Abstract
Background
Staphylococcus aureus is the most commonly isolated organism in periprosthetic joint infection (PJI). Resistant strains such as methicillin-resistant S aureus (MRSA) are on the rise, and many programs have instituted decolonization protocols. There are limited data on the success of S aureus nasal decolonization programs and their impact on PJI.
Questions/purposes
The purposes of this study were to (1) determine the proportion of patients successfully decolonized using a 2-week protocol; (2) compare infection risks between our surveillance and decolonization protocol group against a historical control cohort to evaluate changes in proportions of S aureus infections; and (3) assess infection risk based on carrier type, comparing S aureus carriers with noncarrier controls.
Methods
We retrospectively evaluated a group of 3434 patients who underwent elective primary and revision hip and knee arthroplasty over a 2-year period; each patient in the treatment group underwent a surveillance protocol, and a therapeutic regimen of mupurocin and chlorhexidine was instituted when colonization criteria were met. A 2009 to 2010 comparative historical cohort was chosen as the control group. We compared risks of infection between our treatment group and the historical control cohort. Furthermore, in patients who developed surgical site infections (SSIs), we compared the proportions of each S aureus type between the two cohorts. Finally, we compared infection rates based on carrier status. Surveillance for infection was carried out by the hospital infection control coordinator using the Centers for Disease Control and Prevention (CDC) criteria. During the time period of this study, the CDC defined hospital-acquired infection related to a surgical procedure as any infection diagnosed within 1 year of the procedure. With the numbers available, we had 41% power to detect a difference of 0.3% in infection rate between the treatment and control groups. To achieve 80% power, a total of 72,033 patients would be needed.
Results
Despite the protocol, 22% (26 of 121) of patients remained colonized with MRSA. With the numbers available, there were no differences in infection risk between the protocoled group (27 of 3434 [0.8%]) and the historical control group (33 of 3080 [1.1%]; relative risk [RR], 0.74; 95% confidence interval [CI], 0.44–1.22; p = 0.28). In terms of infecting organism in those who developed SSI, S aureus risk decreased slightly (treatment: 13 of 3434 patients [0.38%]; control: 21 of 3080 patients [0.68%]; RR, 0.56; CI, 0.28–1.11; p = 0.11). Within the protocoled group, carriers had a slightly higher risk of developing SSI (carrier: seven of 644 [1.1%]; noncarrier: 18 of 2763 [0.65%]; RR, 1.77; CI, 0.74–4.24; p = 0.20).
Conclusions
The screening and decolonization protocol enabled a substantial reduction in nasal carriage of MRSA, but some patients remained colonized. However, our nasal decolonization protocol before elective total joint arthroplasty did not demonstrate a decrease in the proportion of patients developing SSI. Future meta-analyses and systematic reviews will be needed to pool the results of studies like these to ascertain whether small improvements in infection risk are achieved by protocols like ours and to determine whether any such improvements warrant the costs and potential risks of surveillance and intervention.
Level of Evidence
Level III, therapeutic study.
Introduction
Periprosthetic joint infection (PJI) after total joint arthroplasty (TJA) is reported in 1% to 2% of patients who have undergone primary TJA and 4% to 6% of patients who have undergone revision TJA, and PJI increases both morbidity and economic costs [2, 11, 32]. The most commonly isolated microorganism in PJI after TJA is Staphylococcus aureus [22, 29]. Resistant strains such as methicillin-resistant S aureus (MRSA) are on the rise [21] and are associated with higher rates of treatment failure [3, 23]. The ecologic niche of S aureus is the nasal passages [30], and the association between nasal carriage of S aureus and surgical site infection (SSI) was established in 1959 [33]. Carriers of S aureus are two to nine times more likely to develop an SSI, and it has been shown that 85% of SSIs can be traced to endogenous colonization of the patients [6, 18, 25, 31, 34]. In 1999, the Centers for Disease Control and Prevention (CDC) recognized nasal colonization of S aureus as a risk factor for SSI [20]. As a result, there has been much focus on S aureus decolonization as a means to reduce the rate of SSI.
Intranasal mupirocin ointment and chlorhexidine soap are established treatments for the decolonization of S aureus [4, 7, 13, 15, 25, 26]. Several studies have been conducted to assess the effectiveness of a decolonization program using intranasal mupirocin ointment, chlorhexidine body washes, or both [1, 9, 10, 16, 19, 27]. Although many studies have assessed the impact of a decolonization protocol on SSI, very few have evaluated the effectiveness and outcomes of the decolonization process itself.
The purposes of our study were to (1) determine the proportion of patients successfully decolonized using a 2-week protocol; (2) compare infection risks between our surveillance and decolonization protocol groups against a historical control cohort to evaluate changes in proportions of S aureus infections; and (3) assess infection risk based on carrier type, comparing S aureus carriers with noncarrier controls.
Patients and Methods
Our retrospective clinical study received approval from our institutional review board. Our cohort included a series of all patients undergoing primary or revision THA or TKA over a 2-year period (January 2012 through December 2013) at a single institution. Patients were excluded if they had a history of infection at the operative site. Our treatment group included 3434 patients who underwent primary or aseptic revision TKA or THA performed by 18 surgeons (Table 1).
Table 1.
Distribution of patient cohort with percentage of patients screening positive for MSSA and MRSA
| Procedure | Number of patients | MSSA colonized | MRSA colonized |
|---|---|---|---|
| TKA | 1824 (53%) | 268 (15%) | 63 (4%) |
| Bilateral TKA | 31 (1%) | 6 (19%) | 2 (6%) |
| UKA | 19 (1%) | 3 (16%) | 0 (0%) |
| Revision TKA | 245 (7%) | 36 (15%) | 22 (9%) |
| THA | 1018 (30%) | 159 (16%) | 53 (5%) |
| Bilateral THA | 11 (0.3%) | 3 (27%) | 0 (0%) |
| Revision THA | 286 (8%) | 33 (12%) | 18 (6%) |
| Total | 3434 (100%) | 508 (15%) | 158 (5%) |
MSSA = methicillin-sensitive Staphylococcus aureus; MRSA = methicillin-resistant S aureus; UKA = unilateral knee arthroplasty.
S aureus Screening Protocol
At the time of the routine preoperative assessment (typically 2 weeks before the intended surgical date), all patients were screened for nasal colonization with methicillin-sensitive Staphylococcus aureus (MSSA) and MRSA. Microbiologic samples were obtained by trained nurses in the preoperative area using a nasal swab on the inside of the nares for 5 seconds in each naris. Samples were sent for rapid polymerase chain reaction (PCR) using GeneXpert® XVI (Cepheid, Sunnyvale, CA, USA) for the detection of MRSA. Standard culture was used for the detection of MSSA. Patients determined to be carriers of either MSSA or MRSA were provided treatment with intranasal 2% mupirocin ointment (Bactroban; GlaxoSmithKline, Middlesex, UK) twice daily for 5 days and daily skin cleansing with 4% chlorhexidine soap (Dyna-Hex 4; Xttrium Laboratories, Chicago, IL, USA) for 5 days, including the day of surgery. Patients who were colonized received a phone call from a preoperative nurse and were provided with instructions on the treatment protocol and literature supporting the use of both products. Patients colonized with MRSA at the initial preoperative visit were rescreened on the day of surgery using the identical screening protocol for MRSA. The results of the day-of-surgery rapid PCR were made available before the start of the procedure.
Antibiotic Prophylaxis
Standard perioperative antibiotic prophylaxis at our institution is achieved with an intraoperative dose of a first-generation cephalosporin (cefazolin) followed by two additional doses postoperatively at 8-hour intervals. In the presence of a well-documented ß-lactam allergy, patients are treated with an intraoperative dose of vancomycin and one additional dose 12 hours postoperatively. Patients colonized with MRSA at either the 2-week preoperative screening visit or on the day-of-surgery screening received a single intraoperative dose of vancomycin in addition to the standard protocol of cefazolin. Patients who remained colonized with MRSA on the day of surgery were placed on isolation precautions during their hospitalization.
Patients were monitored prospectively for SSI by a hospital-employed nurse responsible for quality control and infection prevention. We defined SSI in accordance with the CDC/National Healthcare Safety Network guidelines [12]. Our study treatment protocol went into effect in November 2011. Data analysis for the treatment group began in January 2012, because we felt that the first 2 months would be part of a “learning curve.” We elected to use a patient group from a 2-year period before implementation of our screening and decolonization protocol (January 2009 through December 2010) as a historical control group.
Statistical Analysis
Standard descriptive statistics were reported, including frequencies and proportions. We compared risks of infection between our treatment group and the historical control cohort, stratifying the results in terms of primary and revision cases. Furthermore, in patients who developed SSI, we compared the proportions of each S aureus type (MSSA and MRSA) between the two cohorts. The differences in proportions were assessed using a chi-square test or Fisher’s exact test. Finally, we compared infection rates based on carrier status, calculating relative risk (RR) and 95% confidence intervals (CIs) of infection based on patient status of being a noncarrier, an MRSA carrier, or an MSSA carrier. An a priori significance level of 0.05 was used for statistical tests.
Results
Our study population consisted of 2903 primary cases and 531 revision cases for a total of 3434 patients. Overall, 15% (486 of 3434) and 5% (158 of 3434) of our patient population screened positive for MSSA and MRSA, respectively. The control cohort consisted of 3080 patients from 2009 to 2010, which included 2515 primary cases and 567 revision cases (Table 2).
Table 2.
Comparison of infection rate between treatment group and historical controls
| Procedure | 2009–2010 | 2012–2013 | Relative risk (95% confidence interval) | p value |
|---|---|---|---|---|
| Primary cases | 2513 | 2903 | 0.77 (0.40–1.49) | 0.51 |
| Primary infections | 19 (1%) | 17 (1%) | ||
| Revision cases | 567 | 531 | 0.76 (0.34–1.7) | 0.65 |
| Revision infections | 14 (3%) | 10 (2%) | ||
| All cases | 3080 | 3434 | 0.74 (0.44–1.22) | 0.28 |
| All infections | 33 (1%) | 27 (1%) |
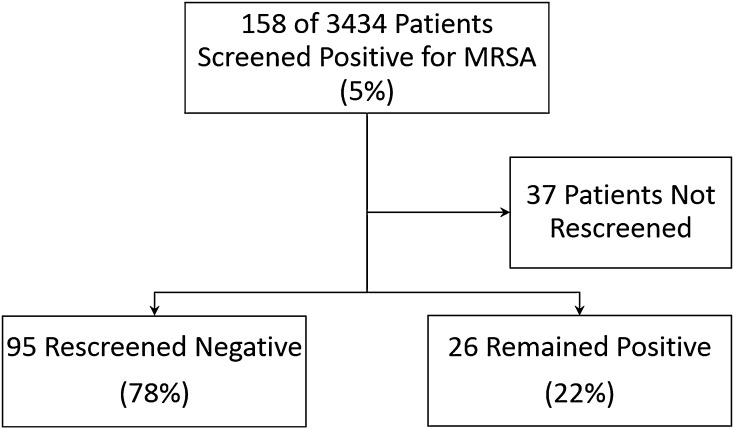
Of the 158 patients who initially tested positive for MRSA, 37 patients were unintentionally not rescreened on the date of surgery and thus were not included in the MRSA eradication analysis.
Of the 121 patients who screened positive for MRSA, 95 (79%) were MRSA-negative after the decolonization protocol and 26 remained MRSA-positive (failed to decolonize; 22%) when rescreened on the day of surgery (Fig. 1).
Fig. 1.
Of the 121 patients who screened positive for MRSA, 95 (78%) were MRSA-negative after the decolonization protocol and 26 remained MRSA-positive (failed to decolonize; 22%) when rescreened on the day of surgery.
With the numbers available, we found no statistically significant differences between the rate of SSI between our 2012 to 2013 treatment group (including all noncolonized patients and colonized patients undergoing the protocol) and the 2009 to 2010 control group; this nondifferent finding persisted with stratification of patients based on primary and revision cases. The rate of SSI in our treatment group (2012–2013) was 1% (27 of 3434 patients) for all patients; 1% (17 of 2903 patients) for primary TJA; and 2% (10 of 531 patients) for revision TJA. In comparison, the rate of SSI in our historical control group (2009–2010) was 1% (33 of 3080 patients) overall, 1% (19 of 2513 patients) for primary TJA, and 3% (14 of 567 patients) for revision TJA. Compared with our historical controls, the RR of SSI was 0.47 (95% CI, 0.44–1.22] overall and 0.77 (0.40–1.49) and 0.76 (0.34–1.70) for primary and revision TJA, respectively (Table 2).
After instituting a decolonization protocol, the relative proportions of S aureus infection (MSSA and MRSA) likewise were not statistically different between our treatment group and the historical control cohort (Table 3). Overall, the proportion of S aureus infection in the historical control group was 0.68% (21 of 3080 patients) compared with 0.38% in the study group (13 of 3434 patients; RR, 0.56; 95% CI, 0.28–1.11; p = 0.11). When subgroup analysis was performed, the proportion of MSSA infection was 0.2% in the historical control group (six of 3080 patients), compared with 0.15% in the study group (five of 3434 patients; RR, 0.75; 95% CI, 0.23–2.45; p = 0.66), and the proportion of MRSA infection was 0.49% in the historical control group (15 of 3080 patients) compared with 0.23% in the study group (eight of 3434 patients; RR, 0.48; 95% CI, 0.20–1.13; p = 0.10).
Table 3.
Comparison of infections by organism
| Organism | 2009–2010 (n = 3080) |
2012–2013 (n = 3434) |
Relative risk | p value |
|---|---|---|---|---|
| MSSA | 6 (0.20%) | 5 (0.15%) | 0.75 (0.23–2.45) | 0.66 |
| MRSA | 15 (0.49%) | 8 (0.23%) | 0.48 (0.20–1.13) | 0.10 |
| Total S aureus | 21 (0.68%) | 13 (0.38%) | 0.56 (0.28–1.11) | 0.11 |
| Coagulase-negative Staphylococcus | 2 (0.06%) | 5 (0.15%) | 2.24 (0.44–11.55) | 0.46 |
| Streptococcus species | 3 (0.10%) | 1 (0.03%) | 0.30 (0.03–2.87) | 0.35 |
| Polymicrobial | 2 (0.06%) | 4 (0.12%) | 1.79 (0.33–9.79) | 0.69 |
| Pseudomonas | 1 (0.03%) | 1 (0.03%) | 0.90 (0.06–14.33) | NA† |
| Enterobacter | 2 (0.06%) | 0 (0.00%) | NA† | NA† |
| Other* | 1 (0.03%) | 4 (0.12%) | 3.59 (0.40–32.14) | 0.38 |
* One each of Citrobacter, Klebsiella, Proteus, Serratia, Staphylococcus lugdunesis; †NA = frequency of events too small; MSSA = methicillin-sensitive Staphylococcus aureus; MRSA = methicillin-resistant S aureus.
Differences in infection risk were not detected with the numbers available comparing carriers of S aureus with noncarriers (carriers = seven of 644 patients [1.1%]; noncarriers = 18 of 2763 patients [0.65%]; RR, 1.77; 95% CI, 0.74–4.24; p = 0.36; Table 4). However, when subgroup analysis was performed, stratifying for carrier type, MRSA carriers were more likely to develop SSI than MSSA carriers (four of 158 patients (2.5%) versus two of 486 patients (0.4%), respectively; RR, 6.15; 95% CI, 1.14–33.65; p = 0.03). MRSA carriers were also more likely to develop SSI than noncarriers (four of 158 patients [2.5%] versus 17 of 2763 patients [0.6%], respectively; RR, 4.11; 95% CI, 1.40–12.08; p = 0.002). All identified infections (27 of 3434 study cohort patients and 33 of 3080 historical cohort patients) required surgical intervention (Table 5). Of the 33 infected cases in the historical control, there were three superficial infections treated with limited irrigation and débridement. The remaining infections were deep infections treated with deep irrigation and débridement without modular component exchange (six cases), deep irrigation and débridement with modular component exchange (17 cases), and removal of the prosthesis and placement of an antibiotic spacer (six cases). One infected case during the historical control period was treated at an outside institution, the details of which were unavailable. Of the 27 infected cases in the study cohort, there was one superficial infection treated with limited irrigation and débridement. The remaining infections were deep infections treated with deep irrigation and débridement without modular component exchange (four cases), deep irrigation and débridement with modular component exchange (12 cases), resection arthroplasty (one case), and removal of the prosthesis and placement of an antibiotic spacer (eight cases). One infected case during the study period was treated at an outside institution, the details of which were unavailable.
Table 4.
Comparison of infection rate by carrier type (stratified)
| Group | Number of patients | Infections | Relative risk (95% CI) |
p value*,† |
|---|---|---|---|---|
| Noncarriers | 2763 | 17 (0.6%) | – | – |
| All carriers | 644 | 7 (1%) | 1.77 (0.74–4.24)‡ | 0.20§ |
| MRSA carriers | 158 | 4 (2.5%) | 4.11 (1.40–12.08)|| | 0.0002¶ |
| MSSA carriers | 486 | 2 (0.4%) | 0.62 (0.13–2.97)** | 0.63†† |
| MRSA carriers versus MSSA carriers | – | (2.5% versus 0.4%) | 6.15 (1.14–33.65)‡‡ | 0.03§§ |
* Chi-square; †Fisher’s exact; †RR of infection of all carriers compared with noncarriers; §chi-square comparing infection rate of all carriers with noncarriers; ||RR of infection of MRSA carriers compared with noncarriers; ¶Fisher’s exact comparing infection rate of MRSA carriers with noncarriers; **RR of infection of MSSA carriers compared with noncarriers; ††Fisher’s exact comparing infection rate of MSSA carriers with noncarriers; ‡‡RR of infection of MRSA carriers with MSSA carriers; §§Fisher’s exact comparing infection rate of MRSA carriers with MSSA carriers; CI = confidence interval; MRSA = methicillin-resistant Staphylococcus aureus; MSSA = methicillin-sensitive S aureus; RR = relative risk.
Table 5.
Breakdown of treatments for infection cases*
| Procedure | Primary THA | Revision THA | Primary TKA | Revision TKA | ||||
|---|---|---|---|---|---|---|---|---|
| Control | Cohort | Control | Cohort | Control | Cohort | Control | Cohort | |
| Superficial I&D | 1 | 1 | 0 | 0 | 2 | 0 | 0 | 0 |
| Deep I&D | 4 | 2 | 1 | 1 | 0 | 1 | 1 | 0 |
| Deep I&D with liner exchange | 3 | 6 | 1 | 1 | 7 | 3 | 6 | 2 |
| Resection arthroplasty | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Antibiotic spacer | 2 | 4 | 1 | 2 | 2 | 1 | 1 | 1 |
* Fifty-eight of 60 infections in both the historical control and study cohort groups are demonstrated. Two cases were reported as infections at outside institutions but details of the treatment were unavailable; I&D = irrigation and débridement.
Discussion
PJI after THA and TKA is associated with substantial patient morbidity and economic burden to the healthcare system. Many resources have been devoted to infection reduction methods. One such strategy is identifying patients who are colonized with S aureus and attempting to decolonize them, because S aureus colonization has been identified as a risk factor for PJI. There are limited data on the success of decolonization protocols and their subsequent effect on PJI. Thus, controversy exists on the use of such screening and treatment programs. The objectives of this study were to (1) assess the rate of successful decolonization using a 5-day protocol for MRSA-colonized patients; (2) to assess the effect of a decolonization protocol on the rate of PJI compared with a historical control group; and (3) to assess infection risk based on carrier type, comparing S aureus carriers with noncarrier controls.
Within the present study, 22% of patients failed to decolonize from MRSA despite the treatment protocol. Additionally, we were unable to show a statistically significant reduction in surgical site infection between the treatment group and a historical control group with the numbers provided in the study. Finally, patients colonized with MRSA were shown to be more likely to develop PJI than noncarriers of MRSA.
Our study had several limitations. First, we were underpowered to detect a statistically significant difference in SSI among any of our comparison groups. Given the very low infection rate, we would have needed more than 72,000 patients in both the treatment and historical control groups to achieve 80% power, which would be very challenging at a single institution. Second, the use of a historical control group has been associated with false outcomes as a result of potential confounding variables [16]. Several confounding variables may have influenced our findings. Most importantly, over time, institutions nationwide (including ours) have adopted infection prevention strategies to reduce the incidence of SSI. The protocols include a perioperative checklist to ensure appropriate dose and timing of antibiotics as well as appropriate discontinuation of antibiotics within 24 hours of surgery (Surgical Care Improvement Project measures). In addition, the inclusion of SSI as a “never event” by the Centers for Medicare & Medicaid Services has brought significant attention toward the prevention of SSI. In addition to antibiotics, these measures include hand washing protocols, isolations protocols, and identification of modifiable risk factors for infection. Many of these protocols were likely implemented over the course of the study. We are unable to ensure the uniformity of these protocols among both groups and therefore the sole treatment effect of the screening and decolonization program could be limited. One could assume that the addition of these infection prevention protocols would have more of an effect on the study group than the historical control groups. As such, the institution of these protocols could account for a proportion of the reduction in SSI among the study group. Third, we were unable to measure patient compliance with the treatment protocol to determine which patients followed the decolonization protocol; however, compliance with a similar protocol has been previously reported to be nearly 95% and 99% with the use of mupirocin ointment and a single chlorhexidine shower, respectively [10]. Finally, monitoring for postoperative infections was performed by an individual nurse employed by a single institution, and it is possible some patients were treated for infection at other institutions; however, we believe this to have been very unlikely given that our institution is a referral center for complex TJAs, including PJIs.
Decolonization with mupirocin nasal ointment and chlorhexidine soap was ineffective at MRSA decolonization in approximately 22% of patients in this study and is similar to the decolonization failures found in a similar study by Kim et al. [17].We believe the two most likely factors associated with decolonization failure are patient noncompliance and the presence of resistant organisms. Although we did not measure patient compliance in our study, there is supporting evidence that compliance is high in this setting and a less likely cause of decolonization failure [10, 17]. The emergence of resistant organisms to intranasal mupirocin has been evaluated [5, 14, 28]. In one study, 19% of swab isolates demonstrated resistance to mupirocin [8]. Although it was not evaluated in this study, we believe that mupirocin resistance may in part explain the decolonization failures in this study.
With the numbers available in this study, we were unable to detect statistically significant differences in SSI between the group receiving our surveillance and treatment protocol and the historical control cohort. Hacek et al. evaluated the use of rapid PCR screening followed by decolonization with intranasal mupirocin and showed a decreased infection rate in their treatment cohort compared with both concurrent and historical controls [9]. Although their study did not subclassify S aureus into MSSA and MRSA, they estimated that their protocol prevented eight infections in their cohort of 1495 patients. Kim et al. [17] were able to demonstrate the effectiveness of their institution’s screening and decolonization program in a cohort of more than 7000 patients undergoing elective spine surgery and TJA. To our knowledge, the study by Kim et al. included the single largest cohort of patients undergoing orthopaedic procedures. They found a decrease in SSI in their treatment group compared with historical controls and also among noncarrier patients compared with patients colonized with MRSA. Hadley et al. were able to show a reduction in SSI compared with a concurrent control group [10]. However, unlike our study, where decolonization treatment was provided only to patients who proved colonized on testing, patients in the study by Hadley et al. were empirically treated, regardless of screening results.
The results of the present study suggest that nasal carriers of S aureus may have an increased risk of PJI when compared with noncarriers. This risk is further elevated for carriers of MRSA. Kim et al. found a statistically significant correlation between MRSA carrier status and PJI when compared with noncarriers. However, this correlation was not statistically significant when comparing MSSA carrier status with noncarriers. Based on these data from Kim et al.’s study and our own, it is our belief that decolonization treatment should be reserved for patients colonized with S aureus as opposed to empiric treatment of all patients. In our study, 19.3% of patients were shown to be carriers of S aureus. Thus, empiric treatment would have resulted in unnecessarily treating 2771 of the 3434 patients in our cohort, which would have added unnecessary costs, possible side effects of the medications, and, potentially, the development of antibiotic resistance.
At our institution, vancomycin is reserved for patients who screen positive for MRSA or have a documented ß-lactam allergy. Because only 5% of the patients in our study screened positive for MRSA, it is our belief that empiric prophylaxis against MRSA would add unnecessary risks and costs to patient care. Thus, we are in agreement with a recent consensus statement that recommended against routine perioperative antibiotic prophylaxis for MRSA in patients undergoing TJA [24].
Although an institution-wide screening and decolonization protocol enables a substantial reduction in nasal carriage of MRSA, 22% of patients in our study remained colonized. Based on the numbers available in our study, nasal decolonization protocols before elective TJA did not demonstrate a statistically significant reduction in the rate of SSIs. Future meta-analyses and systematic reviews may be needed to pool the results of studies like these to ascertain whether small improvements in infection risk are achieved by protocols like ours and to determine whether any such improvements warrant the costs and potential risks of surveillance and intervention. Additionally, novel methods of decolonization may provide further benefit in reduction of SSI and may warrant clinical investigation.
Acknowledgments
All microbiologic samples were obtained by trained nurses in the preoperative area of Novant Health Charlotte Orthopedic Hospital (Charlotte, NC, USA).
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Novant Health Charlotte Orthopedic Hospital, Charlotte, NC, USA.
References
- 1.Bode LG, Wertheim HF, Kluytmans JA, Bogaers-Hofman D, Vandenbroucke-Grauls CM, Roosendaal R, Toelstra A, Box AT, Voss A, van Belkum A, Verbrugh HA, Vos MC. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362:9–17. doi: 10.1056/NEJMoa0808939. [DOI] [PubMed] [Google Scholar]
- 2.Bozic KJ, Ries MD. The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization. J Bone Joint Surg Am. 2005;87:1746–1751. doi: 10.2106/JBJS.D.02937. [DOI] [PubMed] [Google Scholar]
- 3.Bradbury T, Fehring TK, Taunton M, Hanssen A, Azzam K, Parvizi J, Odum SM. The Fate of acute methicillin-resistant Staphylococcus aureus periprosthetic knee infections treated by open débridement and retention of components. J Arthroplasty. 2009;24:101–104. doi: 10.1016/j.arth.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 4.Climo MW, Sepkowitz KA, Zuccotti G, Fraser VJ, Warren DK, Perl TM, Speck K, Jernigan JA, Robles JR, Wong ES. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and healthcare-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med. 2009;37:1858–1865. doi: 10.1097/CCM.0b013e31819ffe6d. [DOI] [PubMed] [Google Scholar]
- 5.Deshpande LM, Fix AM, Pfaller MA, Jones RN, SENTRY Antimicrobial Surveillance Program Participants Group Emerging elevated mupirocin resistance rates among staphylococcal isolates in the SENTRY Antimicrobial Surveillance Program (2000): correlations of results from disk diffusion, Etest and reference dilution methods. Diagn Microbiol Infect Dis. 2000;2002(42):283–290. doi: 10.1016/s0732-8893(01)00328-5. [DOI] [PubMed] [Google Scholar]
- 6.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med. 2001;344:11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 7.Eiselt D. Presurgical skin preparation with a novel 2% chlorhexidine gluconate cloth reduces rates of surgical site infection in orthopaedic surgical patients. Tokyo Ika Shika Daigaku Iyo Kizai Kenkyusho Hokoku. 2009;28:141–145. doi: 10.1097/NOR.0b013e3181a469db. [DOI] [PubMed] [Google Scholar]
- 8.Gilpin DF, Small S, Bakkshi S, Kearney MP, Cardwell C, Tunney MM. Efficacy of a standard methicillin-resistant Staphylococcus aureus decolonisation protocol in routine clinical practice. J Hosp Infect. 2010;75:93–98. doi: 10.1016/j.jhin.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 9.Hacek DM, Robb WJ, Paule SM, Kudrna JC, Stamos VP, Peterson LR. Staphylococcus aureus nasal decolonization in joint replacement surgery reduces infection. Clin Orthop Relat Res. 2008;466:1349–1355. doi: 10.1007/s11999-008-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hadley S, Immerman I, Hutzler L, Slover J, Bosco J. Staphylococcus aureus decolonization protocol decreases surgical site infections for total joint replacement. Arthritis. 2010;2010:924518. doi: 10.1155/2010/924518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanssen AD, Rand JA. Evaluation and treatment of infection at the site of a total hip or knee arthroplasty. Instr Course Lect. 1999;48:111–122. [PubMed] [Google Scholar]
- 12.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Hudson IR. The efficacy of intranasal mupirocin in the prevention of staphylococcal infections: a review of recent experience. J Hosp Infect. 1994;27:81–98. doi: 10.1016/0195-6701(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 14.Hurdle JG, O’Neill AJ, Mody L, Chopra I, Bradley SF. In vivo transfer of high-level mupirocin resistance from Staphylococcus epidermidis to methicillin-resistant Staphylococcus aureus associated with failure of mupirocin prophylaxis. J Antimicrob Chemother. 2005;56:1166–1168. doi: 10.1093/jac/dki387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson AJ, Daley JA, Zywiel MG, Delanois RE, Mont MA. Preoperative chlorhexidine preparation and the incidence of surgical site infections after hip arthroplasty. J Arthroplasty. 2010;25(Suppl):98–102. doi: 10.1016/j.arth.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Kalmeijer MD, Coertjens H, van Nieuwland-Bollen PM, Bogaers-Hofman D, de Baere GA, Stuurman A, van Belkum A, Kluytmans JA. Surgical site infections in orthopedic surgery: the effect of mupirocin nasal ointment in a double-blind, randomized, placebo-controlled study. Clin Infect Dis. 2002;35:353–358. doi: 10.1086/341025. [DOI] [PubMed] [Google Scholar]
- 17.Kim DH, Spencer M, Davidson SM, Li L, Shaw JD, Gulczynski D, Hunter DJ, Martha JF, Miley GB, Parazin SJ, Dejoie P, Richmond JC. Institutional prescreening for detection and eradication of methicillin-resistant Staphylococcus aureus in patients undergoing elective orthopaedic surgery. J Bone Joint Surg Am. 2010;92:1820–1826. doi: 10.2106/JBJS.I.01050. [DOI] [PubMed] [Google Scholar]
- 18.Kluytmans JA, Mouton JW, Ijzerman EP, Vandenbroucke-Grauls CM, Maat AW, Wagenvoort JH, Verbrugh HA. Nasal carriage of Staphylococcus aureus as a major risk factor for wound infections after cardiac surgery. J Infect Dis. 1995;171:216–219. doi: 10.1093/infdis/171.1.216. [DOI] [PubMed] [Google Scholar]
- 19.Lee EW, Kim HT. Early fatigue failures of cemented, forged, cobalt-chromium femoral stems at the neck-shoulder junction. J Arthroplasty. 2001;16:236–238. doi: 10.1054/arth.2001.20542. [DOI] [PubMed] [Google Scholar]
- 20.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20:250–280. [DOI] [PubMed]
- 21.National Nosocomial Infections Surveillance System National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–485. doi: 10.1016/j.ajic.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Noskin GA, Rubin RJ, Schentag JJ, Kluytmans J, Hedblom EC, Smulders M, Lapetina E, Gemmen E. The burden of Staphylococcus aureus infections on hospitals in the United States: an analysis of the 2000 and 2001 Nationwide Inpatient Sample Database. Arch Intern Med. 2005;165:1756–1761. doi: 10.1001/archinte.165.15.1756. [DOI] [PubMed] [Google Scholar]
- 23.Parvizi J, Azzam K, Ghanem E, Austin MS, Rothman RH. Periprosthetic infection due to resistant staphylococci: serious problems on the horizon. Clin Orthop Relat Res. 2009;467:1732–1739. doi: 10.1007/s11999-009-0857-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parvizi J, Gehrke T. International consensus on periprosthetic joint infection: let cumulative wisdom be a guide. J Bone Joint Surg Am. 2014;96:441. doi: 10.2106/JBJS.N.00023. [DOI] [PubMed] [Google Scholar]
- 25.Perl TM, Cullen JJ, Wenzel RP, Zimmerman MB, Pfaller MA, Sheppard D, Twombley J, French PP, Herwaldt LA. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N Engl J Med. 2002;346:1871–1877. doi: 10.1056/NEJMoa003069. [DOI] [PubMed] [Google Scholar]
- 26.Perl TMT, Golub JEJ. New approaches to reduce Staphylococcus aureus nosocomial infection rates: treating S aureus nasal carriage. Ann Pharmacother. 1998;32:S7–S16. doi: 10.1177/106002809803200104. [DOI] [PubMed] [Google Scholar]
- 27.Rao NN, Cannella BAB, Crossett LSL, Yates AJA, McGough RLR, Hamilton CWC. Preoperative screening/decolonization for Staphylococcus aureus to prevent orthopedic surgical site infection: prospective cohort study with 2-year follow-up. J Arthroplasty. 2011;26:1501–1507. doi: 10.1016/j.arth.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 28.Rossney A, O’Connell S. Emerging high-level mupirocin resistance among MRSA isolates in Ireland. Euro Surveill. 2008;13. pii:8084. [PubMed]
- 29.Saadatian-Elahi M, Teyssou R, Vanhems P. Staphylococcus aureus, the major pathogen in orthopaedic and cardiac surgical site infections: a literature review. Int J Surg. 2008;6:238–245. doi: 10.1016/j.ijsu.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Sanford MD, Widmer AF, Bale MJ, Jones RN, Wenzel RP. Efficient detection and long-term persistence of the carriage of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 1994;19:1123–1128. doi: 10.1093/clinids/19.6.1123. [DOI] [PubMed] [Google Scholar]
- 31.Skramm I, Fossum Moen AE, Aroen A, Bukholm G. Surgical site infections in orthopaedic surgery demonstrate clones similar to those in orthopaedic Staphylococcus aureus nasal carriers. J Bone Joint Surg Am. 2014;96:882–888. doi: 10.2106/JBJS.M.00919. [DOI] [PubMed] [Google Scholar]
- 32.Spangehl MJ, Younger AS, Masri BA, Duncan CPC. Diagnosis of infection following total hip arthroplasty. Instr Course Lect. 1998;47:285–295. [PubMed] [Google Scholar]
- 33.Weinstein HJ. The relation between the nasal-staphylococcal-carrier state and the incidence of postoperative complications. N Engl J Med. 1959;260:1303–1308. doi: 10.1056/NEJM195906252602601. [DOI] [PubMed] [Google Scholar]
- 34.Wertheim HFL, Vos MC, Ott A, van Belkum A, Voss A, Kluytmans JA, van Keulen PH, Vandenbroucke-Grauls CM, Meester MH, Verbrugh HA. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet. 2004;364:703–705. doi: 10.1016/S0140-6736(04)16897-9. [DOI] [PubMed] [Google Scholar]