Abstract
Introduction
Alcohol and nicotine are commonly used substances in the U.S., with significant impacts on health. Using both substances concurrently impacts quit attempts. While studies have sought to examine changes in alcohol use co-occurring with tobacco cessation, results have not been consistent. Understanding these changes has clinical implications. The objective of this study is to identify changes in alcohol consumption that occur following tobacco cessation, as well as predictors of alcohol use patterns following a smoking cessation attempt.
Methods
A secondary analysis of a randomized, placebo-controlled trial evaluating the efficacy of five tobacco cessation pharmacotherapies. Participants (N = 1301) reported their smoking and alcohol consumption daily for two weeks prior to, and two weeks after, the target quit date (TQD).
Results
Generally, alcohol use decreased post-TQD. Smokers who reported less pre-quit alcohol use, as well as smokers who were female, non-white, and had a history of alcohol dependence tended to use less alcohol post-quit. Pre- and post-quit alcohol use were more strongly related among men and among those without a history of alcohol dependence.
Conclusions
For most smokers alcohol use decreased following smoking cessation. These results suggest that the expectation should be of decreased alcohol use post cessation. However, attention may be warranted for those who drink higher amounts of alcohol pre-cessation because they may be more likely to drink more in the post-quit period which may influence smoking cessation success.
Keywords: tobacco use, smoking cessation, alcohol use
1. Introduction
Nicotine and alcohol are two addictive drugs that have a substantial impact on public health given that they are both prevalent and related to significant health risks. Tobacco use accounts for nearly half a million premature deaths annually in the United States [1]. Currently, approximately 18% of US adults smoke; however, certain subpopulations, including the less educated and those with psychiatric comorbidities (including alcohol use and other substance use disorders), smoke at even higher rates [2, 3]. Alcohol use is also very prevalent in the United States. Current surveys estimate that about 50% of US adults consume alcohol regularly [4] and that alcohol use accounted for over 25,000 deaths in 2010 [5]. The consequences of alcohol use, however, start occurring below thresholds of substance abuse definitions [6]. The concurrent use of alcohol and smoking is common, and ripe with complications, including a reduced likelihood of trying to quit smoking, a lower success rate for those who make an attempt [7, 8], and an increased rate of relapse back to smoking in the presence of heavy drinking [7].
Given alcohol’s influence on smoking cessation success, one key question regarding the association between drinking and smoking is what happens to the rate of alcohol use when a person quits smoking. Specifically, does quitting smoking increase or decrease drinking, and are certain person characteristics or smoking cessation treatments related to changes in drinking behavior after a smoking cessation attempt? While a variety of studies have attempted to answer these questions, the findings have not been consistent. Older studies suggest that smoking cessation results in an increase in alcohol use [9–11], while more recent epidemiologic studies have found that alcohol use decreases [12–16] and other studies have found that alcohol use does not change [17–22] as a result of smoking cessation. Most recently, Lisha et al. [23] found that alcohol use did not change with smoking cessation; however this study used 90-day recall methods for substance use and the populations studied were either alcohol dependent patients in early recovery or HIV positive patients, representing specific subsets of the general population. The lack of consistent findings in the previous studies could be due to methodologic variability, non-naturalistic settings, limited external validity, recall bias, and the lack of temporal ordering to allow for causal inference. Defining the pattern of non-problematic alcohol use concurrent with smoking cessation in the general population is an important clinical question with potential counseling implications.
The goal of the proposed research is to address the question of changes in drinking behavior following smoking cessation in a manner not subject to the methodological constraints outlined above. The study uses data collected in real-time during the course of a planned smoking cessation attempt from a sample of nicotine-dependent, treatment-seeking smokers, who participated in a smoking cessation clinical trial, but who represented members of the general population. This approach mitigates problems with non-naturalistic settings, recall bias, and the limited generalizability that affected prior studies on this topic.
We will also explore potential predictors of post-quit drinking behavior, with a focus on predictors that could be considered in a clinical setting for counseling purposes. In other words, if clinicians could identify risk factors for drinking during a smoking cessation attempt, which would represent a significant risk for relapsing back to smoking as well as a health risk in and of itself, the clinician would be able to address alcohol use more comprehensively among such patients. The predictors we evaluated include pre-quit alcohol use, gender, age, ethnicity, nicotine dependence and heaviness of cigarette consumption, and history of alcohol abuse or dependence. Finally, we will examine the effects of active smoking cessation treatments on alcohol use.
2. Methods
2.1 Participants
The current project is a secondary analysis of the Wisconsin Smokers’ Health Study, a smoking cessation study that enrolled 1504 adult smokers (58% female, 83% white) from the greater Madison and Milwaukee, Wisconsin area [24]. Inclusion criteria included: smoking more than 9 cigarettes daily for the past 6 months, having an exhaled carbon monoxide (CO) level of at least 9 ppm, and being motivated to quit smoking. Exclusion criteria included: non-cigarette tobacco use, current bupropion use, ongoing psychotic or schizophrenic disorder, any medical contraindications for the pharmacotherapies, a high alcohol consumption rate (greater than 6 drinks daily on more than 6 days each week), a history of seizure or untreated hypertension or an eating disorder, a recent cardiac event, allergies to any of the cessation medications, and pregnancy or breastfeeding. In addition, women were required to take steps to prevent pregnancy during treatment.
Additional methodological details and the full CONSORT diagram are available in Piper et al. [24]. The study was approved by the University of Wisconsin Health Sciences Institutional Review Board.
2.2 Procedures
Participants were screened to determine eligibility, and attended an information session where written informed consent was obtained. Baseline visits were completed to gather vital signs and a carbon monoxide (CO) breath test, as well as demographics, smoking history, and tobacco dependence data (i.e., Fagerstrom Test of Nicotine Dependence [FTND]; [25]). Participants were randomized to treatment groups in a double-blind fashion, stratified by gender and self-reported race (white/nonwhite). Study staff were blinded to treatment assignment. Treatment groups comprised: (1) bupropion SR 150mg twice daily for 9 weeks (1 week pre-quit, 8 weeks post-quit), (2) nicotine lozenge (2mg or 4mg based on smoking within the first 30 minutes of waking) for 12 weeks post-quit, (3) nicotine patch 21mg/14mg/7mg 24-hour patches, titrated down over 8 weeks post-quit, (4) nicotine patch plus nicotine lozenge at doses and durations referenced above, (5) bupropion SR plus nicotine lozenge at doses and durations referenced above, and (6) placebo equivalents for all five active pharmacotherapy groups. Final analyses combined all active pharmacotherapies and compared them to the placebo group. In addition to the pharmacotherapies, all participants received six individual counseling sessions with bachelors-level, trained case managers supervised by a licensed clinical psychologist. Two sessions occurred prior to the quit date, and four sessions occurred post-quit.
Participants were prompted daily by a palmtop computer to record the number of cigarettes smoked and alcoholic drinks consumed during the two weeks prior to, and following, the target quit date (TQD) [26]. Alcohol consumption is reported as the mean number of drinks consumed per day in the pre-quit period, and the mean number of drinks consumed per day in the post-quit period. The primary outcome tested was change in alcohol consumption after the TQD. Use of means allowed flexibility for missing data. For example, if only 12 days pre-quit included alcohol consumption data, the average alcohol consumption for that time period was averaged over 12 days instead of 14. Participants were excluded from this analysis if they did not have any data for pre-quit or post-quit alcohol use, as the change in alcohol consumption could not be calculated (n = 174). They were also excluded if the mean amount of alcohol they reported consuming was more than three standard deviations above the average alcohol use recorded for all participants in the pre-quit period (n = 29). These “heavier drinkers” were excluded to specifically focus on non-problematic alcohol use to reflect changes that may be seen in the general population.
Abstinence status post-quit was assessed by self-report of continuous abstinence, confirmed by exhaled carbon monoxide (defined as CO < 10ppm), from Week 1 post-quit to Week 8. This endpoint was chosen in concordance with recommendations of an initial grace period and use of prolonged abstinence as put forth by the Society for Research on Nicotine & Tobacco workgroup [27].
2.3 Statistical Analysis
All analyses were completed using SAS/STAT software, Version 9.4 of the SAS System for Windows, SAS Institute Inc. (Cary, NC). A paired t-test was used to examine the change in average alcohol use from pre-quit to post-quit. A multivariate linear regression analysis was used to identify significant predictors of post-quit alcohol use with pre-quit alcohol use entered as a covariate. Based on the model selection strategy proposed by Hosmer and Lemeshow [28], each potential predictor was analyzed separately, and those reaching a significance level of p < 0.25 were retained for analysis in a combined multivariate model. Variables not meeting the significance level of p < 0.05 in the multivariate model were successively eliminated, and then interactions between retained variables were analyzed and omitted if they did not reach statistical significance defined as p < 0.05. As a final step, all omitted variables were added en bloc back into the model to ensure that they did not reach statistical significance. Potential predictors examined were: gender, race, history of alcohol abuse and dependence, use of smoking cessation pharmacotherapy, mean pre-quit cigarettes per day as recorded in the palm-top computers, FTND score, packyears of smoking, baseline cigarettes smoked per day, and age. Gender and race were chosen as predictor variables given the differences in alcohol consumption commonly seen in these demographic variables [29]. A history of alcohol abuse and dependence, as diagnosed by DSM-IV [30] was included to control for a past history of heavy and problematic drinking, if present. Amounts of cigarettes consumed and FTND score were used as predictor variables to control for level of nicotine dependence which may influence likelihood of substitution with alcohol use. Likewise, if participants were assigned to an inactive treatment group, they also may have been more likely to substitute with alcohol after their smoking cessation quit date. Age was the final predictor variable used, to account for higher rates of alcohol use in younger age groups [29]. We also included post-quit smoking status as a predictor to examine the relation between relapse and changes in drinking during the post-quit period. After the final model was ascertained, effect sizes were computed as omega-squared [31].
3. Theory
In addition to the pragmatic issues of improved cessation success and general health through reduced drinking discussed above, it is important to consider the theoretical implication of comorbid alcohol and tobacco abuse. Current theories of addiction physiology hold that dopamine deficiency is a driving factor of both alcohol and tobacco withdrawal symptoms (both somatic and affective; [32]). Nicotine increases functional dopamine levels in the brain through its acute effect of activating nicotinic acetylcholine receptors, which produces a surge in dopamine, and through its chronic effect of desensitizing the gamma-aminobutyric acid (GABA) receptors, which causes a decreased inhibitory tone [33–35]. Likewise, alcohol acts to increase dopaminergic tone in the brain by reducing GABA inhibition. Alcohol also acts as a co-agonist to nicotinic acetylcholine receptors to potentiate acetylcholine’s activating effects [36, 37]. Based on the idea that during withdrawal from smoking participants will experience a deficiency in dopaminergic tone, we hypothesize that they will engage in alternate drug use behavior (i.e., drinking) to alleviate that decrease in dopamine.
4. Results
Of the 1504 participants enrolled in the Piper et al. study [24], 1301 participants met criteria for this secondary analysis (see Table 1 for sample characteristics). Overall, 34.7% of the participants were able to maintain continuous smoking abstinence from Week 1 post-quit through Week 8 (38% of males and 32.4% of females; t(1299) = 2.11, p = 0.04). Relapsed participants had more missing alcohol data than abstinent participants (43.6% versus 26.5%, respectively).
Table 1.
Demographics and smoking history (N = 1301).
| Variable | n | Percent |
|---|---|---|
| Women | 765 | 58.8% |
| Married | 589 | 45.5% |
| Employed for wages | 885 | 68% |
| High school education only | 309 | 23.9% |
| Race/ethnicity | ||
| White | 1094 | 84.3% |
| Non-White | 204 | 15.7% |
| Missing | 3 | 0.2% |
| Continuous abstinence from Weeks 1–8 | 452 | 34.7% |
| History of alcohol abuse | 614 | 48.27% |
| History of alcohol dependence | 122 | 9.59% |
| Treatment group | ||
| Placebo | 149 | 11.5% |
| Bupropion | 234 | 18% |
| Lozenge | 218 | 16.8% |
| Patch | 230 | 17.7% |
| Bupropion + Lozenge | 229 | 17.6% |
| Patch + Lozenge | 241 | 18.5% |
| Mean | Standard Deviation | |
| Age (years) | 45.1 | 11 |
| Previous quit attempts | 5.8 | 9.6 |
| FTND total score | 5.4 | 2.1 |
| Pre-quit cigarettes/day | 18.44 | 8.66 |
| Post-quit cigarettes/day | 1.79 | 4.29 |
| Baseline cigarettes smoked/day | 21.3 | 8.9 |
| Packyears smoked | 29.53 | 20.15 |
4.1 Change in Average Daily Alcohol Use
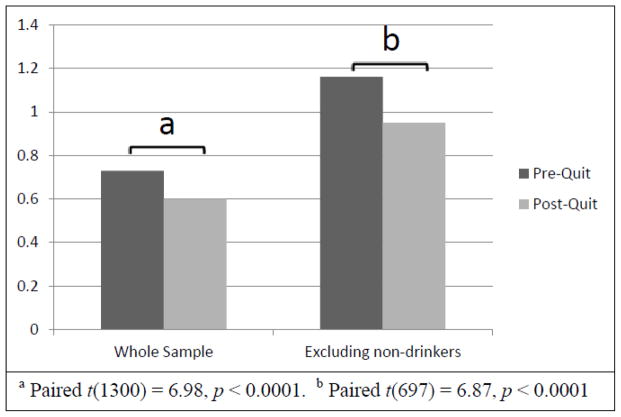
The mean pre-quit alcohol use was 0.73 drinks per day (SD = 0.93, range 0 – 4.29). Excluding the 482 (37%) participants who reported no drinking in the pre-quit period, the average pre-quit alcohol use was 1.16 drinks per day (SD = 0.94, range 0.07 – 4.29). Of those who reported drinking in the pre-quit period, women drank significantly fewer drinks per day than men (M = 1.00 [SD = 0.82] versus 1.35 [SD = 1.04]; t(817) = 5.42, p < 0.001) in the pre-quit period. For the whole sample, the average post-quit alcohol use was 0.6 drinks per day (SD = 0.88, range 0 – 6.6). Excluding the 493 (38%) participants who reported no drinking in the post-quit period, the post-quit average alcohol use was 0.95 drinks per day (SD = 0.94, range 0.07 – 6.6), with females drinking significantly fewer drinks per day than men (M = 0.75 [SD = 0.8] versus M = 1.22 [SD = 1.04]; t(806) = 7.24, p < 0.001). As shown in Figure 1, the decrease in alcohol use during the two weeks post-quit was significant for the sample as a whole, and when non-drinkers were excluded from the analyses. The significant decrease in alcohol use was still observed in the post-quit period when the 29 “heavier drinkers” were included in the sample.
Figure 1.
Change in Alcohol Use after Target Quit Date.
4.2 Prediction Model
To identify variables that predict the amount of alcohol used in the post-quit period, we used post-quit mean drinks per day as the dependent variable, adjusted for pre-quit drinks per day (i.e., pre-quit alcohol use was entered as a covariate in the models). The final model had an R2 = 0.51, accounting for half of the variance in post-quit drinking. The final model predictors included pre-quit alcohol use, gender, race, a history of ever being diagnosed with alcohol dependence, gender by pre-quit alcohol use interaction, and a history of alcohol dependence by pre-quit alcohol use interaction (see Table 2). Specifically, the main effects indicated that higher pre-quit alcohol use, male gender, white race, and not having a history of alcohol dependence were related to higher amounts of post-quit alcohol use. The interaction between gender and pre-quit alcohol use showed pre-quit and post-quit alcohol use were more strongly related for men (r = 0.72, p < 0.0001) than they were for women (r = 0.67, p < 0.0001). Similarly, participants with no history of alcohol dependence had a stronger relation between pre-quit and post-quit alcohol use (r = 0.71, p < 0.0001) compared to those who did carry such a diagnosis (r = 0.62, p < 0.0001). Due to the non-normal distribution of pre- and post-quit alcohol use, the final linear regression model was also tested using a Poisson regression analysis. Results were similar between the two models, except that the interaction between pre-quit alcohol use and a history of alcohol dependence was not statistically significant in the Poisson regression model. When the 29 “heavier drinkers” were included in the sample, the prediction model did not change significantly.
Table 2.
Final Prediction Model
| Post-quit alcohol use = β0 + β1(pre-quit alcohol use) + β2(gender) + β3(alcohol dependence) + β4(race) + β5(gender)(pre-quit alcohol use) + β6(alcohol abuse)(pre-quit alcohol use) | ||||
|---|---|---|---|---|
| Variable | β estimate | F-value | p-value | ω2 |
| Pre-quit alcohol use | 0.704 | 693.97 | < 0.0001 | 0.4997 |
| Gender (Male = 0, Female = 1) | −0.087 | 3.64 | 0.058 | 0.0135 |
| History of Alcohol Dependence (no dependence = 0, dependence = 1) | −0.02 | 0.09 | 0.771 | 0.004 |
| Race (Non-White = 0, White = 1) | 0.098 | 4.22 | 0.04 | 0.0022 |
| Gender x Pre-quit Alcohol Use Interaction | −0.096 | 6.43 | 0.011 | 0.0031 |
| Pre-quit Alcohol Use x History of Alcohol Dependence Interaction | −0.229 | 11.42 | 0.007 | 0.0081 |
5. Discussion
The primary goal of this research was to describe the changes in alcohol use following a smoking cessation attempt in a contemporary sample, representative of the general smoking cessation population without problematic alcohol use, using data collected in real-time. Overall, participants significantly decreased their alcohol use post-quit. These findings do not support the study’s main hypothesis that smoking cessation would result in increased alcohol consumption during the immediate post-quit period. The net decrease in alcohol use among those trying to quit smoking could reflect participants’ motivations to make healthier lifestyle changes. All participants were assumed to be highly motivated to quit, given their participation in a three-year research study and their self-reported desire to quit smoking. The decreased drinking could also reflect the counseling provided to participants that focused on reducing alcohol use, especially during the first few weeks of the cessation attempt, as a means of preventing relapse to smoking [38]. Further, counseling focused on helping participants develop skills for coping with negative affect and cravings, symptoms of nicotine withdrawal that have been shown to influence cessation success [39]. It may be that dopamine deficiency plays a role in the underlying neurobiology of such symptoms [40–42]. However, if the counseling provided to participants in this cessation study was able to bolster participants’ abilities to cope with withdrawal symptoms, perhaps this overcame the temptation to substitute for the lost dopamine by drinking more.
The secondary goal of this analysis was to identify individual characteristics that predict drinking behavior following smoking cessation. The prediction model allowed for an in-depth assessment of the factors related to post-quit alcohol use. These findings showed that while pre-quit alcohol use was the best determinant of post-quit use, this relation was especially strong for men and those with no history of alcohol dependence. In fact, almost all of the 51% of the variance explained by the prediction model was accounted for by pre-quit alcohol use. Being white and male also predicted higher levels of post-quit drinking, but the effect sizes were much smaller. It is interesting that factors related to dependence severity such as the FTND score and cigarettes smoked did not enter into the final model, nor did use of active smoking cessation medications.
Post-quit smoking did not enter into the model. An examination of relapsed participants’ cigarette use after their TQD revealed that the average number of cigarettes smoked per day fell from 18.5 during the two weeks pre-quit to 2.8 during the 2 weeks post-quit – a decrease of 85%. This marked decrease in cigarettes smoked per day may have diminished the difference between alcohol drinking rates between abstinent and relapsed, as, on average, all smokers were deprived of their usual levels of nicotine.
According to the prediction model, factors that were predictive of higher post-quit rates of alcohol use included higher pre-quit alcohol use, being male (especially at higher levels of pre-quit alcohol use), being white, and not having a previous diagnosis of alcohol dependence (again, especially at higher levels of pre-quit alcohol use). Women typically drink less than men [43], so this finding is consistent with current research. It is interesting to note that pre-quit alcohol use is less predictive of post-quit alcohol use among those with a history of alcohol dependence. While the validity of the interaction with pre-quit alcohol use needs to be tempered given that it was not significant in the Poisson regression model, it might suggest that those with and without a history of alcohol dependence have a different approach to drinking when trying to quit smoking. It may be that those with a history of alcohol dependence who have been able to return to controlled drinking are using the strategies they learned to control their drinking while they are trying to quit whereas those without such a history are continuing to drink at similar, if somewhat reduced levels. In this sample, it does appear that those participants with a history of alcohol dependence reduced their post-quit alcohol use more, compared to those without this history (M = 0.32 [SD = 0.63] versus M = 0.62 [SD = 0.9]; t(1271) = 3.66, p < 0.001). The fact that people with a history of alcohol dependence drank less alcohol post-quit is consistent with the body of evidence that tobacco cessation treatment for patients in alcohol treatment programs does not undermine their alcohol cessation efforts [44, 45] and supports the findings of Lisha et al. [23].
While this study has a number of strengths, including a large sample size representative of the general population of smokers seeking cessation treatment and real-time data assessment of both smoking and drinking behavior, there are limitations. If participants had a smoking relapse while drinking during prior quit attempts, study counselors spent time reviewing that relapse and developing alternate coping plans, but no additional visits or counseling time were given to these participants. This focused counseling could reflect strategies that go above and beyond what would be found in general practice. Interestingly, when participants with a history of alcohol dependence (n = 150), who may have had focused counseling regarding risks for relapsing, were excluded from the analysis the results of this study were largely unchanged. The only exception was the race variable in the Poisson regression analysis that was statistically significant (p = 0.044) in the original analysis; this variable was no longer significant (p = 0.084) after excluding the participants with a history of alcohol dependence. This would suggest that the focused counseling did not influence drinking behaviors in a significant manner. Finally, we only examined drinking behavior for the first two weeks post-quit. While the majority of lapses occur in the first two weeks [46] it may be that different drinking patterns could emerge later in the quitting process. Further research using real-time data collection could be undertaken to determine if drinking patterns change after the immediate post-tobacco cessation period.
6. Conclusion
This research provides detailed information about changes in alcohol use when people quit smoking. Overall, smokers trying to quit smoking decreased their alcohol use. The most predictive factor of higher amounts of post-quit alcohol use was higher amounts of pre-quit alcohol use, and this was especially true for men and those with no history of alcohol dependence. Male gender, white race, and not having a history of alcohol dependence all predicted higher levels of post-quit alcohol use. These findings suggest that clinicians should not expect increased use of alcohol with tobacco cessation attempts. However, particular attention and counseling on the risks of post-quit alcohol use may be warranted in those who already consume higher amounts of alcohol, particularly males, as this population appeared the most likely to continue their higher levels of alcohol use post-quit.
HIGHLIGHTS.
Alcohol use decreased in the two weeks following tobacco cessation.
Post-quit alcohol use was positively correlated with pre-quit alcohol use.
Women and those with a history of alcohol dependence drank less post-quit.
Participants who self-identified as non-white drank less post-quit.
Acknowledgments
Role of Funding Source
This research was supported by the Training Tobacco Scientists Mini-Grant through the University of Wisconsin Center for Tobacco Research and Intervention, which is funded from grant 9P50CA143188-11 from the National Cancer Institute. The funding source did not have impact on the planning, design, analysis or interpretation of this secondary analysis.
Footnotes
Contributors
All authors contributed to the design of this secondary analysis. Authors MP, DJ, SS, and MF contributed to the initial Wisconsin Smoker’s Health Study. Author KB conducted the statistical analysis with assistance provided by SS and all authors contributed to and approved the final version of this manuscript.
Conflict of Interest
Author DJ has received research support from Nabi Biopharmaceuticals and Pfizer. Authors KB, MP, MF report no current or recent (five year) potential conflicts. Author SS has received research support as a co-investigator on an investigator-initiated research project funded by Pfizer, Inc. in the past five years.
This research has been presented in earlier forms as a poster at the Society of General Internal Medicine Annual Meeting in May 2012, and at the University of Wisconsin, Department of Medicine Research Day in June 2011.
The contents of this study do not represent the views of the Department of Veterans Affairs or the United States Government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Megan E. Piper, Email: mep@ctri.wisc.edu.
Stevens S. Smith, Email: Sss@ctri.wisc.edu.
Michael C. Fiore, Email: mcf@ctri.wisc.edu.
Douglas E. Jorenby, Email: dej@ctri.wisc.edu.
References
- 1.U.S. Department of Health and Human Services. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Vol. 2014. Atlanta GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center fo rChronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. Printed with corrections, January 2014. [Google Scholar]
- 2.Centers for Disease Control and Prevention. [Accessed August 2013];Adult Cigarette Smoking in the United States: Current Estimate. 2014 Available at: http://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm.
- 3.Grant BF, et al. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61(11):1107–15. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. [Accessed August 2013];Alcohol and Public Health: Alcohol-Related Disease Impact (ARDI) 2010 Available at: http://apps.nccd.cdc.gov/DACH_ARDI/default/default.aspx.
- 5.Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 2013;61(4):1–117. [PubMed] [Google Scholar]
- 6.Saunders JB, Lee NK. Hazardous alcohol use: its delineation as a subthreshold disorder, and approaches to its diagnosis and management. Compr Psychiatry. 2000;41(2 Suppl 1):95–103. doi: 10.1016/s0010-440x(00)80015-2. [DOI] [PubMed] [Google Scholar]
- 7.Cook JW, et al. Relations of alcohol consumption with smoking cessation milestones and tobacco dependence. J Consult Clin Psychol. 2012;80(6):1075–85. doi: 10.1037/a0029931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinberger AH, et al. Changes in smoking for adults with and without alcohol and drug use disorders: longitudinal evaluation in the US population. Am J Drug Alcohol Abuse. 2013;39(3):186–93. doi: 10.3109/00952990.2013.785557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carmelli D, Swan GE, Robinette D. The relationship between quitting smoking and changes in drinking in World War II veteran twins. J Subst Abuse. 1993;5(2):103–16. doi: 10.1016/0899-3289(93)90055-g. [DOI] [PubMed] [Google Scholar]
- 10.Gaudet FJ, Hugli WC., Jr Concomitant habit changes associated with changes in smoking habits: a pilot study. Med Times. 1969;97(4):195–205. [PubMed] [Google Scholar]
- 11.Perkins KA, et al. Effects of smoking cessation on consumption of alcohol and sweet, high-fat foods. J Subst Abuse. 1990;2(3):287–97. doi: 10.1016/s0899-3289(10)80002-1. [DOI] [PubMed] [Google Scholar]
- 12.Dawson DA, Goldstein RB, Grant BF. Prospective correlates of drinking cessation: variation across the life-course. Addiction. 2013;108(4):712–22. doi: 10.1111/add.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289–94. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 14.Karlamangla A, et al. Longitudinal trajectories of heavy drinking in adults in the United States of America. Addiction. 2006;101(1):91–9. doi: 10.1111/j.1360-0443.2005.01299.x. [DOI] [PubMed] [Google Scholar]
- 15.Puddey IB, et al. Haemodynamic and neuroendocrine consequences of stopping smoking--a controlled study. Clin Exp Pharmacol Physiol. 1984;11(4):423–6. doi: 10.1111/j.1440-1681.1984.tb00292.x. [DOI] [PubMed] [Google Scholar]
- 16.Stamford BA, et al. Effects of smoking cessation on weight gain, metabolic rate, caloric consumption, and blood lipids. Am J Clin Nutr. 1986;43(4):486–94. doi: 10.1093/ajcn/43.4.486. [DOI] [PubMed] [Google Scholar]
- 17.Cooney JL, et al. Effects of nicotine deprivation on urges to drink and smoke in alcoholic smokers. Addiction. 2003;98(7):913–21. doi: 10.1046/j.1360-0443.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 18.Kahler CW, et al. Quitting smoking and change in alcohol consumption in the International Tobacco Control (ITC) Four Country Survey. Drug Alcohol Depend. 2010;110(1–2):101–7. doi: 10.1016/j.drugalcdep.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray RP, et al. Longitudinal analysis of the relationship between changes in smoking and changes in drinking in a community sample: the Winnipeg Health and Drinking Survey. Health Psychol. 2002;21(3):237–43. [PubMed] [Google Scholar]
- 20.Murray RP, Istvan JA, Voelker HT. Does cessation of smoking cause a change in alcohol consumption? Evidence from the Lung Health Study. Subst Use Misuse. 1996;31(2):141–56. doi: 10.3109/10826089609045804. [DOI] [PubMed] [Google Scholar]
- 21.Nothwehr F, Lando HA, Bobo JK. Alcohol and tobacco use in the Minnesota Heart Health Program. Addict Behav. 1995;20(4):463–70. doi: 10.1016/0306-4603(95)00008-z. [DOI] [PubMed] [Google Scholar]
- 22.Tang J, et al. Health profiles of current and former smokers and lifelong abstainers. OXCHECK Study Group. OXford and Collaborators HEalth ChecK. J R Coll Physicians Lond. 1997;31(3):304–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Lisha NE, et al. Reciprocal effects of alcohol and nicotine in smoking cessation treatment studies. Addict Behav. 2014;39(3):637–43. doi: 10.1016/j.addbeh.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piper ME, et al. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Arch Gen Psychiatry. 2009;66(11):1253–62. doi: 10.1001/archgenpsychiatry.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heatherton TF, et al. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 26.Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- 27.Hughes JR, et al. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5(1):13–25. [PubMed] [Google Scholar]
- 28.Hosmer DW, LS, Sturdivant RX. Applied Logistic Regression. 3. 2013. [Google Scholar]
- 29.Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: National Health Interview Survey, 2012. National Center for Health Statistics. Vital Health Stat. 2014;10(260) [PubMed] [Google Scholar]
- 30.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: 2000. text rev. [Google Scholar]
- 31.SAS Institute Inc. SAS/STAT 9.3 User’s Guide. SAS Institute Inc; Cary, NC: Effect size measurement for F tests in GLM (experimental) pp. 3223–3228. [Google Scholar]
- 32.Kenny PJ, Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav. 2001;70(4):531–49. doi: 10.1016/s0091-3057(01)00651-7. [DOI] [PubMed] [Google Scholar]
- 33.Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat Neurosci. 2005;8(11):1465–70. doi: 10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- 34.D’Souza MS, Markou A. Neuronal mechanisms underlying development of nicotine dependence: implications for novel smoking-cessation treatments. Addict Sci Clin Pract. 2011;6(1):4–16. [PMC free article] [PubMed] [Google Scholar]
- 35.Picciotto MR, et al. It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84(4):329–42. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soderpalm B, Ericson M. Neurocircuitry involved in the development of alcohol addiction: the dopamine system and its access points. Curr Top Behav Neurosci. 2013;13:127–61. doi: 10.1007/7854_2011_170. [DOI] [PubMed] [Google Scholar]
- 37.Soderpalm B, Lof E, Ericson M. Mechanistic studies of ethanol’s interaction with the mesolimbic dopamine reward system. Pharmacopsychiatry. 2009;42(Suppl 1):S87–94. doi: 10.1055/s-0029-1220690. [DOI] [PubMed] [Google Scholar]
- 38.Fiore MC, Jaen CR, Baker TB, et al. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; May, 2008. Treating Tobacco Use and Dependence: 2008 Update. [Google Scholar]
- 39.Schlam TR, Baker TB. Interventions for tobacco smoking. Annu Rev Clin Psychol. 2013;9:675–702. doi: 10.1146/annurev-clinpsy-050212-185602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baker TB, et al. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- 41.Brown RA, et al. Distress tolerance and duration of past smoking cessation attempts. J Abnorm Psychol. 2002;111(1):180–5. [PubMed] [Google Scholar]
- 42.Brown RA, et al. Distress tolerance and early smoking lapse. Clin Psychol Rev. 2005;25(6):713–33. doi: 10.1016/j.cpr.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schoenborn CA, Adams PF, Peregoy JA. Health behaviors of adults: United States, 2008–2010. Vital Health Stat. 2013;10(257):1–184. [PubMed] [Google Scholar]
- 44.Cooney NL, et al. Concurrent brief versus intensive smoking intervention during alcohol dependence treatment. Psychol Addict Behav. 2007;21(4):570–5. doi: 10.1037/0893-164X.21.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richter KP, Arnsten JH. A rationale and model for addressing tobacco dependence in substance abuse treatment. Subst Abuse Treat Prev Policy. 2006;1:23. doi: 10.1186/1747-597X-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kenford SL, et al. Predicting smoking cessation. Who will quit with and without the nicotine patch. JAMA. 1994;271(8):589–94. doi: 10.1001/jama.271.8.589. [DOI] [PubMed] [Google Scholar]