Abstract
This review discusses recent work on melatonin-mediated circadian regulation and metabolic and molecular signaling mechanisms involved in human breast cancer growth and associated consequences of circadian disruption by exposure to light at night (LEN). The anti-cancer actions of the circadian melatonin signal in human breast cancer cell lines and xenografts heavily involve MT1 receptor-mediated mechanisms. In estrogen receptor alpha (ERα)-positive human breast cancer, melatonin, via the MT1 receptor, suppresses ERα mRNA expression and ERα transcriptional activity. As well, melatonin regulates the transactivation of other members of the nuclear receptor super-family, estrogen metabolizing enzymes, and the expression of core clock and clock-related genes. Furthermore, melatonin also suppresses tumor aerobic metabolism (Warburg effect), and, subsequently, cell-signaling pathways critical to cell proliferation, cell survival, metastasis, and drug resistance. Melatonin demonstrates both cytostatic and cytotoxic activity in breast cancer cells that appears to be cell type specific. Melatonin also possesses anti-invasive/anti-metastatic actions that involve multiple pathways including inhibition of p38 MAPK and repression of epithelial-to-mesenchymal transition. Studies demonstrate that melatonin promotes genomic stability by inhibiting the expression of LINE-1 retrotransposons. Finally, research in animal and human models indicate that LEN induced disruption of the circadian nocturnal melatonin signal promotes the growth, metabolism, and signaling of human breast cancer to drive breast tumors to endocrine and chemotherapeutic resistance. These data provide the strongest understanding and support of the mechanisms underpinning the epidemiologic demonstration of elevated breast cancer risk in night shift workers and other individuals increasingly exposed to LEN.
Keywords: Melatonin, Breast Cancer, Nuclear receptors, Molecular Signaling, Circadian Disruption, Genomic instability, Drug resistance
Introduction
Melatonin (N-acetyl-5-methoxytrypamine) is an indolic hormone identified by Lerner and colleagues as a factor from the mammalian pineal gland that lightens melanocytes (Lerner et al. 1960). Melatonin production is regulated by photoperiod as its synthessis and secretion are repressed by light but induced at night in response to darkness (Brainard et al. 2001). Consequently, a diurnal pattern of melatonin synthesis rising during the night but diminished throughout the day is seen in humans. In addition to the pineal gland, there is evidence for melatonin synthesis in extra-pineal organs including the retina, gastrointestinal tract, skin, bone marrow, and lymphocytes (Acuña-Castroviejo et al. 2014). Furthermore, the “master biological clock” located in the suprachiasmatic nucleus (SCN) of the hypothalamus takes this photoperiodic information and modulates the circadian synthesis and secretion of melatonin (Berson et al. 2002; Hastings et al. 2003). As will be discussed later, melatonin is not only an output of the central circadian clock, but it is also an important modulator/regulator of the central circadian clock and peripheral oscillators in tissues and organs including the breast (Stehle et al. 2003).
Most of the normal physiologic and metabolic processes are temporally organized in response to photoperiod and thus predictably modulated throughout the day and night. Thus, allowing anticipation and adaptation of the organism to environmental changes. Similarly, the multitude of processes governing cancer initiation, promotion, progression including invasion and metastasis are temporally modulated by the host’s circadian rhythmic outputs from the central circadian pacemaker in the SCN (Stehle et al. 2003; Troung et al. 2014). At the same time, the same molecular clock genes and their protein products seen in the master clock are expressed in peripheral tissues both normal and cancerous and termed peripheral oscillators, and these peripheral oscillators are coordinately expressed and regulated in a circadian manner (You et al. 2005). Numerous laboratories are focused on defining the importance of these clock/oscillator genes and proteins are involved in cellular processes including cell cycle regulation, cell proliferation, cell survival, apoptosis, DNA damage/repair, and tumor suppression/promotion (Kohsaka & Bass 2007; Troung et al. 2014). It is now evident that the central circadian system and even peripheral oscillators, play important roles in the regulation of intermediary metabolism and cancer (Blask et al. 2005; Slominski et al. 2012; Kelleher et al. 2014). Melatonin, as a consequence of its ability to be regulated as an output of the central circadian clock as well as its input to both the central clock and peripheral oscillators, also plays an important role in the regulation of intermediary metabolism and cancer (Hastings et al. 2003).
The level of melatonin in the blood is regulated by its synthesis in the pineal gland in response to dark and light as well as by its peripheral degradation primarily in the liver (Kelleher et al. 2014). Three major pathways are involved in melatonin degradation: (1) hepatic biotransformation generating 6-hydroxy-melatonin and secretion in the urine after conjugation with sulphate or glucoronide, (2) by alternative indolic pathways which produce 5-methoxyindole acetic acid or 5-methoxytryptophol, and also (3) through a kynuric pathway that produces N1-acetyl-N2-formyl-5-methoxykynuramine.
A plethora of biological actions have been reported for melatonin however best described is its chronobiotic action as a neurohormone and entrainer to the light/dark cycle, and a synchronizer of peripheral clocks to the SCN (Slominski et al. 2012). Elevated nighttime levels of melatonin in the blood inform the bodies cells and organs that it is nighttime (Slominski et al. 2012) and help organize target organs and organ systems into appropriate homeostatic metabolic rhythms. Alterations in either day length or the timing/phasing of light exposure can desynchronize the SCN activity and consequently the production of melatonin by the pineal gland, a phenomenon referred to as circadian disruption.
Over the last two decades, significant research efforts have been afforded to defining the role of melatonin in the etiology of cancer. In the last few years, disruption of melatonin’s circadian profile by exposure to light at night has been described to play an important role in the development, promotion, and progression of breast cancer (Reiter 1991; Claustrat et al. 2005; Jasser et al. 2006, Reiter et al. 2007; Stevens et al. 2007; Hill et al. 2011). While light exposure at night (LEN)-induced circadian disruption can potentially impact many aspects of both the central circadian clock and peripheral oscillators, including expression of the clock genes period 1 (Per1) and period 2 (Per2), a known tumor suppressor, in tissues and organs such as the breast (You et al. 2005), data also suggests that LEN-induced circadian disruption also dramatically inhibits the amplitude and phase of the nocturnal melatonin circadian signal in the blood. Given melatonin’s role as an oncostatic/anti-cancer agent and its tightly regulated synthesis by the light/dark cycle, it is believed that the impact of LEN-induced circadian disruption on breast cancer may be heavily mediated by the disruption of the nocturnal melatonin signal (Reiter et al. 2007; Hill et al. 2011).
While melatonin production can occur in numerous tissues (e.g., retina, gut), it is primarily synthesized in the pineal gland from the amino acid tryptophan in response to the onset of darkness (Huether 1993). As an indolamine, melatonin is highly lipophilic and rapidly diffuses into the blood stream for delivery to distant organs and the cerebrospinal fluid to bathe the hypothalamus and CNS. The fact that melatonin’s biological activity is mediated via two major mechanisms: G protein-coupled receptor-mediated activity and non-receptor-mediated antioxidant activity, is born out in the literature (Dubocovich et al. 2003; Dubocovich et al. 2010).
The focus of this article is to review the current literature including our own studies on the role of the nocturnal melatonin signal in the inhibition of breast cancer promotion and drug resistnace and metastasis via the regulation of tumor metabolism, signal transduction, gene expression (Dauchy et. al 2014), genomic instability and Line 1 expression (Belancio et al. 2015), reactive oxygen species (ROS) (García et al. 2015), and the control of peripheral oscillators (Xiang et al. 2012) involving in vitro and in vivo models of human breast cancer. As well, we will review recent studies focusing on the impact of circadian disruption of the melatonin nocturnal signal by LEN and dim LEN (dLEN) on breast cancer progression, promotion, and drug resistance.
Melatonin receptors in breast cancer
Many of the anti-cancer effects of melatonin are mediated primarily through binding to the two membrane associated G-protein coupled receptors (GPCRs). These two GPCRs found in humans and mammals are the MT1 melatonin receptor (formerly the Mel1a) encoded by the MTNR1A gene and the MT2 melatonin receptor (formerly the Mel1B) encoded by the MTNR1B gene (Brydon et al. 1999; Dubocovich et al. 2010). Both MT1 and MT2 receptors are expressed in various organs but show different yet overlapping expression profiles and overlap in their variable coupling G proteins and signal transduction pathways. Numerous reports demonstrate that melatonin via binding and activation of the MT1 and MT2 G protein-coupled receptors, modulates the activity of a variety of G proteins, including Gαi2, Gαi3, Gαq, and Gα11 (Kiefer et al. 2002; Lai et al. 2008). The MT1 receptor is coupled to the inhibition of cyclic adenosine monophosphate (cAMP) via pertussis-toxin sensitive inhibitory Gαi proteins (Dinet et al. 2007; Lai et al. 2008). Activation of the MT1 receptor promotes inhibition of forskolin-stimulated cAMP formation, suppression of Protein kinase A (PKA) activity, and phosphorylation of the cAMP-responsive element-binding protein (CREB) (Kiefer et al. 2002; Dinet et al. 2007). Melatonin via activation of the MT1 receptor has also been reported to modulate ion channels in cells (Steffens et al. 2003; Schuster 2007).
The MT2 receptor also couples to G-proteins to inhibit forskolin stimulated cAMP production, modulation of cyclic guanosine monophosphate (cGMP) formation, and increase Protein kinase C (PKC) activity (Witt-Enderby et al. 2003). The complexity of melatonin signaling through melatonin receptors is increased by the fact that MT1 and MT2 receptors can form homo- or hetero-dimers, to modify receptor function and activity (Ayoub et al. 2002). As well, Jockers and co-workers (Levoye et al. 2006) have also identified the G protein coupled receptor 50 (GPR50) as a melatonin-related receptor with 45% identity to the MT1/2 melatonin receptors. The GRP50 is unable to bind melatonin, but can dimerize with MT1 and MT2 receptors to suppress the affinity of MT1, but not MT2 for melatonin.
The activation of the MT1 receptor appears mediates much of melatonin’s oncostatic actions in ERα-positive MCF-7 human breast cancer cells. Employing the MCF-7 human breast cancer cells, we reported that melatonin activated MT1 receptors couple to Gαi2, Gαi3, Gαq, and Gα11 proteins (Lai et al. 2008). Over expression of the MT1 receptor can potentiate the anti-proliferative effects of melatonin on breast cancer cells both in vivo and in vitro, but are reversed by non-selective MT1 and MT2 melatonin receptor antagonists (Yuan et al. 2002; Collins et al. 2003). Furthermore, using confocal microscopy we demonstrated that the MT1 receptor localizes to the cell membrane in breast tumor cells, with some co-localizing with caveolin-1 in membrane-associated signaling lipid raft platforms (Lai et al. 2009). Analysis of MT1 receptor expression in a small sample of 50 breast tumor biopsy specimens revealed a positive correlation between the MT1 receptor and ERα expression. Jablonska et al. (2013) also demonstrated that expression of the MT1 receptor in breast tumors correlates with ERα expression and is an independent prognostic marker in ERα-positive breast tumors for overall survival and event-free survival. Recently, Oprea-Iles et al. (2013) reported a higher incidence of MT1-negative tumors in African American (AA) women (48%) as compared to Caucasian women (11%) in a cohort of 167 triple-negative breast cancers (TNBC). Furthermore, in TNBC, MT1 positivity was associated with a lower stage and smaller tumor size at time of diagnosis, while MT1 negativity in TNBC was significantly associated with a higher risk of disease progression, shorter progression-free survival, and disease-related death regardless of race. To date, a number of laboratories have demonstrated MT1 expression in human breast tumor biopsies in a variety of breast tumor types ranging from ERα-positive (luminal A) to TNBC (basal-like). All studies to date have correlated MT1 expression with an improved prognosis compared to those with MT1-negative breast tumors (Lai et al. 2009; Rögelsperger et al. 2009; Jablonska et al. 2013; Oprea-Ilies et al. 2013).
MT1/MT2 receptor independent actions of melatonin
Melatonin can bind and activate cell membrane associated MT1/MT2 G protein coupled receptors (GPCRs) (You et al. 2005; Kohsaka & Bass 2007; Troung et al. 2014). The lipophilic nature of melatonin also allows it to transverse the cell, nuclear, and even mitochondrial membranes to bind to cytosolic, nuclear, and mitochondrial proteins to elicit a variety of non-receptor mediated effects in breast cancer. Numerous studies have shown that melatonin binds the Ca2+-regulatory protein calmodulin (CaM) leading to decreased sensitivity of adenylate cyclase (AC) in binding to CaM (Dai et al. 2002; Schuster et al. 2005). Repressed AC activity is associated with reduced cAMP levels within cells that can lead to altered PKA, cAMP biding protein (CREB) and p300 coregulator expression/activation, as well as the attenuation of phospho-activation and transactivation of various transcription factors and nuclear receptors (NRs) including ERα (Kiefer et al. 2002; Del Rio et al. 2004; Sánchez-Barceló et al. 2005).
It was originally reported by Becker Andre et al. (1994) that melatonin bound as a ligand to the retinoic acid-related orphan receptors alpha (RORα), members of the NR/steroid receptor superfamily. This report, however, was withdrawn (Erratum 1997), as other laboratories working on RORαs were unable to reproduce melatonin’s binding to these receptors. Unfortunately, the fact that melatonin is not a ligand for the RORα receptor has not been well accepted by all groups studying melatonin and the literature is rife with discussions of melatonin as a ligand for RORα. As will be discussed later, melatonin via activation of its MT1 receptor can in fact modulate RORα transcriptional activity.
Initial reports by Reiter and co-workers (Poeggeler et al. 1993) identifying melatonin as a potent free radical scavenger has been confirmed by many other groups further demonstrating that melatonin impacts quinone reductases to reduce oxidative damage by ROS in various tissues including breast tumor cells. These reports also confirm that this effect of melatonin is not mediated through MT1 or MT2 receptors. Furthermore, Blask et al. (1997) showed that administration of melatonin to MCF-7 and ZR-75-1 breast cancer cells in vitro induced the expression of the potent antioxidants glutathione and glutathione-S-transferase that also promoted inhibition of tumor metabolism leading to suppression of cell proliferation. Other non-receptor mediated effects of melatonin include its immune system modulation (Lissoni et al. 1991; Pawlikowski et al. 2002; Carrillo-Vico et al. 2003) and tumor surveillance (Cos & Sanchez-Barcelo 2000) and its ability to decrease telomerase activity (Leon-Blanco et al. 2003).
Anti-proliferative actions of melatonin in breast cancer
Numerous studies have shown that melatonin exerts oncostatic effects on a variety of malignancies (Hill et al. 2011) with its effects on breast cancer being the most extensively studied. Clinical data as well as animal studies have provided evidence that melatonin reduces the incidence of experimentally induced cancers (Tamarkin et al. 1981; Blask et al. 1991; Teplitzky et al. 2001) and significantly inhibits the growth of some human breast tumors (Hill & Blask 1988; Hill et al. 1992; Blask et al. 2011; Mao et al. 2014). In general, it has been found that melatonin exerts both cytostatic anti-proliferative effects and cytotoxic apoptotic effects in breast cancer cells via a variety of mechanisms (Blask 2009; Mediavilla et. al 2010). We reported in 1988 that ERα-positive MCF-7 breast cancer cells were growth inhibited by physiologic concentrations (1 nM) of melatonin (Hill & Blask 1988). Subsequent studies have validated that melatonin suppresses the proliferation of both ERα-positive and ERα-negative human breast tumor cell lines, as well as various animal models of mammary cancer (Hill et al. 1992; 2011; Mao et al. 2014). Most studies support that the majority of melatonin’s anti-proliferative actions are mediated via activation of the MT1 receptor as MCF-7 cells transfected with the MT1 receptor (Collins et al. 2003; Yuan et al. 2002) show significantly enhanced anti-proliferative activity in response to melatonin. A variety of receptor-mediated mechanisms have been described for melatonin’s anti-proliferative actions including its repression of ERα transcriptional activity in ERα-positive breast tumor cells (Kiefer et al. 2002; Ram et al. 2002), inhibitory actions on Ca2+ signaling and CaM expression (Dai et al. 2002), and induction of p53 expression (Proietti et al. 2013) and its target gene p21 (Mediavilla et al. 1999). Work by Santoro et al. (2013) demonstrated that blockade of MT1/MT2 receptors in MCF-7 cells impairs p53-mediated prevention of DNA damage. Other mechanisms associated with the anti-proliferative actions of melatonin include cell cycle with arrest of breast tumor cells in the G1 phase of the cell cycle (Cos et al. 1991), repression of estrogen synthesis in the gonads (Blask et al. 2011) or the tumor by repression of aromatase (Martínez-Campa et al. 2009), and finally by suppression of tumor metabolism (Warburg effect) via repression of tumor uptake of free fatty acids, particularly linoleic acid and its conversion to 13-hydroxyoctadecadienoic acid (13-HODE) (Reiter 1991; Blask et al. 2014).
Melatonin modulation of the estrogen/ERα-signaling pathway
Early reports that melatonin could suppress the synthesis of gonadal steroids such as estrogen (Woo et al. 2001) were the first indication of cross talk between melatonin and the estrogen signaling pathway. Subsequent studies, including our own, showing that melatonin inhibited estrogen-mediated proliferation of human breast cancer cells in culture (Hill & Blask 1988; Hill et al. 2011) suggested that melatonin influences estrogenic actions on breast and mammary tissue by three key mechanisms: (1) by suppression of gonadal synthesis of steroids (including estrogens) to decrease their circulating levels; (2) by interacting with the ERα as a selective estrogen receptor modulator (SERM) to alter estrogen binding, DNA-binding, and transcriptional activity; and (3) by repressing the activity of other enzymes such as aromatase, sulfatase, and aldo-keto reductases (AKRs) involved in the synthesis of estrogens from cholesterol or other steroids (González et al. 2007; Dauchy et. al 2014).
Initially, melatonin was shown to be a regulator of reproduction in seasonally breeding animals under natural photoperiods (Reiter 1980) through regulation of the hypothalamic-pituitary axis and gonadal activity (Barrett & Bolborea 2012). This regulation of the hypothalamic-pituitary-gonadal axis by melatonin resulted in the suppression of ovarian estrogen synthesis (Bondi et al. 2014). While humans are not seasonal breeders and the impact of melatonin on human reproductive physiology is not completely clear (Tamura et al. 2008), melatonin does exert some modulatory action on steroidogenesis in human granulosa-leuteal cells (Woo et al. 2001). Since estrogen plays a pivotal role in breast cancer etiology, these data suggested melatonin might be an important inhibitor of breast cancer.
Our early studies demonstrated that physiologic concentrations of melatonin (1 nM) were able to suppress the proliferation of ERα-positive human breast cancer cell lines in vitro (Hill & Blask 1988). Subsequently, we and others have found that melatonin inhibits the proliferation of some ERα-negative breast cancer cell lines and that differences in sensitivity to melatonin are evident between different cell lines (Hill et al. 1992; 2011). Employing ERα-positive MCF-7 breast cancer cells, Molis et al. (1994) reported that melatonin-mediated suppression of ERα mRNA. Ram et al. (Kiefer et al. 2002; Ram et al. 2002) was the first to show that melatonin was a potent repressor of estrogen-induced ERα transcriptional activity, inhibiting the expression of numerous estrogen-induced mitogenic and anti-apoptotic genes including Bcl-2, while inducing growth-inhibitory and pro-apoptotic genes including TGF-α and Bax. Lai (Lai et al. 2008) and Kiefer (Kiefer et al. 2002) subsequently demonstrated that melatonin activation of Gαi2 proteins to decrease cAMP/PKA levels and phosphorylation of the ERα serine 236 (s236), a PKA sensitive site, mediates melatonin’s inhibition of ERα transcriptional activity. The involvement of CaM in melatonin’s repression of ERα transcriptional activity, as described by Del Rio et al. (2004), is consistent with the reported modulation of the Ca2+/CaM pathway by PKA (Barrett & Bolborea 2012). Subsequently, Dai et al. (2002) have confirmed that melatonin modulates Ca2+ and CaM expression and signaling in MCF-7 breast cancer cells.
We employed chromatin immunoprecipitation (ChiP) analysis of the cyclin D1 promoter, to show that melatonin blocks 17-β estradiol the recruitment of CaM and p300 to the cyclin D1 promoter (Hill et al. 2011). In the absence of estrogen, this effect is considerably blunted effecting a delay and decreasing to a lesser degree p300 the recruitment of p300 to the cyclin D1 promoter. Over a 90 min. time course melatonin, in the absence of estrogen, induces an oscillation in CaM recruitment to the cyclin D1 promoter that is anti-phase with the recruitment seen in the presence of estrogen. Surprisingly, a daily rhythm if estrogen that peaks in the early morning is evident in the blood (Bao et al. 200; Royston et al. 2014). As plasma melatonin levels typically peak between midnight and 2 am and then decline as estrogen levels begin to rise, this may suggerst that melatonin can differentially influence CaM and p300 recruitment to the cyclin D1 promoter and other promoters to regulate gene transcription during the night (Hill et al. 2011).
The mechanism(s) involved in melatonin’s modulation of ERα transcriptional activity are still not completely resolved but appear to involve changes in phosphorylation of receptor and/or coactivator (e.g. CaM, SRC-1, CBP/p300 etc.) expression and phosphorylation. In a recent report by Dauchy et al. (2014), we demonstrated that dLEN, by its suppression of the nocturnal melatonin circadian signal, induced the phosphorylation of the ERα at s167 and s118 via the induction of extracellular receptor kinase 1 and 2 (ERK1/2), protein kinase B (AKT), and c-SRC kinase. Notably, supplementation with exogenous melatonin during dLEN significantly repressed ERα phosphorylation at these sites. These data strongly suggest that melatonin may have an important influence on gene expression in human breast cancer cells mediated by the actions of the MT1 receptor on specific NRs (e.g., ERα) and their signal transduction pathways.
Melatonin modulates the expression and/or transactivation of other nuclear receptors (NRs) in human breast cancer cells
In addition to its suppression of ERα transactivation, melatonin via it MT1 receptor, also modulates the expression and/or transcriptional activity of other members of the steroid hormone/NR superfamily. For example, melatonin has been shown to repress the ligand-induced transactviation of the glucocorticoid receptor (GR) and RORα in breast cancer cells (Dai et al. 2001; Kiefer et al. 2005). Conversely, melatonin can augment/potentiate the transactivation of other NRs including the retinoic acid receptor alpha (RARα), and retinoic acid X receptor alpha (RXRα) (Kiefer et al. 2005; Hill et al. 2011). We initially reported (Hill et al. 2011) and Proiette et al. (2011) confirmed in breast cancer cells that melatonin potentiates the transactivation of the vitamin D receptor (VDR) and the peroxisome proliferation activating receptor gamma (PPARγ). However, not all NRs are impacted by melatonin, as no effects of melatonin were observed on the ERβ in human breast cancer or HEK293 embryonic kidney cells (Lai et al. 2008). The mechanism(s) involved in melatonin’s modulatory effects on NRs, particularly the ERα, are not completely delineasted but appear to involve direct changes in receptor and/or coactivator (CaM, SRC1, CBP/p300, etc.) expression and phosphorylation. Our recent report that dLEN-mediated circadian melatonin disruption induces - and melatonin administration during dLEN ablates or represses - the expression and/or phospho-activation of a number of kinases (ERK1/2, ATK, PKA, SRC, FAK, etc.) that are well known to regulate the phospho-activation of NRs and other transcription factors (Ap-1, Elk-1, NF-kB, STAT3, etc.) (Dauchy et. al 2014), clearly demonstrates the importance of melatonin in regulating on gene expression in human breast cancer.
The potential clinical significance of melatonin’s regulation of breast cancer NRs in is highlighted in several studies. For example, Eck et al. (1998) demonstrated that pre-treatment of MCF-7 cells with melatonin following theadministration of all trans-retinoic acid, a ligand for both RARα and RXRα, was able to promote apoptosis in MCF-7 breast cancer cells via activation of the RARα coincident with repression of transforming growth factor beta 1 (TGF-β1) and Bcl2 (an anti-apoptotic protein), and increased expression of the pro-apoptotic protein Bax. Follow-up studies by Teplitizky (Teplitzky et al. 2001) and Melancon (Melancon et al. 2005) found that the combination of melatonin and 9-cis retinoic acid was able to repress the development of N-nitroso-N-methylurea (NMU)-induced mammary tumors in rats by more than 90%, as well as induce the regression of 78% of established NMU-mammary tumors, with 54% undergoing complete regression. The interaction between the melatonin and vitamin D/VDR pathways was demonstrated in a report by Proietti et al. (2011) showing that melatonin could promote VDR transcriptional activity and drive MCF-7 breast tumor cells to apoptosis in vitro.
Melatonin modulation of gene expression in breast cancer
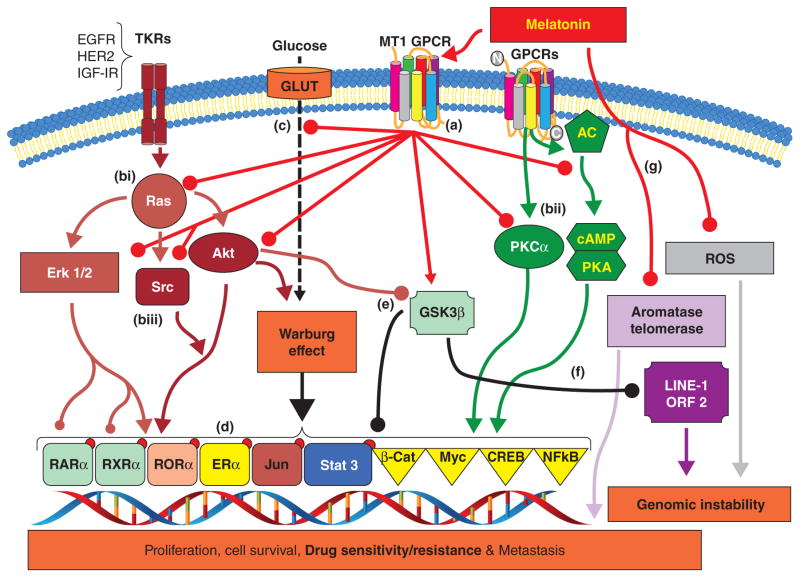
The effects of Melatonin on breast cancer are quite diverse, ranging from antioxidant, immuno-modulatory, and enzyme regulatory, to regulation of various kinases and transcription factors. Via activation of its MT1 receptor in breast cancer, melatonin has been shown to inhibit the expression and/or phospho-activation of numerous kinases (AKT, ERK1/2, PKA, PKC, c-SRC, GSK3β, etc.), transcription factors (ERα, RORα, RAR, RXR, VDR, PPARγ, Ap-1, Elk-1, CREB, NF-kB, and STAT3), and ERα coregulators such as calmodulin (CaM), cAMP binding protein (CBP/p300), and steroid receptor coactivator-1 (SRC-1) known to drive breast cancer promotion and/or progression (Hill et al. 2011; Dauchy et. al 2014).
To further explore the mechanisms by which melatonin suppresses breast cancer cell growth, various laboratories including our own have chosen to employ a systems approach to identify the expression profile of genes regulated by melatonin in MCF-7 cells in vitro. Employing genomic profiling, Lee et al. (2011) evaluated miroRNA (miRNA) and gene expression in MCF-7 breast tumor cells treated with 1 nM and 100 nM concentrations of melatonin for 24 h and found significant differences in both miRNA and gene expression in response to the two different doses. In cells treated with 1 nM melatonin, 5 miRNAs were either up- or down-regulated as compared to 18 miRNAs in cells treated with 100 nM melatonin, with only miR-1207-3p overlapping between the two groups. At the mRNA level, treatment of cells with 1 nM melatonin regulated the expression of twice as many genes as the higher concentration (100 nM) of melatonin. These effects were equally weighted between gene induction and suppression with the majority of genes functionally related to signal transduction, transcription, cell proliferation, and cell transport. This same group (Lee et al. 2013) also evaluated the effects of melatonin on gene methylation and found a number of genes were aberrantly methylated and their expression decreased in response to melatonin, including early growth responsive gene 3 (Egr3) and POU4F2/Brn-3b, both of which are associated with increased invasive and proliferative tumor cell capacity. Conversely, melatonin (1 nM) de-methylated and increased the expression of the tumor and metastasis suppressor glypican-3 (GPC-3).
A genomic profiling analysis by Liu et al. (2013) examining the effect of the carcinogen methyl methanesulfonate on melatonin (1 nM) pre-treated MCF-7 cells showed altered expression of DNA-damage response pathway genes. Further pathway-based bioinformatics analyses revealed that the top functional networks included DNA replication, recombination, DNA repair, and cancer. Among these genes are several with known roles in regulation of DNA repair activity including up regulation of CEP152, a regulator of genomic integrity and cellular response to DNA damage through the ATR-mediated checkpoint signaling pathway (Kalay et al. 2011). N4BP2L2 mRNA was also induced following carcinogen exposure and was further elevated with melatonin pre-treatment. N4BP2L2 encodes a phospho-noformate immune-associated protein that is phosphorylated by ATM or ATR upon DNA damage.
To identify candidate genes that respond directly or indirectly to melatonin via activation of its MT1 receptor we examined the gene expression profile of MCF-7 human breast carcinoma cells transiently transfected with the MT1 receptor and treated with 1 nM melatonin using a limited (8,000–9,000 genes) cDNA microarray analysis. Close to 300 genes were found to have significantly altered levels of expression ≥ 1.4-fold. Of these, melatonin was found to suppress the expression of more than 210 genes and induce the expression of more than 80 genes. A summary list and values of these cancer related genes and their function can be found in Table 1.
Table 1.
Representative list of differentially expressed genes by melatonin in MCF-7 cells
| GENE SYMBOL | GENE NAME | FOLD CHANGE | FUNCTION | ACCESSION NUMBER |
|---|---|---|---|---|
|
Cell-to-cell adhesion and communication
| ||||
| FN1 | Fibronectin 1 | −2.6 | Involves cell adhesion, cell motility, wound healing. Possible role in metastasis | AW385690 |
| DPI, DPII | Desmoplakin (DPI, DPII) | −2.3 | Intercellular junction, adhesion protein | M77830 |
|
| ||||
|
Cytokine and cytokine receptors
| ||||
| BMP7 | Bone morphogenetic protein 7 (osteogenic protein 1) | −2.5 | Member of the transforming growth factor-beta superfamily, induces epithelial cell growth | BE395650 |
|
| ||||
|
Growth factor and receptors
| ||||
| AREG | Amphiregulin (schwannoma-derived growth factor) | −2.0 | A member of the epidermal growth factor family, growth inhibition and stimulations | NM_001657 |
| IGF1R | Insulin-like growth factor 1 receptor | −1.4 | Binds insulin-like growth factor, plays a critical role in transformation events. | X04434 |
|
| ||||
|
Signal transduction-related
| ||||
| S100P | S100 calcium-binding protein P | −2.6 | Calcium-binding protein, mediates Ca2+ signals, overexpressed in breast cancer | BE536069 |
| CALR | Calreticulin | 1.6 | Ca2+ binding protein, modulator of nuclear receptor function, possible role in angiogenesis | M84739 |
| NUCB2 | Nucleobindin 2 | 2.8 | Ca2+ binding protein, possible role in calcium homeostasis | AF052644 |
|
| ||||
|
Chromatin remodeler and coactivator
| ||||
| CITED2 | Cbp/p300-interacting transactivator | minus;1.7 | Coactivator of transcription factors | BF110899 |
| SRC1 | Nuclear receptor coactivator 1 | −1.4 | Transcriptional coactivator for steroid and nuclear hormone receptors. | U59302 |
|
| ||||
|
Transcription factor
| ||||
| EPAS1 | Endothelial PAS domain protein 1 | −2.3 | Transcriptional factor, induces VEGF expression, possible role in angiogenesis | AW377189 |
|
| ||||
|
Cell proliferation-related
| ||||
| Ki-67 | Antigen identified by monoclonal antibody Ki-67 | −1.8 | Possible role in cell proliferation | X65550 |
| p21 | Cyclin-dependent kinase inhibitor 1A (p21, Cip1) | 1.7 | Cyclin-dependent kinase inhibitor, blocking cell cycle progression | U09579 |
|
| ||||
|
Development and differentiation-related
| ||||
| NDRG1 | N-myc downstream regulated | −2.1 | A member of the N-myc downregulated gene family, possible role in growth arrest and cell differentiation, induced by hypoxia | X92845 |
|
| ||||
|
Metastasis-related
| ||||
| IGFBP5 | Insulin-like growth factor binding protein 5 | −3.3 | Growth inhibitor and proapoptotic agent in breast cancer, possible role in metastasis | AA374325 |
| FN1 | Fibronectin 1 | −2.6 | Cell adhesion, cell motility, wound healing. Possible role in metastasis | AW385690 |
| EphA1 | EphA1 | −1.6 | Ephrin receptor subfamily of the protein-tyrosine kinase family, possible role in metastasis | Z27409 |
| TIMP3 | Tissue inhibitor of metalloproteinase 3 | 1.9 | Natural inhibitors of the matrix metalloproteinases, inhibits VEGF binding to VEGFR2 and angiogenesis | AI095372 |
|
| ||||
|
Angiogenesis
| ||||
| EPAS1 | Endothelial PAS domain protein 1 | −2.3 | Transcriptional factor, induces VEGF expression, possible role in angiogenesis | AW377189 |
| NDRG1 | N-myc downstream regulated | −2.1 | A member of the N-myc downregulated gene family, induced by hypoxia | X92845 |
| FN1 | Fibronectin 1 | −2.6 | Involves cell adhesion, cell motility, wound healing. Possible role in metastasis | AW385690 |
| EphA1 | EphA1 | −1.6 | Ephrin receptor subfamily of the protein-tyrosine kinase family, possible role in metastasis | Z27409 |
| CALR | Calreticulin | 1.6 | Ca2+ binding protein, modulator of nuclear receptor function, possible role in angiogenesis | M84739 |
| TIMP3 | Tissue inhibitor of metalloproteinase 3 | 1.9 | Natural inhibitors of the matrix metalloproteinases, inhibits VEGF binding to VEGFR2 and angiogenesis | AI095372 |
Positive number of fold change represents the up-regulation of gene expression by melatonin.
Negative number of fold change represents the down-regulation of gene expression by melatonin.
Key examples include cell-to-cell adhesion and communication molecules including fibronectin (FN1) and desmoplakin that were down regulated by 2.6- and 2.3-fold, respectively; the cytokine bone morphogenic protein 7 (BMP7) was down regulated by 2.5-fold, and the growth factors amphiregulin (AREG) and insulin-like growth factor 1 receptor (IGF1R) were down regulated by 2.0- and 1.4-fold, respectively. The Ca2+ homeostasis-associated S100 calcium-binding protein (S100P) was also down regulated by 2.6-fold, while nucleobindin 2 (NUCB2) was up regulated by 2.8-fold in response to melatonin activation of the MT1 receptor.
Genes associated with chromatin remodeling and transcriptional regulation including steroid receptor coactivator one (SRC-1) and the cAMP binding protein (CBP)/p300-interacting transactivator (CITED2), well-established coregulators of the NR family and other transcription factors, were down regulated by 1.4- and 1.7-fold, respectively, while calreticulin (CALR), a NR interacting protein, was up-regulated by 1.6-fold. The transcription factor Endothelial PAS domain protein 1 (EPAS1) also termed Hypoxia inducible factor 2 alpha (HIF2α), which is associated with increased angiogenesis, was down regulated by 2.3-fold. A variety of proteins associated with cell proliferation were down regulated including Ki-67 (-1.8-fold). However the cell cycle inhibitor p21 was up regulated by 1.7-fold.
Metastasis related genes, including insulin-like growth factor binding protein 5 (IGFBP5), fibronectin (FN1), and ephrin A1 (EFNA1) were down regulated by 3.3-, 2.6-, and 1.6-fold, respectively. However, the tissue inhibitor of metalloproteinase three (TIMP3) was induced by 1.9-fold. Interestingly, TIMP3 has been shown to promote breast tumor sensitivity to tamoxifen and, as we recently reported (Dauchy et. al 2014) the in vivo repression of the circadian melatonin signal by dLEN drives breast tumors to intrinsic resistance to tamoxifen. Angiogenesis is an essential step in tumor formation and plays a critical role in tumor metastasis. A number of angiogenesis-associated genes were down regulated by melatonin including EPAS1, N-Myc downstream regulated (NDRG1), and EFNA1, being decreased by 2.3-, 2.1-, and 1.6-fold, respectively.
Melatonin mediated apoptosis in breast cancer
Melatonin’s anticancer actions in breast cancer can be classified as cytostatic or cytotoxic. While considerable evidence shows that both physiologic and pharmacologic concentrations can inhibit breast tumor proliferation, cytotoxicity has also been reported in response to melatonin in breast cancer in a cell and tumor-specific manner, particularly when pharmacologic concentrations of melatonin are employed (Grant et al. 2009; Hill et al. 2011; Proietti et al. 2013). It should be noted that in other specific types of cancer the cytotoxic/apoptotic actions of melatonin are more frequently observed than in breast cancer. Mediavilla et al. (1999) and other laboratories have found that physiologic concentrations of melatonin reduce the in vitro proliferation of breast cancer cells by elongating cell-cycle length via control of the p53/p21 pathway, independent of promoting apoptosis. We have, however, documented a significant rise in apoptosis in MCF-7 cells in vitro when melatonin was administered together with retinoids (Eck et al. 1998). Some indirect proof of melatonin-induced apoptotic activity is seen in in vivo studies in rat mammary tumors showing a significant increase in caspase-3 activity and DNA fragmentation in tumor samples following melatonin administration (Adb El-Aziz et al. 2005). However, a report by Cucina et al. in 2009 found that under appropriate conditions two distinct apoptotic processes could be triggered by melatonin in MCF-7 cells; including an early response that was independent of TGFβ1 and caspase activity, and a later apoptotic response that was both TGFβ1 and caspases-dependent, with caspases-7 involved as the terminal effector.
More recent work by Proietti et al. (2014) confirms the work of Cucina showing that within 3 h of treatment with 1 nM melatonin a dramatic decrease in murine double minute 2 (MDM2), a regulator of p53 ubiquitination, was observed. Down-regulation of MDM2 allowed elevated expression and acetylation of p53, which increased p21 levels, leading to decreased cell cycle progression and promoted p53-mediated apoptosis. Furthermore, they reported that melatonin decreased the expression of the survival protein silent mating type information regulation 1 homolog (Sirt1) via modulation of the MDM2/murine double minute X (MDMX)-p53 pathway. Both flow cytometry and DNA-fragmentation analyses documented a two-phase apoptotic response to melatonin (early @ 24 h and late @ 96 h). Early apoptosis appears to be caspase-independent, while the later response appears to involve TGF-β1, caspase-7, caspase-9, PARP cleavage, and a down-regulation of the Bcl-2/Bax ratio. These melatonin-mediated apoptotic responses are even more complex and appear to involve p53 and p73 release with p53 activated in the early response and p73 mediating the caspase-dependent late response.
A number of studies support that both in vitro and in vivo models of breast cancer are less responsive to the apoptotic effects of melatonin when it is used as a single agent. However, melatonin appears to amplify the cytotoxic effects induced by other hormones or conventional drugs (Eck et al. 1998; Carrillo-Vico et al. 2003; Dauchy et. al 2014). Although we do not yet have a complete understanding regarding the mechanism(s) by which melatonin exerts its full anti-cancer effects, when used at pharmacologic concentrations melatonin activates responses that involve the intrinsic and/or extrinsic apoptotic pathway in cancer cells, namely through an increase in the p53/MDM2p ratio and down-regulation of Sirt1.
Melatonin effects on tumor metabolic activity
Efficient biosynthesis of the cellular and molecular building blocks required for tumor growth is fueled by a process involving the robust uptake of circulating glucose and its conversion to lactate by cancer cells via glycolytic metabolism in the presence of ample oxygen. This type of glucose metabolism is termed aerobic glycolysis (also referred to as the Warburg effect), and it represents the bioenergetic process preferred by cancer cells over oxidative phosphorylation to accommodate rapidly expanding tumor biomass (Warburg 1925; DeBerardinis et al. 2008; Vander Heiden et al. 2009; Locasale & Cantley 2010). AKT, HIF-1α and c-MYC (Elstrom et al. 2004; Gordan et al. 2008) are important signal transduction and transcriptional networks that drive the Warburg effect to re-route bioenergetics in cancer cells to generate molecular intermediates required to sustain continual cancer cell proliferation (DeBerardinis et al. 2008; Vander Heiden et al. 2009; Locasale & Cantley 2010). In addition to glucose metabolism via the Warburg effect, the cellular uptake of linoleic acid (LA), an essential omega-6 fatty acid (FA) that is the most prevalent polyunsaturated FA in the western diet is also critical for cancer cell proliferation and tumor growth (Sauer & Dauchy 1992; Sauer et al. 1997; 1999). Cancer cells take-up LA by a cAMP-dependent transport mechanism and via activation of the enzyme 15-lipoxygenase-1 (Blask et al. 1999; 2005; Sauer et al. 1999; Dauchy et al. 2004), the activity of which is up-regulated by activation of epidermal growth factor (EGF) and insulin-like growth factor-1 (IGF-1) receptors (Glasgow & Eling et al. 1994; Glasgow et al. 1997), metabolize LA to the mitogenic metabolite 13-HODE. 13-HODE has been shown to exert a positive feedback effect on EGF and IGF-1R growth and survival signaling pathways, in a variety of tumors, including human cancer xenografts (Sauer et al. 1999; Dauchy et al. 2004; Blask et al. 2005), to enhance the downstream phospho-activation of AKT and ERK1/2 leading to the amplification of cell proliferation and survival responses (Hsi et al. 2003; Blask et al. 2011).
The host/cancer balance is maintained by the circadian organization of these daily bioenergetic, metabolic, signaling, and proliferative activities into coordinated rhythms that help to ultimately slow the expansion of tumor biomass. Both host and tumor rhythms are driven by the circadian nocturnal melatonin signal which, by virtue of its intrinsic chronobiotic and oncostatic properties, provides a light/dark cycle-entrained temporal framework for and organization of tumor growth (Blask et al. 2011). For example, in human breast cancer xenografts grown in athymic female nude rats, greatly elevated tumor uptake of LA and metabolism to 13-HODE and aerobic glycolysis during the daytime are involved in promoting a corresponding increase in cell proliferative/survival activities in tumors, whereas during the nighttime, these processes become quiescent as shown by much lower cell proliferative rates and increased cell loss. Therefore, the relatively slow net tumor growth rate in the presence of an intact nocturnal circadian melatonin signal reflects an overall balance in tumor circadian dynamics characterized by a up-regulation of daytime metabolism, signaling, proliferation and cell survival offset by a highly significant down-regulation of these activities during the nighttime (Blask et al. 2014).
Under the conditions of exposure to low intensity light at night and circadian disruption that only suppresses nocturnal melatonin production, both the Warburg effect and the uptake of LA and its metabolism to 13-HODE in breast cancer xenografts become completely arrhythmic and operate at a constitutively high level throughout the entire day (all 24-hrs). This form of circadian disruption not only provokes a melatonin-deficient state, but also compromises normal rhythms of blood glucose, insulin and IGF-1 leading to hyperglycemia and hyperinsulinemia, in addition to persistently high IGF-1 blood levels (Wu et al. 2011). Thus, a chronic lack of circadian-organization in proliferative activity and metabolic signal processing results in a 24-hour per day hyper-metabolic and -proliferative state that culminates in markedly accelerated overall tumor growth rates. Additionally, key tumor signal transduction and transcriptional factors such as cAMP, AKT, and HIF-1α regulation of LA metabolism and the Warburg effect in tumors show melatonin-driven circadian oscillations that are dramatically disrupted by dLEN indicating their unique role in regulating metabolic fluxes in coordination with classical allosteric feedback mechanisms controlling intermediary metabolism in human breast cancer (Blask et al. 2011).
Melatonin regulation of genomic instability
A well-established connection between shift-work associated circadian disruption and increased cancer risk in humans and animal models strongly supports the loss of cellular ability to maintain genome stability in response to extended LEN. This assumption is based on the causative association between genomic instability and cancer. As described in the next section, LEN triggers changes in gene expression and modifications of protein activity relevant to cellular ability to respond to and repair DNA damage. Genomic instability could originate from DNA damage resulting from internal and external sources. It was previously mentioned that melatonin is a powerful antioxidant and can reduce ROS production and accumulation by improving mitochondrial function as well as by stimulating anti-oxidative enzymes.
Another source of genomic instability in mammalian cells is transposable elements, which are represented by non-long terminal repeat retro-elements in the human genome. Using a “copy-and-paste” mechanism of amplification, Long interspersed element-1 (L1) has accumulated to over 500,000 copies, which are distributed throughout the human genome (Lander et al. 2001). About 80–100 of these loci remain functional (Brouha et al. 2002; 2003). Expression of some of these fixed active L1s, as well as a number of polymorphic loci, contribute an estimated 0.04–0.07 de novo inserts per normal cell (neuron) (Evrony et al. 2012). A full-length, functional L1 locus generates an mRNA and two proteins ORF1p and ORF2p. The L1 proteins bind to the L1 mRNA to form a retrotranspositionally competent ribonucleoprotein (RNP) complex and have distinct roles in retrotransposition. The ORF1p serves as a structural protein with nucleic acid chaperon activity (Belancio et al. 2010). The ORF2 protein has an endonuclease, which cuts the host DNA, and a reverse transcriptase, which synthesizes L1 cDNA in the nucleus (Belancio et al. 2010). The L1 integration process is most likely completed with the aid of poorly defined cellular factors. In addition to retrotransposition, L1 can damage genomic DNA via generation of double strand breaks (DSBs) (Belancio et al. 2010), the structure and mutagenic potential of which remain unknown.
The Belancio laboratory has recently reported that melatonin, via the MT1 receptor mediated action, suppresses expression of the endogenous retrotransposon L1 in a tissue-isolated model of human cancer (Deharo et al. 2014). This finding supports that LEN-induced suppression of nocturnal melatonin synthesis activates L1 expression. Our tissue culture based experiments demonstrated that MT1 expression decreases L1 mobilization by about 10-fold in HeLa cells by down regulating L1 ORF1 protein levels (Deharo et al. 2014). These data support that LEN promotes L1 expression and damage in tumor and possibly normal tissues. This previously unanticipated connection between L1 activity and environmental light exposure supports the possibility that L1-induced damage may contribute to the LEN-associated cancer risk.
Effects of light exposure at night (LEN) on melatonin and breast cancer
Based on early studies demonstrating melatonin’s anti-cancer actions in breast cancer, Stevens (Stevens 1987) hypothesized that suppression of nighttime pineal melatonin production in response to light at night might explain the rise in breast cancer rates that have accompanied industrialization and electrification in the United States and other westernized countries. Light exposure at night is a well-recognized environmental disruptor of the central circadian timing system located in the SCN (Reiter 1991; Claustrat et al. 2005; Stevens et al. 2007; Straif et al. 2007). Nighttime production of melatonin by the pineal gland represents a highly reliable output signal of the circadian clock whose suppression by LEN is intensity-, duration-, and wavelength-dependent (Revell & Skene 2007; Rüger et al. 2013). Given that, numerous studies have shown that the circadian melatonin signal regulates metabolic and cell signaling activities to inhibit breast cancer initiation, promotion, and progression (Grant et al. 2009; Hill et al. 2011; Proietti et al. 2013). Following and early epidemiologic studies by Davis and Stevens (Davis et al. 2001), and Shernhammer et al. (2001) using the Harvard Nurses Cohort, the World Health Organization has designated night shift work involving LEN-induced circadian/melatonin disruption as a probable carcinogen (class 2a) and risk factor for the development of breast cancer (Straif et al. 2007).
A paradigm shifting study by Blask and co-workers (Blask et al. 2005) demonstrated that when tissue-isolated breast tumor xenografts in female nude rats were perfused with blood collected from women at night but exposed to dim light (0.2 lux) at night (melatonin-poor), LA uptake and its conversion to 13-HODE, cAMP levels, and the expression of MAPK downstream effectors MEKs/ERKs were induced. Conversely, these same parameters were greatly reduced when tumor xenografts were perfused with nighttime blood taken from women during dark night (melatonin-rich). The inhibitory effect of circadian melatonin during dark night was receptor mediated, as MT1/MT2 receptor antagonists were able to block these effects. Wu et al. (2011) reported increases in tumor growth rates and enhanced ERK1/2, AKT, and AKT-stimulatory 3-phosphoinositide-dependent kinase-1 (PDK1) activity in breast tumor xenografts in response to light exposure during the night or day, by employing the novel tissue-isolated MCF-7 human breast cancer xenograft model in circadian/melatonin-intact female nude rats. Furthermore, they reported that these changes were blocked by melatonin (Wu et al. 2011). Also utilizing the tissue-isolated human breast tumor xenograft model in female nude rats, Mao et al. (2012) observed that the phosphorylation of GSK3β, an enzyme critical in cell metabolism and proliferation/survival, exhibited a circadian rhythm in tumor xenografts. Exposure to light at night by suppressing nocturnal pineal melatonin synthesis induced AKT phospho-activation at serine 473 promoting its inhibitory phosphorylation at serine 9 of GSK3β to block GSK3β activation and ubiquitination activity.
As alluded to above, a recent study by Blask et al. (2014) using tissue-isolated human breast tumor xenografts grown in female nude rats, showed that tumor xenograft LA uptake, metabolism, and proliferation and survival signaling pathways in the tumor were dynamically coordinated within the circadian time structure of the 24-hour light/dark cycle by nocturnal pineal melatonin production driven by the SCN. This work demonstrated that dLEN and its associated suppression of nocturnal circadian melatonin altered the host/cancer balance in numerous cancer promoting signaling pathways driving hyperglycemia and hyper-insulinemia in the rat and hyper/runaway aerobic glycolysis (Warburg effect), and proliferation in the tumor.
Our most recent work examined the effect of dLEN and melatonin on the development of tamoxifen resistance (TAM-R) in breast cancer (Dauchy et. al 2014). Although the majority of this work will be discussed below in the section entitled “Melatonin: a regulator of resistance to endocrine and drug therapy,” this study clearly demonstrates that dLEN via its repression of the nocturnal circadian melatonin signal promotes tumor aerobic glycolysis (Warburg effect) and the expression and/or phospho-activation of key signaling pathways and nodes involved in tumor proliferation and survival that drive resistance in breast cancer cells to endocrine and chemo-therapies. These signaling pathways induced by dLEN include the PI3K/AKT pathway, the EGFR/HER2 and downstream RAS/MAPK/ERK pathways, the p21 activating kinase 1 (PAK1), and PI3K/AKT/pyruvate dehydrogenase kinase one (PDK1)/mTOR/p90 ribosomal S6 kinase (RSK) family members, all of which can drive cancer cells to proliferation, survival, drug resistance, and metastasis (Lee et al. 1992; McCubrey et al. 2007; Li et al. 2008; Romeo et al. 2012; Sims et al. 2013; Roskoski 2014).
Other signaling pathways elevated or activated in response to dLEN-induced circadian/melatonin disruption include c-SRC, FAK, cAMP, PKA, CREB, STAT3, NF-kB, and protein kinase C alpha and delta (PKCα and δ) (Lazennec et al. 2001; Gonzalez-Angulo et al. 2007; Díaz-Bessone et al. 2011; Zhang et al. 2011; Anbalagan et al. 2012). In tissue-isolated tumors grown in a lighting schedule of 12h light/12 h dark (LD 12:12) with nocturnal circadian melatonin elevated during dark night, or in 12:12dLEN schedule but supplemented with melatonin in the nighttime drinking water, melatonin (endogenous or exogenous) was able to block or dramatically suppress the expression and/or phospho-activation of each of these signaling pathways to dramatically suppress tumor cell proliferation and resistance to endocrine and chemotherapies (Dauchy et. al 2014).
Circadian synchronization is controlled, in part, by ambient light decreasing melatonin synthesis and secretion in the pineal gland and coordinated by the SCN of the hypothalamus. Peripheral cell autonomous circadian clocks termed “peripheral oscillators,” controlled by the master clock in the SCN, exist with every cell of the body including the breast, and are comprised of the same genes as the master clock (Sellix 2013). The clock genes in a peripheral oscillator can subsequently regulate clock-controlled genes involved in the cell-cycle including c-Myc, Wee1, cyclin D, and p21 (Wood et al. 2009). Light may directly affect tumor growth via the key oscillator (clock) genes PER1 and PER2, which in turn regulate cell cycle and apoptosis-regulated genes (Moriya et al. 2007; Gery & Hoeffler 2009; Wood et al. 2009). For example, Gery et al. (2007) reported that PER2 plays a role in ERα ubiquitination, while Xiang et al. (2012) demonstrated that re-expression of PER2 in MCF-7 breast tumor cells induces p53 mRNA expression. PER2 has been reported to be a tumor suppressor gene whose expression inhibits the formation of a variety of tumors including breast, prostate, lung, and lymphoma (Gery & Hoeffler 2009). Thus, loss of PER2 could well be involved in the initiation or progression of breast cancer. Repression of PER2 gene expression by methylation of the PER2 promoter or phosphorylation by casein kinase 1ε (Gery et al. 2007) might be involved in the initiation or progression of breast cancer. Interestingly, Xiang et al. (2012) showed that PER2 mRNA expression is non-rhythmic in MCF-7 breast cancer cells in vitro and that serum shock of MCF-7 cells induced the rhythmic expression of most core clock genes except for PER2, a phenomenon that was notably restored by administration of melatonin after serum shock.
In an earlier section entitled “Melatonin modulation of gene expression in breast cancer,” melatonin was reported to modulate the methylation of some genes. For example, Stevenson & Prendergast (2013) demonstrated in seasonally breeding male and female Siberian hamsters that DNA methylation of the proximal promoter for the type III diodinase (dio3) gene in the hamster hypothalamus is reversible and critical for photoperiod time measurement. Furthermore, they showed that short-day photoperiods with winter-like levels of melatonin inhibited hypothalamic DNA methyltransferase expression and reduced dio3 promoter DNA methylation to up regulate dio3 expression and gonadal regression. In a genome-wide profiling study of breast cancer cell lines, Lee et al. (2013) demonstrated that administration of 1 nM melatonin to cells was able to alter DNA methylation patterns and decrease the expression of known oncogenic genes including early growth response gene 3 (EGR3) and POU4F2/Brn-3b while up regulating the expression of the tumor suppressor gene, GPC3. Thus, the circadian melatonin signal can be dramatically altered by LEN and may affect overall DNA methylation and clock gene expression including PER2 to promote tumor progression. Conversely, the presence of nighttime melatonin may inhibit tumor progression via epigenetic regulation of gene expression.
A recent study by Dai et al. (2011), using a univariate logistic regression analysis, has demonstrated that polymorphisms of CLOCK and CRY1 genes were associated with breast cancer risk. Furthermore, we reported (Dai et al. 2001; Dong et al. 2010) that melatonin represses the transcriptional activity of RORα1, a member of the NR/steroid hormone receptor superfamily, a core circadian clock gene, and a transcriptional inducer of the core clock gene BMAL1. In MCF-7 breast cancer and MCF-10A human breast epithelial cells, melatonin administration significantly repressed RORα1 transactivation inhibiting its induction of BMAL1 gene expression. Thus melatonin, via its MT1 receptor, directly suppresses elements of the peripheral oscillator in breast epithelial and cancer cells (Xiang et al. 2012).
Melatonin: a regulator of epithelial-to-mesenchymal transition (EMT) and metastasis
An early study by Cos et al. (1998) noted that the in vitro invasive capacity of ERα-positive MCF-7 breast tumor cells as suppressed by melatonin via regulation of E-cadherin and β-integrin. Unfortunately, the luminal A (ERα-positive/PR-positive) MCF-7 human breast tumor cell line is considered by most to be poorly metastatic (Yang & Kim 2014). However, using MCF-7 human breast tumor cell clones that over-expressed either the ErbB2/Her2-neu oncogene or cytokine receptor CXCR4, or MCF-7 cells serially passaged through nude mice until they developed a metastatic phenotype (MFCF7/6 cells) (Bracke et al. 1991), Mao et al. (2010) demonstrated that melatonin indeed possesses anti-invasive/anti-metastatic action suppressing cell invasion by 60% to 85% in trans-well/matrigel insert assays. In this study Mao demonstrated that the anti-invasive actions of melatonin were at least partially mediated by inhibition of p38 MAPK and matrix metalloproteinases (MMP) 2 and 9, which are involved in degradation of the basement membrane and metastatic cell extravasation.
The cytoskeleton is an important component of the cellular architecture composed of intricate network of fibers, microtubules, microfilaments, and intermediate filaments and their associated proteins (Roberts 1974). The cytoskeleton shows dynamic changes and together with related adhesion proteins, modulates much of the metabolic and signal transduction machinery of the cell (Ingber 2001). Melatonin, via activation of its MT1/MT2 receptors, has been reported to significantly impact microtubule organization and thus cytoskeleton organization in breast cancer cells. Benitiz-King et al. (1990) demonstrated a complex interaction between melatonin and the cytoskeleton in MCF-7 cells. Their studies showed that melatonin induced the formation of focal adhesions in MCF-7 cells and altered the arrangement of microfilaments and stress fibers to form thicker bundles assembled with phospho-vinculin to promote adhesion contacts (Ortíz-López 2009).
Protein kinase C (PKC) via activation and induction of Rho-associated kinase (ROCK) induces stress fiber thickening and decrease focal adhesion events known to promote breast tumor cell migration and invasion (Soto-Vega et al. 2004; Ramirez-Rodriguez et al. 2007). Melatonin has been reported to inhibit stress fiber formation and thickening via the suppression of PKC (Soto-Vega et al. 2004; Ramirez-Rodriguez et al. 2007; Yuan et. al 2008). In new studies currently in press (Hill et al. 2014), we demonstrate that in MCF-7 tissue-isolated tumor xenografts grown in female nude rats housed in a photoperiod of 12h light:12 dLEN PKCα levels were elevated but were dramatically inhibited in tumors supplemented with nighttime melatonin during dLEN (data not shown). Although these tumor xenografts were not analyzed for stress fiber formation, the inhibition of PKCα by melatonin combined with the above reports suggest that melatonin may inhibit the invasive/metastatic capacity of breast cancer cells through inhibition of PKCα-induced stress fiber formation.
The developmental process of EMT in which epithelial cells acquire a mesenchymal phenotype and become migratory (Tomaskovic-Crook et al. 2009), is also seen in cancer cells as they acquire invasive and metastatic phenotypes (Jechlinger et al. 2003; Rubin 2014). EMT in cancer is characterized by cellular changes include loss of cell adhesion proteins, cytoskeleton reorganization, acquisition of mesenchymal cell spindle-like morphology with increased motility and invasiveness. A key hallmark of EMT is the reduced expression of E-cadherin, a structural component of adherent junctions, critical for epithelial cell polarity and adhesion (Hollestelle et al. 2013; Chen et al. 2014).
Also linked to progression of EMT is the Wnt/β-catenin pathway, with β-catenin being a core component of the adherent junctions through its binding to E-cadherin (ten Berge et al. 2008). Upon dissociation of β-catenin from E-cadherin, it is able translocate to the nucleus and complex with the T cell factor/lymphocyte enhancer factor (TCF/LEF) a key transcription factor to promote the expression of Wnt target genes including Snail, Slug, and c-MYC transcriptional repressors of E-cadherin, and vimentin, an intermediate filament protein and well-known marker of mesenchymal cells (Gilles et al. 2003; Zhou et al. 2004; Rubin 2014). The destruction complex of glycogen synthase kinase 3β (GSK3β), axin, adenomatous polyposis coli, and casein kinase 1 promotes the ubiquitination of excess cytoplasmic β-catenin (Gilles et al. 2003; Zhou et al. 2004). GSK3β when activated phosphorylates β-catenin promoting its ubiquitination/degradation. Inhibitory phosphorylation by AKT or WNT as s9 of GSK3β stabilizes β-catenin, allowing its translocation to the nuclear translocation and dimerization with TCF/LEF to promote Wnt target gene expression (Zhou et al. 2004). Interestingly, similar to β-catenin, GSK3β, via phosphorylation mediated mechanisms, can drive the ubiquitination of Snail, Slug, and c-MYC, known repressor of E-cadherin (Zhou et al. 2004). Thus GSK3β, due to its ability to regulate both Snail and β-catenin is a clinically important target in the EMT process.
Employing the tissue-isolated human breast xenograft tumor nude rat model Mao et al. (2012) demonstrated that GSK3β exhibits a circadian rhythm of phosphorylation. Light exposure at night suppresses the nocturnal circadian melatonin to disrupt the circadian rhythm of GSK3β phosphorylation. In the presence of melatonin, GSK3β was activated in breast tumor xenografts via melatonin’s blockade of AKT’s inhibiting-phosphorylation of GSK3β, allowing GSK3β to induce β-catenin ubiquitination and inhibit EMT. Thus, chronic disruption of the circadian melatonin profile by LEN (occupational exposure to light at night or age-related sleep disturbances) and its inhibition GSK3β activity and promotion of EMT, appears to be an important contributor to the metastatic spread in breast cancer patients.
Melatonin: a regulator of resistance to endocrine and drug therapy
Resistance to endocrine therapy and chemotherapy are major impediments to the successful treatment of breast cancer (Ravdin et al. 1992; Sabnis & Brodie 2010). Preclinical and clinical evidence link resistance to anti-estrogen and chemotherapeutic drugs in breast cancer cells with the over-expression and/or activation of various pro-oncogenic tyrosine kinases. Approximately 60%–75% of breast cancers express ERα and PR that are markers and determinants for the use of endocrine therapies including selective ERα modulators such as tamoxifen (TAM) (Ravdin et al. 1992; Sabnis & Brodie 2010). Presently, anywhere from 30% to 50% of patients with ERα-positive breast tumors display intrinsic resistance to TAM while most patients that are initially responsive will eventually develop acquired resistance to TAM (Sabnis & Brodie 2010). The anthracycline doxorubicin (Dox) is one of the most frequently used chemotherapeutic agents for patients whose breast tumors are endocrine resistant or metastatic (Lewis-Wambi & Jordan 2006). However, as with endocrine therapies, many cancers are intrinsically resistant to conventional chemotherapeutic agents, and others that initially respond acquire resistance during treatment (Gariboldi et al. 2003).
Resistance to endocrine or drug therapies may occur through a variety of reported mechanisms (deGraffenried et al. 2004; Vallabhaneni et al. 2011; Burris 2013; Austreid et al. 2014). Cells can become resistant through activation of several key-signaling pathways including the EGFR/HER2/MAPK/ERK and PI3K/AKT pathways, as well as a variety of other signaling pathways (Roskoski 2014). Numerous studies have reported that the expression and/or phospho-activation of a variety of kinases and transcription factors are elevated in human breast cancer cell lines and clinical breast tumor biopsies with both intrinsic and acquired resistance to endocrine and chemotherapies. Furthermore, intrinsic or acquired resistance to endocrine therapies or chemotherapeutic drugs has also been linked to up-regulation of ABC transporter efflux molecules (Gottesman et al. 2002) such as ABCB1 (MDR-1/P-gp), ABCC1 (MRP1), and ABCG2 (BCRP) as well as drug metabolizing enzymes (Kassner et al. 2008; Novotna et al. 2008), all of which lead to diminished levels of active drug within the cancer cells. Finally, mounting evidence supports the concept that dysregulated cellular metabolism (aerobic glycolysis, Warburg effect) is linked to drug resistance in cancer therapy via up regulation of tumor glucose and lactate, and increased cell signaling (Elstrom et al. 2004; Fantin et al. 2006; Robey & Nay 2009; Hua et al. 2014).
Several in vitro studies have provided evidence that melatonin may enhance the efficacy of TAM (Wilson et al. 1992; Lissoni et al. 1995; Lissoni et al. 1999) and Dox (Fan et al. 2010). In our recent studies examining the effects of dLEN mediated disruption of the circadian melatonin signal on breast cancer, we reported that in tissue-isolated MCF-7 breast tumor xenografts from female nude rats housed in a 12h light:12h dLEN environment, key tumor promoting kinases (ERK1/2, AKT, SRC, and FAK) and transcription factors (ERα, CREB, and STAT3) were highly expressed and/or phospho-activated. All of these signaling nodes have been found to be elevated in TAM-R breast cancer. Additionally, tumor xenografts from rats housed in dLEN showed constitutive aerobic glycolysis (Warburg effect) with increased tumor glucose uptake and lactic acid production. These tumor xenografts from rats exposed to dLEN, showed complete TAMR when treated with 4-hydroxy-tamoxifen (4OH-TAM). Conversely, tumor xenografts from rats housed in LD 12:12 with dark night (elevated endogenous melatonin), or in 12L:12 dLEN but supplemented with melatonin during dLEN, showed abolished or greatly diminished expression and/or phospho-activation of these tumor promoting kinases (ERK1/2, SRC, FAK) and transcription factors (CREB, ERα, STAT3), dramatic suppression of the Warburg effect, and greatly enhanced sensitivity to and synergism with 4OH-TAM such that tumor xenografts rapidly and significantly regressed.
Circadian melatonin disruption by dLEN in breast tumor xenografts show that dLEN promotes but melatonin inhibits the phosphorylation of the ERα at key regulatory sites including S118 and S167 (Dauchy et. al 2014). These ERα sites are reported to be phosphorylated in TAM-R tumors and convert the response of the ERα to TAM from antagonist to agonist. As noted earlier, we (Lai et al. 2008), and others (Del Rio et al. 2004), have shown that melatonin can repress estrogen-induced EPα transactivation in vitro and have found significant repression of ERα phosphorylation at S118 and S167 by melatonin (Dauchy et. al 2014). Our most recent studies confirm this effect in vivo and show that melatonin, via repression of key kinase signaling pathways, alters ERα phospho-activation and thus ERα’s responsivity to TAM to ensure TAM acts as an ERα antagonist. In unpublished studies we have found that exposure to dLEN increases the expression of the ABC transporter ABCG2 also termed breast cancer resistance protein (BCRP) a known efflux pump for 4OH-TAM and Endoxifen (Selever et al. 2011). Our data suggest that melatonin, in addition to its regulation of tumor metabolism and cell signaling, can also sensitize the response of breast tumors to TAM by regulating its interaction with the ERα and by repressing the efflux of TAM from breast tumor cells.
In work recently presented at the American Association of Cancer Research meeting on Cancer Prevention (Hill et al. 2014), we reported that MCF-7 tissue-isolated breast tumor xenografts grown in female nude rats housed in dLEN environment showed complete intrinsic resistance to the anthrecycline, Dox. This resistance, as seen with TAM-R, was associated with elevated aerobic glycolysis (Warburg effect) and enhanced expression and/or phospho-activation of key signaling pathways involved in tumor proliferation, survival, and progression. Conversely, tumor xenografts in hosts exposed to dLEN but supplemented with nighttime melatonin, showed inhibition of the Warburg effect and abolished/diminished expression and/or phospho-activation of key kinases and transcription factors involved in Dox-R and tumor progression. These tumor xenografts were highly sensitive to the synergistic actions of melatonin and Dox as evidenced by their rapid regression (data not shown). These studies demonstrate that disruption of the circadian melatonin signal by dLEN is sufficient to regulate tumor metabolism and signaling to drive tumors to complete intrinsic resistance to specific endocrine and drug therapies.
Conclusion
The study of melatonin as an anti-cancer hormone has spanned nearly 5 decades since the early observation by Lapin and Ebels (Lapin & Ebels 1976) that extracts from the pineal gland repressed the growth of different tumors in rats and mice. Numerous studies have documented the oncostatic properties of this indolamine in human breast tumor cell lines in vitro and in animal models including human breast tumor xenografts in athymic nude mice and nude rats, carcinogen-induced mammary tumors in rats, and genetically engineered models of breast cancer in mice. Tremendous knowledge regarding the actions of melatonin on breast cancer has been gained through the concerted efforts of investigators involved in melatonin, cancer, genetic, and epidemiological research. The actions of melatonin in breast cancer appear to be mediated via both growth-inhibitory (cytostatic) and pro-apoptotic (cytotoxic) effects and are clearly complex being mediated through a number of mechanisms involving a multitude of molecular pathways. The majority of studies indicate major involvement of the MT1 receptor for mediating both the cytostatic and pro-apoptotic actions of melatonin. Yet while, melatonin’s actions as a potent endogenous scavenger of reactive oxygen species (ROS) are clearly important to genomic stability and tumor initiation, the extent of their role in breast tumor promotion and progression remains to be determined. In general, melatonin’s cytostatic actions appear to be heavily mediated via its interaction with the estrogen/ERα signaling pathway, although new ERα-independent pathways have been identified in a number of recent studies. However, the cytotoxic actions of melatonin are mediated via a number of pathways including p53, p73, TGF-β1, NRs including RARα, RXRα, and VDR, and even clock genes such as PER2.
A number of new important avenues regarding melatonin’s actions in breast cancer have recently emerged, representing great potential to better understand melatonin’s anti-cancer properties and potential future as a therapeutic agent for breast cancer. First, work by Blask and Dauchy (Blask et al. 2014) have demonstrated that the circadian melatonin signal inhibits aerobic glycolysis (Warburg effect) in breast tumors, blocking tumor uptake of linoleic acid and glucose. Future studies need to define the mechanism(s) involved and the downstream consequences of these metabolic changes on cell signaling and tumor progression. Second, our recent reports combined with studies by Cos (Cos et al. 1998) highlight the exciting, but poorly understood, anti-metastatic actions of melatonin in breast cancer. New in vivo studies are needed in circadian-complete (melatonin producing) animal models of breast cancer to define the mechanism(s) and pathways involved in melatonin’s anti-metastatic action. Third, given the circadian nature of melatonin synthesis and release and its regulation by photoperiod and exposure to light at night, new models need to be developed to begin to fully understand how LEN impacts breast cancer initiation, promotion, and progression. These studies will be essential to identify the interactions and independent effects mediated by melatonin via the central clock and peripheral oscillators in the breast and breast cancer. Fourth, exciting new studies by the Belancio laboratory (Deharo et al. 2014) demonstrating the circadian/melatonin regulation of LINE-1 elements and genomic instability in breast cancer are only the beginning of an exciting new story about melatonin’s role in regulating genomic stability/instability and the initiation of breast cancer. Finally, additional research is required to clarify the consequences of dLEN-induced circadian melatonin disruption on endocrine and chemotherapy resistance in breast cancer. Follow-up studies are needed to define if melatonin, given its potent role as a circadian-regulated kinase and transcription factor inhibitor in cancer, in combination with these therapeutic agents, constitutes a new more efficacious therapy for breast cancer than the multiple-kinase inhibitors in combination with chemotherapeutic agents. The broad action of melatonin on breast cancer including its inhibition of tumor metabolism, signaling, and genomic instability, its activity as a scavenger of ROS, synergism with other cancer therapeutic agents, lack of toxicity, and wide availability and minimal cost, should make its movement into clinical trials a high priority.
Figure 1.
Acknowledgments
Financial Support: This work was supported by the following grants NIH Grant R21CA129875–04 (to DEB) and an American Association for Laboratory Animal Science GLAS grant (RTD and DEB), and Life Extension Foundation (VPB). Part of the cost of this work was defrayed with the support of the Edmond and Lily Safra Endowed Chair for Breast Cancer Research (SMH recipient) at Tulane Cancer Center.
Footnotes
Declaration of interest: The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Acuña-Castroviejo D, Escames G, Venegas C, Díaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan DX, Reiter RJ. Extrapineal melatonin: sources, regluation, and potential functions. Cellular and Molecular Life Sciences. 2014;71:2997–3025. doi: 10.1007/s00018-014-1579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adb El-Aziz MA, Hassan HA, Mohamed MH, Meki AR, Abdel-Ghaffar SK, Hussein MR. The biochemical and morphological alterations following administration of melatonin, retinoic acid and Nigell satvia in mammary carcinoma: an animal model. International. Journal of Experimental Pathology. 2005;86:383–396. doi: 10.1111/j.0959-9673.2005.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anbalagan M, Carrier L, Glodowski S, Hangauer D, Shan B, Rowan BG. KX-01, a novel Src kinase inhibitor directed toward the peptide substrate site, synergizes with tamoxifen in estrogen receptor α positive breast cancer. Breast Cancer Research and Treatment. 2012;132:391–409. doi: 10.1007/s10549-011-1513-3. [DOI] [PubMed] [Google Scholar]
- Austreid E, Lonning PE, Eikesdal HP. The emergence of targeted drugs in breast cancer to prevent resistance to endocrine treatment and chemotherapy. Expert Opinion on Pharmacotherapy. 2014;5:681–700. doi: 10.1517/14656566.2014.885952. [DOI] [PubMed] [Google Scholar]
- Ayoub MA, Couturier C, Lucas-Meunier E, Angers S, Fossier P, Bouvier M, Jockers R. Monitoring of ligand-independent dimerization and ligand-induced conformational changes of melatonin receptors in living cells by bioluminescence resonance energy transfer. Journal of Biological Chemistry. 2002;277:21522–21528. doi: 10.1074/jbc.M200729200. [DOI] [PubMed] [Google Scholar]
- Bao AM, Liu RY, van Someren EJ, Hofman MA, Cao YX, Zhou JN. Diurnal rhythm of free estradiol during the menstrual cycle. European Journal of Endocrinology. 2003;148:227–32. doi: 10.1530/eje.0.1480227. [DOI] [PubMed] [Google Scholar]
- Barrett P, Bolborea M. Molecular pathways involved in seasonal body weight and reproductive responses governed by melatonin. Journal of Pineal Research. 2012;52:376–388. doi: 10.1111/j.1600-079X.2011.00963.x. [DOI] [PubMed] [Google Scholar]
- Becker-André M, Wiesenberg I, Schaeren-Wiemers N, André E, Missbach M, Saurat JH, Carlberg C. Pineal gland hormone melatonin binds and activates an orphan of the nuclear receptor superfamily. Journal Biological Chemistry. 1994;269:28531–28534. [PubMed] [Google Scholar]
- Belancio VP, Blask DE, Deininger P, Hill SM, Jazwinski SM. The aging clock and circadian control of metabolism and genome stability. Frontiers in Genetics. 2015;5:455–463. doi: 10.3389/fgene.2014.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belancio VP, Roy-Engel AM, Deininger PL. All y’all need to know ‘bout retroelements in cancer. Seminars in Cancer Biology. 2010;20:200–210. doi: 10.1016/j.semcancer.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benítez-King G, Huerto-Delgadillo L, Antón-Tay F. Melatonin effects on the cytoskeletal organization of MDCK and neuroblastoma N1E-115 cells. Journal of Pineal Research. 1990;9:209–220. doi: 10.1111/j.1600-079x.1990.tb00709.x. [DOI] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Blask DE, Brainard GC, Dauchy RT, Hanifin JP, Davidson LK, Krause JA, Sauer LA, Rivera-Bermudez MA, Dubocovich ML, Jasser SA, et al. Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Research. 2005;65:11174–11184. doi: 10.1158/0008-5472.CAN-05-1945. [DOI] [PubMed] [Google Scholar]
- Blask DE, Dauchy RT, Dauchy EM, Mao L, Hill SM, Greene MW, Belancio VP, Sauer LA, Davidson L. Light exposure at night disrupts host/cancer circadian regulatory dynamics: impact on the Warburg effect, lipid signaling and tumor growth prevention. PLoS One. 2014;9:e102776. doi: 10.1371/journal.pone.0102776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blask DE, Hill SM, Dauchy RT, Xiang S, Yuan L, Duplessis T, Mao L, Dauchy E, Sauer LA. Circadian regulation of molecular, dietary, and metabolic signaling mechanisms of human breast cancer growth by the nocturnal melatonin signal and the consequences of its disruption by light at night. Journal of Pineal Research. 2011;51:259–269. doi: 10.1111/j.1600-079X.2011.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blask DE, Pelletier DB, Hill SM, Lemus-Wilson A, Grosso DS, Wilson ST, Wise ME. Pineal melatonin inhibition of tumor promotion in the N-nitroso-N-methylurea model of mammary carcinogenesis: potential involvement of antiestrogenic mechanisms in vivo. Journal of Cancer Research and Clinical Oncology. 1991;117:526–532. doi: 10.1007/BF01613283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blask DE, Sauer LA, Dauchy RT, Armstrong BJ, Scalici S. Melatonin inhibition of cancer growth in vivo involves suppression of tumor fatty acid metabolism via melatonin receptor-mediated signal transduction events. Cancer Research. 1999;59:4693–701. [PubMed] [Google Scholar]
- Blask DE, Wilson ST, Zalatan F. Physiological melatonin inhibition of human breast cancer cell growth in vitro: evidence for a glutathione-mediated pathway. Cancer Research. 1997;57:1909–1914. [PubMed] [Google Scholar]
- Blask DE. Melatonin, sleep disturbance and cancer risk. Sleep Medicine Reviews. 2009;13:252–264. doi: 10.1016/j.smrv.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Bondi CD, Alonso-Gonzalez C, Clafshenkel WP, Kotlarczyk MP, Dodda BR, Sanchez-Barcelo E, Davis VL, Witt-Enderby PA. The effect of estradiol, progesterone, and melatonin on estrous cycling and ovarian aromatase expression in intact female mice. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2014;174:80–85. doi: 10.1016/j.ejogrb.2013.11.027. [DOI] [PubMed] [Google Scholar]
- Bracke ME, Larebeke NA, Vyncke BM, Mareel MM. Retinoic acid modulates both invasion and membrane ruffling in MCF-7 human mammary carcinoma cells in vitro. British Journal of Cancer. 1991;63:867–872. doi: 10.1038/bjc.1991.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. Journal of Neuroscience. 2001;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouha B, Badge R, Schustak J, Lutz-Prigge S, Farley AH, Moran JV, Kazazian HH., Jr Active L1 retrotransposons in the human genome. American Journal of Human Genetics. 2002;71:410. [Google Scholar]
- Brouha B, Schustak J, Badge RM, Lutz-Prigge S, Farley AH, Moran JV, Kazazian HH., Jr Hot L1s account for the bulk of retrotransposition in the human population. Proceedings of the National Academy of Sciences USA. 2003;100:5280–5285. doi: 10.1073/pnas.0831042100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon L, Roka F, Petit L, de Coppet P, Tissot M, Barrett P, Morgan PJ, Nanoff C, Strosberg AD, Jockers R. Dual signaling of human Mel1a melatonin receptors via G(i2), G(i3), and G(q/11) proteins. Molecular Endocrinology. 1999;13:2025–2038. doi: 10.1210/mend.13.12.0390. [DOI] [PubMed] [Google Scholar]
- Burris HA. Overcoming acquired resistance to anticancer therapy: focus on the PI3K/AKT/mTOR pathway. Cancer Chemotherapy and Pharmacology. 2013;4:829–842. doi: 10.1007/s00280-012-2043-3. [DOI] [PubMed] [Google Scholar]
- Carrillo-Vico A, García-Mauriño S, Calvo JR, Guerrero JM. Melatonin counteracts the inhibitory effect of PGE2 on IL-2 production in human lymphocytes via its mt1 membrane receptor. FASEB Journal. 2003;17:755–767. doi: 10.1096/fj.02-0501fje. [DOI] [PubMed] [Google Scholar]
- Chen A, Beetham H, Black MA, Priya R, Telford BJ, Guest J, Wiggins GA, Godwin TD, Yap AS, Guilford PJ. E-cadherin loss alters cytoskeletal organization and adhesion in non-malignant breast cells but is insufficient to induce an epithelial-mesenchymal transition. BMC Cancer. 2014;14:552–559. doi: 10.1186/1471-2407-14-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claustrat B, Brun H, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Medicine Reviews. 2005;9:11–24. doi: 10.1016/j.smrv.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Collins A, Yuan Y, Kiefer TL, Cheng Q, Lai L, Hill SM. Over-expression of the MT1 melatonin receptor in MCF-7 human breast cancer cells inhibits mammary tumor formation in nude mice. Cancer Letters. 2003;189:49–57. doi: 10.1016/s0304-3835(02)00502-5. [DOI] [PubMed] [Google Scholar]
- Cos S, Blask DE, Lemus-Wilson A, Hill AB. Effects of melatonin on the cell cycle kinetics and “estrogen-rescue” of MCF-7 human breast cancer cells in culture. Journal of Pineal Research. 1991;10:36–42. doi: 10.1111/j.1600-079x.1991.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Cos S, Fernandez R, Guezmes A, Sánchez-Barceló EJ. Influence of melatonin on invasive and metastatic properties of MCF-7 human breast cancer cells. Cancer Research. 1998;58:4383–4390. [PubMed] [Google Scholar]
- Cos S, Sanchez-Barcelo EJ. Melatonin and mammary pathological growth. Frontiers in Neuroendocrinology. 2000;21:133–170. doi: 10.1006/frne.1999.0194. [DOI] [PubMed] [Google Scholar]
- Cucina A, Proietti S, D’Anselmi F, Coluccia P, Dinicola S, Frati L, Bizzarri M. Evidence for a biphasic apoptotic pathway induced by melatonin in MCF-7 breast cancer cells. Journal of Pineal Research. 2009;46:172–180. doi: 10.1111/j.1600-079X.2008.00645.x. [DOI] [PubMed] [Google Scholar]
- Dai H, Zhang L, Cao M, Song F, Zheng H, Zhu X, Wei Q, Zhang W, Chen K. The role of polymorphisms in circadian pathway genes in breast tumorigenesis. Breast Cancer Research and Treatment. 2011;127:531–540. doi: 10.1007/s10549-010-1231-2. [DOI] [PubMed] [Google Scholar]
- Dai J, Inscho EW, Yuan L, Hill SM. Modulation of intracellular calcium and calmodulin by melatonin in MCF-7 human breast cancer cells. Journal of Pineal Research. 2002;32:112–119. doi: 10.1034/j.1600-079x.2002.1844.x. [DOI] [PubMed] [Google Scholar]
- Dai J, Ram PT, Yuan L, Spriggs L, Hill SM. Transcriptional repression of RORalpha activity in human breast cancer cells by melatonin. Molecular and Cellular Endocrinology. 2001;176:111–120. doi: 10.1016/s0303-7207(01)00449-x. [DOI] [PubMed] [Google Scholar]
- Dauchy RT, Dauchy EM, Sauer LA, Blask DE, Davidson LK, Krause JA, Lynch DT. Differential inhibition of fatty acid transport in tissue-isolated steroid receptor negative human breast cancer xenografts perfused in situ with isomers of conjugated linoleic acid. Cancer Letters. 2004;209:7–15. doi: 10.1016/j.canlet.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Dauchy RT, Xiang S, Mao L, Brimer S, Wren MA, Yuan L, Anbalagan M, Hauch A, Frasch T, Rowan BG, et al. Circadian and melatonin disruption by exposure to light at night drives intrinsic resistance to tamoxifen therapy in breast cancer. Cancer Research. 2014;74:4099–4110. doi: 10.1158/0008-5472.CAN-13-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. Journal of the National Cancer Institute. 2001;93:1557–1562. doi: 10.1093/jnci/93.20.1557. [DOI] [PubMed] [Google Scholar]
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thomposon CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metabolism. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- de Graffenried LA, Chandrasekar B, Friedrichs WE, Donzis E, Silva J, Hidalgo M, Freeman JW, Weiss GR. NF-kappa B inhibition markedly enhances sensitivity of resistant breast tumor cells to tamoxifen. Annals of Oncology. 2004;15:885–890. doi: 10.1093/annonc/mdh232. [DOI] [PubMed] [Google Scholar]
- Deharo D, Kines KJ, Sokolowski M, Dauchy RT, Streva VA, Hill SM, Hanifin JP, Brainard GC, Blask DE, Belancio VP. Regulation of L1 expression and retrotransposition by melatonin and its receptor: implications for cancer risk associated with light exposure at night. Nucleic Acids Research. 2014;42:7694–7707. doi: 10.1093/nar/gku503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio B, Garcia Pedrero JM, Martinez-Campa C, Zuazua P, Lazo PS, Ramos S. Melatonin, an endogenous-specific inhibitor of estrogen receptor alpha via calmodulin. Journal of Biological Chemistry. 2004;279:38294–38302. doi: 10.1074/jbc.M403140200. [DOI] [PubMed] [Google Scholar]
- Díaz-Bessone MI, Berardi DE, Campodónico PB, Todaro LB, Lothstein L, Bal de Kier Joffé ED, Urtreger AJ. Involvement of PKC delta (PKCδ) in the resistance against different doxorubicin analogs. Breast Cancer Research and Treatment. 2011;126:577–587. doi: 10.1007/s10549-010-0956-2. [DOI] [PubMed] [Google Scholar]
- Dinet V, Korf HW. Impact of melatonin receptors on pCREB and clock-gene protein levels in the murine retina. Cell and Tissue Research. 2007;330:29–34. doi: 10.1007/s00441-007-0468-5. [DOI] [PubMed] [Google Scholar]
- Dong C, Yuan L, Dai J, Lai L, Mao L, Xiang S, Rowan B, Hill SM. Melatonin inhibits mitogenic cross-talk between retinoic acid-related orphan receptor alpha (RORalpha) and ERalpha in MCF-7 human breast cancer cells. Steroids. 2010;75:944–951. doi: 10.1016/j.steroids.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Delarange P, Krause DN, Sugden D, Cardinali DP, Olcese J. International union of basic and clinical pharmacology. LXXV Nomenclature, classification, and pharmacology of G-protein-coupled melatonin receptors. Pharmacology Reviews. 2010;62:343–380. doi: 10.1124/pr.110.002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubocovich ML, Rivera-Bermudez MA, Gerdin MJ, Masana MI. Molecular pharmacology, regulation and function of mammalian melatonin receptors. Frontiers in Bioscience. 2003;8:d1093–1108. doi: 10.2741/1089. [DOI] [PubMed] [Google Scholar]
- Eck KM, Yuan L, Duffy L, Ram PT, Ayettey S, Chen I, Cohn CS, Reed JC, Hill SM. A sequential treatment regimen with melatonin and all-trans retinoic acid induces apoptosis in MCF-7 tumor cells. British Journal of Cancer. 1998;77:2129–2137. doi: 10.1038/bjc.1998.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM, et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Research. 2004;64:3892–3899. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM, et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Research. 2004;64:3892–3899. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- Erratum in: Journal of Biological Chemistry. 1997;272:16707.
- Evrony GD, Cai X, Lee E, Hills LB, Elhosary PC, Lehmann HS, Parker JJ, Atabay KD, Gilmore EC, Poduri A, et al. Single-neuron sequencing analysis of l1 retrotransposition and somatic mutation in the human brain. Cell. 2012;151:483–496. doi: 10.1016/j.cell.2012.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan LL, Sun GP, Wei W, Wang ZG, Ge L, Fu WZ, Wang H. Melatonin and doxorubicin synergistically induce cell apoptosis in human hepatoma cell lines. World Journal of Gastroenterology. 2010;16:1473–1481. doi: 10.3748/wjg.v16.i12.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantin VR, St-Pierre L, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- García JJ, Reiter RJ, Ortiz GG, Oh CS, Tang L, Yu BP, Escames G. Melatonin enhances tamoxifen’s ability to prevent the reduction in microsomal membrane fluidity induced by lipid peroxidation. Journal of Membrane Biology. 1988;162:59–65. doi: 10.1007/s002329900342. [DOI] [PubMed] [Google Scholar]
- Gariboldi MB, Ravizza R, Riganti L, Meschini S, Calcabrini A, Marra M, Arancia G, Dolfini E, Monti E. Molecular determinants of intrinsic resistance to doxorubicin in human cancer cell lines. International Journal of Oncology. 2003;22:1057–1064. [PubMed] [Google Scholar]
- Gery S, Koeffler HP. Per2 is a C/EBP target gene implicated in myeloid leukemia. Integrative Cancer Therapeutics. 2009;8:317–320. doi: 10.1177/1534735409352084. [DOI] [PubMed] [Google Scholar]
- Gery S, Virk RK, Chumakov K, Yu A, Koeffler HP. The clock gene Per2 links the circadian system to the estrogen receptor. Oncogene. 2007;26:7916–7920. doi: 10.1038/sj.onc.1210585. [DOI] [PubMed] [Google Scholar]
- Gilles C, Poliette M, Mestdagt M, Nawrocki-Raby B, Ruggeri P, Birembaut P, Foidart JM. Transactivation of vimentin by beta-catenin in human breast cancer cells. Cancer Research. 2003;63:2658–2664. [PubMed] [Google Scholar]
- Glasgow WC, Eling TE. Structure-activity relationship for potentiation of EGF-dependent mitogenesis by oxygenated metabolites of linoleic acid. Archieves of Biochemistry & Biophysics. 1994;311:286–292. doi: 10.1006/abbi.1994.1239. [DOI] [PubMed] [Google Scholar]
- Glasgow WC, Hui R, Everhart AL, Jayawickreme SP, Angerman-Stewart J, Han BB, Eling TE. The linoleic acid metabolite, (13S)-hydroperoxyoctadecadienoic acid, augments the epidermal growth factor receptor signaling pathway by attenuation of receptor dephosphorylation. Differential response in Syrian hamster embryo tumor suppressor phenotypes. Journal of Biological Chemistry. 1997;272:19269–19276. doi: 10.1074/jbc.272.31.19269. [DOI] [PubMed] [Google Scholar]
- González A, Martínez-Campa C, Mediavilla MD, Alonso-González C, Sánchez-Mateos S, Hill SM, Sánchez-Barceló EJ, Cos S. Effects of MT1 melatonin receptor overexpression on the aromatase-suppressive effect of melatonin in MCF-7 human breast cancer cells. Oncology Reports. 2007;17:947–953. doi: 10.3892/or.17.4.947. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Advances in Experimental Medical Biology. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- Gordan JD, Thompson CB, Simon MC. HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell. 2007;12:108–13. doi: 10.1016/j.ccr.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordge PC, Hulme MJ, Clegg RA, Miller WR. Elevation of protein kinase A and protein kinase C activities in malignant as compared with normal human breast tissue. European Journal of Cancer. 1996;32A:2120–2126. doi: 10.1016/s0959-8049(96)00255-9. [DOI] [PubMed] [Google Scholar]
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nature Reviews Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- Grant SG, Melan MA, Latimer JJ, Witt-Enderby PA. Melatonin and breast cancer: cellular mechanisms, clinical studies and future perspectives. Expert Reviews in Molecular Medicine. 2009;11:1–15. doi: 10.1017/S1462399409000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings M, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nature Reviews Neuroscience. 2003;4:649–661. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- Hill SM, Blask DE, Xiang S, Yuan L, Mao L, Dauchy RT, Dauchy EM, Frasch T, Duplesis T. Melatonin and associated signaling pathways that control normal breast epithelium and breast cancer. Journal of Mammary Gland Biology and Neoplasia. 2011;16:235–245. doi: 10.1007/s10911-011-9222-4. [DOI] [PubMed] [Google Scholar]
- Hill SM, Blask DE. Effects of the pineal hormone melatonin on the proliferation and morphological characteristics of human breast cancer cells (MCF-7) in culture. Cancer Research. 1988;48:6121–6126. [PubMed] [Google Scholar]
- Hill SM, Dauchy RT, Xiang S. Frontiers in Cancer Prevention Research. American Association for Cancer Research; New Orleans: 2014. Circadian/melatonin disruption by dim light at night drives chemotherapy resistance in breast cancer; p. 89. Abstract #PR13. [Google Scholar]
- Hill SM, Spriggs LL, Simon MA, Muraoka H, Blask DE. The growth inhibitory action of melatonin on human breast cancer cells is linked to the estrogen response system. Cancer Letters. 1992;64:249–256. doi: 10.1016/0304-3835(92)90050-6. [DOI] [PubMed] [Google Scholar]
- Hollestelle A, Peeters JK, Smid M, Timmermans M, Verhoog LC, Westenend PJ, Heine AA, Chan A, Sieuwerts AM, Wiemer EA, et al. Loss of E-cadherin is not a necessity for epithelial to mesenchymal transition in human breast cancer. Breast Cancer Research and Treatment. 2013;138:47–57. doi: 10.1007/s10549-013-2415-3. [DOI] [PubMed] [Google Scholar]
- Hsi LC, Wilson LC, Eling TE. Opposing effects of 15-lipoxygenase-1 and -2 metabolites on MAPK signaling in prostate. Alteration in peroxisome proliferator-activated receptor gamma. Journal of Biological Chemistry. 2002;277:40549–56. doi: 10.1074/jbc.M203522200. [DOI] [PubMed] [Google Scholar]
- Hua G, Liu Y, Li X, Xu P, Luo Y. Targeting glucose metabolism in chondrosarcoma cells enhances the sensitivity to doxorubicin through the inhibition of lactate dehydrogenase-A. Oncology Reports. 2014;31:2727–2734. doi: 10.3892/or.2014.3156. [DOI] [PubMed] [Google Scholar]
- Huether G. The contribution of extrapineal sites of melatonin synthesis to circulating melatonin levels in higher vertebrates. Experientia. 1993;49:665–670. doi: 10.1007/BF01923948. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Tensegrity II. How structural networks influence cellular information processing networks. Journal of Cell Science. 2001;116:1379–1408. doi: 10.1242/jcs.00360. [DOI] [PubMed] [Google Scholar]
- Jablonska K, Pula B, Zemia A, Owczarek T, Wojnar A, Rys J, Ambicka A, Podhorska-Okolow M, Ugorski M, Dziegiel P. Expression of melatonin receptor MT1 in cells of human invasive ductal breast carcinoma. Journal of Pineal Research. 2013;54:334–345. doi: 10.1111/jpi.12032. [DOI] [PubMed] [Google Scholar]
- Jasser SA, Blask DE, Brainard GC. Light during darkness and cancer: relationships in circadian photoreception and tumor biology. Cancer Causes and Control. 2006;17:515–523. doi: 10.1007/s10552-005-9013-6. [DOI] [PubMed] [Google Scholar]
- Jechlinger M, Grunert S, Tamir IH, Janda E, Lundemann S, Waerner T, Seither P, Weith A, Beug H, Kraut N. Expression profiling of epithelial plasticity in tumor progression. Oncogene. 2003;22:7155–7169. doi: 10.1038/sj.onc.1206887. [DOI] [PubMed] [Google Scholar]
- Kalay E, Yigit G, Aslan Y, Brown KE, Pohl E, Bicknell LS, Kayserili H, Li Y, Tüysüz B, Nürnberg G, et al. CEP152 is a genomic maintenance protein disrupted in Seckel syndrome. Nature Genetics. 2011;43:23–26. doi: 10.1038/ng.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassner N, Huse K, Martin HJ, Gödtel-Armbrust U, Metzger A, Meineke I, Brockmöller J, Klein K, Zanger UM, Maser E, et al. Carbonyl reductase 1 is a predominant doxorubicin reductase in the human liver. Drug Metabolism & Disposition. 2008;36:2113–2120. doi: 10.1124/dmd.108.022251. [DOI] [PubMed] [Google Scholar]
- Kelleher FC, Rao A, Maguire A. Circadian molecular clocks and cancer. Cancer Letters. 2014;342:9–18. doi: 10.1016/j.canlet.2013.09.040. [DOI] [PubMed] [Google Scholar]
- Kiefer T, Yuan L, Ram P, Hill SM. Melatonin inhibits estrogen receptor transactivation and cAMP levels in breast cancer cells. Breast Cancer Research and Treatment. 2002;71:37–45. doi: 10.1023/a:1013301408464. [DOI] [PubMed] [Google Scholar]
- Kiefer TL, Lai L, Yuan L, Dong C, Burrow ME, Hill SM. Differential regulation of estrogen receptor alpha, glucocorticoid receptor and retinoic acid receptor alpha transcriptional activity by melatonin is mediated via different G proteins. Journal of Pineal Research. 2005;38:231–239. doi: 10.1111/j.1600-079X.2004.00198.x. [DOI] [PubMed] [Google Scholar]
- Kohsaka A, Bass J. A sense of time: how molecular clocks organize metabolism. Trends in Endocrinology and Metabolism. 2007;18:4–11. doi: 10.1016/j.tem.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Lai L, Yuan L, Chen Q, Dong C, Mao L, Rowan B, Frasch T, Hill SM. The Galpha i and Galpha q proteins mediate the effects of melatonin on steroid/thyroid hormone receptor transcriptional activity and breast cancer cell proliferation. Journal of Pineal Research. 2008;45:476–488. doi: 10.1111/j.1600-079X.2008.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai L, Yuan L, Cheng Q, Dong C, Mao L, Hill SM. Alteration of the MT1 melatonin receptor gene and its expression in primary human breast tumors and breast cancer cell lines. Breast Cancer Research and Treatment. 2009;118:293–305. doi: 10.1007/s10549-008-0220-1. [DOI] [PubMed] [Google Scholar]
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Lapin V, Ebels I. Effects of some low molecular weight sheep pineal fractions and melatonin on different tumors in rats and mice. Oncology. 1976;33:110–113. doi: 10.1159/000225117. [DOI] [PubMed] [Google Scholar]
- Lazennec G, Thomas JA, Katzenellenbogen BS. Involvement of the cAMP response element binding protein (CREB) and estrogen receptor phosphorylation in the synergistic action of the estrogen receptor by estradiol and protein kinase activators. Journal of Steroid Biochemistry Molecular Biology. 2001;77:193–203. doi: 10.1016/s0960-0760(01)00060-7. [DOI] [PubMed] [Google Scholar]
- Lee SA, Karaszkiewicz JW, Anderson WB. Elevated level of nuclear protein kinase C in multidrug-resistant MCF-7 human breast carcinoma cells. Cancer Research. 1992;52:3750–3759. [PubMed] [Google Scholar]
- Lee SE, Kim SJ, Yoon HJ, Yu SY, Yang H, Jeong SI, Hwang SY, Park CS, Park YS. Genome-wide profiling in melatonin-exposed human breast cancer cell lines identifies differentially methylated genes involved in the anticancer effect of melatonin. Journal of Pineal Reseach. 2013;54:80–88. doi: 10.1111/j.1600-079X.2012.01027.x. [DOI] [PubMed] [Google Scholar]
- Lee SE, Kim SJ, Yoon JP, Hwang SY, Park CS, Park YS. MircroRNA and gene expression analysis of melatonin-exposed human breast cancer cell lines indicating involvement of the anticancer effect. Journal of Pineal Research. 2011;51:345–352. doi: 10.1111/j.1600-079X.2011.00896.x. [DOI] [PubMed] [Google Scholar]
- Leon-Blanco MM, Guerrero JM, Reiter RJ, Calvo JR, Pozo D. Melatonin inhibits telomerase activity in the MCF-7 tumor cell line both in vivo and in vitro. Journal of Pineal Research. 2003;35:204–211. doi: 10.1034/j.1600-079x.2003.00077.x. [DOI] [PubMed] [Google Scholar]
- Lerner AB, Case JD, Takahashi Y. Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands. Journal of Biological Chemistry. 1960;235:1992–1997. [PubMed] [Google Scholar]
- Levoye A, Dam J, Ayoub MA, Guillaume JL, Couturier C, Delagrange P, Jockers R. The orphan GPR50 receptor specifically inhibits MT1 melatonin receptor function through heterodimerization. EMBO Journal. 2006;25:3012–3023. doi: 10.1038/sj.emboj.7601193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis-Wambi JS, Jordan VC. Treatment of Postmenopausal Breast Cancer with Selective Estrogen Receptor Modulators (SERMs) Breast Disease. 2006;24:93–105. doi: 10.3233/bd-2006-24108. [DOI] [PubMed] [Google Scholar]
- Li Q, Mullins SR, Sloane BF, Mattingly PR. P21-activated kinase 1 coordinates aberrant cell survival and pericellular proteolyisis in a three-demensional culture model for premalignant progression of human breast cancer. Neoplasia. 2008;10:314–328. doi: 10.1593/neo.07970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissoni P, Barni S, Mandalà M, Ardizzoia A, Paolorossi F, Vaghi M, Longarini R, Malugani F, Tancini G. Decreased toxicity and increased efficacy of cancer chemotherapy using the pineal hormone melatonin in metastatic solid tumour patients with poor clinical status. European Journal of Cancer. 1999;35:1688–1692. doi: 10.1016/s0959-8049(99)00159-8. [DOI] [PubMed] [Google Scholar]
- Lissoni P, Barni S, Meregalli S, Fossati V, Cazzaniga M, Esposti D, Tancini G. Modulation of cancer endocrine therapy by melatonin: a phase II study of tamoxifen plus melatonin in metastatic breast cancer patients progressing under tamoxifen alone. British Journal of Cancer. 1995;71:854–856. doi: 10.1038/bjc.1995.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissoni P, Tisi E, Brivio F, Crispino S, Barni S, Tancini G, Conti A, Maestroni GJ. Modulation of interleukin-2-induced macrophage activation by the pineal hormone melatonin in cancer patients. Journal of Biological Regulation and Homeostatic Agents. 1991;5:154–156. [PubMed] [Google Scholar]
- Liu R, Fu A, Hoffman AE, Zhu Y. Melatonin enhances DNA repair capacity possibly by affecting genes involved in DNA damage responsive pathways. BMC Cell Biology. 2013;14:1–6. doi: 10.1186/1471-2121-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locasale JW, Cantley LC. Altered metabolism in cancer. BMC Biology. 2010;8:88. doi: 10.1186/1741-7007-8-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Dauchy RT, Blask DE, Slakey LM, Xiang S, Yuan L, Dauchy EM, Shan B, Brainard GC, Hanifin JP, et al. Circadian gating of epithelial-to-mesenchymal transition in breast cancer cells via melatonin regulation of GSK3β. Molecular Endocrinology. 2012;26:1808–1820. doi: 10.1210/me.2012-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Yuan L, Slakey LM, Jones FE, Burow ME, Hill SM. inhibition of breast cancer cell invasion by melatonin is mediated through regulation of p38 mitogen-activated protien kinase signaling pathway. Breast Cancer Research. 2010;12:R107. doi: 10.1186/bcr2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Yuan L, Xiang S, Zeringue SB, Dauchy RT, Blask DE, Hauch A, Hill SM. Molecular deficiency(ies) in MT1 melatonin signaling pathway underlies the melatonin-unresponsive phenotype in MDA-MB-231 human breast cancer cells. Journal of Pineal Research. 2014;56:246–253. doi: 10.1111/jpi.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Campa C, González A, Mediavilla MD, Alonso-González C, Alvarez-García V, Sánchez-Barceló EJ, Cos S. Melatonin inhibits aromatase promoter expression by regulating cyclooxygenases expression and activity in breast cancer cells. British Journal of Cancer. 2009;101:1613–1619. doi: 10.1038/sj.bjc.6605336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochimica et Biophysica Acta Molecular Cell Research. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mediavilla MD, Cos S, Sanchez-Barcelo EJ. Melatonin increases p53 and p21waf1 expression in MCF-7 human breast cancer cells in vitro. Life Sciences. 1999;65:415–420. doi: 10.1016/s0024-3205(99)00262-3. [DOI] [PubMed] [Google Scholar]
- Mediavilla MD, Sanchez-Barcelo EJ, Tan DX. Basic Mechanisms involved in the anti-cancer effects of melatonin. Current Medicinal Chemistry. 2010;17:4462–4481. doi: 10.2174/092986710794183015. [DOI] [PubMed] [Google Scholar]
- Melancon K, Cheng Q, Kiefer TL, Dai J, Lai L, Dong C, Yuan L, Collins A, Thiyagarajah A, Long S, et al. Regression of NMU-induced mammary tumors with the combination of melatonin and 9-cis-retinoic acid. Cancer Letters. 2005;227:39–48. doi: 10.1016/j.canlet.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Molis TM, Spriggs LL, Hill SM. Modulation of estrogen receptor mRNA expression by melatonin in MCF-7 human breast cancer cells. Molecular Endocrinology. 1994;8:1681–1690. doi: 10.1210/mend.8.12.7708056. [DOI] [PubMed] [Google Scholar]
- Moriya T, Hiraishi K, Horie N, Mitome M, Shinohara K. Correlative association between circadian expression of mousePer2 gene and the proliferation of the neural stem cells. Neuroscience. 2007;146:494–498. doi: 10.1016/j.neuroscience.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Novotna R, Wsol V, Xiong G, Maser E. Inactivation of the anticancer drugs doxorubicin and oracin by aldo-keto reductase (AKR)1C3. Toxicology Letters. 2008;181:1–6. doi: 10.1016/j.toxlet.2008.06.858. [DOI] [PubMed] [Google Scholar]
- Oprea-Ilies G, Haus E, Sackett-Lundeen L, Liu Y, McLendon L, Busch R, Adams A, Cohen C. Expression of melatonin receptors in triple negative breast cancer (TNBC) in African American and Caucasian women: relation to survival. Breast Cancer Research and Treatment. 2013;137:677–687. doi: 10.1007/s10549-012-2371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortíz-López L, Morales-Mulia S, Ramírez-Rodríguez G, Benítez-King G. ROCK-regulated cytoskeletal dynamics participate in the inhibitory effect of melatonin on cancer cell migration. Journal of Pineal Research. 2009;46:15–21. doi: 10.1111/j.1600-079X.2008.00600.x. [DOI] [PubMed] [Google Scholar]
- Pawlikowski M, Winczyk K, Karasek M. Oncostatic action of melatonin: facts and question marks. Neuroendocrinology Letters. 2002;23:24–29. [PubMed] [Google Scholar]
- Poeggeler B, Reiter RJ, Tan DX, Chen LD, Manchester LC. Melatonin, hydroxyl radical-mediated oxidative damage, and aging: a hypothesis. Journal of Pineal Research. 1993;14:151–168. doi: 10.1111/j.1600-079x.1993.tb00498.x. [DOI] [PubMed] [Google Scholar]
- Proietti S, Cucina A, D’Anselmi F, Dinicola S, Pasqualato A, Lisi E, Bizzarri M. Melatonin and vitamin D3 synergistically down-regulate Akt and MDM2 leading to TGFβ-1-dependent growth inhibition of breast cancer cells. Journal of Pineal Research. 2011;50:150–158. doi: 10.1111/j.1600-079X.2010.00824.x. [DOI] [PubMed] [Google Scholar]
- Proietti S, Cucina A, Dobrowoly G, D’Anselmi F, Dinicola S, Masiello MG, Pasqualato A, Palombo A, Morini V, Reiter RJ, et al. Melatonin down-regulates MDM2 gene expression and enhances p53 acetylation in MCF-7 cells. Journal of Pineal Research. 2014;57:120–129. doi: 10.1111/jpi.12150. [DOI] [PubMed] [Google Scholar]
- Proietti S, Cucina A, Reiter R, Bizzarri M. Molecular mechanisms of melatonin’s inhibitory actions on breast cancer. Cellular and Molecular Life Sciences. 2013;70:2139–2157. doi: 10.1007/s00018-012-1161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram PT, Dai J, Yuan L, Dong C, Kiefer TL, Lai L, Hill SM. Involvement of the mt1 melatonin receptor in human breast cancer. Cancer Letters. 2002;179:141–150. doi: 10.1016/s0304-3835(01)00873-4. [DOI] [PubMed] [Google Scholar]
- Ramirez-Rodriguez G, Ortiz-Lopez L, Benitez-King G. Melatonin increases stress fibers and focal adhesions in MDCK cells: participation of Rho-associated kinase and protein kinase C. Journal of Pineal Research. 2007;42:180–190. doi: 10.1111/j.1600-079X.2006.00404.x. [DOI] [PubMed] [Google Scholar]
- Ravdin PM, Green S, Dorr TM, McGuire WL, Fabian C, Pugh RP, Carter RD, Rivkin SE, Borst JR, Belt RJ, et al. Prognostic significance of progesterone receptor levels in estrogen receptor-positive patients with metastatic breast cancer treated with tamoxifen: results of a prospective Southwest Oncology Group study. Journal of Clinical Oncology. 1992;8:1284–1291. doi: 10.1200/JCO.1992.10.8.1284. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Korkmaz A, Erren TC, Piekarski C, Tamura H, Manchester LC. Light at night, chronodisruption, melatonin suppression, and cancer risk: a review. Critical Reviews in Oncogenesis. 2007;13:303–328. doi: 10.1615/critrevoncog.v13.i4.30. [DOI] [PubMed] [Google Scholar]
- Reiter RJ. The pineal and its hormones in the control of reproduction in mammals. Endocrine Reviews. 1980;1:109–131. doi: 10.1210/edrv-1-2-109. [DOI] [PubMed] [Google Scholar]
- Reiter RJ. Melatonin: the chemical expression of darkness. Molecular and Cellular Endocrinology. 1991;79:C153–158. doi: 10.1016/0303-7207(91)90087-9. [DOI] [PubMed] [Google Scholar]
- Revell VL, Skene DJ. Light-induced melatonin suppression in humans with polychromatic and monochromatic light. Chronobiology International. 2007;24:1125–1137. doi: 10.1080/07420520701800652. [DOI] [PubMed] [Google Scholar]
- Roberts K. Cytoplasmic microtubules and their functions. Progress in Biophysical Molecular Biology. 1974;28:371–420. doi: 10.1016/0079-6107(74)90022-4. [DOI] [PubMed] [Google Scholar]
- Robey RB, Nay JR. Is Akt the “Warburg kinase”?-Akt-energy metabolism interactions and oncogenesis. Seminars in Cancer Biology. 2009;19:25–31. doi: 10.1016/j.semcancer.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rögelsperger O, Ekmekcioglu C, Jäger W, Klimpfinger M, Königsberg R, Krenbek D, Sellner F, Thalhammer T. Coexpression of the melatonin receptor 1 and nestin in human breast cancer specimens. Journal of Pineal Research. 2009;46:422–432. doi: 10.1111/j.1600-079X.2009.00679.x. [DOI] [PubMed] [Google Scholar]
- Romeo Y, Zhang X, Roux PP. Regulation and function of the RSK family of protein kinases. Biochemical Journal. 2012;441:553–569. doi: 10.1042/BJ20110289. [DOI] [PubMed] [Google Scholar]
- Roskoski R. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacology Research. 2014;79:34–74. doi: 10.1016/j.phrs.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Royston SE, Yasui N, Kondilis AG, Lord SV, Katzenellenbogen JA, Mahoney MM. ESR1 and ESR2 differentially regulate daily and circadian activity rhythms in female mice. Endocrinology. 2014;155:2613–23. doi: 10.1210/en.2014-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin MA. Insights into the mechanism of organ-specific cancer metastasis. Cancer Discovery. 2014;4:1262–1264. doi: 10.1158/2159-8290.CD-14-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüger M, St Hilaire MA, Brainard GC, Khalsa SB, Kronauer RE, Czeisler CA, Lockley SW. Human phase response curve to a single 6.5 h pulse of short-wavelength light. The Journal of Physiology. 2013;591:353–363. doi: 10.1113/jphysiol.2012.239046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabnis G, Brodie A. Understanding resistance to endocrine agents: molecular mechanisms and potential for intervention. Clinical Breast Cancer. 2010;18:E6. doi: 10.3816/CBC.2010.n.014. [DOI] [PubMed] [Google Scholar]
- Sánchez-Barceló EJ, Cos S, Mediavilla D, Martínez-Campa C, González A, Alonso-González C. Melatonin-estrogen interactions in breast cancer. Journal of Pineal Research. 2005;38:217–222. doi: 10.1111/j.1600-079X.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- Santoro R, Mori F, Marani M, Grasso G, Cambria MA, Blandino G, Muti P, Strano S. Blockage of melatonin receptors impairs p53-medited prevention of DNA damage accumulation. Carcionogenesis. 2013;34:1051–1061. doi: 10.1093/carcin/bgt025. [DOI] [PubMed] [Google Scholar]
- Sauer LA, Dauchy RT, Blask DE, Armstrong BJ, Scalici S. 13-Hydroxyoctadecadienoic acid is the mitogenic signal for linoleic acid-dependent growth in rat hepatoma 7288CTC in vivo. Cancer Research. 1999;59:4688–4692. [PubMed] [Google Scholar]
- Sauer LA, Dauchy RT, Blask DE. Dietary linoleic acid intake controls the arterial blood plasma concentration and the rates of growth and linoleic acid uptake and metabolism in hepatoma 7288CTC in Buffalo rats. Journal of Nutrition. 1997;127:1412–1421. doi: 10.1093/jn/127.7.1412. [DOI] [PubMed] [Google Scholar]
- Sauer LA, Dauchy RT. The effect of omega-6 and omega-3 fatty acids on 3H-thymidine incorporation in hepatoma 7288CTC perfused in situ. Bristish Journal of Cancer. 1992;66:297–303. doi: 10.1038/bjc.1992.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Colditz GA. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. Journal of the National Cancer Institute. 2001;93:1563–1568. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- Schuster C, Williams LM, Morris A, Morgan PJ, Barrett P. The human MT1 melatonin receptor stimulates cAMP production in the human neuroblastoma cell line SH-SY5Y cells via a calcium-calmodulin signal transduction pathway. Journal of Neuroendocrinology. 2005;17:170–178. doi: 10.1111/j.1365-2826.2005.01288.x. [DOI] [PubMed] [Google Scholar]
- Schuster C. Sites and mechanisms of action of melatonin in mammals: the MT1 and MT2 receptors. Journal of Social Biology. 2007;201:85–96. doi: 10.1051/jbio:2007010. [DOI] [PubMed] [Google Scholar]
- Selever J, Gu G, Lewis MT, Beyer A, Herynk MH, Covington KR, Tsimelzon A, Dontu G, Provost P, Di Pietro A, et al. Dicer-mediated up regulation of BCRP confers tamoxifen resistance in human breast cancer cells. Clinical Cancer Research. 2011;17:6510–6521. doi: 10.1158/1078-0432.CCR-11-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellix MT. Clocks underneath: the role of peripheral clocks in the timing of female reproductive physiology. Frontiers in Endocrinology. 2013;4:91. doi: 10.3389/fendo.2013.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims JT, Ganguly SS, Bennett H, Friend JW, Tepe J, Plattner R. Imatinib reverses doxorubicin resistance by affecting activation of STAT3-dependent NF-κB and HSP27/p38/AKT pathways and by inhibiting ABCB1. PLoSOne. 2013;8:e55509. doi: 10.1371/journal.pone.0055509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski RM, Reiter RJ, Schlabritz-Loutsevithc N, Ostrom RS, Slominski AT. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Molecular and Cellular Endocrinology. 2012;351:152–166. doi: 10.1016/j.mce.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Vega E, Meza I, Ramírez-Rodríguez G, Benitez-King G. Melatonin stimulates calmodulin phosphorylation by protein kinase C. Journal of Pineal Research. 2004;37:98–106. doi: 10.1111/j.1600-079X.2004.00141.x. [DOI] [PubMed] [Google Scholar]
- Steffens F, Zhou XB, Sausbier U, Sailer C, Motejlek K, Ruth P, Olcese J, Korth M, Wieland T. Melatonin receptor signaling in pregnant and non-pregnant rat uterine myocytes as probed by large conductance Ca2+-activated K+ channel activity. Molecular Endocrinology. 2003;17:2103–2115. doi: 10.1210/me.2003-0047. [DOI] [PubMed] [Google Scholar]
- Stehle JH, von Gall C, Korf HW. Melatonin: a clock-output, a clock-input. Journal of Neuroendocrinology. 2003;15:383–389. doi: 10.1046/j.1365-2826.2003.01001.x. [DOI] [PubMed] [Google Scholar]
- Stevens RG, Blask DE, Brainard GC, Hansen J, Lockley SW, Provencio I, Rea MS, Reinlib L. Meeting report: the role of environmental lighting and circadian disruption in cancer and other diseases. Environmental Health Perspectives. 2007;115:1357–1362. doi: 10.1289/ehp.10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RG. Electric power used and breast cancer: a hypothesis. American Journal of Epidemiology. 1987;125:556–561. doi: 10.1093/oxfordjournals.aje.a114569. [DOI] [PubMed] [Google Scholar]
- Stevenson TJ, Prendergast BJ. Reversible DNA methylation regulates seasonal photoperiodic time measurement. Proceedings of the National Academy of Sciences USA. 2013;110:16651–16666. doi: 10.1073/pnas.1310643110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, Altieri A, Benbrahim-Tallaa L, Cogliano V. Carcinogenicity of shift work, painting, and fire fighting. Lancet Oncology. 2007;8:1065–1066. doi: 10.1016/S1470-2045(07)70373-X. [DOI] [PubMed] [Google Scholar]
- Tamarkin L, Cohen M, Roselle D, Reichert C, Lippman M, Chabner B. Melatonin inhibition and pinealectomy enhancement of 7,12-dimethylbenz(a)anthracene-induced mammary tumors in the rat. Cancer Research. 1981;41:4432–4446. [PubMed] [Google Scholar]
- Tamura H, Nakamura Y, Terron MP. Melatonin and pregnancy in the human. Reproductive Toxicology. 2008;25:291–303. doi: 10.1016/j.reprotox.2008.03.005. [DOI] [PubMed] [Google Scholar]
- ten Berge D, Koole W, Fuerer C, Fish M, Eroglu E, Nusse R. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell. 2008;3:508–518. doi: 10.1016/j.stem.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teplitzky SR, Kiefer TL, Cheng Q, Dwivedi PD, Moroz K, Myers L, Anderson MB, Collins A, Dai J, Yuan L, et al. Chemoprevention of NMU-induced rat mammary carcinoma with the combination of melatonin and 9-cis-retinoic acid. Cancer Letters. 2001;168:155–163. doi: 10.1016/s0304-3835(01)00548-1. [DOI] [PubMed] [Google Scholar]
- Tomaskovic-Crook E, Thompson EW, Thiery JP. Epithelial to mesenchymal transition and breast cancer. Breast Cancer Research. 2009;11:213. doi: 10.1186/bcr2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong T, Liquet B, Menegaux F, Plancoulaine S, Laurent-Puig P, Mulot C, Cordina-Duverger E, Sanchez M, Arveux P, Kerbrat P, et al. Breast cancer risk, nightwork, and circadian clock gene polymorphisms. Endocrine Related Cancer. 2014;2:629–638. doi: 10.1530/ERC-14-0121. [DOI] [PubMed] [Google Scholar]
- Vallabhaneni S, Nair BC, Cortez V, Challa R, Chakravarty D, Tekmal RR, Vadlamudi RK. Significance of ER-Src axis in hormonal therapy resistance. Breast Cancer Research and Treatment. 2011;130:377–385. doi: 10.1007/s10549-010-1312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O. Uber den Stoffwechsel der Carcinomzelle. Klinische Wochenschrift. 1925;4:534–536. [Google Scholar]
- Wilson ST, Blask DE, Lemus-Wilson AM. Melatonin augments the sensitivity of MCF-7 human breast cancer cells to tamoxifen in vitro. Journal of Clinical Endocrinology & Metabolism. 1992;75:669–670. doi: 10.1210/jcem.75.2.1639964. [DOI] [PubMed] [Google Scholar]
- Witt-Enderby PA, Bennett J, Jarzynka MJ, Firestine S, Melan MA. Melatonin receptors and their regulation: biochemical and structural mechanisms. Life Sciences. 2003;72:2183–2198. doi: 10.1016/s0024-3205(03)00098-5. [DOI] [PubMed] [Google Scholar]
- Woo MM, Tai CJ, Kang SK, Nathwani PS, Pang SF, Leung PC. Direct action of melatonin in human granulosa-luteal cells. Journal of Clinical Endocrinology & Metabolism. 2001;86:4789–4797. doi: 10.1210/jcem.86.10.7912. [DOI] [PubMed] [Google Scholar]
- Wood PA, Yang X, Hrushesky WJ. Clock genes and cancer. Integrative Cancer Therapeutics. 2009;8:303–308. doi: 10.1177/1534735409355292. [DOI] [PubMed] [Google Scholar]
- Wu J, Dauchy RT, Tirrell PC, Wu SS, Lynch DT, Jitawatanarat P, Burrington CM, Dauchy EM, Blask DE, Greene MW. Light at night activates IGF-1R/PDK1 signaling and accelerates tumor growth in human breast cancer xenografts. Cancer Research. 2011;71:2622–2631. doi: 10.1158/0008-5472.CAN-10-3837. [DOI] [PubMed] [Google Scholar]
- Xiang S, Mao L, Duplessis T, Yuan L, Dauchy R, Dauchy E, Blask DE, Frasch T, Hill SM. Oscillation of clock and clock controlled genes induced by serum shock in human breast epithelial and breast cancer cells: regulation by melatonin. Breast Cancer. 2012;6:137–150. doi: 10.4137/BCBCR.S9673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Kim HM. ROCK inhibition activates MCF-7 cells. PLoSOne. 2014;9:e88489. doi: 10.1371/journal.pone.0088489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You P, Wood PA, Xiong Y, Kobayashi M, Du-Quiton J, Hrushesky WJ. Daily coordination of cancer growth and circadian clock gene expression. Breast Cancer Research and Treatment. 2005;91:47–60. doi: 10.1007/s10549-004-6603-z. [DOI] [PubMed] [Google Scholar]
- Yuan J, Shi GX, Shao Y, Dai G, Wei JN, Chang DC, Li CJ. Calmodulin bound to stress fibers but not microtubules involves regulation of cell morphology and motility. International Journal of Biochemistry & Cell Biology. 2008;40:284–293. doi: 10.1016/j.biocel.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Yuan L, Collins AR, Dai J, Dubocovich ML, Hill SM. MT(1) melatonin receptor overexpression enhances the growth suppressive effect of melatonin in human breast cancer cells. Molecular and Cellular Endocrinology. 2002;192:147–156. doi: 10.1016/s0303-7207(02)00029-1. [DOI] [PubMed] [Google Scholar]
- Zhang W, Ding W, Chen Y, Feng M, Ouyang Y, Yu Y, He Z. Up-regulation of breast cancer resistance protein plays a role in HER2-mediated chemoresistance through PI3K/Akt and nuclear factor-kappa B signaling pathways in MCF7 breast cancer cells. Acta Biochimica et Biophysica Sinica. 2011;43:647–653. doi: 10.1093/abbs/gmr050. [DOI] [PubMed] [Google Scholar]
- Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nature Cell Biology. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]