Abstract
Objectives
To analyze kidney injury molecule-1 (KIM-1) and neutrophil gelatinase-associated lipocalin (N-GAL) excretion post-intravenous contrast enhanced-CT (CE-CT) in patients with chronic kidney disease (CKD).
Methods
Patients were enrolled in a trial on hydration regimes to prevent contrast-induced acute kidney injury (CI-AKI). Blood and urine samples were taken at baseline, 4 – 6, and 48 – 96 h post CE-CT. Urinary KIM-1 and N-GAL values were normalized for urinary creatinine levels, presented as medians with 2.5 – 97.5 percentiles.
Results
Of the enrolled 511 patients, 10 (2 %) were lost to follow-up. CI-AKI occurred in 3.9 % of patients (20/501). Median KIM-1 values were 1.2 (0.1 – 7.7) at baseline, 1.3 (0.1 – 8.6) at 4 – 6 h, and 1.3 ng/mg (0.1 – 8.1) at 48 – 96 h post CE-CT (P = 0.39). Median N-GAL values were 41.0 (4.4 – 3,174.4), 48.9 (5.7 – 3,406.1), and 37.8 μg/mg (3.5 – 3,200.4), respectively (P = 0.07). The amount of KIM-1 and N-GAL excretion in follow-up was similar for patients with and without CI-AKI (P-value KIM-1 0.08, P-value N-GAL 0.73). Neither patient characteristics at baseline including severe CKD, medication use, nor contrast dose were associated with increased excretion of KIM-1 or N-GAL during follow-up.
Conclusion
KIM-1 and N-GAL excretion were unaffected by CE-CT both in patients with and without CI-AKI, suggesting that CI-AKI was not accompanied by tubular injury.
Key Points
• KIM-1 and N-GAL excretion were unaffected by intravenous contrast-enhanced CT (CE-CT).
• Patient or procedure characteristics were not associated with increased KIM-1 or N-GAL excretion.
• Performance of CE-CT in CKD patients is likely to be safe.
Keywords: Acute kidney injury; Contrast media; Renal insufficiency, chronic; Biological markers; Multidetector computed tomography
Introduction
Contrast-induced acute kidney injury (CI-AKI) is an acute decline in renal function following administration of iodinated contrast media [1, 2]. CI-AKI occurs in 5 – 6 % of patients undergoing intravenous contrast enhanced computed tomography (CE-CT), a very common procedure worldwide [3, 4]. Although the definition of CI-AKI is the subject of debate, all proposed criteria are based on changes in serum creatinine within a few days following contrast administration [5]. However, serum creatinine is regarded as a non-specific marker for CI-AKI since several mechanisms (i.e. use of medication, hemodynamics, and comorbidity such as peripheral artery disease or diabetes) can also influence its value [1, 6, 7]. The clinical significance of serum creatinine changes post CE-CT has become disputable after publication of studies suggesting that fluctuations in serum creatinine occur as frequent in patients undergoing CE-CT, as in those not receiving contrast media [8, 9]. However, physicians are still concerned about the risk of CI-AKI and are hesitant to use CE-CT in their diagnostic workup, especially in patients with pre-existing chronic kidney disease (CKD) [8]. Knowledge about the risk of renal injury post-CE-CT is, therefore, of clinical importance and may be derived from studies measuring biomarkers of acute kidney injury in the context of CI-AKI, such as kidney injury molecule-1 (KIM-1) and neutrophil gelatinase-associated lipocalin (N-GAL). These biomarkers have been proven to be predictive of CI-AKI in the setting of percutaneous coronary interventions requiring intra-arterial contrast administrations [10–16]. Yet, although N-GAL excretion has been analyzed in a small cohort of patients undergoing CE-CT, these biomarkers have not been studied thoroughly within this population. Hence, the aim of our study was to investigate KIM-1 and N-GAL excretion and their association with the occurrence CI-AKI after CE-CT in patients with pre-existing CKD.
Methods
Study patients were enrolled in a randomized controlled trial on CI-AKI preventing hydration regimes between January 2010 and June 2012 in four Dutch hospitals (Leiden University Medical Center, Leiden, Bronovo Hospital, The Hague, St. Lucas Andreas Hospital, Amsterdam, and St. Antonius Hospital, Nieuwegein). Patients electively underwent CE-CT and were randomized to either 250 ml 1.4 % sodium bicarbonate hydration 1 h prior to CE-CT or standard treatment with 1,000 ml 0.9 % saline during 4 to 12 h prior to and after CE-CT. No other CI-AKI preventive treatments were used, such as administration of N-acetylcysteine. Patients were 18 years or older, eligible to receive the fluid challenge of saline hydration, and had an estimated glomerular filtration rate (eGFR) < 60 ml/min. eGFR values were calculated using the abbreviate modification of diet in renal disease (MDRD) formula [17]. Exclusion criteria were documented allergy for iodinated contrast media, pregnancy, previous contrast administrations within 7 days prior to CE-CT, and hemodynamic instability. In addition to the general exclusion criteria for the trial, patients in whom CT was cancelled or unexpectedly performed without intravenous contrast administration were also excluded for the current analysis. Type of contrast media used at time of CE-CT was according to clinical practice in the participating hospitals and varied between Iomeprol (Iomeron, Bracco Imaging, Milan, Italy), Iobitridol (Xenetix, Guerbet, Aulnay-sous-Bois, France), or Iodixanol (Visipaque, GE Healthcare, Chalfort St. Giles, UK). An independent data and safety monitoring board periodically reviewed study outcomes. All patients provided written informed consent and the protocol of the trial was approved by the institutional review boards of each of the participating centres. The trial was performed according to the declaration of Helsinki and was registered with the Netherlands Trial Register, NTR 2149.
Materials
Serum and urine samples were collected prior to hydration, and once between 4 – 6 and 48 – 96 h post-CE-CT for analyses of serum creatinine and urinary creatinine, KIM-1 and N-GAL levels. Urinary creatinine levels were used to correct KIM-1 and N-GAL values for dilution as a result of the two hydration regimes [18]. Therefore, analyses were made using N-GAL/urine creatinine and KIM-1/urine creatinine ratios, which will be revered to as KIM-1 (in ng/mg) and N-GAL (μg/mg) excretion. Serum and urine samples were also collected 2 months post-CE-CT for patients developing CI-AKI, to determine whether renal function had recovered. Furthermore, information on patient characteristics at baseline, use of nephrotoxic medication and injected contrast volumes were collected.
Outcomes and Definitions
Primary outcomes of our study were relative changes in KIM-1 and N-GAL excretion post CE-CT as compared with baseline. Furthermore, we studied whether changes in KIM-1 and N-GAL excretion post-CE-CT correlated to changes in serum creatinine or the occurrence of CI-AKI. CI-AKI was defined by an increase in serum creatinine over 25 % or 44 μmol/L (0.5 mg/L) within 48 – 96 h post-CE-CT. Renal function was considered recovered if the increase in serum creatinine 2 months post-CE-CT no longer exceeded these thresholds.
Laboratory Methods
All samples were stored at -80 °C. Serum and urine creatinine levels were analyzed using Roche Diagnostics, Mannheim, Germany. Urinary KIM-1 and N-GAL values were analyzed using ELISA (R&D systems, DuoSets Human TIM-1/KIM-1/HAVCR and Lipocalin-2/N-GAL, MN, USA).
Statistical Analyses
KIM-1 and N-GAL excretion at 4 – 6 and 48 – 96 h post-CE-CT were compared with baseline using linear mixed models based on log-transformed KIM-1 and N-GAL values as they were non-normally distributed among the population. The base linear mixed model contained random subject effects and fixed effects of time (categorical), randomization, and their interaction. To study whether baseline conditions or contrast dose were associated with changes in KIM-1 and N-GAL excretion during follow-up, these factors and their interaction with time were added to the base model. Correlation studies were performed on the association between changes in serum creatinine and in KIM-1 and N-GAL excretion at 4 – 6 and 48 – 96 h post-CE-CT compared with baseline, using Spearman’s correlation tests, and reported as correlation terms with corresponding P-values. The area under the receiver operating curve (ROC) was calculated under nonparametric assumption, to analyze the predictive value of KIM-1 and N-GAL excretion for the occurrence of CI-AKI measured at baseline, 4 – 6 and 48 – 96 h post-CE-CT. P-values < 0.05 were considered statistically significant. All statistical analyses were performed using SPSS version 20.
Results
Study population
A total of 570 patients provided written informed consent. Of those, 22 patients withdrew their consent and CT was performed without intravenous contrast administration or cancelled in 37 patients. As a result, the total population for this analysis comprises 511 patients of whom 10 (2.0 %) where lost to follow-up for the endpoint of CI-AKI. Patient characteristics at baseline are reported in Table 1. Mean relative increase in serum creatinine 48 – 96 h post-CE-CT compared with baseline was 1.2 % (SD 13.3 %). CI-AKI occurred in 3.9 % of patients (20/501, 95 % CI 2.6 – 6.1 %). Renal function recovered within 2 months post-CE-CT in 13 out of 19 (68.0 %) patients diagnosed with CI-AKI and one patient was lost to follow-up. None of the patients developing CI-AKI had a need for renal replacement therapy within 2 months after CE-CT.
Table 1.
Patient characteristics at baseline
| N | 511 | |
|---|---|---|
| Mean age, years | 72.3 | (9.4) |
| Sex, male | 308 | (60.3) |
| Outpatients | 478 | (93.5) |
| Mean eGFR ml/min | 50.7 | (13.8) |
| eGFR > 45 ml/min | 354 | (69.3) |
| eGFR 30-45 ml/min | 121 | (23.7) |
| eGFR < 30 ml/min | 36 | (7.0) |
| Hypertension | 256 | (50.1) |
| Diabetes mellitus | 137 | (26.8) |
| Peripheral artery disease | 168 | (32.9) |
| Coronary artery disease | 175 | (34.2) |
| Congestive heart failure | 87 | (17.0) |
| Primary renal or urological disease | 224 | (43.8) |
| Medication | ||
| NSAIDs | 26 | (5.1) |
| Diuretics | 234 | (45.8) |
| ACE-inhibitors | 205 | (40.1) |
| Angiotensin II receptor blockers | 101 | (19.8) |
| Chemotherapy | 11 | (2.2) |
| Preprocedural stop of medication | 117 | (22.9) |
| Type of CT-scan | ||
| CT abdomen | 167 | (32.7) |
| CT thorax | 46 | (9.0) |
| CT angiography | 190 | (37.2) |
| CT thorax-abdomen | 29 | (5.7) |
| CT kidney-pelvis | 43 | (8.4) |
| Other | 36 | (7.05) |
| Mean contrast volume, ml | 105.2 | (21.3) |
Data are presented as mean (SD), or n (%)
eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease; NSAIDs, non-steroidal anti-inflammatory drugs
N-GAL and KIM-1 excretion
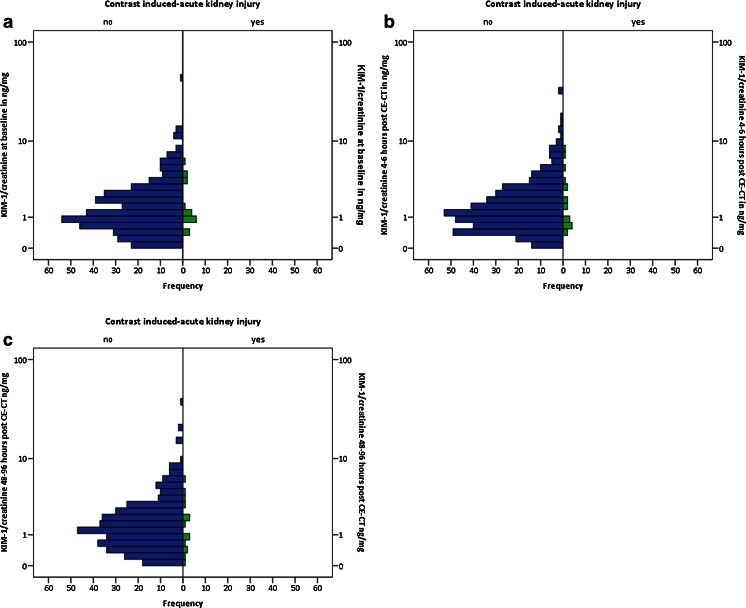
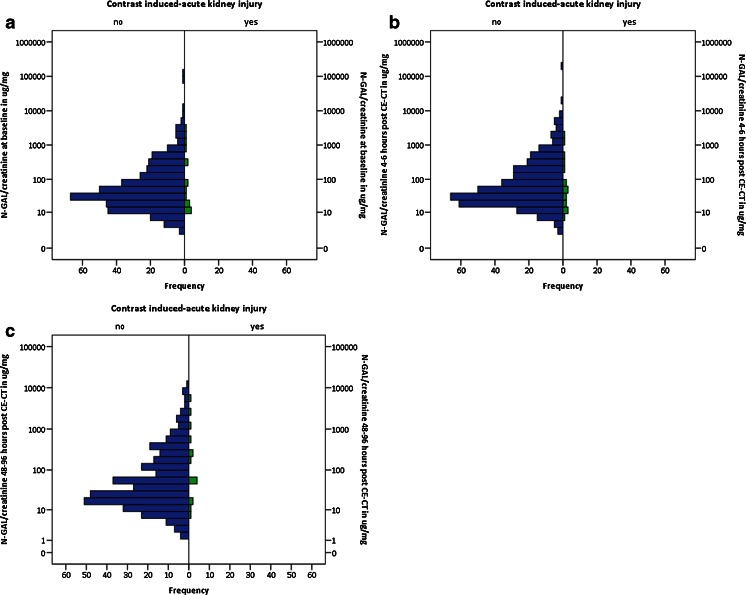
Baseline KIM-1 and N-GAL values correlated well with KIM-1 (Spearman’s rho 0.83, P < 0.001) and N-GAL excretion adjusted for dilution by urine creatinine (Spearman’s rho 0.87, P < 0.001) (Fig. 1 a, b). Median KIM-1 and N-GAL excretion at baseline, 4 – 6 h and 48 – 96 h post-CE-CT are reported in Table 2. Figures 2 and 3 reflect KIM-1 and N-GAL excretion in patients with and patients without CI-AKI at A) baseline, B) 4 – 6 h, and C) 48 – 96 h post-intravenous contrast-enhanced computed tomography. Both KIM-1 and N-GAL excretion remained unchanged during follow-up within the total population. Additionally, the relative change in KIM-1 and N-GAL excretion during follow-up was similar for patients with and without CI-AKI (P-value for KIM-1 excretion = 0.08 and for N-GAL excretion = 0.73), suggesting that CI-AKI post-CE-CT was not associated with tubular epithelial damage. No correlations were found between an increase in serum creatinine and N-GAL excretion 4 – 6 h (correlation term -0.12, P = 0.18), and 48 – 96 h post-CE-CT (correlation term -0.04, P = 0.51), or between an increase in serum creatinine and KIM-1 excretion at the same time points (correlation term 0.07, P = 0.17, and 0.08, P = 0.12, respectively). Regression analysis was performed to identify subgroups of patient susceptible for changes in KIM-1 and N-GAL excretion post-CE-CT, the results of which are reported in Table 3. Neither patient characteristics at baseline including severe CKD (i.e. eGFR < 30 ml/min), medication use, type of hydration regime, nor contrast dose were associated with changes in KIM-1 or N-GAL excretion during follow-up.
Fig. 1.
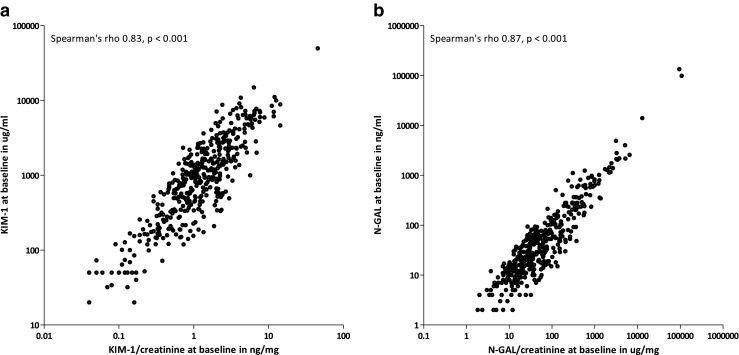
Correlation between A) KIM-1 and KIM-1/urine creatinine at baseline, and B) N-GAL and N-GAL/urine creatinine at baseline
Table 2.
KIM-1 and N-GAL excretions during follow-up
| Baseline | 4 – 6 h post-CE-CT | 48 – 96 h post-CE-CT | P-value | ||||
|---|---|---|---|---|---|---|---|
| KIM-1 ratio in ng/mg* | 1.2 | (0.1 – 7.7) | 1.3 | (0.1 – 8.6) | 1.3 | (0.1 – 8.1) | 0.39 |
| N-GAL in ug/mg † | 41.0 | (4.4 – 3,174.4) | 48.9 | (5.7 – 3,406.1) | 37.8 | (3.5 – 3,200.4) | 0.07 |
Data are presented as median with 2.5 – 97.5 percentiles
*Values available at baseline in 431/511, at 4 – 6 h in 440/511, and at 48 – 96 h post-CE-CET in 401/511
†Values available at baseline in 417/511, at 4 – 6 h in 419/511, and at 48 – 96 h post-CE-CT in 388/511
Fig. 2.
Figures reflect KIM-1 excretions in patients with (in green) and patients without (in blue) contrast-induced acute kidney injury at A) baseline, B) 4 – 6 h, and C) 48 – 96 h post-intravenous contrast-enhanced computed tomography
Fig. 3.
Figures reflect N-GAL excretions in patients with (in green) and patients without (in blue) contrast-induced acute kidney injury at A) baseline, B) 4 – 6 h, and C) 48 – 96 h post-intravenous contrast-enhanced computed tomography
Table 3.
Effects of comorbidity, medication, and contrast dose on KIM-1 and N-GAL excretion
| Variable | Median KIM-1 ratio (2.5-97.5 percentiles) | P-value | Median N-GAL ratio (2.5-97.5 percentiles) | P-value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 4-6 h | 48-96 h | Baseline | 4-6 h | 48-96 h | |||||||||
| Age | ||||||||||||||
| > 75 years | 1.2 | (0.1-6.3) | 1.2 | (0.1-8.1) | 1.4 | (0.1-7.9) | 0.58 | 44.4 | (5.0-3403.0) | 54.8 | (6.9-4336.2) | 41.9 | (3.1-3867.3) | 1.0 |
| < 75 years | 1.2 | (0.1-8.3) | 1.3 | (0.1-10.4) | 1.3 | (0.1-13.9) | 39.25 | (4.1-2965.0) | 43.1 | (4.5-2614.1) | 36.0 | (3.7-3122.8) | ||
| Severe CKD | ||||||||||||||
| Present | 1.9 | (0.1-11.7) | 1.3 | (0.2-8.9) | 1.9 | (0.1-9.3) | 0.16 | 239.1 | (7.2-3734.5) | 190.3 | (13.2-4486.3) | 176.5 | (10.3-5341.3) | 0.84 |
| Absent | 1.2 | (0.1-6.9) | 1.3 | (0.1-8.6) | 1.3 | (0.1-8.0) | 38.1 | (4.3-2523.9) | 44.1 | (5.6-2529.1) | 33.9 | (3.4-2251.1) | ||
| Diabetes mellitus | ||||||||||||||
| Present | 1.4 | (0.1-6.6) | 1.3 | (0.2-8.0) | 1.4 | (0.1-6.9) | 0.41 | 51.6 | (7.0-2714.5) | 49.9 | (8.6-2661.9) | 43.7 | (6.4-2010.2) | 0.66 |
| Absent | 1.1 | (0.1-11.2) | 1.3 | (0.1-1.3) | 1.3 | (0.1-8.9) | 39.5 | (3.9-3224.1) | 48.5 | (5.6-3702.9) | 32.6 | (2.8-4584.2) | ||
| Congestive heart failure | ||||||||||||||
| Present | 1.5 | (0.1-6.6) | 1.6 | (0.1-9.9) | 1.9 | (0.1-7.7) | 0.98 | 39.5 | (5.9-1162.2) | 45.5 | (2.4-1199.4) | 43.5 | (2.5-722.7) | 0.71 |
| Absent | 1.2 | (0.1-8.9) | 1.2 | (0.1-8.6) | 1.3 | (0.1-8.7) | 41.1 | (4.2-3291.3) | 49.9 | (6.0-4086.2) | 37.1 | (3.7-3927.8) | ||
| Coronary artery disease | ||||||||||||||
| Present | 1.3 | (0.1-6.4) | 1.3 | (0.1-8.3) | 1.5 | (0.2-7.7) | 0.56 | 34.9 | (4.8-4012.5) | 42.6 | (5.2-4198.7) | 32.3 | (2.5-2003.4) | 0.80 |
| Absent | 1.1 | (0.1-11.7) | 1.3 | (0.1-10.6) | 1.3 | (0.1-9.1) | 44.5 | (4.3-3216.7) | 50.3 | (6.0-3304.7) | 42.2 | (4.6-4477.4) | ||
| Peripheral artery disease | ||||||||||||||
| Present | 1.3 | (0.1-6.3) | 1.4 | (0.1-7.7) | 1.3 | (0.1-7.2) | 0.99 | 35.0 | (3.9-760.8) | 44.6 | (5.8-1383.1) | 32.1 | (2.6-1140.9) | 0.77 |
| Absent | 1.2 | (0.1-10.5) | 1.2 | (0.1-10.5) | 1.3 | (0.1-8.5) | 42.4 | (4.4-3703.5) | 49.2 | (5.7-4593.8) | 39.5 | (3.9-4680.3) | ||
| NSAIDs use | ||||||||||||||
| Present | 1.2 | (0.1-2.4) | 1.0 | (0.1-8.4) | 0.8 | (0.1-4.2) | 0.81 | 43.2 | (5.0-614.5) | 49.6 | (3.5-842.4) | 49.1 | (5.4-633.1) | 0.91 |
| Absent | 1.2 | (0.1-7.7) | 1.3 | (0.1-8.5) | 1.4 | (0.1-8.1) | 40.1 | (4.3-3216.7) | 48.7 | (5.7-3600.9) | 37.1 | (3.4-3741.2) | ||
| Diuretic use | ||||||||||||||
| Present | 1.4 | (0.1-7.6) | 1.4 | (0.2-8.2) | 1.5 | (0.1-7.0) | 0.98 | 38.6 | (5.0-1511.4) | 36.7 | (6.0-2499.2) | 31.5 | (3.2-2688.5) | 0.71 |
| Absent | 1.1 | (0.1-11.1) | 1.2 | (0.1-10.7) | 1.3 | (0.1-9.1) | 41.7 | (3.9-3976.3) | 56.8 | (5.4-4785.5) | 43.7 | (3.8-3916.6) | ||
| Contrast dose* | ||||||||||||||
| High | 2.1 | (0.2-11.9) | 1.6 | (0.2-18.7) | 1.9 | (0.2-12.0) | 0.29 | 68.4 | (5.4-7919.6) | 88.1 | (8.5-5885.2) (8.5 | 55.3 | (6.7-8300.8) | 0.72 |
| Low | 1.1 | (0.1-6.8) | 1.2 | (0.1-8.4) | 1.3 | (0.1-7.9) | 38.3 | (4.3-2417.1) | 43.6 | (5.2-2514.1) | 36.5 | (3.4-2157.7) | ||
Diagnostic test characteristics
We calculated the diagnostic value of KIM-1 and N-GAL excretion at baseline, 4 – 6 and 48 – 96 h post-CE-CT for the endpoint of CI-AKI (Table 4). Overall, the performance of the biomarkers was poor, with the highest area under the ROC for N-GAL excretion measured between 48 – 96 h post-CE-CT (area 0.63, 95 % CI 0.45 – 0.80).
Table 4.
Test characteristics of KIM-1 and N-GAL excretion for the diagnosis of contrast-induced acute kidney injury after 48 – 96 h
| Biomarker | AUC | 95 % CI |
|---|---|---|
| KIM-1 ratio | ||
| Baseline | 0.47 | (0.34-0.60) |
| 4-6 h post-CE-CT | 0.52 | (0.36-0.66) |
| 48-96 h post-CE-CT | 0.50 | (0.34-0.65) |
| N-GAL ratio | ||
| Baseline | 0.55 | (0.36-0.65) |
| 4-6 h post-CE-CT | 0.53 | (0.35-0.70) |
| 48-96 h post-CE-CT | 0.63 | (0.45-0.80) |
AUC area under the curve; CE-CT intravenous contrast media-enhanced CT
Discussion
In the present study, we show that KIM-1 and N-GAL excretion were unaffected by CE-CT in CKD patients both with and without CI-AKI. This finding suggests that CE-CT was not accompanied by tubular injury.
The American College of Radiology and the European Society of Urology and Radiology guidelines on CI-AKI prevention state that the risk of CI-AKI is lower after CE-CT compared with intra-arterial contrast administration and that therefore, preventive hydration can be withheld in either all CKD patients [19], or at least in those with CKD stage 3A or less (i.e. eGFR > 45 – 60 ml/min) [2] undergoing CE-CT. The level of evidence for these guideline recommendations is low, as they were based on retrospective studies comparing patients undergoing CE-CT with a control group not receiving iodinated contrast media, showing similar risks of acute kidney injury in both groups [9, 20–23]. However, these studies were prone for confounding by factors associated with the indication for contrast media use despite their attempts to adjust for this matter, and their results are, therefore, difficult to generalize into clinical practice [24].
The current understanding of the pathophysiology of CI-AKI is based on tubular injury due to direct toxic effects of contrast agents and secondary tubular injury due to ischemia. We used a valid method to study contrast media induced tubular injury, by measuring KIM-1 and N-GAL excretion post-CE-CT, two well-known biomarkers of CI-AKI as shown by several studies among patients undergoing percutaneous coronary interventions [11, 13–16]. Our study results suggest that in both CKD patients with and without CI-AKI, signals for tubular injury post-CE-CT are lacking. Hence, changes in serum creatinine post-CE-CT might be harmless, and possibly the result of interplay between contrast media-induced vasoconstriction [1], daily intra-individual variations in serum creatinine [24], and co-morbidity affecting serum creatinine values. Our study, therefore, challenges the creatinine-based definition of CI-AKI.
Only two prior studies reported on N-GAL expression post CE-CT and found significant increases in urinary N-GAL after 6 and 8 h, respectively [25, 26]. However, in the first study, CI-AKI occurred in 23/60 (23 %) patients, which is much higher than the 5 – 6 % reported by two meta-analyses and the 3.9 % observed in our study [4, 27]. It is, therefore, questionable whether this study is representative of the general CKD population undergoing CE-CT in daily practice. The second study only included inpatients (n = 47), who might be more prone to develop AKI due to other causes than contrast media administration, which was also reflected by the 8.5 % incidence of CI-AKI [26].
The KIM-1 receptor has been proposed to have two different functions [28]. First, KIM-1 serves as a phagocytic receptor on tubular cells contributing to repair processes [28, 29]. Second, KIM-1 might also have an inflammatory modulating role attracting macrophages, monocytes, and fibroblast to the region where epithelium cells are damaged [28]. The exact working mechanism of N-GAL is unclear, although it has been proposed that N-GAL limits tubular injury by modulation of cellular responses such proliferation, apoptosis, and differentiation [30, 31].
In contrast with our results, studies on KIM-1 and N-GAL excretion after intra-arterial contrast injections (not intravenous such as for CE-CT) did find increased levels of these biomarkers during follow-up [11, 13–16]. One of these studies reported absence of an association between N-GAL excretion and adverse clinical outcomes after coronary angiography, whereas the other studies did not report results on the association between increased KIM-1 or N-GAL excretion and adverse clinical outcomes. Therefore, the clinical relevance of the increased KIM-1 or N-GAL excretion found after intra-arterial contrast administration in terms of long-term changes in renal function remains unclear.
Our study results show no increase in KIM-1 and N-GAL excretion post-CE-CT and absence of a predictive value of these biomarkers for the development of CI-AKI, which is in contradiction to the literature on N-GAL and KIM-1 excretion after intra-arterial contrast injections. This can be explained as follows. First, percutaneous coronary intervention and other procedures requiring intra-arterial contrast administrations bring along procedural related risk factors for AKI, such as cholesterol emboli shedding into the renal vasculature by catheterization of the descending aorta [32–34]. Second, patients undergoing percutaneous coronary interventions might experience hemodynamic instability as a pre-renal cause of AKI, since acute myocardial infarction is one of the main indications for this procedure. Third, suprarenally injected contrast boluses, as used for radial percutaneous coronary interventions, might be more concentrated and, therefore, nephrotoxic than intravenously injected boluses when they arrive at the level of the kidney. Fourth, patients undergoing CE-CT might differ from patients undergoing intra-arterial contrast administrations in terms of the presence of comorbidity at risk of developing CI-AKI, such as diabetes mellitus [35]. These differences in patient characteristics may also affect KIM-1 and N-GAL expression after administration of iodinated contrast media. Although the results of our study can’t designate by which mechanism the risk of tubular injury varies after intravenous and intra-arterial contrast administration, they do suggest that the performance of CE-CT in CKD patients is likely to be safe. This finding endorses the recommendation of the CI-AKI guideline of the American College of Radiology to withhold preventive hydration in CKD patients undergoing CE-CT.
Our study, therefore, adds clinically relevant information to the research field. To our knowledge, this is the first study analyzing both KIM-1 and N-GAL expression post-CE-CT. We analyzed these two established biomarkers of CI-AKI within adequate time frames, as indicated by previous studies [11, 14]. Moreover, due to the prospective study design, all relevant information on co-morbidity influencing our outcomes was available for analysis. However, some aspects of our study require comment. We included a population with mainly moderate CKD since only 7 % of patients had a baseline eGFR value of less than 30 ml/min. Therefore, we can’t exclude the possibility of some extent of tubular injury post CE-CT in patients with severe CKD. However, that group reflects only 10 % of CKD patients undergoing CE-CT in daily practice [36]. Moreover, our sample size was too small to study differences in KIM-1 and N-GAL expression between different types of contrast media.
In conclusion, KIM-1 and N-GAL excretion were unaffected by CE-CT in both patients with and without CI-AKI. This study suggests that CE-CT can probably be safely performed in patients with CKD, and challenges the creatinine based definition of CI-AKI.
Acknowledgments
The scientific guarantor of this publication is Professor A.J. Rabelink. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. One of the authors has significant statistical expertise. Institutional review board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Methodology: prospective, randomised controlled trial, multicenter study.
References
- 1.Seeliger E, Sendeski M, Rihal CS, Persson PB. Contrast-induced kidney injury: mechanisms, risk factors, and prevention. Eur Heart J. 2012;33:2007–2015. doi: 10.1093/eurheartj/ehr494. [DOI] [PubMed] [Google Scholar]
- 2.Stacul F, van der Molen AJ, Reimer P, Webb JA, Thomsen HS, Morcos SK, et al. Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2011;21:2527–2541. doi: 10.1007/s00330-011-2225-0. [DOI] [PubMed] [Google Scholar]
- 3.Kooiman J, Pasha SM, Zondag W, Sijpkens YW, van der Molen AJ, Huisman MV, et al. Meta-analysis: serum creatinine changes following contrast enhanced CT imaging. Eur J Radiol. 2012;81:2554–2561. doi: 10.1016/j.ejrad.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 4.Moos SI, van Vemde DN, Stoker J, Bipat S (2013) Contrast induced nephropathy in patients undergoing intravenous (IV) contrast enhanced computed tomography (CECT) and the relationship with risk factors: A meta-analysis. Eur J Radiol 2013 [DOI] [PubMed]
- 5.Slocum NK, Grossman PM, Moscucci M, Smith DE, Aronow HD, Dixon SR, et al. The changing definition of contrast-induced nephropathy and its clinical implications: insights from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2) Am Heart J. 2012;163:829–834. doi: 10.1016/j.ahj.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Russo D, Minutolo R, Cianciaruso B, Memoli B, Conte G, De NL. Early effects of contrast media on renal hemodynamics and tubular function in chronic renal failure. J Am Soc Nephrol. 1995;6:1451–1458. doi: 10.1681/ASN.V651451. [DOI] [PubMed] [Google Scholar]
- 7.Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol. 2009;20:672–679. doi: 10.1681/ASN.2008070669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katzberg RW, Newhouse JH. Intravenous contrast medium-induced nephrotoxicity: is the medical risk really as great as we have come to believe? Radiology. 2010;256:21–28. doi: 10.1148/radiol.10092000. [DOI] [PubMed] [Google Scholar]
- 9.McDonald RJ, McDonald JS, Bida JP, Carter RE, Fleming CJ, Misra S, et al. Intravenous contrast material-induced nephropathy: causal or coincident phenomenon? Radiology. 2013;267:106–118. doi: 10.1148/radiol.12121823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alharazy SM, Kong N, Saidin R, Abdul Gafor AH, Maskon O, Mohd M, et al (2013) Neutrophil Gelatinase-Associated Lipocalin as an Early Marker of Contrast-Induced Nephropathy After Coronary Angiography. Angiology [DOI] [PubMed]
- 11.Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, Malyszko JS, Pawlak K, Mysliwiec M, et al. Could neutrophil-gelatinase-associated lipocalin and cystatin C predict the development of contrast-induced nephropathy after percutaneous coronary interventions in patients with stable angina and normal serum creatinine values? Kidney Blood Press Res. 2007;30:408–415. doi: 10.1159/000109102. [DOI] [PubMed] [Google Scholar]
- 12.Lenhard DC, Pietsch H, Sieber MA, Ernst R, Lengsfeld P, Ellinghaus P, et al. The osmolality of nonionic, iodinated contrast agents as an important factor for renal safety. Invest Radiol. 2012;47:503–510. doi: 10.1097/RLI.0b013e318258502b. [DOI] [PubMed] [Google Scholar]
- 13.Ling W, Zhaohui N, Ben H, Leyi G, Jianping L, Huili D, et al. Urinary IL-18 and NGAL as early predictive biomarkers in contrast-induced nephropathy after coronary angiography. Nephron Clin Pract. 2008;108:c176–c181. doi: 10.1159/000117814. [DOI] [PubMed] [Google Scholar]
- 14.Malyszko J, Bachorzewska-Gajewska H, Poniatowski B, Malyszko JS, Dobrzycki S. Urinary and serum biomarkers after cardiac catheterization in diabetic patients with stable angina and without severe chronic kidney disease. Ren Fail. 2009;31:910–919. doi: 10.3109/08860220903216113. [DOI] [PubMed] [Google Scholar]
- 15.McCullough PA, Williams FJ, Stivers DN, Cannon L, Dixon S, Alexander P, et al. Neutrophil gelatinase-associated lipocalin: a novel marker of contrast nephropathy risk. Am J Nephrol. 2012;35:509–514. doi: 10.1159/000339163. [DOI] [PubMed] [Google Scholar]
- 16.Shaker OG, El-Shehaby A, El-Khatib M. Early diagnostic markers for contrast nephropathy in patients undergoing coronary angiography. Angiology. 2010;61:731–736. doi: 10.1177/0003319710373093. [DOI] [PubMed] [Google Scholar]
- 17.Kooiman J, Sijpkens YW, de Vries JP, Brulez HF, Hamming JF, van der Molen AJ, et al (2014) A randomized comparison of 1-h sodium bicarbonate hydration versus standard peri-procedural saline hydration in patients with chronic kidney disease undergoing intravenous contrast-enhanced computerized tomography. Nephrol Dial Transplant [DOI] [PubMed]
- 18.Ralib AM, Pickering JW, Shaw GM, Devarajan P, Edelstein CL, Bonventre JV, et al. Test characteristics of urinary biomarkers depend on quantitation method in acute kidney injury. J Am Soc Nephrol. 2012;23:322–333. doi: 10.1681/ASN.2011040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American College of Radiology (2012). Manual on Contrast Media v8
- 20.McDonald JS, McDonald RJ, Comin J, Williamson EE, Katzberg RW, Murad MH, et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology. 2013;267:119–128. doi: 10.1148/radiol.12121460. [DOI] [PubMed] [Google Scholar]
- 21.Bansal GJ, Darby M. Measurement of change in estimated glomerular filtration rate in patients with renal insufficiency after contrast-enhanced computed tomography: a case-control study. J Comput Assist Tomogr. 2009;33:455–459. doi: 10.1097/RCT.0b013e31818160a3. [DOI] [PubMed] [Google Scholar]
- 22.Bruce RJ, Djamali A, Shinki K, Michel SJ, Fine JP, Pozniak MA. Background fluctuation of kidney function versus contrast-induced nephrotoxicity. AJR Am J Roentgenol. 2009;192:711–718. doi: 10.2214/AJR.08.1413. [DOI] [PubMed] [Google Scholar]
- 23.McDonald JS, McDonald RJ, Carter RE, Katzberg RW, Kallmes DF, Williamson EE (2014) Risk of Intravenous Contrast Material-mediated Acute Kidney Injury: A Propensity Score-matched Study Stratified by Baseline-estimated Glomerular Filtration Rate. Radiology 130775 [DOI] [PubMed]
- 24.Newhouse JH, Kho D, Rao QA, Starren J. Frequency of serum creatinine changes in the absence of iodinated contrast material: implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol. 2008;191:376–382. doi: 10.2214/AJR.07.3280. [DOI] [PubMed] [Google Scholar]
- 25.Lacquaniti A, Buemi F, Lupica R, Giardina C, Mure G, Arena A, et al. Can neutrophil gelatinase-associated lipocalin help depict early contrast material-induced nephropathy? Radiology. 2013;267:86–93. doi: 10.1148/radiol.12120578. [DOI] [PubMed] [Google Scholar]
- 26.Filiopoulos V, Biblaki D, Lazarou D, Chrisis D, Fatourou M, Lafoyianni S, et al. Plasma neutrophil gelatinase-associated lipocalin (NGAL) as an early predictive marker of contrast-induced nephropathy in hospitalized patients undergoing computed tomography. Clin Kidney J. 2013;28:8. doi: 10.1093/ckj/sft109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kooiman J, Pasha SM, Zondag W, Sijpkens YW, van der Molen AJ, Huisman MV, et al (2011) Meta-analysis: Serum creatinine changes following contrast enhanced CT imaging. Eur J Radiol [DOI] [PubMed]
- 28.Waanders F, van Timmeren MM, Stegeman CA, Bakker SJ, van Goor H. Kidney injury molecule-1 in renal disease. J Pathol. 2010;220:7–16. doi: 10.1002/path.2642. [DOI] [PubMed] [Google Scholar]
- 29.Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest. 2008;118:1657–1668. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543. doi: 10.1097/01.ASN.0000088027.54400.C6. [DOI] [PubMed] [Google Scholar]
- 31.Smertka M, Chudek J. Using NGAL as an early diagnostic test of acute kidney injury. Ren Fail. 2012;34:130–133. doi: 10.3109/0886022X.2011.623500. [DOI] [PubMed] [Google Scholar]
- 32.Fukumoto Y, Tsutsui H, Tsuchihashi M, Masumoto A, Takeshita A. The incidence and risk factors of cholesterol embolization syndrome, a complication of cardiac catheterization: a prospective study. J Am Coll Cardiol. 2003;42:211–216. doi: 10.1016/S0735-1097(03)00579-5. [DOI] [PubMed] [Google Scholar]
- 33.Saklayen MG, Gupta S, Suryaprasad A, Azmeh W. Incidence of atheroembolic renal failure after coronary angiography. A prospective study. Angiology. 1997;48:609–613. doi: 10.1177/000331979704800707. [DOI] [PubMed] [Google Scholar]
- 34.Vuurmans T, Byrne J, Fretz E, Janssen C, Hilton JD, Klinke WP, et al. Chronic kidney injury in patients after cardiac catheterisation or percutaneous coronary intervention: a comparison of radial and femoral approaches (from the British Columbia Cardiac and Renal Registries) Heart. 2010;96:1538–1542. doi: 10.1136/hrt.2009.192294. [DOI] [PubMed] [Google Scholar]
- 35.Kooiman J, Le Haen PA, Gezgin G, de Vries JP, Boersma D, Brulez HF, et al. Contrast-induced acute kidney injury and clinical outcomes after intra-arterial and intravenous contrast administration: risk comparison adjusted for patient characteristics by design. Am Heart J. 2013;165:793–799. doi: 10.1016/j.ahj.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Kim SM, Cha RH, Lee JP, Kim DK, Oh KH, Joo KW, et al. Incidence and outcomes of contrast-induced nephropathy after computed tomography in patients with CKD: a quality improvement report. Am J Kidney Dis. 2010;55:1018–1025. doi: 10.1053/j.ajkd.2009.10.057. [DOI] [PubMed] [Google Scholar]