Abstract
Background
Activities of daily living (ADL) are important for quality of life. They are indicators of cognitive health status and their assessment is a measure of independence in everyday living. ADL are difficult to reliably assess using questionnaires due to self-reporting biases. Various sensor-based (wearable, in-home, intrusive) systems have been proposed to successfully recognize and quantify ADL without relying on self-reporting. New classifiers required to classify sensor data are on the rise. We propose two ad-hoc classifiers that are based only on non-intrusive sensor data.
Methods
A wireless sensor system with ten sensor boxes was installed in the home of ten healthy subjects to collect ambient data over a duration of 20 consecutive days. A handheld protocol device and a paper logbook were also provided to the subjects. Eight ADL were selected for recognition. We developed two ad-hoc ADL classifiers, namely the rule based forward chaining inference engine (RBI) classifier and the circadian activity rhythm (CAR) classifier. The RBI classifier finds facts in data and matches them against the rules. The CAR classifier works within a framework to automatically rate routine activities to detect regular repeating patterns of behavior. For comparison, two state-of-the-art [Naïves Bayes (NB), Random Forest (RF)] classifiers have also been used. All classifiers were validated with the collected data sets for classification and recognition of the eight specific ADL.
Results
Out of a total of 1,373 ADL, the RBI classifier correctly determined 1,264, while missing 109 and the CAR determined 1,305 while missing 68 ADL. The RBI and CAR classifier recognized activities with an average sensitivity of 91.27 and 94.36%, respectively, outperforming both RF and NB.
Conclusions
The performance of the classifiers varied significantly and shows that the classifier plays an important role in ADL recognition. Both RBI and CAR classifier performed better than existing state-of-the-art (NB, RF) on all ADL. Of the two ad-hoc classifiers, the CAR classifier was more accurate and is likely to be better suited than the RBI for distinguishing and recognizing complex ADL.
Electronic supplementary material
The online version of this article (doi:10.1186/s12938-015-0050-4) contains supplementary material, which is available to authorized users.
Keywords: Naïve Bayes, Random Forest, Activities of daily living, ADL recognition, Wireless sensor, Forward chaining inference engine, Circadian activity rhythm, Rule based inference, Classifiers
Background
Activities of daily living (ADL) is an umbrella term that refers to self-care, comprising activities or tasks that people undertake routinely in their everyday life. ADL are the essential activities a person needs to perform to be able to live independently. Existing literature identified the importance of ADL such as bathing, toileting, eating [1] as indicators of the physical and cognitive abilities of elderly individuals. ADL have been shown to predict the functional capacity in healthy adults and elderly people [1] and admission to a nursing home [2].
Activities of daily living assessment measures individuals’ ability to formulate and plan goals in interaction with the environment in which they live and are routinely used by physicians/clinicians. Decline in ADL performance was shown to reflect cognitive impairment, neurobehavioral dysfunction or other neurological disorder/injury [3]. Measuring impairments in ADL performance is important, but difficult to be assessed and recognized outside the native environment of a person (e.g. doctor’s office). Moreover, it is an important information for professional caregivers to optimize medication and to personalize care [4].
Activities of daily living are traditionally assessed with questionnaires like the Katz Index of Independence in Activities of Daily Living (Katz ADL) [1], Stanford Health Assessment Questionnaire [5] and the Barthel ADL Index [6]. The Katz ADL [1] ranks adequacy of performance in the six functions of bathing, dressing, toileting, transferring, continence and feeding. Questionnaire based ADL assessments are challenging as they rely on informant information. In addition, self-reported data are subject to bias and errors due to cognitive impairments or lack of insight.
The automatic assessment of ADL is a fundamental problem in elderly care. Several groups have proposed sensor-based systems to recognize and quantify ADL [7–11] in the patient’s home. In so-called smart homes, several sensors, e.g. accelerometers, microphone arrays, pressure sensitive mats, gas sensors and cameras are installed in the proximity of older patients to determine specific activities and to monitor their ability of coping with ADL [8, 9]. Other types of methods used to detect and recognise activities within a home include wearable sensors [12, 13], as well as data received from a residential power line [14, 15]. Wearable sensors are usually used to gather physiological and movement data [12], while cameras are used to detect a variety of activities, including sign language recognition, human gait recognition, sitting, standing and walking behaviors.
Irrespective of the sensor-type, all sensor systems require processing and classification of the massive amount of collected data to derive information regarding the ADL. Different algorithms have been used to classify and recognize ADL from sensor streams, such as probabilistic based [16], rule based [15], Gaussian Mixture Models [7], K-means clustering [15], Support Vector Machine (SVM) [17], Hidden Markov Models (HMM) [13, 18] and the Naïve Bayes (NB) classifier [18, 19]. The majority of these algorithms are training-based. Despite being quite simple, NB often delivers good results making it a good state-of-the art algorithm for first data assessments. Furthermore, the performance of NB gives better insight into the “complexity” of the underlying patterns than SVM or HMM. SVM and HMM are already designed to work with complicated data. The Random Forest (RF) [20] belongs to the new generation of classification schemes and has been used in clinical applications [21].
This paper describes the two ad-hoc classifier algorithms (rule based, pattern based) we developed and evaluates them using wireless sensor data collected from the homes of ten healthy subjects. The performance of the ad-hoc classifiers is also validated using the NB and RF classifiers. The choice of the state-of-the-art classifiers was based on common practice of first data assessment with the NB, and on novelty and resistance to over-fitting performance, shown by many data mining and machine learning researchers, for RF [20]. Firstly, we introduce the rule based forward chaining inference engine (RBI) classifier that finds facts in data and matches them against rules until a conclusion is reached. Inferencing in the context of rule-based system means to process the supplied data and the stored knowledge, in order to produce correct conclusions. A rule based inference engine using forward chaining searches the inference rules until it finds one where the rules match. When such a rule is found, the engine can conclude or infer the new fact to be added to the rule database. Forward chaining starts with the available data and uses inference rules to extract more data until a goal is reached.
Secondly, we introduce the circadian activity rhythm (CAR) classifier, which works within a framework to automatically rate routine activities and detect regular patterns of behavior. The human body keeps track of time in a section of the brain called the suprachiasmatic nucleus [22]. Clinical observations have shown that human functions follow periodical variations regulated by internal biological rhythms. When the period of the cycle approximates 24 h, it is qualified as circadian. Hunger, for instance, represents a circadian rhythm that is regulated by hormones and activity. Our hypothesis was that measuring the circadian variability of the ADL could improve the recognition accuracy of complex ADL.
Methods
Sensor system
The wireless sensor system [23] comprises of ten sensors boxes and a central computing unit (CCU). Five sensors which capture ambient values [i.e. temperature (°C) (DS18B20, Dallas Inc.), humidity (g/m3) (SHT21P, SENSIRION), luminescence (l×) (AMS302, Panasonic Inc.), passive infrared radiation (V) (EKMB1101111, Panasonic Inc.) and acceleration (m/s2) (ADXL345, Analog Device)] are assembled within one sensor box (l × w × h = 15 mm × 30 mm × 60 mm, weight = 80 g). A commercially available laptop, running customized Microsoft Windows 7 software, serves as the CCU and acts as a local data server. The ad-hoc classifiers implemented in Matlab R2007b (The MathWorks, Inc.) and state-of-the-art classifier implemented in KNIME (https://www.knime.org/knime.org) run on the CCU too. A receiver unit attached to the CCU collects the data packets transmitted from the ten sensor boxes. Besides the five ambient sensor values, each data packet includes a date, timestamp, sensor node number, supply voltage, status word with even-parity error handling and a handshake word to detect frame collision as shown in Figure 1. A wireless protocol device [23] built in a housing with a wearable belt clip and fitted with switches corresponding to the selected ADL serves as an electronic logbook.
Figure 1.

Data packet. The contents of the data packet transmitted by the sensor boxes to the central computing unit. The Box ID contains the room information while the 4 bytes of the acceleration represent the node acceleration, acceleration in x-axis, y-axis and z-axis.
Subject recruitment
The data collection was carried out in accordance with the latest version of the Declaration of Helsinki and was approved by the Ethics Committee of the Canton of Bern, Switzerland. All procedures related to the study were explained to the participants and a written informed consent was obtained prior to participation. No compensation for participation was provided. Healthy subjects were recruited via advertisement in the local newspaper and in the Senior University of Bern. Demographic details were collected using standard questionnaires. Subjects were assessed for neuropsychological details with standardized paper–pencil test battery, which included the Montreal Cognitive Assessment (MoCA) [24, 25], the Trail Making Test A and B (TMT-A, TMT-B) [26] and the timed “Up & Go” test [27, 28]. Subjects with cognitive impairment (MoCA score ≤26), or significant motor impairment (timed “Up & Go” ≥12 s) or sharing the house with others or not living alone were excluded. Ten healthy subjects (6 women, 4 men; age 28–79 years) were included in the study. The data were collected continuously for 20 consecutive days per subject.
ADL selection
Activities of daily living such as sleeping, grooming, toileting, getting ready for bed, cooking, eating, watching TV and seated activity were chosen for this study. The eight ADL (Table 1) and their definition were used to define the parameters required for classification.
Table 1.
Definition of the eight ADL selected for recognition
| ADL | Included activities | Excluded activities |
|---|---|---|
| Sleeping | Resting at night, a nap either in bed on couch | Lying down for recovery |
| Grooming | Personal hygiene | Simple toileting and hand washing |
| Toileting | Simple toileting and washing hands | Other or additional personal hygiene |
| Getting ready for bed | Personal hygiene before bedtime | Pre-bedtime rituals |
| Cooking | Preparing food in the kitchen | Cutting pizza from delivery service, making popcorn, etc., making tea or coffee |
| Eating | Having a meal (also delivered food) | Snack or having just a cup of coffee or a glass of water |
| Watching TV | Watching TV with main focus on the TV | Other activities while the TV is just on |
| Seated activity | Sitting at a table or in an easy chair | Taking a nap in a chair |
ADL activity of daily living.
System setup
The sensor boxes and the CCU were installed in the home of the ten healthy subjects. For each room, one sensor box was fixed at a height of approximately 2 m, facing towards the middle of the room. Additional sensor boxes were placed in the kitchen (on the fridge door) and in the bathroom (on the flush handle). Once the sensor system was set up and initialized, it recorded the five ambient environmental values autonomously at a rate of 0.2 Hz and continuously for a duration of 20 days in the participant’s home. For validation, the subjects were instructed to protocol their ADL using the wireless protocol device and a paper–pencil log book.
Data handling
Prior to classification, the collected data were stratified by rooms in the apartment [23] using a Bucketsort algorithm [29]. The sensor node number recorded during data acquisition provides the room information. The data for each room were then sorted in a chronological order by using a Radixsort algorithm [30].
Data analysis: ADL classification
Data analysis was based both on existing classification (NB and RF) algorithms and two ad-hoc (RBI and CAR), in-house developed algorithms. The goal of classification is to assign the sorted ambient sensor values to a given set of ADL. The supervised training approach based classifiers such as the NB and RF use a training-set data to predict the classification. Our ad-hoc classification algorithm is based on the assumption that irrespective of the daily routine of the subject, specific patterns with specific duration and timing occur every day [31].
Naïve Bayes (NB) classifier
This classifier is based on Bayes theorem [32], which assumes that the features are independent. Bayes conditional probability model is then combined with a decision rule, which picks the most probable hypothesis. This is done by maximising the posterior probability, and thereby assigning a class-label to a given input vector.
Random Forest (RF) classifier
The RF [33] belongs to one of the newest algorithm and is a non-probabilistic decision-tree based classifier. The algorithm generates a number of decision-trees. For each tree, only a random subset of the available data is considered. Additionally at each node, only a random subset of all features is used for the split. No pruning is performed on the final trees. To classify new data, it is feed into each tree, so that a majority vote over all trees decides which class-label is assigned. The advantages of the RF classifier is its resistance to overfitting which guarantees generalisation to new data.
Rule based inference (RBI) classifier
The RBI classifier is built on (1) a database of facts, (2) a rule-repository and (3) a forward chaining inference engine [23]. The database of facts is a collection of data sorted by the Bucketsort [29] and Radixsort [30] algorithms, but also previously classified ADL data (historical). The rule-repository provides the forward chaining inference engine with a set of parameterized behavioral knowledge ). The behavioral knowledge was defined by our medical team which consists of parameters such as minimum duration required for classification of the ADL sleeping or time defined for routine meal activities. A parser translates the parameterized behavioral knowledge into a lookup table disposable in the Random-Access Memory of the CCU. The forward chaining inference engine (Figure 2) then checks if the available data fulfills the defined rules and behaviors to be classified as a specific ADL and runs using three basic components:
Figure 2.
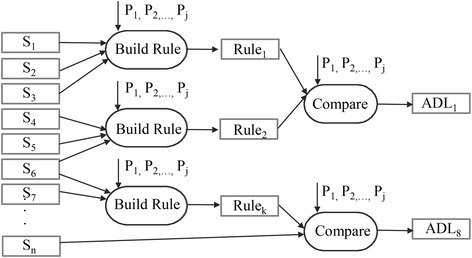
Forward chaining inference engine. Block diagram showing the forward chaining inference engine of the rule based inference (RBI) classifier. The forward chaining engine receives the sorted data from the database of facts and behavioural parameters (P 1, P 2, P 3, P j) the rule repository. The sorted data from the database of facts are converted to ambient value matrices (S i). The rules (Rule 1, Rule 2, Rule 3,…, Rule k) are built upfront using the behavioural parameters.
For each potential ADL, an ambient value matrix (Si) is composed from the sorted raw data. Each ambient value matrix, Si is then compared to a set of rules defined upfront for each of the eight ADL. The rules are defined manually and are the same for all subjects. For example, the values of the humidity sensor must increase to detect the action of grooming in the bathroom. The rules are built under the premise of the behavioral parameters. For example, cooking can only take place in the kitchen. Data not captured in the kitchen can be neglected when it comes to the determination of the ADL cooking. The rule “cooking can only take place in the kitchen” is a must and hence a very top level rule for an ad-hoc classifier. On the other end there are very low level rules like “switching of lights when leaving the kitchen”. After cooking, switching of the lights is the most likely case, but it is not necessary. Hence, “switching of lights when leaving kitchen” is likely, but not a requirement for the ADL cooking.
A conflict resolution strategy is implemented within the forward chaining inference engine to decide the order of information processing. Depending on the complexity of the ADL classified, n-steps of iteration were used. By processing the daily routine with the RBI classifier, the system allots specific patterns to one of the eight ADL. The output of the RBI classifier is the recognized ADL a subject performed at a given time on a day.
Circadian activities rhythm (CAR) classifier
Biological circadian rhythms are mainly based on daylight and are characterized by their amplitude, period and phase [22]. The daily activities of humans also periodically fluctuate and are dependent on the circadian rhythms. The CAR classifier is based on measuring the circadian variability of an activity by recognizing rhythmic patterns with small fluctuations for an activity. It is built on the idea of pattern recognition with a core algorithm, which analyses sequences [34] of ambient value matrices (Si). Figure 3 shows the sequence of data processing and analysis in the CAR classifier.
Figure 3.
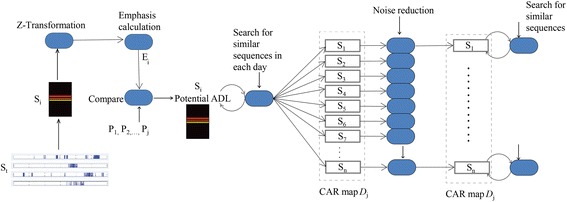
CAR classifier schematic. Schematic of data processing sequences in a circadian activity rhythm (CAR) classifier. The sorted data is composed into ambient value matrices (S i) and transformed using z-transformation. The emphasis of the matrices S i are calculated before detecting regular patterns in each of them.
After sortation, the ambient values samples (St) are composed into ambient value matrices (Si). This is done by summing up the St chronologically as long as the next sample is not null.
As each subjects performs or behaves differently in his or her home environment, there is inter-subject variability leading to unknown parameters for Si. For further computation, the Si are standardized by z-Transformation:
Next, the emphasis of the Si are calculated to detect data sequences with unique or pronounced patterns.
The obtained emphasis Ei is then compared to a set of parameterized behavioural knowledge defined by our medical team. If the emphasis Ei of a Si matches the parameters of the behavioural knowledge base, the Si is stated as a potential ADL. Based on this information, similar Si or Si sequences can be found throughout each day. By assembling these similar Si sequences according to their apportionment a CAR map Dj is obtained:
In order to reduce noise and eliminate out layers, brought in by sensor fluctuations and transmission errors, a Gaussian broad band filter is applied:
In a final step, similar sequences throughout each day (Figure 4) are analysed and determined. By analysing the occurrence and duration of each sequence in relation to other sequences, one of the eight ADL is allotted.
Figure 4.
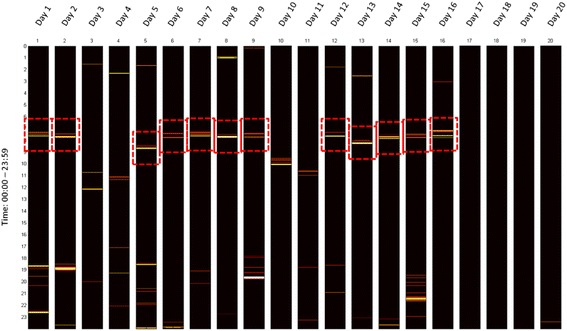
Regular patterns: CAR maps. The CAR classifier recognises similar patterns throughout each day in the ambient value matrices (S i) and checks for the time of occurrence, duration, and duration w.r.t to other sequences/patterns to recognise the ADL.
Threshold to remove false positives
To remove false positives, we implemented a simple thresholding approach which removed all predicted activities that lasted less than 20 s.
Classification performance
To evaluate the activity recognition performance of the NB and RF classifiers, a leave-one-out [35] cross-validation was performed on the ADL data. Leave-one-out is a special case of the k-folds validation. During each step of cross-validation, we trained our system with the data of nine subjects. We then used the trained system on the remaining (tenth) subject, to label the ADL. This cross-validation of training and testing was performed for all of the ten possible combinations.
The activity log from the wireless protocol device and the paper–pencil log book were merged to a single journalized ADL log which served as the ground truth for comparison with the classifier’s output (Figure 5). The performances (sensitivity and specificity) were calculated by cross-validating the output of the four classifiers with the journalized ADL log.
Figure 5.
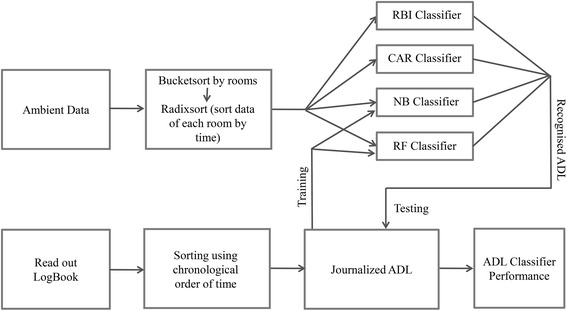
Validation schematic. Cross-validation of ADL classifier output with the wireless protocol box data/paper–pencil log book. The sensitivity and specificity of the classifiers are calculated using the logged data as the ground truth.
Activity maps
The activity map is a visualization technique which makes it possible to analyse the complete whole data at once. It is both a visual technique which can make use of the human eye’s ability to recognize patterns, and a quantitative one, in the sense that it introduces coloured sequences which quantify the information contained in the data patterns. For the RBI and CAR classifier, the recognized ADL for each subject were plotted against the time period of 20 days to generate an activity map.
Results
Demographics
The demographics of the healthy subjects are summarized in Table 2. The MoCA score, TMT-A, TMT-B and timed “Up & Go” scores were in a normal, non-pathological range.
Table 2.
Demographics and clinical parameters of the healthy subjects
| Demographics | Healthy subjects, n = 10 |
|---|---|
| Age (years) (mean ± SD [range]) | 48.8 ± 20.0 [28–79] |
| Gender (m/f) (% male) | 4/6 (40) |
| MoCA score (max = 30) (mean ± SD [range]) | 29.1 ± 1.14 [28–30] |
| Timed Up & Go (s) (mean ± SD [range]) | 8.2 ± 1.3 [7.0–9.0] |
| Trial Making Test A (s) (mean ± SD [range]) | 39.1 ± 20.0 [15–66] |
| Trial Making Test B (s) (mean ± SD [range]) | 62.6 ± 32.3 [21–97] |
MoCA Montreal Cognitive Assessment.
Data acquisition and transmission
The wireless sensors distributed in the home of the ten subjects (20 days/subject) successfully captured 33,939,441 data packets (543,031,056 environmental values). A small percentage (0.47%, 160,269 data packets) of the captured data packets were lost due to transmission errors, hence leading to an overall transmission reliability of 99.53%.
ADL classification and performance comparison
For the ad-hoc classifiers, the sorting and analysis of the captured data packets retrieved a total of 1,373 ADL. The RBI classifier correctly determined 1,264 ADL and missed 109 ADL, while the CAR classifier determined 1,305 ADL and missed 68 ADL. Additional file 1: Table S1 shows the number of missed and correctly classified ADL by the different classifiers. The state-of-the-art algorithms retrieved a total of 18,214 ADL datasets, whereof 5,032 were correctly recognised by NB and 13,971 by RF classifiers.
The difference in sensitivity and specificity for the individual ADL between the classifiers are shown in Table 3. The RF classifier with an average specificity of 97.07% and sensitivity of 60.36% performed better than the NB classifier whose mean specificity was 90.61%. The CAR classifier achieved high sensitivity for grooming (97.78%) and toileting (96.09%). Cooking proved to be the most challenging activity for the RBI classifier while both cooking and eating were challenging for the CAR. The CAR classifier performed better than the RBI (sensitivity 91.27%, specificity 92.52%) [23] with a result of 94.36% for sensitivity and 98.17% for specificity.
Table 3.
Performance of state-of the-art and ad-hoc classifiers
| Activities of daily living | State-of the-art classifiers | Ad hoc classifiers | ||||||
|---|---|---|---|---|---|---|---|---|
| Naive Bayes | Random Forest | Rule based inference | Circadian activity rhythm | |||||
| Sens. | Spec. | Sens. | Spec. | Sens. | Spec. | Sens. | Spec. | |
| Sleeping | 91.85 | 40.99 | 98.08 | 84.71 | 93.64 | 85.77 | 95.95 | 95.58 |
| Grooming | 27.03 | 96.67 | 55.58 | 99.62 | 94.07 | 96.98 | 97.78 | 98.91 |
| Toileting | 23.95 | 96.92 | 52.53 | 99.61 | 94.79 | 91.54 | 96.09 | 98.23 |
| Getting ready for bed | 9.53 | 98.52 | 43.03 | 99.86 | 92.38 | 94.48 | 95.24 | 98.39 |
| Cooking | 26.88 | 98.83 | 47.28 | 99.92 | 84.29 | 90.92 | 91.43 | 98.23 |
| Eating | 1.34 | 99.40 | 47.35 | 99.97 | 87.78 | 94.83 | 90.00 | 98.85 |
| Watching TV | 18.41 | 95.31 | 85.36 | 93.81 | 93.17 | 90.63 | 95.96 | 98.28 |
| Seated activity | 12.90 | 98.25 | 53.67 | 99.05 | 90.06 | 94.98 | 92.40 | 98.92 |
| Mean | 26.49 | 90.61 | 60.36 | 97.07 | 91.27 | 92.52 | 94.36 | 98.17 |
All values are represented as %.
Sens. sensitivity, Spec. specificity.
The activity map of a healthy female subject (age 75, MoCA 29) shown in Figure 6, displays the correctly recognised ADL from the data collected over 20 days using our ad-hoc classifiers. There are subtle differences in the visualised activity map of the same subject using the RBI and CAR classifiers.
Figure 6.
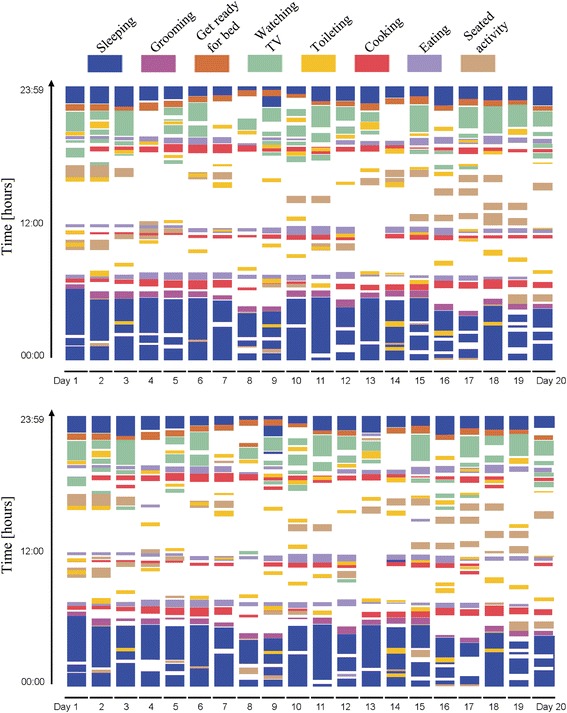
Activity maps. Activity maps are qualitative means to visualize the recognized ADL. This activity map visualizes the ADL recognized from sensor data of a healthy female subject (age 75, Montreal Cognitive Assessment score 29) using CAR (above) in comparison with the RBI [23] (below) classifier.
Discussion
Ambient assisted living applications are on the rise [36] and hence evaluation of classifiers are important for their deployment. In addition, validation of classifiers should be considered as part of establishing the clinical validity of the recognition technique [37]. We evaluated the NB, RF, RBI and CAR classifiers and demonstrated that all classifiers recognize ADL from data collected via the wireless sensor system. The comparison between the classifiers indicated that the best classification results were achieved using the CAR classifier. The CAR classifier does not require the training data, which makes it promising for easy and fast deployment. A good classifier is a vital parameter for a sensor system [38] and thus selecting the right classifier is important.
Some ADL are not exclusively room related and evoke only slightly different ambient values as reported earlier [16, 17, 39]. In our study, the spatial information is obtained from the sensor node number which recognizes the room of ADL. Spatial information helps increasing the accuracy of classification which is justified with our results. Highest accuracies were achieved for room specific ADL such as grooming with RBI and CAR and for toileting, cooking, eating with NB and RF. However, our algorithms have not taken the temporal information (limiting activity to time of the day) into consideration. This is an advantage, useful to extend our application to different kinds of patients and applications.
Specificity and accuracy of ADL recognition have been reported before and our results are in line with them [16, 17]. The SVM models used to recognise specific ADL using data captured from video cameras and microphones, wearable kinetic sensors achieved a sensitivity of 97.80% [17]. Single and combined classifiers have been compared before for specificity and sensitivity of ADL detection and shown that combined classifiers outperform single classifiers [40]. Specificity (99.6%) for routine ADL have been reported using a waist-worn wireless tri-axial accelerometer with an ensemble of classifiers [40]. The CAR classifier results are in line with these results, while the RBI classifier performance falls below this level.
Accurate models of ADL require labelled examples of ADL for training, however comparable ADL inevitably vary between households and between individuals. The protocol box and log book provided ADL labels for cross-validation and were used for training in the NB and RF classifiers, though not for training for the ad-hoc classifiers. However, we should not rule out the possibility of subjects reporting a wrong activity or missing an activity. This compliance in logging ADL negatively affects the performance of ADL recognition and may be one of the reason that performance for some ADL are low.
The majority of the algorithms reported earlier are usually embedded in other algorithms and make up only part of the final results, which makes a comparison of the different algorithms difficult. It is possible that one classifier can obtain very high accuracy for one ADL and average accuracy for another ADL, while the other classifier obtains moderate accuracy for both ADL. When such a condition arises it is difficult to predict the best classifier. In our case, the CAR classifier performed better than RBI for all ADL which makes the comparison easier. The RBI classifier can handle unusual data by adding another rule. But with the manual implementation of a rule-based algorithm there is a risk for over fitting and the performance on new data is non-predictable. The CAR classifier uses the fact that a person’s behavior during the daily cycle tends to fall into regular patterns and variation in these circadian patterns can be pre-markers of upcoming diseases. CAR based approaches have great potential in monitoring activity using ambient sensor systems and are expected to make “aging in place” a possibility for elderly people and patients with dementia [10, 11].
Activity maps can provide information about the variations or transitions in ADL patterns. This may help to benchmark the physical and cognitive abilities of patients [1]. The difference in activity maps between the two ad-hoc classifiers are may be too small and can be ignored by the human eye, however, changing the threshold window size the small differences between patients can be tuned in the activity map. An activity like sleeping, characterized by longer duration, can be best viewed with the activity maps. In addition, using threshold window size to exclude activities of very short duration improves classification performance.
The advantages of our wireless sensor system lie in its low maintenance, quick and easy installation, small discrete shape, non-intrusiveness into privacy of subjects and model-independent data collection. The sensor system further does not draw attention from the user and can be used discreetly. The classification performance in our study relied on the protocol compliance (wireless protocol box and paper–pencil log book) of the subjects. One possible downfall of this approach is that subjects might have forgotten to protocol an activity or logged a wrong ADL, which in turn may have affected the classification performance. While using the log book approach is far from flawless, it seems that the classifier performances reported in this paper actually represent a worst case scenario. Moreover, the ADL recognized and reported here are activities performed by subjects in a free-living context, where subjects made natural transitions between activities. We have classified a wide range of activities ranging from static “sleeping” to the more dynamic “cooking” compared to most studies which limit themselves to two or three ADL. The accuracies obtained with the wide range of ADL in our study is promising. State-of-the-art classifiers as well as custom built algorithms can be used with our wireless sensor system. As for limitations, we collected data using a small sample of participants. Future studies would benefit from using multiple demographic participant designs, as daily activity patterns vary with individuals.
Conclusion
The non-intrusive wireless sensor system can be used to acquire environmental data essential for the classification of ADL. Both the ad-hoc classifiers performed better than the state-of-art classifiers. The pattern recognition based CAR classifier performed better than the RBI and we surmise that it is better suited for complex ADL. Variations in regular circadian patterns are thus measurable by monitoring activity and hold great promise in early detection of disorders.
Authors’ contributions
TN, RMM and UPM were responsible for the conception of the research project and study design. The measurements were organised and carried out by PU, RS MB, and LR. RS and MB were involved in the data analysis and data mining. PU, LR, TN, RMM, UPM contributed to the interpretation of the data. PU was involved in drafting the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors would like to thank the subjects who volunteered for this study. We would also like to thank the Senior University of Bern, Switzerland, for their help with recruiting subjects.
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Funding This research was partially funded by the Bangerter-Rhyner Stiftung, Switzerland. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- ADL
activities of daily living
- RBI
rule based forward chaining inference engine
- CAR
circadian activity rhythm
- NB
Naïve Bayes
- RF
Random Forest
- SVM
Support Vector Machine
- HMM
Hidden Markov Models
Additional files
Additional file 1: Table S1 Confusion matrix for the classification of data.
Contributor Information
Prabitha Urwyler, Email: prabitha.urwyler@artorg.unibe.ch.
Luca Rampa, Email: luca.rampa@gef.be.ch.
Reto Stucki, Email: reto.stucki@artorg.unibe.ch.
Marcel Büchler, Email: marcel-buechler@bluewin.ch.
René Müri, Email: rene.mueri@insel.ch.
Urs P Mosimann, Email: urs.mosimann@gef.be.ch.
Tobias Nef, Email: tobias.nef@artorg.unibe.ch.
References
- 1.Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721–727. doi: 10.1111/j.1532-5415.1983.tb03391.x. [DOI] [PubMed] [Google Scholar]
- 2.Branch LG, Jette AM. A prospective-study of long-term care institutionalization among the aged. Am J Public Health. 1982;72(12):1373–1379. doi: 10.2105/AJPH.72.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Árnadóttir G. The brain and behavior: assessing cortical dysfunction through activities of daily living (ADL) St. Louis: Mosby; 1990. [Google Scholar]
- 4.Juchli L. Praxis und Theorie der Gesundheits- und Krankenpflege. Stuttgart: Thieme; 1994. [Google Scholar]
- 5.Pincus T, Summey JA, Soraci SA, Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26(11):1346–1353. doi: 10.1002/art.1780261107. [DOI] [PubMed] [Google Scholar]
- 6.Wade DT, Collin C. The Barthel ADL Index: a standard measure of physical disability. Disabil Rehabil. 1988;10(2):64–67. doi: 10.3109/09638288809164105. [DOI] [PubMed] [Google Scholar]
- 7.Almudevar A, Leibovici A, Tentler A. Home monitoring using wearable radio frequency transmitters. Artif Intell Med. 2008;42(2):109–120. doi: 10.1016/j.artmed.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Arcelus A, Jones MH, Goubran R, Knoefel F. Integration of smart home technologies in a health monitoring system for the elderly. In: IEEE conference. IEEE; 2007. p. 820–825.
- 9.Popescu M, Chronis G, Ohol R, Skubic M, Rantz M. A health recording system for predictive health assessment. In: IEEE conference. IEEE; 2011.
- 10.Rowe M, Lane S, Phipps C. CareWatch: a home monitoring system for use in homes of persons with cognitive impairment. Top Geriatr Rehabil. 2007;23(1):3–8. doi: 10.1097/00013614-200701000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wood A, Virone G, Doan T, Cao Q, Selavo L, Wu Y, et al. ALARM-NET: wireless sensor networks for assisted-living and residential monitoring. In: University of Virginia Computer Science Report, vol. 13; 2006.
- 12.Leutheuser H, Schuldhaus D, Eskofier BM. Hierarchical, multi-sensor based classification of daily life activities: comparison with state-of-the-art algorithms using a benchmark dataset. PLoS One. 2013;8(10):e75196. doi: 10.1371/journal.pone.0075196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stikic M, Huynh T, Van Laerhoven K, Schiele B. ADL recognition based on the combination of RFID and accelerometer data. In: International conference on pervasive computing technologies for healthcare. 2008.
- 14.Noury N, Berenguer M, Teyssier H, Bouzid MJ, Giordani M. Building an index of activity of inhabitants from their activity on the residential electrical power line. IEEE Trans Inf Technol Biomed. 2011;15(5):758–766. doi: 10.1109/TITB.2011.2138149. [DOI] [PubMed] [Google Scholar]
- 15.Berenguer M, Giordani M, Giraud-By F, Noury N. Automatic detection of activities of daily living from detecting and classifying electrical events on the residential power line. In: 10th IEEE international conference on e-health networking, applications and services (HealthCom 200). Singapore: IEEE; 2008. p. 29–32.
- 16.Bang SL, Kim M, Song S, Park SJ. Toward real time detection of the basic living activity in home using a wearable sensor and smart home sensors. In: IEEE conference. IEEE; 2008. p. 5200–5203. [DOI] [PubMed]
- 17.Fleury A, Vacher M, Noury N. SVM-based multimodal classification of activities of daily living in health smart homes. Inf Technol Biomed. 2010;14(2):274–283. doi: 10.1109/TITB.2009.2037317. [DOI] [PubMed] [Google Scholar]
- 18.van Kasteren TLM, Englebienne G, Kröse BJA. An activity monitoring system for elderly care using generative and discriminative models. Personal Ubiquitous Comput. 2010;14:489–498. doi: 10.1007/s00779-009-0277-9. [DOI] [Google Scholar]
- 19.Tapia EM, Intille SS, Haskell W, Larson K, Wright J, King A, et al. Real-time recognition of physical activities and their intensities using wireless accelerometers and a heart rate monitor. In: 11th IEEE international symposium on wearable computers. 2007. p. 37–40.
- 20.Witten IH, Frank E, Hall MA. Data mining: practical machine learning tools and techniques. 3. Burlington: Morgan Kaufmann; 2011. [Google Scholar]
- 21.Wang Y, Guo Y, Kuang Q, Pu X, Ji Y, Zhang Z, et al. A comparative study of family-specific protein-ligand complex affinity prediction based on random forest approach. J Comput Aided Mol Des. 2015;29(4):349–360. doi: 10.1007/s10822-014-9827-y. [DOI] [PubMed] [Google Scholar]
- 22.Vitaterna MH, Takahashi JS, Turek FW. Overview of circadian rhythms. Alcohol Res Health. 2001;25(2):85–93. [PMC free article] [PubMed] [Google Scholar]
- 23.Stucki RA, Urwyler P, Rampa L, Muri R, Mosimann UP, Nef T. A web-based non-intrusive ambient system to measure and classify activities of daily living. J Med Internet Res. 2014;16(7):e175. doi: 10.2196/jmir.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freitas S, Simoes MR, Marôco J, Alves L, Santana I. Construct validity of the Montreal Cognitive Assessment (MoCA) J Int Neuropsychol Soc. 2012;1(1):1–9. doi: 10.1017/S1355617711001573. [DOI] [PubMed] [Google Scholar]
- 25.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 26.Reitan RM. Trail making test. Tucson: Reitan Neuropsychology Laboratory; 1992. [Google Scholar]
- 27.Bischoff HA, Stähelin HB, Monsch AU, Iversen MD, Weyh A, von Dechend M, et al. Identifying a cut-off point for normal mobility: a comparison of the timed ‘up and go’ test in community-dwelling and institutionalised elderly women. Age Ageing. 2003;32(3):315–320. doi: 10.1093/ageing/32.3.315. [DOI] [PubMed] [Google Scholar]
- 28.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 29.Corwin E, Logar A. Sorting in linear time––variations on the bucket sort. J Comput Small Coll. 2004;20(1):197–202. [Google Scholar]
- 30.Blelloch GE, Leiserson CE, Maggs BM, Plaxton CG, Smith SJ, Zagha M. A comparison of sorting algorithms for the connection machine CM-2. In: Parallel algorithms and architectures. South Carolina: ACM; 1991. p. 3–16.
- 31.Virone G, Alwan M, Dalal S, Kell SW, Turner B, Stankovic JA, et al. Behavioral patterns of older adults in assisted living. IEEE Trans Inf Technol Biomed. 2008;12(3):387–398. doi: 10.1109/TITB.2007.904157. [DOI] [PubMed] [Google Scholar]
- 32.Bayes T, Price R. An essay towards solving a problem in the doctrine of chances. by the late rev. mr. bayes, frs communicated by mr. price, in a letter to john canton, amfrs. Philos Trans. 1963;1683–1775:380–418. [Google Scholar]
- 33.Breiman L. Random forests. Mach Learn. 2001;45(1):5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- 34.Bao L, Stephen SI. Activity recognition from user-annotated acceleration data. In: Ferscha A, Mattern F, editors. Pervasive computing. Berlin: Springer; 2004. p. 1–17 (Lecture Notes in Computer Science, vol. 3001).
- 35.van Kasteren TLM, Englebienne G, Kröse BJA. Human activity recognition from wireless sensor network data: benchmark and software. In: Chen L, Nugent CD, Biswas J, Hoey J, editors. Activity recognition in pervasive intelligent environments. Amsterdam: Atlantis Press; 2010. pp. 165–186. [Google Scholar]
- 36.Boulos MN, Wheeler S, Tavares C, Jones R. How smartphones are changing the face of mobile and participatory healthcare: an overview, with example from eCAALYX. Biomed Eng Online. 2011;10:24. doi: 10.1186/1475-925X-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foster KR, Koprowski R, Skufca JD. Machine learning, medical diagnosis, and biomedical engineering research––commentary. Biomed Eng Online. 2014;13:94. doi: 10.1186/1475-925X-13-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bersch SD, Azzi D, Khusainov R, Achumba IE, Ries J. Sensor data acquisition and processing parameters for human activity classification. Sensors. 2014;14(3):4239–4270. doi: 10.3390/s140304239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bouten CV, Koekkoek KT, Verduin M, Kodde R, Janssen JD. A triaxial accelerometer and portable data processing unit for the assessment of daily physical activity. IEEE Trans Biomed Eng. 1997;44(3):136–147. doi: 10.1109/10.554760. [DOI] [PubMed] [Google Scholar]
- 40.Yuwono M, Moulton BD, Su SW, Celler BG, Nguyen HT. Unsupervised machine-learning method for improving the performance of ambulatory fall-detection systems. Biomed Eng Online. 2012;11:9. doi: 10.1186/1475-925X-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


