SUMMARY
The increase in multi-drug resistant bacteria is limiting the effectiveness of currently approved antibiotics, leading to a renewed interest in antibiotics with distinct chemical scaffolds. We have solved the structures of the Thermus thermophilus 70S ribosome with A-, P- and E-site tRNAs bound, and in complex with either the aminocyclitol-containing antibiotic hygromycin A (HygA) or the nucleoside antibiotic A201A. Both antibiotics bind at the peptidyl transferase center and sterically occlude the CCA-end of the A-tRNA from entering the A-site of the peptidyl transferase center. Single-molecule Förster resonance energy transfer (smFRET) experiments reveal that HygA and A201A specifically interfere with full accommodation of the A-tRNA, leading to the presence of tRNA accommodation intermediates, and thereby inhibiting peptide bond formation. Thus, our results provide not only insight into the mechanism of action of HygA and A201A, but also into the fundamental process of tRNA accommodation during protein synthesis.
Keywords: Hygromycin A, A201A, antibiotic, 70S ribosome, X-ray structure, inhibition of translation, peptidyl transferase inhibitors, tRNA accommodation intermediate, single-molecule FRET
Graphical abstract
INTRODUCTION
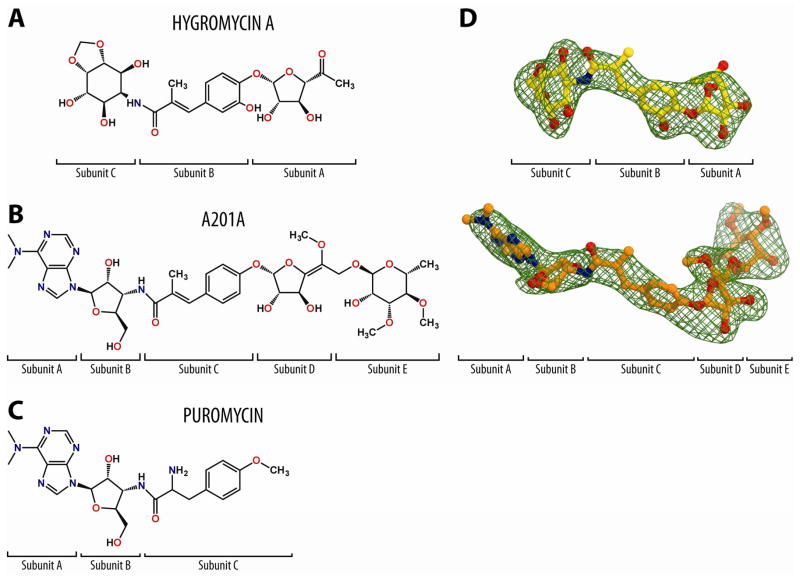
The process of protein synthesis in a bacterial cell is one of the major targets for antibiotics, with more than half of clinically used drugs binding to the ribosome (Wilson, 2009, 2014). However, the increase in multi-drug resistance within many pathogenic bacteria has limited the utility of many of the currently available antibiotics, which has renewed interest in the discovery and characterization of small-molecule compounds with novel chemical scaffolds (Sutcliffe, 2011). Two such compounds are hygromycin A (HygA, Fig. 1A) and A201A (Fig. 1B), which bear some resemblance to each other, as well as to the well-characterized nucleoside antibiotic puromycin (PMN) (Fig. 1C).
Figure 1. Chemical structures and electron density maps of aminocyclitol antibiotic HygA and nucleoside antibiotics A201A and PMN.
Chemical structures of (A) hygromycin A (HygA), (B) A201A, and (C) puromycin (PMN). (D) Difference Fourier maps of HygA and A201A in complex with the T. thermophilus 70S ribosome (green mesh). The refined models of the drugs are displayed in their respective electron densities before refinement. The unbiased (Fobs − Fcalc) difference electron density maps are contoured at 3.0σ. Carbon atoms are coloured yellow for HygA and orange for A201A, nitrogens are blue, and oxygens are red.
HygA was first discovered in the 1950’s as a secondary metabolite produced by Streptomyces hygroscopicus (Mann et al., 1953; Pittenger et al., 1953). Biosynthetic studies have revealed that HygA is assembled from three independently synthesized subunits, 5-dehydro-α-L-fucofuranose (subunit A), (E)-3-(3,4-dihydroxyphenyl)-2-methylacrylic acid (subunit B), and the aminocyclitol, 2L-2-amino-2-deoxy-4,5-O-methylene-neo-inositol (subunit C) (Kakinuma et al., 1976; Mann and Woolf, 1957) (Fig. 1A). HygA is, therefore, structurally distinct from the well-characterized aminoglycoside antibiotic hygromycin B, which was subsequently isolated from the same organism (Mann and Bromer, 1958). HygA has broad-spectrum activity against Gram-positive and, to a lesser extent, Gram-negative bacteria (Hayashi et al., 1997; Mann et al., 1953; Wakisaka et al., 1980). HygA also displays strong in vitro potency against Serpulina (Treponema) hyodysenteriae (Nakagawa et al., 1987; Omura et al., 1987), the causative agent of swine dysentery, an economically significant muco-hemorrhagic disease of pigs. In addition, HygA and its derivatives have been reported to have herbicidal (Kim et al., 1990; Lee et al., 2003) as well as immunosuppressant properties (Uyeda et al., 2001; Yoshida et al., 1986). Biochemical studies indicate that HygA inhibits translation by binding to the peptidyl transferase center (PTC) on the large ribosomal subunit and preventing the binding of aminoacyl-tRNA to the A-site (Guerrero and Modolell, 1980; Polacek et al., 2002; Poulsen et al., 2000).
A201A is an aminoacyl-nucleoside antibiotic that was first isolated from Streptomyces capreolus NRRL 3817 (Kirst et al., 1985) and more recently from the deep-sea marine actinomycete Marinactinospora thermotolerans SCSIO 00652 (Zhu et al., 2012). A201A is comprised of five subunits (Fig. 1B): 6-N-dimethylaminopurine (A), 3′-amino-3′-deoxyribose (B), α-methyl-p-coumaric acid (C), an unnamed hexofuranose (D), and 3,4-di-O-methyl-D-rhamnose (E), connected linearly via one amide and three glycosidic linkages (Kirst et al., 1985). Subunits A-C of A201A are structurally similar to PMN, whereas subunits C and D of A201A are structurally similar to subunits A and B of HygA (Fig. 1A–C). A201A is active against Gram-positive aerobic and anaerobic bacteria, and most Gram-negative anaerobic species (Epp and Allen, 1976). In contrast, it is weakly toxic to aerobic Gram-negative bacteria, certain fungi and mammals (Ensminger and Wright, 1976). A201A has been reported to inhibit peptide-bond formation on bacterial ribosomes (Epp and Allen, 1976), but is less effective against eukaryotic ribosomes (Jiménez and Vázquez, 1983).
The total synthesis of HygA (Chida et al., 1989; Donohoe et al., 2009) and, more recently, A201A (Nie et al., 2014) as well as the ability to generate biosynthetic precursors with biological activity (Dhote et al., 2009; Habib el et al., 2003; Palaniappan et al., 2006; Palaniappan et al., 2009), has further opened the way to developing successive generations of these antibiotics with improved antimicrobial properties. However, to fully understand the structure-activity relationships of HygA, A201A and analogs thereof, insights into the molecular modes by which these antibiotics interact with the ribosome and inhibit translation are required.
Here we present X-ray crystal structures of HygA in complex with the Thermus thermophilus (Tth) vacant 70S ribosome as well as HygA and A201A in complex with Tth 70S ribosome bearing A-, P- and E-site tRNAs, at resolutions ranging between 2.6–3.1Å (Table 1). These structures reveal that HygA and A201A bind at a common site within the PTC, in a position overlapping with that of aminoacylated-A76 of an A-tRNA. The presence of HygA and A201A sterically blocks the accommodation of A-tRNA at the PTC, causing local distortions of the tRNA acceptor arm and CCA-end. Consistent with these observations, single-molecule Förster resonance energy transfer (smFRET) imaging revealed that HygA and A201A do not interfere with initial binding of the ternary complex but specifically slow the proofreading phase of A-tRNA selection by as much as 1000-fold by preventing A-tRNA accommodation into the PTC.
Table 1.
Data collection and refinement statistics
| Crystals | 70S-HygA | 70S-HygA with A-, P- and E-tRNAs | 70S-A201A with A-, P- and E-tRNAs |
|---|---|---|---|
|
Diffraction data
| |||
| Space Group | P212121 | P212121 | P212121 |
| Unit Cell Dimensions, Å (a x b x c) | 209.51 × 448.29 × 619.37 | 208.39 × 444.58 × 619.20 | 208.89 × 446.22 × 619.48 |
| Wavelength, Å | 1.1000 | 0.9795 | 0.9795 |
| Resolution range (outer shell), Å | 310-3.10 (3.18-3.10) | 361-2.60 (2.67-2.60) | 262-2.65 (2.72-2.65) |
| I/σI (outer shell with I/σI=1) | 7.75 (1.01) | 7.32 (1.04) | 6.34 (1.03) |
| Resolution at which I/σI=1, Å | 3.10 | 2.60 | 2.65 |
| Resolution at which I/σI=2, Å | 3.35 | 2.79 | 2.85 |
| CC(1/2) at which I/σI=1, % | 26.8 | 19.0 | 27.2 |
| Completeness (outer shell), % | 99.9 (99.9) | 99.3 (99.5) | 98.5 (99.3) |
| Rmerge (outer shell)% | 22.7 (211.8) | 18.6 (150.3) | 15.0 (120.3) |
| No. of crystals used | 1 | 1 | 1 |
| No. of Reflections Used: | |||
| Observed | 5,754,441 | 9,880,724 | 5,648,754 |
| Unique | 1,041,680 | 1,725,274 | 1,628,435 |
| Redundancy | 5.52 | 5.73 | 3.47 |
| Wilson B-factor, Å2 | 81.2 | 51.7 | 50.7 |
|
| |||
|
Refinement
| |||
| Rwork/Rfree, % | 23.2/28.3 | 23.3/28.3 | 22.6/27.1 |
| No. of Non-Hydrogen Atoms | |||
| RNA | 192,347 | 200,165 | 200,193 |
| Protein | 91,479 | 90,982 | 90,982 |
| Ions (Mg, K, Zn, Fe) | 1,746 | 2,331 | 2,322 |
| Waters | 2,922 | 3,530 | 3.713 |
| Ramachandran Plot | |||
| Favored regions, % | 95.52 | 93.39 | 93.04 |
| Allowed regions, % | 4.02 | 5.56 | 5.72 |
| Outliers, % | 0.45 | 1.06 | 1.24 |
| Deviations from ideal values (RMSD) | |||
| Bond, Å | 0.003 | 0.005 | 0.005 |
| Angle, degrees | 0.678 | 0.965 | 0.945 |
| Chirality | 0.031 | 0.041 | 0.040 |
| Planarity | 0.003 | 0.005 | 0.005 |
| Dihedral, degrees | 14.709 | 15.330 | 15.055 |
| Average B-factor (overall), Å2 | 82.3 | 58.2 | 59.0 |
Rmerge = Σ|I−<I>|/Σ I, where I is the observed intensity and <I> is the average intensity from multiple measurements.
Rwork = Σ|Fobs − Fcalc|/Σ Fobs. For calculation of Rfree, 5% of the truncated dataset was excluded from the refinement.
RESULTS AND DISCUSSION
Structure of HygA in complex with the 70S ribosome
To determine the binding site of HygA on the ribosome, vacant Tth 70S ribosomes were crystallized in the presence of 100 μM HygA and a structure of the complex was determined by X-ray crystallography at 3.1 Å resolution (Table 1). An unbiased difference Fourier map, which was calculated using the observed amplitudes from the crystal and the amplitudes and phases derived from a model of the ribosome without the bound antibiotic, revealed positive density peaks resembling characteristic features of the HygA chemical structure (Fig. 1D). A single binding site for HygA was observed on the ribosome within the PTC of the large ribosomal subunit (Fig. 2A–C).
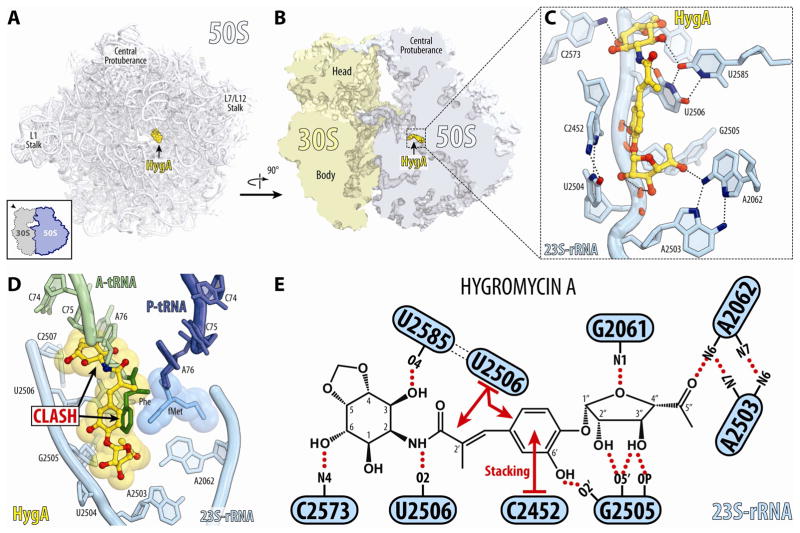
Figure 2. Structures of HygA on the ribosome.
(A, B) Overview of HygA binding sites on the 70S ribosome (30S, light yellow; 50S, light blue). (C) Interactions of HygA (yellow) with 23S rRNA nucleotides (cyan) within the PTC. (D) HygA (yellow) binding site relative to Phe-tRNA in the A-site (green with Phe moiety coloured dark green) and fMet-tRNA in the P-site (dark blue with fMet moiety coloured blue) (Polikanov et al., 2014b). (E) Schematic representation of the interactions shown in (C). Potential H-bond interactions are indicated with dashed lines, stacking interactions are shown with red arrows. See also Figures S1 and S2.
In agreement with the ability of HygA to inhibit binding of PMN, aminoacylated tRNAs and tRNA fragments to the ribosomal A site (Guerrero and Modolell, 1980; Polacek et al., 2002; Poulsen et al., 2000), the binding site for HygA overlaps with the aminoacyl moiety and nucleotide A76 of an A-tRNA (Fig. 2D). Unlike PMN, which contains an alpha-amino mimic on subunit C (Fig. 1C), HygA has a methyl group in the equivalent position (Fig. 1A) and therefore cannot act as an acceptor of the amino acid or nascent chain attached to the P-tRNA (Fig. 2D). Thus, while PMN inhibits translation by promoting premature termination and release of the nascent polypeptide chain, HygA acts by sterically occluding the binding of A-site ligands.
Consistent with reports that HygA competes with chemically distinct PTC inhibitors for ribosome binding (Guerrero and Modolell, 1980), the binding site of HygA overlaps with those of chloramphenicol (Bulkley et al., 2010; Dunkle et al., 2010), linezolid (Ippolito et al., 2008; Wilson et al., 2008), and clindamycin (Dunkle et al., 2010; Schlünzen et al., 2001; Tu et al., 2005) (Fig. S1A–D). The competition of HygA with 16-membered macrolide antibiotics, such as spiramycin, tylosin or carbomycin, but not with 14-membered macrolides, such as erythromycin, is in accordance with the suggestion that the mycarose sugar moiety of the 16-membered macrolides (absent in erythromycin) encroaches upon the HygA binding site (Poulsen et al., 2000) (Fig. S2A–D).
Interactions of HygA at the PTC
HygA binds within the A-site cleft on the large ribosomal subunit with its aromatic subunit B sandwiched between U2506 and C2452, and is oriented such that the methylenedioxy ring of subunit C protrudes towards C2573, while the furanose ring of subunit A (at the other end of HygA) extends into the ribosome exit tunnel (Fig. 2C, D). Binding of HygA appears to induce a rotation of U2506 towards the drug, compared to the position of U2506 in the Tth 70S lacking A- and P-tRNAs (Polikanov et al., 2012). In this position, U2506 forms base-pairing interactions with U2585, which explains protection of these bases from chemical modification in the presence of HygA (Poulsen et al., 2000).
HygA is highly hydrophilic and its many hydroxyl groups establish numerous hydrogen bonding (H-bond) interactions with nucleotides of the 23S rRNA (Fig. 2C, E). The C3 and C6 hydroxyls of the aminocyclitol ring of subunit C of HygA are within 3.1 Å and 2.7 Å from the O4 of U2585 and the N4 of C2573, respectively. These interactions are likely to be important, since HygA derivatives, where the C3 and C6 hydroxyls are methylated, exhibit reduced antimicrobial activity (Hecker et al., 1992). Although the methylenedioxy ring of subunit C of HygA has also been reported to be critical for biological activity (Chida et al., 1990; Hecker et al., 1992; Palaniappan et al., 2009), it does not appear to contribute directly to the interaction of HygA with the ribosome (Fig. 2C). This observation agrees with the suggestion that the methylenedioxy ring is important to induce the twisted-boat conformation of the adjacent aminocyclitol ring (Chida et al., 1990; Hecker et al., 1992), which, in turn, orients the C3 and C6 hydroxyls to establish the observed H-bond interactions (Fig. 2C, E). The C6′ hydroxyl in subunit B of HygA is within H-bond distance (2.9 Å) from the ribose 2′-OH of G2505. Substitutions of the C6′ hydroxyl generally lead to decreased antimicrobial activity (Hecker et al., 1993), highlighting the importance of HygA interaction with G2505. The hydroxyls of the furanose moiety of subunit A also potentially form H-bonds with A2062, U2504 and G2505 and are thus likely to contribute to HygA binding affinity. Consistent with this notion, HygA analogs lacking the furanose ring (i.e., subunit BC compounds) are devoid of antimicrobial activity (Jaynes et al., 1993). The furanose sugar can, however, be substituted with simple lipophilic alkyl esters without loss of activity (Jaynes et al., 1993; Jaynes et al., 1992), indicating the possibility for development of better HygA derivatives via alterations within the subunit C region of the drug.
HygA resistance mechanisms
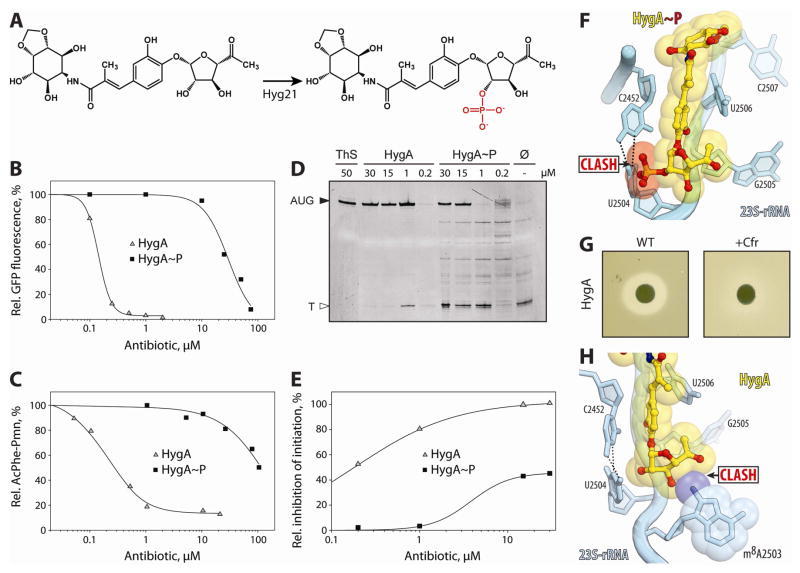
The strain of S. hygroscopicus that produces HygA is thought to obtain self-resistance to HygA using at least two mechanisms: (i) efflux of the drug by the putative proton gradient-dependent efflux pumps Hyg19 and Hyg28 (Dhote et al., 2009); (ii) inactivation of HygA through the action of the phosphotransferase Hyg21 (Dhote et al., 2008). The latter catalyzes the transfer of the γ-phosphoryl group from ATP to the ribose 2″-OH of subunit A of HygA (Fig. 3A), rendering the drug inactive (Dhote et al., 2008). To ascertain whether the inactivity of phosphorylated-HygA (HygA~P) could be attributed to the abrogation of translation inhibition, rather than other factors such as decreased membrane penetration, we performed in vitro translation of green fluorescent protein (GFP) in the presence of increasing concentrations of HygA and HygA~P (Fig. 3B). Consistent with previous studies (Dhote et al., 2009; Guerrero and Modolell, 1980; Palaniappan et al., 2009), HygA displayed strong inhibitory properties, inhibiting GFP production with a half inhibitory concentration (IC50) of 0.2 μM (Fig. 3B). In contrast, HygA~P was over 50-fold less effective, displaying an IC50 of 12 μM (Fig. 3B). An even larger difference between HygA and HygA~P was observed using the puromycin assay (Fig. 3C), which monitors the formation of AcPhe-PMN when PMN is added to a ribosomal complex containing AcPhe-tRNAPhe in the P-site. The IC50 of HygA for the puromycin assay was 0.5 μM, whereas HygA~P was 200 times less effective, exhibiting only 50% inhibition at 100 μM (Fig. 3C). Toe-printing assays revealed that HygA stalls ribosomes at the mRNA start codon, whereas HygA~P is much less effective as an inhibitor, allowing ribosomes to initiate and even enter the translation elongation stage (Fig. 3D, E). Modelling the phosphate group onto the subunit A ribose 2″-OH showed a steric clash with U2504 of the 23S rRNA, providing a structural basis for the poor antimicrobial activity observed with HygA~P (Fig. 3F).
Figure 3. Resistance to HygA.
(A) Schematic diagram illustrating the conversion of HygA to phosphorylated HygA (HygA~P) catalyzed by Hyg21 O-phosphotransferase (Dhote et al., 2008). (B) In vitro translation of green fluorescence protein (GFP) in the presence of increasing concentrations of HygA or HygA~P. (C) Puromycin reactivity of a ribosomal complex containing AcPhe-tRNA bound at the P-site in the presence of increasing concentrations of HygA or HygA~P. (D) Toe-printing assay monitoring translation in the presence of increasing concentrations of HygA or HygA~P. Additionally, control reactions without antibiotic (Ø) or including thiostrepton (ThS) are shown. AUG designates location of the ribosomes stalled at the start codon, T denotes the position of the unique Thr codon, which stalls ribosomes because of the lack of Thr amino acid in the reaction mixture. (E) Quantitation of (D) where the inhibition efficiency was calculated as a percentage of stalling at AUG relative to combined intensities of AUG and T. (F) Molecular modelling of HygA~P reveals a clash between the phosphate group and nucleobase of U2504 of the 23S rRNA. (G) Disc-diffusion assay monitoring growth of wild-type E. coli strain lacking Cfr (WT, left) and the same strain expressing Cfr (+Cfr, right) in the presence of HygA (5 nmol). (H) Molecular model of C8-methylation of A2503 by Cfr revealing a clash with the ribose of subunit A of HygA. See also Figure S3.
Resistance to many peptidyl transferase inhibitors, such as chloramphenicol, lincomycin and linezolid, is conferred by Cfr, a methyltransferase that monomethylates the C8 position of A2503 of the 23S rRNA (Long et al., 2006). To assess whether Cfr also confers resistance to HygA, we employed a disc-diffusion assay (Fig. 3G). As expected, a ring of growth inhibition for the parental E. coli strain was observed around the origin where HygA was spotted onto the disc. In contrast, the growth inhibition was alleviated when the same strain contained a plasmid expressing Cfr, indicating acquired resistance to HygA (Fig. 3G). Moreover, the toe-printing assay revealed that HygA is less effective as a translation initiation inhibitor when using Cfr-modified ribosomes (Fig. S3A). Modelling the C8-methylation of A2503 shows a steric clash with the ribose of subunit A of HygA, providing a structural basis for the Cfr-mediated HygA resistance (Fig. 3H).
HygA allows initial binding but prevents full accommodation of A-tRNA
To investigate the impact of HygA on the mechanism of aminoacyl-tRNA (aa-tRNA) selection, we employed pre-steady state single-molecule Förster resonance energy transfer (smFRET) imaging methods that enable real-time measurements of inter-tRNA distance during EF-Tu-catalyzed delivery of aa-tRNA to surface-immobilized ribosomes (Blanchard et al., 2004; Geggier et al., 2010). Here, the time-evolution of FRET was monitored at a 10 ms/frame time resolution within individual 70S ribosomes bound with (Cy3-s4U8) fMet-tRNAiMet in the P site upon stopped-flow injection of ternary complex containing EF-Tu, GTP and (Cy5-acp3U47) Phe-tRNAPhe (Fig. 4A).
Figure 4. smFRET analysis of the HygA effect on tRNA accommodation.
(A) Schematic diagram illustrating the delivery of EF-Tu•GTP•tRNA ternary complex containing cognate Phe-tRNAPhe(Cy5) to the A site of E. coli 70S ribosomes containing tRNAiMet(Cy3) in the P site, leading to intermediate (0.35) FRET during initial steps of codon recognition, and high (0.63) FRET upon A-site tRNA accommodation. (B–E) Single-molecule FRET imaging of aa-tRNA selection performed under direct 532 nm excitation, using either a (B, C) 10 ms/frame or (D, E) 250 ms/frame time resolution in the absence of drugs (B, D), or with 20 μM HygA (C, E). The two-dimensional histograms were generated by superimposing all individual FRET trajectories, aligned to the initial time point of non-zero FRET efficiency. See also Figure S4.
As expected from previous investigations in the absence of the drug (Geggier et al., 2010), productive FRET events leading to the incorporation of aa-tRNA at the A site evolved from a low- (~0.2) to high- (~0.63) FRET via the reversible transit of at least one intermediate- (~0.35) FRET configuration (Fig. 4B). The intermediate FRET value reflects the “A/T” state (Blanchard et al., 2004; Geggier et al., 2010), where ternary complex is bound within the ribosomal A site such that the tRNA anticodon is paired with mRNA in the small subunit and the acceptor stem interacts with EF-Tu (central panel in Fig. 4A) (Schuette et al., 2009). Consistent with the rapid tRNA progression through the selection process, the time delay between the initial observation of low FRET and formation of the stable, high-FRET state, corresponding to the fully accommodated, classically configured (A/A) tRNA position (right panel in Fig. 4A), was ~60 ms.
In the presence of saturating concentrations of HygA (20 μM), aa-tRNA progression into the ribosome was strongly and specifically blocked at what appeared to be an intermediate state in the selection process that exhibited a FRET value (~0.54) in between those of the GTPase-activated A/T (~0.35 FRET) and fully accommodated (A/A) (~0.63 FRET) positions (Fig. 4C). By contrast, early steps in the selection process were unaffected, including the rate and efficiency with which this state was achieved (Fig. 4C and Fig. S4A).
Notably, the inspection of fluorescence and FRET trajectories from single ribosomes revealed that the HygA-stalled ribosomes exhibited highly anti-correlated fluctuations in donor and acceptor intensities, reflecting rapid, reversible fluctuations between intermediate- (~0.35) and high-(~0.63) FRET states (Fig. S4B). Analogous fluctuations were also observed on drug-free ribosomes (Fig. S4B and (Geggier et al., 2010)), albeit for a much shorter period of time before stable accommodation. This important observation indicates that the FRET value observed at the population level (~0.54) for the stalled complex (Fig. 4C) does not reflect a defined tRNA accommodation intermediate on the pathway between the A/T and A/A state, but rather a weighted average of the intermediate- and high-FRET states. The average high-FRET value observed in the presence of HygA (~0.54) resembled that for the inhibited fully-accommodated aa-tRNA (~0.63), indicating that HygA blocks a very late stage of aa-tRNA accommodation. Imaging experiments performed at a lower time resolution (250 ms/frame) indicated that the HygA-induced stall in tRNA selection persisted for ~60 seconds prior to complete accommodation of aa-tRNA at the PTC (Fig. 4D, E and Fig. S4C, D). At present, it is unclear whether EF-Tu remains bound to the ribosome during these processes.
Structure of 70S complex with HygA and bound A-site tRNA
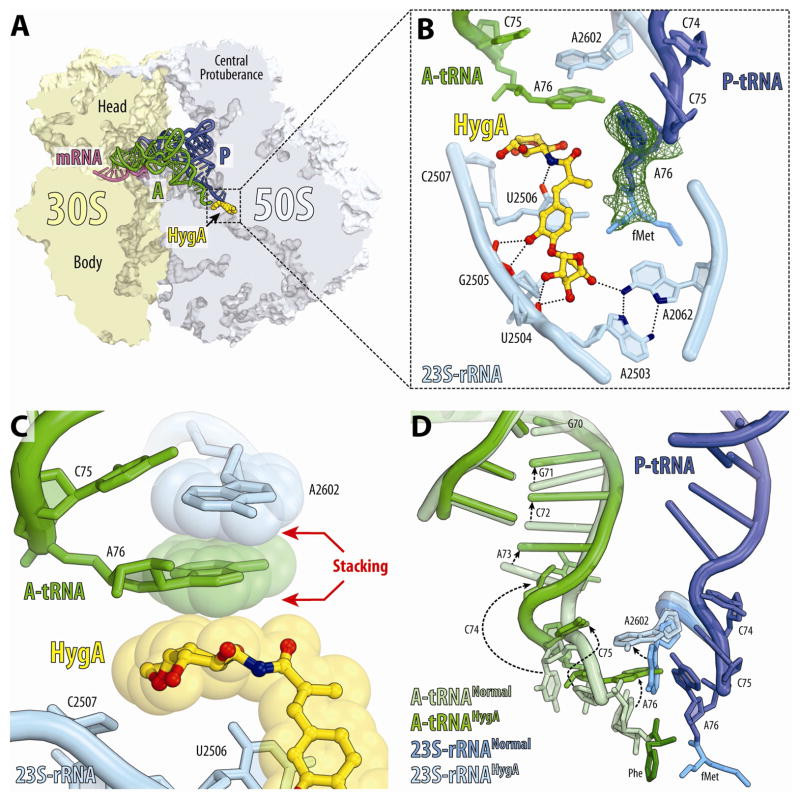
The observation from the FRET data that HygA and a partially accommodated tRNA-intermediate can co-exist on the ribosome suggested that it might be possible to investigate the structure of this state. To accomplish this, Tth 70S ribosomes containing mRNA and fMet-tRNAiMet in the P-site were co-crystallized with 100 μM HygA as well as with the cognate Phe-tRNAPhe. The presence of A-, P- and E-tRNAs significantly improved the X-ray diffraction quality compared to that of the vacant 70S ribosomes, enabling a structure of the complex to be determined at 2.6 Å resolution (Fig. 5A, Table 1). In this complex, the binding site of HygA (Fig. 5B, Fig. S5A, Movie S1) was identical to that observed in the absence of tRNAs (Fig. 2C, E), with two main exceptions: (i) HygA appears to protect the P-tRNA from hydrolysis, leading to well-defined density for the fMet moiety of the P-site tRNA (Fig. 5B); (ii) the aminocyclitol ring of HygA establishes additional stacking interactions with the nucleobase of A76 of the A-tRNA (Fig. 5C, Movie S1).
Figure 5. Structure of HygA-70S ribosome complex with A- and P-tRNAs.
(A) Transverse section of 70S ribosome (30S, light yellow; 50S, light blue) showing HygA (yellow) relative to A-site tRNA (green), P-site tRNA (blue) and mRNA (magenta). (B) Binding of HygA (yellow) to the ribosome prevents hydrolysis of the amino acid from the P-site tRNA and leading to clearly visible electron density for the fMet moiety (green mesh). (C) Stacking interactions between A76 of A-tRNA (green), A2602 (cyan) and the aminocyclitol ring of HygA. (D) Comparison of the position of A-tRNA in the presence of HygA (green, A-tRNAHygA) with the canonical position of A-tRNA when fully accommodated in the A-site (light green, A-tRNANormal). Relative positions of fMet-tRNA (blue) in the P-site, and A2602 in the presence (cyan) and absence (blue) of HygA are also shown. See also Figures S5 and S7.
As expected, the presence of HygA precludes normal accommodation of A76 of A-tRNA within the PTC, and causes A76 to adopt a previously unseen position, which is rotated by ~120° from its canonical position (Fig. 5D, Movie S1). This rotated orientation of A76 induces a corresponding rotation in A2602, which thereby stacks upon the nucleobase of A76 (Fig. 5D). The most dramatic consequences, however, are observed for nucleotides of the 3′-strand of the acceptor arm of the A-tRNA, which readjust their positions to account for the unaccommodated A76 position. This readjustment requires large-scale movements, including rotation of C74 and C75, as well as register shifts in the acceptor arm nucleotides that are propagated as far as G70 of the A-tRNA (Fig. 5D).
A201A prevents full accommodation of the A-site tRNA
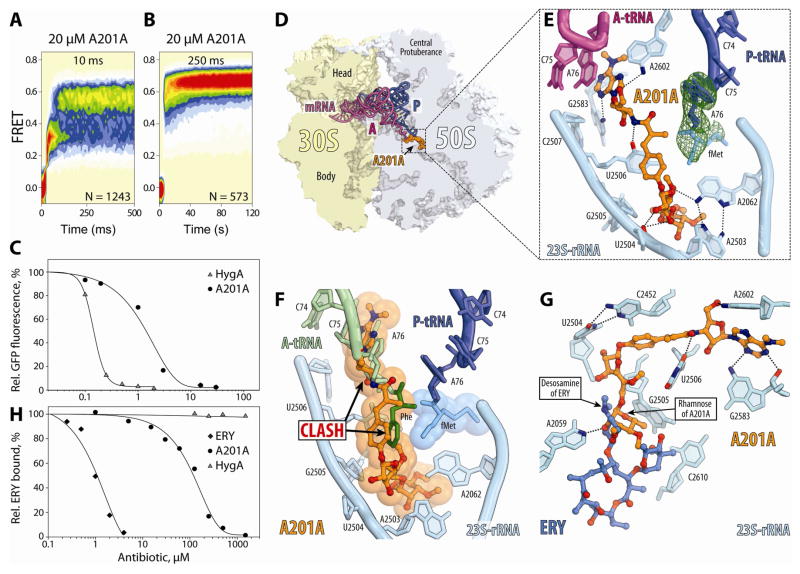
Since A201A and HygA are both peptidyl transferase inhibitors and share a number of related chemical features (Fig. 1A, B), we employed the smFRET method to investigate whether A201A also interferes with the A-tRNA accommodation process (Fig. 4). In the presence of saturating concentrations of A201A (20 μM), a delay was observed in the transition of the aa-tRNA into the high-(~0.63) FRET state (Fig. 6A). As observed for HygA, fast, reversible fluctuations were observed between intermediate- and high-FRET states (Fig. S4), however, the average FRET value exhibited by the A201A-stalled complex was ~0.56, modestly higher than that observed for HygA. Such dynamics persisted for ~5 seconds before completion of the selection process finally occurred (Fig. 6B). This relatively modest delay in the selection process compared to that observed for HygA (Fig. 4C), is consistent with its 10-fold increased IC50 (2 μM) for translation inhibition (Fig. 6C).
Figure 6. Effect of A201A on A-tRNA accommodation observed via smFRET and structurally.
(A, B) Single-molecule FRET imaging of aa-tRNA selection performed under direct 532 nm excitation, using either a (A) 10 ms/frame or (B) 250 ms/frame time resolution in the presence of 20 μM A201A. (C) In vitro translation of green fluorescent protein (GFP) in the presence of increasing concentrations of HygA or A201A. (D) Transverse section of 70S ribosome (30S, light yellow; 50S, light blue) showing the A201A (orange) relative to A-site tRNA (red), P-site tRNA (blue) and mRNA (magenta). (E) Interactions of A201A (orange) with 23S rRNA nucleotides (cyan), A-tRNA (red) and fMet-tRNA (dark blue with fMet moiety coloured light blue). Electron density for the fMet moiety is shown as a green mesh. (F) A201A relative to Phe-tRNA in the A site (light green with Phe moiety coloured dark green) (Polikanov et al., 2014b). (G) Fucofuranose of A201A interacts with A2059 of the 23S rRNA and overlaps with the binding site of the desosamine sugar of ERY (blue). (H) Competition assay showing binding of radiolabelled ERY to 70S ribosomes in the presence of increasing concentrations of HygA, A201A or unlabeled ERY. Binding of radiolabeled ERY in the absence of other drugs is defined as 100%. See also Figures S3, S5, S6 and S7.
Structure of A201A-70S complex in the presence of A-site tRNA
To further investigate the interplay between A201A and the A-tRNA, we also determined a structure of A201A (100 μM) in complex with the Tth 70S ribosome with bound A- and P-tRNAs at 2.65 Å resolution (Fig. 6D, Table 1). As expected, A201A binds in the A-site pocket of the PTC (Fig. 6E, Movie S2), overlapping with the binding site of A-tRNA (Fig. 6F). Specifically, the 6-N-dimethyl-3′-amino-3′-deoxyadenosyl moiety of A201A overlaps with the binding position of A76 of the A-tRNA (Fig. 6F). Consistent with this observation, the toe-printing assay revealed that A201A, like HygA, causes stalling of the ribosomes at the start codon of mRNA, albeit less efficiently than HygA (Fig. S3B). Similar to HygA, A201A has a methyl group on subunit C, where PMN contains the alpha-amino mimic and, therefore, A201A cannot act as a peptide-bond acceptor, as PMN does. The α-methyl-p-coumaric acid and the unsaturated furanose moieties of A201A, which are also present in HygA, make a similar network of H-bond interactions (Fig. 6E and Fig. S5B), except that A201A lacks one hydroxyl group (that is present on subunit B of HygA, Fig. 1A, B) and, therefore, cannot form the H-bonds with G2505 as observed for HygA (Fig. 2C, E and Fig. 5B). In addition to the efflux mechanism (Barrasa et al., 1995), self-resistance to A201A of S. capreolus NRRL 3817 is obtained via Ard2-mediated phosphorylation of the ribose 2′-OH of subunit D of A201A (Barrasa et al., 1997). Analogous to HygA, phosphorylation at this position could prevent drug binding by clashing with U2504 of the 23S rRNA. Moreover, disc-diffusion assays demonstrated that Cfr also confers resistance against A201A (Fig. S3C).
Unlike HygA, A201A contains a unique 3,4-di-O-methyl-D-rhamnose moiety glycosidically linked to the furanose moiety, which extends deeper into the ribosomal tunnel and comes within H-bond distance of A2059 (Fig. 6G). The position of the D-rhamnose moiety of A201A overlaps with the desosamine sugar of the macrolide erythromycin (Fig. 6G, Movie S2), which also contacts A2059 (Bulkley et al., 2010; Dunkle et al., 2010; Schlünzen et al., 2001; Tu et al., 2005). Consistent with this observation, we could demonstrate that A201A competes with erythromycin for ribosome binding, whereas HygA (which lacks the D-rhamnose) does not (Fig. 6H).
Because the nucleoside moiety of A201A occupies the position at the PTC which is normally taken by aminoacyl-A76 of the A-tRNA (Fig. 6F), rearrangements of the acceptor arm of the A-tRNA are necessary to accommodate the binding of the A-tRNA to the ribosome (Fig. S6). The conformation of the A-tRNA CCA-end observed in the presence of A201A is distinct from that observed with HygA (Fig. S6). While these rearrangements are reminiscent of those observed for P-tRNA in the presence of the antibiotic blasticidin S (Svidritskiy et al., 2013) and for deacylated tRNA bound at the E site (Schmeing et al., 2003; Selmer et al., 2006) (Fig. S6), in these latter cases the conformational changes are local and do not disturb the overall conformation of the acceptor arm.
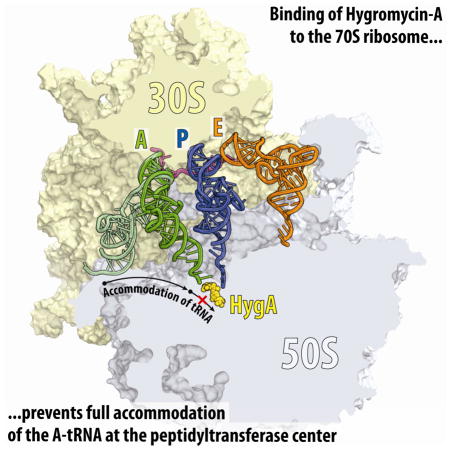
CONCLUSIONS
Collectively, our structural and biochemical results lead us to propose a model for the mechanism of translation inhibition by HygA and A201A (Fig. 7). Both HygA and A201A allow binding of the initiator fMet-tRNAiMet to the P site of the ribosome (Fig. 7A, B). This is consistent with the toe-printing experiments showing that both HygA and A201A inhibit translation when the ribosome still resides at the start codon (Fig. 3D, E and Fig. S3B). Structurally, we observe that the binding of either drug to the PTC leads to protection of the fMet-tRNAiMet from hydrolysis (Fig. 5B and Fig. 6E). However, we cannot rule out the possibility that HygA and A201A can also bind to post-translocational ribosomal complexes (that also have a free A site) and, thus, prevent translation during later elongation stages. Indeed, HygA has been shown to bind to ribosomes with Phe-tRNAPhe in the P-site (Guerrero and Modolell, 1980; Polacek et al., 2002; Poulsen et al., 2000) and also to promote binding of CACCA-AcLeu tRNA fragments to the ribosomal P site (Guerrero and Modolell, 1980), indicating that HygA binding and inhibition is not specific for P-site fMet-tRNAiMet, which was used in our studies.
Figure 7. Model for action of HygA and A201A during translation.
(A–E) Schematic diagram illustrating the mechanism of action of HygA and A201A to prevent full-accommodation of the A-tRNA during translation.
Our smFRET experiments (Fig. 4 and Fig. 6A, B) reveal that the delivery and initial binding of the EF-Tu-GTP-aminoacyl-tRNA ternary complex to the ribosomal A site remains unaffected by the presence of HygA or A201A (Fig. 7B, C). In contrast, both drugs interfere with late steps in tRNA selection where the aa-tRNA rapidly transitions back and forth between A/T-like and accommodated positions (Fig. 7D). These reversible FRET dynamics correspond to kinetic steps (k4/k−4) associated with tRNA movements between the GTPase-activated and accommodated states accompanying the proofreading phase of selection process (Geggier et al., 2010; Whitford et al., 2010). Hence, these findings suggest that HygA specifically acts to sterically interfere with the productive docking of the CCA-end of the A-tRNA at the PTC upon successful navigation of the accommodation corridor (Sanbonmatsu et al., 2005; Whitford et al., 2010). Further experiments will be required to explore the relative timing of these A-tRNA movements with inorganic phosphate and EF-Tu release, which are also associated with proofreading, and to what extent HygA and A201A influence this process. The partially accommodated A-tRNA states observed in our X-ray structures in the presence of HygA or A201A have a fully-accommodated elbow region and therefore would be consistent with the high FRET state observed in the smFRET experiments performed in the presence of the drugs (Fig. 4A–E and Fig. 6A, B). However, we note that in both cases the tRNAs appeared to be deacylated, most likely due to the hydrolysis during crystallization. Nevertheless, in silico modelling the aminoacyl-moiety back into the structures indicates that it would be possible to bind an aminoacylated-tRNA in the partially accommodated states (Fig. S7). Unlike the crystal structures in which only distinct partially accommodated states are observed, the smFRET experiments reveal that HygA and A201A cause the A-tRNA to oscillate between the A/T-like configuration and partially accommodated states for extended periods of time (5–60 s) (Movies S1 and S2). Stall durations of this magnitude (~100–1000-fold longer than the uninhibited selection process of ~50 ms) are significant, especially when compared to the rate of translation (~10–20 aa/s), and may therefore explain why these drugs are effective translation inhibitors.
In conclusion, we have revealed the mode of interaction of the PTC inhibitors HygA and A201A with the ribosome, providing two novel scaffolds upon which to develop new improved antibiotics. Our studies uncovered the mechanism of action of HygA and A201A, both of which allow delivery and initial binding of the A-tRNA but prevent its full accommodation at the PTC. These data expand our knowledge of the mechanisms by which peptidyltransferase inhibitors impact tRNA selection and suggest the existence of functional states during proofreading that have yet to be identified.
EXPERIMENTAL PROCEDURES
Materials for biochemical experiments
Hygromycin A and derivatives were purified from wild-type or mutant S. hygroscopicus NRRL 2388 as described previously (Palaniappan et al., 2006). A201A was kindly provided by Dr. Antonio Jimenez. Erythromycin and radiolabelled erythromycin were obtained from Sigma Aldrich and Perkin Elmer, respectively. Puromycin dihydrochloride and tRNAPhe were obtained from Sigma Aldrich, radiolabeled L-[2,3,4,5,6-3H]phenylalanine was obtained from Amersham Biosciences. E. coli ribosomes and AcPhe-tRNAPhe were prepared as described previously (Blaha et al., 2000; Marquez et al., 2004). Cfr-modified ribosomes were prepared from an E. coli strain overexpressing Cfr. For crystallization, the 70S ribosomes from Tth and the unmodified tRNAiMet and tRNAPhe from E. coli were purified and aminoacylated as previously described (Polikanov et al., 2014b) and the synthetic mRNA with the sequence 5′-GGC AAG GAG GUA AAA AUG UUC UAA-3′ was obtained from Integrated DNA Technologies (Coralville, IA).
In vitro translation and binding assays
The inhibition of green fluorescent protein (GFP) synthesis by HygA, HygA~P or A201A was assessed using E. coli lysate-based transcription-translation coupled assay (RTS100, 5Prime) and as described previously (Starosta et al., 2010; Starosta et al., 2009). The peptidyl transferase activity in the presence or absence of antibiotic was measured using the puromycin reaction as described previously (Mamos et al., 2013). Binding of HygA, A201A and erythromycin (ERY) to an empty E. coli ribosome was monitored using a competition assay with radiolabelled [14C]-erythromycin (170 dpm/pmol) as described before (Karahalios et al., 2006; Petropoulos et al., 2009; Starosta et al., 2010). The position of the ribosome on the mRNA was monitored using a toe-printing assay based on the in vitro coupled transcription-translation system using the PURExpress Δaa ΔtRNA kit (New England Biolabs) as described previously (Starosta et al., 2014). Further details on the in vitro translation and binding assays are provided in the Supplemental Experimental Procedures
Disc-diffusion assay
Disc-diffusion assays were performed as described by (Bailey et al., 2008). Briefly, the E. coli strain SQ110DTC (ΔtolC), which is hyper-sensitive to antibiotics (Orelle et al., 2013), was transformed with the plasmid pBglII expressing Cfr (Kehrenberg et al., 2005), or the parental plasmid pBluescript II SK+ (Stratgene), and plated onto LB plates. A 5-mm in diameter sterile Whatmann 3MM paper disc spotted with 10 μL of 500 μM HygA (or A201A) was placed in the center of the plate, which was then incubated overnight at 37°C. Antibiotic resistance was measured by monitoring the ring of growth inhibition around the antibiotic-containing disc.
Single-molecule FRET imaging
The smFRET experiments were performed as described previously (Blanchard et al., 2004; Munro et al., 2007) and are described in detail in the Supplemental Experimental Procedures. To increase the FRET imaging duration and overall signal-to-noise ratio of imaging, an intra-molecularly photostabilized derivative of Cy5 was employed in combination with a cocktail of solution protective agents (Juette et al., 2014; Zheng et al., 2014). To minimize the contribution of hybrid state tRNA configurations formed after peptide bond formation, experiments were performed using complexes lacking ribosomal protein L1 (Munro et al., 2007). Fluorescence and FRET traces were selected for analysis using semi-automated smFRET analysis software implemented in Matlab (The MathWorks) as previously described (Geggier et al., 2010).
Crystallographic structure determination
For details of structure determination refer to Supplemental Experimental Procedures. Briefly, ribosome complexes with mRNA and tRNAs were formed essentially as described previously (Polikanov et al., 2014a; Voorhees et al., 2009). In the co-crystallization experiments with either HygA or A201A, the antibiotic was added to a final concentration of 100 μM. Crystals were grown by the vapor diffusion method in sitting drops at 19°C and stabilized as described previously (Polikanov et al., 2012; Polikanov et al., 2014a) with the antibiotics included in the stabilization buffers (100 μM HygA or 100 μM A201A). Each structure was solved and refined as described previously (Polikanov et al., 2014a).(Adams et al., 2010; Emsley and Cowtan, 2004; McCoy et al., 2007) The statistics of data collection and refinement for each complex are compiled in Table 1.
Supplementary Material
HIGHLIGHTS.
X-ray structures of antibiotics Hygromycin A and A201A on the ribosome
Structural basis for Hygromycin A and A201A resistance mechanisms
tRNA accommodation intermediates with Hygromycin A/A201A observed by FRET
X-ray structures of partially accommodated A-tRNAs with Hygromycin A/A201A
Acknowledgments
We thank Dr. A. Jimenez for kindly providing A201A compound, Dr. B. Vester for the Cfr-expressing plasmid, Dr. A.S. Mankin for providing the SQ110DTC strain, M. Stavropoulou and U. Antczak for technical help, R.L. Grodzicki for preparation of the unmodified tRNAs (for structural studies). We also thank the staff at the Advanced Photon Source (beamline 24ID), supported by award GM103403 from the National Center for Research Resources at the National Institutes of Health, the staff at the National Synchrotron Light Source (beamline X25) for help during data collection and the staff at the Richards Center at Yale University for computational support. This research was supported by grants from the National Institutes of Health (GM022778 to T.A.S., GM095737 to K.R. and D.N.W., and GM079238 and GM098859 to S.C.B.), HFSP (to D.N.W and S.C.B), the Deutsche Forschungsgemeinschaft (FOR1805, WI3285/3-1 and GRK1721 to D.N.W.) and the German Academic Exchange Service (DAAD postdoctoral fellowship to M.F.J.).
Footnotes
AUTHOR CONTRIBUTIONS
Y.S.P. designed and performed X-ray crystallography experiments. A.L.S. performed toe-printing, in vitro translation, binding and disc-diffusion assays. M.F.J., R.B.A., D.S.T., B.J.B and S.C.B designed and performed smFRET experiments. W.L. and K.R. isolated and purified HygA. All authors interpreted data. Y.S.P., S.C.B, T.A.S and D.N.W wrote the manuscript.
ACCESSION NUMBERS
Coordinates and structure factors were deposited in the RCSB Protein Data Bank with accession codes 4Z3R for the Tth 70S ribosome in complex with HygA; 4Z3Q for the Tth 70S ribosome with HygA, A-, P- and E-site tRNAs, and 4Z3S for the Tth 70S ribosome in complex with A201A, A-, P- and E-site tRNAs.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey M, Chettiath T, Mankin AS. Induction of erm(C) expression by noninducing antibiotics. Antimicrob Agents Chemother. 2008;52:866–874. doi: 10.1128/AAC.01266-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrasa MI, Tercero JA, Jimenez A. The aminonucleoside antibiotic A201A is inactivated by a phosphotransferase activity from Streptomyces capreolus NRRL 3817, the producing organism. Isolation and molecular characterization of the relevant encoding gene and its DNA flanking regions. Eur J Biochem. 1997;245:54–63. doi: 10.1111/j.1432-1033.1997.00054.x. [DOI] [PubMed] [Google Scholar]
- Barrasa MI, Tercero JA, Lacalle RA, Jimenez A. The ard1 gene from Streptomyces capreolus encodes a polypeptide of the ABC-transporters superfamily which confers resistance to the aminonucleoside antibiotic A201A. Eur. J Biochem. 1995;228:562–569. doi: 10.1111/j.1432-1033.1995.tb20295.x. [DOI] [PubMed] [Google Scholar]
- Blaha G, Stelzl U, Spahn CM, Agrawal RK, Frank J, Nierhaus KH. Preparation of functional ribosomal complexes and effect of buffer conditions on tRNA positions observed by cryoelectron microscopy. Methods Enzymol. 2000;317:292–309. doi: 10.1016/s0076-6879(00)17021-1. [DOI] [PubMed] [Google Scholar]
- Blanchard SC, Gonzalez RL, Kim HD, Chu S, Puglisi JD. tRNA selection and kinetic proofreading in translation. Nat Struct Mol Biol. 2004;11:1008–1014. doi: 10.1038/nsmb831. [DOI] [PubMed] [Google Scholar]
- Bulkley D, Innis CA, Blaha G, Steitz TA. Revisiting the structures of several antibiotics bound to the bacterial ribosome. Proc Natl Acad Sci USA. 2010;107:17158–17163. doi: 10.1073/pnas.1008685107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida N, Nakazawa K, Ohtsuka M, Suzuki M, Ogawa S. Total synthesis of methoxyhygromycin and its 5-epimer. Chem Lett. 1990:423–426. [Google Scholar]
- Chida N, Ohtsuka M, Nakazawa K, Ogawa S. Total synthesis of hygromycin A. J Chem Soc Chem Comm. 1989:436–438. [Google Scholar]
- Dhote V, Gupta S, Reynolds KA. An O-phosphotransferase catalyzes phosphorylation of hygromycin A in the antibiotic-producing organism Streptomyces hygroscopicus. Antimicrob Agents Chemother. 2008;52:3580–3588. doi: 10.1128/AAC.00157-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhote V, Starosta AL, Wilson DN, Reynolds KA. The final step of hygromycin A biosynthesis, oxidation of C-5″-dihydrohygromycin A, is linked to a putative proton gradient-dependent efflux. Antimicrob Agents Chemother. 2009;53:5163–5172. doi: 10.1128/AAC.01069-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe TJ, Flores A, Bataille CJ, Churruca F. Synthesis of (−)-hygromycin A: application of Mitsunobu glycosylation and tethered aminohydroxylation. Angew Chem Int Ed Engl. 2009;48:6507–6510. doi: 10.1002/anie.200902840. [DOI] [PubMed] [Google Scholar]
- Dunkle JA, Xiong L, Mankin AS, Cate JH. Structures of the Escherichia coli ribosome with antibiotics bound near the peptidyl transferase center explain spectra of drug action. Proc Natl Acad Sci USA. 2010;107:17152–17157. doi: 10.1073/pnas.1007988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Ensminger PW, Wright WE. A201A, a new antibiotic produced by Streptomnyces cupreolus. II. Biological studies. 16th Intersci. Conf Antimicrob. Agents Chemother; Chicago. 1976. [Google Scholar]
- Epp JK, Allen NE. A201A, a new antibiotic produced by Streptomyces capreolus, IV. Mode of action studies. 16th Intersci. Con$ Antimicrob. Agents Chemothel; Chicago. 1976. p. 63. [Google Scholar]
- Geggier P, Dave R, Feldman MB, Terry DS, Altman RB, Munro JB, Blanchard SC. Conformational sampling of aminoacyl-tRNA during selection on the bacterial ribosome. J Mol Biol. 2010;399:576–595. doi: 10.1016/j.jmb.2010.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero MD, Modolell J. Hygromycin A, a novel inhibitor of ribosomal peptidyl transferase. Eur J Biochem. 1980;107:409–414. doi: 10.1111/j.1432-1033.1980.tb06044.x. [DOI] [PubMed] [Google Scholar]
- Habib el SE, Scarsdale JN, Reynolds KA. Biosynthetic origin of hygromycin A. Antimicrob Agents Chemother. 2003;47:2065–2071. doi: 10.1128/AAC.47.7.2065-2071.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi SF, Norcia LJL, Seibel SB, Silvia AM. Structure-activity relationships of hygromycin A and its analogs: Protein synthesis inhibition activity in a cell free system. J Antibiot. 1997;50:514–521. doi: 10.7164/antibiotics.50.514. [DOI] [PubMed] [Google Scholar]
- Hecker SJ, Lilley SC, Minich ML, Werner KM. Application of hygromycin A structure-activity relationships to the antibiotic A201A. Bioorg Med Chem Lett. 1993;3:295–298. [Google Scholar]
- Hecker SJ, Lilley SC, Werner KM. Hygromycin A - preparation of aminocyclitol analogs defining the minimum functionality required for biological activity. Bioorg Med Chem Lett. 1992;2:1043–1046. [Google Scholar]
- Ippolito JA, Kanyo ZF, Wang D, Franceschi FJ, Moore PB, Steitz TA, Duffy EM. Crystal structure of the oxazolidinone antibiotic linezolid bound to the 50S ribosomal subunit. J Med Chem. 2008;51:3353–3356. doi: 10.1021/jm800379d. [DOI] [PubMed] [Google Scholar]
- Jaynes BH, Cooper CB, Hecker SJ, Blair KT, Elliott NC, Lilley SC, Minich ML, Schicho DL, Werner KM. Synthesis and in vitro antibacterial activity of hygromycin A analogs modified at the C4′ aryl position. Bioorg Med Chem Lett. 1993;3:1531–1536. [Google Scholar]
- Jaynes BH, Elliott NC, Schicho DL. Semisynthetic hygromycin A analogs - synthesis and antibacterial activity of derivatives lacking the furanose moiety. J Antibiot. 1992;45:1705–1707. doi: 10.7164/antibiotics.45.1705. [DOI] [PubMed] [Google Scholar]
- Jiménez A, Vázquez D. Novel inhibitors of translation in eukaryotic systems. In Modes and mechanisms of microbial growth inhibitors. In: Hahn FE, editor. Antibiotics. Berlin: Springer-Verlag; 1983. pp. 248–254. [Google Scholar]
- Juette MF, Terry DS, Wasserman MR, Zhou Z, Altman RB, Zheng Q, Blanchard SC. The bright future of single-molecule fluorescence imaging. Curr Opin Chem Biol. 2014;20:103–111. doi: 10.1016/j.cbpa.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakinuma K, Kitahara S, Watanabe K, Sakagami Y, Fukuyasu T, Shimura M, Ueda M, Sekizawa Y. Structure of hygromycin location of a methylene substituent and anomeric configuration of arabino-hexoside moiety. J Antibiot. 1976;29:771–773. doi: 10.7164/antibiotics.29.771. [DOI] [PubMed] [Google Scholar]
- Karahalios P, Kalpaxis DL, Fu H, Katz L, Wilson DN, Dinos GP. On the mechanism of action of 9-O-arylalkyloxime derivatives of 6-O-mycaminosyltylonolide, a new class of 16-membered macrolide antibiotics. Mol Pharmacol. 2006;70:1271–1280. doi: 10.1124/mol.106.026567. [DOI] [PubMed] [Google Scholar]
- Kehrenberg C, Schwarz S, Jacobsen L, Hansen LH, Vester B. A new mechanism for chloramphenicol, florfenicol and clindamycin resistance: methylation of 23S ribosomal RNA at A2503. Mol Microbiol. 2005;57:1064–1073. doi: 10.1111/j.1365-2958.2005.04754.x. [DOI] [PubMed] [Google Scholar]
- Kim SD, Kweon MH, Kim CJ, Yoo ID. Hygromycin, a plant growth inhibitor. Korean J Biochem. 1990;23 [Google Scholar]
- Kirst HA, Dorman DE, Occolowitz JL, Jones ND, Paschal JW, Hamill RL, Szymanski EF. The structure of A201A, a novel nucleoside antibiotic. J Antibiot. 1985;38:575–586. doi: 10.7164/antibiotics.38.575. [DOI] [PubMed] [Google Scholar]
- Lee HB, Kim CJ, Kim JS, Hong KS, Cho KY. A bleaching herbicidal activity of methoxyhygromycin (MHM) produced by an actinomycete strain Streptomyces sp8E-12. Lett Appl Microbiol. 2003;36:387–391. doi: 10.1046/j.1472-765x.2003.01327.x. [DOI] [PubMed] [Google Scholar]
- Long KS, Poehlsgaard J, Kehrenberg C, Schwarz S, Vester B. The Cfr rRNA methyltransferase confers resistance to phenicols, lincosamides, oxazolidinones, pleuromutilins, and streptogramin A antibiotics. Antimicrob Agents Chemother. 2006;50:2500–2505. doi: 10.1128/AAC.00131-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamos PL, Krokidis MG, Papadas AT, Karahalios P, Starosta AL, Wilson DN, Kalpaxis DL, Dinos GP. On the use of the antibiotic chloramphenicol to target polypeptide chain mimics to the ribosomal exit tunnel. Biochemie. 2013 doi: 10.1016/j.biochi.2013.06.004. in press. [DOI] [PubMed] [Google Scholar]
- Mann RL, Bromer WW. The isolation of a 2nd antibiotic from Streptomyces hygroscopicus. J Am Chem Soc. 1958;80:2714–2716. [Google Scholar]
- Mann RL, Gale RM, Van Abeele FR. Hygromycin. II Isolation and properties. Antibiot Chemother. 1953;3:1279–1282. [PubMed] [Google Scholar]
- Mann RL, Woolf DO. Hygromycin. III Structure studies. J Am Chem Soc. 1957;79:120–126. [Google Scholar]
- Marquez V, Wilson DN, Tate WP, Triana-Alonso F, Nierhaus KH. Maintaining the ribosomal reading frame: the influence of the E site during translational regulation of release factor 2. Cell. 2004;118:45–55. doi: 10.1016/j.cell.2004.06.012. [DOI] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J Appl Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro JB, Altman RB, O’Connor N, Blanchard SC. Identification of two distinct hybrid state intermediates on the ribosome. Mol Cell. 2007;25:505–517. doi: 10.1016/j.molcel.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa A, Fujimoto T, Omura S, Walsh JC, Stotish RL, George B. Hygromycin A, an antitreponemal substance. II Therapeutic effect for swine dysentery. J Antibiot. 1987;40:1627–1635. doi: 10.7164/antibiotics.40.1627. [DOI] [PubMed] [Google Scholar]
- Nie S, Li W, Yu B. Total synthesis of nucleoside antibiotic A201A. J Am Chem Soc. 2014;136:4157–4160. doi: 10.1021/ja501460j. [DOI] [PubMed] [Google Scholar]
- Omura S, Nakagawa A, Fujimoto T, Saito K, Otoguro K, Walsh JC. Hygromycin A, an antitreponemal substance. I Screening method and therapeutic effect for Treponema hyodysenteriae-caused infection in CF-1 mice. J Antibiot. 1987;40:1619–1626. doi: 10.7164/antibiotics.40.1619. [DOI] [PubMed] [Google Scholar]
- Orelle C, Carlson S, Kaushal B, Almutairi MM, Liu H, Ochabowicz A, Quan S, Pham VC, Squires CL, Murphy BT, Mankin AS. Tools for characterizing bacterial protein synthesis inhibitors. Antimicrob Agents Chemother. 2013;57:5994–6004. doi: 10.1128/AAC.01673-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniappan N, Ayers S, Gupta S, Habib el SE, Reynolds KA. Production of hygromycin A analogs in Streptomyces hygroscopicus NRRL 2388 through identification and manipulation of the biosynthetic gene cluster. Chem Biol. 2006;13:753–764. doi: 10.1016/j.chembiol.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Palaniappan N, Dhote V, Ayers S, Starosta AL, Wilson DN, Reynolds KA. Biosynthesis of the aminocyclitol subunit of hygromycin A in Streptomyces hygroscopicus NRRL 2388. Chem Biol. 2009;16:1180–1189. doi: 10.1016/j.chembiol.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Petropoulos AD, Kouvela EC, Starosta AL, Wilson DN, Dinos GP, Kalpaxis DL. Time-resolved binding of azithromycin to Escherichia coli ribosomes. J Mol Biol. 2009;385:1179–1192. doi: 10.1016/j.jmb.2008.11.042. [DOI] [PubMed] [Google Scholar]
- Pittenger RC, Wolfe RN, Hoehn PN, Daily WA, McGuire JM. Hygromycin. I Preliminary studies in the production and biologic activity on a new antibiotic. Antibiot Chemother. 1953;3:1268–1278. [PubMed] [Google Scholar]
- Polacek N, Swaney S, Shinabarger D, Mankin AS. SPARK - a novel method to monitor ribosomal peptidyl transferase activity. Biochemistry. 2002;41:11602–11610. doi: 10.1021/bi026040s. [DOI] [PubMed] [Google Scholar]
- Polikanov YS, Blaha GM, Steitz TA. How hibernation factors RMF, HPF, and YfiA turn off protein synthesis. Science. 2012;336:915–918. doi: 10.1126/science.1218538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polikanov YS, Osterman IA, Szal T, Tashlitsky VN, Serebryakova MV, Kusochek P, Bulkley D, Malanicheva IA, Efimenko TA, Efremenkova OV, et al. Amicoumacin A inhibits translation by stabilizing mRNA interaction with the ribosome. Mol Cell. 2014a;56:531–540. doi: 10.1016/j.molcel.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polikanov YS, Steitz TA, Innis CA. A proton wire to couple aminoacyl-tRNA accommodation and peptide-bond formation on the ribosome. Nat Struct Mol Biol. 2014b;21:787–793. doi: 10.1038/nsmb.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen SM, Kofoed C, Vester B. Inhibition of the ribosomal peptidyl transferase reaction by the mycarose moiety of the antibiotics carbomycin, spiramycin and tylosin. J Mol Biol. 2000;304:471–481. doi: 10.1006/jmbi.2000.4229. [DOI] [PubMed] [Google Scholar]
- Sanbonmatsu KY, Joseph S, Tung CS. Simulating movement of tRNA into the ribosome during decoding. Proc Natl Acad Sci USA. 2005;102:15854–15859. doi: 10.1073/pnas.0503456102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlünzen F, Zarivach R, Harms J, Bashan A, Tocilj A, Albrecht R, Yonath A, Franceschi F. Structural basis for the interaction of antibiotics with the peptidyl transferase center in eubacteria. Nature. 2001;413:814–821. doi: 10.1038/35101544. [DOI] [PubMed] [Google Scholar]
- Schmeing TM, Moore PB, Steitz TA. Structures of deacylated tRNA mimics bound to the E site of the large ribosomal subunit. RNA. 2003;9:1345–1352. doi: 10.1261/rna.5120503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuette JC, Murphy FV, Kelley AC, Weir JR, Giesebrecht J, Connell SR, Loerke J, Mielke T, Zhang W, Penczek PA, et al. GTPase activation of elongation factor EF-Tu by the ribosome during decoding. EMBO J. 2009;28:755–765. doi: 10.1038/emboj.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmer M, Dunham CM, Murphy FVt, Weixlbaumer A, Petry S, Kelley AC, Weir JR, Ramakrishnan V. Structure of the 70S ribosome complexed with mRNA and tRNA. Science. 2006;313:1935–1942. doi: 10.1126/science.1131127. [DOI] [PubMed] [Google Scholar]
- Starosta AL, Karpenko VV, Shishkina AV, Mikolajka A, Sumbatyan NV, Schluenzen F, Korshunova GA, Bogdanov AA, Wilson DN. Interplay between the ribosomal tunnel, nascent chain, and macrolides influences drug inhibition. Chem Biol. 2010;17:1–10. doi: 10.1016/j.chembiol.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Starosta AL, Lassak J, Peil L, Atkinson GC, Virumae K, Tenson T, Remme J, Jung K, Wilson DN. Translational stalling at polyproline stretches is modulated by the sequence context upstream of the stall site. Nucleic Acids Res. 2014;42:10711–10719. doi: 10.1093/nar/gku768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starosta AL, Qin H, Mikolajka A, Leung GY, Schwinghammer K, Nicolaou KC, Chen DY, Cooperman BS, Wilson DN. Identification of distinct thiopeptide-antibiotic precursor lead compounds using translation machinery assays. Chem Biol. 2009;16:1087–1096. doi: 10.1016/j.chembiol.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JA. Antibiotics in development targeting protein synthesis. Ann N Y Acad Sci. 2011;1241:122–152. doi: 10.1111/j.1749-6632.2011.06323.x. [DOI] [PubMed] [Google Scholar]
- Svidritskiy E, Ling C, Ermolenko DN, Korostelev AA. Blasticidin S inhibits translation by trapping deformed tRNA on the ribosome. Proc Natl Acad Sci USA. 2013;110:12283–12288. doi: 10.1073/pnas.1304922110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu D, Blaha G, Moore PB, Steitz TA. Structures of MLSBK antibiotics bound to mutated large ribosomal subunits provide a structural explanation for resistance. Cell. 2005;121:257–270. doi: 10.1016/j.cell.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Uyeda M, Mizukami M, Yokomizo K, Suzuki K. Pentalenolactone I and hygromycin A, immunosuppressants produced by Streptomyces filipinensis and Streptomyces hygroscopicus. Biosci Biotechnol Biochem. 2001;65:1252–1254. doi: 10.1271/bbb.65.1252. [DOI] [PubMed] [Google Scholar]
- Voorhees RM, Weixlbaumer A, Loakes D, Kelley AC, Ramakrishnan V. Insights into substrate stabilization from snapshots of the peptidyl transferase center of the intact 70S ribosome. Nat Struct Mol Biol. 2009;16:528–533. doi: 10.1038/nsmb.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakisaka Y, Koizumi K, Nishimoto Y, Kobayashi M, Tsuji N. Hygromycin and epihygromycin from a bacterium, Corynebacterium equi No 2841. J Antibiot. 1980;33:695–704. doi: 10.7164/antibiotics.33.695. [DOI] [PubMed] [Google Scholar]
- Whitford PC, Geggier P, Altman RB, Blanchard SC, Onuchic JN, Sanbonmatsu KY. Accommodation of aminoacyl-tRNA into the ribosome involves reversible excursions along multiple pathways. RNA. 2010;16:1196–1204. doi: 10.1261/rna.2035410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DN. The A-Z of bacterial translation inhibitors. Crit Rev Biochem Mol Biol. 2009;44:393–433. doi: 10.3109/10409230903307311. [DOI] [PubMed] [Google Scholar]
- Wilson DN. Ribosome-targeting antibiotics and bacterial resistance mechanisms. Nat Rev Microbiol. 2014;12:35–48. doi: 10.1038/nrmicro3155. [DOI] [PubMed] [Google Scholar]
- Wilson DN, Schluenzen F, Harms JM, Starosta AL, Connell SR, Fucini P. The oxazolidinone antibiotics perturb the ribosomal peptidyl transferase center and effect tRNA positioning. Proc Natl Acad Sci USA. 2008;105:13339–13344. doi: 10.1073/pnas.0804276105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Takahashi E, Uozumi T, Beppu T. Hygromycin A and methoxyhygromycin, novel inhibitors of K88-antigen synthesis of enterotoxic Escherichia coli strain. Agr Biol Chem Tokyo. 1986;50:143–149. [Google Scholar]
- Zheng Q, Juette MF, Jockusch S, Wasserman MR, Zhou Z, Altman RB, Blanchard SC. Ultra-stable organic fluorophores for single-molecule research. Chem Soc Rev. 2014;43:1044–1056. doi: 10.1039/c3cs60237k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Li J, Ma J, Luo M, Wang B, Huang H, Tian X, Li W, Zhang S, Zhang C, Ju J. Discovery and engineered overproduction of antimicrobial nucleoside antibiotic A201A from the deep-sea marine actinomycete Marinactinospora thermotolerans SCSIO 00652. Antimicrob Agents Chemother. 2012;56:110–114. doi: 10.1128/AAC.05278-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.