Abstract
The colonic and intestinal epithelium are renewed every 3 days. In the intestine there are at least two principal stem cell pools. The first contains rapid cycling crypt based columnar (CBC) Lgr5+ cells, while the second is comprised of slower cycling Bmi1-expressing cells at the +4 position above the crypt base. In the colon, however, the identification of Lgr5-negative stem cell pools has proven more challenging. Here, we demonstrate that the intermediate filament, keratin-19 (Krt19), marks long-lived, radiation resistant cells above the crypt base that generate Lgr5+ CBCs in the colon and intestine. In colorectal cancer models, Krt19+ cancer initiating cells are also radioresistant while Lgr5+ stem cells are radiosensitive. Moreover, Lgr5+ stem cells are dispensable in both the normal and neoplastic colonic epithelium, as ablation of Lgr5+ stem cells results in their regeneration from Krt19 expressing cells. Thus, Krt19+ stem cells are a discrete target relevant for cancer therapy.
Keywords: Krt19, intestine, colon, stem cells, cancer
Graphical Abstract
Introduction
Adult tissue stem cells are characterized by multipotentiality and the capacity to self-renew (Li and Clevers, 2010). In the mouse small intestine and colon, the simple columnar epithelium is rapidly renewed every 3 days. Genetic inducible fate mapping studies suggest that epithelial cells in the small intestine are replaced from at least two principal stem cell pools, comprising both rapidly cycling crypt based columnar (CBC) Lgr5-expressing cells, and slower cycling Bmi1-expressing stem cells situated at position +4 above the crypt base (Barker et al., 2007; Sangiorgi and Capecchi, 2008). In the small intestine, the stem cell markers Sox-9 and Hes1 are also expressed in actively cycling Lgr5+ cells, while Bmi-1, mTert, Hopx and Lrig1 are expressed in relatively quiescent stem cells, confirming the existence of more than one stem cell pool (Fre et al., 2011; Furuyama et al., 2011; Montgomery et al., 2011; Powell et al., 2012; Takeda et al., 2011). Moreover, Lgr5+ cells are dispensable in the small intestine, with Bmi1+ stem cells able to regenerate Lgr5+ cells and their lineages (Tian et al., 2011).
The epithelial lining of the colon is also comprised of a single layer of columnar epithelial cells that are constantly renewed by a pool of committed stem cells. These colonic stem cells give rise to progeny that terminally differentiate into a number of lineages that include colonocytes, mucus-secreting goblet cells, and enteroendocrine cells. Most recent studies have suggested that colonic stem cells are located at the crypt base throughout the colon, and a number of markers for colon stem cells have been proposed, including Lgr5 (Barker et al., 2007), Lrig1 (Powell et al., 2012), Sox9 (Ramalingam et al., 2012), and EphB2 (Jung et al., 2011). Lgr5 has been the best studied, with in vivo lineage-tracing showing that Lgr5-expressing cells at the colonic crypt base are capable of self-renewal and able to differentiate into all three colonic lineages. In the colon, however, it has been more challenging to identify the stem cells that reside above the crypt base. Bmi1+ cells, for example, do not exist in the colon. Thus, it is not known whether more than one distinct stem cell pool exists in the colon.
Tumors are postulated to arise from tissue stem or progenitor cells, but the relative contribution of different stem cell pools to tumorigenesis remains unknown (Barker et al., 2009). In addition, our current understanding of colon cancer is based on a model of clonal evolution, whereby early adenomas advance to invasive carcinomas through stepwise acquisition of mutations (Fearon and Vogelstein, 1990). In rapidly proliferating tissues such as the intestine or colon, however, this model of tumorigenesis implies that only stem cells are sufficiently long-lived to accumulate the requisite mutations. Indeed, the contribution of Lgr5+ stem cells to intestinal tumorigenesis has been demonstrated by the formation of adenomas upon targeted mutation of the Apc gene specifically in Lgr5+ cells (Barker et al., 2009). Nonetheless, the contribution of additional Lgr5-negative stem cells to the cellular origin of both colonic and intestinal cancer has not been clarified.
To determine if an Lgr5-negative stem cell contributes to colonic homeostasis and tumor initiation, we established a genetic fate-mapping system for labeling keratin-19 (Krt19) expressing progenitor/stem cells. Cytokeratins are a multigene family of intermediate filaments, critical in the maintenance of the cytoskeleton but expressed in different lineages within the epithelium (Moll R et al, Cell 1982). Cytokeratin 19 or Krt19 is the smallest known acid keratin (~40kDa), and is epithelial specific, found in a broad range of epithelial tissues. In the gastrointestinal tract, Krt19 expression is restricted to the proliferating compartments of the stomach, small intestine and colon, as well as the pancreatic ducts of the adult pancreas and the hepatobiliary ducts (Brembeck et al., 2001). Krt19 is expressed in the stem cell zone of the hair follicle (Brembeck et al., 2001; Lapouge et al., 2011; Means et al., 2008), is amplified in many solid tumors and, as we demonstrate here, is expressed near the presumptive progenitor/stem cell zone of both the colon and intestine. More specifically, we examined Krt19 because it is expressed at position +4 extending up to the isthmus, thus allowing us to selectively label a population of cells that included transit amplifying cells, progenitors and long-lived stem cells, yet exclude rapidly cycling CBC Lgr5+ stem cells.
We compared Krt19+ cells above the crypt base to Lgr5+ CBC cells with respect to their response to epithelial injury and cancer initiating ability. Krt19-expressing cells identify long-lived progenitors/stem cells distinct from Lgr5+ cells, and additionally render Lgr5+ stem cells dispensable in both the colon and intestine. Under conditional loss of the Apc gene, Krt19+ stem cells also display cancer initiating ability, yet are functionally distinct from Lgr5+ cancer initiating cells by their relative radioresistance.
Results
Krt19 transcript localizes to the stem cell zone above the crypt base and marks both colonic and intestinal stem cells
To localize Krt19 mRNA and protein expression, we performed in situ hybridization for Krt19 mRNA and immunofluorescence staining for Krt19 protein. Krt19 RNA was completely absent from the colonic crypt base, and was detected primarily in the isthmus (i.e. area of crypt narrowing) that included cells extending down near the presumptive crypt progenitor/stem cell zone (Figure 1A, S1A). In contrast, Krt19 protein showed minimal overlap with Krt19 RNA, and was localized predominantly in differentiated cells (Figure S1B). Similarly, in the intestine Krt19 RNA was detected primarily in the isthmus and not the intestinal crypt base (Figure 1B, S1B), while Krt19 protein expression localized to differentiated cells of the intestinal villus (Figure S1D). We have previously reported a progenitor/stem cell marker in the stomach that similarly displayed a discrepancy in the pattern between RNA versus protein expression (Quante et al., 2010), so we sought to examine whether Krt19 also marked a stem cell population. We developed a Krt19-BAC-mApple (Krt19-mApple) reporter mouse (Figure S1I) confirming that Krt19 gene expression was limited only to cells located well above the crypt base in both the colon (Figure 1C) and intestine (Figure 1D). Notably, the absence of both Krt19 mRNA and protein expression from the crypt base (Figure 1A–D and S1A–H) afforded us the unique opportunity to selectively label and compare a progenitor/stem cell pool situated above the crypt base (position +4) to the well described Lgr5+ CBC cells (Barker et al., 2007), and the more recently reported Lrig1+ stem cells (Powell et al., 2012) found at the crypt base.
Figure 1. Krt19 mRNA localizes to the colonic and intestinal stem cell zone and marks long-lived stem cells.
Krt19 mRNA is expressed in the isthmus extending down to the +4 position of the colonic (A) and (B) intestinal crypt. High magnification images of the crypt base are shown as insets. Krt19-mApple+ cells in the colon (C) and small intestine (D) of Krt19-mApple reporter mice show expression similar to in situ. 24 h post tamoxifen, β-gal+ colonic (E) and intestinal (F) crypts in Krt19-CreERT;R26RLacZ mice also show expression identical to in situ. Lineage tracing in the colon (G) of Krt19-CreERT;R26RLacZ mice. High magnification images of the crypt base are shown at 24 hours and 52 weeks following tamoxifen (6 mg p.o.). Low magnification images of lineage tracing in the colon (H) of Krt19-CreERT;R26RLacZ 26 weeks following tamoxifen (n ≥ 7 per group). Bright-field (6 and 24 hours after culture) (I) and 2-photon images (24 hours and 9 days following tamoxifen (6 mg p.o.)) (J) of colonic crypts from Krt19-CreERT; R26-mT/mG mice (n ≥ 4 per group) cultured in vitro. Lineage tracing in the intestine (K) in Krt19-CreERT/R26RLacZ mice with high magnification images of the crypt base shown at 24 hours to 52 weeks following tamoxifen (6 mg p.o.). Note black arrows that show Krt19-derived CBCs at 7 days and Paneth cells at 16 and 52 weeks. Low magnification images of lineage tracing in the intestine (L) of Krt19-CreERT/R26RLacZ 26 weeks following tamoxifen (n ≥ 7 per group). See also Figure S1 and S2.
To identify an Lgr5-negative progenitor/stem cell pool in the colon, we established a genetic fate-mapping system for labeling Krt19. We generated a Krt19-BAC-CreERT2 (Krt19-CreERT) transgenic line (Figure S1J–K) that was crossed to R26-LacZ (R26-LacZ) and ROSA26-mT/mG (R26-mT/mG) reporters in order to perform genetic lineage tracing experiments in homeostasis, inflammation and cancer. Shortly following tamoxifen induction, β-gal+ cells were localized to the colonic crypt (Figure 1E) in a pattern identical to Krt19-expressing cells detected by in situ and Krt19-mApple+ cells detected using a Krt19-mApple transgenic reporter mouse. Twenty-four (24) hours after tamoxifen, recombination was evident in the colonic isthmus extending down to the +4 position, but distinctly above the crypt base (Fig. 1G and S2A). One week (1 wk) following tamoxifen, recombined cells derived from Krt19+ cells extended downward to include Lgr5+ cells at the colonic crypt base (Figure 1G, and S2A). Sixteen weeks (16 wk) post-induction, Krt19+ cells traced all epithelial cell lineages in the colon (Figure 1G and S2A), and completely labeled glands were detected without any loss of labeling beyond 52 weeks (Figure 1G and S2A–D), consistent with Krt19 labeling long-lived stem cells.
In vitro, two-photon florescence microscopy of colonic crypts isolated from Krt19- CreERT;R26-mT/mG mice 12 h after a single dose of tamoxifen also revealed recombination (GFP+) in a number of Krt19-expressing cells above the crypt base (Figure 1I–J; n=6, colon). These cells contained bona fide stem cells and gradually replaced all epithelial cells over 9–10 days (colon: Figure 1I–J).
Similarly, in the intestine, genetic lineage tracing experiments revealed that shortly following tamoxifen induction, Krt19 labeled β-gal+ cells located clearly above the crypt base in the intestinal crypt (Figure 1K and S2E). These cells eventually traced all intestinal epithelial cell lineages, including Lgr5+ cells at the crypt base (Figure 1K and S2E–F), and again, consistent with the labeling of long lived stem cells, we observed no loss of labeling beyond 52 weeks (Figure 1C–D). Furthermore, in intestinal enteroids grown in vitro, a few Krt19+ cells above the crypt base could be detected 12h following tamoxifen (Figure S2G–I; n=4, intestine) and these cells expanded over 9–10 days to replace the entire crypt-villus column (Figure S2G–I). Single cell culture of Krt19-mApple+ cells isolated from the intestine of Krt19-mApple reporter mice further confirmed the stem cell capacity of Krt19+ cells (Figure S2J) at ~1% clonogenic efficiency compared to 5% for Lgr5-GFP+ cells.
Krt19+ potential stem cells above the crypt base are distinct from Lgr5+ CBCs
To measure the overlap of Krt19+ cells with Lgr5+ cells, we performed Krt19 in situ hybridization on colonic (Figure 2A) tissues of Lgr5-EGFP-IRES-CreERT2 mice. We confirmed that Krt19 mRNA expression was not detectable in CBC stem cells marked by Lgr5, and overlapped rarely with a very small subset of Lgr5-GFP positive cells located much higher in the colonic crypt (average position +7) (Figure 2C). Similarly, Krt19 mRNA expression was not detectable in Lgr5+ CBCs in the intestine (Figure 2B), and again overlapped rarely with a very small subset of Lgr5-GFP positive cells located much higher in the crypt (average position +7) (Figure 2C). The majority of Krt19+ cells were also distinct from Lgr5+ cells with respect to their proliferation status, as most Lgr5+ cells were located immediately below the proliferation zone, whereas Krt19+ cells predominated within the proliferation zone (~12% Lgr5+/Ki67+ versus ~50% Krt19+/Ki67+ cells) (Figure 2D–F and S3A–J). Importantly, when we generated Krt19-mApple;Lgr5-EGFP-IRES-CreERT2 dual reporter mice, Krt19-mApple+/Lgr5-GFP+ double positive cells were only detected in extremely rare cells located higher in the colonic crypt (Figure 2G) and comprised <0.05% of total epithelial cells and <6% of Lgr5-GFP+ cells as detected by FACS (Figure 2I–J, S3K). Similarly, in the intestine, <0.01% of total epithelial cells, and <5% of Lgr5-GFP+ cells were Krt19-mApple+/Lgr5-GFP+ double positive cells as determined by imaging (Figure 2H) and FACS analysis (Figure 2J and S3K). Thus, Krt19 and Lgr5 identify largely distinct populations (Figure 2K), with only rare overlap near the +7 cell region.
Figure 2. Krt19+ cells are located above Lgr5+ crypt base columnar cells in the colon and intestine.
Colocalization of Krt19 mRNA expressing cells (red) detected by in situ and Lgr5-GFP+ cells (green) in the colon (A) and small intestine (B) of Lgr5-EGFP-IRES-CreERT2 mice. White arrows denote double positive cells. (C) Average cell position of Krt19 mRNA expressing (red) and Lgr5-EGFP+ (green) cells within the colonic (top panel) and intestinal (bottom panel) crypt. Colocalization of Ki67 and Lgr5-EGFP+ cells (D) versus Ki67 and Krt19-EGFP+ cells 12 h after tamoxifen (E) in Lgr5-EGFP-IRES-CreERT2 or Krt19-CreERT;ROSA26-mG/mT mice, respectively. Yellow arrow shows a rare double positive (Ki67+, Lgr5-GFP+) cells and white arrow shows a rare Krt19+, Ki67− cells. Quantification of double positive Ki67+Krt19+ cells (red bars) versus Ki67+Lgr5-GFP+ cells (green bars) (F). Representative images of the colon (G) and SI (H) of Krt19-mApple;Lgr5-EGFP-IRES-CreERT2 dual reporter mice showing no overlap of Krt19-mApple+ and Lgr5-GFP+ cells in the colon and rare Krt19-mApple+/Lgr5-GFP+ double positive cells higher in the crypt of the SI. FACS plot (I) and quantification (J) of colonic and intestinal Krt19-mApple+ and Lgr5-GFP+ positive cells from Krt19-mApple;Lgr5-EGFP-IRES-CreERT2 mice. Schematic diagram of the intestinal crypt demonstrating the location of Krt19-expressing cells in relation to Lgr5+ crypt based columnar cells (K). mRNA expression levels of + 4 stem cell (BMi1, Hopx and Lrig1) and progenitor (Dll1) markers among Krt19-mApple+, Lgr5-GFP+ and Krt19-mApple+/Lgr5-GFP+ double positive cell populations (L). See also Figure S3.
Interestingly, RNA expression analysis revealed that the Krt19-mApple+/Lgr5-GFP+ double positive intestinal cells displayed significant enrichment for the known “+4” intestinal stem cell markers Bmi1, Hopx, Lrig1, as well as the intestinal progenitor marker Dll1 (Figure 2L), whereas Lgr5-negative Krt19-mApple+ cells showed relatively low or undetectable levels of both “+4” stem cell and progenitor markers (Figure 2L). Remarkably, despite the heterogeneity of Krt19+ cells posing a potential confounding factor in this RNA expression analysis, we confirmed using microscopy that only rare Krt19+/Bmi1+ cells are detected in the crypts of Krt19-CreERT;ROSA26-Tomato mice crossed to Bmi1-GFP mice (Figure S3L).
Thus, given the infrequent overlap of Krt19 and Lgr5, we sought to definitively distinguish between Krt19+ versus Lgr5+ cells. We generated Lgr5-DTR-EGFP;Krt19-CreERT;R26-Tomato mice to conditionally ablate Lgr5+ cells following administration of diphtheria toxin, as previously described (Tian et al., 2011). First, we confirmed at twenty-four hours following tamoxifen induction that Krt19-Tomato+ cells were located above the crypt base, and almost entirely distinct from Lgr5-GFP+ CBCs, (Figure 3A–B). Next, we administered diphtheria toxin (DT) three days prior to tamoxifen in order to ablate Lgr5+ cells before the start of Krt19 lineage tracing (Figure 3C). Interestingly, Krt19+ colonic stem cells continued to lineage trace in the colon, and gave rise to new Lgr5-GFP+ cells when the diphtheria toxin was stopped (Figure 3D). Importantly, the efficiency of Krt19+ cell lineage tracing was unchanged despite Lgr5+ cell ablation effectively eliminating both the Lgr5-GFP+ CBCs, as well as the rare “+4” Krt19-mApple+/Lgr5-GFP+ double positive cell populations (Figure 3E).
Figure 3. Krt19+ stem cells render Lgr5+ stem cells dispensable in the colon and small intestine.
Tamoxifen protocol (A) used to analyze Krt19+ and Lgr5+ cells in Lgr5-DTR-EGFP;Krt19-CreERT/R26RTomato mice 24h post tamoxifen. Images of the colon and SI 24h post tamoxifen are shown (B). Diphtheria toxin (DT) ablation regimen (C) used in Lgr5-DTR-EGFP;Krt19-CreERT/R26RTomato mice showing Krt19+ stem cells (red) render Lgr5+ cells (green) dispensable in the colon and SI (D). Quantification of Krt19+ stem cell lineage tracing efficiency in the presence or absence of Lgr5+ stem cells (E) is shown. DT induced Lgr5+ cells ablation and 5-FU induced transit amplifying (TA) cell ablation regimen (F) used in Lgr5-DTR-EGFP;Krt19-CreERT/R26RTomato mice. (G) Representative high power view of Ki67+ cells in the colon of control (left) versus 5-FU treated (right) mice. Quantification of Ki67+ cells in the colon or intestine of control versus 5-FU treated mice (H). Krt19+ stem cells (red) render Lgr5+ cells (green) dispensable in the colon and SI (J) in spite of 5-FU ablation of TA cells. Quantification of Krt19+ stem cell lineage tracing efficiency in the presence or absence of DT and/or 5-FU stem cells (I) is shown (n ≥ 6 per group). See also Figure S4 and S5.
Similarly, Krt19+ cells in the intestine continued to lineage trace and display resilency in the face of Lgr5-GFP+ cell ablation (Figure 3D). From these data, we conclude that Krt19+ lineage tracing in the intestine was not due to overlap with the Dll1+ progenitor population for several reasons. First, Dll1 RNA expression was predominantly detected within the rare “+4” Krt19-mApple+/Lgr5-GFP+ double positive and Lgr5-GFP+ CBC populations, rather than Krt19-mApple+ cells. Moreover, Dll1+ progenitors are reported to show no lineage tracing capacity when irradiated 2 weeks after tamoxifen (van Es et al., 2012), and additionally, are unable to form intestinal enteroids in the absence of Wnt3a (van Es et al., 2012). In contrast, Krt19+ cells formed intestinal enteroids in the absence of Wnt3a (Figure S4A–C) and also showed lineage tracing capacity in vivo when irradiated 2 weeks after tamoxifen (Figure 4K–L).
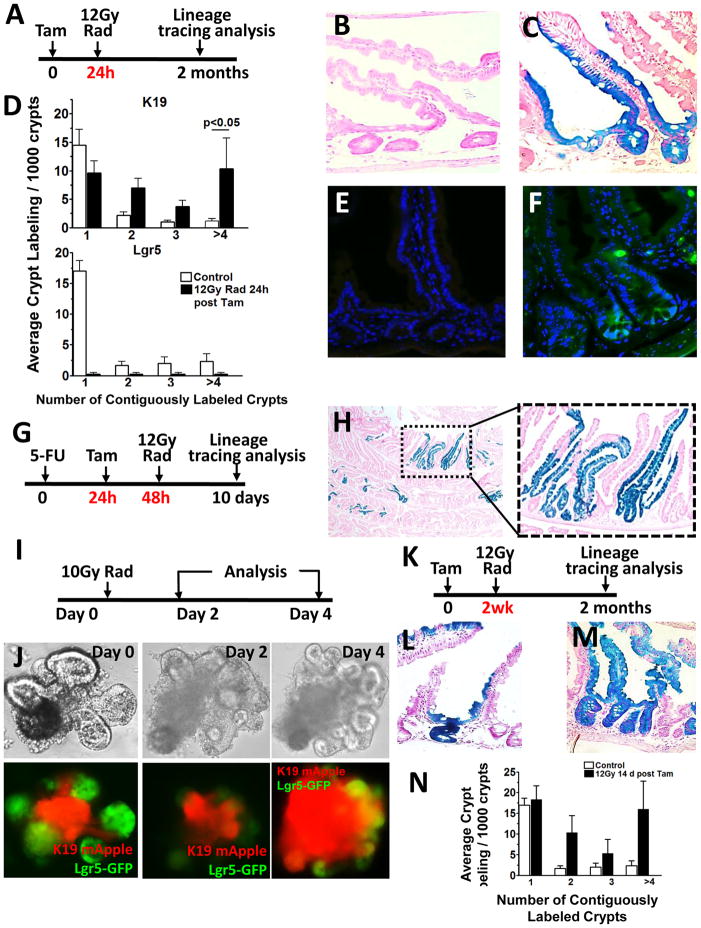
Figure 4. Krt19+ cells expand in response to injury and display relative radioresistance compared to Lgr5+ stem cells.
To examine the radiosensitivity of Krt19+ and Lgr5+ stem cells, mice were irradiated 24 h after tamoxifen and lineage tracing examined 2 months following tamoxifen (A). Representative β-gal+ intestinal crypts in Lgr5-EGFP-IRES-CreERT2;R26RLacZ (B) versus Krt19-CreERT;R26RLacZ (C) mice irradiated (12 Gy) 24 h post tamoxifen. Quantification of contiguously labeled β-gal+ Krt19 (top) versus Lgr5 (bottom) labeled crypts following irradiation 24h after tamoxifen (D). Representative small intestinal crypt-villus image of Lgr5-EGFP-IRES-CreERT2;R26RLacZ mice following high dose radiation exposure demonstrating the disappearance of Lgr5-EGFP+ crypt based columnar cells 24 h following irradiation (E) and re-emergence of EGFP+ CBCs 7 days following irradiation (F). In vivo 5-FU (150 mg/kg) protocol used to examine the effects of TA cell ablation on Krt19+ stem cell lineage tracing (G). Representative low (left) and high (right) power images of Krt19+ cell lineage tracing in Krt19-CreERT;R26RLacZ mice treated with 5-FU and examined 8 d post radiation (H). In vitro radiation protocol used to examine the effects of radiation injury on intestinal Krt19 and Lgr5 stem cell populations (I). Bright-field (top) and fluorescent (bottom) images of intestinal enteroids from Krt19-mApple+/Lgr5-GFP+ double transgenic mice cultured in vitro pre and post radiation (10Gy) (J). Radiation protocol used to examine Lgr5 derived lineage tracing in Lgr5-EGFP-IRES-CreERT2/R26RLacZ mice two weeks after tamoxifen (K). β-gal+ intestinal crypts from control (L) versus irradiated (M) Lgr5-EGFP-IRES-CreERT2;R26RLacZ mice. Quantification of contiguously labeled β-gal+ Lgr5 labeled crypts following irradiation 2 weeks after tamoxifen (N); (n ≥ 5 per group). See also Figure S6.
Krt19+ lineage tracing capacity was not due to overlap with Krt19+ transit amplifying (TA) cells. We used 5-fluorouracil (5-FU) to target the rapidly proliferating TA cell population as previously described (Doetsch et al., 1999; Stange et al., 2013), and confirmed that this treatment eliminated nearly all (> 95%) of the proliferating TA cells in both the colon and intestine (Figure 3F–H, S4D). Krt19+ cells lineage traced with the same efficiency in both the colon and intestine regardless of TA cell ablation alone, or TA cell plus Lgr5+ cell ablation (Figure 3J–I). Taken together, these data prove that Krt19 identifies a novel Lgr5(−) Krt19-expressing potential stem cell population in both the colon and intestine. Interestingly, Krt19 and Lgr5 additionally label distinct cell populations during development. Using a newly generated, constitutive Krt19-BAC-CRE transgenic mouse, we observed that Krt19 marked the early gastrointestinal endoderm, raising the possibility that Krt19 may also label a stem cell population in development (Figure S5A–G). This is in contrast to Lgr5-GFP+ cells which were first detected in the intestine as weakly GFP+ cells on post-natal day 5 (Figure S5H–I).
Krt19+ cells show relative radioresistance and are functionally distinct from Lgr5+ stem cells and Dll1+ progenitors
Radiation injury initiates intestinal stem cell division during epithelial repair (May et al., 2008; Yan et al., 2012), but Lgr5+ stem cells have been proposed to be radiosensitive (van Es et al., 2012; Yan et al., 2012), whereas Bmi1+ stem cells radioresistant (Yan et al., 2012). Thus, we sought to compare Krt19+ versus Lgr5+ stem cells with respect to their sensitivity to radiation. Krt19-CreERT;R26-LacZ and Lgr5-EGFP-IRES-CreERT2;R26-LacZ mice were irradiated (12 Gy) 24 h following tamoxifen labeling of each cell population (Figure 4A). When Lgr5-EGFP-IRES-CreERT2;R26-LacZ mice were irradiated 24 h after tamoxifen, absence of lineage tracing (Figure 4B and 4D and S6A) immediately following and in the early post radiation period confirmed that Lgr5+ cells were radiosensitive (Yan et al., 2012). Consistent with Lgr5+ cell radiosensitivity, we observed a loss of Lgr5-GFP expression immediately following radiation (Figure 4E). In contrast, radioresistant Krt19+ cells continued to lineage trace in irradiated Krt19-CreERT;R26-LacZ mice (Figure 4C and S6B), and we detected an increase in contiguously labeled Krt19+ crypts, consistent with crypt fission (Figure 4D) and stem cell expansion through symmetric division as previously described (Park et al., 1995). Moreover, the conclusion that radioresistance of Krt19+ cells was due to the labeling of stem cells, rather than TA cells, was supported by our observations that targeting of TA cells with 5-FU prior to radiation did not alter the lineage tracing capacity of Krt19+ cells (Figure 4G–H). Krt19+ cells also showed longevity (> 18 months) well beyond the two week life-span of Dll1+ progenitors and importantly, showed lineage tracing capacity even when irradiated 2 weeks following tamoxifen induction (Figure S6E–F).
To confirm our in vivo observations, we examined the effects of radiation on intestinal enteroid growth and stem cell function in vitro (Figure 4I). Following 10 Gy irradiation, intestinal enteroids from Krt19-mApple+/Lgr5-GFP+dual reporter mice showed a marked reduction in Lgr5-GFP+ stem cells associated with the loss of crypt budding suggestive of crypt injury (Figure 4I–J). In contrast, the same enteroids showed that Krt19-mApple expressing cells remained radioresistant and survived radiation injury. Indeed, during the regenerative state post radiation, newly budding crypts arose from radioresistant Krt19–mApple labeled cells (Figure 4J). These data again confirmed our in vivo observations that Krt19+ cells show relative radioresistance when compared to Lgr5+ stem cells.
Recently, it was shown that interconversion can occur between Hopx+ and Lgr5+ cells in vitro (Science 2012), and that Dll1+ progenitors can also revert back to an Lgr5+ state in vitro (van Es et al., 2012). Thus, we examined whether radiosensitive Lgr5+ stem cells could give rise to radioresistant stem cells in vivo. When we allowed for Lgr5 lineage tracing to occur for up to two weeks prior to radiation (12Gy) exposure, we observed a significant increase in the number of contiguously labeled Lgr5 traced SI crypts in Lgr5-EGFP-IRES-CreERT2;R26-LacZ mice after radiation (Figure 4K–N and S6C–D). Lineage tracing from Lgr5+ cells was only observed when tamoxifen was administered at least two weeks prior to radiation, suggesting that Lgr5+ cells can over time give rise to a radioresistant stem cell population such as Krt19+ cells. We demonstrated that Krt19+ cells give rise to Lgr5+ cells (Figure 1G, 1K, S2A and S2E); thus, to our knowledge, this is the first in vivo evidence that stem cell interconversion readily occurs between radioresistant (Krt19+) and radiosensitive (Lgr5+) states.
Radioresistant Krt19+ cancer initiating cells are functionally distinct from Lgr5+ cells
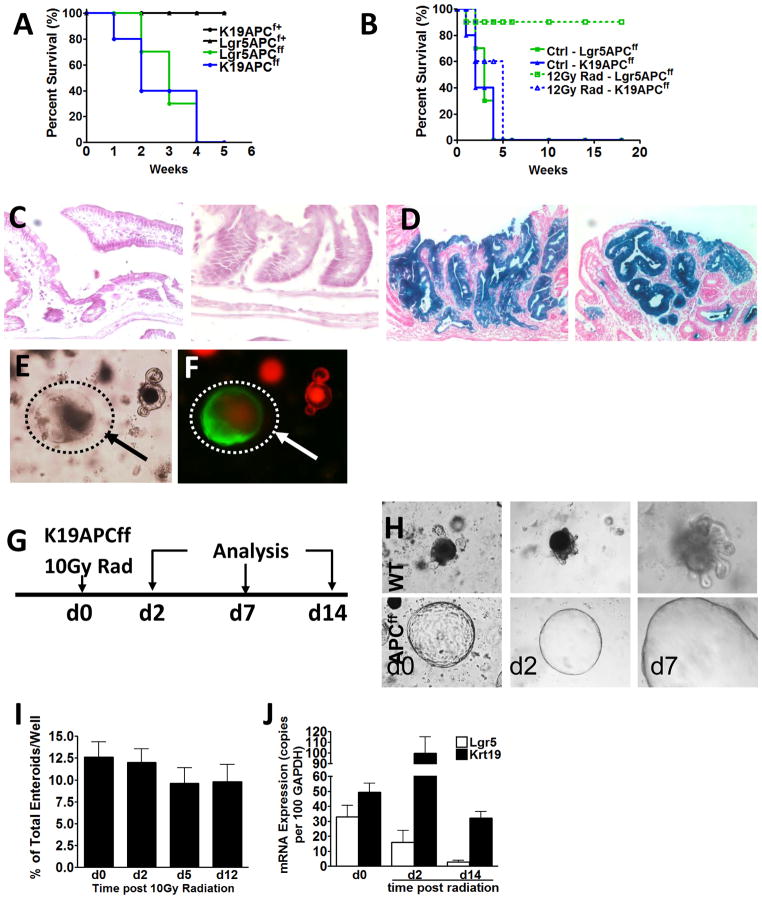
The contribution of Lgr5+ stem cells to early tumor development has previously been demonstrated by the formation of intestinal adenomas upon targeted mutation of the Apc gene in this lineage (5). However, the contribution of additional stem cell pools to the origin of cancer remains unknown. To determine whether Krt19+ cells can also function as cancer initiating cells in the colon and intestine, we generated Krt19-CreERT;R26-LacZ;ApcF/F mice in which conditional expression of a truncated form of Apc occurs in Krt19+ cells following tamoxifen induction. Analogous to Lgr5+ stem cells, Krt19+ cells initiated intestinal tumorigenesis following Apc deletion, resulting in rapid mortality (Figure 5A). To functionally distinguish between Krt19+ and Lgr5+ cancer-initiating cells, however, we further compared the susceptibility of these two stem cell pools to radiation injury. Interestingly, when irradiated 24h after tamoxifen, Lgr5-EGFP-IRES-CreERT2;R26-LacZ;ApcF/F mice showed no mortality (Figure 5B) and normal non-lineage traced colon and intestine (Figure 5C), whereas similarly treated Krt19-CreERT;R26-LacZ;ApcF/F mice continued to display rapid mortality (Figure 5B) from numerous lineage traced colonic and intestinal tumors (Figure 5D). Taken together, these observations provide evidence that Krt19+ stem cells are cancer initiating cells distinct from Lgr5+ cells.
Figure 5. Radioresistant Krt19+ cancer initiating cells are functionally distinct from Lgr5+ stem cells.
Krt19+ and Lgr5+ stem cells both serve as cancer initiating cells resulting in rapid mortality in Krt19-CreERT;R26-LacZ;ApcF/F or Lgr5-EGFP-IRES-CreERT2;R26-LacZ;ApcF/F mice (A). Lgr5-EGFP-IRES-CreERT2;R26-LacZ;ApcF/F mice irradiated 24h after tamoxifen show no mortality (B) and normal non-lineage traced intestine (left) and colon (right) (C). In contrast, Krt19-CreERT;R26-LacZ;ApcF/F mice irradiated 24h after tamoxifen continue to show rapid mortality (B) from intestinal (left) and colonic (right) tumors (D); (n ≥ 6 per group). Bright-field (E) and fluorescent (F) images of intestinal crypts from Krt19-CreERT;R26-mT/mG;ApcF/F cultured in vitro 24 h after tamoxifen. Note arrows point to recombined GFP+ Apc floxed Krt19+ cells appearing as spheroid structures. In vitro radiation protocol used to examine the effects of radiation injury on intestinal Krt19+ cell derived Apc floxed tumor populations (G). Krt19+ cell derived Apc floxed tumor populations pre (d0) and post (day 2 and day 7) radiation (10 Gy) (H). Quantification of surviving Krt19+ cell derived APC floxed enteroids pre and post radiation injury (I). Krt19 and Lgr5 mRNA expression levels in Krt19+ cell derived Apc floxed enteroids pre and post radiation injury (J).
Furthermore, when crypts from Krt19-CreERT; R26-mT/mG;ApcF/F mice were cultured in vitro 24 h after tamoxifen, recombined Apc floxed Krt19+ cells appeared as GFP+ spheroid structures (Figure 5E–F), which were easily distinguishable from non-recombined crypts that remained Tomato+ and formed normal budding crypt structures. Consistent with our in vivo observations, following in vitro irradiation (10 Gy), Apc floxed Krt19+ spheroids were radioresistant with no change in growth, and non-recombined Apc wild-type crypts showed radiosensitivity only within the budding crypts that contain Lgr5+ stem cells (Figure 5G–I). Post-radiation, there was neither in vitro nor in vivo Lgr5 mRNA expression, while Krt19 mRNA actually increased (Figure 5J and 6A). Thus, radiosensitive Lgr5+ stem cells are dispensable in both normal and Apc mutated crypts. Similarly, in Apc floxed tumors of Lgr5-EGFP-IRES-CreERT2;R26-LacZ;ApcF/F mice, we detected reduced Lgr5-GFP+ cells, but unchanged Krt19 protein positive cells 24 h post radiation (Figure 6B–C).
Figure 6. Lgr5+ stem cells are radiosensitive within colonic tumors unlike Krt19+ cells that are radioresistant.
In vivo Krt19 and Lgr5 mRNA in Apc floxed tumors pre or post (24h and 7 d) radiation (A); * indicates p<0.05 vs control; (n ≥ 4 per group). Lgr5-GFP+ (B) and Krt19 immunopositive (C) cells in Apc floxed tumors pre or post radiation induced targeting of Lgr5+ cells. In vitro Lgr5+ cell ablation protocol used to examine the dispensability of Lgr5+ cells in Krt19+ cell derived Apc floxed enteroids from Lgr5-EGFP-DTR;Krt19-CreERT;ApcF/F mice (D). Bright-field images of intestinal enteroids from Lgr5-EGFP-DTR;Krt19-CreERT;ApcF/F mice pre (day 0) and post (day 2 and day 7) DT ablation of Lgr5+ cells (E). Quantification of surviving Krt19+ cell derived WT and APC floxed enteroids pre and post Lgr5+ cell ablation (F). Krt19 and Lgr5 mRNA expression levels in Krt19+ cell derived APC floxed enteroids pre and post (48 h) Lgr5+ cell ablation (G); ** indicates p<0.01 vs control; (n ≥ 4 per group). Bright-field images of Krt19+ cell derived APC floxed intestinal enteroids from Lgr5-EGFP-DTR;Krt19-CreERT;ApcF/F mice cultured in the presence (−DT) or absence (+DT) of Lgr5+ cell ablation (H). Quantification of surviving Krt19+ cell derived APC floxed enteroids pre and post Lgr5+ cell ablation and cultured in the presence/absence of growth factors (I). Krt19 and Lgr5 mRNA expression levels in Krt19+ cell derived APC floxed enteroids grown in the presence or absence of standard growth factors (R-spondin and noggin), pre and post (48 h) Lgr5+ cell ablation (J); (n ≥ 4 per group).
When we additionally performed DT ablation of Lgr5+ cancer stem cells in crypts from Lgr5-DTR-EGFP;Krt19-CreERT;R26-LacZ;ApcF/F mice, we observed continued growth of many, but not all, Krt19+ Apc floxed enteroids (Figure 6D–F). We confirmed the efficacy of DT ablation of Lgr5+ cells by the absence of Lgr5 mRNA expression (Figure 6G), which again was associated with a corresponding increase in Krt19 mRNA expression (Figure 6G). Importantly, Krt19+ Apc floxed enteroids could also be maintained in culture in the absence of R-spondin, and were completely unaffected even by Lgr5+ cell ablation in this setting (Figure 6H–I). These data prove, for the first time, that Krt19+ cell derived Apc floxed enteroids are heterogeneous, with Lgr5+ cancer stem cells being completely dispensable in these enteroids (Figure 6I–J).
Discussion
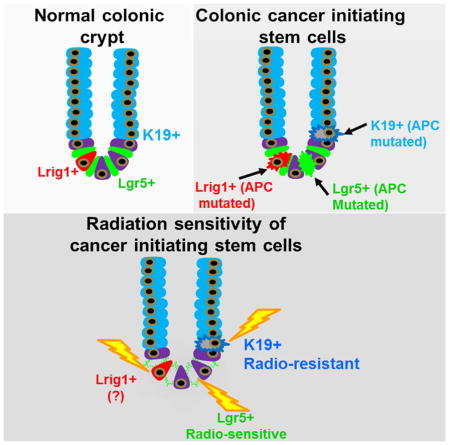
In contrast to the intestine, the paucity of stem cell markers in the colon has hampered our ability to identify and adequately characterize Lgr5-negative stem cell pools in normal and neoplastic colonic crypts. Here, we show that Krt19-expressing cells, extending from the +4 position to the crypt isthmus, include unique long-lived stem cells that are distinct from Lgr5+ CBCs. The distinct nature of Krt19+ versus Lgr5+ stem cells was confirmed by the observation that Krt19+ cells continue to lineage trace crypts despite ablation of Lgr5+ stem cells in both the colon and intestine. Krt19+ cells actively contribute to normal epithelial maintenance and are also clearly functionally distinct from Lgr5+ stem cells by their relative radioresistance. The radioresistance of Krt19+ cells holds true not only in the colon, but also in the intestine. Recognizing that Krt19-expressing cells comprise a heterogeneous population that also includes progenitor and TA cells, we confirmed that the differences in radiation response were nonetheless attributable to Krt19+ stem cells. Indeed, the combination of TA cell targeting by 5-FU and DT ablation of Lgr5+ cells confirmed that a unique population of Krt19+ stem cells continue to lineage trace and expand following radiation injury. Notably, in the intestine, it has been shown that Dll1+ progenitors and Bmi1+ stem cells are radioresistant (van Es et al., 2012; Yan et al., 2012) while Lgr5+ stem cells are radiosensitive. Interestingly, our observations regarding Krt19+ cell radioresistance now extend these findings to the colon, where Bmi1+ and Dll1+ cells are not found (Sangiorgi and Capecchi, 2008; van Es et al., 2012). Moreover, Krt19+ cells lineage trace independent of Lgr5+ cells following radiation injury, as Lgr5+ stem cells are radiosensitive when irradiated 24 h following tamoxifen induction. Taken together with the recent observations of Metcalfe et al., the rapid regeneration of Lgr5+ cells in the immediate post radiation period is essential for epithelial repair and likely to occur from a radioresistant Krt19+ population (Metcalfe et al., 2014). Thus, long-lived radioresistant Krt19+ cells are functionally distinct from radiosensitive Lgr5+ CBCs in both the colon and intestine.
Recent work by Takeda and colleagues (Takeda et al., 2011) suggested that interconversion between two or more stem cell pools occurs in enteroid cultures. Here, we demonstrate that Krt19+ stem cells give rise to Lgr5+ CBCs in both the colon and intestine, and that the reverse is also true. That is, radiosensitive Lgr5+ stem cells give rise to Krt19+ radioresistant cells, given enough time to interconvert following tamoxifen. Therefore, although previously speculated to be true, we provide the first in vivo evidence that Lgr5+ cells can indeed give rise to a radioresistant stem cell population (Figure 4 and S6).
Although the colon was the predominant focus of the current study, the potential overlap of Krt19 with other +4 intestinal stem cell markers or Dll1+ progenitors raises the possibility that some of our observations in the intestine could be attributed to overlap with these cell populations. It is important to note, however, that intestinal Krt19+ cells are long-lived, survive well beyond 18 months, and show lineage tracing capacity even when irradiated 2 weeks following tamoxifen induction. This is true, not only in the colon, but also in the intestine, where this is in sharp contrast to intestinal Dll1+ progenitors that are short-lived and do not display any lineage tracing capacity when irradiated beyond 24h following tamoxifen (van Es et al., 2012). Moreover, Krt19+ cells remained capable of sustaining intestinal enteroids in vitro, despite ablation of Lgr5+ stem cells and the absence of Wnt3a (a factor recently shown to be essential for Dll1+ progenitor reversion to stem cells). Furthermore, our RNA expression analysis revealed that the overlap of Krt19 with the various +4 intestinal stem cell markers, as well as Dll1, was only true of rare Krt19+/Lgr5+ double positive cells above the CBCs, yet DT ablation of all Lgr5+ cells including this overlapping population had no effect on Krt19+ stem cell lineage tracing activity. Taken together, these data prove that overlap with Dll1+ progenitors and Bmi1+ stem cells cannot solely explain our observations regarding radioresistance of Krt19+ cells in the intestine.
Ritsma and colleagues recently suggested that Lgr5+ cells display heterogeneity based on their “border” versus “central” position within the intestinal crypt (Ritsma et al., 2014). Our own observations that a rare subset of Lgr5+ cells expresses Krt19, while the majority of Lgr5+ cells do not, supports the premise of heterogeneity among Lgr5+ cells, and additionally, leads one to speculate whether Krt19-mApple+/Lgr5-GFP+ double positive cells identify a unique subset of Lgr5+ cells with “potential” stem cell activity as previously described (Kozar et al., 2013).
Conditional expression of a truncated form of Apc additionally confirmed that Krt19+ cells include a population of cancer initiating cells. That cancer initiation in Lgr5-EGFP-IRES-CreERT2;R26-LacZ;ApcF/F mice was completely suppressed in the colon as well as the intestine by high dose radiation 24h following tamoxifen confirmed that Lgr5+ cancer initiating cells were radiosensitive. In contrast, similarly treated Krt19-CreERT;R26-LacZ;ApcF/F mice developed many colonic and intestinal tumors despite irradiation, again demonstrating the functional distinction of Krt19+ versus Lgr5+ cancer initiating cells. Thus, we provide the first definitive evidence of an Krt19+/Lgr5(−) radioresistant cancer initiating cell population in both the colon and intestine. In view of the high prevalence of colon cancer and inflammatory conditions affecting the colon, the identification of colonic Krt19+/Lgr5(−) cancer initiating stem cells is highly relevant to our understanding and treatment of human disease. Additionally, we now demonstrate that Lgr5+ cancer stem cells are dispensable in Apc floxed tumors, particularly in R-spondin independent conditions. In view of the recent findings that R-spondin fusion proteins activate Wnt signaling in a subset of human colorectal tumors, our observations may have important implications for Lgr5+ cell targeted therapy in subsets of colorectal cancer patients (Seshagiri et al. 2012).
In summary, we identify a novel population of colonic Krt19+ cells that give rise to Lgr5+ CBC cells. Radioresistant Krt19+ cells located above the crypt base can initiate cancer and are functionally distinct from radiosensitive Lgr5+ CBCs. These findings have important clinical relevance for future cancer therapy targeting colonic stem cell populations.
Materials & Methods
Generation of Krt19-CreERT2 transgenic mice
For BAC recombineering, the K19 containing BAC clone (BAC RP-23-24N13) was transformed into SW105 competent cells and a Krt19-BAC-CreERT2 construct generated by BAC recombineering. See Supplementary Information for further details.
Lineage tracing analysis and assessment and immunofluorescence
Intestinal and colonic tissues were prepared as swiss rolls and sections fixed with 4% paraformaldehyde and β-galactosidase labeling assessed by X-gal staining of frozen sections taken from R26rLacZ mice. Mice were sacrificed at various time points post tamoxifen and analyzed at the time points specified. We similarly analyzed tissues from R26-mT/mG reporter mice for EGFP positive cells and their progeny at various time points post tamoxifen. Further details are outlined in the supplementary methods. For immunostaining, we performed staining of frozen sections with antibodies according to the methods detailed in the Supplementary Information.
Flow Cytometry
Single-cell suspensions were stained with antibodies and analyzed on FACS Calibur, Aria III (BD) or Gallios (Beckman Coulter). FlowJo software (Ashland) was used for data analysis. Detailed methods and antibodies are described in the Supplemental Information.
In situ hybridization
Using a cRNA probe constructed and labeled for Krt19, paraformaldehyde fixed SI and colonic tissues were hybridized with the probe. Detailed methods and antibodies are described in the Supplemental Information.
Statistical analysis
Statistical analysis was performed with Student’s t-test or Mann-Whitney when comparing two groups or standard ANOVA analysis with Bonferri correction. Values of * (P<0.05) and ** (P<0.01) were considered statistically significant.
Enteroid in vitro cultures
Intestinal or colonic glands units were isolated from mouse as previously described by Bjerknes and Cheng with some modifications, and cultured in the presence of EGF 50 ng/ml (Invitrogen), mNoggin 100 ng/ml (Peprotech), and R-Spondin 1 ug/ml as previously described (Sato et al., 2009). Detailed protocols are described in the Supplemental Information.
Supplementary Material
Acknowledgments
The authors thank the members of the Irving Cancer Research Center Core Microscopy Core Facility for their technical assistance. We also thank Genentech for their generosity in providing the Lgr5-GFP-DTR knock-in mice used for this study. The authors also recognize the technical assistance of Yagnesh Tailor, Karan Nagar, Chintan Kapadia and Kelly Betz. Dr. Samuel Asfaha is supported by a CIHR Clinician Scientist Phase I Award and AHFMR Clinical Fellowship Award. This work was supported by NIH UO1 DK103155, NIH R37 DK052778 and NIH RO1 DK097016 to Timothy C. Wang, and NIH R01 DK056645, Hansen Foundation and National Colon Cancer Research Alliance (AKR) and NIH P30 DK 050306 (AKR) and its Mouse Core Facility.
Footnotes
The authors do not have any conflicts of interests to declare.
Author Contributions:
| Samuel Asfaha: | study concept and design, acquisition of data, analysis and interpretation of data; writing of manuscript |
| Yoku Hayakawa: | acquisition of data, analysis |
| Ashlesha Muley | acquisition of data, analysis |
| Sarah Stokes: | acquisition of data, analysis |
| Trevor Graham: | analysis and interpretation of data |
| Russell Ericksen: | acquisition of data, analysis |
| Christoph B. Westphalen: | acquisition of data, analysis |
| Johannes von Burstin: | acquisition of data, analysis |
| Teresa L. Mastracci: | acquisition of data, analysis |
| Daniel L. Worthley: | analysis and writing of manuscript |
| Chandan Guha | analysis and interpretation of data |
| Michael Quante: | analysis and interpretation of data |
| Anil K. Rustgi: | analysis and writing of manuscript |
| Timothy C. Wang: | study concept, analysis and interpretation of data; writing of manuscript |
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Brembeck FH, Moffett J, Wang TC, Rustgi AK. The keratin 19 promoter is potent for cell-specific targeting of genes in transgenic mice. Gastroenterology. 2001;120:1720–1728. doi: 10.1053/gast.2001.24846. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Fre S, Hannezo E, Sale S, Huyghe M, Lafkas D, Kissel H, Louvi A, Greve J, Louvard D, Artavanis-Tsakonas S. Notch lineages and activity in intestinal stem cells determined by a new set of knock-in mice. PloS one. 2011;6:e25785. doi: 10.1371/journal.pone.0025785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, Koizumi M, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nature genetics. 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- Jung P, Sato T, Merlos-Suarez A, Barriga FM, Iglesias M, Rossell D, Auer H, Gallardo M, Blasco MA, Sancho E, et al. Isolation and in vitro expansion of human colonic stem cells. Nature medicine. 2011;17:1225–1227. doi: 10.1038/nm.2470. [DOI] [PubMed] [Google Scholar]
- Kozar S, Morrissey E, Nicholson AM, van der Heijden M, Zecchini HI, Kemp R, Tavare S, Vermeulen L, Winton DJ. Continuous clonal labeling reveals small numbers of functional stem cells in intestinal crypts and adenomas. Cell stem cell. 2013;13:626–633. doi: 10.1016/j.stem.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Lapouge G, Youssef KK, Vokaer B, Achouri Y, Michaux C, Sotiropoulou PA, Blanpain C. Identifying the cellular origin of squamous skin tumors. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7431–7436. doi: 10.1073/pnas.1012720108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May R, Riehl TE, Hunt C, Sureban SM, Anant S, Houchen CW. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem cells. 2008;26:630–637. doi: 10.1634/stemcells.2007-0621. [DOI] [PubMed] [Google Scholar]
- Means AL, Xu Y, Zhao A, Ray KC, Gu G. A CK19(CreERT) knockin mouse line allows for conditional DNA recombination in epithelial cells in multiple endodermal organs. Genesis. 2008;46:318–323. doi: 10.1002/dvg.20397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe C, Kljavin NM, Ybarra R, de Sauvage FJ. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell stem cell. 2014;14:149–159. doi: 10.1016/j.stem.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk ME, Henderson DE, Baffour-Awuah NY, Ambruzs DM, Fogli LK, Algra S, et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:179–184. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Goodlad RA, Wright NA. Crypt fission in the small intestine and colon. A mechanism for the emergence of G6PD locus-mutated crypts after treatment with mutagens. The American journal of pathology. 1995;147:1416–1427. [PMC free article] [PubMed] [Google Scholar]
- Powell AE, Wang Y, Li Y, Poulin EJ, Means AL, Washington MK, Higginbotham JN, Juchheim A, Prasad N, Levy SE, et al. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 2012;149:146–158. doi: 10.1016/j.cell.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quante M, Marrache F, Goldenring JR, Wang TC. TFF2 mRNA transcript expression marks a gland progenitor cell of the gastric oxyntic mucosa. Gastroenterology. 2010;139:2018–2027. e2012. doi: 10.1053/j.gastro.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalingam S, Daughtridge GW, Johnston MJ, Gracz AD, Magness ST. Distinct levels of Sox9 expression mark colon epithelial stem cells that form colonoids in culture. American journal of physiology Gastrointestinal and liver physiology. 2012;302:G10–20. doi: 10.1152/ajpgi.00277.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritsma L, Ellenbroek SI, Zomer A, Snippert HJ, de Sauvage FJ, Simons BD, Clevers H, van Rheenen J. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature. 2014;507:362–365. doi: 10.1038/nature12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nature genetics. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Stange DE, Koo BK, Huch M, Sibbel G, Basak O, Lyubimova A, Kujala P, Bartfeld S, Koster J, Geahlen JH, et al. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell. 2013;155:357–368. doi: 10.1016/j.cell.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334:1420–1424. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es JH, Sato T, van de Wetering M, Lyubimova A, Nee AN, Gregorieff A, Sasaki N, Zeinstra L, van den Born M, Korving J, et al. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nature cell biology. 2012;14:1099–1104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR, et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:466–471. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.