Abstract
Background
MS is an immune-mediated inflammatory disease of the CNS. B cells have been strongly implicated in disease pathogenesis based on clinical trials with B cell ablation. There is a growing body of evidence linking microRNAs with regulation of the immune system. Dicer, a key enzyme involved in microRNA biogenesis, is necessary for normal B cell function.
Objective
To determine whether Dicer expression is impaired in B cells and is linked to increased expression co-stimulatory molecules in MS patients.
Methods
B cells were separated from MS patients and healthy subjects. Expression of Dicer and co-stimulatory molecules CD80 and CD86 was tested. The effect of Dicer modulation on CD80 and CD86 expression in B cells was studied.
Results
Dicer expression was decreased in B cells but not in monocytes of MS patients compared to healthy subjects. CD80 and CD86 expression was increased on B cells of MS patients compared to healthy subjects. Inhibition of Dicer expression in B cells by small interfering RNA led to increased expression of CD80.
Conclusion
Dicer expression is decreased and is mechanistically linked to increased expression of co-stimulatory molecule CD80 in B cells of MS patients. This may contribute to activation of immune responses in MS.
Keywords: multiple sclerosis, Dicer, B cells, CD80
1. Introduction
The contribution of B cells and their products to the pathogenesis of multiple sclerosis (MS) is well established. B cell accumulation, clonal expansion and the production of oligoclonal IgG in the cerebrospinal fluid (CSF) of patients with MS have been repeatedly documented (1-3). The elimination of B cells with anti-CD20 monoclonal antibodies suppresses disease activity in patients with relapsing-remitting MS (RRMS) (4-7). Notably, it is not accompanied by reduction in CSF immunoglobulins (3). In addition, T cell responses are reduced in MS patients with depleted B cells (8). B cells regulate T cell response via cytokines (8-9) or co-stimulatory molecules (10-11) and can efficiently present myelin antigen to T cells (12). Co-stimulatory molecules CD80 and CD86 are critical in activating T cell immune response (13-14) and are over-expressed on B cells in MS patients (15).
MicroRNAs (miRNAs) are gene regulatory molecules that influence the output of protein-coding genes in mammals (16-17). Dicer (aka Dicer 1 in humans), a member of the RNase III family of enzymes, is closely involved in miRNA biogenesis (16). Dicer and miRNA expression have a crucial role in immune regulation (18). For example, mice with a conditional deletion of Dicer in B cells have a complete block in B cell development and an alteration in antibody repertoire (19). Dicer is also involved in the establishing of B cell tolerance. The self-reactive auto-antibodies are increased in Dicer-deficient mice (20).
We hypothesized that Dicer expression could be impaired in B cells of MS patients and linked to increased co-stimulatory function of B cells. Here we report that expression of Dicer is selectively decreased in B cells of patients with MS and is mechanistically linked to increased expression of co-stimulatory molecule CD80.
2. Materials and Methods
2.1. Patients and healthy controls
Control healthy donors (HD) and patients diagnosed with clinically definite relapsing-remitting MS (RRMS)_(21)_or clinically isolated syndrome (CIS) with brain MRI suggestive of MS defined as two or more clinically silent lesions that were at least 3 mm in diameter on MRI scans with at least one lesion had to be periventricular or ovoid (22) were included in the study approved by an institutional review committee. The patients had not been taking any disease-modifying drugs for at least 3 months prior to their enrollment in the study. 14 out of 17 enrolled MS patients had at least one clinical relapse in the year before sample collection. The average annual relapse rate among these 14 active patients was 1.5 relapses/year. Additional exclusion criteria included steroid treatment in the month prior to the blood draw; presence of other disorders that may be associated with abnormal immune response; pregnancy; baseline Expanded Disability Status Scale score greater than 5 (23); and clinical signs or history suggesting infection within 2 weeks prior to the blood draw. The age (mean ± SEM) of five healthy subjects (3 females/2 male) and six patients (4 females/2 males) tested in gene expression experiments were 36.4 ± 5.2 and 40 ± 4.6, respectively. The age (mean ± SEM) of six healthy subjects (5 females/1 male) and five patients (4 females/1 males) tested in Fig. 2 were 32.33 ± 4.5 and 35.6 ± 4.2, respectively. The age of 16 healthy subjects (11 females/5 males) and 19 patients (14 females/5 males) tested in Fig. 1 were 35.19 ± 2.59 and 35.42 ± 1.9, respectively.
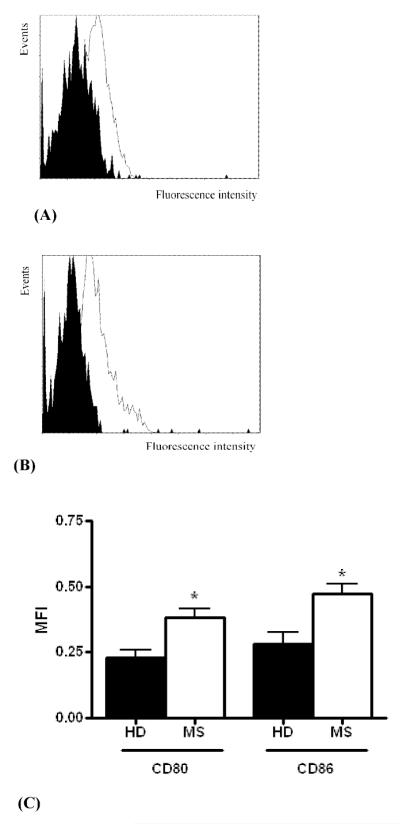
Figure 2. Expression of CD80 and CD86 on B cells is increased in MS patients.
PBMC cells were separated from patients with multiple sclerosis (MS) and healthy donors (HD). CD80 and CD86 expression was analyzed among CD19 positive (gated) B cells. Fig. 2A and 2B depict overlap histograms of CD80 (Fig 2A) and CD86 (Fig 2B) from a representative healthy donor (black histogram) and a patient with MS (white histogram). The Mean Fluorescent Intensity (MFI) of CD80 and CD86 on B cells separated from six healthy donors and five MS patients is shown in Fig. 2C. * − p< 0.02
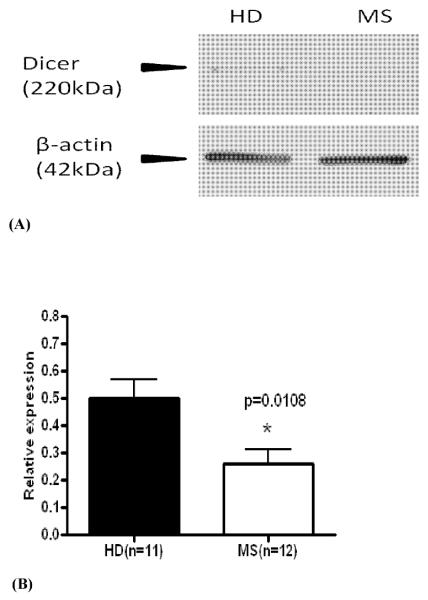
Figure 1. Dicer protein expression is decreased in B cells of MS patients.
B cells were separated from 12 patients with multiple sclerosis (MS) and 11 healthy donors (HD) and tested for Dicer expression by Western blot as described in Material and Methods. Dicer protein expression was normalized against endogenous beta-actin protein. Figure 1A depicts Western blot from a representative HD and a patient with MS. The mean relative expression of Dicer is shown in Figures 1B. * − p = 0.0108.
Study Approval: The study was approved by the Rutgers Biomedical and Health Sciences Institutional Review Board (New Brunswick/Piscataway Campus) and all subjects gave written informed consent.
2.2. Separation of cell subsets
Peripheral blood mononuclear cells (PBMC) were separated by Ficoll density gradient (MediaTech Inc). Separation of B cells and monocytes was done by negative immunomagnetic sorting using B cell isolation kit II (Miltenyi Biotec, Cat # 130 091 151) and monocyte isolation kit II (Cat # 130-091-153), respectively, according to the manufacturer’s protocol. More than 95% of separated B cells were CD19-positive. More than 85% of separated monocytes were CD14-positive. Separated cell subsets were immediately resuspended in RNA cell protect reagent solution (Qiagen) for gene expression analysis or frozen at −80°C for Western blot experiments.
2.3. Cell culture and modulation of Dicer expression with small interfering RNA
Ramos B lymphocytes (cat#CRL-1596, ATCC) were cultured with complete medium (RPMI plus 10% FBS and L-glutamine). Cells were transfected with 50 nM of Dicer small interfering RNA (siRNA) (Cat#SI02655492, Qiagen) or Allstars negative control (Cat# SI03650318, Qiagen) using TransIT-TKO transfection reagent (Cat#MIR2154, Mirus Bio) for 72 hours according to manufacturer’s instructions. CD80 and CD86 expressions of siRNA treated Ramos cells were analyzed by Flow cytometry. Similar experiments were done with B cells separated from healthy controls (1×106) transfected with 100 nM of Dicer small interfering RNA (siRNA) (Cat#SI02655492, Qiagen) or Allstars negative control (Cat# SI03650318, Qiagen)
2.4. Flow Cytometry analysis
PBMC separated from healthy donors and MS patients were stained with PE-labeled anti-human CD19 mAb (Biolegend), and FITC-labeled anti-CD80 mAb, anti-CD86 mAb, anti-CD40 mAb or mouse IgG1 isotype control (Biolegend) followed by a two-color flow cytometry analysis on FC500 flow cytometer (Beckman Coulter). Results were analyzed with CXP software (Beckman Coulter). To analyze the effect of silencing Dicer on CD80 and CD86 expressions, Ramos cells were stained with FITC-labeled anti-CD80 mAb or anti-CD86 mAb (Biolegend). Mean fluorescence intensity (MFI) of CD80 (or CD86) was calculated as a difference between MFI of cell samples stained with anti-CD80 mAb minus MFI of cell samples stained with isotype control Ab. CD80 and CD86 expression change on transfected cells was evaluated by the fold change of MFI, calculated as the ratio of MFI of cells transfected with Dicer siRNA/MFI of cells transfected with negative control siRNA.
2.4. Gene expression analysis
Gene expression analysis was determined by Real-time quantitative polymerase chain reaction (RT-qPCR) as described earlier (24) using TaqMan Gene Expression Assays (the set of primers and probes) for Dicer 1 and endogenous control HPRT1 purchased from Applied Biosystems, CA.
2.5. Western blot
1,000,000 cells were lysed by sonication in Nupage LDS Sample buffer (4×) (Invitrogen, Cat #NP0007) with the addition of DTT (50mM) and heated at 70°C for 10 minutes. Cell lysates were loaded on Nupage 4-12% Bis-Tris gels (Invitrogen, Cat #NP0323BOX) and transferred to PVDF membrane (Biorad, Cat #162-0255) by electroblotting. The membranes were blocked in Tris/Tween-20 buffer containing 5% Non-fat dry milk (Biorad, Cat #170-6404) for one hour at room temperature and then probed with anti-Dicer antibody (Cat# 3363, Cell Signaling) for overnight at 4°C. Membranes were washed three times with Tris/Tween-20 buffer (50mM Tris (pH7.4), 150mM NaCl, 0.1% Tween-20 and probed with anti-rabbit IgG-HRP (Sigma-Aldrich, Cat #A6154) for one hour at room temperature. The enhanced chemiluminescence agent (Pierce, Cat #32106) was used to detect proteins. The intensities of protein bands were measured with Image J software (NIH). Membranes were stripped with Restore Western blot stripping buffer (Pierce, Cat #21059) and re-probed with mouse monoclonal anti-Beta-actin (Sigma-Aldrich, Cat # A5441) followed by anti-mouse IgG-HRP (Cat# A4416) for protein normalization.
2.6. Statistical analysis
Figures were generated and statistical analysis was performed with a GraphPad Prism 4 package from GraphPad Software, Inc., La Jolla, CA. The results are presented as Mean ± SEM. The paired or unpaired t test using two-tailed p-values were utilized as appropriate.
3. Results
3.1. Decreased Dicer expression in B cells of MS patients
As shown in Figure 1, Dicer protein expression was significantly decreased in B cells of patients with MS (0.2616 ± 0.0505 relative units, n = 12) compared to healthy donors (0.4989 ± 0.0695, n = 11), p = 0.0108. In contrast to B cells, Dicer expression was not significantly altered in monocytes of patients with MS (0.2379 ± 0.0833 relative units) compared to healthy controls (0.1998 ± 0.0563), p = 0.7107. We also evaluated Dicer 1 gene expression in five healthy donors and six MS patients by qRT-PCR. Dicer gene expression in MS patients (0.5462 ± 0.0301) was significantly lower than in healthy donors (0.8433 ± 0.0929), p = 0.0094.
3.2. Increased expressions of CD80 and CD86 in B cells of MS patients
We hypothesized that decreased Dicer expression is linked to altered expression of co-stimulatory molecules on B cells in MS. Co-stimulatory molecules CD80 and CD86 are expressed by B cells and other antigen-presenting cells and promote activation of T cells implicated in MS pathogenesis. As a first step, we studied expression of CD80 and CD86 on B cells in our human subjects. As shown in Figure 2C, Mean Fluorescence Intensity (MFI) of CD80 on B cells of MS patients (0.3828 ± 0.0324) was significantly increased compared to healthy subjects (0.228 ± 0.0329), p = 0.0091. MFI of CD86 was also increased in MS patients (0.4736 ± 0.0378) compared to healthy subjects (0.2808 ± 0.0467), p = 0.0125. CD40 expression on resting B cells was not changed in MS patients (7.726 ± 0.3407) compared to healthy subjects (7.559 ± 05418).
3.3. Inhibition of Dicer leads to increased expression of CD80
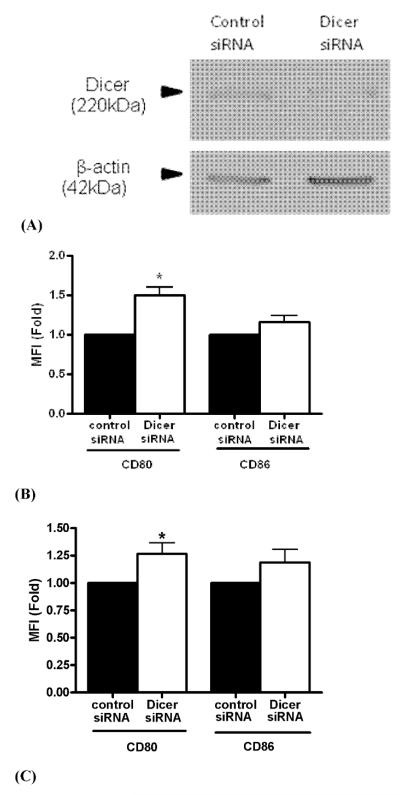
To evaluate the effect of inhibition of Dicer expression in B cells, we conducted transfection experiments with Dicer-specific or control nonspecific siRNA in-vitro. Transfection of Ramos B cells with Dicer-specific siRNA decreased Dicer protein expression as shown in Fig. 3A. CD80 and CD86 expressions were analyzed in transfected cells by flow cytometry. Inhibition of Dicer expression in cells transfected with Dicer siRNA led to 1.49 fold increased CD80 expression, p = 0.0167, compared to cell transfected with control siRNA (Fig. 3B). In contrast, the expression of CD86 was not significantly affected by Dicer modulation. Transfection experiments with Dicer-specific siRNA were also conducted with primary human B cells from healthy subjects. Similar to Ramos B cells, decreased Dicer expression led to increased CD80 expression, p ≡ 0.027 (Fig. 3C).
Figure 3. CD80 expression is increased upon silencing Dicer.
Ramos B cells or B cells from healthy human subjects were transfected with Dicer siRNA or control siRNA. Dicer expression was tested by Western blot. CD80 and CD86 expression was examined by Flow cytometry as described in Material and Methods. Fig. 3A depicts Ramos B cells Western blots from a representative experiment. The average fold change of CD80 and CD86 expression from six different experiments with Ramos B cells is shown in Fig. 3B (* − p = 0.0167). The average fold change of CD80 and CD86 expression from experiments with six different human subjects is shown in Fig. 3C (* − p = 0.027).
4. Discussion
CD80 and CD86 positive lymphocytes were increased in peripheral blood of MS patients with active disease compared to healthy donors (15). In contrast to lymphocytes, expression of CD80 or CD86 on monocytes was not increased in MS (15). In line with this, up-regulation of CD80 expression in MS plaques (25) and increased concentration of CD80 positive B cells in CSF of MS patients (26) were also reported. CD80 and CD86 enhance antigen-presenting function of B cells and were required for T cell activation (14),(27-28), and lack of CD80/CD86 co-stimulation could lead to T cell anergy (28). Notably, inhibition of CD80 activity with anti-CD80 antibody could prevent the development of experimental autoimmune encephalitis (EAE), a T cell-dependent disease, an animal model of MS. Interestingly, anti-CD86 antibodies could increase disease severity of EAE (29). CD80 and CD86 expressed on B cells are required for T-cell dependent immunoglobulin production (30). Here, we describe increased expression of CD80 and CD86 on B cells of MS patients. Furthermore, we demonstrated that CD80 expression, but not CD86, was modulated by Dicer. The exact mechanism of decreased Dicer expression in patients with MS is not clear at this time. Dicer was found to be critical in many biological processes which are also important for MS pathogenesis. One may hypothesize that decreased Dicer expression leads to impaired miRNA biogenesis and abnormal expression of protein-coding genes involved in immune response regulation and cell traffic to the CNS in patients with MS. For example, inhibition of Dicer in tumor cells was associated with enhanced expression of metallic matrix proteases MMP-2 and MMP-9 (31), enzymes implicated in increased permeability of blood-brain barrier and MS pathogenesis (32). Based on our results, Dicer was selectively decreased in B cells but not in monocytes in patients with MS (Figure 1). It is known that Dicer expression may be cell lineage-specific. For example, Dicer expression is increased in melanocytes that occurs via direct transcriptional targeting of Dicer by the melanocyte master transcriptional regulator (MITF) (33).
In summary, we have found that Dicer expression is selectively decreased in B cells of patients with relapsing form of MS and is linked to increased expression of co-stimulatory molecule CD80. This may contribute to increased activation of T cell responses in MS. The cause of decreased Dicer expression in B cells of patients with MS remains to be identified.
Acknowledgements
The study was supported in part by grant number K23NS052553 from the National Institute of Neurological Disorders and Stroke. We thank Joan Moore for proof reading of the article.
Footnotes
Conflict of Interest Statement: The authors have no commercial conflicts of interests or relationships which will be affected by the article. Dr. Balashov has served as a consultant for Bayer, Novartis and Sandoz. He has been funded by NIH, NMSS, and has received research support from Biogen Idec, TEVA Neuroscience, and Patterson Trust.
References
- 1.Kuenz B, Lutterotti A, Ehling R, Gneiss C, Haemmerle M, Rainer C, et al. Cerebrospinal fluid B cells correlate with early brain inflammation in multiple sclerosis. PLoS One. 2008;3(7):e2559. doi: 10.1371/journal.pone.0002559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franciotta D, Salvetti M, Lolli F, Serafini B, Aloisi F. B cells and multiple sclerosis. Lancet Neurol. 2008 Sep;7(9):852–8. doi: 10.1016/S1474-4422(08)70192-3. [DOI] [PubMed] [Google Scholar]
- 3.Cross AH, Waubant E. MS and the B cell controversy. Biochim Biophys Acta. 2011 Feb;1812(2):231–8. doi: 10.1016/j.bbadis.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 4.Bar-Or A, Calabresi PA, Arnold D, Markowitz C, Shafer S, Kasper LH, et al. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol. 2008 Mar;63(3):395–400. doi: 10.1002/ana.21363. [DOI] [PubMed] [Google Scholar]
- 5.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008 Feb 14;358(7):676–88. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 6.Kappos L, Li D, Calabresi PA, O’Connor P, Bar-Or A, Barkhof F, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet. 2011 Nov 19;378(9805):1779–87. doi: 10.1016/S0140-6736(11)61649-8. [DOI] [PubMed] [Google Scholar]
- 7.Naismith RT, Piccio L, Lyons JA, Lauber J, Tutlam NT, Parks BJ, et al. Rituximab add-on therapy for breakthrough relapsing multiple sclerosis: a 52-week phase II trial. Neurology. 2010 Jun 8;74(23):1860–7. doi: 10.1212/WNL.0b013e3181e24373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bar-Or A, Fawaz L, Fan B, Darlington PJ, Rieger A, Ghorayeb C, et al. Abnormal B-cell cytokine responses a trigger of T-cell-mediated disease in MS? Ann Neurol. 2010 Apr;67(4):452–61. doi: 10.1002/ana.21939. [DOI] [PubMed] [Google Scholar]
- 9.Bouaziz JD, Calbo S, Maho-Vaillant M, Saussine A, Bagot M, Bensussan A, et al. IL-10 produced by activated human B cells regulates CD4(+) T-cell activation in vitro. Eur J Immunol. 2010 Oct;40(10):2686–91. doi: 10.1002/eji.201040673. [DOI] [PubMed] [Google Scholar]
- 10.Thompson CB, Lindsten T, Ledbetter JA, Kunkel SL, Young HA, Emerson SG, et al. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1333–7. doi: 10.1073/pnas.86.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002 Feb;2(2):116–26. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 12.Harp CT, Ireland S, Davis LS, Remington G, Cassidy B, Cravens PD, et al. Memory B cells from a subset of treatment-naive relapsing-remitting multiple sclerosis patients elicit CD4(+) T-cell proliferation and IFN-gamma production in response to myelin basic protein and myelin oligodendrocyte glycoprotein. Eur J Immunol. 2010 Oct;40(10):2942–56. doi: 10.1002/eji.201040516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Good KL, Avery DT, Tangye SG. Resting human memory B cells are intrinsically programmed for enhanced survival and responsiveness to diverse stimuli compared to naive B cells. J Immunol. 2009 Jan 15;182(2):890–901. doi: 10.4049/jimmunol.182.2.890. [DOI] [PubMed] [Google Scholar]
- 14.Bar-Or A, Oliveira EM, Anderson DE, Krieger JI, Duddy M, O’Connor KC, et al. Immunological memory: contribution of memory B cells expressing costimulatory molecules in the resting state. J Immunol. 2001 Nov 15;167(10):5669–77. doi: 10.4049/jimmunol.167.10.5669. [DOI] [PubMed] [Google Scholar]
- 15.Genc K, Dona DL, Reder AT. Increased CD80(+) B cells in active multiple sclerosis and reversal by interferon beta-1b therapy. J Clin Invest. 1997 Jun 1;99(11):2664–71. doi: 10.1172/JCI119455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010 Sep;11(9):597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 17.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004 Jan 23;116(2):281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 18.O’Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. 2010 Feb;10(2):111–22. doi: 10.1038/nri2708. [DOI] [PubMed] [Google Scholar]
- 19.Koralov SB, Muljo SA, Galler GR, Krek A, Chakraborty T, Kanellopoulou C, et al. Dicer ablation affects antibody diversity and cell survival in the B lymphocyte lineage. Cell. 2008 Mar 7;132(5):860–74. doi: 10.1016/j.cell.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 20.Belver L, de Yebenes VG, Ramiro AR. MicroRNAs prevent the generation of autoreactive antibodies. Immunity. 2010 Nov 24;33(5):713–22. doi: 10.1016/j.immuni.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol. 2011 Feb;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobs LD, Beck RW, Simon JH, Kinkel RP, Brownscheidle CM, Murray TJ, et al. CHAMPS Study Group. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. N Engl J Med. 2000 Sep 28;343(13):898–904. doi: 10.1056/NEJM200009283431301. [DOI] [PubMed] [Google Scholar]
- 23.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983 Nov;33(11):1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 24.Balashov KE, Aung LL, Vaknin-Dembinsky A, Dhib-Jalbut S, Weiner HL. Interferon-beta inhibits toll-like receptor 9 processing in multiple sclerosis. Ann Neurol. 2010 Dec;68(6):899–906. doi: 10.1002/ana.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Windhagen A, Newcombe J, Dangond F, Strand C, Woodroofe MN, Cuzner ML, et al. Expression of costimulatory molecules B7-1 (CD80), B7-2 (CD86), and interleukin 12 cytokine in multiple sclerosis lesions. J Exp Med. 1995 Dec 1;182(6):1985–96. doi: 10.1084/jem.182.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svenningsson A, Dotevall L, Stemme S, Andersen O. Increased expression of B7-1 costimulatory molecule on cerebrospinal fluid cells of patients with multiple sclerosis and infectious central nervous system disease. J Neuroimmunol. 1997 May;75(1-2):59–68. doi: 10.1016/s0165-5728(96)00234-2. [DOI] [PubMed] [Google Scholar]
- 27.Linsley PS, Ledbetter JA. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- 28.Gimmi CD, Freeman GJ, Gribben JG, Gray G, Nadler LM. Human T-cell clonal anergy is induced by antigen presentation in the absence of B7 costimulation. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6586–90. doi: 10.1073/pnas.90.14.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuchroo VK, Das MP, Brown JA, Ranger AM, Zamvil SS, Sobel RA, et al. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995 Mar 10;80(5):707–18. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 30.Lumsden JM, Williams JA, Hodes RJ. Differential requirements for expression of CD80/86 and CD40 on B cells for T-dependent antibody responses in vivo. J Immunol. 2003 Jan 15;170(2):781–7. doi: 10.4049/jimmunol.170.2.781. [DOI] [PubMed] [Google Scholar]
- 31.Han L, Zhang A, Zhou X, Xu P, Wang GX, Pu PY, et al. Downregulation of Dicer enhances tumor cell proliferation and invasion. Int J Oncol. 2010 Aug;37(2):299–305. doi: 10.3892/ijo_00000678. [DOI] [PubMed] [Google Scholar]
- 32.Leppert D, Lindberg RL, Kappos L, Leib SL. Matrix metalloproteinases: multifunctional effectors of inflammation in multiple sclerosis and bacterial meningitis. Brain Res Brain Res Rev. 2001 Oct;36(2-3):249–57. doi: 10.1016/s0165-0173(01)00101-1. [DOI] [PubMed] [Google Scholar]
- 33.Levy C, Khaled M, Robinson KC, Veguilla RA, Chen PH, Yokoyama S, et al. Lineage-specific transcriptional regulation of DICER by MITF in melanocytes. Cell. 2010 Jun 11;141(6):994–1005. doi: 10.1016/j.cell.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]