Abstract
Acylation of peptide has been reported for a number of peptides and proteins during release from polymers comprising of lactide and glycolide. We hypothesize that reversible hydrophobic ion-pairing (HIP) complex may minimize octreotide acylation during release. Sodium dodecyl sulfate (SDS), dextran sulfate A (DSA, Mw 9–20kDa) and dextran sulfate B (DSB, Mw 36–50kDa) were selected as ion-pairing agents to prepare reversible HIP complex with octreotide. Complexation efficiency was optimized with respect to the mole ratio of ion-pairing agent to octreotide to achieve 100% complexation of octreotide. Dissociation studies suggested that DSA-octreotide and DSB-octreotide complexes dissociate completely at physiological pH in presence of counter ions unlike SDS-octreotide complex. DSA-octreotide and DSB-octreotide complex encapsulated PLGA microparticles (DSAMPs and DSBMPs) were prepared using the S/O/W emulsion method. Entrapment efficiencies for DSAMPs and DSBMPs were 74.7±8.4% and 81.7±6.3%, respectively. In vitro release of octreotide was performed by suspending MPs in gel. A large fraction of peptide was released in chemically intact form and <7% was acylated from DSAMPs and DSBMPs in gel over 55 days. Therefore, HIP complexation could be a viable strategy to minimize acylation of peptides and proteins during extended release from lactide and glycolide based polymers.
Keywords: Acylation, peptide, Dextran sulfate, Sodium dodecyl sulfate
1. Introduction
There has been a substantial rise in the number of protein and peptide biologics in clinical trials for various diseases (Vaishya et al., 2014; Vaishya and Mitra, 2014). Recent advancements in protein engineering allow for rapid development of new peptide/protein therapeutics along with improved understanding of pharmacokinetics and pharmacodynamics. Unfortunately, most therapeutic proteins/peptides do not possess the physicochemical characteristics of an ideal drug candidate, such as lipophilicity and permeability (Mitragotri et al., 2014). Biotherapeutics suffer from a myriad of delivery-related issues, such as short half-life, poor bioavailability, low permeability across biological membranes and stability due to hydrophilicity and high molecular weight (Vaishya et al., 2014). Short half-life requires frequent parenteral administrations, which are not patient compliment. Sustained and controlled delivery of biologics may overcome these challenges to realize full potential as biotherapeutics.
Among various approaches investigated for sustained delivery of biologics, nano- and micro-particles (e.g., Sandostatin LAR® depot containing octreotide) have been effective and clinically approved for various ailments (Patel et al., 2014b; Vaishya et al., 2014). Octreotide is a semisynthetic cyclic octapeptide and a somatostatin analog indicated for the treatment of acromegaly (De Martino et al., 2010). It is also recommended for symptomatic relief by suppressing severe diarrhea and flushing episodes associated with metastatic carcinoid tumors (De Martino et al., 2010; Feelders et al., 2009; Modlin et al., 2006). It has a short half-life, ~100 min, following subcutaneous (SC) and intra-muscular (IM) administrations (Chanson et al., 1993); hence, it is a suitable candidate for sustained delivery formulation. It is marketed as a solution for SC injection (lactate buffer, pH 4.2) and as a microparticle depot form for IM administration. MPs are formulated with PLGA-glucose polymer for delivery over a period of 4 weeks. Lactide- and glycolide-based biodegradable polymers such as poly(D,L-lactide-coglycolide) (PLGA) and poly(D,L-lactide) (PLA) are FDA-approved and have been widely investigated for the sustained release of therapeutic peptides and proteins (Vaishya et al., 2014; Vaishya and Mitra, 2014). However, these polymers may not be suitable for delivering peptide and protein biologics as recent studies indicate that peptides/proteins are susceptible to acylation within the microenvironment of particles during release (Ghassemi et al., 2012; Ibrahim et al., 2005; Murty et al., 2003). Acylation at lysine residue of peptide and protein is catalyzed by low pH resulting from degraded products of PLGA polymers (Fig. 1a). In addition, thiol (−SH) and alcohol (−OH) groups may also act as nucleophiles. It has been well documented that chemical stability of octreotide is compromised during release from Sandostatin LAR® depot (Ghassemi et al., 2012). Nearly 60% of the octreotide was acylated and <20% of native octreotide was released during the In vitro release over a period of 3 months. Similar results has been reported for other peptides and proteins such as bovine serum albumin, human atrial natriuretic peptide, human parathyroid hormone, leuprolide, insulin and salmon calcitonin (Ghalanbor et al., 2012; Ibrahim et al., 2005; Lucke et al., 2002; Na et al., 2003b; Zhang and Schwendeman, 2012).
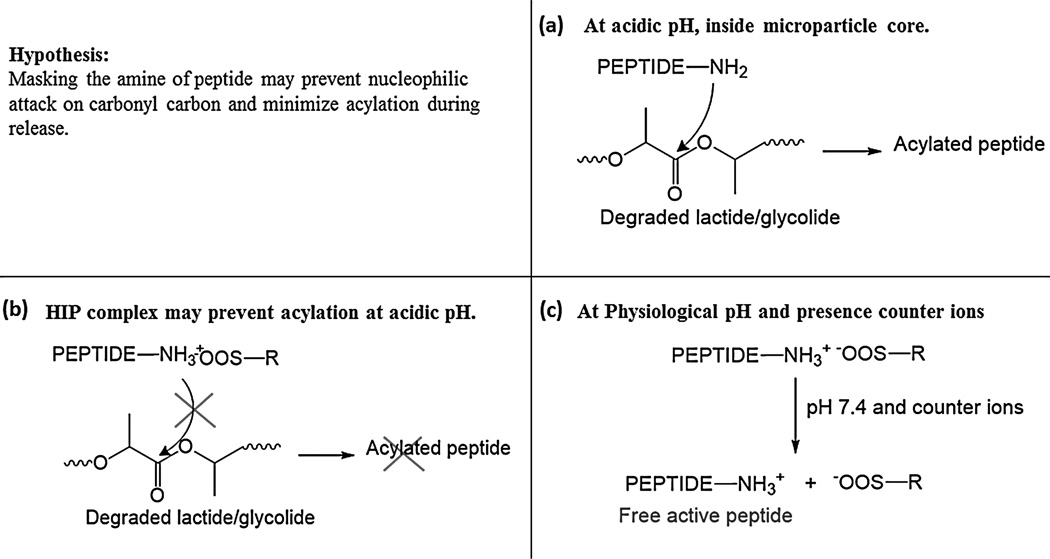
Figure 1.
(a) chemical derivatization of peptide occurs in MPs core, largely via nucleophilic attack of amine on carbonyl carbon of degraded polymer oligomers. (b) HIP complex may not dissociate at lower pH, preventing acylation. (c) At physiological pH and in presence of counter ions, the HIP complex may dissociate to produce free native peptide.
Several approaches have been investigated to minimize peptide acylation with some degree of success. Some noteworthy strategies include polymer modifications, chemical modification of octreotide and encapsulation of divalent cations (Ahn et al., 2011; Ghassemi et al., 2012; Lucke et al., 2002; Qi et al., 2015; Zhang and Schwendeman, 2012). Ahn JH et al and Na DH et al chemically modified octreotide at reactive amines to minimize acylation by maleic anhydride and PEG. Ahn JH et al showed that maleic anhydride conjugated octreotide inhibited acylation of peptide and only 10% peptide underwent acylation from PLGA films (Ahn et al., 2011). Na DH et al reported that PEGlyation significantly lowered the adsorption of peptide on PLGA surface and may minimize acylation of peptide. However, in all these studies peptide derivatives were physically incubated with PLGA in solution instead of encapsulating derivatives in microparticles (Na and DeLuca, 2005; Na et al., 2005). Zhang Y et al and Sophocleous et al showed that simple encapsulation of divalent cationic salts in PLGA MPs prevents peptide sorption on PLGA and thereby minimize octreotide acylation, nonetheless there was a limited success in preventing peptide acylation (Sophocleous et al., 2009; Zhang et al., 2009). Ghassemi AH et al modified polymer Poly(D,L-lactide-co-hydroxymethyl glycolide) to minimize nucleophilic attack of octreotide amine on glycolide by steric hindrance and were able to obtain more than 70% octreotide in native form during in vitro release (Ghassemi et al., 2012). Thus, acylation of peptide in lactide- and glycolide-based polymers is a significant roadblock preventing the development of clinically suitable sustained-release formulations for protein and peptide biologics.
Hydrophobic ion-paring (HIP) complexation involves the formation of a reversible complex. This process can increase hydrophobicity of biologics thereby improving entrapment efficiency in polymeric formulations. It has been shown that peptide/protein retains activity and conformational stability with HIP complex in various studies (Gaudana et al., 2013; Gaudana et al., 2011; Patel et al., 2014a; Shi et al., 2008). We hypothesize that acylation of peptides may be prevented or minimized by masking the reactive nucleophile amine with reversible HIP complex. HIP complex, formed by charge-charge interaction, may be stable at lower pH and thus prevent the nucleophilic attack of amines on PLGA degradation products inside MPs core (Fig. 1b). HIP complex may dissociate to release native peptides at physiological pH and in presence of counter ions (Fig. 1c). A schematic presentation of overall hypothesis is depicted in Fig. 1. Hence, the aim of our current study was to prepare and characterize HIP complex of octreotide using various ion-pairing agents and evaluate acylation of octreotide during release from PLGA microspheres.
2. Material and methods
2.1. Materials
Octreotide acetate (D-Phe-Cys-Phe-D-Trp-Lys-Thr-Cys-Thr-ol; MW 1019.23 Da) was procured from ChinaPeptides Co., Ltd (Shanghai, China). Poly(D, L-lactic-co-glycolide) (50:50, Mw 40–70 kDa), dextran sulfate sodium-salt (Mw 9–20 kDa) and sodium dodecyl sulfate (SDS) were obtained from Sigma Aldrich (St Louis, MO). Dextran sulfate sodium-salt (Mw 36–50 kDa) was obtained from MP Biomedicals (Illkirch, France). MicroBCA™ protein assay kit was purchased from Thermo Scientific (Rockford, IL). All solvents were of analytical reagent grade.
2.2. Methods
2.2.1. Preparation of HIP complex
HIP complex was prepared by simple mixing of aqueous solutions containing ion-pairing agent and octreotide (Gaudana et al., 2013; Gaudana et al., 2011; Patel et al., 2014a). Briefly, stock solutions of octreotide and ion-pairing agents were prepared in 10 mM citrate buffer at pH 4. Solutions were then mixed in calculated proportions and vortexed. After 3 h, water insoluble complex was separated by centrifugation at 12,000 rpm for 10 min. HIP complex pellet was freeze-dried and stored at −20°C until further use. Supernatant was analyzed for the amount of free peptide using microBCA™ assay following the protocol provided by the manufacturer (Na et al., 2003a). The percentage of octreotide complexed (complexation efficiency (%CE)), was calculated according to equation 1.
| (1) |
Mt= Amount of octreotide in supernatant
Mo= Initial amount of octreotide
2.2.2. Effect of mole ratio of ion-pairing agent to octreotide on complexation efficiency
Three ion-pairing agents, dextran sulfate (9–20kDa, DSA), dextran sulfate (36–50kDa, DSB) and SDS, were employed to prepare HIP complex with octreotide acetate. HIP complex was prepared following a method explained earlier (section 2.2.1). The solutions were mixed at various mole ratios of ion-pairing agent to octreotide to get charge ratio of 0.25, 0.5, 1, 2 and 4.
2.2.3. Dissociation of HIP complex
HIP complex of octreotide with ion-pairing agents was prepared at optimal mole ratio producing maximum %CE following the method explained earlier. Freeze-dried HIP complex was incubated in an isotonic phosphate buffer saline (IPBS) at pH 7.4 to study the dissociation under simulated physiological condition. Mechanistic studies were carried out to investigate the effects of ionic strength and pH on complex dissociation. HIP complex was incubated in 10 mM phosphate buffer (NaH2PO4 (monobasic) and Na2HPO4 (dibasic), pH 7.4) containing various strengths of NaCl (10 mM, 100 mM and 154 mM), DDI water and 10 mM citrate buffer (100 mM NaCl, pH 4.2) to delineate the influence of concentration of counter-ions and pH on complex dissociation. Following 30 min incubation, the insoluble complexes were separated by centrifugation at 12,000 rpm for 10 min. Amount of dissociated octreotide in supernatant was quantitated using microBCA™ assay and the percentage of dissociated octreotide was calculated according to equation 2.
| (2) |
Ms= Amount of octreotide in supernatant
Mi= Initial amount of octreotide in HIP complex
2.2.4. Particle size and zeta potential determination for DSA-octreotide and DSB-octreotide HIP complexes
Freeze-dried HIP complex of DSA-octreotide and DSB-octreotide were studied for particle size and zeta potential. Mean particle size and zeta potential of HIP complex were determined using Zeta Sizer (Zetasizer Nano ZS, Malvern Instruments Ltd, Worcestershire, UK) at RT (n=3). For size determination, HIP complex (DSA-octreotide or DSB-octreotide) was suspended in DDI water and tip-sonicated for 30 s at output of 2 W. Freeze-dried HIP complex was suspended in 1 mM citrate buffer to measure zeta potential.
2.2.5. Preparation of microparticles
HIP complexes of octreotide were prepared using ion-pairing agents DSA and DSB at optimal mole ratio following the method described previously. MPs encapsulating HIP-complexed octreotide were prepared by solid/oil/water (S/O/W) emulsion solvent evaporation method. Briefly, oil phase (O) consisted of 100 mg PLGA (50/50, Mw 40–70kDa) in 900 µL DCM. The oil phase was added to a vial containing 10 mg octreotide equivalent HIP complex and vortexed for 2 min. The resulting suspension was tip-sonicated for 30 s at power output of 2 W to form S/O suspension. The suspension was added drop-wise to constantly stirred water phase (5 mL) containing 2% w/v PVA as a stabilizer to form S/O/W emulsion. The resulting emulsion was kept in the hood under constant stirring to evaporate the organic solvent. After 6 h, the resulting MPs were separated by centrifugation at 13,000 rpm for 10 min. MPs were washed with DDI water and centrifuged twice to remove un-entrapped peptide. MPs were suspended in solution containing 2% w/v mannitol for freeze-drying. Freeze-dried particles were then store at −20°C until further use. Ghost MPs without octreotide were also prepared in an identical manner where equivalent amount of ion-pairing agent were suspended in oil phase.
2.2.6. Drug loading (%) and entrapment efficiency (%)
Encapsulation efficiency was determined by UV spectroscopy using Nanodrop™ (Thermo Fisher Scientific Inc.). Briefly, five mg of freeze-dried particles (n=3) were dissolved in 200 µL DMSO. Ghost MPs containing corresponding ion-pairing agents served as blank. Standard of octreotide were prepared in DMSO ranging from 2.72 to 0.043 mg/mL. UV absorbance was measured at 280 nm. Entrapment efficiency & drug loading were calculated by equation 3 & 4, respectively.
| (3) |
| (4) |
2.2.7. Scanning electron microscopy
Scanning electron microscopy (SEM) analysis was performed to analyze particle morphology (size and shape) of MPs and HIP complex (DSA-octreotide and DSB-octreotide). Freeze-dried MPs or HIP complex were applied to carbon film positioned on an aluminum stub. Surface of particles on carbon film was coated with Au-Pd under centrifugation followed by sample analysis in Phenom Pro desktop scanning electron microscope.
2.2.8. In vitro release of octreotide from MPs-in-gel composite formulation
Freeze-dried MPs were subjected to in vitro release study in PBS buffer (pH 7.4) at 37°C. PLGA MPs encapsulating HIP-complexed octreotide (DSA and DSB) were suspended in 400 µL of thermosensitive gel solution (20 wt%) maintained at 4°C. Pentablock polymer PLA250-PCL1250-PEG1500-PCL1250-PLA250 was utilized to prepare thermoreversible gel solution. Preparation and characterization of gelling polymer has been published earlier from our laboratory (Patel et al., 2014d). Resulting MPs-in-gel suspension was incubated at 37°C for 2 min to form hydrogel followed by slow addition of 1.5 mL of PBS (pH 7.4) which was pre-incubated at 37°C. At predefined time intervals, 1 mL of clear supernatant was collected and replaced with equal volume of fresh PBS. Release samples were evaluated for native and chemically modified octreotide using ultra-fast liquid chromatography (HPLC) assay. The experiments were carried out in triplicate and plotted as cumulative octreotide released (%) with respect to %EE vs. time.
2.2.9. High-performance liquid chromatography assay
The octreotide concentration in released media was quantified by HPLC assay. A Shimadhu (Shimadzu Scientific Instruments, Columbia, MD, USA) HPLC system coupled with pumps having built-in system controller (LC-20AT), degasser (DGU-20A3R), DAD detector (SPD-20AV) and autosampler (SIL-20AHT) were employed. Phenomenax column (Phenomenex C18 kinetex column 100×4.6 mm, 5µm) along with a guard column (Phenomenex SecuritGuard Catridges, C18, 4×2 mm) was used at a flow rate of 0.5 mL/min. A gradient elution method was employed. Mobile Phase A (HPCL water with 0.1% formic acid) at 10% and mobile phase B (ACN with 0.1% formic acid) at 90% were ran for first 2 min followed by a linear gradient to reach 100% of Phase B at 18 min. Standards were prepared in PBS buffer ranging from 3.1 to 100 µg/mL. DAD detector was set at 280 nm to determine UV absorbance. Injection volumes was 50 µL.
2.2.10. HPLC-MS analysis
HPLC-MS analysis was performed with electrospray ionization (ESI) in a positive ion mode on QTrap® API-3200 mass spectrometer equipped with Shimadzu quaternary pump, vacuum degasser, DAD detector and autosampler (Shimadzu Scientific Instruments, Columbia, MD, USA). Data acquisition and data processing were performed by Analyst 1.4.2 software package (Applied Biosystems, Foster City, CA, USA). LC conditions including column and gradient composition remains same as explained in the earlier HPLC assay. Injection volumes were 30 µL for all samples. UV detector was set on 280 nm and MS was set in a range of 200 to 1700 amu. Total ion chromatogram was extracted for acylated peptide m/z to produce extracted ion chromatogram (EIC) and was compared with UV chromatogram to identify the native and acylated octreotide adducts.
3. Result and discussion
3.1. Preparation of HIP complex
We investigated SDS and dextran sulfate as the complex forming agents. Dextran sulfate of molecular weights 9–20k and 36–50k Da were examined. Dextran sulfate has been shown to be safe and tolerable in vivo and had been used as antilipemic and anti-HIV (Maderich and Sugita, 1996). In a human clinical trial, dextran sulfate was found to be safe following oral administration (Abrams et al., 1989). In addition, the amount of dextran sulfate that we have used in this study is low (0.4 mg dextran sulfate for each mg of octreotide). Therefore, we do not anticipate any toxicity associated with dextran sulfate use. Reversible HIP complex was prepared by simple mixing of solutions containing octreotide and ion-pairing agents at low pH. Citrate buffer (pH 4) was selected to dissolve octreotide and ion-pairing agents based on published reports from our laboratory (Gaudana et al., 2013; Patel et al., 2014a). Octreotide has two pKa, 7 and 10.15 associated with amine of lysine and guanidine group of terminal arginine. As a result, a large fraction of amines is ionized at pH 4. The ion-pairing agents have pKa of <2 due to sulfonic acid group, resulting in complete ionization at pH 4. Water insoluble HIP complex is formed via ionic interactions between oppositely charged amine and sulfonate groups. Ionization of charged groups is primarily responsible for aqueous solubility of octreotide and ion-pairing agents. Hence, following charge neutralization, the complex can no longer remain in aqueous solution resulting in precipitation.
3.2. Effect of mole ratio of ion-pairing agent to octreotide on complexation efficiency
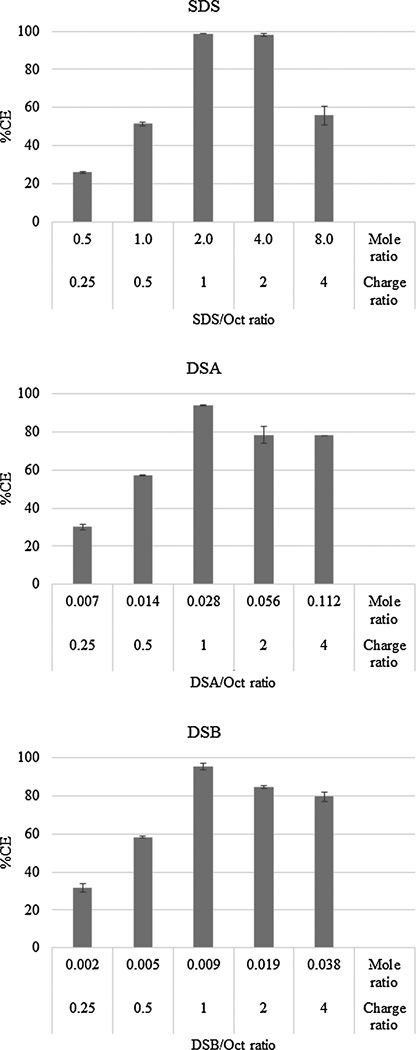
Stoichiometric balance i.e., charge ratio and corresponding mole ratio of ion-pairing agent to octreotide is an important parameter that may directly influence the efficiency of complex formation. The aim of this experiment was to identify mole ratio producing complete complexation of octreotide to avoid loss of uncomplexed peptide. Effect of mole ratio of ion-pairing agent to octreotide on complexation efficiency is illustrated in Fig. 2. Mole ratios were selected based on charge ratio of ion-pairing agent to octreotide. For SDS, DSA and DSB, fraction of octreotide complexed reached maximum at mole ratios of 2, 0.028 and 0.009, respectively and nearly all octreotide was complexed. At these optimal mole ratios, the charge ratio also approaches to 1:1 for all ion-pairing agents resulting in near complete binding Fig. 2. However, fraction of octreotide complexed declined upon further increment in mole ratio. Similar results were observed with lysozyme and IgG-FAB with these ion-pairing agents (Gaudana et al., 2013; Gaudana et al., 2011; Patel et al., 2014a). This may be due to incomplete charge neutralization in DSA and DSB at higher mole ratio. In case of SDS, it may be attributed to formation of micelles, which subsequently solubilized hydrophobic SDS-octreotide complex (Shi et al., 2008).
Figure 2.
Effect of charge/mole ratio of ion-pairing agent/octreotide on % octreotide complexation. Data represented as mean±SD, n=3.
3.3. Dissociation of HIP complex
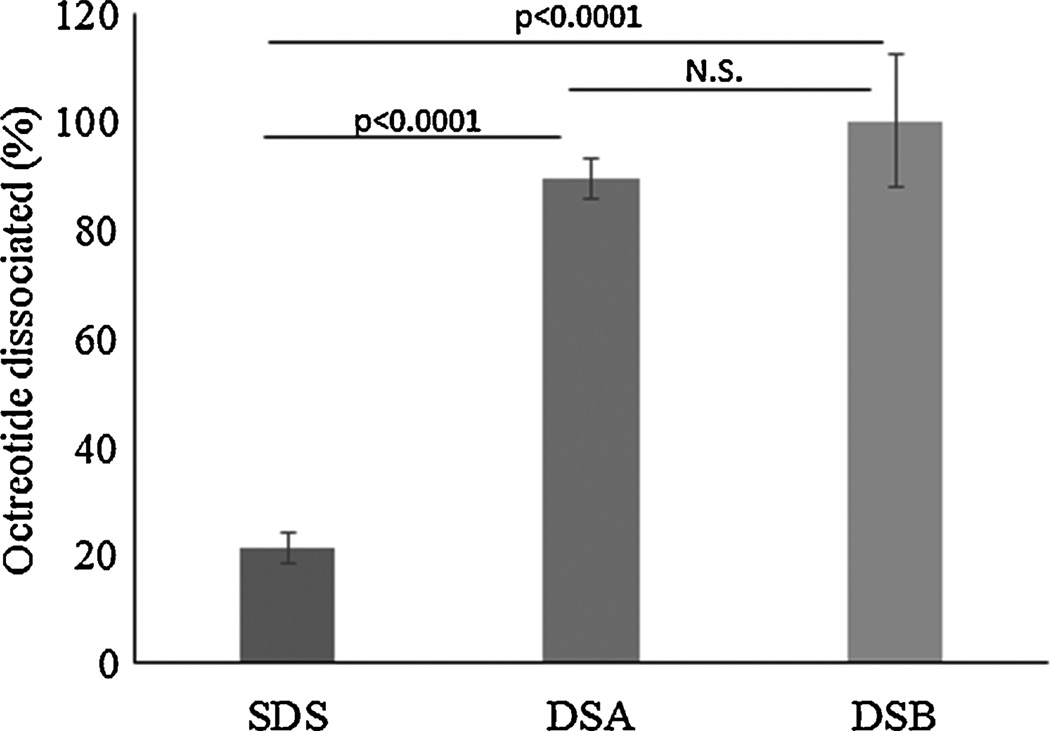
HIP complexes of octreotide with SDS, DSA and DSB were incubated in IPBS buffer (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4; pH 7.4) and free octreotide in solution was quantified for dissociated octreotide. Dextran sulfate complexes resulted in significantly higher dissociation compared to SDS-octreotide complex (t-test p<0.001) (Fig. 3). Percent of octreotide dissociated was 90% and 100% with DSA and DSB (t-test, one tailed p=0.11), respectively. Incomplete dissociation (20%) was observed for SDS-octreotide complex. Similar incomplete dissociation with SDS-IgG-FAB complex has been reported earlier (Patel et al., 2014a). HIP complex formation is governed by electrostatic interactions between opposite charges. Nonetheless, hydrophobic interaction may also play a key role in stabilization of water insoluble complex. If an ionic interaction was the only force responsible for the stabilization of HIP complex, then SDS-octreotide complex should have dissociated completely. Instead, only a fraction of octreotide was dissociated suggesting the role of hydrophobic interaction in stabilization of SDS-octreotide complex.
Figure 3.
Dissociation of SDS-octreotide, DSA-octreotide and DSB-octreotide HIP complex in IPBS buffer at pH 7.4. Data represented as mean±SD, n=3.
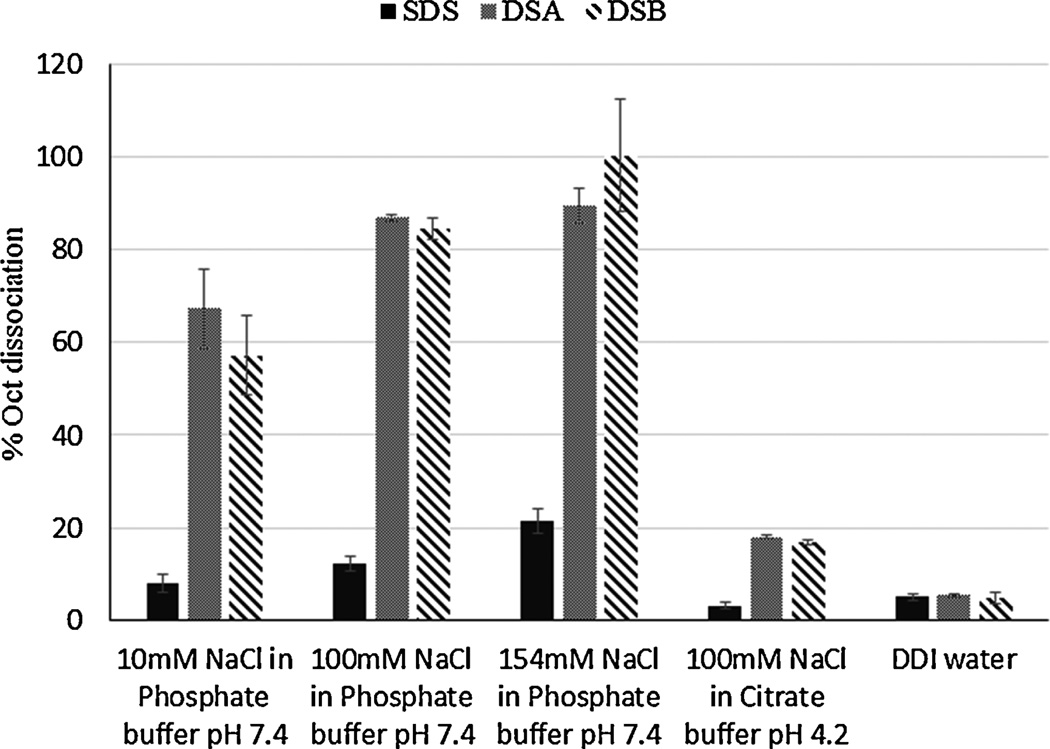
For successful application of HIP complex to minimize acylation of peptide, the complex should remain stable at lower pH (Fig. 1b). Following release, the complex must dissociate completely to produce native peptide (Fig. 1c). Thus, the dissociation of complex directly affects bioavailability of peptide. Therefore, mechanistic studies were performed to further investigate the factors influencing dissociation phenomenon. We examined the influence of concentration of counter ion on dissociation in phosphate buffer (pH 7.4) containing increasing concentration of NaCl. The complex was incubated in DDI water, which acted as a negative control with absence of counter ions. As shown in Fig. 4, less than 5% octreotide dissociated upon incubation in DDI water because of the absence of counter ions. A proportional increase in fraction of dissociated peptide was observed with an increase in concentration of counter ion at pH 7.4 in phosphate buffer. Dissociation of complex in phosphate buffer (Fig. 4) and IPBS (Fig. 3) clearly indicates that the dissociation of complex depends on concentration of counter ions. Again, near complete dissociation was observed in cases of DSA and DSB compared to SDS at 154 mM NaCl. Dissociation of HIP complexes at acidic condition was also studied by incubating complex in 10 mM citrate buffer with 100 mM NaCl at pH 4. Importance of pH on dissociation of complex is evident because a substantially low fraction of complex was dissociated at pH 4 despite presence of counter ions. Based on these results, it can be inferred that HIP complex may be able to mask reactive amines groups in acidic microenvironment inside microparticle core and prevent acylation of peptide (Fig. 1c).
Figure 4.
Dissociation of SDS-octreotide, DSA-octreotide and DSB-octreotide HIP complex at various ionic strength and pH.
3.4. Particle size, zeta potential and surface morphology of HIP complex
DSA-octreotide and DSB-octreotide resulted in complete dissociation of complex at physiological pH and hence they were further studied for particle size, morphology and zeta potential. Scanning electron micrograph of freeze dried complex showed that the particles were irregular shaped (Supplementary Fig. 1). Freeze-dried complex was suspended in DDI water and tip-sonicated for 30 s at output of 2 W to disperse the HIP complex for particle size determination. Particle size was found to be 796.6±204.8 nm and 3154±596.7 nm for DSA-octreotide and DSB-octreotide complexes, respectively. Higher size of DSB-octreotide complex could be attributed to its larger molecular weight. Zeta potential for DSA-octreotide and DSB-octreotide were found to be −45.8±2.45 mV and −39.6±2.01 mV, respectively. Negative potential could be attributed to free carboxylic group of octreotide in HIP complex.
3.5. Preparation and characterization of MPs
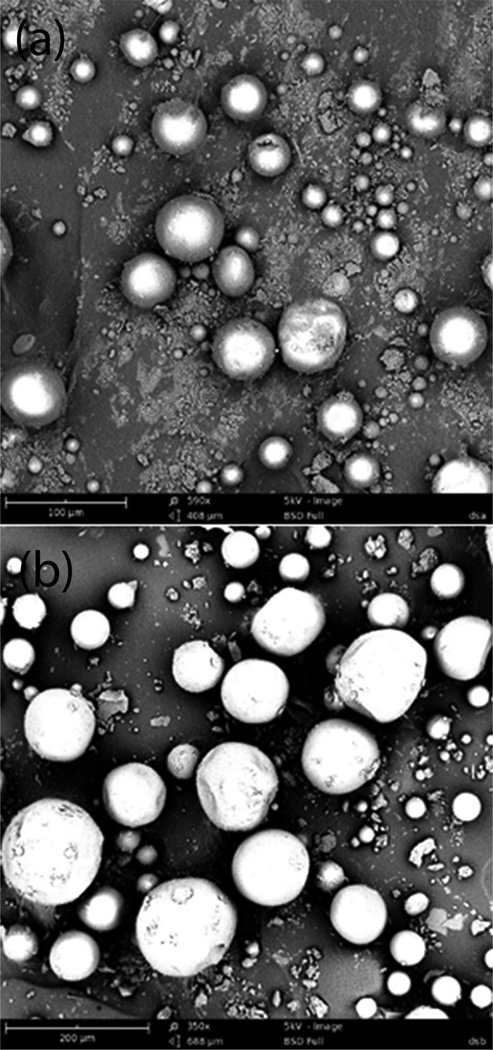
Ion-pairing agents DSA and DSB were selected for further studies based on the dissociation of complex at simulated physiological condition and stability at low pH. MPs were prepared by S/O/W method and characterized for octreotide loading and encapsulation efficiency following direct method of analysis. PLGA 50/50 at polymer to drug weight ratio of 10:1 was utilized. Excellent entrapment efficiency and drug loading for both DSA and DSB ion-pairing agents were achieved with S/O/W method (Table 1). MPs were characterized for particle size and morphology with scanning electron microscopy. MPs were spherical in shape with smooth surfaces (Fig. 5). DSAMPs were slightly polydispersed and had overall size less than 100 µm. On the other hand, DSBMPs appears to be less polydispersed with a size of 200 µm or less. Larger size of DSBMPs could be attributed to higher molecular weight of DSB (36–50kDa) compared to DSA (9–20kDa), which resulted in larger size DSB-Oct complex.
Table 1.
Summary of entrapment efficiency and drug loading for DSAMPs and DSBMPs.
| Polymer | Complexing agent |
Polymer:octreotide weight ratio |
%EE | %DL |
|---|---|---|---|---|
| PLGA (50/50) | DSA | 10:1 | 74.7±8.4 | 7.8±0.3 |
| PLGA (50/50) | DSB | 10:1 | 81.7±6.3 | 7.9±0.5 |
Data represented as mean+SD, n=3.
Figure 5.
Scanning electron micrograph showing surface morphology for (a) DSAMPs and (b) DSBMPs.
3.6. Mechanism and kinetics of release
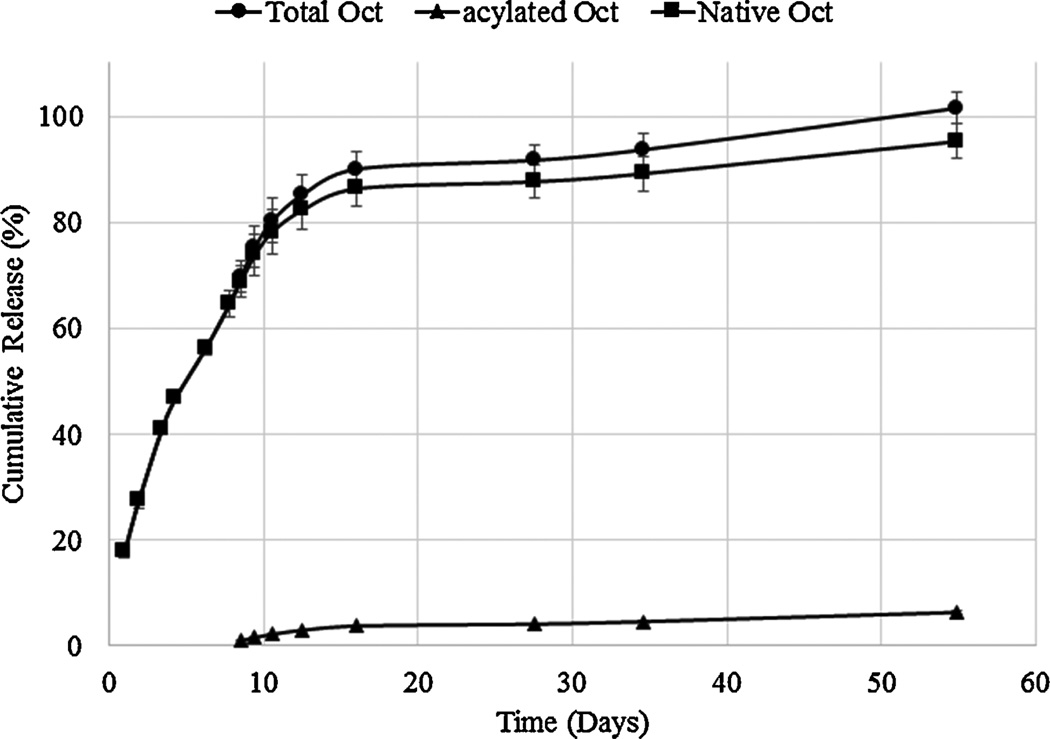
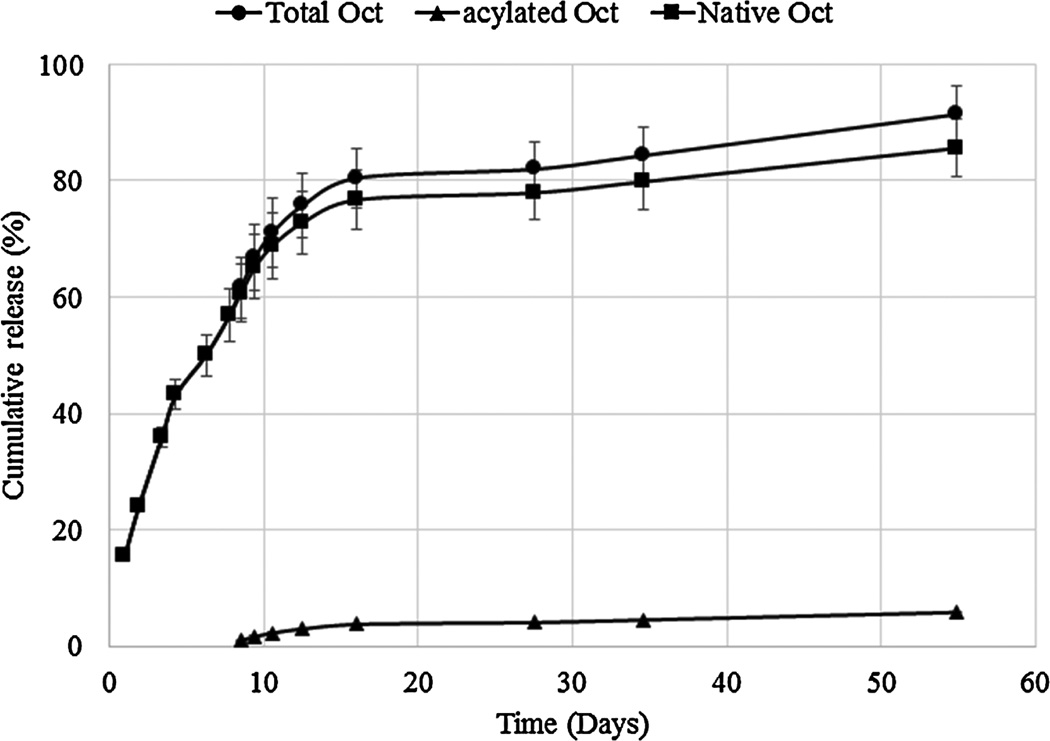
In vitro release studies were carried out to investigate the mechanism and kinetics of octreotide release from DSAMPs-in-gel and DSBMPs-in-gel in PBS buffer at 37°C. Samples were analyzed by HPLC to determine the concentration of released peptide (native and acylated). Release profiles depicting the release of total, native and acylated octreotide from DSAMPs-in-gel and DSBMPs-in-gel are illustrated in Fig. 6 and 7. Our laboratory specializes in particle-in-gel formulations (Khurana et al., 2014; Mishra et al., 2011; Mitra and Mishra, 2011; Patel et al., 2014c; Patel et al., 2015; Patel et al., 2014d). Pentablock thermoresponsive polymer (PLA250-PCL1250-PEG1500-PCL1250-PLA250) was utilized to sustain the release of octreotide. Preparation and characterization of gelling polymer has been published earlier from our laboratory (Patel et al., 2014d). MPs were suspended in an aqueous solution of pentablock polymer (20% w/w), which phase transform to gel at 37 °C to entrap MPs within gel matrix. We have previously shown that burst release from particles can be efficiently prevented by using particle-in-gel approach (Patel et al., 2014c; Patel et al., 2014d). Suspending the MPs in gel creates a depot where particles remains localized during release. The hydrogel also provides an additional diffusion barrier to peptide released from MPs and thus may extend duration of release (Patel et al., 2014d; Tamboli et al., 2013).
Figure 6.
In vitro release profiles of total, native and acylated octreotide from DSAMPs-in-gel composite formulation.
Figure 7.
In vitro release profiles of total, native and acylated octreotide from DSBMPs-in-gel composite formulation.
DSAMPs-in-gel and DSBMPs-in-gel sustained release of octreotide over a period of 55 days. Near complete release was observed for both DSAMPs and DSBMPs in gel. No significant difference in initial burst release was observed for DSAMPs-in-gel (17.8±1.1%) and DSBMPsin-gel (15.6±0.5%) (t-test p=0.146). Mechanism of release for total octreotide was delineated by fitting the data in mechanistic models including Higuchi, Korsmeyer-Peppas, and Hixson-Crowell models (Higuchi, 1963; Hixson and Crowell, 1931; Korsmeyer et al., 1983). Table 2 summarizes the coefficient of determination (R2) and associated parameter for all the models. Best fit was observed for Korsmeyer-Peppas model based on the R2 value for both formulations among all three models (Table 2). The n-value for both formulations were found to be 0.64 suggesting the release mechanism to be non-Fickian (Korsmeyer et al., 1983). This mechanism suggests that the release of peptide was both diffusion and degradation controlled. Data was also fitted to zero- and first-order equations to determine the order of release. Process of release was first order kinetics indicated by R2 values for both DSAMPs and DSBMPs in gel (Table 2). First order kinetic suggests that the release rate is proportional to the concentration. As a result, slower release was observed for both MPs-in-gel in the later phase of release (Fig. 6 and 7). Compared to DSBMPs-in-gel, release rate was faster for DSAMPs-in-gel, as indicated by first order rate constant. This result can be attributed to accelerated erosion of polymer matrix due to smaller particle size.
Table 2.
Summary of fit for kinetic models and associated parameters for release of octreotide from DSAMPs and DSBMPs in gel formulations.
| MPs | Mechanistic and kinetic models | ||||||
|---|---|---|---|---|---|---|---|
| Higuchi Model |
Korsmeyer-Peppas model |
Hixson- Crowell model |
Zero Order |
First order | |||
| R2 | R2 | n | R2 | R2 | R2 | Rate constant k (Day−1) |
|
| DSAMPs | 0.5988 | 0.9964 | 0.6403 | 0.7884 | 0.5821 | 0.9124 | 0.107 |
| DSBMPs | 0.6224 | 0.9935 | 0.6378 | 0.7582 | 0.5948 | 0.8385 | 0.040 |
3.7. Acylation of peptide during release
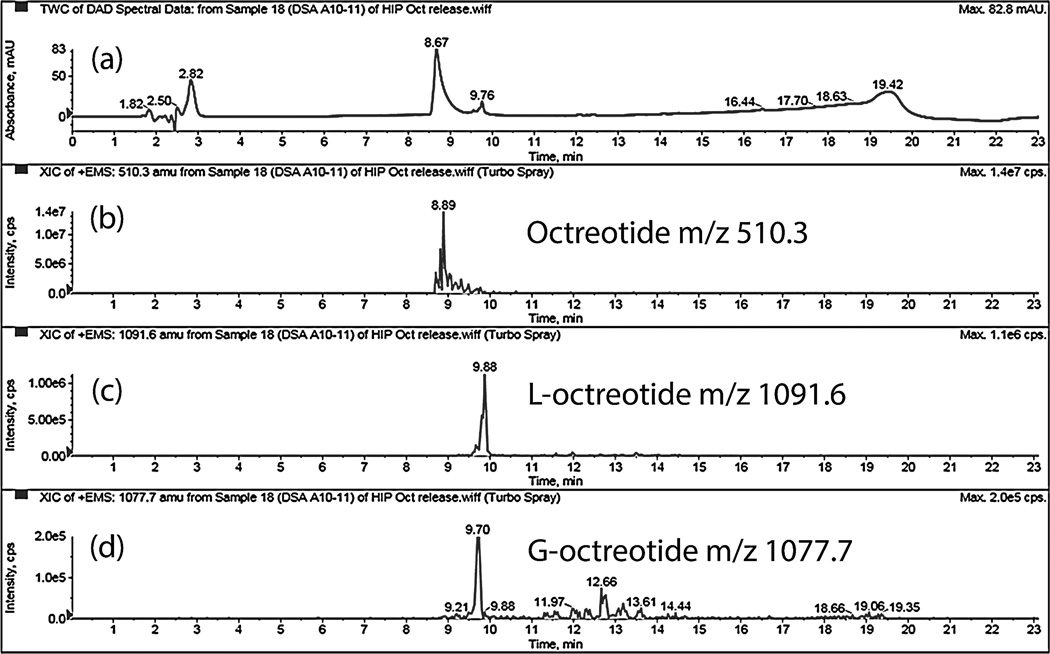
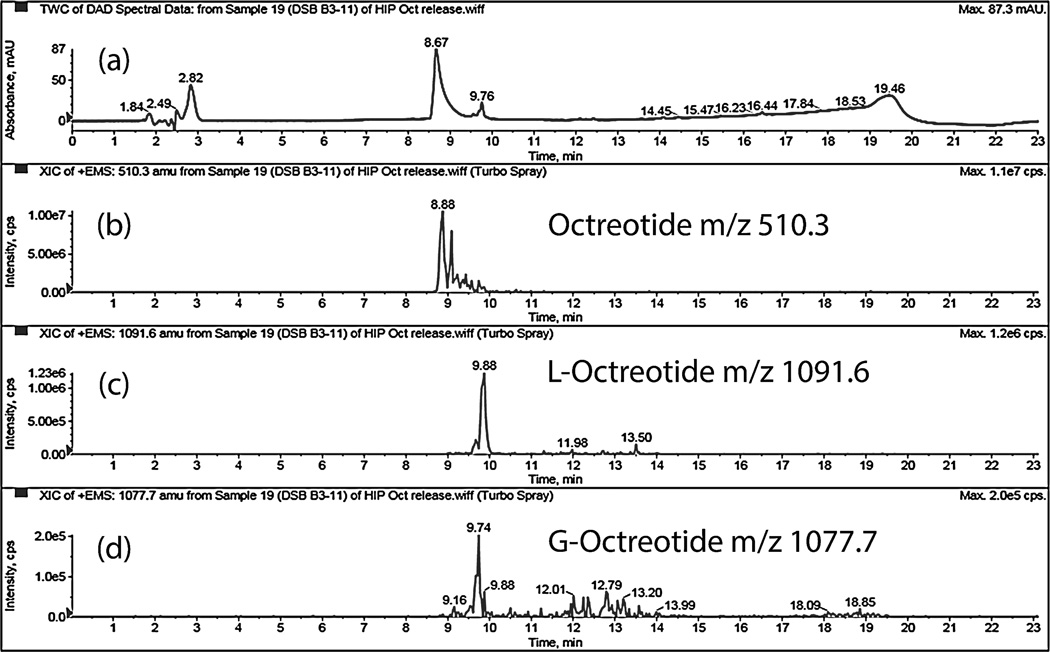
Dissociation of HIP complex under acidic pH suggests that peptide complex remains stable at acidic pH inside MPs and may prevent acylation of peptide during release. Amount of native and acylated peptide released from DSAMPs and DSBMPs in gel were quantified by HPLC. Compared to marketed PLGA MPs formulation of octreotide (Sandostatin LAR® depot) where ~60% of octreotide is acylated during in vitro release (Ghassemi et al., 2012), only 6.3% and 5.8% of peptide were acylated for DSAMPs and DSBMPs in gel, respectively, in our case. This small amount of acylation may be explained by dissociation of small fraction of peptide at acidic pH (Fig. 4). Significantly large fractions of octreotide, 85% from DSBMPs-in-gel and 95% DSAMPs-in-gel, were chemically intact following the release relative to Sandostatin LAR® depot where <20% was release in native form (Ghassemi et al., 2012). HPLC-MS analysis was performed to identify the peaks associated with native and acylated peptide in the LC chromatogram. HPLC-MS profile for release samples on day 16 from DSAMPs-in-gel and DSBMPs-in-gel are represented in Fig. 8 and 9. Native octreotide eluted at 8.89 min in extracted ion-chromatogram as m/z of 510.3 corresponding to peak at 8.67 min in UV chromatogram (Fig. 8a and 9a). Parent ion for native octreotide at m/z 1019.3 was also observed (Data not shown). Presence of 1019.3 and 510.3 m/z suggest that the peptide maintained its native cyclic chemical structure. In both MPs, a very small amount of lactoyl-octreotide (m/z 1091.6, 9.8 min) and glycoyl-octreotide (m/z 1077.7, 9.7 min) adducts were observed as acylated species (Fig. 8b–8d and Fig. 9b–9d), which eluted following native peptide due to higher hydrophobicity. Similar findings have been reported by other investigators (Na et al., 2003a). Table 3 depicts the chemically modified species observed in the release samples for DSAMPs and DSBMPs. The observations in Table 3 are based on HPLC-MS analysis of release samples on day 1, 9 and 16. The gelling polymer contained polycaprolactone block but caprolactoyl-octreotide (m/z 1133.5) was not detected. Moreover, di-latoyl and di-glycoyl adducts were not observed (Table 3).
Figure 8.
HPLC-MS spectrum of release sample (day 16) from DSAMPs-in-gel. (a) UV chromatogram. Extracted ion chromatogram (EIC) for (b) octreotide, m/z 510.3 (c) Lactoyloctreotide, m/z 1091.6 and (d) Glycoyl-octreotide, m/z 1077.7.
Figure 9.
HPLC-MS spectrum of release sample (day 16) from DSBMPs-in-gel. (a) UV chromatogram. Extracted ion chromatogram (EIC) for (b) octreotide, m/z 510.3 (c) Lactoyloctreotide, m/z 1091.6 and (d) Glycoyl-octreotide, m/z 1077.7.
Table 3.
Summary of octreotide adducts identified using UFLC-MS analysis in release samples.
| MPs | Acylated species | M+H/Z | Retention time (min) |
|---|---|---|---|
| DSAMPs | L-Octreotide | 1091.6 | 9.8 |
| G-Octreotide | 1077.7 | 9.7 | |
| LL-Octreotide | 1163.2 | ND | |
| GG-Octreotide | 1135.5 | ND | |
| C-Octreotide | 1133.5 | ND | |
| DSBMPs | L-Octreotide | 1091.6 | 9.8 |
| G-Octreotide | 1077.7 | 9.7 | |
| LL-Octreotide | 1163.2 | ND | |
| GG-Octreotide | 1135.5 | ND | |
| C-Octreotide | 1133.5 | ND |
C-Caprolactyl, L-Lactoyl, G-Glycoyl, ND-Not detected.
The DSA-octreotide and DSB-octreotide HIP complex resulted in nearly 100% dissociation at physiological condition. In addition, MPs with both DSA and DSB HIP complexes resulted in high drug loading and these MPs were very similar to each other in regards to minimizing acylation of peptide and release rate. However, particle size of DSAMPS was less than that of DSBMPs. The small size particles will be easy to inject in the clinical settings and hence DSAMPs would be preferred over DSBMPs.
4. Conclusions
Process of HIP complex formation was very simple and straightforward involving simple mixing of solutions. Complexation efficiency of nearly 100% was achieved with all ion-pairing agents. HIP complex with dextran sulfate was reversible unlike SDS at physiological pH. HIP complex also resulted in excellent encapsulation and loading efficiency in MPs due to increased hydrophobicity. MPs-in-gel composite formulation resulted in sustained release of peptide. More than 95% of released peptide maintained native chemical structure and less than 7% octreotide was acylated with DSA-octreotide HIP complex. Reversible HIP complex is a viable strategy to maintain chemical stability of peptide during long-term delivery from lactide and glycolide based polymers.
Supplementary Material
Acknowledgements
This research was supported by grants R01 EY 09171-14 and R01 EY 10659-12 from the National Institutes of Health. The authors would like to thank Dr. James Murowchick from the School of Arts and Sciences, University of Missouri-Kansas City for XRD analysis and Ashwin Parenky from Department of Pharmaceutical Sciences, Mercer University for SEM analysis. We are thankful to Anal Shah at Department of Pharmacotherapy and Outcomes Science, School of Pharmacy, Virginia Commonwealth University for proof reading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
The authors report no declarations of interest.
References
- Abrams DI, Kuno S, Wong R, Jeffords K, Nash M, Molaghan JB, Gorter R, Ueno R. Oral dextran sulfate (UA001) in the treatment of the acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. Annals of internal medicine. 1989;110:183–188. doi: 10.7326/0003-4819-110-3-183. [DOI] [PubMed] [Google Scholar]
- Ahn JH, Park EJ, Lee HS, Lee KC, Na DH. Reversible blocking of amino groups of octreotide for the inhibition of formation of acylated peptide impurities in poly(lactide-coglycolide) delivery systems. AAPS Pharm Sci Tech. 2011;12:1220–1226. doi: 10.1208/s12249-011-9694-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanson P, Timsit J, Harris AG. Clinical pharmacokinetics of octreotide. Therapeutic applications in patients with pituitary tumours. Clinical pharmacokinetics. 1993;25:375–391. doi: 10.2165/00003088-199325050-00004. [DOI] [PubMed] [Google Scholar]
- De Martino MC, Hofland LJ, Lamberts SW. Somatostatin and somatostatin receptors: from basic concepts to clinical applications. Progress in brain research. 2010;182:255–280. doi: 10.1016/S0079-6123(10)82011-4. [DOI] [PubMed] [Google Scholar]
- Feelders RA, Hofland LJ, van Aken MO, Neggers SJ, Lamberts SW, de Herder WW, van der Lely AJ. Medical therapy of acromegaly: efficacy and safety of somatostatin analogues. Drugs. 2009;69:2207–2226. doi: 10.2165/11318510-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Gaudana R, Gokulgandhi M, Khurana V, Kwatra D, Mitra AK. Design and evaluation of a novel nanoparticulate-based formulation encapsulating a HIP complex of lysozyme. Pharmaceutical development and technology. 2013;18:752–759. doi: 10.3109/10837450.2012.737806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudana R, Khurana V, Parenky A, Mitra AK. Encapsulation of Protein-Polysaccharide HIP Complex in Polymeric Nanoparticles. J Drug Deliv. 2011;2011:458128. doi: 10.1155/2011/458128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghalanbor Z, Korber M, Bodmeier R. Protein release from poly(lactide-co-glycolide) implants prepared by hot-melt extrusion: thioester formation as a reason for incomplete release. International journal of pharmaceutics. 2012;438:302–306. doi: 10.1016/j.ijpharm.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Ghassemi AH, van Steenbergen MJ, Barendregt A, Talsma H, Kok RJ, van Nostrum CF, Crommelin DJ, Hennink WE. Controlled release of octreotide and assessment of peptide acylation from poly(D,L-lactide-co-hydroxymethyl glycolide) compared to PLGA microspheres. Pharmaceutical research. 2012;29:110–120. doi: 10.1007/s11095-011-0517-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi T. Mechanism of Sustained-Action Medication. Theoretical Analysis of Rate of Release of Solid Drugs Dispersed in Solid Matrices. Journal of pharmaceutical sciences. 1963;52:1145–1149. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- Hixson AW, Crowell JH. Dependence of Reaction Velocity upon surface and Agitation. Industrial & Engineering Chemistry. 1931;23:923–931. [Google Scholar]
- Ibrahim MA, Ismail A, Fetouh MI, Gopferich A. Stability of insulin during the erosion of poly(lactic acid) and poly(lactic-co-glycolic acid) microspheres. Journal of controlled release : official journal of the Controlled Release Society. 2005;106:241–252. doi: 10.1016/j.jconrel.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Khurana V, Patel SP, Agrahari V, Pal D, Mitra AK. Novel Pentablock Copolymer Based Nanoparticles Containing Pazopanib: A Potential Therapy for Ocular Neovascularization. Recent Patents on Nanomedicine. 2014;4:57–68. See more at: http://www.eurekaselect.com/125019/article#sthash.gPVmIUVb.dpuf. [Google Scholar]
- Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. International journal of pharmaceutics. 1983;15:25–35. [Google Scholar]
- Lucke A, Fustella E, Tessmar J, Gazzaniga A, Gopferich A. The effect of poly(ethylene glycol)-poly(D,L-lactic acid) diblock copolymers on peptide acylation. Journal of controlled release : official journal of the Controlled Release Society. 2002;80:157–168. doi: 10.1016/s0168-3659(02)00020-2. [DOI] [PubMed] [Google Scholar]
- Maderich AB, Sugita ET. Absorption enhancement of dextran sulfate after enteral administration in a dispersion. International journal of pharmaceutics. 1996;137:85–94. [Google Scholar]
- Mishra GP, Tamboli V, Mitra AK. Effect of hydrophobic and hydrophilic additives on sol-gel transition and release behavior of timolol maleate from polycaprolactone-based hydrogel. Colloid and polymer science. 2011;289:1553–1562. doi: 10.1007/s00396-011-2476-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra AK, Mishra GP. Pentablock Polymers. Google Patents. 2011 [Google Scholar]
- Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nature reviews. Drug discovery. 2014;13:655–672. doi: 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlin IM, Latich I, Kidd M, Zikusoka M, Eick G. Therapeutic options for gastrointestinal carcinoids. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2006;4:526–547. doi: 10.1016/j.cgh.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Murty SB, Goodman J, Thanoo BC, DeLuca PP. Identification of chemically modified peptide from poly(D,L-lactide-co-glycolide) microspheres under in vitro release conditions. AAPS Pharm Sci Tech. 2003;4:E50. doi: 10.1208/pt040450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na DH, DeLuca PP. PEGylation of octreotide: I. Separation of positional isomers and stability against acylation by poly(D,L-lactide-co-glycolide) Pharmaceutical research. 2005;22:736–742. doi: 10.1007/s11095-005-2589-4. [DOI] [PubMed] [Google Scholar]
- Na DH, Lee KC, DeLuca PP. PEGylation of octreotide: II. Effect of N-terminal mono-PEGylation on biological activity and pharmacokinetics. Pharmaceutical research. 2005;22:743–749. doi: 10.1007/s11095-005-2590-y. [DOI] [PubMed] [Google Scholar]
- Na DH, Murty SB, Lee KC, Thanoo BC, DeLuca PP. Preparation and stability of poly(ethylene glycol) (PEG)ylated octreotide for application to microsphere delivery. AAPS Pharm Sci Tech. 2003a;4:E72. doi: 10.1208/pt040472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na DH, Youn YS, Lee SD, Son MW, Kim WB, DeLuca PP, Lee KC. Monitoring of peptide acylation inside degrading PLGA microspheres by capillary electrophoresis and MALDI-TOF mass spectrometry. Journal of controlled release : official journal of the Controlled Release Society. 2003b;92:291–299. doi: 10.1016/s0168-3659(03)00366-3. [DOI] [PubMed] [Google Scholar]
- Patel A, Gaudana R, Mitra AK. A novel approach for antibody nanocarriers development through hydrophobic ion-pairing complexation. Journal of microencapsulation. 2014a;31:542–550. doi: 10.3109/02652048.2014.885606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Patel M, Yang X, Mitra AK. Recent advances in protein and Peptide drug delivery: a special emphasis on polymeric nanoparticles. Protein and peptide letters. 2014b;21:1102–1120. doi: 10.2174/0929866521666140807114240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SP, Vaishya R, Mishra GP, Tamboli V, Pal D, Mitra AK. Tailor-made pentablock copolymer based formulation for sustained ocular delivery of protein therapeutics. J Drug Deliv. 2014c;2014:401747. doi: 10.1155/2014/401747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SP, Vaishya R, Pal D, Mitra AK. Novel pentablock copolymer-based nanoparticulate systems for sustained protein delivery. AAPS Pharm Sci Tech. 2015;16:327–343. doi: 10.1208/s12249-014-0196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SP, Vaishya R, Yang X, Pal D, Mitra AK. Novel thermosensitive pentablock copolymers for sustained delivery of proteins in the treatment of posterior segment diseases. Protein and peptide letters. 2014d;21:1185–1200. doi: 10.2174/092986652111141001122054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi F, Yang L, Wu J, Ma G, Su Z. Microcosmic Mechanism of Dication for Inhibiting Acylation of Acidic Peptide. Pharmaceutical research. 2015 doi: 10.1007/s11095-015-1622-5. [DOI] [PubMed] [Google Scholar]
- Shi K, Cui F, Yamamoto H, Kawashima Y. Investigation of drug loading and in vitro release mechanisms of insulin-lauryl sulfate complex loaded PLGA nanoparticles. Die Pharmazie. 2008;63:866–871. [PubMed] [Google Scholar]
- Sophocleous AM, Zhang Y, Schwendeman SP. A new class of inhibitors of peptide sorption and acylation in PLGA. Journal of controlled release : official journal of the Controlled Release Society. 2009;137:179–184. doi: 10.1016/j.jconrel.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamboli V, Mishra GP, Mitra AK. Novel pentablock copolymer (PLA-PCL-PEGPCL- PLA) based nanoparticles for controlled drug delivery: Effect of copolymer compositions on the crystallinity of copolymers and in vitro drug release profile from nanoparticles. Colloid and polymer science. 2013;291:1235–1245. doi: 10.1007/s00396-012-2854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishya R, Khurana V, Patel S, Mitra AK. Long-term delivery of protein therapeutics. Expert opinion on drug delivery. 2014:1–26. doi: 10.1517/17425247.2015.961420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishya R, Mitra AK. Future of sustained protein delivery. Therapeutic delivery. 2014;5:1171–1174. doi: 10.4155/tde.14.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Schwendeman SP. Minimizing acylation of peptides in PLGA microspheres. Journal of controlled release : official journal of the Controlled Release Society. 2012;162:119–126. doi: 10.1016/j.jconrel.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sophocleous AM, Schwendeman SP. Inhibition of peptide acylation in PLGA microspheres with water-soluble divalent cationic salts. Pharmaceutical research. 2009;26:1986–1994. doi: 10.1007/s11095-009-9914-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.