Abstract
We have previously developed mouse models of HER-2-positive cervical cancer. Tumors in nude mice had histological structures similar to the original tumor and were stained by anti-HER-2 antibody in the same pattern as the patient’s cancer. We have also previously developed tumor-targeting Salmonella typhimurium A1-R and have demonstrated its efficacy against patient-derived tumor mouse models, both alone and in combination. In the current study, we determined the efficacy of S. typhimurium A1-R in combination with trastuzumab on a patient-cancer nude-mouse model of HER-2 positive cervical cancer. Mice were randomized to 5 groups and treated as follows: (1) no treatment; (2) carboplatinum (30 mg/kg, ip, weekly, 5 weeks); (3) trastuzumab (20 mg/kg, ip, weekly, 5 weeks); (4) S. typhimurium A1-R (5 × 107 CFU/body, ip, weekly, 5 weeks); (5) S. typhimurium A1-R (5 × 107 CFU/body, ip, weekly, 5 weeks) + trastuzumab (20 mg/kg, ip, weekly, 5 weeks). All regimens had significant efficacy compared to the untreated mice. The relative tumor volume of S. typhimurium A1-R + trastuzumab-treated mice was smaller compared to trastuzumab alone (p = 0.007) and S. typhimurium A1-R alone (p = 0.039). No significant body weight loss was found compared to the no treatment group except for carboplatinum-treated mice (p = 0.021). Upon histological examination, viable tumor cells were not detected, and replaced by stromal cells in the tumors treated with S. typhimurium A1-R + trastuzumab. The results of the present study suggest that S. typhimurium A1-R and trastuzumab in combination are highly effective against HER-2-expressing cervical cancer.
Introduction
Cervical cancer is the second most common cancer in women [1]. There were 454,000 cases and 200,000 deaths in 2010 worldwide and 11,000 new cases and 3,870 deaths from cervical carcinoma in the U.S. [2, 3]. Paclitaxel, carboplatin, cisplatinum, bleomycin, mitomycin-C, vincristine and irinotecan are used for cervical cancer [4]. However, there is no standard treatment for cervical cancer.
The incidence of HER-2 positivity in cervical cancer was reported from 1% to 21% [5], and overexpression of HER-2 has been associated with more advanced stages and a worse prognosis [6, 7].
We previously developed mouse models of HER-2-positive patient cervical cancer [8]. Our laboratory has also previously developed a genetically-modified bacteria strain, Salmonella typhimurium A1-R, selected for tumor-targeting in vivo. S. typhimurium A1-R is auxotrophic for leu and arg [9]. The strain targets and grows in tumors. In contrast, normal tissue is cleared of these bacteria even in immunodeficient athymic mice.
S. typhimurium A1-R is effective against prostate cancer [10], breast cancer [11, 12], pancreatic cancer [13–16], glioma [17, 18], lung cancer [19], fibrosarcoma [20, 21], osteosarcoma [22] and ovarian cancer [23].
In the present study, we demonstrate the efficacy of S. typhimurium A1-R in combination with trastuzumab on mouse models of patient cervical cancer expressing HER-2.
Materials and Methods
Ethics Statement
All animal studies were conducted with an AntiCancer Institutional Animal Care and Use Committee (IACUC)-protocol specifically approved for this study and in accordance with the principals and procedures outlined in the National Institute of Health Guide for the Care and Use of Animals under Assurance Number A3873-1. In order to minimize any suffering of the animals the use of anesthesia and analgesics were used for all surgical experiments. Animals were anesthetized by intramuscular injection of a 0.02 ml solution of 20 mg/kg ketamine, 15.2 mg/kg xylazine, and 0.48 mg/kg acepromazine maleate. The response of animals during surgery was monitored to ensure adequate depth of anesthesia. Ibuprofen (7.5 mg/kg orally in drinking water every 24 hours for 7 days post-surgery) was used in order to provide analgesia post-operatively in the surgically-treated animals. The animals were observed on a daily basis and humanely sacrificed by CO2 inhalation when they met the following humane endpoint criteria: prostration, skin lesions, significant body weight loss, difficulty breathing, epistaxis, rotational motion and body temperature drop. The use of animals was necessary to understand the in vivo efficacy, in particular, anti-metastatic efficacy of the agents tested. Animals were housed with no more than 5 per cage. Animals were housed in a barrier facility on a high efficiency particulate air (HEPA)-filtered rack under standard conditions of 12-hour light/dark cycles. The animals were fed an autoclaved laboratory rodent diet (Supp. Information S1).
Animals
Female athymic (nu/nu) nude mice (AntiCancer, Inc., San Diego, CA), 4–6 weeks old, were used in this study. Mice were kept in a barrier facility under HEPA filtration. Mice were fed with autoclaved laboratory rodent diet. All mouse surgical procedures and imaging were performed with the animals anesthetized by intramuscular injection of a 0.02 ml solution of 20 mg/kg ketamine, 15.2 mg/kg xylazine, and 0.48 mg/kg acepromazine maleate. All animal studies were conducted with an AntiCancer Institutional Animal Care and Use Committee (IACUC)-protocol specifically approved for this study and in accordance with the principals and procedures outlined in the National Institute of Health Guide for the Care and Use of Animals under Assurance Number A3873-1.
Specimen collection
The patient provided written informed consent and the tumor specimen was procured under the approval of the Institutional Review Board of the University of California San Diego.
Subcutaneous implantation of patient cervical cancer
Tumor tissues were obtained from the HER-2-positive cervical cancer patient at surgery and cut into fragments (3-mm3) and transplanted subcutaneously in nude mice [8].
Tissue histology
Tumor tissue was removed along with surrounding normal tissues at the time of resection. The tissues were fixed in 10% formalin and embedded in paraffin before sectioning and staining. Tissue sections (3 μm) were deparaffinized in xylene and rehydrated in an ethanol series. Hematoxylin and eosin (H&E) staining was performed according to standard protocols. For immunohistochemistry, sections (5 μm) were then treated for 30 min with hydrogen peroxide (0.3%) to block endogenous peroxidase activity. The sections were subsequently washed with PBS and unmasked in citrate antigen-unmasking solution (Mitsubishi Kagaku Iatron, Inc., Tokyo, Japan) in a water bath for 40 min at 98°C. After incubation with 10% normal goat serum, the sections were incubated with anti-HER-2/ErbB2 (1:100; Cell Signaling Technology, Inc., Danvers, MA, USA) at 4°C overnight. The binding of primary antibodies was detected using anti-rabbit secondary antibodies and an avidin/biotin/horseradish peroxidase complex (DAKO Cytomation, Kyoto, Japan) for 30 min at room temperature. The labeled antigens were visualized with the DAB kit (DAKO Cytomation). Finally, the sections were counterstained with hematoxylin and examined with a BH-2 microscope (Olympus, Tokyo, Japan) equipped with an INFINITY1 2.0 megapixel CMOS digital camera (Lumenera Corporation, Ottawa, Canada). All images were acquired using INFINITY ANALYZE software (Lumenera Corporation) without post-acquisition processing.
Treatment of patient cervical cancer growing in nude mice
Six weeks after implantation, the mice were randomized to 5 groups and treated as follows: (1) no treatment; (2) carboplatinum (Selleck Chemicals, Houston, TX, USA, 30 mg/kg, ip, weekly, 5 weeks); (3) trastuzumab (Genentech, Inc., South San Francisco, CA, USA, 20 mg/kg, ip, weekly, 5 weeks); (4) S. typhimurium A1-R (5 × 107 CFU/body, ip, weekly, 5 weeks); and (5) S. typhimurium A1-R (5 × 107 CFU/body, ip, weekly, 5 weeks) + trastuzumab (20 mg/kg, ip, weekly, 5 weeks) were co-administered. Each treatment arm comprised 6 tumor-bearing mice. Tumor size was evaluated every 3 or 4 days by caliper measurements and the approximate volume of the tumor was calculated using the formula 4/3π (d/2)2 D/2; where d is the minor tumor axis and D is the major tumor axis. Body weight of the mice was measured on a balance every 3 or 4 days. Relative tumor volume and body weight were calculated by comparison to day-1 values. Tumors were imaged with a Canon EOS 60D digital camera with an EF–S18–55 IS lens (Canon, Tokyo, Japan) and harvested for analysis.
Evaluation of histopathological response
Histopathological response to chemotherapy was defined according to Evans’s grading scheme: Grade I, little (<10%) or no tumor cell destruction is evident; Grade II a, destruction of 10%-50% of tumor cells; Grade II b, destruction of 51%-90% of tumor cells; Grade III, few (<10%) viable-appearing tumor cells are present; Grade IV, no viable tumor cells are present [24, 25].
Statistical analysis
PASW Statistics 18.0 (SPSS, Inc) was used for all statistical analyses. Final tumor volumes (at day-36) in each treatment group were compared to the untreated control using a 2-tailed Student’s t-test. A p-value of ≤ 0.05 was considered statistically significant for all comparisons.
Results and Discussion
Histology of the original tumor is preserved in the mouse
Sheet-like growth without gland formation and stromal tissue with fibroblastic proliferation, which penetrated into nests of cancer cells was observed in the H&E stained sections of the original tumor (Fig 1A). Oval- to spindle-shaped cancer cells with high nuclear/cytoplasmic ratio were found in high magnification images (Fig 1B). In the immunostained sections with anti-HER-2 antibody, the membrane and the cytoplasm of cancer cells were strongly stained, but no staining was found in the stromal tissue (Fig 1C and 1D). All mouse-grown cervical patient-derived tumor had histological structures similar to the original tumor and were stained by anti-human HER-2 antibody (Fig 1E–1H), suggesting that the model recapitulates the biological behavior of the original tumor [8].
Fig 1. Tumor histology and immunohistochemistry.
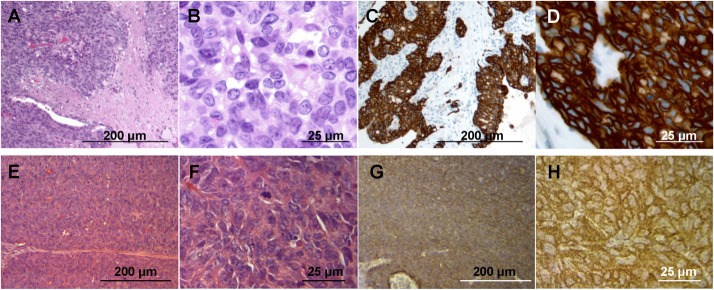
(A) H & E-stained section of the original patient tumor. (B) High magnification image of (A). (C) Immunostained section of the original patient tumor using anti-HER-2 antibody. (D) High magnification image of (C). (E) H & E-stained section of the mouse-grown tumor. (B) High magnification image of (F). (G) Immunostained section of the mouse-grown tumor using an anti-HER-2 antibody. (H) High magnification image of (G). Scale bars: 200 μm (A, C, E and G) and 25 μm (B, D, F and H).
S. typhimurium A1-R + trastuzumab combination is effective against patient-derived cervical cancer growing in nude mice
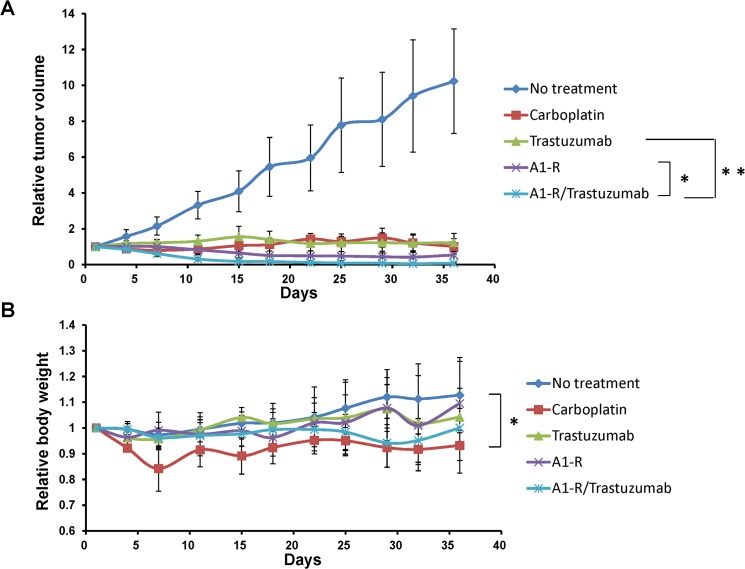
The relative tumor volumes at day-36, compared to day-1, of each group were as follows: (1) no treatment: 10.23 ± 2.91; (2) carboplatinum: 1.02 ± 0.43; (3) trastuzumab: 1.23 ± 0.50; (4) S. typhimurium A1-R: 0.54 ± 0.40; (5) S. typhimurium A1-R + trastuzumab: 0.08 ± 0.04 (Fig 2A). All regimens had significant efficacy compared to the untreated mice. The relative tumor volume of S. typhimurium A1-R + trastuzumab-treated mice was smaller than with trastuzumab treatment alone (p = 0.007) or S. typhimurium A1-R treatment alone (p = 0.039) (Fig 2A).
Fig 2. S. typhimurium A1-R, trastuzumab and combination drug treatment of the patient cervical tumor grown in nude mice.
(A) Growth curves of the subcutaneous tumor treated with various drugs. The values are mean relative tumor volumes ± S.D. (bars) of five different tumors. * p < 0.05, ** p < 0.01. (B) Body weight curves of the mice with the subcutaneous tumors treated with the indicated drugs. The values are mean relative body weights ± S.D. (bars) of five different mice.
The relative body weight at day-36, compared to day-1, of each group was as follows: (1) no treatment: 1.13 ± 0.14; (2) carboplatinum: 0.93 ± 0.06; (3) trastuzumab: 1.04 ± 0.22; (4) S. typhimurium A1-R: 1.09 ± 0.06; (5) S. typhimurium A1-R + trastuzumab: 1.00 ± 0.08. No significant body weight loss was found compared to the untreated mice, except for carboplatinum-treated mice (p = 0.021) (Fig 2B).
S. typhimurium A1-R + trastuzumab combination eradicates HER-2-positive cervical cancer cells in mice as observed in histological sections
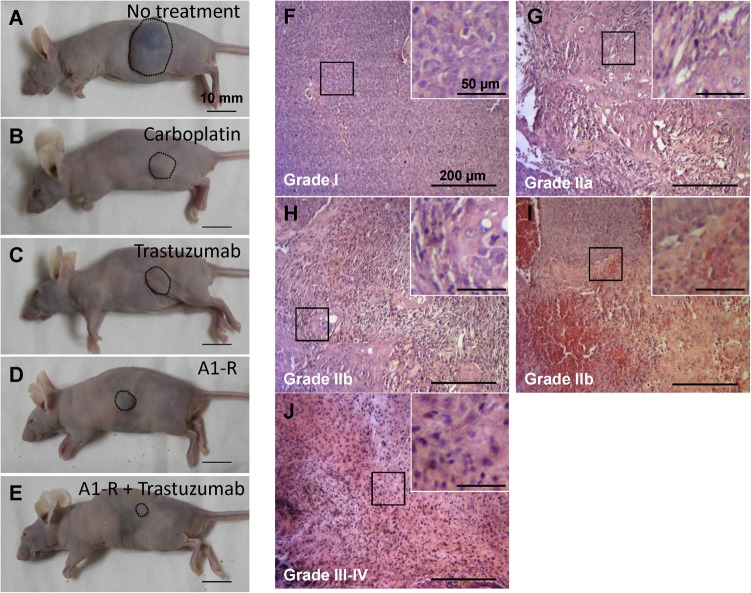
Histopathological response to treatment was defined according to Evans’s grading scheme. In tissue sections from untreated mice, numerous cancer cells were observed (Fig 3F). Approximately 30% of the cancer cells were destroyed and replaced by stromal cells in tissues sections from mice treated with carboplatinum (Fig 3G). Seventy percent of the cancer cells were destroyed in the tissue sections from mice treated with trastuzumab (Fig 3H). Sixty percent of the cancer cells were destroyed in the tissue sections from mice treated with S. typhimurium A1-R (Fig 3I). In the tissue sections from mice treated with S. typhimurium A1-R + trastuzumab, viable cancer cells were not detected and were replaced by stromal cells (Fig 3J). The untreated control was judged as grade I; carboplatinum treatment as IIa; trastuzumab treatment as IIb; S. typhimurium A1-R treatment as IIb; and S. typhimurium A1-R + trastuzumab treatment as III—IV. These results suggest the S. typhimurium A1-R + trastuzumab combination can eradicate HER-2-positive cervical cancer in mice.
Fig 3. Efficacy of S. typhimurium A1-R, trastuzumab and combination treatment on tumor size and histology.
(A) Untreated control. (B) Carboplatinum-treated mice. (C) Trastuzumab-treated mice. (D) S. typhimurium A1-R-treated mice. (E) S. typhimurium A1-R + trastuzumab-treated mice. These images were obtained at day-36. Scale bars: 10 mm. Histopathological response to treatment was defined according to Evans’s grading scheme. Treatment effect of untreated control (F) was judged as grade I; carboplatinum (G) as IIa; trastuzumab (H) as IIb; S. typhimurium A1-R (I) as IIb; and S. typhimurium A1-R + trastuzumab (J) as III—IV. Scale bars: 200 μm.
The mechanisms of the strong efficacy of the combination of S. typhimurium A1-R and trastuzumab remains to be elucidated. One contributing factor could be the ability of S. typhimurium A1-R to stimulate (decoy) the cell cycle of quiescent, resistant cells, which then begin to cycle and become sensitive to trastuzumab [26]. Having found the strong efficacy of the combination of S. typhimurium A1-R and trastuzumab in subcutaneous models, future experiments will utilize orthotopic models, using the surgical orthotopic implantation (SOI) method our laboratory has developed for patient tumors [27–32]. Access of S. typhimurium A1-R to orthotopic tumors has been previously demonstrated by us [8–24]. Access to trastuzumab therapy in orthotopic models has already been demonstrated by us as well [33]. The orthothopic models of a series of HER-2 positive and mixed HER-2 expressing cervical cancer will allow efficacy testing of the combination of S. typhimurium A1-R and trastuzumab against primary and metastatic disease and further characterization of stromal and cancer cells before and after treatment, as well as the effects of leukocyte-depleting regimens following or before the treatment. SOI requires surgery and since ketamine may affect the immune system, different anesthetics will be compared for any effect on treatment response in future experiments.
Acknowledgments
This paper is dedicated to the memory of A. R. Moossa, M.D.
Data Availability
All data underlying the findings in this study are freely available in the paper. Any queries may be directed to the authors (all@anticancer.com).
Funding Statement
This study was supported in part by National Cancer Institute grant numbers CA CA132971 and CA142669 and JSPS KAKENHI grant numbers 26830081 to YH, 26462070 to IE and 24592009 to KT. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wang CW, Wu TI, Yu CT, Wu YC, Teng YH, Chin SY, et al. Usefulness of p16 for differentiating primary pulmonary squamous cell carcinoma from cervical squamous cell carcinoma metastatic to the lung. Am J Clin Pathol 2009;131:715–722. 10.1309/AJCPTPBC6V5KUITM [DOI] [PubMed] [Google Scholar]
- 2. Sabatier R, Roussin C, Riviere JP, Jalaguier A, Jacquemier J, Bertucci F. Breast metastasis of a squamous cell carcinoma of the uterine cervix mimicking inflammatory breast cancer. Case Rep Oncol 2012;5:464–470. 10.1159/000342255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gamez RG, Jessurun J, Berger MJ, Pambuccian SE. Cytology of metastatic cervical squamous cell carcinoma in pleural fluid: report of a case confirmed by human papillomavirus typing. Diagn Cytopathol 2009;37:381–387. 10.1002/dc.21027 [DOI] [PubMed] [Google Scholar]
- 4. Hashimoto K, Yonemori K, Katsumata N, Hirakawa A, Hirata T, Yamamoto H, et al. Use of squamous cell carcinoma antigen as a biomarker of chemotherapy response in patients with metastatic cervical carcinoma. Eur J Obstet Gynecol Reprod Biol 2011;159:394–398. 10.1016/j.ejogrb.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 5. Yan M, Parker BA, Schwab R, Kurzrock R. HER-2 aberrations in cancer: Implications for therapy. Cancer Treat Rev 2014;40:770–780. 10.1016/j.ctrv.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 6. Costa MJ, Walls J, Trelford JD. c-erbB-2 oncoprotein overexpression in uterine cervix carcinoma with glandular differentiation. A frequent event but not an independent prognostic marker because it occurs late in the disease. Am J Clin Pathol 1995;104:634–642. [DOI] [PubMed] [Google Scholar]
- 7. Kihana T, Tsuda H, Teshima S, Nomoto K, Tsugane S, Sonoda T, et al. Prognostic significance of the overexpression of c-erbB-2 protein in adenocarcinoma of the uterine cervix. Cancer 1994;73:148–153. [DOI] [PubMed] [Google Scholar]
- 8.Hiroshima Y, Zhang Y, Zhang M, Maawy AA, Mii S, Yamamoto M, et al. Establishment of a patient-derived orthotopic xenograph (PDOX) model of HER-2-positive cervical cancer expressing the clinical metastatic pattern. PLOS ONE, in press. [DOI] [PMC free article] [PubMed]
- 9. Zhao M, Yang M, Li XM, Jiang P, Baranov E, Li S, et al. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium . Proc Natl Acad Sci USA 2005;102:755–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao M, Geller J, Ma H, Yang M, Penman S, Hoffman RM. Monotherapy with a tumor-targeting mutant of Salmonella typhimurium cures orthotopic metastatic mouse models of human prostate cancer. Proc Natl Acad Sci USA 2007;104:10170–10174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao M, Yang M, Ma H, Li X, Tan X, Li S, et al. Targeted therapy with a Salmonella typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice. Cancer Res 2006;66:7647–7652. [DOI] [PubMed] [Google Scholar]
- 12. Zhang Y, Tome Y, Suetsugu A, Zhang L, Zhang N, Hoffman RM, et al. Determination of the optimal route of administration of Salmonella typhimurium A1-R to target breast cancer in nude mice. Anticancer Res 2012;32:2501–2508. [PubMed] [Google Scholar]
- 13. Nagakura C, Hayashi K, Zhao M, Yamauchi K, Yamamoto N, et al. Efficacy of a genetically-modified Salmonella typhimurium in an orthotopic human pancreatic cancer in nude mice. Anticancer Res 2009;29:1873–1878. [PubMed] [Google Scholar]
- 14. Yam C, Zhao M, Hayashi K, Ma H, Kishimoto H, McElroy M, et al. Monotherapy with a tumor-targeting mutant of S. typhimurium inhibits liver metastasis in a mouse model of pancreatic cancer. J Surg Res 2010;164:248–255. 10.1016/j.jss.2009.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hiroshima Y, Zhao M, Zhang Y, Maawy A, Hassanein MK, Uehara F, et al. Comparison of efficacy of Salmonella typhimurium A1-R and chemotherapy on stem-like and non-stem human pancreatic cancer cells. Cell Cycle 2013;12:2774–2780. 10.4161/cc.25872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hiroshima Y, Zhao M, Maawy A, Zhang Y, Katz MH, Fleming JB, et al. Efficacy of Salmonella typhimurium A1-R versus chemotherapy on a pancreatic cancer patient-derived orthotopic xenograft (PDOX). J Cell Biochem 2014;115:1254–1261. 10.1002/jcb.24769 [DOI] [PubMed] [Google Scholar]
- 17. Kimura H, Zhang L, Zhao M, Hayashi K, Tsuchiya H, Tomita K, et al. Targeted therapy of spinal cord glioma with a genetically-modified Salmonella typhimurium . Cell Prolif 2010;43:41–48. 10.1111/j.1365-2184.2009.00652.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Momiyama M, Zhao M, Kimura H, Tran B, Chishima T, Bouvet M, et al. Inhibition and eradication of human glioma with tumor-targeting Salmonella typhimurium in an orthotopic nude-mouse model. Cell Cycle 2012;11:628–632. 10.4161/cc.11.3.19116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao M, Suetsugu A, Ma H, Zhang L, Liu F, Zhang Y, et al. Efficacy against lung metastasis with a tumor-targeting mutant of Salmonella typhimurium in immunocompetent mice. Cell Cycle 2012;11:187–193. 10.4161/cc.11.1.18667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, et al. Cancer metastasis directly eradicated by targeted therapy with a modified Salmonella typhimurium . J Cell Biochem 2009;106:992–998. 10.1002/jcb.22078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miwa S, Zhang Y, Baek K-E, Uehara F, Yano S, Yamamoto M, et al. Inhibition of spontaneous and experimental lung metastasis of soft-tissue sarcoma by tumor-targeting Salmonella typhimurium A1-R. Oncotarget 2014;5:12849–12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, et al. Systemic targeting of primary bone tumor and lung metastasis of high-grade osteosarcoma in nude mice with a tumor-selective strain of Salmonella typhimurium . Cell Cycle 2009;8:870–875. [DOI] [PubMed] [Google Scholar]
- 23. Matsumoto Y, Miwa S, Zhang Y, Hiroshima Y, Yano S, Uehara F, et al. Efficacy of tumor-targeting Salmonella typhimurium A1-R on nude mouse models of metastatic and disseminated human ovarian cancer. J Cell Biochem 2014;115:1996–2003. 10.1002/jcb.24871 [DOI] [PubMed] [Google Scholar]
- 24. Hiroshima Y, Zhao M, Maawy A, Zhang Y, Katz MH, Fleming JB, et al. Efficacy of Salmonella typhimurium A1-R versus chemotherapy on a pancreatic cancer patient-derived orthotopic xenograft (PDOX). J Cell Biochem 2014;115:1254–1261. 10.1002/jcb.24769 [DOI] [PubMed] [Google Scholar]
- 25. Evans DB, Rich TA, Byrd DR, Cleary KR, Connelly JH, Levin B, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg 1992;127:1335–1339. [DOI] [PubMed] [Google Scholar]
- 26. Yano S, Zhang Y, Zhao M, Hiroshima Y, Miwa S, Uehara F, et al. Tumor-targeting Salmonella typhimurium A1-R decoys quiescent cancer cells to cycle as visualized by FUCCI imaging and become sensitive to chemotherapy. Cell Cycle 2014;13:3958–3963. 10.4161/15384101.2014.964115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fu X, Besterman JM, Monosov A, Hoffman RM. Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. Proc Natl Acad Sci USA1991;88:9345–9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically from histologically intact patient specimens. Proc Natl Acad Sci USA 1992;89:5645–5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang X, Fu X, Hoffman RM. A new patient-like metastatic model of human lung cancer constructed orthotopically with intact tissue via thoracotomy in immunodeficient mice. Int. J. Cancer 1992;51:992–995. [DOI] [PubMed] [Google Scholar]
- 30. Fu X, Hoffman RM. Human ovarian carcinoma metastatic models constructed in nude mice by orthotopic transplantation of histologically-intact patient specimens. Anticancer Res 1993;13:283–286. [PubMed] [Google Scholar]
- 31. Fu X, Le P, Hoffman RM. A metastatic-orthotopic transplant nude-mouse model of human patient breast cancer. Anticancer Res 1993;13:901–904. [PubMed] [Google Scholar]
- 32. Furukawa T, Kubota T, Watanabe M, Kitajima M, Hoffman RM. Orthotopic transplantation of histologically intact clinical specimens of stomach cancer to nude mice: correlation of metastatic sites in mouse and individual patient donors. Int J Cancer 1993;53:608–612. [DOI] [PubMed] [Google Scholar]
- 33. Gros S, Kurschat N, Dohrmann T, Reichelt U, Dancau A-M, Peldschus K, et al. Effective therapeutic targeting of the overexpressed HER-2 receptor in a highly metastatic orthotopic model of esophageal carcinoma. Mol Cancer Ther 2010;9:2037–2045. 10.1158/1535-7163.MCT-10-0209 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data underlying the findings in this study are freely available in the paper. Any queries may be directed to the authors (all@anticancer.com).