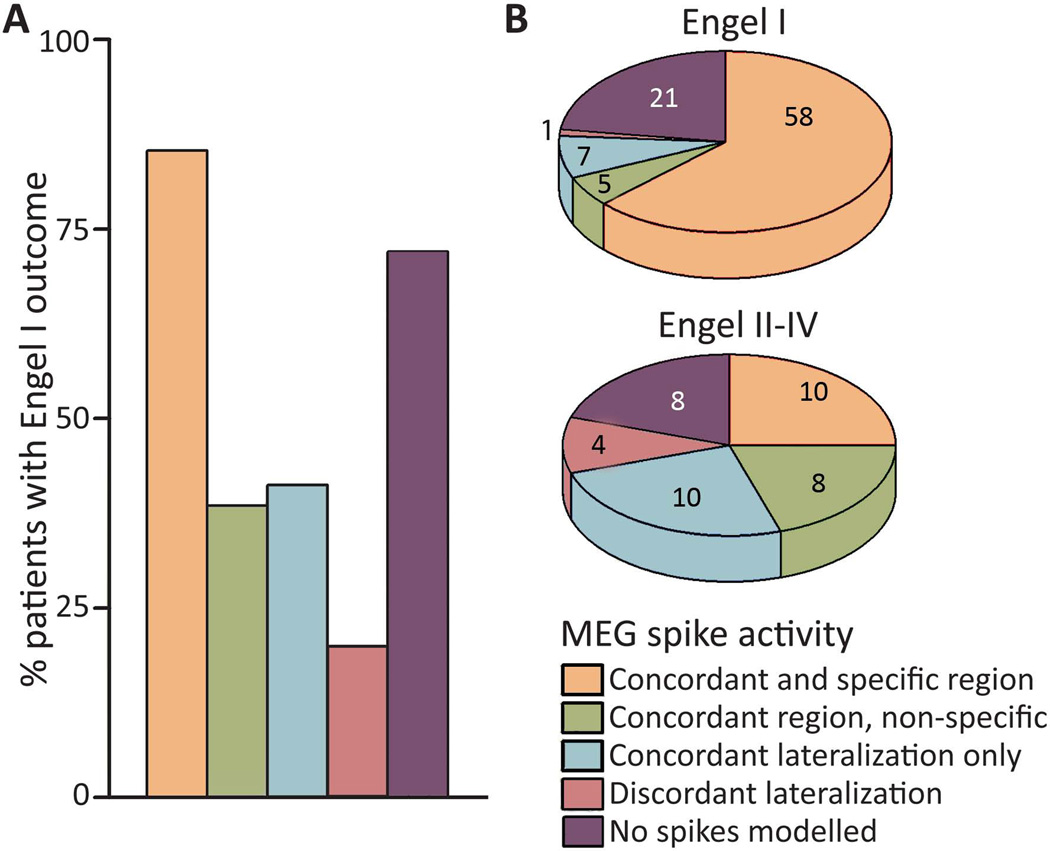
After surgery, 92 (70%) patients were free of disabling seizures (Engel IA-D), including 67 (51%) individuals who achieved complete seizure-freedom (Engel IA). Other seizure outcomes were Engel II, Engel III, or Engel IV in 13 (10%), 17 (13%), and 10 (8%) patients, respectively. We examined the relationship between post-operative seizure outcome and MEG concordance with the area of resection (Fig. 3). Among patients with successful dipole modelling, Engel I outcome was achieved by 85% of 68 individuals with concordant/specific MEG findings, but in only 37% of 35 patients with spike dipoles that were lateralized-only, non-specific, or discordant (χ2 = 26.4, p < 0.001). Of note, concordant/specific MEG was associated with seizure-freedom in both patients with localized ictal scalp EEG (n = 79, χ2 = 6.4, p = 0.02) and with non-localized EEG (n = 50, χ2 = 9.8, p = 0.002), suggesting that MEG contributes useful diagnostic information in patients with ambiguous EEG findings. Only one of five (20%) patients with MEG spike lateralization discordant with the area of resection became seizure-free. Overall, concordant and specific MEG findings predicted seizure freedom with an odds ratio of 5.11 (2.23–11.8, 95% CI). These findings suggest that seizure freedom is significantly more likely when resection is performed in agreement with dipole localization than in cases without concordance between the lobe of resection and MEG.
Figure 3. Relationship between MEG findings and seizure outcome.
A) Post-operative seizure-freedom (Engel I outcome) was significantly more common in patients with concordant and specific MEG (85% seizure-free) than in patients with non-specific, lateralized-only, or discordant MEG (37% seizure-free overall, χ2 = 26.4, p < 0.001). Among patients with no spikes modelled, 72% were seizure-free after surgery. B) MEG findings stratified by seizure outcome. For details of these categories, see Fig. 2 legend. N = 132 patients. MEG: magnetoencephalography.
Seizure outcomes were stratified across various other factors of interest, as listed in Table 3. Although no relationship between demographics and seizure outcome was observed (Table 3A), patients who became seizure-free had tried slightly fewer anti-epileptic drugs than those with persistent seizures (Table 3B). Also, fewer patients with a history of generalized tonic-clonic seizures became seizure-free (62%) than those with only partial seizures (82%). Seizure freedom was more common among individuals with lesional pathologies – such as mesial temporal sclerosis (84%), focal cortical dysplasia (79%), and tumor (77%) – than among patients with the non-specific finding of gliosis only (55%). Examining pre-operative diagnostic studies, individuals with a localized ictal EEG were more likely to achieve Engel I outcome than those with non-localized EEG (Table 3C). Finally, among those patients with a well-defined radiological lesion, outcomes were more favorable with gross-total over subtotal resection (Table 3D).
Discussion
Here we report MEG dipole mapping results and long-term seizure outcomes in 132 patients who underwent MEG prior to resective epilepsy surgery at our institution. Interictal MEG spike activity was observed in 78% of patients, and spike localization was concordant with and specific to the lobe of resection in two-thirds of these individuals, with discordant lateralization in 5% of cases. Post-operative seizure-freedom was achieved in 85% of patients with MEG findings concordant with and specific to the region of resection. In contrast, only 37% of individuals became seizure-free when resection was performed in the setting of non-specific or discordant MEG results. Finally, MEG predicted seizure freedom on multivariate analysis, along with localized ictal scalp EEG and a lack of generalized seizures. Our results suggest that although MEG dipole mapping is not without limitations, it represents a valuable non-invasive tool to help localize the epileptogenic zone in pre-surgical epilepsy patients, and may assist with outcome prognostication.
Previous studies have examined the sensitivity and accuracy of MEG in epilepsy surgery planning. In the largest series of MEG recordings in epilepsy, Stefan and colleagues reported a 70% sensitivity of MEG for epileptic activity across 455 patients, with localization of the correct lobe to be treated in 89% of 131 surgical cases.16 Some groups have reported that MEG may be more likely to localize to the epileptogenic zone than interictal or ictal scalp EEG.14; 15 Other investigators have reported good agreement between MEG and invasive electrodiagnostic modalities in patient series with implanted grid/strip electrodes or stereo-electroencephalography.19; 25; 26 Also, some authors have described an important role of MEG in planning surgical placement of intracranial electrodes.27; 28 Finally, various groups have reported clinical utility of MEG in planning resection for focal neocortical epilepsy,17; 29; 30 hemispheric epilepsy,31 and re-operation after failed epilepsy surgery.32 In our study, MEG dipole modelling was found to have high concordance with MRI and ECoG findings, and was also found to have similar reliability in both patients with or without localized findings on long-term scalp EEG. Our results confirm the utility of MEG spike localization described in these prior reports, and extend these findings by demonstrating the value of MEG in predicting long-term surgical outcome.
MEG in temporal lobe epilepsy
Given that source localization may be more challenging with deeper regions - including the mesial temporal structures - some have argued that MEG has diminished clinical utility in TLE.20; 21 Our group previously reported a series of 25 patients with mesial TLE, in which MEG interictal spikes were observed in 86% of patients, including well-localized spikes in two-thirds of individuals with a non-localized MRI.33 Other studies have also demonstrated favorable dipole localization with MEG in TLE patients.34; 35 In the present series, concordance between MEG dipoles and the lobe of resection was comparable between TLE and extra-temporal lobe epilepsy patients, and between patients with mesial temporal sclerosis versus other pathologies. However, our study does not address the localizing value of mesial versus lateral temporal dipoles in patients with mesial TLE, as temporal lobectomies in our series were performed anatomically, including resection of both mesial structures and lateral temporal cortex. Also, MEG referrals for typical cases of mesial temporal sclerosis are less common in our practice, which may contribute to selection bias in this study. While our results suggest MEG can be a clinically useful in confirming TLE, the predictive value of mesial temporal spike localization warrants further attention. Potentially, this issue could be addressed by examining mesial versus lateral temporal MEG spikes in patients who receive selective amygdalo-hippocampectomy with preservation of the lateral temporal cortex.
Technique limitations and future directions
While our results support a role for MEG interictal dipole mapping, there are limitations to the technique, highlighted by patients in which spikes were not encountered, and those with non-specific localization. MEG has higher spatial resolution without signal loss by the skull and scalp compared to scalp EEG, but MEG is typically limited to interictal recordings, and thus cannot replace video/EEG monitoring of ictal events. Localization of interictal spikes with either MEG or electrodiagnostic studies can help to identify the irritative zone, but this may not always coincide with the epileptogenic zone required for seizure generation.36 Furthermore, it is important to recognize that in the present study, MEG was typically performed late in the pre-surgical evaluation process, in cases without clear lineation of the epileptogenic zone after scalp EEG and MRI. This precludes direct comparison of the clinical utility of MEG with that of EEG or MRI. Also, although MEG is noninvasive and allows whole-brain coverage, direct recordings with invasive ECoG remain the gold standard for high-resolution and reliable delineation of the seizure focus.6 As with other diagnostic modalities, MSI dipole modelling requires specialized training and is prone to interpretation error. Although advanced adaptive spatial filtering techniques can be employed for automated spike localization, further validation is required before such methods can replace manual dipole fitting.37 Beyond dipole mapping, other potential roles for MEG in epilepsy evaluation may include the investigation of high frequency oscillations (HFOs), of which the localizing value has become increasingly appreciated in epilepsy,38 and MEG-based functional connectivity analysis, which have been used successfully to explore resting-state network disturbances in numerous brain disorders.39
Study design limitations
The most notable limitation of the present study design is its non-randomized, retrospective nature. While we can ascertain the statistical relationship between MEG results and seizure outcome in hindsight, we cannot directly access the effect that MEG had on planning the resection, and thus, on seizure outcome. Furthermore, selection bias is likely an important limitation of our study, given that only a subset of surgical epilepsy patients – typically those with an epileptogenic zone that is more difficult to localize – received MEG at our center. This must be carefully considered in the interpretation of our results. A randomized, controlled trial examining the effects of pre-surgical MEG use on seizure outcome in epilepsy surgery would be the definitive study to address the clinical value of this modality. In such a study, patients with medically-refractory epilepsy presumed to be both localization-related and non-eloquent after long-term video-EEG monitoring and high-resolution MRI would be randomized to receive or not receive MEG prior to final determination of the surgical plan (resection or staged placement of invasive electrodes). However, ethical concerns may limit the feasibility of such a trial, particularly given that in our experience, final selection of a surgical target is sometimes not achieved without a convincing cluster of dipoles on MEG. The strength of our present study, however, is that it is the largest series to date examining MEG results in epilepsy patients with long-term seizure outcomes.
Conclusions
MEG represents a useful tool for interictal spike localization in pre-surgical epilepsy patients, given high spatio-temporal resolution and lack of signal deterioration by the skull and scalp. Although interictal spikes are not always observed with MEG, dipole modelling was successful in 78% patients in the present study, providing accurate and specific localization of the region for resection in two-thirds of those individuals. Seizure freedom was common (85%) in patients with localizing and specific MEG findings, but uncommon (37%) when the resection was performed with non-specific or discordant MEG results. Although the technique has its limitations, MEG dipole mapping can provide valuable information for surgical planning, and other potential uses for MEG in the evaluation of epilepsy patients warrant further attention.
Key Bullet Points.
-
-
MEG is a valuable tool for non-invasive interictal spike mapping in epilepsy surgery.
-
-
In particular, MEG may be useful in patients with non-localized findings on long-term EEG monitoring.
-
-
Localization of the epileptogenic zone using MEG is associated with improved seizure outcomes.
Acknowledgements
This was supported by the National Institutes of Health (F32-NS086353 to DJE). We thank all members of the UCSF Comprehensive Epilepsy Center and the Biomagnetic Imaging Lab for their support of this work, and for continued excellence in patient care.
Footnotes
Disclosures of Conflicts of Interest
The authors have no conflicts of interest to disclose.
Ethical publication
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Cascino GD. When drugs and surgery don't work. Epilepsia. 2008;49(Suppl 9):79–84. doi: 10.1111/j.1528-1167.2008.01930.x. [DOI] [PubMed] [Google Scholar]
- 2.Englot DJ, Lee AT, Tsai C, et al. Seizure types and frequency in patients who "fail" temporal lobectomy for intractable epilepsy. Neurosurgery. 2013;73:838–844. doi: 10.1227/NEU.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 3.Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7:525–537. doi: 10.1016/S1474-4422(08)70109-1. [DOI] [PubMed] [Google Scholar]
- 4.Englot DJ, Breshears JD, Sun PP, et al. Seizure outcomes after resective surgery for extra-temporal lobe epilepsy in pediatric patients. J Neurosurg Pediatr. 2013;12:126–133. doi: 10.3171/2013.5.PEDS1336. [DOI] [PubMed] [Google Scholar]
- 5.Englot DJ, Rolston JD, Wang DD, et al. Seizure outcomes after temporal lobectomy in pediatric patients. J Neurosurg Pediatr. 2013;12:134–141. doi: 10.3171/2013.5.PEDS12526. [DOI] [PubMed] [Google Scholar]
- 6.Yuan J, Chen Y, Hirsch E. Intracranial electrodes in the presurgical evaluation of epilepsy. Neurol Sci. 2012;33:723–729. doi: 10.1007/s10072-012-1020-2. [DOI] [PubMed] [Google Scholar]
- 7.Englot DJ, Han SJ, Rolston JD, et al. Epilepsy surgery failure in children: a quantitative and qualitative analysis. J Neurosurg Pediatr. 2014;14:386–395. doi: 10.3171/2014.7.PEDS13658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Englot DJ, Raygor KP, Molinaro AM, et al. Factors associated with failed focal neocortical epilepsy surgery. Neurosurgery. 2014;75:648–656. doi: 10.1227/NEU.0000000000000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirsch HE, Mantle M, Nagarajan SS. Concordance between routine interictal magnetoencephalography and simultaneous scalp electroencephalography in a sample of patients with epilepsy. J Clin Neurophysiol. 2007;24:215–231. doi: 10.1097/WNP.0b013e3180556095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tovar-Spinoza ZS, Ochi A, Rutka JT, et al. The role of magnetoencephalography in epilepsy surgery. Neurosurg Focus. 2008;25:E16. doi: 10.3171/FOC/2008/25/9/E16. [DOI] [PubMed] [Google Scholar]
- 11.Zumer JM, Attias HT, Sekihara K, et al. A probabilistic algorithm integrating source localization and noise suppression for MEG and EEG data. Neuroimage. 2007;37:102–115. doi: 10.1016/j.neuroimage.2007.04.054. [DOI] [PubMed] [Google Scholar]
- 12.Funke M, Constantino T, Van Orman C, et al. Magnetoencephalography and magnetic source imaging in epilepsy. Clin EEG Neurosci. 2009;40:271–280. doi: 10.1177/155005940904000409. [DOI] [PubMed] [Google Scholar]
- 13.Stefan H, Rampp S, Knowlton RC. Magnetoencephalography adds to the surgical evaluation process. Epilepsy Behav. 2011;20:172–177. doi: 10.1016/j.yebeh.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Paulini A, Fischer M, Rampp S, et al. Lobar localization information in epilepsy patients: MEG--a useful tool in routine presurgical diagnosis. Epilepsy Res. 2007;76:124–130. doi: 10.1016/j.eplepsyres.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Pataraia E, Simos PG, Castillo EM, et al. Does magnetoencephalography add to scalp video-EEG as a diagnostic tool in epilepsy surgery? Neurology. 2004;62:943–948. doi: 10.1212/01.wnl.0000115122.81621.fe. [DOI] [PubMed] [Google Scholar]
- 16.Stefan H, Hummel C, Scheler G, et al. Magnetic brain source imaging of focal epileptic activity: a synopsis of 455 cases. Brain. 2003;126:2396–2405. doi: 10.1093/brain/awg239. [DOI] [PubMed] [Google Scholar]
- 17.Mu J, Rampp S, Carrette E, et al. Clinical relevance of source location in frontal lobe epilepsy and prediction of postoperative long-term outcome. Seizure. 2014;23:553–559. doi: 10.1016/j.seizure.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Fischer MJ, Scheler G, Stefan H. Utilization of magnetoencephalography results to obtain favourable outcomes in epilepsy surgery. Brain. 2005;128:153–157. doi: 10.1093/brain/awh333. [DOI] [PubMed] [Google Scholar]
- 19.Knowlton RC, Elgavish R, Howell J, et al. Magnetic source imaging versus intracranial electroencephalogram in epilepsy surgery: a prospective study. Ann Neurol. 2006;59:835–842. doi: 10.1002/ana.20857. [DOI] [PubMed] [Google Scholar]
- 20.Leijten FS, Huiskamp GJ, Hilgersom I, et al. High-resolution source imaging in mesiotemporal lobe epilepsy: a comparison between MEG and simultaneous EEG. J Clin Neurophysiol. 2003;20:227–238. doi: 10.1097/00004691-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Shigeto H, Morioka T, Hisada K, et al. Feasibility and limitations of magnetoencephalographic detection of epileptic discharges: simultaneous recording of magnetic fields and electrocorticography. Neurol Res. 2002;24:531–536. doi: 10.1179/016164102101200492. [DOI] [PubMed] [Google Scholar]
- 22.Deuschl G, Eisen A. Recommendations for the practice of clinical neurophysiology: guidelines of the International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:1–304. [PubMed] [Google Scholar]
- 23.Engel J, Van Ness P, Rasmussen T, et al. Outcome with respect to epileptic seizures. In: Engel J, editor. Surgical Treatment of the Epilepsies. ed 2. New York: Raven Press; 1993. pp. 609–621. [Google Scholar]
- 24.Blumcke I, Thom M, Aronica E, et al. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2011;52:158–174. doi: 10.1111/j.1528-1167.2010.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung J, Bouet R, Delpuech C, et al. The value of magnetoencephalography for seizure-onset zone localization in magnetic resonance imaging-negative partial epilepsy. Brain. 2013;136:3176–3186. doi: 10.1093/brain/awt213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agirre-Arrizubieta Z, Huiskamp GJ, Ferrier CH, et al. Interictal magnetoencephalography and the irritative zone in the electrocorticogram. Brain. 2009;132:3060–3071. doi: 10.1093/brain/awp137. [DOI] [PubMed] [Google Scholar]
- 27.Knowlton RC, Razdan SN, Limdi N, et al. Effect of epilepsy magnetic source imaging on intracranial electrode placement. Ann Neurol. 2009;65:716–723. doi: 10.1002/ana.21660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutherling WW, Mamelak AN, Thyerlei D, et al. Influence of magnetic source imaging for planning intracranial EEG in epilepsy. Neurology. 2008;71:990–996. doi: 10.1212/01.wnl.0000326591.29858.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim H, Kankirawatana P, Killen J, et al. Magnetic source imaging (MSI) in children with neocortical epilepsy: surgical outcome association with 3D post-resection analysis. Epilepsy Res. 2013;106:164–172. doi: 10.1016/j.eplepsyres.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Mamelak AN, Lopez N, Akhtari M, et al. Magnetoencephalography-directed surgery in patients with neocortical epilepsy. J Neurosurg. 2002;97:865–873. doi: 10.3171/jns.2002.97.4.0865. [DOI] [PubMed] [Google Scholar]
- 31.Torres CV, Fallah A, Ibrahim GM, et al. The role of magnetoencephalography in children undergoing hemispherectomy. J Neurosurg Pediatr. 2011;8:575–583. doi: 10.3171/2011.8.PEDS11128. [DOI] [PubMed] [Google Scholar]
- 32.Mohamed IS, Otsubo H, Ochi A, et al. Utility of magnetoencephalography in the evaluation of recurrent seizures after epilepsy surgery. Epilepsia. 2007;48:2150–2159. doi: 10.1111/j.1528-1167.2007.01271.x. [DOI] [PubMed] [Google Scholar]
- 33.Kaiboriboon K, Nagarajan S, Mantle M, et al. Interictal MEG/MSI in intractable mesial temporal lobe epilepsy: spike yield and characterization. Clin Neurophysiol. 2010;121:325–331. doi: 10.1016/j.clinph.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baumgartner C, Pataraia E, Lindinger G, et al. Neuromagnetic recordings in temporal lobe epilepsy. J Clin Neurophysiol. 2000;17:177–189. doi: 10.1097/00004691-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Stephen JM, Ranken DM, Aine CJ, et al. Differentiability of simulated MEG hippocampal, medial temporal and neocortical temporal epileptic spike activity. J Clin Neurophysiol. 2005;22:388–401. [PubMed] [Google Scholar]
- 36.Ray A, Tao JX, Hawes-Ebersole SM, et al. Localizing value of scalp EEG spikes: a simultaneous scalp and intracranial study. Clin Neurophysiol. 2007;118:69–79. doi: 10.1016/j.clinph.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Kirsch HE, Robinson SE, Mantle M, et al. Automated localization of magnetoencephalographic interictal spikes by adaptive spatial filtering. Clin Neurophysiol. 2006;117:2264–2271. doi: 10.1016/j.clinph.2006.06.708. [DOI] [PubMed] [Google Scholar]
- 38.Bragin A, Engel J, Jr, Staba RJ. High-frequency oscillations in epileptic brain. Curr Opin Neurol. 2010;23:151–156. doi: 10.1097/WCO.0b013e3283373ac8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guggisberg AG, Honma SM, Findlay AM, et al. Mapping functional connectivity in patients with brain lesions. Ann Neurol. 2008;63:193–203. doi: 10.1002/ana.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]