Abstract
Objective
To develop an electronic surveillance tool for catheter-associated urinary tract infections (CAUTIs) and assess its performance.
Methods
The study was conducted at a 947-bed tertiary care center. Subjects included adults aged ≥18 years, admitted to an intensive care unit (ICU) between January 10 and June 30, 2012 with an indwelling urinary catheter during their admission. We identified CAUTIs using four methods: (1) Traditional Surveillance (TS): manual chart review by Infection Control Practitioners, (2) an Electronic Surveillance (ES) tool, (3) Augmented Electronic Surveillance (AES): ES with chart review on a subset of cases, and (4) Reference Standard (RS): A subset of CAUTIs originally ascertained by TS or ES, confirmed by review. We assessed performance characteristics to RS for reviewed cases.
Results
We identified 417 candidate CAUTIs in 308 patients; 175 (42.0%) of these candidate CAUTIs were selected for review, yielding 32 confirmed CAUTI in 22 patients (RS). Compared with RS, the sensitivities of TS, ES, and AES were 43.8% (95% confidence interval [CI]: 26.4–62.3%), 100.0% (95% CI: 89.1–100.0%), and 100.0% (89.1–100.0%). Specificities were 82.5% (95% CI: 75.3–88.4%), 2.8% (95% CI: 0.8–7.0%), and 100.0% (95% CI: 97.5–100.0%).
Discussion
Traditional methods of CAUTI surveillance are error-prone and resource-intensive. We developed a highly sensitive electronic surveillance tool.
Conclusion
Electronic CAUTI surveillance offers a streamlined approach to improve reliability and resource burden of surveillance.
Keywords: catheter-associated urinary tract infection, electronic surveillance, infection control, NHSN
INTRODUCTION
In 2012, the Joint Commission for Accreditation and Certification of Healthcare Organizations adopted catheter-associated urinary tract infection (CAUTI) prevention as a National Patient Safety Goal.[1] Beginning January 1, 2012, as a condition of participation in repayment programs, the Centers for Medicare and Medicaid Services (CMS) mandated CAUTI reporting from all intensive care units (ICUs) to the National Healthcare Safety Network (NHSN), a patient and healthcare personnel safety surveillance system managed by the Centers for Disease Control and Prevention (CDC). At present, this mandated reporting requires trained personnel, usually Infection Control Practitioners (ICPs), to review the charts of all ICU patients to identify those meeting criteria for CAUTI, a process that has proven exceedingly time- and labor-intensive, demanding substantial staffing adjustments.[2–6]
With chart review continuing to serve as the gold standard for CAUTI surveillance, efficient identification of CAUTI by hospitals remains elusive and serves as an impediment to improvement in patient outcomes and infection control practices.[4] We developed an efficient and practical mechanism for CAUTI identification.
MATERIALS AND METHODS
Massachusetts General Hospital (MGH) is a 947-bed teaching hospital in Boston, Massachusetts with 8 adult ICUs (~166 ICU beds). The MGH study population included all adult patients (≥18 years) with an indwelling urinary catheter at any time while admitted to an ICU between January 10 and June 30, 2012.
At the time of the study, the CDC and NHSN defined a CAUTI as a symptomatic UTI or asymptomatic bacteremic UTI occurring in any patient from the time an indwelling urinary catheter is inserted until 48 hours beyond removal (Table 1).
Table 1.
2012 Centers for Disease Control and Prevention/National Healthcare Safety Network Surveillance Definition of Catheter-Associated Urinary Tract Infection in Adults
| Symptomatic CAUTI with High Colony Count |
Symptomatic CAUTI with Intermediate Colony Count |
Asymptomatic Bacteremic CAUTI | |
|---|---|---|---|
| Indwelling urinary cathetera in place or removed within 48 hours of the time of specimen collection | |||
| Signs and | Fever (>38ºC) with no other | Fever (>38ºC) with no other | Patient must have NO signs or symptoms |
| Symptomsb | recognized cause, suprapubic tenderness, urgency, frequency, dysuria, or CVA pain | recognized cause, suprapubic tenderness, urgency, frequency, dysuria, or CVA pain | (EXCEPTION: if age ≥65 years, fever does not disqualify) |
| Urine culture | |||
| Colony count | ≥105 CFU/ml | ≥103 and <105 CFU/ml | ≥105 CFU/ml |
| No. species | ≤2 | ≤2 | ≤2 |
| Urinalysis | Not required | + leukocyte esterase or nitrite, pyuriac or + gram stain | Not required |
| Blood culture | Not required | Not required | Positive blood culture with ≥1 uropathogend matching urine culture |
Abbreviations: CFU, colony-forming units; CVA, costovertebral angle
An indwelling urinary catheter is defined as a drainage tube that is inserted into the urinary bladder through the urethra and is left in place while connected to a drainage bag.
At least one sign or symptom must be present with no other recognized cause. The following symptoms are taken into account only after the catheter has been removed: urgency, frequency, dysuria.
Pyuria: urine specimen with ≥10 WBC/mm3 or ≥3 WBC/high power field of unspun urine
Allowable uropathogens per NHSN definition: Gram-negative bacilli, Staphylococcus spp., Yeast, Beta-hemolytic Streptococcus spp., Enterococcus spp., G. vaginalis, Aerococcusurinae, Corynebacterium (urease-positive)
We identified candidate CAUTI using four methods: (1) Traditional Surveillance (TS): manual chart review by trained ICPs, (2) Electronic Surveillance (ES): an electronic surveillance tool designed to query multiple hospital databases and intended to generate a list of candidate CAUTI for further chart review, (3) Augmented Electronic Surveillance (AES): independent, blinded chart review by two study investigators of a subset of candidate CAUTIs found by ES, and (4) Reference Standard (RS): a consensus list of investigator-reviewed candidate CAUTIs initially detected by either TS or ES. The performance of the three other surveillance methods was compared to RS.
For all surveillance methods, the eligible patient pool with indwelling urinary catheters was identified using a computerized nursing database (QuadraMed; Reston, VA). Bedside nurses use this software to document the presence or absence of an indwelling urinary catheter. Previously, Infection Control staff demonstrated >95% concordance between this electronic record and in-person surveillance rounds of indwelling urinary catheters.
Traditional Surveillance (TS)
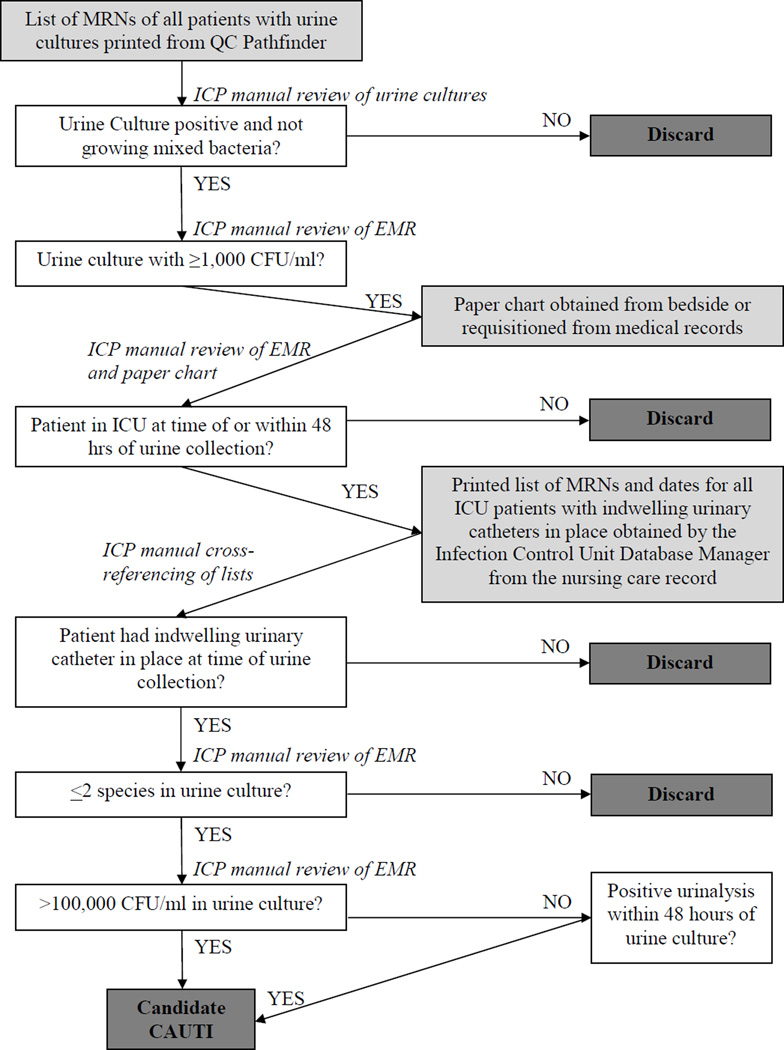
Prospective surveillance using the NHSN CAUTI definition was implemented as of January 1, 2012 by five ICPs within the MGH Infection Control Unit. The ICPs are registered nurses with 7 to 28 years of infection control experience and certified in infection prevention and control. TS involved manual review and cross-referencing of microbiological, laboratory, and nursing care records, along with daily progress notes and vital signs from paper charts (Appendix Figure A).
Appendix Figure A. Flow Diagram of Traditional Surveillance.
Appendix Figure A is a flow diagram demonstrating the ICPs’ protocol for performing traditional CAUTI surveillance without the assistance of the electronic surveillance system. Boxes shaded in light grey indicate electronic or paper records obtained by the ICPs. White boxes and arrows describe steps taken in the ICPs’ workflow. Dark grey boxes indicate stopping points in the workflow (e.g., when a potential CAUTI is “screened out” and discarded or is deemed to be a candidate CAUTI). To begin, on a daily basis, a designated ICP would query the Infection Control Unit’s web-based repository of clinical microbiological data (QC Pathfinder, Vecna Technologies, Cambridge, MA), generating a hospital-wide list of medical record numbers (MRNs) for all patients with urine cultures processed by the microbiology lab within 48 hours. The ICP manually reviewed this list and selected all positive urine cultures, excluding those with “mixed bacteria.” Next, the designated ICP searched the patients’ electronic medical record to determine which positive urine cultures met NHSN criteria for colony growth. For cultures meeting colony growth criteria, the ICP then reviewed the patient’s history in the electronic medical record and paper chart to determine whether or not the patient was in an ICU at the time or within 48 hours of urine culture specimen collection. For all potential CAUTI, the ICP printed separate reports for each ICU and distributed these lists to the staff member covering the unit. Each ICP then provided the list of MRNs and dates to the Infection Control Unit database manager, who accessed the computerized nursing care record and generated a list of dates on which each patient had an indwelling urinary catheter in place. The ICPs then manually cross-referenced the printed lists of positive urine cultures by MRN with the list of patient catheter-days. For all patients with co-occurring indwelling urinary catheters and positive urine cultures, the ICP reviewed the electronic medical record, the microbiological and urinalysis data, as well as the paper chart to determine whether a potential case met NHSN CAUTI criteria. All identified cases meeting CAUTI criteria were reported to the NHSN’s secure internet-based surveillance system.
Electronic Surveillance (ES)
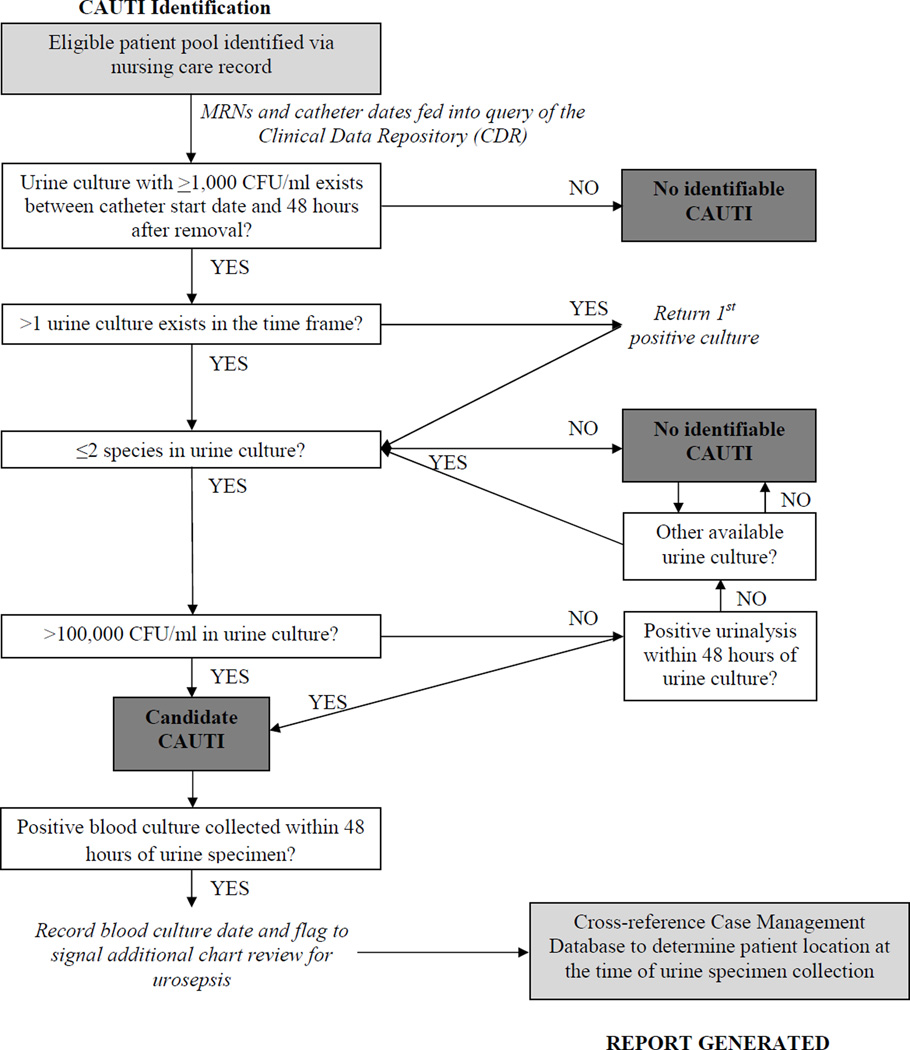
We developed an ES system using a computerized algorithm to automate identification of candidate CAUTIs by linking daily electronic documentation of indwelling urinary catheter presence from the computerized nursing care record with electronic microbiological data, other laboratory results, and electronic case management records on admissions, room assignments, and discharges (Appendix Figure B). The algorithm was designed to run on a weekly basis using data collected within the prior 14 days to ensure inclusion of all finalized urine culture results. The algorithm was automated to rapidly identify the CAUTI-candidate population rather than to function as an independent, automated CAUTI detection system.
Appendix Figure B. Flow Diagram of Electronic Algorithm for Candidate.
Appendix Figure B is a flow diagram demonstrating the electronic algorithm’s protocol for processing information from multiple electronic databases. Boxes shaded in light grey indicate electronic databases accessed by the algorithm. White boxes and arrows describe sequential steps processed by the algorithm. Dark grey boxes indicate stopping points in the algorithm (e.g., when a potential CAUTI is “screened out” and discarded or is deemed to be a candidate CAUTI).As in traditional surveillance, the eligible patient pool of catheterized patients is identified via the electronic nursing care record. The MRNs and indwelling urinary catheter documentation dates for the eligible patient pool then form the basis of an automated query of the hospital’s Clinical Data Repository (CDR), a real-time data warehouse of clinical and microbiological data collected by multiple hospital systems. Based on the 2012 NHSN surveillance definition for CAUTI, we defined a patient’s duration of CAUTI development eligibility from the first urinary catheter presence documentation date during an admission through 48 hours beyond the last documentation date. Catheterization time windows separated by more than 2 calendar days were treated as unique catheterization events. If more than one urine culture existed during the time frame for each report generated by the electronic algorithm, the query returned the first positive culture that met the NHSN microbiological and laboratory data requirements.
The electronic algorithm determined hospital unit of assignment by selecting the inpatient location of the patient 48 hours prior to the urine culture collection date. If a patient was classified as a non-inpatient at this time, the candidate CAUTI was assigned to the patient’s first inpatient location per NHSN guidelines, and the case was flagged for an additional review for possible UTI presence on admission. In addition, the electronic algorithm also generated a note of the number and dates of positive cultures occurring between 14 days prior to admission and 2 days after admission to aid in chart review determination of whether or not a UTI was preexisting. Supplementary elements of the algorithm-generated report included patient demographics, the start and end dates of a patient’s indwelling urinary catheter placement time period, patient location at the time of urine specimen collection, urine culture and urinalysis results, and an indication of whether or not a blood culture was performed within 48 hours of the positive urine culture.
Augmented Electronic Surveillance (AES)
Two study investigators (HEH and ESS), both certified in surveillance for hospital-acquired infections by NHSN, performed independent and blinded retrospective medical record reviews (e.g., nurse/doctor clinical notes, flowsheets, microbiology reports, diagnostic studies, medication records) on a selection of the list of candidate CAUTIs generated by the ES system.
Reference Standard (RS)
Two study investigators (HEH and ESS) also independently reviewed a random selection of charts for CAUTI identified by TS. For the review of TS-identified charts, the investigators were blinded to each other’s decision and to the initial identification method. Taken together, chart review occurred for a selection of 175 of the 417 candidate CAUTIs (42.0%) initially identified by either TS or ES. Discordance between the two investigators on case assignment triggered a second chart review, discussion, and consensus. The 175 investigator-reviewed cases form the reference standard (RS).
Candidate CAUTI and confirmed CAUTI case ascertainment
We calculated the frequencies and percentages of candidate CAUTIs ascertained by TS, ES, and AES, as well as the number of RS CAUTI cases confirmed by investigator chart review. For the candidate CAUTIs selected for investigator review, we calculated a kappa statistic to assess agreement between the two study investigators’ CAUTI designations. Interpretation of kappa values for inter-rater agreement were defined a priori, with <0.20 considered poor, 0.21–0.40 considered fair, 0.41–0.60 considered moderate, 0.61–0.80 considered good, and 0.81–1.00 considered very good.[7]
To compare candidate CAUTI case designations by method of surveillance with RS, we constructed 2×2 contingency tables and a summary contingency table comparing all methods. We determined the sensitivity, specificity, and positive and negative predictive values of all methods compared to RS for the investigator-reviewed cases. For all test performance characteristics, we report exact binomial 95% confidence intervals.
We constructed a 2×2 table to compare case designations by TS and ES among the subset of candidate CAUTIs reviewed by study investigators and calculated a kappa statistic to assess the influence of investigator versus ICP status on final CAUTI determination.
Validation of the Electronic Algorithm
Using data collected during the study period, the electronic algorithm was developed over multiple iterations, with the goal of >95% ascertainment of cases identified by TS. From July 1 to September 30, 2012, we conducted a separate, formal validation process to determine the electronic algorithm’s ability to ascertain cases identified by TS in the absence of revisions to the algorithm. Only candidate CAUTIs identified by TS that were determined on investigator review to meet the NHSN definition were included in calculations of the percentage of traditional surveillance cases ascertained by the electronic algorithm.
Protection of Human Subjects
This study was approved by the Partners Human Subjects Research Office (2011P001471).
RESULTS
Candidate CAUTI and Confirmed CAUTI Case Ascertainment
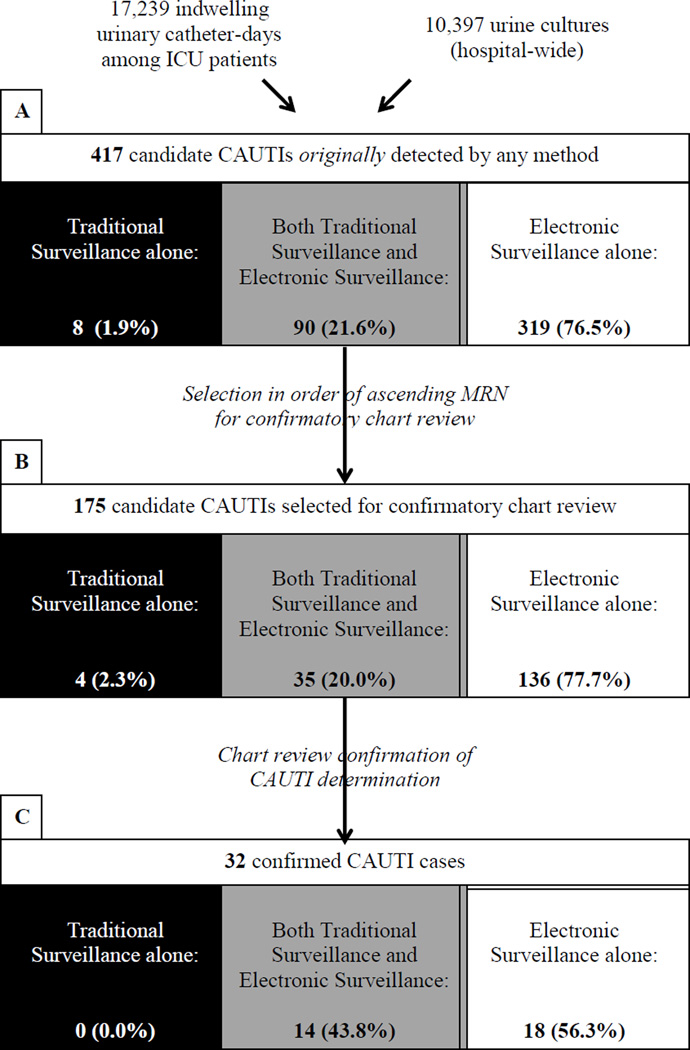
From January 10 through June 30, 2012, 10,397 urine cultures were obtained hospital-wide, and 17,239 indwelling urinary catheter-days were recorded among 1,683 ICU patients. Using a combination of TS and ES, we identified 417 candidate CAUTIs in 308 patients during the study period (Figure 1). TS alone identified 8 potential cases which were not identified by ES (8/417;1.9%), while 90 (90/417; 21.6%) were detected by both TS and ES. ES alone generated the majority of the candidate CAUTIs that were not identified by TS (319/417; 76.5%).
Figure 1. Case Ascertainment Flow Diagram.
Figure 1 is a flow diagram that indicates the number and proportion of candidate and confirmed CAUTI identified by alternative case ascertainment methods. Black shading indicates CAUTIs identified by traditional surveillance alone; gray shading indicates CAUTIs detected by both traditional surveillance and electronic surveillance; and white shading indicates CAUTIs identified by electronic surveillance alone. Section A shows the proportion of candidate CAUTI detected by traditional surveillance alone, electronic surveillance alone, or both methods (N=417). Section B and Section C indicate the breakdown of these ascertainment methods for candidate CAUTI selected for confirmatory chart review (N=175) and confirmed CAUTI (N=32), respectively. Abbreviations: ICU, intensive care unit; CAUTI, catheter-associated urinary tract infection; MRN, medical record number
Of the 417 candidate CAUTIs originally detected, a random subset of 175 candidate CAUTIs were selected for chart review by study investigators (Figure 1). Within this subset of investigator-reviewed candidate CAUTIs, 4 were identified by TS alone (4/175; 2.3%) while 35 were identified by both TS and ES (35/175; 20.0%), and 136 were detected by ES alone (136/175; 77.7%), reflecting similar distributions to the overall sample. Investigator review confirmed 32 CAUTIs in 22 patients (RS). Agreement on case designation between study investigators (HEH and ESS) on initial review was considered good (kappa 0.71; 95% CI 0.57–0.85), with concordance achieved for 92% of cases (161/175). Fourteen cases (8%) required a second review and discussion by the study investigators prior to final case designation.
Characteristics of RS-Confirmed CAUTI Cases
The majority of patients with confirmed CAUTI on investigator review were female (77.3%) and median age was 72.5 years (range: 32–94 years). Median length of stay was 13.0 days (range: 4.6–77.7 days); the number of indwelling urinary catheter-days for each period of catheterization ranged from 3 to 39 days (median: 8 days). Three patients with confirmed ICU-based CAUTI died during the hospitalization (13.6%).
Comparisons of Surveillance Methods to the Reference Standard
Traditional surveillance
TS detected 14 of 32 CAUTIs identified by the RS. Overall concordance of case classification between TS and RS was 75.4% (132/175; Table 2). Compared with RS, the sensitivity of TS was 43.8% (14/32; 95% confidence interval: 26.4–62.3%), while specificity was 82.5% (118/143; 95% confidence interval: 75.3–88.4%) (Table 3).
Table 2.
Comparison of Alternative Methods of CAUTI Surveillance with the Reference Standard
| Reference Standard | |||
|---|---|---|---|
| CAUTI | Not CAUTI | ||
| Traditional Surveillance | |||
| Candidate CAUTI | 14 | 25 | 39 |
| Not CAUTI | 18 | 118 | 136 |
| 32 | 143 | ||
| Electronic Surveillance | |||
| Candidate CAUTI | 32 | 139 | 171 |
| Not CAUTI | 0 | 4 | 4 |
| 32 | 143 | ||
| Augmented Electronic Surveillance | |||
| Candidate CAUTI | 32 | 0 | 32 |
| Not CAUTI | 0 | 143 | 143 |
| 32 | 143 | ||
Table 3.
Performance Characteristics of Alternative Methods of CAUTI Surveillance Compared with the Reference Standard: Sensitivity, Specificity, and Positive and Negative Predictive Values
| Sensitivity (95% CI*) |
Specificity (95% CI) |
Positive Predictive Value (95% CI) |
Negative Predictive Value (95% CI) |
|
|---|---|---|---|---|
| Traditional Surveillance | ||||
| 43.8% (26.4 – 62.3%) | 82.5% (75.3 – 88.4%) | 35.9% (21.2 – 52.8%) | 86.8% (79.9 – 92.0%) | |
| Electronic Surveillance | ||||
| 100.0% (89.1 – 100.0%) | 2.8% (0.8 – 7.0%) | 18.7% (13.2 – 25.4%) | 100.0% (39.8 – 100.0%) | |
| Augmented Electronic Surveillance | ||||
| 100.0% (89.1 – 100.0%) | 100.0% (97.5% – 100.0%) | 100.0% (89.1 – 100.0%) | 100.0% (97.5 – 100.0%) | |
Abbreviations: CI – confidence interval
Electronic Surveillance
ES identified all 32 investigator-confirmed CAUTIs, but properly classified only 4 of 143 candidate CAUTIs as true negatives (Table 2). Overall concordance of case classification was 20.6% (36/175). Compared with RS, the sensitivity of ES was 100.0% (95% confidence interval: 89.1 to 100.0%), while specificity was 2.8% (4/143; 95% confidence interval: 0.8 to 7.0%, Table 3). If fever, as documented in the paper chart and recorded in the investigator chart review, were an electronically searchable criterion and could be incorporated into the electronic algorithm, the specificity of the algorithm alone would increase to 100.0% (95% confidence interval: 97.5 to 100.0%), while sensitivity would decrease to 78.1% (95% confidence interval: 60.0 to 90.7%).
Augmented Electronic Surveillance
AES achieved complete concordance with RS, classifying 32 of 32 true positive investigator-confirmed CAUTI cases and 143 of 143 true negatives (Table 2). Thus, when compared to RS, the sensitivity of AES was 100.0% (95% confidence interval: 89.1 to 100.0%), and specificity was 100.0% (95% confidence interval: 97.5 to 100%) (Table 3).
Comparison of TS with ES
Of the 39 candidate CAUTIs identified by TS selected for confirmatory investigator review, four potential cases were not ascertained by ES alone. Upon review of these four cases, two were found to have a urinalysis outside of the algorithm’s 48 hour time window, one had a urine culture that was >1 week removed from the CAUTI event date, and one was attributed to the wrong medical record number (MRN). Chart review by ICPs and study investigators achieved 75.4% concordance (132/175), but agreement was only considered fair (kappa 0.24; 95% confidence interval: 0.07 – 0.41).
Less than half of all confirmed CAUTI (43.75%; n=14) were detected by all 3 alternative surveillance methods: TS, ES, and AES. Over 80% of the candidate CAUTI detected by the ES system were neither detected by TS nor confirmed by investigator review as part of AES (Table 4).
Table 4.
Summary of CAUTI Designations by the Alternative Surveillance Methods
| Reference Standard |
Traditional Surveillance |
Electronic Surveillance |
Augmented Electronic Surveillance |
N (%) |
|---|---|---|---|---|
| CAUTI (n=32) | CAUTI | CAUTI | CAUTI | 14 (43.8%) |
| CAUTI | CAUTI | Not CAUTI | 0 (0%) | |
| CAUTI | Not CAUTI | CAUTI | 0 (0%) | |
| CAUTI | Not CAUTI | Not CAUTI | 0 (0%) | |
| Not CAUTI | CAUTI | CAUTI | 18 (56.3%) | |
| Not CAUTI | CAUTI | Not CAUTI | 0 (0%) | |
| Not CAUTI | Not CAUTI | CAUTI | 0 (0%) | |
| Not CAUTI | Not CAUTI | Not CAUTI | 0 (0%) | |
| Not CAUTI (n=143) | CAUTI | CAUTI | CAUTI | 0 (0%) |
| CAUTI | CAUTI | Not CAUTI | 21 (14.7%) | |
| CAUTI | Not CAUTI | CAUTI | 0 (0%) | |
| CAUTI | Not CAUTI | Not CAUTI | 4 (2.8%) | |
| Not CAUTI | CAUTI | CAUTI | 0 (0%) | |
| Not CAUTI | CAUTI | Not CAUTI | 118 (82.5%) | |
| Not CAUTI | Not CAUTI | CAUTI | 0 (0%) | |
| Not CAUTI | Not CAUTI | Not CAUTI | 0 (0%) | |
Validation of the ES System
From July 1 through September 30, 2012 (after the initial enrollment period), TS identified 46 additional candidate CAUTIs, while ES identified 206. Upon review of the 46 TS candidates by study investigators, five were determined not to meet NHSN criteria. Of the 41 candidates reported by TS and confirmed to meet NHSN microbiological and laboratory criteria by investigator review, ES detected 40 cases (97.6%). The single case missed by ES was due to the a correction of a urine culture logging-error made by the microbiology laboratory.
DISCUSSION
Traditional CAUTI surveillance methods – grounded in bedside rounding, manual patient chart review, and the subjective implementation of multi-dimensional and often evolving NHSN case definitions – remain the current standard in hospital-acquired infection identification. These methods are error-prone, time consuming, and susceptible to assessment bias in case ascertainment.[2–6] As electronic medical records become more robust, investigators and practitioners have sought to develop computer -aided surveillance systems either to completely automate infection detection or to automate the identification of patients with a high probability of infection.[4, 8–15] For CAUTI surveillance in particular, investigators at several institutions have created electronic algorithms to aid in CAUTI identification. To date, these efforts have incorporated either International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9) coding[16] or traditional surveillance by manual chart review[17, 18] as the reference standard, both of which are not only retrospective but have also been shown to misrepresent the “true” CAUTI rate.[2–6, 19]
We developed an electronic algorithm-based surveillance tool to streamline prospective CAUTI identification at a large, urban teaching hospital. Application of our ES system to query multiple hospital databases to identify CDC/NHSN defined CAUTIs achieved >97% ascertainment of confirmed CAUTI reported by traditional surveillance during both the study time frame and a period of external validation.
Our ES system was never intended to function in isolation as a fully automated surveillance system, and its specificity of <5% reflects this design. Rather, the computerized algorithm was developed with the goal of producing a list of candidate CAUTIs for targeted chart review, at which point signs and symptoms of infection as defined by the CDC/NHSN could be assessed. When combined with confirmatory chart review, the ES system achieved nearly perfect sensitivity and specificity compared to the RS, more than doubling the positive predictive value of TS. Moreover, these improvements in surveillance method performance characteristics complemented substantial reductions in time and the complexity of manual chart review and data documentation tasks. During the study period, ICPs evaluated a total of 10,397 urine cultures and manually cross-checked these cultures with patients’ indwelling urinary catheter status, reviewing 17,239 indwelling urinary catheter-days. If our ES system had been available during this time period, the number of urine cultures that the ICPs would need to review would decrease by 25-fold to 409 cultures and the system would obviate the need for manual review of catheter-days. The reduction in resources utilized with this alternative approach, while not possible to quantify with precision, is nevertheless likely to be substantial given the sheer reduction in volume of patient-level results to review. We anticipate that the impact on resource utilization will expand as regulatory mandates expand: beginning in January 2015, CAUTI surveillance in all adult and pediatric medical and surgical wards will be required for participation in the CMS Hospital Inpatient Quality Reporting Program.
Both full and partial automation of surveillance systems for healthcare-associated infections offer several potential advantages over traditional surveillance.[20] Following a development and validation period, fully-automated algorithms require minimal input from infection control staff beyond occasional quality control audits or adjustments to the program due to changing surveillance definitions. Moreover, fully automated systems that search only objective electronic data may virtually eliminate reliance on subjective aspects of surveillance definitions. By making case criteria completely explicit and standardizing case-finding methodologies, automation increases consistency across surveillance sites and practitioners, thus minimizing assessment bias in case ascertainment.[3] This increases the reliability of comparisons between healthcare settings and improves the ability to evaluate trends over time.
Unless all subjective criteria are eliminated from the NHSN CAUTI case definition, there will continue to be a human dimension to surveillance, Although fully-automated systems may claim more absolute efficiency and standardization, partial automation of surveillance similarly takes advantage of available technology for time saving and error reduction purposes, while preserving the clinical judgment component of manual chart review. As evidenced by our experience, partial automation of surveillance can improve surveillance sensitivity compared to traditional surveillance.
Manual chart review is an expensive endeavor in a time with limited resources in which hospitals are tasked with an increasing number of reportable measures, many of which involve infection prevention. CAUTI surveillance represents a single task in an ever-expanding ICP job description. ICPs’ valuable time may be better spent reviewing candidate cases among an already-defined eligible patient pool or potentially intervening at the bedside to mitigate potential morbidity and mortality with the aid of prospective surveillance systems.
Despite the advantages of the AES in its current form, this study has several potential limitations. First, the reference standard used for comparison of the alternative methods of CAUTI surveillance may not necessarily be a gold standard, a common problem that plagues efforts to improve hospital-acquired infection surveillance.[4] Given the nature of certain components of the NHSN CAUTI definition, even with confirmatory chart review by two study investigators, identified cases may have been misclassified. In many instances, the surveillance definition may bias review towards misclassification of clinically identified CAUTI given the high prevalence of asymptomatic bacteriuria in catheterized patients,[21] as well as persistent fever with unknown source in ICU patients.[22] A second limitation of this study is the fact that we both developed and evaluated the performance of the electronic algorithm within the same study period, potentially manufacturing 100% sensitivity of the algorithm as we incorporated changes to the program. We addressed this limitation by conducting a separate validation outside of the study period, which confirmed >97% ascertainment of cases identified by traditional surveillance.
CONCLUSIONS
We developed a highly sensitive, computerized surveillance tool for CAUTI based on objective criteria from the NHSN/CDC CAUTI surveillance definition.
Many of the objective data defining the infection-susceptible population are readily available electronically. The development of electronic tools to aid in identification of hospital-acquired infections is crucial given the potential for impact on patient care and increasing internal and external demands for augmented surveillance and reporting. These more efficient approaches to case identification show promise for increasing the amount of time available to ICPs for implementation of prevention efforts.
Highlights.
Traditional methods of CAUTI surveillance are error-prone and resource-intensive
We developed a successful electronic surveillance system for CAUTIs
The system achieved >97% ascertainment of CAUTIs
ACKNOWLEDGEMENTS
The authors would like to thank the Director of the Massachusetts General Hospital Infection Control Unit during the time the study was conducted, Paula Wright, RN, BSN, CIC, along with all of the Infection Control Practitioners – Kathleen Hoffman, RN, CIC, Fred Hawkins, RN, MHR, CIC, Heidi Schleicher, RN, BSN, CIC, and Nancy Swanson, RN, BSN, CIC – as well as Infection Control Database Manager Irene Goldenshtein, MS, for their guidance, patience, and tireless efforts. The authors would like to thank Erin E. Ryan, MPH, of the MGH Medical Practice Evaluation Center (MPEC) for assistance in manuscript preparation.
The study was approved by the Partners Human Research Committee (protocol number 2011-P-001471); requirement for informed consent was waived.
FUNDING
This work was supported by a grant from the Doris Duke Charitable Foundation to Harvard Medical School to fund Clinical Research Fellow (HEH), Massachusetts General Hospital Departmental Funds (DCH, ESS, WW, DK, HL, PZ), a National Institutes of Health Training Grant T32 A107061 (ESS), K01 AI110524 (ESS), and the Harvard Center for AIDS Research (RPW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Commission J. Catheter-associated urinary tract infection. R3 Report. 2011;(2) [Google Scholar]
- 2.Lin MY, et al. Quality of traditional surveillance for public reporting of nosocomial bloodstream infection rates. JAMA. 2010;304(18):2035–2041. doi: 10.1001/jama.2010.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin MY, Bonten MJ. The dilemma of assessment bias in infection control research. Clin Infect Dis. 2012;54(9):1342–1347. doi: 10.1093/cid/cis016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klompas M, Yokoe DS. Automated surveillance of health care-associated infections. Clin Infect Dis. 2009;48(9):1268–1275. doi: 10.1086/597591. [DOI] [PubMed] [Google Scholar]
- 5.Emori TG, et al. Accuracy of reporting nosocomial infections in intensive-care-unit patients to the National Nosocomial Infections Surveillance System: a pilot study. Infect Control Hosp Epidemiol. 1998;19(5):308–316. doi: 10.1086/647820. [DOI] [PubMed] [Google Scholar]
- 6.Stevenson KB, et al. Administrative coding data, compared with CDC/NHSN criteria, are poor indicators of health care-associated infections. Am J Infect Control. 2008;36(3):155–164. doi: 10.1016/j.ajic.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Altman DG. Practical statistics for medical research. 1st ed. London ; New York: Chapman and Hall; 1991. p. xii.p. 611. [Google Scholar]
- 8.Klompas M, Kleinman K, Platt R. Development of an algorithm for surveillance of ventilator-associated pneumonia with electronic data and comparison of algorithm results with clinician diagnoses. Infect Control Hosp Epidemiol. 2008;29(1):31–37. doi: 10.1086/524332. [DOI] [PubMed] [Google Scholar]
- 9.Woeltje KF, et al. Automated surveillance for central line-associated bloodstream infection in intensive care units. Infect Control Hosp Epidemiol. 2008;29(9):842–846. doi: 10.1086/590261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright MO, et al. The electronic medical record as a tool for infection surveillance: successful automation of device-days. Am J Infect Control. 2009;37(5):364–370. doi: 10.1016/j.ajic.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Platt R, Yokoe DS, Sands KE. Automated methods for surveillance of surgical site infections. Emerg Infect Dis. 2001;7(2):212–216. doi: 10.3201/eid0702.010212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yokoe DS, et al. Simplified surveillance for nosocomial bloodstream infections. Infect Control Hosp Epidemiol. 1998;19(9):657–660. doi: 10.1086/647894. [DOI] [PubMed] [Google Scholar]
- 13.Kahn MG, et al. An expert system for culture-based infection control surveillance. Proc Annu Symp Comput Appl Med Care. 1993:171–175. [PMC free article] [PubMed] [Google Scholar]
- 14.Trick WE, et al. Computer algorithms to detect bloodstream infections. Emerg Infect Dis. 2004;10(9):1612–1620. doi: 10.3201/eid1009.030978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans RS, et al. Computer surveillance of hospital-acquired infections and antibiotic use. JAMA. 1986;256(8):1007–1011. [PubMed] [Google Scholar]
- 16.Landers T, et al. A comparison of methods to detect urinary tract infections using electronic data. Jt Comm J Qual Patient Saf. 2010;36(9):411–417. doi: 10.1016/s1553-7250(10)36060-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choudhuri JA, et al. An electronic catheter-associated urinary tract infection surveillance tool. Infect Control Hosp Epidemiol. 2011;32(8):757–762. doi: 10.1086/661103. [DOI] [PubMed] [Google Scholar]
- 18.Goris A, et al. Automated surveillance for catheter-associated urinary tract infection [abstract 322]. International Conference on Healthcare-Associated Infections; Atlanta, GA. 2010. [Google Scholar]
- 19.Meddings J, Saint S, McMahon LF. Hospital-acquired catheter-associated urinary tract infection: documentation and coding issues may reduce financial impact of Medicare's new payment policy. Infect Control Hosp Epidemiol. 2010;31(6):627–633. doi: 10.1086/652523. [DOI] [PubMed] [Google Scholar]
- 20.Woeltje K, Lautenbach E. Informatics and epidemiology in infection control. Infect Dis Clin North Am. 2011;25(1):261–270. doi: 10.1016/j.idc.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Nicolle LE, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40(5):643–654. doi: 10.1086/427507. [DOI] [PubMed] [Google Scholar]
- 22.Horowitz HW. Fever of unknown origin or fever of too many origins? N Engl J Med. 2013;368(3):197–199. doi: 10.1056/NEJMp1212725. [DOI] [PubMed] [Google Scholar]