Abstract
Suppressor of cytokine signaling-1 (SOCS-1) is a member of the suppressor of cytokine signaling family of proteins and an inhibitor of interleukin-6 (IL-6) signaling. SOCS-1 has been shown to protect cells from cellular damage and apoptosis induced by tumor necrosis factor (TNF), lipopolysaccharide (LPS), and interferon gamma (IL-γ). However, it is not known whether increased SOCS-1 is protective during pulmonary oxidative stress. Therefore, we hypothesized that increased SOCS-1 in the lungs of mice would be protective in the setting of hyperoxic lung injury. We administered SOCS-1 adenovirus (Ad-SOCS-1) intratracheally into the lungs and exposed the mice to 100% O2. Mice infected with GFP adenovirus (Ad-GFP) were used as controls. Mice treated with Ad-SOCS-1 had enhanced survival in 100% oxygen compared to Ad-GFP-administered mice. After 3 days of hyperoxia, Ad-GFP mice were ill and tachypnic and died after 4 days. In contrast, all Ad-SOCS-1-treated mice survived for at least 6 days in hyperoxia and 80% survived beyond 7 days. Ad-SOCS-1 transfection protected mouse lungs from injury as indicated by lower lung wet/dry weight, alveolar-capillary protein leakage, reduced infiltration of inflammatory cells, and lower content of thiobarbituric acid-reactive substances in lung homogenate. Our results also indicated that Ad-SOCS-1 significantly inhibits hyperoxia-induced ASK-1 (apoptosis signal-regulating kinase 1) expression. Taken together, these findings show that increased expression of adenovirus-mediated SOCS-1 in the lungs of mice significantly protects against hyperoxic lung injury.
Keywords: Adenovirus, acute lung injury (ALI), suppressor of cytokine signaling-1 (SOCS-1), hyperoxia, inflammation, apoptosis signal-regulating kinase-1 (ASK-1)
Introduction
Acute lung injury (ALI) is a major clinical problem in the United States with an estimated incidence rate of 262,500 patients and 40-50% mortality [1-3]. In experimental animals, detrimental effects of hyperoxia manifest as ALI. Hyperoxia therapy is a necessary part of treatment for patients with acute and chronic cardiovascular and pulmonary diseases [4-8]. However, prolonged exposure to hyperoxia may lead to ALI. ALI is characterized by severe alveolar damage resulting from an acute inflammatory response that leads to immune cell infiltration and edema [9,10]. The fundamental mechanism of this serious condition evolves from an imbalance between proinflammatory and anti-inflammatory cytokines, and the simultaneous induction of apoptosis activators [1,2,11]. Hence, a viable approach to prevent ALI would be to resolve this imbalance. It is known that cytokine signaling is mediated via the Janus Kinases-Signal Transducer and Activator of Transcription (JAK-STAT) signaling pathway, an important contributor to the production of inflammatory cytokines [12]. The suppressor of cytokine signaling-1 (SOCS-1) protein is a physiological regulator of cytokine production and has been shown to be the most efficient inhibitor of JAK-STAT pathway [13].
SOCS-1 is an anti-apoptotic and potent anti-inflammatory, negative regulator of the IL-6-mediated JAK-STAT signaling pathway [14,15]. It has also been reported that SOCS-1 exerts its protective effects against apoptosis induced by TNF-α, INF-γ and LPS [16-21]. Our recent report suggests that IL-6 cytoprotection against hyperoxic acute lung injury (HALI) is associated with enhanced SOCS-1 expression [4]. However, the therapeutic role of SOCS-1 under oxidative stress is not yet known.
Apoptosis signal-regulating kinase-1 (ASK-1) is one of the key mitogen-activated protein kinases (MAPKs) required for reactive oxygen species (ROS) and TNF-α induced cell death and inflammation [21]. Recent studies suggest the possibility that inhibitors of ASK-1 have potential benefits in the management of ALI [22-25] and suggest that ASK-1 is significantly activated and involved in HALI [4]. The presented evidence indicates that Ad-SOCS-1 can protect against hyperoxic injury and is associated in the suppression of ASK-1.
Materials and Methods
Reagents and antibodies
The following antibodies were used: SOCS-1 (Immuno-Biological Laboratories, Minneapolis, MN), GAPDH (Cell Signaling Technology, Inc., Beverly, MA), ASK-1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and caspase-3 (Cell Signaling Technology, Inc., Beverly, MA). The protein concentration of cell lysates was quantified using a BCA assay kit (Thermo scientific, Rockford, IL). The SOCS-1 adenovirus (Ad-SOCS-1) and GFP adenovirus (Ad-GFP) were gifts kindly provided by Dr. Akihiko Yoshimura (Japan). All other reagents were purchased from Sigma (St Louis, MO).
Mice
The approval of these animal procedures was obtained from the University of South Florida Institutional Animal Care and Use Committee (IACUC) and all mice were maintained in a specific-pathogen-free animal facility at the University of South Florida. C57BL/6J (Wild type, 6 weeks old, 50% male and 50% female; n = 20) mice were purchased from Harlan laboratories (Indianapolis, IN).
Transfection protocols
Cells were treated with Phosphate Buffered Saline (PBS), Ad-GFP, or Ad-SOCS-1 as mentioned previously [26]. After reaching confluence, cells were transduced with adenoviral stock containing 108 Plaque Forming units (PFU) according to the manufacturer’s protocol (Millipore, Billerica, MA). Within 10-13 days, this treatment yielded greater than 95% of stable transductants. Adenoviral vectors were used to attain in vivo stable transduction of mice. C57BL/6 mice were intraperitoneally anesthetized with a ketamine/xylazine mixture. Prior to injection, the ventral area of the neck was sprayed with alcohol. Small incision was made in the ventral neck skin area to expose the trachea of each mouse. The adenovirus (108 PFU) in 50 μl of PBS was injected into the trachea. The incision was closed with wound closures and the mice were monitored until they recovered from anesthesia. Infected animals were maintained in separate cages for 72 h before hyperoxic exposure. Experimental groups Ad-SOCS-1 (n = 20), Ad-GFP (n = 20) and control group PBS (n = 20) were studied.
Hyperoxia exposure
Six-wk-old mice (n = 20) were placed in cages in a chamber (75 × 50 × 50 cm) and exposed to 100% O2 for 72 h. The controls were exposed to room air. Concentration of O2 in the chamber was regulated and monitored with proOx P100 sensor (BioSpherix) as previously described (2-4).
Bronchoalveolar Lavage (BAL) fluid collection
Mice were anesthetized with an intraperitoneal injection of ketamine/xylazine mixture. After cervical dislocation, the trachea was surgically exposed in the ventral neck area, and a 0.6 mm catheter was inserted into the trachea through a small incision [2,5,27]. Bronchoalveolar lavage (BAL) fluid was collected by perfusing the lungs with sterile PBS as previously described [27]. The BAL fluid perfusion was repeated three times for each mouse. The cell-free BAL fluid was stored at −80°C until analysis.
Lung perfusion and tissue collection
After BAL fluid collection, the abdominal cavity was opened and lungs were perfused through the right ventricle using 10% formalin in PBS at pH 7.40. The left lobe of the lung was fixed in 0.5 ml of 10% neutral buffered formalin; then it was separated from the cavity for histological processing and paraffin embedding (FFPE) [2,5,27]. The remaining pieces of lungs were stored at −80°C until analysis. The paraffin embedded lung tissue sections were stained with hematoxylin and eosin to evaluate the extent of lung injury.
ELISA
Levels of IL-1β (eBioscience, San Diego, CA), IL-6 (BD Bioscience, San Diego, CA), TNF-α RayBiotech Inc., Norcross, GA), and MCP-1 (eBioscience, San Diego, CA) in BAL fluid were measured using commercial ELISA kits as per the manufacturer’s instructions.
Lung injury evaluation
To quantitatively examine lung edema, we recorded wet/dry weight ratios by removing six lungs per group from the hilum as previously described [28]. The lungs were dry blotted and weighed to determine the wet weight. Then the lungs were desiccated overnight by 130°C incubation in a vacuum oven and reweighted to obtain the dry weight. We then calculated the wet/dry ratio [28]. The remaining portion of the lungs were dissected out carefully, frozen in liquid nitrogen, and stored at −80 °C until analysis.
Alveolar fluid clearance (AFC)
AFC was measured as previously described [29] AFC was calculated by: AFC = [(Vi−Vf)/Vi)] × 100%Vf = (Vi×Ei)/Ef; where Vi represents the volume of injected albumin solution and Vf represents the volume of the final alveolar fluid, and E represents the (Ei) injected and (Ef) final concentrations of the Evans Blue-labeled 5% albumin solution.
Survival Study
Mice treated with Ad-SOCS-1 (n = 20) or Ad-GFP (n = 20) were exposed to continuous 100% O2 exposure (hyperoxia) for evaluation of survival. The number of surviving mice was determined at 24-h intervals until the last mouse died.
Analysis of BAL Fluid
Entire BAL fluid (~2-3 mL) was centrifuged at 200 G for 10 min at 4°C. The supernatants were stored at −80°C until analysis; and the cell pellets were resuspended with ice-cold sterile PBS (1 mL). The total number of cells in cell suspension was counted using a glass haemocytometer. Aliquots of 100-300 μl of each cell suspension were centrifuged onto glass slides at 800 rpm for 3 min in a cytocentrifuge (Shandon Cytospin 2, Pittsburgh, PA). Cytospined cells were stained with Diff-Quik stain set (Andwin Scientific, Schaumburg, IL) and differential white blood cell count was performed on a minimum of 200 cells under a microscope at 200X magnification [27].
Immuno cytochemical staining
Cells in 35 mm dishes were fixed with 4% paraformaldehyde for 5 minutes and permeabilized with 1% Triton X-100 in PBS for 10 minutes. They were then incubated with primary antibodies overnight and probed with fluorescent conjugated secondary antibody for 2 h. After repeated washing, cells were air dried and Hoechst stain containing mounting medium was used to mount the slides. The slides were viewed with the Olympus IX81 inverted microscope fitted with the 3i Yokogawa spinning disk scanner and CCD cameras with revolving laser arrays.
Real time PCR
Total RNA was isolated from mouse lung lysates and alveolar macrophages using Trizol according to the manufacturer’s protocols (QIAGEN, Valencia, CA). Total RNA was used for cDNA synthesis by iScript cDNA synthesis kit (BioRad Laboratories, Hercules, CA) according to the manufacturer’s recommendations. Quantitative PCR was performed by using a BioRad iCycler with SYBR green supermix (BioRad Laboratories, Hercules, CA) and ribonuclease-free water. qRT-PCR reactions were performed using the following conditions: amplification at 95°C for 3 min followed by 40 cycles at 95°C for 15 sec and 60°C for 30 sec. The mRNA expression of Ad-SOCS-1 was measured using specific primers: (forward) 5′-TCC GTT CGC ACG CCG ATT AC-3′ and (reverse) 5′-TCA AAT CTG GAA GGG GAA GG-3′. Fluorescence readings determined the amount of amplification at the end of each cycle. 18S rRNA ribosomal peak intensity was used to assess the RNA quality and the ΔCT method was used to estimate the relative amount of RNA in sample.
Western blot
Equal amount of protein was prepared for SDS-PAGE; and protein was transferred to a polyvinylidene difluoride membrane by following the electrophoresis method mentioned in study [30]. The membrane was blocked in 5% nonfat dry milk in PBS containing 0.05% TWEEN 20 (PBS/T) for 1 h at room temperature. Primary and secondary antibodies were diluted to 1000 fold in PBS/T with 2.5% nonfat milk. Probing with antibodies and washing steps on the membrane were followed as previously mentioned [30]. A Pierce ECL Western blotting substrate kit (Thermo scientific Rockford, IL, USA) was utilized to detect the protein as per manufacturer instructions and exposed in a ChemiDoc XRS (Biorad, Hercules, CA, USA).
Statistics
In survival experiments, n equals 20 in each group. Values are expressed as means ± SE. Statistical significance was assessed by using GraphPad Prism version 10.00 for Windows (GraphPad Software, San Diego, CA). Group differences were determined using one- and two-way ANOVA or Student’s unpaired t-test. P < 0.05 was considered statistically significant. Kaplan-Meier analysis was utilized for survival analysis and survival differences between groups were assessed using Ψ2 analysis.
Results
In vivo Ad-SOCS-1 treatment and lung distribution of Ad-SOCS-1
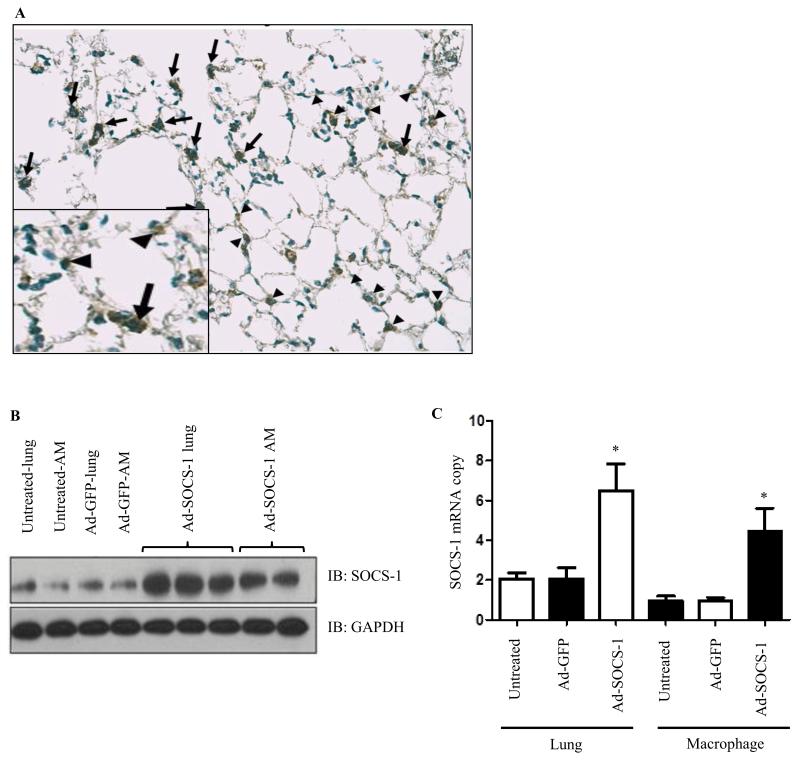
Each mouse (n = 20) was intratracheally treated with 108 plaque-forming units (PFU) of recombinant SOCS-1 adenovirus (Ad-SOCS-1) or GFP adenovirus (Ad-GFP) and allowed to recover for 72 hours. As a first step, we confirmed the adenoviral gene delivery and transfection by immunocytochemical staining for SOCS-1 in lungs and protein expression of Ad-SOCS-1 in lung lysates and alveolar macrophages (AM). Immunocytochemistry confirms that Ad-SOCS-1 reached the lung parenchyma and was distributed throughout the lung including the small distal airways and alveolar region (proximal and distal) (Fig. 1A). Western blot analysis of Ad-SOCS-1 in lung lysates and AM showed a significant increase in Ad-SOCS-1 protein expression compared to the Ad-GFP-transfected controls (Fig. 1B). Adenoviral transfection was further verified by using real-time PCR analysis of lung lysates and AM (Fig. 1C). The mRNA expression of Ad-SOCS-1 was higher in the lung and AM isolates obtained from mice transfected with adenovirus carrying the recombinant SOCS-1 compared to their littermate controls. The presence of Ad-SOCS-1 was detected in airway epithelium (44%), alveolar epithelial cells (22.1%) and macrophages (33.9%) of total immuno positive cells. These results suggest that the adenoviral-mediated gene delivery of recombinant SOCS-1 was efficient.
Figure 1. In vivo Ad-SOCS-1 treatment and lung distribution of SOCS-1.
Mice (n = 20) were treated with recombinant Ad-SOCS-1 and Ad-GFP. (A) Immunocytochemical staining was performed to confirm adenoviral delivery in the lungs. (B) Western blot analysis of PBS (untreated), GFP-transfected, or SOCS-1-transfected cells were obtained from lung lysates and alveolar macrophages. GAPDH was used as an internal control. (C) Further verification of adenoviral transfection is shown by qRT-PCR analysis of lung lysates and alveolar macrophages. Data is representative of at least 3 independent experiments. *P < 0.05 when compared between Ad-GFP- and Ad-SOCS-1-transfected mice.
Supplementation of mice with Ad-SOCS-1 dampens hyperoxia-induced ALI
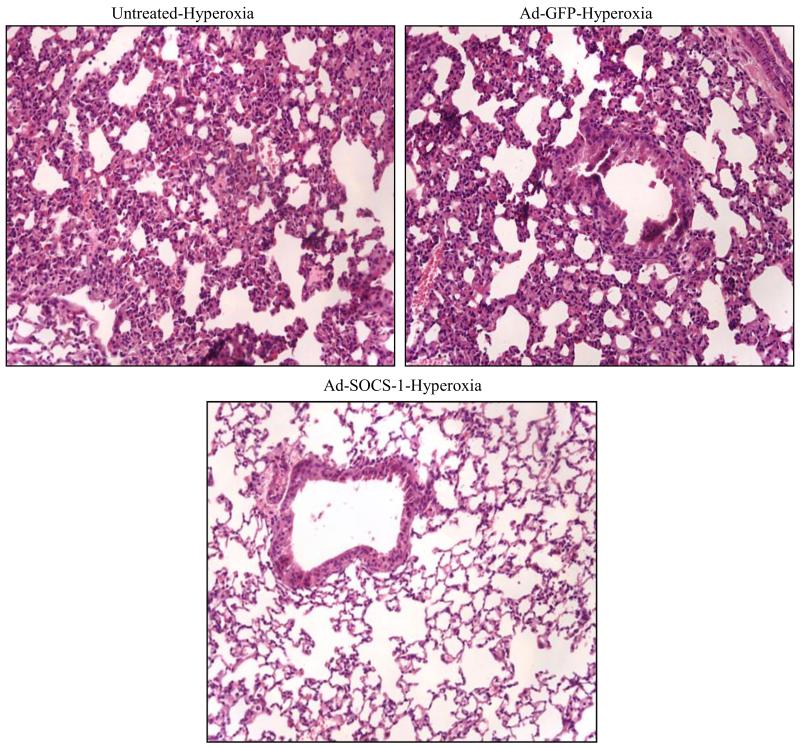
To check if Ad-SOCS-1 can induce protection against hyperoxia-induced ALI, we administered mice with Ad-SOCS-1 or Ad-GFP (control) and exposed mice to hyperoxia for 72 hours. We then analyzed lung morphology by H&E staining (Fig. 2). The results demonstrated a significant infiltration of immune cells and damage in alveoli in untreated or Ad-GFP-treated mice when compared to Ad-SOCS-1-treated mice. Further, gross examination of lungs in the Ad-GFP-administered mice appeared boggy and hemorrhagic with edema fluid upon transection of the trachea. In contrast, these changes were suppressed and unchanged from baseline in lungs of Ad-SOCS-1-administered mice. These results suggest that administration of Ad-SOCS-1 attenuates hyperoxia-induced ALI and confers protection in mice exposed to hyperoxia.
Figure 2. Supplementation of mice with Ad-SOCS-1 dampens hyperoxia-induced ALI.
SOCS-1-treated mice show decreased damage against hyperoxia-induced ALI. Mice treated with PBS (untreated), Ad-GFP, and Ad-SOCS-1 were exposed to hyperoxia for 72 h. The extent of lung injury was evaluated from hematoxylin and eosin (H&E) stained lung tissue sections. Original Magnification: 200X
Increased survival rates in hyperoxia-exposed mice treated with Ad-SOCS-1
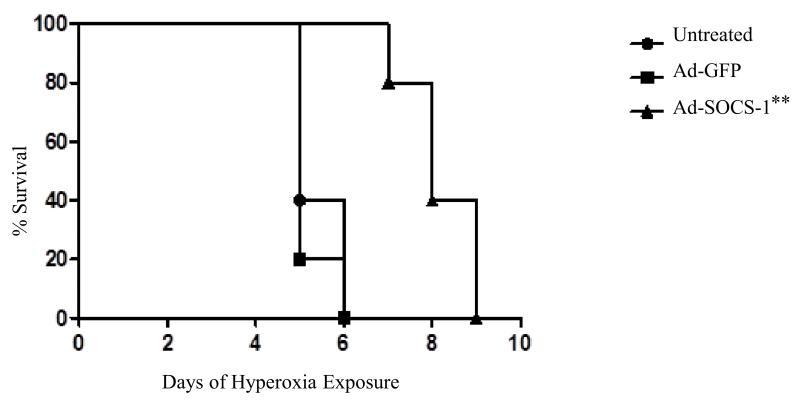
We checked whether Ad-SOCS-1 can induce extended survival rates in mice exposed to hyperoxia and analyzed the rate of survival. Ad-SOCS-1-administered mice exposed to 100% oxygen showed enhanced survival compared to Ad-GFP control mice. After 3 days of hyperoxia, Ad-GFP mice were ill and were manifested by tachypnea. In contrast, all Ad-SOCS-1-treated mice survived for at least 6 days in hyperoxia and 80% of them survived even beyond 7 days (Fig. 3). Interestingly, Ad-SOCS-1 mice exhibited normal levels of activity and normal respiratory rate throughout their hyperoxic exposure. These results confirmed that Ad-SOCS-1 expression prolonged survival rate in mice exposed to hyperoxia.
Figure 3. Increased survival rates in hyperoxia-exposed mice treated with Ad-SOCS-1.
SOCS-1 can induce extended survival rates at 100% hyperoxic exposure. Kaplan-Meier plot of survival shows percent survival of untreated (circle), Ad-GFP (squares), and Ad-SOCS-1 (triangles) mice subjected to 100% hyperoxia for 72 h. The number of surviving mice was counted at 24-h intervals. **P < 0.01 when compared between Ad-GFP- and Ad-SOCS-1-transfected mice under hyperoxia.
Hyperoxia-induced immune cell accumulation was suppressed in mice administered with Ad-SOCS-1
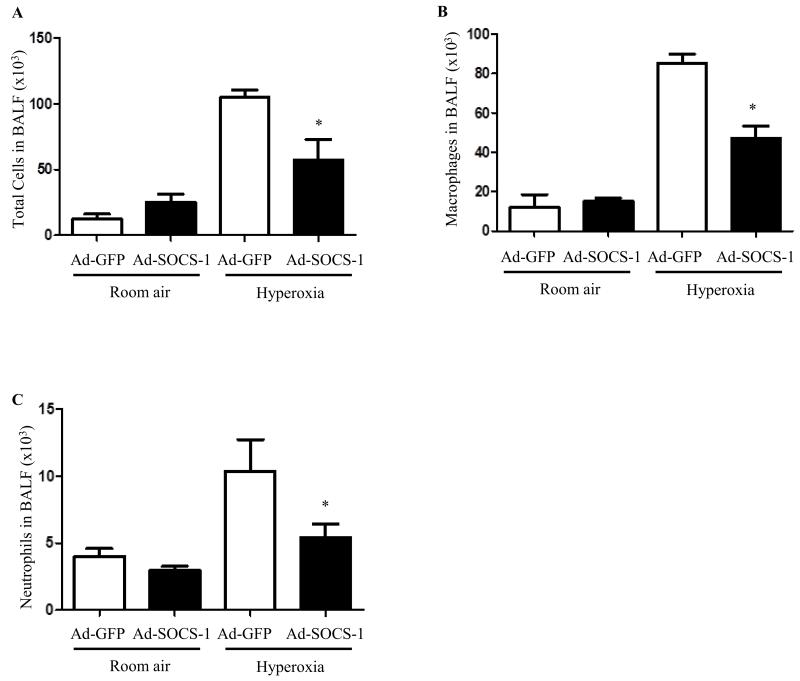
We exposed mice to hyperoxia for 72 hours post administration with Ad-SOCS-1 or the control Ad-GFP. BALF was extracted from these mice and was analyzed for total cells, macrophages, and neutrophils. Quantification of total number of cells recovered from BAL fluid is useful to evaluate alveolitis in hyperoxia-induced murine models. Our results showed a significant decrease in the total number of cells in mice treated with Ad-SOCS-1 when compared to mice administered with Ad-GFP. No significant difference was found between the two groups of mice (Ad-SOCS-1 and Ad-GFP) under normoxic conditions (Fig. 4A). Neutrophil infiltration and macrophage accumulation into the pulmonary interstitium and alveoli is one of the key features of ALI. Macrophage counts were significantly low in mice which received Ad-SOCS-1 compared to mice with Ad-GFP under hyperoxia. Total amount of macrophages remained the same in both the groups of mice under room air (Fig. 4B). We then evaluated neutrophil infiltration in mice which received Ad-SOCS-1 or control Ad-GFP exposed to hyperoxia. Neutrophil accumulation was significantly decreased in mice that received Ad-SOCS-1 compared to Ad-GFP controls. Total number of neutrophils remained the same in mice (Ad-SOCS-1 and Ad-GFP) exposed to normoxia (Fig. 4C). Taken together, these results suggest that SOCS-1 plays a key role in immune cell accumulation and infiltration in hyperoxia-induced ALI.
Figure 4. Hyperoxia-induced immune cell accumulation was suppressed in mice administered with Ad-SOCS-1.
Male and female C57Bl/6 mice transfected with recombinant Ad-SOCS-1 and Ad-GFP were exposed to room air (Normoxia) or 100% O2 (Hyperoxia) at 72 h as described in Material and Methods. After 72 h exposure, BALF was extracted from mice and analyzed for (A) total cells, (B) macrophages, and (C) neutrophils by manual count. *P < 0.05 when compared between Ad-GFP- and Ad-SOCS-1-transfected mice under hyperoxia.
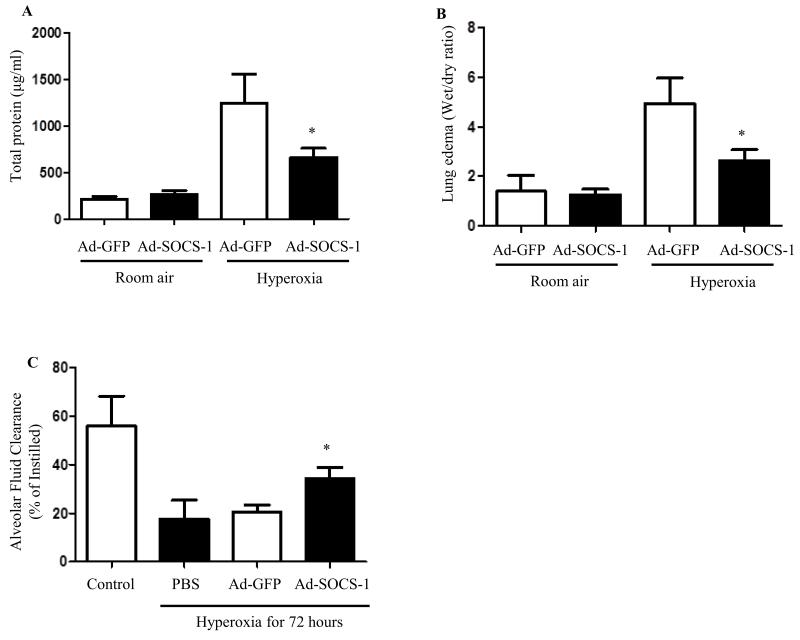
Hyperoxia-mediated lung edema and alveolar leak were suppressed in Ad-SOCS-1-treated mice
Development of pulmonary edema and alveolar protein leak is a hallmark of ALI that ultimately leads to respiratory failure. We analyzed total protein content in mice treated with Ad-SOCS-1 or Ad-GFP under normoxia and hyperoxia. Our results indicated a significant drop in the total protein content in mice treated with Ad-SOCS-1 exposed to hyperoxia when compared with Ad-GFP controls. No significant difference was found in the protein levels when both the groups were exposed to room air (Fig. 5A). We assessed lung edema by obtaining wet/dry ratio in mice administered with Ad-SOCS-1 or Ad-GFP under hyperoxia and room air. Wet/dry ratio levels were significantly lower in mice that received Ad-SOCS-1 when compared to mice administered with Ad-GFP under hyperoxia. Lung edema content remained the same in both the groups exposed to room air (Fig. 5B). These results indicate that Ad-SOCS-1 suppresses lung edema formation, alveolar leak.
Figure 5. Hyperoxia-mediated lung edema and alveolar leak were suppressed in Ad-SOCS-1-treated mice and Ad-SOCS-1 induced alveolar fluid clearance in mice exposed to hyperoxia.
Male and female C57Bl/6 mice transfected with recombinant Ad-SOCS-1 and Ad-GFP were exposed to room air (Normoxia) or 100% O2 (Hyperoxia) at 72 h as described in Material and Methods. (A) Total protein content and (B) lung edema were determined by BCA assay, wet/dry ratio, and spectrophotometric assay, respectively, in Ad-SOCS-1-treated mice and Ad-GFP-treated mice exposed to room air or hyperoxic conditions. (C) Ad-SOCS-1- or Ad-GFP-transfected mice were exsanguinated and alveolar fluid clearance was measured by tracheal instillation of I131 albumin. *P < 0.05 when compared between Ad-GFP- and Ad-SOCS-1-transfected mice under hyperoxia.
Ad-SOCS-1-induced alveolar fluid clearance in mice exposed to hyperoxia
Since our results showed that Ad-SOCS-1-mediated protection against ALI and associated with reduced lung edema (wet/dry ratio), we further analyzed whether Ad-SOCS-1 is involved in alveolar fluid transport in hyperoxia-induced ALI. Mice were administered with Ad-SOCS-1 or control Ad-GFP. Following 72 hours post-hyperoxic exposure, animals were exsanguinated, and distal airspace fluid clearance was measured over 30 minutes by tracheal instillation of I131 albumin. Mice treated with PBS and exposed to room air acted as controls. Our results indicated a significant increase in alveolar fluid clearance in Ad-SOCS-1-administered mice compared to their Ad-GFP controls under hyperoxia (Fig. 5C). These results suggest that Ad-SOCS-1 restores alveolar fluid clearance in hyperoxia-induced ALI.
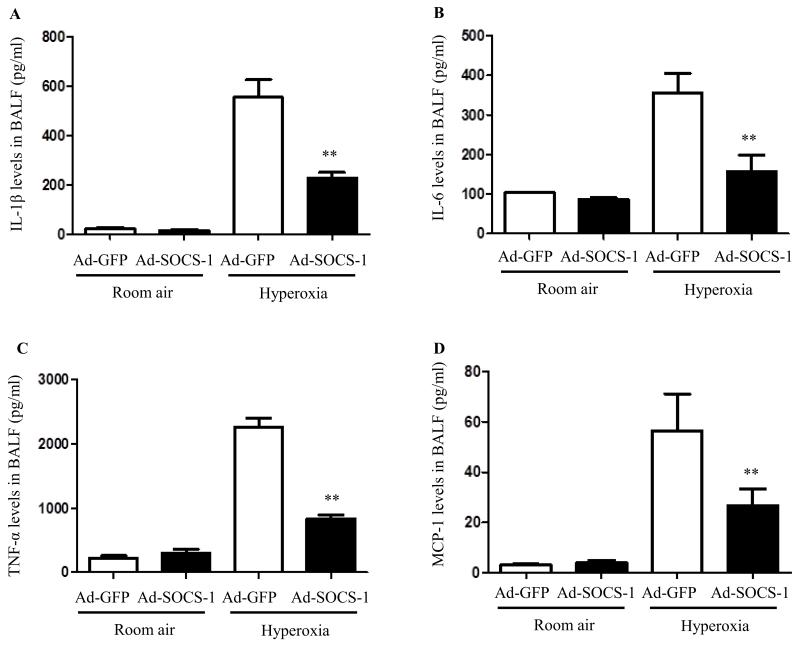
Ad-SOCS-1 administration reduces inflammatory cytokines in BAL in mice exposed to hyperoxia
IL-1β is one of the most potent cytokines in the lungs of early ALI patients. Secreted IL-1β can further induce a cytokine storm exacerbating the inflammatory response. We checked the levels of IL-1β in BALF of mice exposed to room air and hyperoxia that were pretreated with Ad-SOCS-1 or Ad-GFP. Results show that IL-1β was significantly suppressed in BALF of mice which received Ad-SOCS-1 compared to control mice under hyperoxia. No IL-1β induction was observed when mice were exposed to room air (Fig. 6A). Additionally, we also measured the levels of another proinflammatory cytokine IL-6. Ad-SOCS-1-treated mice showed significantly low levels of IL-6 compared to their Ad-GFP controls under hyperoxia. No such difference was observed when mice were exposed to room air (Fig. 6B). TNF-α is the most widely studied proinflammatory cytokine member of the TNF super family and is recognized as a mediator of the pulmonary inflammatory response. Levels of TNF-α in BAL fluid obtained from Ad-SOCS-1-treated mice and Ad-GFP-treated mice exposed to 100% O2 for 72 hours and room air was evaluated. Results show that hyperoxia-induced TNF-α elevation was significantly suppressed in mice that received Ad-SOCS-1 compared to their Ad-GFP controls (Fig. 6C). However, no such difference was observed in mice exposed to room air. MCP-1 is known to be a potent chemo-attractant mainly acting on monocytes/macrophages. MCP-1 concentrations in BAL fluid obtained from Ad-SOCS-1-treated mice and Ad-GFP-treated mice exposed to hyperoxia and room air was evaluated. MCP-1 levels were significantly low in mice treated with Ad-SOCS-1 compared to controls when mice were exposed to 100% oxygen for 72 hours. No such differences were found in mice exposed to room air (Fig. 6D). These results suggest that Ad-SOCS-1 diminishes the secretion of hyperoxia-induced proinflammatory cytokines like IL-1β, IL-6, TNF-α, and MCP-1.
Figure 6. Ad-SOCS-1 administration reduces inflammatory cytokines in BAL in mice exposed to hyperoxia.
Male and female C57Bl/6 mice transfected with recombinant Ad-SOCS-1 and Ad-GFP were exposed to room air (Normoxia) or 100% O2 (Hyperoxia) at 72 h as described in Material and Methods. Proinflammatory cytokine levels (A) IL-1β, (B) IL-6, (C) TNF-α and (D) MCP-1 of SOCS-1- and GFP-treated mice under normoxic and hyperoxic conditions were analyzed using ELISA. **P < 0.01 when compared between Ad-GFP- and Ad-SOCS-1-transfected mice under hyperoxia.
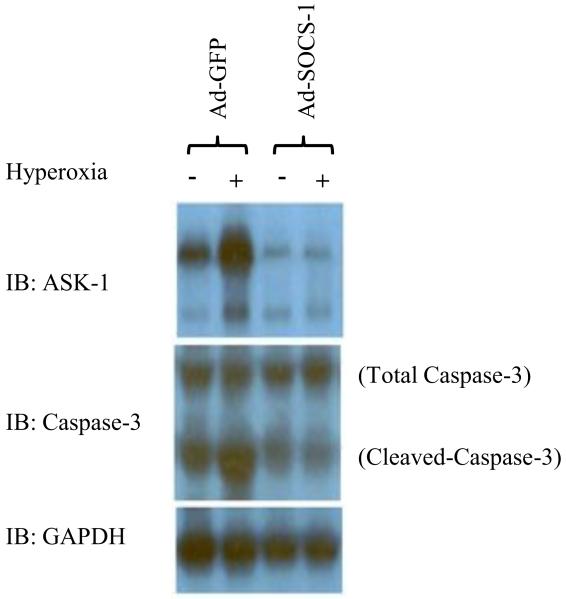
Ad-SOCS-1-mediated protection in ALI may be due to suppression of ASK-1
It has been shown that SOCS-1 protects against TNF-α-induced and ASK-1-mediated cell death. Thus, we hypothesized that the protective effects of Ad-SOCS-1 mice could be in part due to its ability to suppress this ASK-1-mediated apoptotic response. We analyzed the levels of ASK-1 and caspase-3 expression by immunoblot analysis. Our results showed a significant decrease in ASK-1 protein expression in mice lysates treated with Ad-SOCS-1 when compared to Ad-GFP controls under hyperoxia (Fig. 7). Further, we checked for the expression levels of total caspase-3 and cleaved caspase-3 in these lysates and our results strongly supported our hypothesis indicating that ASK-1 protein expression is significantly inhibited in the Ad-SOCS-1 mice even in room air and hyperoxia which is further associated with a significant decrease in cleaved caspase-3 when compared to Ad-GFP control mice.
Figure 7. Ad-SOCS-1-mediated protection in ALI may be due to suppression of ASK-1.
Male and female C57Bl/6 mice transfected with recombinant Ad-SOCS-1 and Ad-GFP were exposed to room air (Normoxia) or 100% O2 (Hyperoxia) at 72 h as described in Material and Methods. Western blot analysis of ASK-1 and caspase-3 (total and cleaved) from cell lysates was obtained from Ad-SOCS-1- or Ad-GFP-treated cells under hyperoxic or normoxic conditions. GAPDH was used as an internal control. Data is representative of at least 3 independent experiments.
Discussion
The purpose of this study was to investigate whether direct overexpression of Ad-SOCS-1 could provide protection from lethal hyperoxia. The present study shows that (i) Ad-SOCS treatment attenuates hyperoxia-induced ALI and confers protection to mice exposed to hyperoxia; (ii) Ad-SOCS-1-treated mice shows extended survival when compared to Ad-GFP-treated mice; (iii) hyperoxia-induced immune cell infiltration was suppressed in mice administered with Ad-SOCS-1; (iv) Ad-SOCS-1 administration suppressed hyperoxia-induced infiltration of inflammatory cytokines; (v) Ad-SOCS-1 administration also suppressed hyperoxia-mediated lung edema, and alveolar leak; (vi) Ad-SOCS-1 administration restores alveolar fluid clearance; (vii) Ad-SOCS-1-mediated protection is associated with the suppressed ASK-1 protein expression.
Tracheal instillation was used as the choice of delivery over vascular administration since it is potentially appropriate to clinical local gene therapy for the lung. Based on the staining, our method using adenoviral delivery of SOCS-1 locally to the lung showed that it reaches to the epithelium and sufficient to protect from hyperoxic injury. It has also been demonstrated that intratracheal transduction of iNOS adenovirus decreases vasoconstrictor responses in rats [31]. In addition, staining of lungs of iNOS-transfected animals showed transgenic expression in the alveolar wall cells [31].
Several in vitro and in vivo studies demonstrated that SOCS-1 plays as an anti-apoptotic and anti-inflammatory molecule in immunological disorders [14,15]. It has been shown that SOCS-1 plays an important role in protecting against Chlamydia pneumonia-induced lethal inflammation but hinders effective clearance of the bacteria [32]. SOCS-1-deficient mice die within 3 weeks of birth with severe inflammation and lung damage through infiltration of inflammatory cells [15-19], which is strikingly similar to the clinical manifestation of ALI. It has also been documented that intracellular protein therapy with SOCS-3 inhibits inflammation and apoptosis [20]. Previously, our studies showed that SOCS-1 overexpression protects from H2O2-induced cell death [4]. In continuation to these studies, the therapeutic role of SOCS-1 overexpression against lethal hyperoxia is demonstrated in the present investigation.
It is known that persistent hyperoxic exposure can lead to ALI and result in inflammation and alveolar damage [10], which is consistent with our results. Based on our histological evaluation, lung tissue exposed to hyperoxia showed extreme change to the alveoli with many hemorrhagic sites of edema in Ad-GFP control mice. However, these changes were significantly ameliorated in mice administered with SOCS-1. Our observations suggest that hyperoxia modulates the inflammatory pathways in the lung and SOCS-1 suppresses these changes as evident in our results of suppressed infiltration of immune cells and cytokines. In addition, mice expressing the Ad-SOCS-1 gene have enhanced survival in 100% hyperoxia and showed normal levels of activity and normal respiratory rate. Although no Ad-GFP mice remained by the sixth day, 80% of Ad-SOCS-1 mice still survived by the seventh day. This extended survival may be the result of the suppressed infiltration of immune cells and alveolar damage as seen in Ad-SOCS-1 mice. SOCS-1 has been shown to inhibit JAK kinase activity and suppresses infiltration of monocytes and neutrophils [33]. Neutrophils and macrophages are important components of the inflammatory response that characterize ALI. Flooding of neutrophils into the bronchoalveolar space can be associated in endothelial and epithelial injury as well as lung edema [34].
Neutrophils are a major factor in the development of ALI and ARDS since neutrophil activation and transmigration is a key effect in the development of ALI and ARDS [34]. In experimental models, the elimination of neutrophils significantly decreases the severity of ALI [35]. In the present study, our BAL fluid analysis reveals a significant decrease in total cells, macrophages, and neutrophils in Ad-SOCS-1-administered mice exposed to hyperoxia when compared to control mice. These results suggest that SOCS-1 is associated in the regulation of immune cell accumulation and infiltration in oxygen toxicity.
It is well established that IL-6 plays an important role in the pathophysiology of acute lung injury [36] and patients with ALI were found to have high plasma IL-6 levels [37]. In our study, Ad-SOCS-1 related mice had significantly reduced IL-6 levels in BAL fluid. This suggests that SOCS-1 has protective effects in part due to attenuated IL-6 levels. In addition, levels of MCP-1, which is known to play a key part in selectively recruiting cytokines such as neutrophils and monocytes [38], were significantly reduced in BAL fluid of Ad-SOCS-1-administered mice. This suggests that SOCS-1 suppresses infiltration of cytokines which help inhibit infiltration of inflammatory cytokines.
It is recognized that air exchange between the circulatory system and the alveolar space is important for respiration [39]. In ALI, failure of the lung epithelial barrier leads to airspace flooding significantly decreasing the efficiency of gas exchange that exacerbates the severity of ALI. In addition, earlier studies demonstrated that resolution of pulmonary edema and lung inflammation is important in the recovery from ALI [40]. Accordingly, our results indicate that Ad-SOCS-1 suppresses lung edema formation and alveolar leak exposed to hyperoxia. This suggests that SOCS-1 protects against alveolar cell damage which eliminates leaking into the airspace.
Earlier studies suggested that patients with maximal alveolar fluid clearance have a lower mortality in ALI [41]. Our study for the first time shows that administration of Ad-SOCS-1 significantly induces clears the alveolar fluid in the lungs of mice exposed to hyperoxia. These results suggest that SOCS-1 not only inhibits leaking but also returns alveolar clearance to its normal state.
Previous clinical studies reveal that inflammatory cytokines and chemokines work together in mediating, amplifying, and perpetuating the process of lung injury [42]. Moreover, inflammatory cytokines like IL-1β can induce the production of other chemotactic cytokines like IL-8. The cytokine IL-1β is also suppressed by SOCS-1 [43]. Our data show that overexpression of Ad-SOCS-1 can significantly inhibit the production of proinflammatory cytokines including IL-1β in BALF of mice exposed to oxygen toxicity.
Recent studies show tolerance of LPS is decreased in SOCS-1−/− cells [44]. SOCS-1 deficient cells have impaired endotoxin tolerance [44]. However, in our previous studies we showed that epithelial cells exposed to H2O2 (which induce oxidative stress) is unable to induce SOCS-1 protein expression levels [4]. Either hyperoxia exposure or H2O2 exposure causes significant ASK-1 activation [4]. Further previous studies revealed that ASK-1 regulates both apoptosis and inflammation [45,46]. According to these findings, we examined if ASK-1 levels changed in Ad-SOCS-1-overexpressed mice compared to control. Our results indicate that ASK-1 levels were significantly suppressed in Ad-SOCS-1-overexpressed mice exposed to hyperoxia compared to controls. These results are further supported by significant decrease in cleaved caspase-3. These results suggest that SOCS-1 can protect cell death in part by suppressing ASK-1-mediated apoptosis. This is supported by our previous study as we documented that the mechanism of SOCS-1-mediated protection against ROS-induced cell death in vitro occurs through ASK-1 degradation [4].
Since oxidative injury is a key element in the pathogenesis of a wide variety of pulmonary diseases and disorders, the present findings are significant from this severe oxidative stress animal model because we showed that SOCS-1 is able to protect from this severe oxidative stress-induced ALI model. Our studies demonstrate, for the first time, that the protective effects of SOCS-1 in the setting of HALI are associated with a significant decrease in inflammation and apoptosis. Our data suggest that SOCS-1 is protective against oxidant-induced injury via ASK-1 degradation. Since oxidative stress plays a critical role in the pathogenesis of many other diseases, the role of SOCS-1 in our model will offer clues to its role in other oxidative stress-related diseases.
In summary, we demonstrate for the first time that adenovirus-mediated SOCS-1 overexpression in mice lungs significantly protects from hyperoxic lung injury when compared to a control adenovirus. Decreased damage is seen by suppressed permeability of alveolar epithelium and inhibition of immune cell accumulation. SOCS-1 protection against ALI is further supported by ASK-1 suppression under Ad-SOCS-1 treatment. These findings reveal that SOCS-1 may have great protective effects against lethal oxidant lung injury. Clinically, the overexpression of SOCS-1 may be a novel approach to suppress ALI and ASK-1-mediated inflammatory diseases.
Highlights.
Delivery of Ad-SOCS-1 to mice dampens hyperoxia-induced ALI
Increased survival rates in hyperoxia-exposed mice treated with Ad-SOCS-1
Hyperoxia-induced immune cell accumulation, lung edema, and alveolar leak were suppressed in Ad-SOCS-1-treated mice
Ad-SOCS-1-induced reduction of inflammatory cytokines in BAL and alveolar fluid clearance in mice exposed to hyperoxia
Ad-SOCS-1-mediated protection in ALI may be due to suppression of ASK-1
Acknowledgements
NK was funded by the American Heart Association National Scientist Development Grant 09SDG2260957 and National Institutes of Health R01 HL105932 and the Joy McCann Culverhouse Endowment to the Division of Allergy and Immunology. SC was funded by the American Heart Association National Scientist Development Grant 11SDG7590063 and National Institutes of Health 1K23AI110731.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Malhotra A. Low-tidal-volume ventilation in the acute respiratory distress syndrome. N Engl J Med. 2007;357:1113–1120. doi: 10.1056/NEJMct074213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waxman AB, Einarsson O, Seres T, Knickelbein RG, Warshaw JB, et al. Targeted lung expression of interleukin-11 enhances murine tolerance of 100% oxygen and diminishes hyperoxia-induced DNA fragmentation. J Clin Invest. 1998;101:1970–1982. doi: 10.1172/JCI1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 4.Kolliputi N, Waxman AB. IL-6 cytoprotection in hyperoxic acute lung injury occurs via suppressor of cytokine signaling-1-induced apoptosis signal-regulating kinase-1 degradation. Am J Respir Cell Mol Biol. 2009;40:314–324. doi: 10.1165/rcmb.2007-0287OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward NS, Waxman AB, Homer RJ, Mantell LL, Einarsson O, et al. Interleukin-6-induced protection in hyperoxic acute lung injury. Am J Respir Cell Mol Biol. 2000;22:535–542. doi: 10.1165/ajrcmb.22.5.3808. [DOI] [PubMed] [Google Scholar]
- 6.He CH, Waxman AB, Lee CG, Link H, Rabach ME, et al. Bcl-2-related protein A1 is an endogenous and cytokine-stimulated mediator of cytoprotection in hyperoxic acute lung injury. J Clin Invest. 2005;115:1039–1048. doi: 10.1172/JCI23004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolliputi N, Waxman AB. IL-6 cytoprotection in hyperoxic acute lung injury occurs via PI3K/Akt-mediated Bax phosphorylation. Am J Physiol Lung Cell Mol Physiol. 2009;297:L6–16. doi: 10.1152/ajplung.90381.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolliputi N, Shaik RS, Waxman AB. The inflammasome mediates hyperoxia-induced alveolar cell permeability. J Immunol. 2010;184:5819–5826. doi: 10.4049/jimmunol.0902766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waxman AB, Mahboubi K, Knickelbein RG, Mantell LL, Manzo N, et al. Interleukin-11 and interleukin-6 protect cultured human endothelial cells from H2O2-induced cell death. Am J Respir Cell Mol Biol. 2003;29:513–522. doi: 10.1165/rcmb.2002-0044OC. [DOI] [PubMed] [Google Scholar]
- 10.Lagishetty V, Parthasarathy PT, Phillips O, Fukumoto J, Cho Y, et al. Dysregulation of CLOCK gene expression in hyperoxia-induced lung injury. Am J Physiol Cell Physiol. 2014;306:C999–c1007. doi: 10.1152/ajpcell.00064.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27:337–349. doi: 10.1055/s-2006-948288. [DOI] [PubMed] [Google Scholar]
- 12.O’Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002;109(Suppl):S121–131. doi: 10.1016/s0092-8674(02)00701-8. [DOI] [PubMed] [Google Scholar]
- 13.Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J Cell Sci. 2004;117:1281–1283. doi: 10.1242/jcs.00963. [DOI] [PubMed] [Google Scholar]
- 14.Mansell A, Smith R, Doyle SL, Gray P, Fenner JE, et al. Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat Immunol. 2006;7:148–155. doi: 10.1038/ni1299. [DOI] [PubMed] [Google Scholar]
- 15.Morita Y, Naka T, Kawazoe Y, Fujimoto M, Narazaki M, et al. Signals transducers and activators of transcription (STAT)-induced STAT inhibitor-1 (SSI-1)/suppressor of cytokine signaling-1 (SOCS-1) suppresses tumor necrosis factor alpha-induced cell death in fibroblasts. Proc Natl Acad Sci U S A. 2000;97:5405–5410. doi: 10.1073/pnas.090084797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marine JC, Topham DJ, McKay C, Wang D, Parganas E, et al. SOCS1 deficiency causes a lymphocyte-dependent perinatal lethality. Cell. 1999;98:609–616. doi: 10.1016/s0092-8674(00)80048-3. [DOI] [PubMed] [Google Scholar]
- 17.Alexander WS, Starr R, Fenner JE, Scott CL, Handman E, et al. SOCS1 is a critical inhibitor of interferon gamma signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell. 1999;98:597–608. doi: 10.1016/s0092-8674(00)80047-1. [DOI] [PubMed] [Google Scholar]
- 18.Nakashima T, Yokoyama A, Onari Y, Shoda H, Haruta Y, et al. Suppressor of cytokine signaling 1 inhibits pulmonary inflammation and fibrosis. J Allergy Clin Immunol. 2008;121:1269–1276. doi: 10.1016/j.jaci.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 19.He Y, Zhang W, Zhang R, Zhang H, Min W. SOCS1 inhibits tumor necrosis factor-induced activation of ASK1-JNK inflammatory signaling by mediating ASK1 degradation. J Biol Chem. 2006;281:5559–5566. doi: 10.1074/jbc.M512338200. [DOI] [PubMed] [Google Scholar]
- 20.Jo D, Liu D, Yao S, Collins RD, Hawiger J. Intracellular protein therapy with SOCS3 inhibits inflammation and apoptosis. Nat Med. 2005;11:892–898. doi: 10.1038/nm1269. [DOI] [PubMed] [Google Scholar]
- 21.Matsuzawa A, Saegusa K, Noguchi T, Sadamitsu C, Nishitoh H, et al. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat Immunol. 2005;6:587–592. doi: 10.1038/ni1200. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Min W. Thioredoxin promotes ASK1 ubiquitination and degradation to inhibit ASK1-mediated apoptosis in a redox activity-independent manner. Circ Res. 2002;90:1259–1266. doi: 10.1161/01.res.0000022160.64355.62. [DOI] [PubMed] [Google Scholar]
- 23.Lu Q, Harrington EO, Rounds S. Apoptosis and lung injury. Keio J Med. 2005;54:184–189. doi: 10.2302/kjm.54.184. [DOI] [PubMed] [Google Scholar]
- 24.Tang PS, Mura M, Seth R, Liu M. Acute lung injury and cell death: how many ways can cells die? Am J Physiol Lung Cell Mol Physiol. 2008;294:L632–641. doi: 10.1152/ajplung.00262.2007. [DOI] [PubMed] [Google Scholar]
- 25.Yamada T, Iwasaki Y, Nagata K, Fushiki S, Nakamura H, et al. Thioredoxin-1 protects against hyperoxia-induced apoptosis in cells of the alveolar walls. Pulm Pharmacol Ther. 2007;20:650–659. doi: 10.1016/j.pupt.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Waxman AB, Kolliputi N. IL-6 protects against hyperoxia-induced mitochondrial damage via Bcl-2-induced Bak interactions with mitofusins. Am J Respir Cell Mol Biol. 2009;41:385–396. doi: 10.1165/rcmb.2008-0302OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukumoto J, Fukumoto I, Parthasarathy PT, Cox R, Huynh B, et al. NLRP3 deletion protects from hyperoxia-induced acute lung injury. Am J Physiol Cell Physiol. 2013;305:C182–189. doi: 10.1152/ajpcell.00086.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sue RD, Belperio JA, Burdick MD, Murray LA, Xue YY, et al. CXCR2 is critical to hyperoxia-induced lung injury. J Immunol. 2004;172:3860–3868. doi: 10.4049/jimmunol.172.6.3860. [DOI] [PubMed] [Google Scholar]
- 29.Qi D, He J, Wang D, Deng W, Zhao Y, et al. 17ss-estradiol suppresses lipopolysaccharide-induced acute lung injury through PI3K/Akt/SGK1 mediated up-regulation of epithelial sodium channel (ENaC) in vivo and in vitro. Respir Res. 2014;15:1512. doi: 10.1186/s12931-014-0159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajanbabu V, Pan CY, Lee SC, Lin WJ, Lin CC, et al. Tilapia hepcidin 2-3 peptide modulates lipopolysaccharide-induced cytokines and inhibits tumor necrosis factor-alpha through cyclooxygenase-2 and phosphodiesterase 4D. J Biol Chem. 2010;285:30577–30586. doi: 10.1074/jbc.M110.137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chicoine LG, Tzeng E, Bryan R, Saenz S, Paffett ML, et al. Intratracheal adenoviral-mediated delivery of iNOS decreases pulmonary vasoconstrictor responses in rats. J Appl Physiol (1985) 2004;97:1814–1822. doi: 10.1152/japplphysiol.00193.2004. [DOI] [PubMed] [Google Scholar]
- 32.Yang T, Stark P, Janik K, Wigzell H, Rottenberg ME. SOCS-1 protects against Chlamydia pneumoniae-induced lethal inflammation but hampers effective bacterial clearance. J Immunol. 2008;180:4040–4049. doi: 10.4049/jimmunol.180.6.4040. [DOI] [PubMed] [Google Scholar]
- 33.Bullen DV, Darwiche R, Metcalf D, Handman E, Alexander WS. Neutralization of interferon-gamma in neonatal SOCS1−/− mice prevents fatty degeneration of the liver but not subsequent fatal inflammatory disease. Immunology. 2001;104:92–98. doi: 10.1046/j.0019-2805.2001.01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011;17:293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31:S195–199. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 36.Cross LJ, Matthay MA. Biomarkers in acute lung injury: insights into the pathogenesis of acute lung injury. Crit Care Clin. 2011;27:355–377. doi: 10.1016/j.ccc.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolters PJ, Wray C, Sutherland RE, Kim SS, Koff J, et al. Neutrophil-derived IL-6 limits alveolar barrier disruption in experimental ventilator-induced lung injury. J Immunol. 2009;182:8056–8062. doi: 10.4049/jimmunol.0801323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Overgaard CE, Mitchell LA, Koval M. Roles for claudins in alveolar epithelial barrier function. Ann N Y Acad Sci. 2012;1257:167–174. doi: 10.1111/j.1749-6632.2012.06545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol. 2005;33:319–327. doi: 10.1165/rcmb.F305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 42.Goodman RB, Pugin J, Lee JS, Matthay MA. Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev. 2003;14:523–535. doi: 10.1016/s1359-6101(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 43.Masters SL, Mielke LA, Cornish AL, Sutton CE, O’Donnell J, et al. Regulation of interleukin-1beta by interferon-gamma is species specific, limited by suppressor of cytokine signalling 1 and influences interleukin-17 production. EMBO Rep. 2010;11:640–646. doi: 10.1038/embor.2010.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kinjyo I, Hanada T, Inagaki-Ohara K, Mori H, Aki D, et al. SOCS1/JAB is a negative regulator of LPS-induced macrophage activation. Immunity. 2002;17:583–591. doi: 10.1016/s1074-7613(02)00446-6. [DOI] [PubMed] [Google Scholar]
- 45.Yamagishi S, Yamada M, Koshimizu H, Takai S, Hatanaka H, et al. Apoptosis-signal regulating kinase-1 is involved in the low potassium-induced activation of p38 mitogen- activated protein kinase and c-Jun in cultured cerebellar granule neurons. J Biochem. 2003;133:719–724. doi: 10.1093/jb/mvg092. [DOI] [PubMed] [Google Scholar]
- 46.Choi JR, Heo H, Lang Y, Shin KS, Kang SJ. Apoptosis signal-regulating kinase 1 regulates the expression of caspase-11. FEBS Lett. 2009;583:3016–3020. doi: 10.1016/j.febslet.2009.08.014. [DOI] [PubMed] [Google Scholar]