Abstract
Background
It is not known whether parasympathetic outflow simultaneously acts on bronchial tone and cardiovascular system waxing and waning both systems in parallel, or, alternatively, whether the regulation is more dependent on local factors and therefore independent on each system. The aim of this study was to evaluate the simultaneous effect of different kinds of stimulations, all associated with parasympathetic activation, on bronchomotor tone and cardiovascular autonomic regulation.
Methods
Respiratory system resistance (Rrs, forced oscillation technique) and cardio-vascular activity (heart rate, oxygen saturation, tissue oxygenation index, blood pressure) were assessed in 13 volunteers at baseline and during a series of parasympathetic stimuli: O2 inhalation, stimulation of the carotid sinus baroreceptors by neck suction, slow breathing, and inhalation of methacholine.
Results
Pure cholinergic stimuli, like O2 inhalation and baroreceptors stimulation, caused an increase in Rrs and a reduction in heart rate and blood pressure. Slow breathing led to bradycardia and hypotension, without significant changes in Rrs. However slow breathing was associated with deep inhalations, and Rrs evaluated at the baseline lung volumes was significantly increased, suggesting that the large tidal volumes reversed the airways narrowing effect of parasympathetic activation. Finally inhaled methacholine caused marked airway narrowing, while the cardiovascular variables were unaffected, presumably because of the sympathetic activity triggered in response to hypoxemia.
Conclusions
All parasympathetic stimuli affected bronchial tone and moderately affected also the cardiovascular system. However the response differed depending on the nature of the stimulus. Slow breathing was associated with large tidal volumes that reversed the airways narrowing effect of parasympathetic activation.
Introduction
In humans, airway smooth muscle (ASM) tone is largely determined by parasympathetic cholinergic control, which is operated by the vagus that innervates the large airways [1].
The central autonomic control of the ASMs tone is a complex and interconnected system with multiple parallel pathways that contribute to regulate the parasympathetic cholinergic outflow in multiple organs and systems. Dysfunction or dysregulation of the autonomic control of ASMs contributes to the pathogenesis of asthma and chronic obstructive pulmonary disease, and may also produce the respiratory symptoms associated with cardiovascular diseases [2–5].
A comprehensive knowledge of the mechanisms regulating ASMs tone would be crucial for a better understanding and treatment of obstructive pulmonary diseases. However, there are still tremendous gaps in our understanding of airway neural control, even in the healthy lung. One of these gaps is related to the central control of autonomic tone and to the integration of different afferent inputs.
Due to the limited availability of methods capable of simultaneously assessing ASMs tone and cardiovascular regulation, the interaction between these two systems remains poorly studied. In particular, it is not known whether parasympathetic activation acts on the bronchial tone and cardiovascular system in parallel, or, alternatively, whether the regulation of the two systems is more determined by local factors acting on each system independently. Moreover it is unclear whether all stimuli associated with parasympathetic activation have similar effects or whether different stimuli might have different effects according to their specific nature. We hypothesized that interventions that stimulate the cardiovascular and respiratory systems through parasympathetic activation would cause both cardiovascular depression and airway narrowing, while interventions associated with a selective direct stimulation of either system would produce independent responses.
To this aim we evaluated the simultaneous effects of different kinds of stimulations, all associated to parasympathetic activation, on bronchomotor tone and cardiovascular autonomic regulation to test whether the control of the cardiovascular and respiratory systems acts separately or in parallel, according to their different needs. In particular we evaluated the effect of neck suction, oxygen inhalation, slow breathing (common in Yoga and other similar practices) and methacoline (Mch) administration in a group of healthy volunteers. Neck suction is a pure carotid baroreceptors stimulation [6–9]. Oxygen inhalation increases cardiac parasympathetic activity [10–12], but may also alter gas exchange and consequently affect ventilation. Slow breathing increases the vagal arm of the cardiac baroreflex [13–16], but also modifies gas exchange [17,18] and, through the increase in tidal volume, may also affect bronchial tone [19]. Finally, inhaled MCh induces airway narrowing as a result of parasympathetic activation, but may also activate sympathetic reflexes in other systems due to the associated hypoxemia [20].
Materials and Methods
Subjects
The study was conducted in 13 healthy subjects whose characteristics are reported in Table 1. None of them was taking any treatment at the time of the study. The study was approved by the Ethical Committee of the S. Croce and Carle Hospital (FPResp 13, 26/7/13, Cuneo, Italy), and written informed consent was obtained from each subject prior to the study.
Table 1. Subjects’ anthropometric characteristics and main lung functional data.
| Sex, M/F | 8/6 |
| Age, yr | 41±14 |
| Height, cm | 172±8 |
| BMI, kg·m-2 | 22±3 |
| FEV1, % of predicted | 108±9 |
| FVC, % of predicted | 114±10 |
| FEV1/FVC, % | 80 ± 9 |
BMI, body mass index; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity. Data are mean ± SD.
Pre-study evaluations
The subjects underwent a spirometric test and methacholine challenge to identify the dose causing a 20% decrease of FEV1 (PD20FEV1).
Measurements
Detailed description of methodology and data analysis is reported in S1 Supporting Information.
Electrocardiogram was measured by placing three electrodes on the patient's anterior chest wall. Oxygen saturation (SaO2) was measured at the finger with a pulse oxymeter and expired carbon dioxide (CO2) by a capnograph. Oxygenation perfusion at the tissue level was estimated at the left forearm by a Near Infrared Spectroscope. Values are expressed as tissue oxygen saturation index (TO2I). Continuous noninvasive arterial blood pressure was monitored via cuffs positioned on the middle finger of the right arm held at the heart level. All signals were simultaneously acquired at 400 Hz on a Macintosh laptop (Apple, Coupertino, CA) with a 12 channel acquisition system.
Airway mechanics was measured by multiple frequency forced oscillation technique (FOT) at 5, 11, and 19 Hz [21–23]. Respiratory system resistance was computed by a least squares algorithm [24,25] at 5 Hz (R5) and 19 Hz (R19).
Parasympathetic stimuli
Oxygen (O2) inhalation was obtained by breathing supplemental O2 for 11 min. O2 supplementation was obtained by mixing 5 L·min-1 of air and 5 L·min-1 O2 using a douglas bag. Therefore the percent of O2 in the inspired gas was 60%. Measurements were taken during the last 5 min.
Baroreceptors stimulation was obtained by sinusoidal suction applied to a lead collar positioned around the neck by a vacuum system via a computer-controlled valve which produced a controlled suction loss [26]. Breathing was paced at a fixed breathing frequency of 15 breath·min-1 imposed by a metronome in order to avoid any entraining effect on breathing by the frequency of neck suction. Measurements were taken during 2 min of sinusoidal suction from 0 to -30 mmHg at 0.1Hz, and 2 min at 0.2 Hz [9]. The control condition was represented by tidal breathing at 15 breath·min-1. Slow deep breathing (SB) was obtained by imposing a fixed frequency of 6 breath·min-1 (5 sec inhalation, 5 sec exhalation) and leaving the subjects regulate their tidal volume as needed for 2 min, during which measurements were taken.
MCh challenge was performed by administering the PD20FEV1 estimated in the pre-study day. Two min after the end of the inhalation, measurements were taken during 2 min of spontaneous breathing.
Protocol
The study was conducted in the sitting position. All measurements described above were performed at baseline conditions for 4 min, and then in random order during O2 breathing, slow deep breathing, and neck suction at 0.1 and 0.2 Hz. The bronchial challenge was always performed at the end of study to avoid the long-lasting effects of the constrictor agent on airway tone control.
Data Analysis
The effects of the parasympathetic activity on the cardiovascular system were estimated from the RR interval, systolic (SBP) and diastolic blood pressures (DBP) and baroreflex sensitivity (BRS). The latter was the mean value computed from seven different tests [27, 28].
Average heart rate (HR) was calculated for each sequence. Heart rate variability was analyzed in terms of SD of each RR sequence and in terms of root mean square of successive differences (RMSSD). At all conditions BRS, HR and HR variability were compared with resting baseline values.
The parasympathetic effects of neck suction were estimated from the power spectra of the RR interval and blood pressure signals evaluated at 0.1 Hz, when the neck suction was timed at 0.1 Hz, and from the power spectra at 0.2 Hz, when the neck suction was timed at 0.2 Hz. Since breathing frequency was fixed at 15 breath·min-1, this approach allowed separating the effects of ventilation, which are more complex than the simple baroreflex effect, from the pure baroreflex stimulus within the respiratory range.
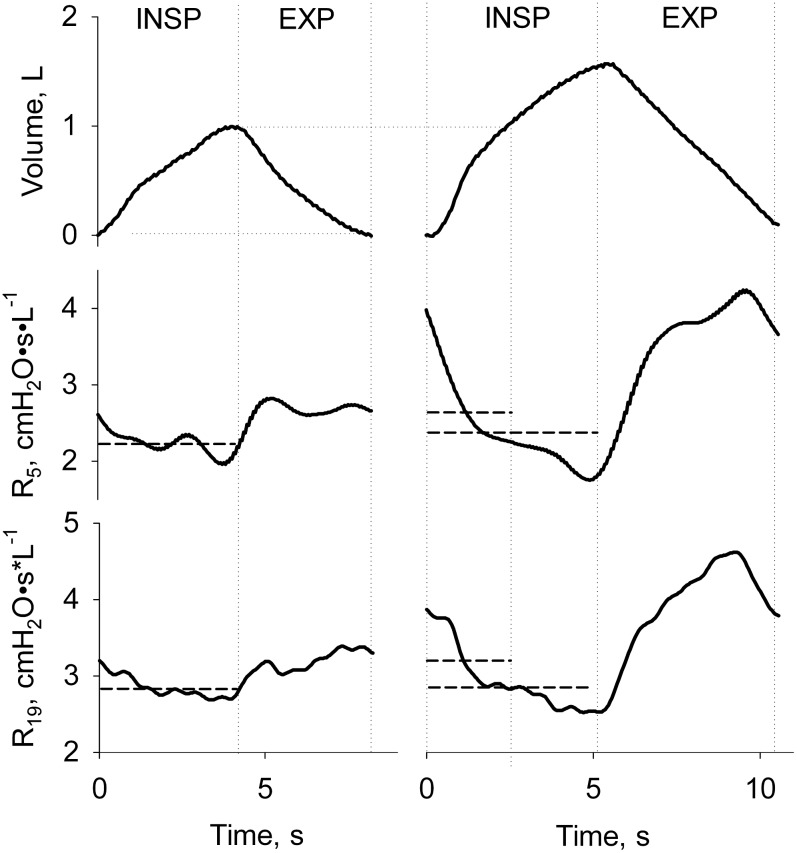
R5 and R19, evaluated during tidal inspiration, were taken as indexes of total and central airways size, respectively [21]. For SB conditions R5 and R19 were also measured over the segment of inspiration corresponding to control tidal volume (R5-IsoVol and R19-IsoVol), as shown in Fig 1. This allowed separating the effects of the parasympathetic stimuli from depth of breathing on bronchomotor tone.
Fig 1. Typical example of measurements of airway resistance during tidal (left panels) and slow deep breathing experimental conditions (right panels).
Tidal volume and resistance at 5 (R5) and 19 Hz (R19) are shown as a function of time in the upper, mid, and lower panels, respectively. Direction of the inspiratory and expiratory phases is indicated. Vertical grey dotted lines from left to right identify the beginning and the end of tidal breathing as well as mid volume for the large breath. Horizontal gray continuous lines are average R5 and R19 measured over the whole inspiration or the portion of volume corresponding to tidal breathing. The latter are named R5-IsoVol and R19-IsoVol in the text.
The effects of the different interventions on gas exchange and O2 delivery to the tissues were estimated from the changes in SaO2 and TO2I.
Statistical analysis
Differences between groups were tested for statistical significance by a one-way analysis of variance (ANOVA) with Holm-Sidak post-hoc test for multiple-comparisons or paired t-test wherever applicable. Values of p<0.05 were considered statistically significant. Data are presented as mean ± standard deviation (SD).
Results
The main anthropometric and functional parameters of the subjects are shown in Table 1. PD20FEV1 was 2400 μg for all subjects but one who responded at 200 μg.
Effect of O2 inhalation
Supplemental O2 caused a marked increase in RR interval (p<0.001), a slight decrease in SBP (p = 0.051) and no relevant changes in BRS and RMSSD. R5 and R19 slightly but significantly increased by similar amount. Minute Ventilation ( E) slightly but significantly decreased (p = 0.020). SaO2 but not TO2I significantly increased (p<0.001). Collectively, these findings suggest that supplemental O2 caused mild depression of the cardiovascular system and central airways narrowing. The main data are reported in Table 2.
Table 2. Main cardiovascular and FOT parameters at control (room air) and during O2 (5 L∙min-1) breathing conditions.
| Control (Room air) | Quiet breathing (O2 5 L∙min-1) | Paired t-test | |
|---|---|---|---|
| Mean RR interval, ms | 814 (109) | 872 (98) | < 0.001 |
| RR-SD, ms | 50 (28) | 44 (24) | 0.204 |
| RMSSD, ms | 47 (26) | 40 (18) | 0.172 |
| SBP, mm Hg | 133 (26) | 121 (25) | 0.051 |
| DBP, mm Hg | 75 (13) | 72 (15) | 0.173 |
| BRS, ms∙mmHg-1 | 12.2 (6.8) | 13.0 (7.7) | 0.582 |
| SaO2, % | 98 (1) | 99 (1) | < 0.001 |
| TO2I, % | 65 (10) | 65 (12) | 0.959 |
| R5, cm H2O∙s∙L-1 | 1.70 (0.55) | 2.12 (0.82) | 0.006 |
| R19, cm H2O∙s∙L-1 | 1.87 (0.61) | 2.45 (0.88) | < 0.001 |
| VT, L | 0.93 (0.17) | 0.76 (0.17) | 0.209 |
| E, L∙min-1 | 9.98 (2.01) | 8.06 (2.60) | 0.020 |
| CO2-ET, mm Hg | 40.5 (4.02) | 34.69 (5.35) | < 0.001 |
RR interval: interval between two RR wave peaks; RR-SD: standard deviation of RR interval; SBP and DBP: systolic and diastolic pressures, respectively; SaO2: oxygen saturation; TO2I: Tissue Oxygen Index; R5 and R19, inspiratory resistance at 5 and 19 Hz, respectively; VT: tidal volume; E: minute ventilation; CO2-ET: carbon dioxide end-tidal. Data are mean ± SD.
Effect of neck suction
Neck suction at 0.1 Hz and at 0.2 Hz significantly increased the oscillations in the RR interval power spectrum at 0.1 Hz (p<0.001) and at 0.2 Hz (p = 0.05). Neck suction at both frequencies was associated with an increase in both R5 and R19, though not statistically significant. Tidal Volume (Vt), E, SaO2 and TO2I remained unmodified. Data are reported in Table 3.
Table 3. Main cardiovascular and FOT parameters at baseline (room air) and neck suction at 0.1 and 0.2 Hz. For all conditions breathing frequency is 15 breaths•min-1.
| Control (15 bpm) | Neck suction (0.1 Hz) | Neck suction (0.2 Hz) | Paired t-test or ANOVA | |
|---|---|---|---|---|
| RR0.1 Hz, ln-Power | 5.96 (0.65) | 7.02 (0.92) | - | < 0.001 |
| RR0.2 Hz, ln-Power | 5.98 (1.09) | - | 6.37 (1.10) | 0.042 |
| Mean RR interval, ms | 769 (102)* | 792 (89)* | 782 (93) | 0.031 |
| RR-SD, ms | 31 (13)* | 47 (19)* § | 36 (13)§ | < 0.001 |
| RMSSD, ms | 32 (13)* | 44 (15)* § | 36 (12)§ | 0.003 |
| SBP, mm Hg | 124 (23) | 126 (24) | 123 (19) | 0.711 |
| DBP, mm Hg | 69 (17) | 72 (14) | 70 (15) | 0.593 |
| SaO2, % | 98 (1) | 98 (1) | 98 (1) | 0.937 |
| TO2I, % | 64 (8) | 64 (6) | 64 (6) | 0.721 |
| R5, cm H2O∙s∙L-1 | 2.00 (0.68) | 2.18 (0.62) | 2.24 (0.67) | 0.059 |
| R19, cm H2O∙s∙L-1 | 2.22 (0.76) | 2.35 (0.63) | 2.43 (0.66) | 0.128 |
| VT, L | 1.23 (0.51) | 1.28 (0.44) | 1.30 (0.49) | 0.424 |
| E L∙min-1 | 18.74 (7.59) | 19.09 (6.45) | 8.06 (7.24) | 0.451 |
| CO2-ET, mm Hg | 33.51 (3.81)¶ | 32.65 (3.49)§ | 30.70 (4.71)§ ¶ | < 0.001 |
RR interval: interval between two RR wave peaks; RR-SD: standard deviation of RR interval; SBP and DBP: systolic and diastolic pressures, respectively; SaO2: oxygen saturation; TO2I: Tissue Oxygen Index; R5 and R19, inspiratory resistance at 5 and 19 Hz, respectively; VT: tidal volume; E: minute ventilation; CO2-ET: carbon dioxide end-tidal. Data are mean ± SD. RR0.1 Hz and RR0.2 Hz are expressed as natural logarithm of power spectra of RR interval at 0.1 and 0.2 Hz, respectively. t-test was used to compare RR0.1 between control and Neck suction (0.1 Hz), and to compare RR0.2 between control and Neck suction (0.2 Hz). ANOVA was used for all the other parameters that were compared for the three conditions.
*: p<0.05 between Neck suction (0.1 Hz) and Control by post-hoc analysis
¶: p<0.05 between Neck suction (0.2 Hz) and Control by post-hoc analysis
§: p<0.05 between Neck suction (0.1 Hz) and Neck suction (0.2 Hz) by post-hoc analysis.
Effect of slow deep breathing
Slow breathing was achieved by doubling Vt (p<0.001). E remained constant. This was associated with an increase in RR-SD (p<0.001) but no significant changes in RR, BRS, RMSSD or blood pressure. R5-IsoVol and R19-Iso-Vol increased by similar amount (p<0.001 for both). These findings suggest mild depression of the cardiovascular system and central airways narrowing. However, R5 and R19 remained similar to baseline conditions. SaO2 and TO2I slightly but significantly increased (p<0.05 for both). Data are reported in Table 4.
Table 4. Main cardiovascular and respiratory and FOT parameters during slow breathing conditions.
| Control (Room air) | 6 breaths∙min-1 | Paired t-test | |
|---|---|---|---|
| Mean RR interval, ms | 814 (109) | 814 (94) | 0.990 |
| RR-SD, ms | 50 (28) | 79 (25) | < 0.001 |
| RMSSD, ms | 47 (26) | 48 (16) | 0.817 |
| SBP, mm Hg | 133 (26) | 132 (24) | 0.483 |
| DBP, mm Hg | 75 (13) | 71 (17) | 0.133 |
| BRS, ms∙mmHg-1 | 12.2 (6.8) | 13.7 (7.4) | 0.263 |
| SaO2, % | 98 (1) | 98 (1) | 0.017 |
| TO2I, % | 65 (10) | 66 (10) | 0.053 |
| R5, cm H2O∙s∙L-1 | 1.70 (0.55) | 1.85 (0.50) | 0.579 |
| R5-IsoVol, cm H2O∙s∙L-1 | - | 2.58 (0.82) | < 0.001 |
| R19, cm H2O∙s∙L-1 | 1.87 (0.61) | 2.09 (0.59) | 0.443 |
| R19-IsoVol, cm H2O∙s∙L-1 | - | 2.59 (0.78) | < 0.001 |
| VT, L | 0.93 (0.17) | 1.92 (0.60) | < 0.001 |
| E, L∙min-1 | 9.98 (2.01) | 11.21 (3.49) | 0.110 |
| CO2-ET, mm Hg | 40.5 (4.02) | 38.82 (3.48) | 0.403 |
RR interval: interval between two RR wave peaks; RR-SD: standard deviation of RR interval; SBP and DBP: systolic and diastolic pressures, respectively; SaO2: oxygen saturation; TO2I: Tissue Oxygen Index; R5 and R19, inspiratory resistance at 5 and 19 Hz, respectively; VT: tidal volume; E: minute ventilation; CO2-ET: carbon dioxide end-tidal. Data are mean ± SD. R5-IsoVol and R19-IsoVol: resistance at 5 and 19 Hz measured at control lung volume.
Effect of inhaling metacholine
Inhaling MCh caused a significant increase in bronchial tone as documented by remarkable increments in R5 and R19 (p<0.001 for both). The increase of the former over the latter (p<0.001) is consistent with heterogeneous distribution of airway narrowing across the lungs. In contrast, all cardiovascular parameters remained unmodified. Vt slightly but significantly decreased (p<0.001). E tended to decrease. SaO2 and TO2I significantly decreased (p<0.001 for both). Data are reported in Table 5.
Table 5. Main cardiovascular and FOT parameters at baseline (room air) and after methacholine (MCh).
| Control | Mch | Paired t-test | |
|---|---|---|---|
| Mean RR interval, ms | 814 (109) | 798 (125) | 0.189 |
| RR-SD, ms | 50 (28) | 39 (24) | 0.040 |
| RMSSD, ms | 47 (26) | 38 (20) | 0.069 |
| SBP, mm Hg | 133 (26) | 130 (19) | 0.709 |
| DBP, mm Hg | 75 (13) | 74 (15) | 0.892 |
| BRS, ms∙mmHg-1 | 12.2 (6.8) | 10.5 (7.8) | 0.184 |
| SaO2, % | 98 (1) | 94 (3) | 0.001 |
| TO2I, % | 65 (10) | 61 (11) | < 0.001 |
| R5, cm H2O∙s∙L-1 | 1.70 (0.55) | 4.57 (1.26) | < 0.001 |
| R19, cm H2O∙s∙L-1 | 1.87 (0.61) | 3.47 (0.93) | < 0.001 |
| VT, L | 0.93 (0.17) | 0.64 (0.14) | 0.001 |
| E, L∙min-1 | 9.98 (2.01) | 8.26 (2.51) | 0.090 |
| CO2-ET, mm Hg | 40.5 (4.02) | 34.46 (3.51) | < 0.001 |
RR interval: interval between two RR wave peaks; RR-SD: standard deviation of RR interval; SBP and DBP: systolic and diastolic pressures, respectively; SaO2: oxygen saturation; TO2I: Tissue Oxygen Index; R5 and R19, inspiratory resistance at 5 and 19 Hz, respectively; VT: tidal volume; E: minute ventilation; CO2-ET: carbon dioxide end-tidal. Data are mean ± SD.
Discussion
We evaluated the effects of different interventions, expected to activate parasympathetic system, on bronchial tone and cardiovascular regulation. For all interventions the observed cardiovascular response was in agreement with previously published works [7,13,15,29–33]. This was considered as a proof that all interventions actually produced a parasympathetic activation. Moreover, all the interventions were also associated with tonic activation of the ASMs, but with important differences in terms of change in airways resistance among different stimuli. This result is consistent with a different balance between the tonic activation mediated by the parasympathetic system and local factors such as the mechanical action operated by ventilation on the airways walls. On the other hand, inhaled MCh caused airway narrowing but not cardiovascular depression.
The simultaneous assessment of the cardiovascular regulation and bronchial tone during different parasympathetic stimulations was possible thanks to the use of FOT for the evaluation of bronchial tone. This technique is very sensitive to changes in airway caliber [34–36] and allows the assessment of the respiratory system resistance without altering the breathing pattern, including spontaneous, paced and slow breathing. Moreover, using a within-breath approach, respiratory resistance can be assessed at any lung volume, allowing the discrimination between the contribution of ASMs tone and lung volume dependence to changes in lung mechanics. Finally FOT does not require the execution of special respiratory maneuvers, like deep inhalation in spirometry, which may themselves counteract the effect of bronchocontricting interventions [19].
Determinants of ASMs tone
The most relevant determinant of baseline cholinergic tone of ASMs is ventilation: increasing or decreasing respiratory rate increases and decreases baseline cholinergic tone, respectively. Ventilation is also the primary antagonist of ASMs tone with tidal breaths cyclically tethering the airways walls counterbalancing the effects of parasympathetic activation [37]. Within tidal breathing range, the tonic activation mediated by the parasympathetic system prevails over the mechanical action operated by ventilation, keeping the airways slightly contracted [38]. Thanks to the presence of a basal cholinergic tone, contractions and relaxations can be mediated reflexively [39–41]. Reflex-mediated contractions of ASMs can be initiated by different stimuli delivered both to the airways and lungs as well as to the heart, arterial baroreceptors and chemoreceptors [39–41]. Smooth muscle contraction can evoke a further reflex-mediated contraction of the ASMs. Therefore, bronchoconstrictors evoke bronchospasm at least in part by initiating parasympathetic-cholinergic reflexes [38].
Specific response to the different parasympathetic stimuli
Supplemental O2 is known to evoke vagal reflexes in disease conditions, and to a lesser extent in healthy subjects. This is due both to local and systemic factors. Vasoconstriction, due to the direct effect of O2 and O2 reactive species on the vascular smooth muscles, is thought to be the primary response [42]. Vasoconstriction is associated with hypertension, while bradycardia is a secondary effect associated with the baroreflex activation. Moreover, at a lung level an excess of free radicals presumably stimulates the parasympathetic lung afferents [11,43]. In our study the bronchomotor and cardiovascular responses to O2 inhalation are consistent with parasympathetic activation. The increase in both R5 and R19 suggests that narrowing involved the central airways where the cholinergic receptors are mostly expressed. Heart rate decreased, blood pressure slightly decreased, though this was not associated with an increase in BRS. This result combined with the lack of increase in TO2I despite the increase in SaO2, would suggest that O2 induced peripheral vasoconstriction, preventing O2 from flowing to the tissues.
Neck suction is a “pure” autonomic stimulus that is not associated with any hemodynamic effect other than reflex [7]. The neck suction at 0.2 Hz (close to but different from the respiratory frequency at 0.25 Hz) causes selective vagal modulation, whereas at 0.1 Hz causes both vagal and sympathetic modulation [7,44]. The efficacy of the technique in causing selective vagal modulation at the cardiovascular level in our study is documented by a prevalent increase in the RR interval variability with no significant changes in systolic and diastolic blood pressure. Differently from supplemental O2, sinusoidal neck suction induces a modulation in autonomic function rather than steady-state changes. The mean values of most variables remained unchanged while RR interval fluctuations coherent with the frequency of neck suction increased. At the bronchial level, the neck suction was associated with a significant increase in R5 and with only a slight not significant increase in R19, suggesting that narrowing occurred at a slightly more peripheral site than with O2 inhalation.
Breathing at 6 cycles/min, RR fluctuations merge at the rate of respiration and their amplitude increases relative to blood pressure changes, enhancing the vagal arm of baroreflex [14–16]. At lung level the increase in tidal volume derived by slow breathing directly increases vagal activity and reduces the sympathetic activity via the Hering–Breuer reflex [13], which in turn reduces the chemoreflex sensitivity and thus might further enhance the baroreflex. Additionally, slow breathing increases O2 saturation as a result of the decrease of physiologic dead space and improved ventilation/perfusion matching [18]. In the present study slow breathing led to cardiovascular inhibition and airway narrowing, as suggested by an increase in BRS and R5-IsoVol and R19-Iso-Vol. However slow breathing was associated with large tidal volumes that reversed the airways narrowing effect of parasympathetic activation due to the well known inverse relationship between lung volume and airway size [45], that leads to direct bronchodilatation at large tidal volumes [19]. Therefore, slow breathing per se caused some constrictive response (suggested by the increase in R5-IsoVol and R19-IsoVol) but this was ablated by larger tidal volumes.
Inhaling MCh induced marked airway narrowing (R5 and R19) and increase in dyspnea [38]. Despite airway narrowing, none of the cardiovascular parameters exhibited significant changes relative to control. We speculate that hypoxemia due to airway narrowing elicited sympathetic reflexes [20] which counteracted the cardiovascular depressive effects of the vagal stimulation.
Clinical implications
Yoga, Zen and physical training are proved to be beneficial for the cardiovascular function [46–49], as they modify the autonomic balance toward a parasympathetic predominance and they strengthen baroreflex, resulting in reduced HR, reduced blood pressure, increased HR variability and increased baroreflex sensitivity, which in turn reduce the load on the heart and cardiovascular system in general. The results of the present study confirm that, and help understand why, these natural stimuli lead to favorable cardiovascular effects without hampering airway function.
Conclusions
All parasympathetic stimuli tested in the present study affected the bronchial tone and moderately affected also the cardiovascular system. When the stimuli were carried by complex interventions that modified breathing pattern or gas exchange the responses were ablated at either level. Slow breathing modulated the cardiovascular activity but left bronchial tone unmodified, likely as a result of the increased depth of breathing.
Supporting Information
(DOCX)
Acknowledgments
We thank Ms. Gloria Arrara for help in data collection, Dr. Michele Neri (Ambiter, Parma, Italy) for writing the software for data acquisition.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
LB is recipient of a grant from the Signe and Ane Gyllenberg Foundation (Helsinki, Finland).
References
- 1. Coleridge HM CJ (1986) Reflexes evoked from tracheobronchial tree and lungs Handbook of Physiology. pp. 395–429. [Google Scholar]
- 2. Proskocil BJ, Fryer AD (2005) Beta2-agonist and anticholinergic drugs in the treatment of lung disease. Proc Am Thorac Soc 2: 305–310; discussion 311–312. 10.1513/pats.200504-038SR [DOI] [PubMed] [Google Scholar]
- 3. Molfino NA, Slutsky AS, Julià-Serdà G, Hoffstein V, Szalai JP, Chapman KR, et al. (1993) Assessment of airway tone in asthma. Comparison between double lung transplant patients and healthy subjects. Am Rev Respir Dis 148: 1238–1243. [DOI] [PubMed] [Google Scholar]
- 4. Benichou M, Lorino AM, Lorino H, Macquin-Mavier I, Istin N, Harf A, et al. (1990) Influence of tidal volume on histamine-induced bronchoconstriction in guinea pigs. J Appl Physiol 68: 1634–1639. [DOI] [PubMed] [Google Scholar]
- 5. Belmonte KE (2005) Cholinergic pathways in the lungs and anticholinergic therapy for chronic obstructive pulmonary disease. Proc Am Thorac Soc 2: 297–304; discussion 311–312. 10.1513/pats.200504-043SR [DOI] [PubMed] [Google Scholar]
- 6. Bartels MN, Gonzalez JM, Kim W, De Meersman RE (2000) Oxygen supplementation and cardiac-autonomic modulation in COPD. 691–696 p. [DOI] [PubMed] [Google Scholar]
- 7. Bernardi L, Bianchini B, Spadacini G, Leuzzi S, Valle F, Marchesi E, et al. (1995) Demonstrable cardiac reinnervation after human heart transplantation by carotid baroreflex modulation of RR interval. Circulation 92: 2895–2903. [DOI] [PubMed] [Google Scholar]
- 8. Brown CM, Dütsch M, Michelson G, Neundörfer B, Hilz MJ (2002) Impaired cardiovascular responses to baroreflex stimulation in open-angle and normal-pressure glaucoma. Clin Sci (Lond) 102: 623–630. [DOI] [PubMed] [Google Scholar]
- 9. Piepoli M, Sleight P, Leuzzi S, Valle F, Spadacini G, Passino C, et al. (1997) Origin of respiratory sinus arrhythmia in conscious humans. An important role for arterial carotid baroreceptors. Circulation 95: 1813–1821. [DOI] [PubMed] [Google Scholar]
- 10. Sjöberg F, Singer M (2013) The medical use of oxygen: A time for critical reappraisal. J Intern Med 274: 505–528. 10.1111/joim.12139 [DOI] [PubMed] [Google Scholar]
- 11. Marczak M, Pokorski M (2004) Oxygen breathing and ventilation. J Physiol Pharmacol 55: 127–134. [PubMed] [Google Scholar]
- 12. Waring WS, Thomson AJ, Adwani SH, Rosseel AJ, Potter JF, Webb DJ, et al. (2003) Cardiovascular effects of acute oxygen administration in healthy adults. J Cardiovasc Pharmacol 42: 245–250. [DOI] [PubMed] [Google Scholar]
- 13. Bernardi L, Gabutti A, Porta C, Spicuzza L (2001) Slow breathing reduces chemoreflex response to hypoxia and hypercapnia, and increases baroreflex sensitivity. J Hypertens 19: 2221–2229. [DOI] [PubMed] [Google Scholar]
- 14. Bernardi L, Porta C, Spicuzza L, Bellwon J, Spadacini G, Frey AW, et al. (2002) Slow Breathing Increases Arterial Baroreflex Sensitivity in Patients With Chronic Heart Failure. Circulation 105: 143–145. [DOI] [PubMed] [Google Scholar]
- 15. Joseph CN, Porta C, Casucci G, Casiraghi N, Maffeis M, Rossi M, et al. (2005) Slow breathing improves arterial baroreflex sensitivity and decreases blood pressure in essential hypertension. Hypertension 46: 714–718. [DOI] [PubMed] [Google Scholar]
- 16. Raupach T, Bahr F, Herrmann P, Luethje L, Heusser K, Hasenfuss G, et al. (2008) Slow breathing reduces sympathoexcitation in COPD. Eur Respir J Off J Eur Soc Clin Respir Physiol 32: 387–392. [DOI] [PubMed] [Google Scholar]
- 17. Bernardi L, Rosengård-Bärlund M, Sandelin A, Mäkinen VP, Forsblom C, Groop PH, et al. (2011) Short-term oxygen administration restores blunted baroreflex sensitivity in patients with type 1 diabetes. Diabetologia 54: 2164–2173. 10.1007/s00125-011-2195-4 [DOI] [PubMed] [Google Scholar]
- 18. Bernardi L, Spadacini G, Bellwon J, Hajric R, Roskamm H, Frey AW, et al. (1998) Effect of breathing rate on oxygen saturation and exercise performance in chronic heart failure. Lancet 351: 1308–1311. [DOI] [PubMed] [Google Scholar]
- 19. Pellegrino R, Sterk PJ, Sont JK, Brusasco V (1998) Assessing the effect of deep inhalation on airway calibre: a novel approach to lung function in bronchial asthma and COPD. Eur Respir J Off J Eur Soc Clin Respir Physiol 12: 1219–1227. [DOI] [PubMed] [Google Scholar]
- 20. Xing T, Pilowsky PM FA (2014) Mechanism of sympathetic activation and blood pressure elevation in humans and animals following acute intermittent hypoxia. Prog Brain Res 209: 131–146. 10.1016/B978-0-444-63274-6.00007-2 [DOI] [PubMed] [Google Scholar]
- 21. Dellacà RL, Pompilio PP, Walker PP, Duffy N, Pedotti A, Calverley PMA, et al. (2009) Effect of bronchodilation on expiratory flow limitation and resting lung mechanics in COPD. Eur Respir J 33: 1329–1337. 10.1183/09031936.00139608 [DOI] [PubMed] [Google Scholar]
- 22. Dellacà RL, Santus P, Aliverti A, Stevenson N, Centanni S, Macklem PT, et al. (2004) Detection of expiratory flow limitation in COPD using the forced oscillation technique. Eur Respir J 23: 232–240. [DOI] [PubMed] [Google Scholar]
- 23. Gobbi A, Milesi I, Govoni L, Pedotti A, Dellaca’ RL (2009) A New Telemedicine System for the Home Monitoring of Lung Function in Patients with Obstructive Respiratory Diseases 2009 Int Conf eHealth, Telemedicine, Soc Med: 117–122. [Google Scholar]
- 24. Kaczka DW, Barnas GM, Suki B, Lutchen KR (n.d.) Assessment of time-domain analyses for estimation of low-frequency respiratory mechanical properties and impedance spectra. Ann Biomed Eng 23: 135–151. [DOI] [PubMed] [Google Scholar]
- 25. Kaczka DW, Ingenito EP, Lutchen KR Technique to determine inspiratory impedance during mechanical ventilation: implications for flow limited patients. Ann Biomed Eng 27: 340–355. [DOI] [PubMed] [Google Scholar]
- 26. Furlan R, Diedrich A, Rimoldi A, Palazzolo L, Porta C, Diedrich L, et al. (2003) Effects of unilateral and bilateral carotid baroreflex stimulation on cardiac and neural sympathetic discharge oscillatory patterns. Circulation 108: 717–723. [DOI] [PubMed] [Google Scholar]
- 27. Bernardi L, De Barbieri G, Rosengård-Bärlund M, Mäkinen V-P, Porta C, Groop PH, et al. (2010) New method to measure and improve consistency of baroreflex sensitivity values. Clin Auton Res 20: 353–361. 10.1007/s10286-010-0079-1 [DOI] [PubMed] [Google Scholar]
- 28. Bertinieri G, di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G, et al. (1985) A new approach to analysis of the arterial baroreflex. J Hypertens Suppl 3: S79–S81. [PubMed] [Google Scholar]
- 29. Dean JB, Mulkey DK, Henderson RA, Potter SJ, Putnam RW (2004) Hyperoxia, reactive oxygen species, and hyperventilation: oxygen sensitivity of brain stem neurons. J Appl Physiol 96: 784–791. [DOI] [PubMed] [Google Scholar]
- 30. Ruan T, Ho C-Y, Kou YR (2003) Afferent vagal pathways mediating respiratory reflexes evoked by ROS in the lungs of anesthetized rats. J Appl Physiol 94: 1987–1998. [DOI] [PubMed] [Google Scholar]
- 31. Lund VE, Kentala E, Scheinin H, Klossner J, Helenius H, Sariola-Heinonen K, et al. (1999) Heart rate variability in healthy volunteers during normobaric and hyperbaric hyperoxia. Acta Physiol Scand 167: 29–35. [DOI] [PubMed] [Google Scholar]
- 32. Bernardi L (2002) Slow Breathing Increases Arterial Baroreflex Sensitivity in Patients With Chronic Heart Failure. Circulation 105: 143–145. [DOI] [PubMed] [Google Scholar]
- 33. Bernardi L, Valenti C, Wdowczyck-Szulc J, Frey AW, Rinaldi M, Spadacini G, et al. (1998) Influence of type of surgery on the occurrence of parasympathetic reinnervation after cardiac transplantation. Circulation 97: 1368–1374. [DOI] [PubMed] [Google Scholar]
- 34. Navajas D, Farré R (1999) Oscillation mechanics. Eur Respir Mon 12: 112–140. [Google Scholar]
- 35. Peslin R, Fredberg J (1986) Oscillation mechanics of the respiratory system Handbook of Physiology. pp. 145–166. [Google Scholar]
- 36. Smith H, Reinhold P, Goldman M (2005) Forced oscillation technique and impulse oscillometry. Eur Respir Mon 31: 72–105. [Google Scholar]
- 37. Stein JF, Widdicombe JG (1975) The interaction of chemo- and mechanoreceptor signals in the control of airway calibre. Respir Physiol 25: 363–376. 10.1016/0034-5687(75)90010-9 [DOI] [PubMed] [Google Scholar]
- 38. Canning BJ (2006) Reflex regulation of airway smooth muscle tone. J Appl Physiol 101: 971–985. 10.1152/japplphysiol.00313.2006 [DOI] [PubMed] [Google Scholar]
- 39. Karlsson JA, Sant’Ambrogio G, Widdicombe J (1988) Afferent neural pathways in cough and reflex bronchoconstriction. J Appl Physiol 65: 1007–1023. [DOI] [PubMed] [Google Scholar]
- 40. Coleridge HM, Coleridge JC, Schultz HD (1989) Afferent pathways involved in reflex regulation of airway smooth muscle. Pharmacol Ther 42: 1–63. [DOI] [PubMed] [Google Scholar]
- 41. Canning BJ, Fischer A (2001) Neural regulation of airway smooth muscle tone. Respir Physiol 125: 113–127. [DOI] [PubMed] [Google Scholar]
- 42. Weaver LK, Howe S, Snow GL, Deru K (2009) Arterial and pulmonary arterial hemodynamics and oxygen delivery/extraction in normal humans exposed to hyperbaric air and oxygen. J Appl Physiol 107: 336–345. 10.1152/japplphysiol.91012.2008 [DOI] [PubMed] [Google Scholar]
- 43. Budzinska K, Ilasz R (2008) Superoxide dismutase mimetic modulates hyperoxic augmentation of the diaphragmatic response to poikilocapnic hypoxia in non-vagotomized rats. J Physiol Pharmacol 59 Suppl 6: 163–172. [PubMed] [Google Scholar]
- 44. Keyl C, Schneider A, Hobbhahn J, Bernardi L (2002) Sinusoidal neck suction for evaluation of baroreflex sensitivity during desflurane and sevoflurane anesthesia. 1629–1636 [DOI] [PubMed] [Google Scholar]
- 45. Hughes J, Hoppin FJ, Mead J (1972) Effect of lung inflation on bronchial length and diameter in excised lungs. J Appl Physiol 32: 25–35. [DOI] [PubMed] [Google Scholar]
- 46. Krishna BH, Pal P, G K P, J B, E J, et al. (2014) Effect of yoga therapy on heart rate, blood pressure and cardiac autonomic function in heart failure. J Clin Diagn Res 8: 14–16. 10.7860/JCDR/2014/7472.4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Muralikrishnan K, Balasubramanian K, Balakrishnan B, Visnegarawla F (2012) Measurement of the effect of Isha Yoga on cardiac autonomic nervous system using short-term heart rate variability. J Ayurveda Integr Med 3: 91 10.4103/0975-9476.96528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Iellamo F, Legramante JM, Massaro M, Raimondi G, Galante A (2000) Effects of a residential exercise training on baroreflex sensitivity and heart rate variability in patients with coronary artery disease: A randomized, controlled study. 2588–2592 p. [DOI] [PubMed] [Google Scholar]
- 49. Hautala AJ, Kiviniemi AM, Tulppo MP (2009) Individual responses to aerobic exercise: The role of the autonomic nervous system. Neurosci Biobehav Rev 33: 107–115. 10.1016/j.neubiorev.2008.04.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.