Abstract
USP18 (Ubiquitin-like specific protease 18) is an enzyme cleaving ubiquitin from target proteins. USP18 plays a pivotal role in antiviral and antibacterial immune responses. On the other hand, ubiquitination participates in the regulation of several ion channels and transporters. USP18 sensitivity of transporters has, however, never been reported. The present study thus explored, whether USP18 modifies the activity of the peptide transporters PEPT1 and PEPT2, and whether the peptide transporters are sensitive to the ubiquitin ligase Nedd4-2. To this end, cRNA encoding PEPT1 or PEPT2 was injected into Xenopus laevis oocytes without or with additional injection of cRNA encoding USP18. Electrogenic peptide (glycine-glycine) transport was determined by dual electrode voltage clamp. As a result, in Xenopus laevis oocytes injected with cRNA encoding PEPT1 or PEPT2, but not in oocytes injected with water or with USP18 alone, application of the dipeptide gly-gly (2 mM) was followed by the appearance of an inward current (Igly-gly). Coexpression of USP18 significantly increased Igly-gly in both PEPT1 and PEPT2 expressing oocytes. Kinetic analysis revealed that coexpression of USP18 increased maximal Igly-gly. Conversely, overexpression of the ubiquitin ligase Nedd4-2 decreased Igly-gly. Coexpression of USP30 similarly increased Igly-gly in PEPT1 expressing oocytes. In conclusion, USP18 sensitive cellular functions include activity of the peptide transporters PEPT1 and PEPT2.
Introduction
The Ubiquitin-like specific protease 18 (USP18), a de-ubiquitin enzyme [1, 2], interacts with interferon α (IFNα)- mediated signalling [3–13] and thus plays a decisive role in the anti-viral [1, 8, 14–16] and antibacterial [5, 17] immune response as well as autoimmune disease [18]. USP18 is mainly located in the cytosol whereas USP18-sf, an isoform of USP18 is distributed in both cytosol and nucleus [4]. USP18 is in part effective by modulating the transcription factors NF-κB and NFAT [19, 20]. Moreover, USP18 competitively inhibits IFN-α/β-induced JAK/STAT activation [13] and upregulates epidermal growth factor receptor (EGFR) expression [21]. USP18 counteracts apoptosis [11, 12, 22] and contributes to the signalling of tumorigenesis and anti-tumor immune response [3, 8, 23–25].
Ubiquitination plays a pivotal role in the regulation of several transport processes [26, 27]. Ubiquitination controls endocytosis and turnover of transport proteins [27]. An ubiquitin ligase particularly important for the regulation of channels and transporters is Nedd4-2 (neuronal precursor cell expressed developmentally downregulated 4–2), which down-regulates a wide variety of transport processes [26, 28–35]. At least in theory, those transporters could be targeted by de-ubiquitination enzymes. As a matter of fact, some transient receptor potential (TRP) channels are regulated by the de-ubiquitinating enzyme cylindromatosis (CYLD) [36]. Surprisingly, little is known about effects of de-ubiquitinating enzymes on other transport systems. Specifically, to the best of our knowledge, a role of altered transport across the cell membrane in the pleotropic effects of USP18 has never been shown.
The present study explored whether USP18 influences the activity of peptide transporters 1 (PEPT1) and 2 (PEPT2), which accomplish electrogenic cellular uptake of di- and tripeptides [37–39] including peptide-like drugs [37, 38]. Regulators of peptide transporters include glucocorticoids [40], leptin [41] and growth hormone [42].
In order to test for an effect of USP18 on peptide transporters, cRNA encoding PEPT1 and PEPT2 were injected into Xenopus laevis oocytes with or without additional injection of cRNA encoding USP18. Subsequently, peptide transport was derived from peptide induced current.
Materials and Methods
Ethics Statement
All animal experiments were conducted according to the recommendations of the Guide for Care and Use of Laboratory Animals of the National Institutes of Health as well as the German law for the welfare of animals, and reviewed and approved by the respective government authority of the state Baden-Württemberg (Regierungspräsidium) prior to the start of the study (Anzeige für Organentnahme nach §6). The Xenopus oocytes were explanted from adult Xenopus laevis (NASCO, Fort Atkinson, USA). The frogs were anaesthesized by a 0.1% Tricain solution. After confirmation of anaesthesia and disinfection of the skin, a small abdominal incision was made and oocytes were removed, followed by closure of the skin by sutures. All efforts were made to minimize animal suffering.
Constructs
Constructs encoding rabbit PEPT1 or PEPT2 [43], human USP18 [14], human USP30 [44] and human KCNQ1/E1 or Kv7.1 [45] were used for generation of cRNA as described previously [43, 46].
Voltage clamp
Xenopus oocytes were prepared as previously described [47]. Where not indicated otherwise, 10 ng cRNA encoding PEPT1, 20 ng cRNA encoding PEPT2, 12 ng cRNA encoding KCNQ1/E1 (9 ng KCNQ1 + 3 ng KCNE1), 10 ng cRNA encoding USP18 or 10 ng cRNA encoding USP30 were injected on the same day after preparation of the oocytes [48]. The oocytes were maintained at 17°C in ND96 solution containing (in mM): 88.5 NaCl, 2 KCl, 1 MgC12, 1.8 CaC12, 2.5 NaOH, 5 HEPES (pH 7.4), sodium pyruvate (C3H3NaO3), Gentamycin (100 mg/l), Tetracycline (50 mg/l), Ciprofloxacin (1.6 mg/l), and Theophiline (90 mg/l). The voltage clamp experiments were performed at room temperature 3 days after injection of cRNA encoding PEPT1 and USP18 or 4 days after injection of cRNA encoding PEPT2 and USP18. Two-electrode voltage-clamp recordings were performed at a holding potential of -70 mV. The data were filtered at 10 Hz and recorded with a Digidata A/D-D/A converter (1322 Axon Instruments) and Clampex 9.2 software for data acquisition and analysis (Axon Instruments) [49]. The control superfusate (ND96) contained (in mM): 93.5 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, 2.5 NaOH and 5 HEPES, pH 7.4. [50]. Glycine-glycine was added to the solutions at a concentration of 2 mM, unless otherwise stated [51]. The flow rate of the superfusion was approx. 20 ml/min, and a complete exchange of the bath solution was reached within about 10 s [52]. The peptide induced current was in preliminary experiments 2.5 ± 0.9 nA (n = 9) 3 days and 13.1 ± 1.5 nA (n = 16) 4 days after injection of 20 ng cRNA encoding PEPT2. The peptide induced current was in preliminary experiments 3.9 ± 1.3 nA (n = 8) and 12.3 ± 1.9 nA (n = 16) 4 days after injection of 10 ng or 20 ng, respectively, cRNA encoding PEPT2.
Detection of PEPT2-HA cell surface expression by chemiluminescence
To determine PEPT2-HA cell surface expression by chemiluminescence [53], defolliculated oocytes were first injected with 20 ng cRNA encoding either PEPT2-HA and/or 10 ng cRNA encoding USP18. After 4 days of incubation, oocytes were blocked with 1% BSA in ND96 solution for 20 minutes and then incubated with anti-HA-HRP antibody (diluted 1:1000, Miltenyi Biotec, Germany). Next, oocytes were washed three times 10 minutes each with 1% BSA in ND96 solution followed by three times 10 minutes each in ND96 solution. Individual oocytes were placed in 96 well plates with 20 μl of SuperSignal ELISA Femto Maximum Sensitivity Substrate (Pierce, Rockford, IL, USA), and chemiluminescence of single occytes was quantified in a luminometer (Walter Wallac 2 plate reader, Perkin Elmer, Juegesheim, Germany) by integrating the signal over a period of 1 s. Results display normalized relative light units.
Statistical analysis
Data are provided as means ± SEM, n represents the number of oocytes investigated. All voltage clamp experiments were repeated with at least 3 batches of oocytes; in all repetitions qualitatively similar data were obtained. Data were tested for significance using ANOVA (Tukey test or Kruskal-Wallis test) or t-test, as appropriate. Results with p < 0.05 were considered statistically significant.
Results
In order to test for an effect of USP18 on the activity of the peptide transporters, the peptide transporters PEPT1 or PEPT2 were expressed in Xenopus laevis oocytes with or without additional expression of USP18. The inward current observed following addition of the dipeptide glycine-glycine (2 mM) to the bath solution (Igly-gly) was taken as a measure of peptide transport.
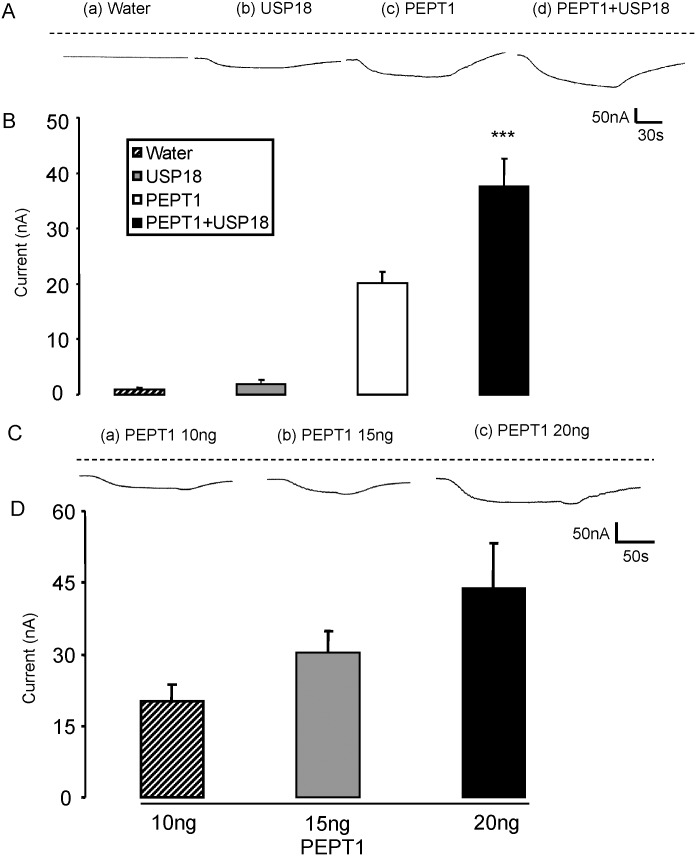
Igly-gly was negligible in water-injected Xenopus laevis oocytes (Fig 1A and 1B). Similarly, injection of cRNA encoding USP18 alone (Fig 1A and 1B) did not yield appreciable Igly-gly. Accordingly, neither in the presence nor in the absence of USP18, Xenopus laevis oocytes expressed sizable endogenous electrogenic glycine-glycine transport. In contrast, addition of glycine-glycine to the bath resulted in the appearance of Igly-gly in Xenopus laevis oocytes injected with cRNA encoding PEPT1. Igly-gly increased with increasing amounts of cRNA injected (Fig 1C and 1D). The additional injection of cRNA encoding wild type USP18 significantly increased Igly-gly in PEPT1 expressing Xenopus oocytes (Fig 1A and 1B).
Fig 1. Coexpression of USP18 increases electrogenic peptide transport in PEPT1-expressing Xenopus laevis oocytes.
A: Representative original tracings showing Igly-gly in Xenopus laevis oocytes injected with water (a), expressing USP18 alone (b) or expressing PEPT1 without (c) or with additional coexpression of wild type USP18 (d). B: Arithmetic means ± SEM (n = 12–18) of Igly-gly in Xenopus laevis oocytes injected with water (striped bar), or expressing USP18 alone (grey bar) or expressing PEPT1 without (white bar) or with (black bar) USP18. C: Representative original tracings showing glycine-glycine (2 mM)—induced current (Igly-gly) in Xenopus laevis oocytes injected with (a) 10 ng, (b) 15ng, or (c) 20ng cRNA encoding PEPT1. D: Arithmetic means ± SEM (n = 9–10) of Igly-gly in Xenopus laevis oocytes injected with 10 ng (striped bar) or 15 ng (grey bar) or 20 ng (black bar) cRNA encoding PEPT1. *** (p<0.001) indicates statistically significant difference from the absence of USP18.
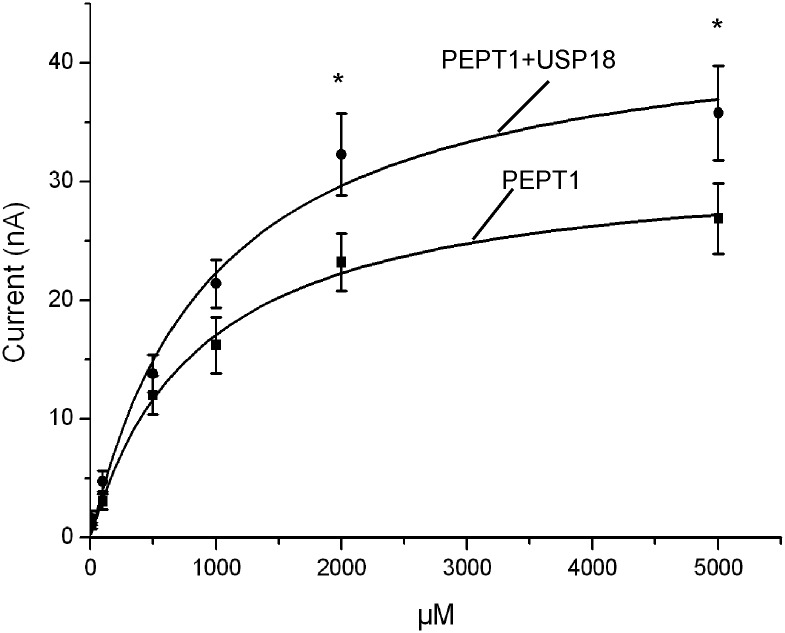
In order to test whether USP18 was effective by modifying maximal transport rate and/or affinity of PEPT1, the oocytes were exposed to glycine-glycine concentrations ranging from 10 μM to 5 mM. As shown in Fig 2 an increase of the bath peptide concentration was followed by an increase of Igly-gly in both, Xenopus oocytes expressing PEPT1 alone and Xenopus oocytes expressing PEPT1 and USP18. The increase of Igly-gly was, however, larger in Xenopus laevis oocytes expressing PEPT1 with USP18 than in Xenopus laevis oocytes expressing PEPT1 alone. Kinetic analysis yielded apparent maximal currents, which were significantly (p<0.05) higher in Xenopus laevis oocytes expressing both, PEPT1 and USP18 (47.7 ± 5.5 nA, n = 11–12) than in Xenopus laevis oocytes expressing PEPT1 alone (32.5 ± 3.3 nA, n = 11–12). The glycine-glycine concentrations required for half maximal current (KM) were not significantly different between Xenopus laevis oocytes expressing PEPT1 alone (1297 ± 379 μM, n = 11–12) and in Xenopus laevis oocytes expressing PEPT1 together with USP18 (1038 ± 160 μM, n = 11–12). Thus, USP18 did not significantly modify the affinity of PEPT1.
Fig 2. Coexpression of USP18 increases maximal electrogenic peptide transport in PEPT1-expressing Xenopus laevis oocytes.
Arithmetic means ± SEM (n = 11–12) of Igly-gly as a function of glycine-glycine concentration in Xenopus laevis oocytes expressing PEPT1 without (black squares), or with (black circles) additional coexpression of wild type USP18. *(p<0.05) indicates statistically significant difference from the absence of USP18.
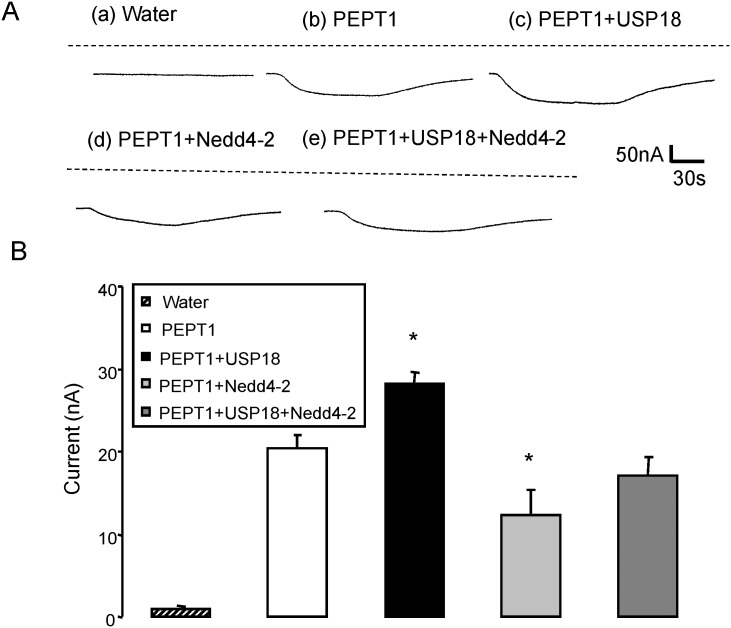
Additional experiments addressed the sensitivity of Igly-gly to the ubiquitin ligase Nedd4-2. To this end, PEPT1 or PEPT1 + USP18 were expressed without and with additional expression of Nedd4-2. As illustrated in Fig 3, coexpression of Nedd4-2 significantly (p<0.05) decreased Igly-gly in both, PEPT1 and USP18 expressing Xenopus laevis oocytes and in Xenopus laevis oocytes expressing PEPT1 without USP18.
Fig 3. Coexpression of Nedd4-2 decreases electrogenic peptide transport in PEPT1-expressing Xenopus laevis oocytes.
A: Representative original tracings showing Igly-gly in Xenopus laevis oocytes injected with water (a), expressing PEPT1 alone (b) or with USP18 (c), Nedd4-2 (d), or with USP18 and Nedd4-2 (e). B: Arithmetic means ± SEM (n = 13–15) of Igly-gly in Xenopus laevis oocytes injected with water (striped bar) or expressing PEPT1 without (white bar) or with USP18 (black bar), with Nedd4-2 (light grey bar), or with USP18 and Nedd4-2 (dark grey bar). *(p<0.05) indicates statistically significant difference from oocytes expressing PEPT1 alone.
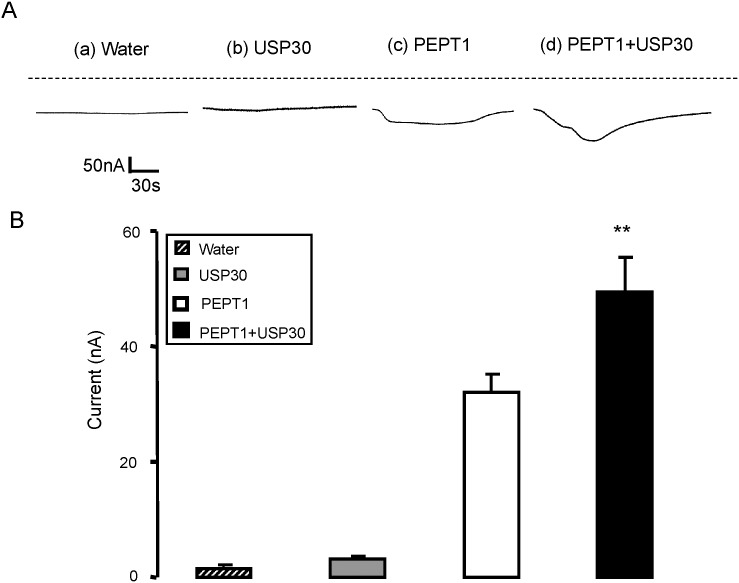
A separate series of experiments explored the effect of USP30, another member of deubiquitinating protease family of enzymes, on the peptide transporter PEPT1. In Xenopus oocytes expressing PEPT1, the addition of glycine-glycine to the bath was again followed by the appearance of Igly-gly, which was significantly (p<0.01) enhanced by additional injection of cRNA encoding USP30 (Fig 4).
Fig 4. Coexpression of USP30 increases electrogenic peptide transport in PEPT1-expressing Xenopus laevis oocytes.
A: Representative original tracings showing Igly-gly in Xenopus laevis oocytes injected with water (a), expressing USP30 alone (b) or expressing PEPT1 without (c) or with additional coexpression of wild type USP30 (d). B: Arithmetic means ± SEM (n = 14–16) of Igly-gly in Xenopus laevis oocytes injected with water (striped bar), or expressing USP30 alone (grey bar) or expressing PEPT1 without (white bar) or with (black bar) USP30. ** (p<0.01) indicates statistically significant difference from the absence of USP30.
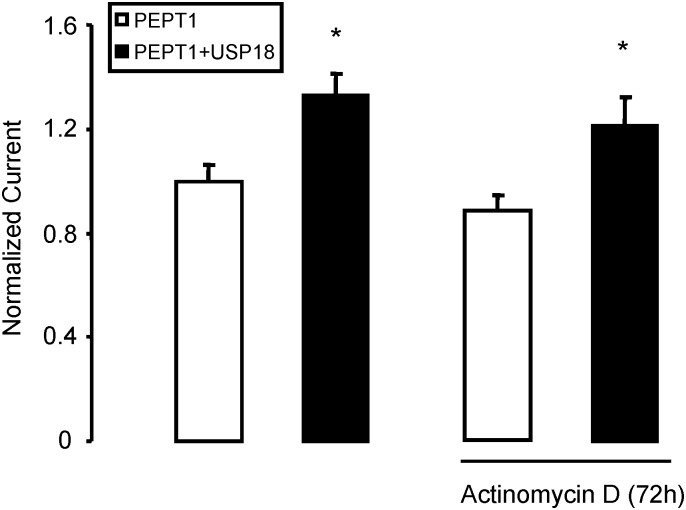
In order to test whether the effect of USP18 on Igly-gly required transcription, experiments were performed in the presence of actinomycin D (50 nM, added 72 hours prior to the experiment). Inhibition of transcription by incubation (72 hours) with actinomycin D (50 nM) did not significantly modify Igly-gly in presence or absence of USP18 (Fig 5).
Fig 5. Effect of USP18 on PEPT1 in absence and presence of Actinomycin D.
Arithmetic means ± SEM (n = 15–16) of Igly-gly in Xenopus oocytes injected PEPT1 without (white bar) or with (black bar) wild type USP18 in the absence (left bars) and presence (right bars) of 50 nM Actinomycin D 72 hours prior to measurement. *(p<0.05) indicates statistically significant difference from the absence of USP18.
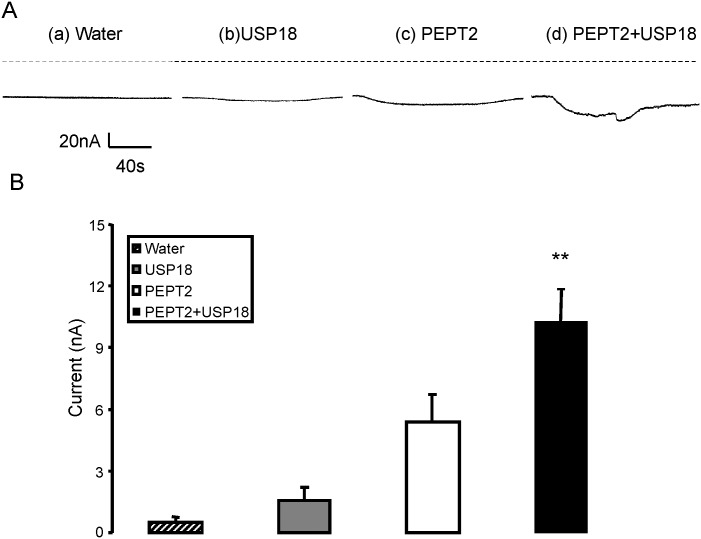
A further series of experiments explored the putative effects of USP18 on the peptide transporter isoform PEPT2. Similar to what has been observed in Xenopus oocytes expressing PEPT1, in Xenopus oocytes expressing PEPT2 addition of glycine-glycine to the bath was followed by the appearance of Igly-gly (5.4 ± 1.3 nA), which was significantly (p<0.05) enhanced by additional injection of cRNA encoding USP18 (10.2 ± 1.6 nA) (Fig 6).
Fig 6. Coexpression of USP18 increases electrogenic peptide transport in PEPT2-expressing Xenopus laevis oocytes.
A: Representative original tracings showing Igly-gly in Xenopus laevis oocytes injected with water (a) or expressing USP18 alone (b) or expressing PEPT2 without (c) or with additional coexpression of wild type USP18 (d). B: Arithmetic means ± SEM (n = 8–9) of Igly-gly in Xenopus oocytes injected with water (striped bar), expressing USP18 alone (grey bar), or expressing PEPT2 without (white bar) or with (black bar) wild type USP18. **(p<0.01) indicates statistically significant difference from the absence of USP18.
Chemiluminescence was employed to quantify PEPT2 protein abundance in the cell membrane. The protein abundance in the oocytes was, however, similar in oocytes co-expressing PEPT2-HA with USP18 (1.04 ± 0.05 relative light units, n = 113) and in oocytes expressing PEPT2-HA alone (1.00 ± 0.03 relative light units, n = 116).
A further series of experiments explored, whether the voltage gated K+ channel KCNQ1/E1 was sensitive to USP18. As a result, the current at +80mV was negligible in Xenopus laevis oocytes injected with water (1.2 ± 2.2 nA, n = 139) and was similarly high in Xenopus laevis oocytes injected with KCNQ1/E1 alone (190.3 ± 17.5 nA, n = 19) and in Xenopus laevis oocytes injected KCNQ1/E1 and USP18 (180.1 ± 10.7 nA, n = 19).
Discussion
The present study reveals a completely novel function of the de-ubiquitin enzyme USP18, i.e. the up-regulation of the peptide transporter isoforms, PEPT1 and PEPT2. The effect was mimicked by USP30 and may thus be a hitherto unknown function of several USP isoforms.
It is tempting to speculate that USP18 is effective by reversing the ubiquitination and subsequent degradation of the carrier protein. Accordingly, USP18 apparently enhances the maximal transport rate. A role of ubiquitination in the regulation of peptide transporters is further suggested by the experiments with Nedd4-2. Coexpression of the ubiquitin ligase Nedd4-2 tended to down-regulate the peptide transporter PEPT1. Comparison of the currents in Xenopus laevis oocytes expressing PEPT1 and USP18 with Xenopus laevis oocytes expressing PEPT1, USP18 and Nedd4-2 suggest that the effect of USP18 might be overridden by the effect of Nedd4-2. It must be kept in mind, though, that the effect of USP18 may be unrelated to that of Nedd4-2.
USP18 did not appreciably modify the protein PEPT2 protein abundance in the cell membrane. It cannot be excluded that USP18 is effective by mechanisms other than the de-ubiquination of the carrier protein. The effect of USP18 on peptide transport apparently does not require transcription. In theory, USP18 could modify peptide transporters by modifying the activity of proteins regulating peptide transporters. Signalling involved in the regulation of peptide transporters include phosphoinositide (PI) 3 kinase [54], phosphoinositide dependent kinase PDK1 [54], serum & glucocorticoid inducible kinase SGK1 [55] and AMP activated kinase [56].
The peptide transporters PEPT1 and PEPT2 accomplish the cellular uptake of di- and tripeptides [37–39] and several drugs [37, 56–58] including beta-lactam antibiotics, angiotensin-converting enzyme inhibitors, antiviral drugs, and anti-cancer agents [38, 59–63]. The carriers are expressed in a variety of cells including proximal renal tubules [64], enterocytes [38, 61], cancer cells [65, 66], and immune cells, such as macrophages [67–69]. The in vivo relevance of USP18 in the regulation of peptide transport remains, however, to be shown. It would be particularly interesting to learn, whether or not USP18 sensitivity of cellular peptide transport is relevant for antiviral and antibacterial activity of USP18.
In conclusion, USP18 has the potency to enhance the activity of the peptide transporters PEPT1 and PEPT2. Further experiments are needed to define the in vivo significance of USP18 sensitive peptide transport.
Acknowledgments
The authors acknowledge the meticulous preparation of the manuscript by Lejla Subasic and technical support by Elfriede Faber.
This work was funded by Deutsche Forschungsgemeinschaft (URL: http://www.dfg.de/): GRK 1302, SFB 773 B4/A1, La 315/13-3. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
We confirm that all findings in our study are freely available and shown within the manuscript.
Funding Statement
This work was funded by Deutsche Forschungsgemeinschaft (URL: http://www.dfg.de/): GRK 1302, SFB 773 B4/A1, La 315/13-3. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ait-Ali T, Wilson AW, Finlayson H, Carre W, Ramaiahgari SC, Westcott DG, et al. Functional analysis of the porcine USP18 and its role during porcine arterivirus replication. Gene. 2009;439(1–2):35–42. 10.1016/j.gene.2009.02.021 . [DOI] [PubMed] [Google Scholar]
- 2. Schwer H, Liu LQ, Zhou L, Little MT, Pan Z, Hetherington CJ, et al. Cloning and characterization of a novel human ubiquitin-specific protease, a homologue of murine UBP43 (Usp18). Genomics. 2000;65(1):44–52. 10.1006/geno.2000.6148 . [DOI] [PubMed] [Google Scholar]
- 3. Burkart C, Arimoto K, Tang T, Cong X, Xiao N, Liu YC, et al. Usp18 deficient mammary epithelial cells create an antitumour environment driven by hypersensitivity to IFN-lambda and elevated secretion of Cxcl10. EMBO molecular medicine. 2013;5(7):967–82. 10.1002/emmm.201201864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burkart C, Fan JB, Zhang DE. Two independent mechanisms promote expression of an N-terminal truncated USP18 isoform with higher DeISGylation activity in the nucleus. The Journal of biological chemistry. 2012;287(7):4883–93. 10.1074/jbc.M111.255570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dauphinee SM, Richer E, Eva MM, McIntosh F, Paquet M, Dangoor D, et al. Contribution of increased ISG15, ISGylation and deregulated type I IFN signaling in Usp18 mutant mice during the course of bacterial infections. Genes and immunity. 2014. 10.1038/gene.2014.17 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Francois-Newton V, Livingstone M, Payelle-Brogard B, Uze G, Pellegrini S. USP18 establishes the transcriptional and anti-proliferative interferon alpha/beta differential. The Biochemical journal. 2012;446(3):509–16. 10.1042/BJ20120541 . [DOI] [PubMed] [Google Scholar]
- 7. Francois-Newton V, Magno de Freitas Almeida G, Payelle-Brogard B, Monneron D, Pichard-Garcia L, Piehler J, et al. USP18-based negative feedback control is induced by type I and type III interferons and specifically inactivates interferon alpha response. PloS one. 2011;6(7):e22200 10.1371/journal.pone.0022200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hong B, Li H, Lu Y, Zhang M, Zheng Y, Qian J, et al. USP18 is crucial for IFN-gamma-mediated inhibition of B16 melanoma tumorigenesis and antitumor immunity. Molecular cancer. 2014;13:132 10.1186/1476-4598-13-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Malakhov MP, Malakhova OA, Kim KI, Ritchie KJ, Zhang DE. UBP43 (USP18) specifically removes ISG15 from conjugated proteins. The Journal of biological chemistry. 2002;277(12):9976–81. 10.1074/jbc.M109078200 . [DOI] [PubMed] [Google Scholar]
- 10. Murray EJ, Burden F, Horscroft N, Smith-Burchnell C, Westby M. Knockdown of USP18 increases alpha 2a interferon signaling and induction of interferon-stimulating genes but does not increase antiviral activity in Huh7 cells. Antimicrobial agents and chemotherapy. 2011;55(9):4311–9. 10.1128/AAC.00644-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Potu H, Sgorbissa A, Brancolini C. Identification of USP18 as an important regulator of the susceptibility to IFN-alpha and drug-induced apoptosis. Cancer research. 2010;70(2):655–65. 10.1158/0008-5472.CAN-09-1942 . [DOI] [PubMed] [Google Scholar]
- 12. Santin I, Moore F, Grieco FA, Marchetti P, Brancolini C, Eizirik DL. USP18 is a key regulator of the interferon-driven gene network modulating pancreatic beta cell inflammation and apoptosis. Cell death & disease. 2012;3:e419 10.1038/cddis.2012.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sarasin-Filipowicz M, Wang X, Yan M, Duong FH, Poli V, Hilton DJ, et al. Alpha interferon induces long-lasting refractoriness of JAK-STAT signaling in the mouse liver through induction of USP18/UBP43. Molecular and cellular biology. 2009;29(17):4841–51. 10.1128/MCB.00224-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Honke N, Shaabani N, Zhang DE, Iliakis G, Xu HC, Haussinger D, et al. Usp18 driven enforced viral replication in dendritic cells contributes to break of immunological tolerance in autoimmune diabetes. PLoS pathogens. 2013;9(10):e1003650 10.1371/journal.ppat.1003650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim JH, Luo JK, Zhang DE. The level of hepatitis B virus replication is not affected by protein ISG15 modification but is reduced by inhibition of UBP43 (USP18) expression. Journal of immunology. 2008;181(9):6467–72. . [DOI] [PubMed] [Google Scholar]
- 16. Ritchie KJ, Hahn CS, Kim KI, Yan M, Rosario D, Li L, et al. Role of ISG15 protease UBP43 (USP18) in innate immunity to viral infection. Nature medicine. 2004;10(12):1374–8. 10.1038/nm1133 . [DOI] [PubMed] [Google Scholar]
- 17. Richer E, Yuki KE, Dauphinee SM, Lariviere L, Paquet M, Malo D. Impact of Usp18 and IFN signaling in Salmonella-induced typhlitis. Genes and immunity. 2011;12(7):531–43. 10.1038/gene.2011.38 . [DOI] [PubMed] [Google Scholar]
- 18. Malhotra S, Morcillo-Suarez C, Nurtdinov R, Rio J, Sarro E, Moreno M, et al. Roles of the ubiquitin peptidase USP18 in multiple sclerosis and the response to interferon-beta treatment. European journal of neurology: the official journal of the European Federation of Neurological Societies. 2013;20(10):1390–7. 10.1111/ene.12193 . [DOI] [PubMed] [Google Scholar]
- 19. Liu X, Li H, Zhong B, Blonska M, Gorjestani S, Yan M, et al. USP18 inhibits NF-kappaB and NFAT activation during Th17 differentiation by deubiquitinating the TAK1-TAB1 complex. The Journal of experimental medicine. 2013;210(8):1575–90. 10.1084/jem.20122327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu D, Lillico SG, Barnett MW, Whitelaw CB, Archibald AL, Ait-Ali T. USP18 restricts PRRSV growth through alteration of nuclear translocation of NF-kappaB p65 and p50 in MARC-145 cells. Virus research. 2012;169(1):264–7. 10.1016/j.virusres.2012.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Duex JE, Sorkin A. RNA interference screen identifies Usp18 as a regulator of epidermal growth factor receptor synthesis. Molecular biology of the cell. 2009;20(6):1833–44. 10.1091/mbc.E08-08-0880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Manini I, Sgorbissa A, Potu H, Tomasella A, Brancolini C. The DeISGylase USP18 limits TRAIL-induced apoptosis through the regulation of TRAIL levels: Cellular levels of TRAIL influences responsiveness to TRAIL-induced apoptosis. Cancer biology & therapy. 2013;14(12):1158–66. 10.4161/cbt.26525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duex JE, Comeau L, Sorkin A, Purow B, Kefas B. Usp18 regulates epidermal growth factor (EGF) receptor expression and cancer cell survival via microRNA-7. The Journal of biological chemistry. 2011;286(28):25377–86. 10.1074/jbc.M111.222760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim YH, Kim WT, Jeong P, Ha YS, Kang HW, Yun SJ, et al. Novel combination markers for predicting survival in patients with muscle invasive bladder cancer: USP18 and DGCR2. Journal of Korean medical science. 2014;29(3):351–6. 10.3346/jkms.2014.29.3.351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shahidul Makki M, Cristy Ruteshouser E, Huff V. Ubiquitin specific protease 18 (Usp18) is a WT1 transcriptional target. Experimental cell research. 2013;319(5):612–22. 10.1016/j.yexcr.2012.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rotin D, Staub O. Role of the ubiquitin system in regulating ion transport. Pflugers Archiv: European journal of physiology. 2011;461(1):1–21. 10.1007/s00424-010-0893-2 . [DOI] [PubMed] [Google Scholar]
- 27. Miranda M, Sorkin A. Regulation of receptors and transporters by ubiquitination: new insights into surprisingly similar mechanisms. Molecular interventions. 2007;7(3):157–67. 10.1124/mi.7.3.7 . [DOI] [PubMed] [Google Scholar]
- 28. Baines D. Kinases as targets for ENaC regulation. Current molecular pharmacology. 2013;6(1):50–64. . [DOI] [PubMed] [Google Scholar]
- 29. Bongiorno D, Schuetz F, Poronnik P, Adams DJ. Regulation of voltage-gated ion channels in excitable cells by the ubiquitin ligases Nedd4 and Nedd4-2. Channels. 2011;5(1):79–88. . [DOI] [PubMed] [Google Scholar]
- 30. Eaton DC, Malik B, Bao HF, Yu L, Jain L. Regulation of epithelial sodium channel trafficking by ubiquitination. Proceedings of the American Thoracic Society. 2010;7(1):54–64. 10.1513/pats.200909-096JS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Laedermann CJ, Decosterd I, Abriel H. Ubiquitylation of voltage-gated sodium channels. Handbook of experimental pharmacology. 2014;221:231–50. 10.1007/978-3-642-41588-3_11 . [DOI] [PubMed] [Google Scholar]
- 32. Lang F, Eylenstein A, Shumilina E. Regulation of Orai1/STIM1 by the kinases SGK1 and AMPK. Cell calcium. 2012;52(5):347–54. 10.1016/j.ceca.2012.05.005 . [DOI] [PubMed] [Google Scholar]
- 33. Snyder PM. Down-regulating destruction: phosphorylation regulates the E3 ubiquitin ligase Nedd4-2. Science signaling. 2009;2(79):pe41 10.1126/scisignal.279pe41 . [DOI] [PubMed] [Google Scholar]
- 34. Soundararajan R, Lu M, Pearce D. Organization of the ENaC-regulatory machinery. Critical reviews in biochemistry and molecular biology. 2012;47(4):349–59. 10.3109/10409238.2012.678285 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang B, Kumar S. Nedd4 and Nedd4-2: closely related ubiquitin-protein ligases with distinct physiological functions. Cell death and differentiation. 2010;17(1):68–77. 10.1038/cdd.2009.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stokes A, Wakano C, Koblan-Huberson M, Adra CN, Fleig A, Turner H. TRPA1 is a substrate for de-ubiquitination by the tumor suppressor CYLD. Cellular signalling. 2006;18(10):1584–94. 10.1016/j.cellsig.2005.12.009 . [DOI] [PubMed] [Google Scholar]
- 37. Inoue M, Terada T, Okuda M, Inui K. Regulation of human peptide transporter 1 (PEPT1) in gastric cancer cells by anticancer drugs. Cancer Lett. 2005;230(1):72–80. [DOI] [PubMed] [Google Scholar]
- 38. Rubio-Aliaga I, Daniel H. Peptide transporters and their roles in physiological processes and drug disposition. Xenobiotica. 2008;38(7–8):1022–42. 10.1080/00498250802491654 [DOI] [PubMed] [Google Scholar]
- 39. Ingersoll SA, Ayyadurai S, Charania MA, Laroui H, Yan Y, Merlin D. The role and pathophysiological relevance of membrane transporter PepT1 in intestinal inflammation and inflammatory bowel disease. Am J Physiol GastrointestLiver Physiol. 2012;302(5):G484–G92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rexhepaj R, Dermaku-Sopjani M, Gehring EM, Sopjani M, Kempe DS, Foller M, et al. Stimulation of electrogenic glucose transport by glycogen synthase kinase 3. Cell Physiol Biochem. 2010;26(4–5):641–6. [DOI] [PubMed] [Google Scholar]
- 41. Yarandi SS, Hebbar G, Sauer CG, Cole CR, Ziegler TR. Diverse roles of leptin in the gastrointestinal tract: modulation of motility, absorption, growth, and inflammation. Nutrition. 2011;27(3):269–75. 10.1016/j.nut.2010.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alteheld B, Evans ME, Gu LH, Ganapathy V, Leibach FH, Jones DP, et al. Alanylglutamine dipeptide and growth hormone maintain PepT1-mediated transport in oxidatively stressed Caco-2 cells. JNutr. 2005;135(1):19–26. [DOI] [PubMed] [Google Scholar]
- 43. Hosseinzadeh Z, Dong L, Bhavsar SK, Warsi J, Almilaji A, Lang F. Upregulation of peptide transporters PEPT1 and PEPT2 by Janus kinase JAK2. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2013;31(4–5):673–82. 10.1159/000350086 . [DOI] [PubMed] [Google Scholar]
- 44. Cunningham CN, Baughman JM, Phu L, Tea JS, Yu C, Coons M, et al. USP30 and parkin homeostatically regulate atypical ubiquitin chains on mitochondria. Nature cell biology. 2015;17(2):160–9. 10.1038/ncb3097 . [DOI] [PubMed] [Google Scholar]
- 45. Strutz-Seebohm N, Henrion U, Schmitt N, Schulze-Bahr E, Seebohm G. A common structural component for beta-subunit mediated modulation of slow inactivation in different KV channels. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2013;31(6):968–80. 10.1159/000350115 . [DOI] [PubMed] [Google Scholar]
- 46. Munoz C, Pakladok T, Almilaji A, Elvira B, Decher N, Shumilina E, et al. Up-regulation of Kir2.1 (KCNJ2) by the serum & glucocorticoid inducible SGK3. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2014;33(2):491–500. 10.1159/000358629 . [DOI] [PubMed] [Google Scholar]
- 47. Fezai M, Elvira B, Borras J, Ben-Attia M, Hoseinzadeh Z, Lang F. Negative regulation of the creatine transporter SLC6A8 by SPAK and OSR1. Kidney & blood pressure research. 2014;39(6):546–54. 10.1159/000368465 . [DOI] [PubMed] [Google Scholar]
- 48. Almilaji A, Sopjani M, Elvira B, Borras J, Dermaku-Sopjani M, Munoz C, et al. Upregulation of the creatine transporter Slc6A8 by Klotho. Kidney & blood pressure research. 2014;39(6):516–25. 10.1159/000368462 . [DOI] [PubMed] [Google Scholar]
- 49. Warsi J, Dong L, Elvira B, Salker MS, Shumilina E, Hosseinzadeh Z, et al. SPAK dependent regulation of peptide transporters PEPT1 and PEPT2. Kidney & blood pressure research. 2014;39(4):388–98. 10.1159/000368451 . [DOI] [PubMed] [Google Scholar]
- 50. Pakladok T, Almilaji A, Munoz C, Alesutan I, Lang F. PIKfyve sensitivity of hERG channels. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2013;31(6):785–94. 10.1159/000350096 . [DOI] [PubMed] [Google Scholar]
- 51. Warsi J, Hosseinzadeh Z, Elvira B, Bissinger R, Shumilina E, Lang F. Regulation of ClC-2 activity by SPAK and OSR1. Kidney & blood pressure research. 2014;39(4):378–87. 10.1159/000355816 . [DOI] [PubMed] [Google Scholar]
- 52. Elvira B, Munoz C, Borras J, Chen H, Warsi J, Ajay SS, et al. SPAK and OSR1 dependent down-regulation of murine renal outer medullary K channel ROMK1. Kidney & blood pressure research. 2014;39(4):353–60. 10.1159/000355812 . [DOI] [PubMed] [Google Scholar]
- 53. Warsi J, Elvira B, Bissinger R, Shumilina E, Hosseinzadeh Z, Lang F. Downregulation of peptide transporters PEPT1 and PEPT2 by oxidative stress responsive kinase OSR1. Kidney & blood pressure research. 2014;39(6):591–9. 10.1159/000368469 . [DOI] [PubMed] [Google Scholar]
- 54. Rexhepaj R, Rotte A, Pasham V, Gu S, Kempe DS, Lang F. PI3 kinase and PDK1 in the regulation of the electrogenic intestinal dipeptide transport. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2010;25(6):715–22. [DOI] [PubMed] [Google Scholar]
- 55. Boehmer C, Palmada M, Klaus F, Jeyaraj S, Lindner R, Laufer J, et al. The peptide transporter PEPT2 is targeted by the protein kinase SGK1 and the scaffold protein NHERF2. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2008;22(5–6):705–14. [DOI] [PubMed] [Google Scholar]
- 56. Pieri M, Christian HC, Wilkins RJ, Boyd CA, Meredith D. The apical (hPepT1) and basolateral peptide transport systems of Caco-2 cells are regulated by AMP-activated protein kinase. AmJPhysiol GastrointestLiver Physiol. 2010;299(1):G136–G43. 10.1152/ajpgi.00014.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mitsuoka K, Kato Y, Miyoshi S, Murakami Y, Hiraiwa M, Kubo Y, et al. Inhibition of oligopeptide transporter suppress growth of human pancreatic cancer cells. EurJPharmSci. 2010;40(3):202–8. [DOI] [PubMed] [Google Scholar]
- 58. Tsume Y, Hilfinger JM, Amidon GL. Enhanced cancer cell growth inhibition by dipeptide prodrugs of floxuridine: increased transporter affinity and metabolic stability. MolPharm. 2008;5(5):717–27. 10.1021/mp800008c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Newstead S. Towards a structural understanding of drug and peptide transport within the proton-dependent oligopeptide transporter (POT) family. BiochemSocTrans. 2011;39(5):1353–8. 10.1042/BST0391353 [DOI] [PubMed] [Google Scholar]
- 60. Brandsch M. Transport of drugs by proton-coupled peptide transporters: pearls and pitfalls. ExpertOpinDrug Metab Toxicol. 2009;5(8):887–905. [DOI] [PubMed] [Google Scholar]
- 61. Meredith D. Review. The mammalian proton-coupled peptide cotransporter PepT1: sitting on the transporter-channel fence? PhilosTransRSocLond B BiolSci. 2009;364(1514):203–7. 10.1098/rstb.2008.0139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kamal MA, Keep RF, Smith DE. Role and relevance of PEPT2 in drug disposition, dynamics, and toxicity. Drug Metab Pharmacokinet. 2008;23(4):236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nakamura T, Yamamori M, Sakaeda T. Pharmacogenetics of intestinal absorption. CurrDrug Deliv. 2008;5(3):153–69. [DOI] [PubMed] [Google Scholar]
- 64. Terada T, Inui K. Recent advances in structural biology of peptide transporters. Current topics in membranes. 2012;70:257–74. 10.1016/B978-0-12-394316-3.00008-9 . [DOI] [PubMed] [Google Scholar]
- 65. Gonzalez DE, Covitz KM, Sadee W, Mrsny RJ. An oligopeptide transporter is expressed at high levels in the pancreatic carcinoma cell lines AsPc-1 and Capan-2. Cancer Res. 1998;58(3):519–25. [PubMed] [Google Scholar]
- 66. Tai W, Chen Z, Cheng K. Expression Profile and Functional Activity of Peptide Transporters in Prostate Cancer Cells. Mol Pharm. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ayyadurai S, Charania MA, Xiao B, Viennois E, Merlin D. PepT1 expressed in immune cells has an important role in promoting the immune response during experimentally induced colitis. Laboratory investigation; a journal of technical methods and pathology. 2013;93(8):888–99. 10.1038/labinvest.2013.77 . [DOI] [PubMed] [Google Scholar]
- 68. Sun D, Wang Y, Tan F, Fang D, Hu Y, Smith DE, et al. Functional and molecular expression of the proton-coupled oligopeptide transporters in spleen and macrophages from mouse and human. Molecular pharmaceutics. 2013;10(4):1409–16. 10.1021/mp300700p [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yang XD, Ma JY, Barger MW, Ma JK. Transport and utilization of arginine and arginine-containing peptides by rat alveolar macrophages. Pharmaceutical research. 2002;19(6):825–31. doi: 12134953 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We confirm that all findings in our study are freely available and shown within the manuscript.