Abstract
In vertebrates and invertebrates, morphological and functional features of gastrointestinal (GI) tracts generally reflect food chemistry, such as content of carbohydrates, proteins, fats, and material(s) refractory to rapid digestion (e.g., cellulose). The expression of digestive enzymes and nutrient transporters approximately matches the dietary load of their respective substrates, with relatively modest excess capacity. Mechanisms explaining differences in hydrolase activity between populations and species include gene copy number variations and single-nucleotide polymorphisms. Transcriptional and posttranscriptional adjustments mediate phenotypic changes in the expression of hydrolases and transporters in response to dietary signals. Many species respond to higher food intake by flexibly increasing digestive compartment size. Fermentative processes by symbiotic microorganisms are important for cellulose degradation but are relatively slow, so animals that rely on those processes typically possess special enlarged compartment(s) to maintain a microbiota and other GI structures that slow digesta flow. The taxon richness of the gut microbiota, usually identified by 16S rRNA gene sequencing, is typically an order of magnitude greater in vertebrates than invertebrates, and the interspecific variation in microbial composition is strongly influenced by diet. Many of the nutrient transporters are orthologous across different animal phyla, though functional details may vary (e.g., glucose and amino acid transport with K+ rather than Na+ as a counter ion). Paracellular absorption is important in many birds. Natural toxins are ubiquitous in foods and may influence key features such as digesta transit, enzymatic breakdown, microbial fermentation, and absorption
Introduction
The gastrointestinal (GI) tract of animals can serve multiple functions including digestion, osmoregulation, and protection (e.g., by detoxification or immune function). The primary functions considered in this article are the extraction of nutrients and toxins from diverse foods consumed by vertebrates and invertebrates. Our review complements and updates many earlier reviews (248, 249) to provide broader taxonomic coverage, and incorporates increased molecular information to characterize further the mechanistic bases of patterns of change within and across species. Where sufficient information is available, phylogenetically informed analyses are included to provide better evidence of evolutionary trajectories and stronger inferences about the adaptive nature of certain traits. We include a new analysis of interactions between digestive physiology and naturally occurring toxins [e.g., plant secondary metabolites (SMs)] because these biochemicals are nearly ubiquitous in foods consumed by wild animals and many of their effects are mediated through interactions with the gut.
We begin with an overview of the architecture of animals’ guts, including a description of simple integrative models that have advanced understanding of how gut size, digesta flow, and biochemical capacity are matched to food intake to achieve efficient nutrient extraction. This overview also introduces the economy of nature as an evolutionary organizing principle that can be used to predict and explain many patterns. Subsequent sections cover mechanisms and patterns of variation across taxa in chemical digestion by animals and their microbiota, and absorption of breakdown products. Two sections focus on enzymatic and transport changes within animals during development and when they switch diets, and the final section is on interactions with natural toxins in foods.
Digestive Designs That Match GI Architecture to Food Composition and Intake Rate
Variation in food chemistry drives diversification of digestive systems
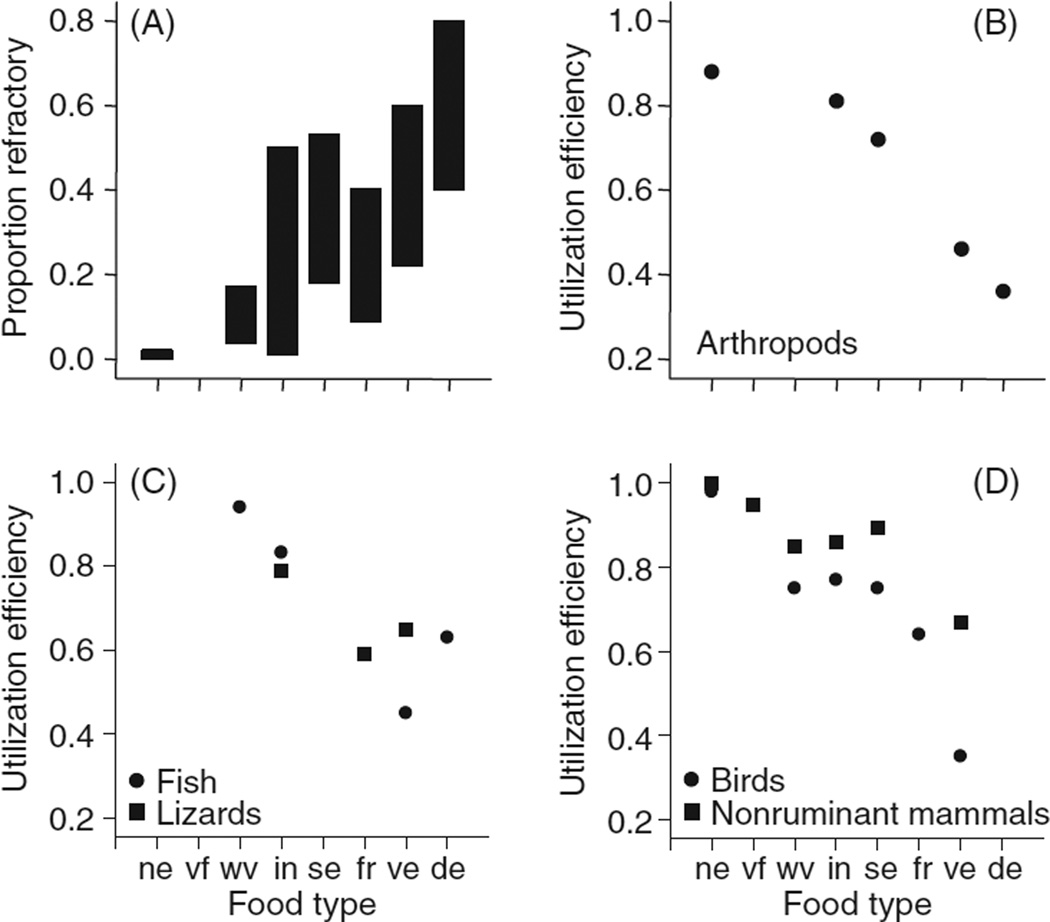
Features of food chemistry ultimately drive diversification of digestive system morphology, physiology, and biochemistry, and account for a lot of the variation among animals in efficiency of digestion (proportion retained/consumed). For example, food types can be ranked in terms of increasing amount of material that is refractory to rapid digestion with endogenous enzymes (i.e., localized to the digestive tract), such as plant cell-wall or arthropod cuticle/chitin (Fig. 1 A). Although within any single food category, there can be tremendous variation, some generalities emerge. Animal foods tend to have the lowest amounts of refractory material (e.g., hair, feathers, bone, and cuticle), seeds and fruits have intermediate levels [measured here as neutral detergent fiber (248)], and herbage has the highest levels (especially mature leaves and structural parts). Detritus, which typically contains a lot of refractory material although it has not been analyzed in a strictly comparable fashion to the other food types, is included as a food type because ecologists have found that it may support over half the animal production in some ecosystems (38). As one looks across animal taxa (Fig. 1B–D), one sees that although there are not data for every food type in each taxon, mean digestive efficiency for food types is inversely related to the relative amount of refractory material in the foods.
Figure 1.
As a general rule, digestive efficiency on a food type declines with increasing amount of refractory material in food. (A) Food types can be ranked according to their relative content of refractory material, which in this case is based largely on neutral detergent fiber (248). Ranges are given for the following food types: ne, nectar; vf, vertebrate flesh; wv, whole vertebrates; in, whole invertebrates; se, seeds; fr, fruit; ve, vegetation (grass, dicot leaves, and twigs); de, detritus. (B-D) Mean utilization efficiencies for animals in different taxa eating different types of food. The data sources and sample sizes for mammals, birds, and lizards are from (315), for immature arthropods, with permission, from reference (410), and for fish, with permission, from reference (37, 40). The efficiencies plotted in figure B–D are a mix of values of dry matter and energy digestibilities, but these measures tend to be close to each other and highly correlated (248).
Among animals that consume foods with low amounts of refractory material(s), a key feature of digestive design for efficiency is hydrolytic and absorptive capacities matched to the relative amounts of carbohydrates, protein, and fats in their diets, as discussed in subsequent sections. Among animals that consume refractory food types there are multiple strategies. Within many taxonomic groups one can identify species that “skim the cream” and assimilate cell contents or other nonrefractory materials and mainly pass the refractory material undigested. Abe and Higashi (1) called them cytoplasm consumers and contrasted them with other species called cell-wall consumers that extract a lot of energy from refractory materials. Among herbivorous mammals, these two extremes are well exemplified by, respectively, Giant pandas (Ailuropoda melanoleuca), which digest less than 10% of cellulose and hemicellulose in ingested bamboo (122) and gorillas, which can digest 45% to 70% of cell-wall material in their herbivorous diet (377). Among birds, examples of cytoplasm consumers would be “plant cutters” (genus Phytotoma) that feed almost exclusively on young leaves (with low cell-wall contents) (46) whereas hoatzins (Ophistocomus hoazin) and some species of grouse consume leaves, buds, and tips of woody twigs and may digest a lot of the cell-wall material (195). A continuum of feeders/digesters bounded by these two strategies can be found among invertebrate taxa as well. Most foliage and grass feeding insects assimilate the easily used compounds (sugars, starch, protein, etc.) and void the remainder including cellulose [e.g., the locust Chortoicetes terminifera (92) and the grasshopper Aracris flavolineata (152)] in contrast to insect species that feed on wood and which exhibit a number of features that enable them to extract energy from cell-wall material [e.g., many termites, some cockroaches, silverfish, and firebrats (128)]. Among herbivorous land crabs, species range from digestion of little cell-wall material up to nearly 100% (295).
Cellulose, a glucose polymer linked by beta 1–4 bonds, is the most abundant carbohydrate in terrestrial ecosystems, but is a challenge to use as an energy source because it is degraded very slowly by enzymatic hydrolysis, often taking many hours (220). The production of intrinsic cellulases by arthropods (insects), crustaceans (crayfish), and nematodes has been firmly established (463), but this capability is apparently absent from all vertebrates. Whether or not the animal has intrinsic cellulolytic capability, it appears that fermentative symbioses with microbes and fungi are generally important for cellulose degradation in animals (see Section “Microbial transformation of digestively-intractable food constituents to compounds that are readily used by the animal”). The microbiota breakdown cellulose and other cell-wall material relatively slowly, and if herbivores retain material in their gut for less than 4 to 8 h the extent of cell-wall digestion is relatively low. Thus, key digestive adaptations of most herbivores besides special compartment(s) to maintain a microbiota are adjustments in digestive compartment sizes and possession of other GI structures that slow the flow of digesta through the tract. We refer the reader to reviews of these features in both vertebrates and invertebrates [e.g., references (246, 248, 419)]. A remarkable report (209) of acquisition of a feature for digesting plants describes the rapid appearance in 36 years (ca. 30 generations) of cecal valves, which slow down food passage and provide for fermenting chambers, among lizards (Podarcis melisellensis) that were introduced onto an island where they consumed eight times more vegetation than did individuals in their source population.
Basic designs of digestive tracts
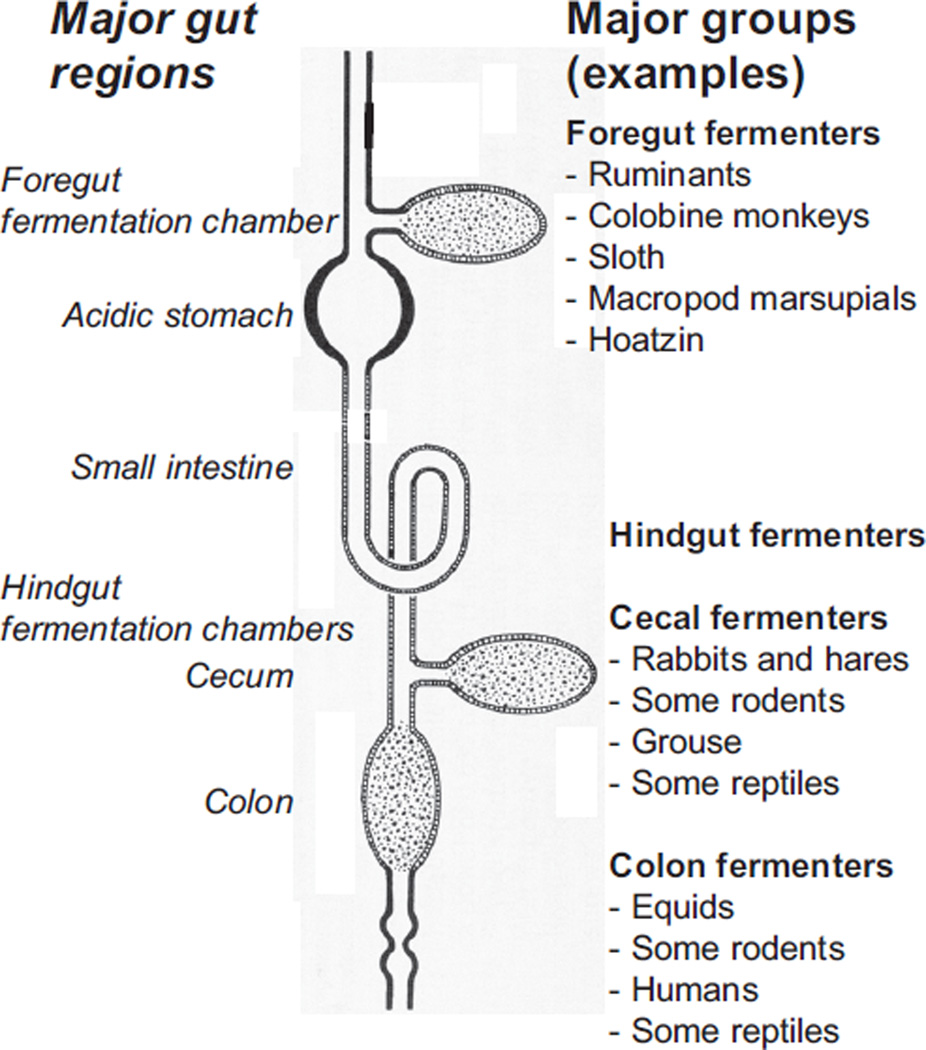
Notwithstanding the diversification of digestive systems caused by diversity among foods, Jumars and Penry (1987) pointed out that most guts can be analyzed as one of three categories of ideal chemical reactors, or combinations of them: batch reactors (e.g., the gastric cavity of a hydra and the blindended cecum of a rabbit), plug-flow reactors (PFRs; e.g., the tubular intestine of many invertebrates and all vertebrates), and continuous-flow stirred tank reactors (CSTRs; e.g., the rumen of a cow or the hindgut of a termite) (Fig. 2). They used mass-balance equations to determine the ideal gut-reactor configuration for two basic types of digestive reactions. In catalytic (i.e., enzymatic) reactions, reaction rate is a function of concentration according to the Michaelis-Menten equation. In autocatalytic (e.g., microbial fermentation) reactions, reaction rate is a complex function of substrate concentration and the concentration of the microbes. In autocatalytic reactions, the maximal rate of reaction occurs at an intermediate, rather than at the highest, reactant concentration. Penry and Jumars (361) concluded that because PFRs maintain a gradient in reactant concentrations and thus of reaction rates from higher values near the reactor entrance to lower values near the exit, they are a better design for digestive processes that rely on catalytic enzymatic reactions. They suggested that this is the reason why tubular guts predominate among complex, multicellular animals. However, they also concluded that if, in addition to catalytic reactions, fermentation autocatalytic reactions are important, then fermentation production rate is maximized when a portion of the gut is a CSTR. These theoretical distinctions explain our separation of sections of this review devoted to digesters that rely largely on intrinsic enzymes to digest relatively nonrefractory materials in foods and sections devoted to digesters that typically ferment relatively refractory materials with the aid of symbiotic microbes. Among the latter group, some species are foregut fermenters in whom the microbial fermentation chamber resides proximal to the small intestine, and some are hindgut fermenters in whom the fermentation chamber resides distal to the host’s stomach and small intestine (248) (Fig. 2).
Figure 2.
Basic design of vertebrate gut. All vertebrates have a small intestine, but vary as to whether they possess other compartments such as crop, forestomach, stomach, cecum, and large intestine/colon. As a general rule, catalytic enzymatic reactions occur in the small intestine, whereas microbial fermentation can occur in the forestomach, cecum, and large intestine/colon (shown with dotted areas). Foregut fermentation occurs in four major clades of mammals and in at least one avian species (the hoatzin). Hindgut fermentation, either in the cecum or large intestine/colon, occurs in many clades of mammals, birds, and reptiles.
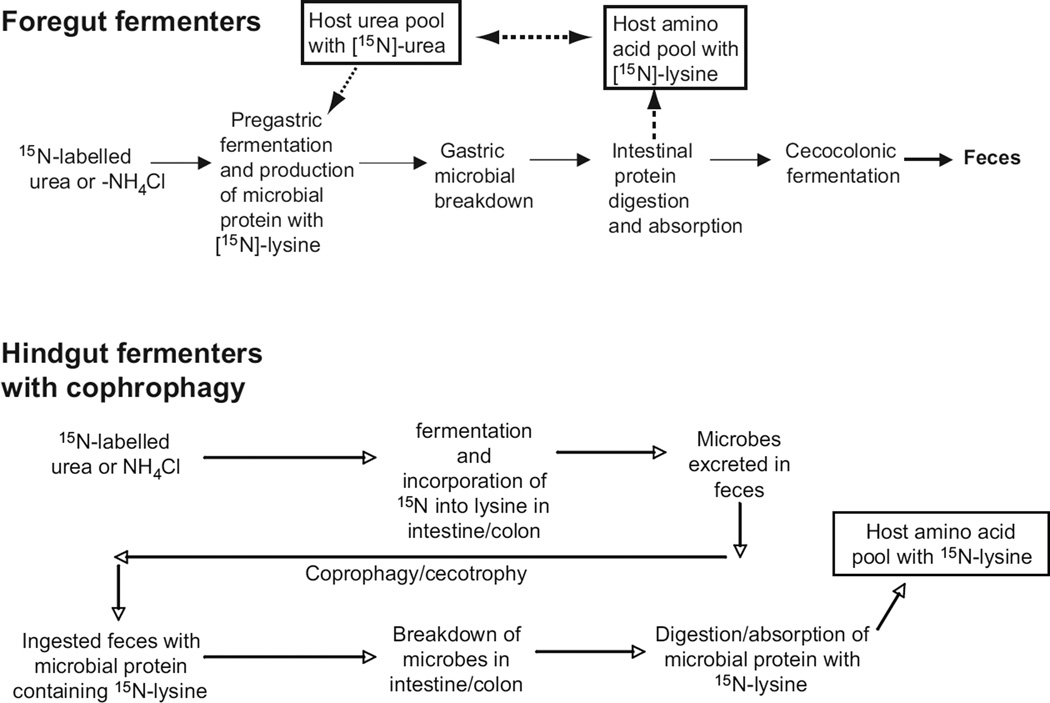
Another feature of overall gut design relates to the recovery processes of material(s) from the gut microbiota. The short-chain fatty acids (SCFAs; also called volatile fatty acids), such as acetic, propionic, and butyric acid, that are generated during fermentation can be absorbed in many regions of the gut by transcellular nonionic diffusion when they are protonated [(246); see also Section “Transcellular pathways for lipid absorption”). But, microbes potentially provide their hosts more than those energy-rich fermentation products. They also synthesize nutrients, including essential amino acids, that may be released from living cells or when microbial cells are digested by the host. A powerful way to study these recovery processes is to track isotopically labeled compounds (168). For example, after urea containing the nitrogen-15 isotope is administered orally to cows, lysine containing that same isotope is found in proteins within tissues of those animals (Fig. 3, top). Because cows cannot synthesize lysine de novo, microbes in the rumen must have converted the labeled urea into lysine, which then is incorporated into microbial protein. When the microbes are moved with digesta from the rumen into the acidic part of the cow stomach and then to the intestine, cow enzymes digest the protein, enabling the animals to absorb the nitrogen-15 lysine.
Figure 3.
Pathways of amino acid recycling depend on gut design and animal behavior. In foregut fermenting herbivores (top schematic), ingested sources of nitrogen (N) can be incorporated into host protein as essential amino acids such as lysine because the microbes can synthesize this amino acid (the vertebrate host cannot). The host breaks down the microbial wall with lysozyme and digestion and absorption of microbial protein occurs in the small intestine, followed by absorption of the amino acid, which enters the host’s amino acid pool. In hindgut fermenters (lower figure), such recycling can occur if the host reingests the feces (called coprophagy or cecotrophy), breaks down the microbes perhaps with intestinal lysozyme, and then digests and absorbs microbial protein that contains the new essential amino acids. Many details remain to be elaborated, such as the location and magnitude of lysozyme capacity. Also, work with pigs (438) and humans (168) that do not reingest feces demonstrates that there is another unknown pathway for absorption of microbially produced essential nutrients.
Nonruminant animals such as rats depend on the microbial community in the cecum and colon to incorporate urea-nitrogen into lysine. When rats reingest feces (coprophagy, or cecotrophy in rabbits), they digest and absorb labeled amino acid from those microbial proteins (Fig. 3, bottom). As predicted, germ-free rats cannot incorporate urea-nitrogen into lysine.
In theory, humans cannot incorporate lysine that might derive from isotope-labeled urea through proteins that the hindgut microbial community produces because they are hindgut fermenters and do not reingest feces. Remarkably, however, nitrogen-15 labeled lysine appears in human plasma proteins hours after labeled urea is administered (168). Thus, amino acids and perhaps other nitrogen-containing compounds may be cycling by currently undefined pathways between humans and their microbiota, a process that potentially could reduce dietary requirements for those nutrients. However, it remains to be resolved whether the fluxes of those amino acids or other essential nutrients between microbes and humans are great enough to influence dietary requirements.
Models help in understanding the diversity of digestive systems and guide mechanistic, integrative research
The gut models derived from chemical reactor theory and applied to both invertebrates and vertebrates have been useful research tools that delineate the important digestive features, show the direction and strength of their interactions, and help achieve the desired integration by relating the features and their interactions to whole-animal feeding rate and extraction efficiency. Application of their basic principles can also explain why animals processing different types of food may exhibit differences in their overall digestive strategy.
The models focus attention on a few characteristics that we list here to provide context for detailed material presented subsequently: (i) reaction rates for substrate breakdown (e.g., by native enzymes or microbial processes) and for monomer absorption; (ii) digesta retention time; (iii) volume of the gut reactor or reactants; and (iv) flow rate of digesta. As a first approximation, conversion or extraction efficiency can be expressed as:
| (1) |
Digesta retention time can be measured using inert markers fed to both vertebrates and invertebrates (248). This equation can be used only as a first approximation because it assumes constancy in many parameters that can be relatively complicated functions of each other [see references (239, 361), for examples of these functions]. But, it illustrates that conversion or extraction efficiency should be reciprocally related to initial concentration and gut volume, and positively related to both retention time and reaction rate. Food intake rate and excreta egestion rate are related to the flow rate of digesta through the gut/reactor that, in relation to its size, determines retention time:
| (2) |
Thus, conversion or extraction efficiency should be reciprocally related to flow rate.
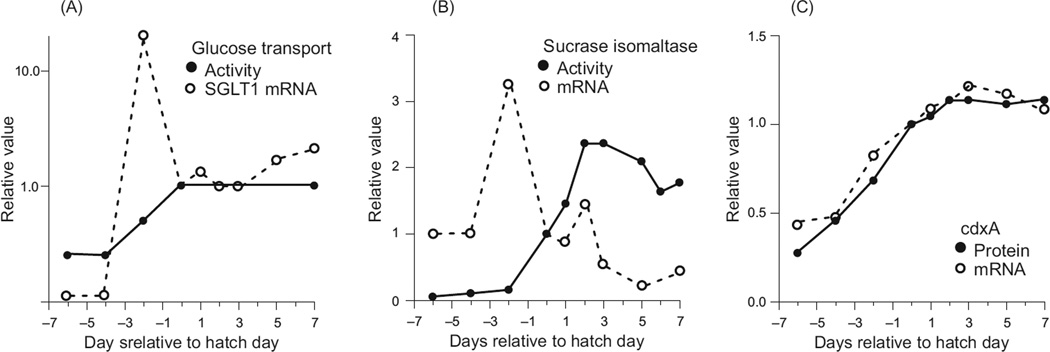
In some animals, these predicted patterns are nicely borne out, as exemplified in nestling house sparrows (Passer domesticus) during growth in the laboratory when fed a diet of constant composition (Table 1). Growth of the gut was complete by day 7 after hatch, and because food intake continued to increase, one would predict from Eq. (2) that digesta retention time should decline, which it did. With shorter retention time in conjunction with the same or lower enzymatic capacity, one would predict from Eq. (1) that overall digestive efficiency should decline, which it did. In both cases, the observed declines were smaller than those predicted, which may reflect some spare volumetric and enzymatic capacity relative to intake rate, but the integrated analysis suggests that the models [Eq. (1) and (2)] are conceptually sound in this case.
Table 1.
Digestive Features in House Sparrow Nestlings of Two Ages, and Comparison of Predicted and Observed Changes in Digesta Retention Time and Overall Digestive Efficiency*
| Feature | Nestling age Days 7–8 |
Nestling age Days 10–11 |
Signif?c | Observed change, % |
Predicted changed, % |
|---|---|---|---|---|---|
| Body mass, g | 17 | 22 | Yes | ||
| Relative gut sizea | 100% | 100% | No | ||
| Relative food intake, % | 65% | 100% | Yes | ||
| Relative retention time | 126% | 100% | Yes | −21% | −35% |
| Enzymatic capacity | 85%b | 100% | Yes/no | ||
| Digestive efficiency, % | 64% | 61% | Yes | −4% | −7% |
Based on masses of stomach, small intestine.
For intestinal aminopeptidase-N and maltase and pancreatic trypsin, chymotrypsin and amylase. In each case, the capacities were calculated based on organ mass and tissue specific activities. Some capacities were significantly lower in younger nestlings (range 75%–100% of that in older nestlings), and the mean was 85% of the value at day 10–11.
Difference between ages was statistically significant.
In many animals, when the proportion of the diet that is refractory to digestion is increased, many of the digestive features change in coordinated fashion enabling the animals to maintain their required intake of digestible dry matter or energy (20). Even for animals that can partially digest the refractory material, the overall digestive efficiency declines as the concentration of refractory material in food increases. To compensate, they must eat increasing amounts of dry matter, and GI tract size typically increases and/or digesta mean retention time may decrease to accommodate this [Eq. (2)]. These adjustments can occur within individuals in a wide variety of herbivorous animals, including endothermic mammals and birds (246, 296) and ectothermic insects (482), and crabs (295), and perhaps in cockles (Cerastoderma edule) switched from phytoplankton to detritus (338). Across species, herbivores tend to have more voluminous mass-corrected digestive tracts than carnivores in fish (136, 379, 458), mammals, birds, reptiles, and amphibians (248, 419), and insects (94).
Another general pattern interpretable in terms of Eqs. (1) and (2), is the response to increases in energy demand as occurs in endothermic birds and mammals when temperature is reduced, or during reproduction. Consideration of Eqs. (1) and (2) suggests any of several responses to higher feeding rate (i.e., higher flow of digesta) on a constant diet: (i) higher digesta flow through a GI tract with little spare digestive capacity would cause shorter retention time and thus result in poorer nutrient extraction efficiency; (ii) if the GI tract enlarges, the retention time might be unchanged as would extraction efficiency; and (iii) if there were no change in gut size, increased biochemical reaction rates per unit gut might compensate for the reduction in retention time, leaving extraction efficiency unchanged. Effective discrimination of these alternatives requires simultaneous measurement of all the variables, as has been done in a number of studies with birds and mammals (248). Typically, the results match option (ii). The most important adjustment to the higher feeding rate is an increase in mass of the GI tract (and liver too), which has two important effects. First, it keeps retention time relatively constant in the face of higher digesta flow (i.e., intake rate). Second, although intestinal tissue-specific rates of hydrolysis and nutrient absorption typically do not change significantly, the total hydrolytic and absorptive capacity of the small intestine does increase because of the increase in intestinal mass. The combined net effect of these changes is to hold digestive efficiency relatively constant even though intake may increase 200 to 300 percent [Eq. (1) and (2)]. A recent meta-analysis (339) underscores aspects of this general response in more than two dozen studies of laboratory mice and rats.
Integrated analysis of digestive strategy using reactor models has been usefully applied in studies with fish as well (175, 216) but other kinds of models, for example, compartment models, are also useful (90). There are modes of digestion that may not be characterized well by the reactor models, such as phagocytosis and pinocytosis followed by intracellular enzymatic hydrolysis that may predominate in some invertebrates [e.g., ticks and mites (345)]. However, modeling approaches have still guided research and enhanced understanding in some taxa that have specialized features of digestion that are not necessarily captured in the simplest reactor models. Some notable examples include evaluation of the “glandular” digestion path in lamellibranch bivalves that involves both intracellular digestion and extracellular digestion in the gut lumen (360), or compartmentalization imparted by the peritrophic envelope and enzyme recycling thought to occur in insects (34).
Modeling has facilitated research that links digestive physiology with whole animal nutrition in production agriculture with vertebrates (380, 384) and aquaculture with invertebrates (376), and with ecological phenomena such as foraging ecology (298, 468) and community structure (353, 469). Modeling has also contributed to understanding impacts of temperature change (297, 474) that could improve predictions of animal responses to climate change (13).
Digestive system design is in accord with the economy of nature
The examples described above illustrate that the digestive system can be viewed as economical in design, achieving a good match to food intake. In an uneconomical match, the enzymatic and absorptive capacities would be in great excess relative to the typical load (i.e., the flow rate of primary nutrient) and/or retention time would be routinely in great excess in relation to reaction rates. But, excessive retention time would either limit food intake rate or impose costly increase in size of the GI tract, or both, and this would be selected against in animals maximizing their growth or reproductive rate. It has been estimated that the digestive tract and liver of a vertebrate accounts for 20% to 25% of the whole animal’s respiration (66, 308). Within species, increases in size of the alimentary organs are associated with increases in basal metabolic rate (265, 364). Probably, because of these costs, there has been selection for the size and performance of the digestive system to be matched to food intake and quality (248).
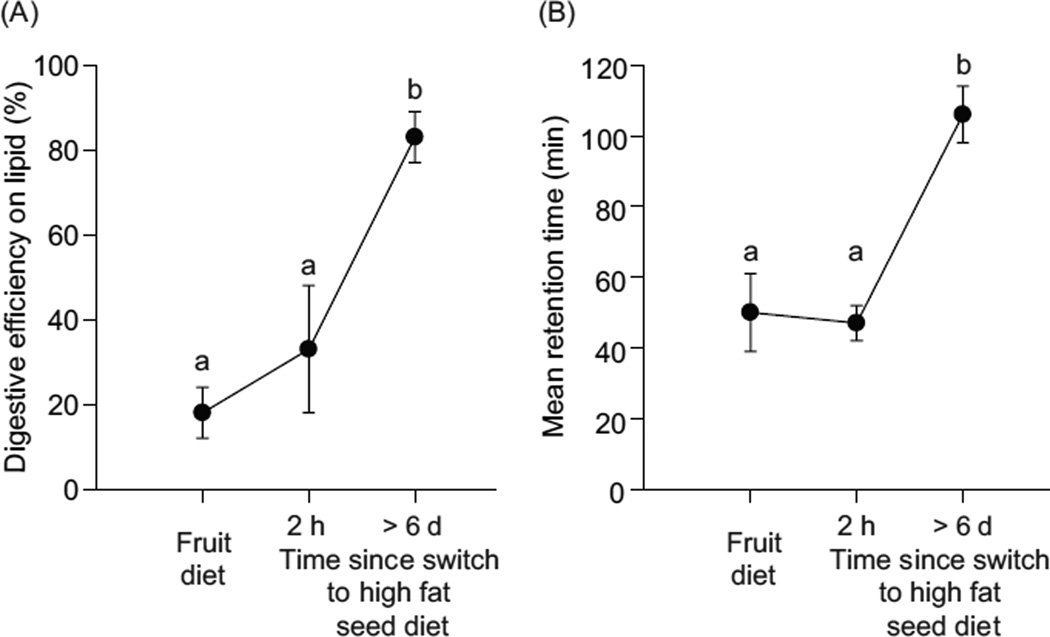
Many examples exist of apparent economy of design in digestive features. In intermittent feeders, such as seasonally dormant mammals (68), reptiles (439), fish (180), and invertebrates (171) the mass of the digestive system is reversibly decreased and increased when intake goes down and later returns to higher levels. A similar pattern occurs for some snakes over days to weeks between meals (395, 417), and for migratory birds that may fast during flights and then feast during migratory stopover (252). In some social ants and wasps in which adults feed larvae proteinaceous food and then ingest larval amino-acid-rich excretions, the levels of protease activities in the adults’ guts are extremely low (159). This seems consistent with theory, because excessive capacity would waste energy and material in synthesis of little used proteins, and the space available for membrane-bound proteins might be limiting (117, 118). In yet another example, omnivorous birds maintained on sugary fruit and then switched to higher fat diets seem initially poorly matched digestively, as reflected in low lipid extraction efficiencies (4, 287), until compensatory adjustments occur in increased digesta retention (4, 288) (Fig. 4) and in pancreatic lipase activity (289). These changes are predicted by the integrative model [Eq. (1)], which assumes that conversion/extraction efficiency will decline when reactant concentration increases unless compensatory changes occur in retention time and/or hydrolysis/absorption rate.
Figure 4.
When digestive features are not well matched to dietary substrate(s), digestion is inefficient. Yellow-rumped warblers, habituated to a sugary fruit-based diet, were transferred to a high fat seed diet. (A) Efficiency of [14C]glycerol trioleate absorption. (B) Mean retention time of digesta measured with [3H]glycerol triether, a nondigestible lipid marker. Within each figure, points that share the same lower case letters do not differ significantly in mean value [Fig. 1, with permission, from reference (243)].
Considerations of evolutionary economic design suggest that enzymatic and absorptive capacities should be modestly in excess of their corresponding loads (enough but not too much) (117, 118). Although measuring the magnitude of these matches and the corresponding “spare capacity,” measured as the ratio of capacity to load, is plagued by a number of problems (66, 435, 466), estimates by a variety of methods in mammals and birds imply that immediate spare capacity (i.e., prior to any acclimation or acclimatization), is less than two (250). Absorptive capacity may be limiting in some developing animals because of scarcity of certain transporters (148). Nestlings of song thrushes (Turdus philomelos) and house sparrows removed from their nests could be overfed less than 20% as compared with controls (controls = nestlings fed amounts that yielded a growth rate similar to that of wild nestlings), and their modest increases in food intake were offset by statistically significant or near-significant declines in digestive efficiency as compared with controls (266, 286). These experimental data are consistent with an inference in the above discussion about Table 1 that house sparrow nestlings have only modest spare digestive capacity.
Catalytic Chemical Breakdown by Intrinsic Enzymes
There is large variation among foods in both types and amounts of main nutritional substrates (e.g., simple and complex carbohydrates, proteins, and fats), and also variation in composition within each substrate type (e.g., specific bond linkages and chain length differences). Different substrate types require different particular complements of secretions and enzymes for their breakdown and particular mechanisms for the absorption of their breakdown products (Table 2). A number of reviews provide many details of the enzymes’ structure, pH dependence, function and distribution among vertebrate and invertebrate taxa (88, 246, 419, 428, 429, 457). Many advances have relied on new molecular techniques. For example, chymotrypsin-like serine proteases (SPs) are important in protein digestion in insects, but may also play roles in immune response and molting. The enzymes important for digestion can be clarified based on cDNA sequence (e.g., particular catalytic motifs), tissue localization (by fluorescent in situ hybridization of mRNA and immunohistochemistry of protein), and developmental and induced expression (e.g., during feeding vs. nonfeeding stages) (487, 488). Structure-function relationships (415) and evolutionary relationships (102) among enzyme isoforms can be discerned as well.
Table 2.
Diet Items, Some of Their Key Chemical Components and Enzymes Required to Break Them Down*
| Diet item | Refractory materials or chemical(s) |
Less refractory chemical(s) |
Enzyme activitiesa |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |||
| Nectar | Nil | Simple sugars | ♦ | ♦ | ♦ | ||||||||
| Milk | Nil | Lactose | ♦ | ♦ | ♦ | ||||||||
| Animal flesh | Nil | Glycogen | ♦ | ♦ | ♦ | ♦ | |||||||
| Insects, zooplankton | Cuticle, chitin | Glycogen, trehalose | ♦ | ♦ | ♦ | ♦ | |||||||
| Bacteria | Peptidoglycan in G(+) bacterial cell walls | Soluble polysaccharides | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | |||||
| Terrestrial plant material (flowers, seeds, fruits, leaves, twigs) | Cellulosesb, lignin, insoluble starchesc | Sucrose, starch | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ||||
| Aquatic/marine plant materials (green and brown, diatoms, seaweeds | Celluloses, mannanes, xylans, agarose | Starch, laminarin and chrysolaminarind | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | |||
| Plant exudates (saps, resins, latexes, gums) | Phenols and terpene derivatives, hemicellulose, other complex β-linked polysaccharides | Sucrose | ♦ | ♦ | ♦ | ♦ | ♦ | ||||||
| Fungi and lichens | Chitin, N-acetyl-β-D-glucoaminides, N bound to cell-wall components; | ♦ | ♦ | ♦ | ♦ | ♦ | |||||||
| Detritus | Celluloses, lignin, xylans, mannanes, | Starches, α-glucans | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | |||
This table is not comprehensive but lists mainly types of food items discussed in this article. The diet items are ranked (top to bottom) in approximate order of the relative amounts of material in them that is refractory to digestion (low to high). From reference (249), based on multiple sources (248,295).
-
1.Proteases (such as pepsins, trypsins, and chymotrypsins) and peptidases (e.g., carboxypeptidases and aminopeptidases).
-
2.Ester bond hydrolases (e.g., lipase and phospholipase).
-
3.α-amylases (hydrolyzes starch from plants and glycogen from animals).
-
4.α-glucosidases (e.g., maltase [hydrolyzes the oligosaccharides that are formed by amylase], sucrase [hydrolyzes sucrose from plants], oligodisaccharidases).
-
5.Trehalase (hydrolyzes trehalose, the principal blood sugar in insects).
-
6.Lactase.
-
7.Cellulase (cellulose is hydrolyzed by the concerted action of three types of cellulases: endocellulases, exocellulases, and β-glucosidases).
-
8.Xylanase and pectinase.
-
9.Laminarinase.
-
10.Chitinases.
-
11.Lysozyme [hydrolyzes peptidoglycan in G(+) bacterial cell walls (141)].
Cellulose and hemicellulose.
The crystalline pattern of starch seems to determine its susceptibility to hydrolysis (139).
β-1,3 glucan storage products (laminarin) (236).
Dietary and phylogenetic correlates of catalytic enzyme activity
Based on arguments of the economy of nature (above), a number of patterns are predicted for animals adapted to particular diet features. For dietary components such as nonstructural carbohydrates (e.g., sugars and starch), protein and lipids, a positive relationship is predicted between their level in the natural diet and the presence or amount of gut enzymes and transporters necessary for their breakdown and absorption (245, 248).
Earlier review of scores of investigations in many taxa identified patterns that were consistent with these predictions (246). For example, many of the carbohydrate-degrading enzymes are correlated positively with dietary carbohydrate level in fish, birds, and mammals (246), crustaceans (235, 236, 389), oligochaetes (110), and possibly insects (94). Although in total these studies are consistent with the adaptational hypotheses, a number of features of the studies in the past decade strengthen the analysis, and we will focus on these studies in the paragraphs that follow.
Knowledge about diets and digestive systems continually increases with the inclusion of information on new taxa of animals, especially invertebrates, eating an ever enlarging variety of diets. Mites that consume plant materials have higher levels of glycosidases (examples in Table 2) than those that live on animal secretions or blood (345), which is a pattern analogous to the correlation postulated above between carbohydrate-digesting enzymes and dietary carbohydrate. Other mites that eat and grow on bacteria have higher activity levels of lysozyme, which breaks down bacterial cell walls (141).
A second feature that strengthens the analysis is a larger number of species measured by uniform methodology and subjected to phylogenetically informed statistical analyses. This is a great improvement over the earliest studies that were sometimes two-species comparisons, which are plagued with a number of difficulties as regards inference about correlated evolution of diet and physiological traits (172). Inclusion of phylogenetic considerations [e.g., by phylogenetically independent contrasts (147)] can improve the analyses because species closely related by evolutionary descent arguably are not statistically independent, which can lead to pseudoreplication (248). Also, researchers on digestive systems of insects (428) and fish (77, 177, 178) have emphasized that, unless phylogenetic relationships are taken into account in comparative studies, important biological information may be overlooked (e.g., phylogenetic “signals” and constraints) or the phylogenetic pattern(s) in the data may obscure pattern(s) of dietary specialization.
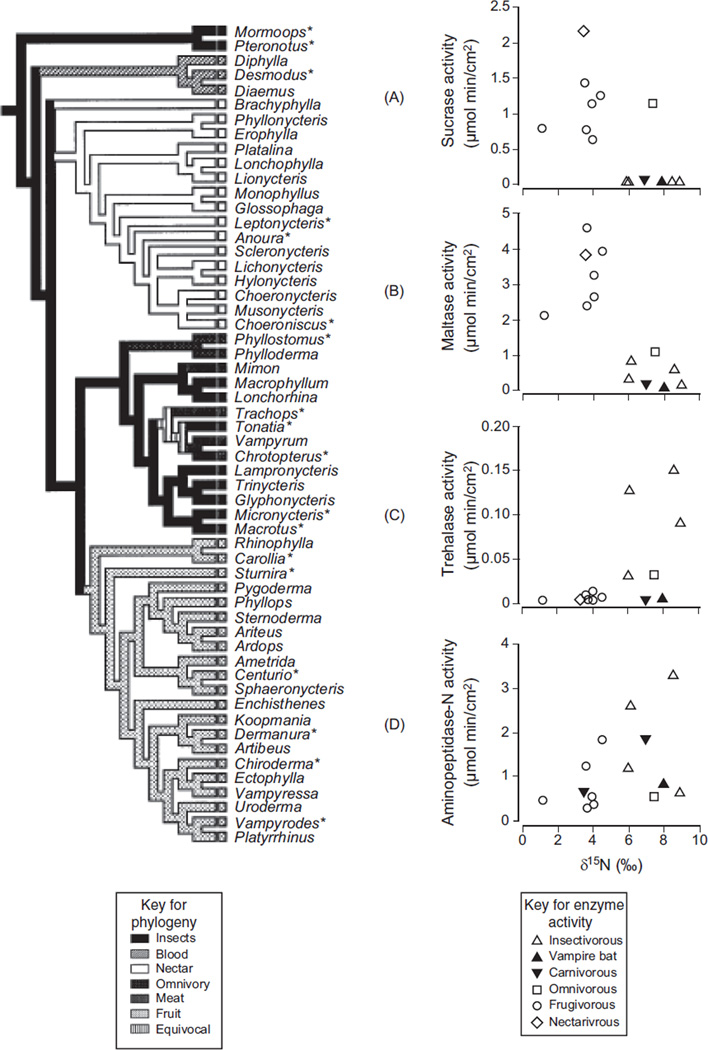
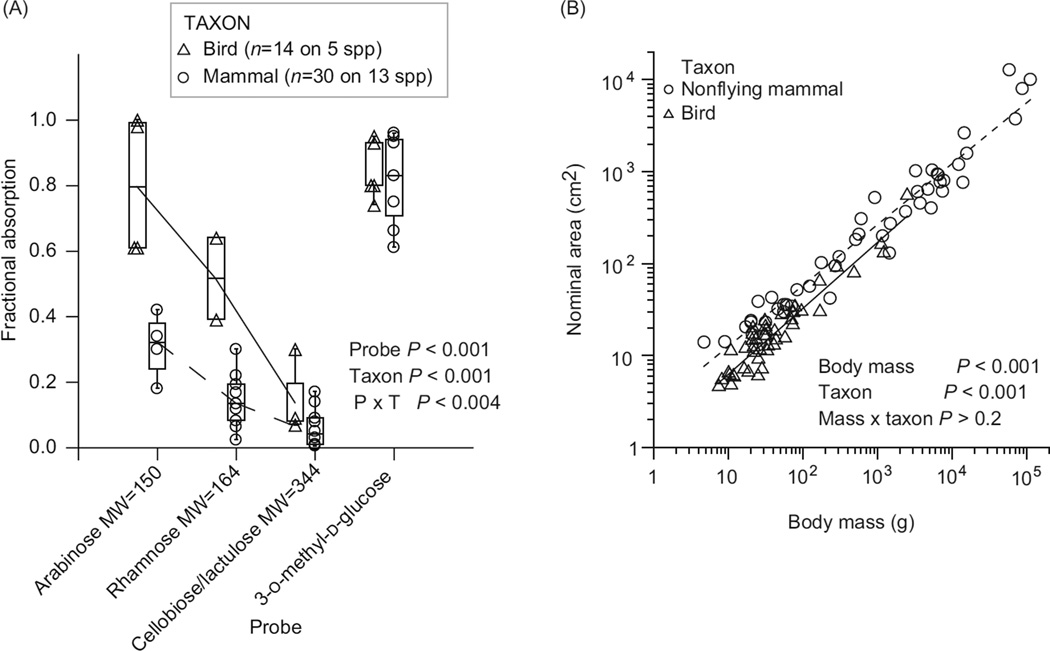
Recent studies with fish, birds, and mammals exemplify these improvements. Schondube et al. (392) used a phylogeny for New World bats (family Phyllostomidae) to analyze the correlation between diet and digestive enzymes in 14 species (Fig. 5). They used the 15N level of the bats’ blood to characterize their diets, which were composed of insects, nectar, fruit, or blood, because the natural abundance of 15N increases with trophic level. Twenty a priori predictions about patterns in sucrase, trehalase, maltase, and aminopeptidase N were borne out. For example, a shift from insectivory to sanguinivory and carnivory (i.e., reduction of insect trehalose in the diet) was accompanied by a tenfold to 15-fold decrease in trehalase activity (Fig. 5C). A shift from insectivory to nectarivory or frugivory (addition of plant sugars to the diet) was accompanied by a significant increase in sucrase (Fig. 5A) and maltase activity (Fig. 5B), a decrease in trehalase activity, and no change in aminopeptidase activity (Fig. 5D), because bats in all diet groups digest protein. The probability of such high concordance with predictions is so infinitesimally low that the authors concluded that evolutionary changes in diet in phyllostomid bats were indeed accompanied by adaptive shifts in digestive enzymes.
Figure 5.
Within the New World bat family Phyllostomidae, the evolutionary shift from insectivory to nectarivory or frugivory was accompanied by changes in digestive enzyme activity. An increase in sucrase (A; top right figure) and maltase (B; second from top) activity (which digest plant sugars in the diet), a decrease in trehalase (C; third from top) activity (digests insect sugar trehalose in the diet), and no change in aminopeptidase (D; bottom right) activity (because bats in all diet groups digest protein). In these plots, increasing animal matter in the bats’ natural diet is indicated by increasing δ15N in the bats’ tissue, and points are species means. The evidence that these correlations represent evolutionary transitions is based on the bats’ diets mapped onto their hypothesized phylogeny, shown on the left. The genera marked with asterisk were included in the data set. Two of the bat genera (Mormoops and Pteronotus) are in a sister family, Mormoopidae. Adapted from reference (248) (Fig. 4.24), with permission; redrawn, with permission, from reference (392).
In an another phylogenetically informed analysis, German et al. (179) constructed a phylogeny for ten minnow species (Cyprinidae), which they incorporated into their tests for digestive system matches to diets composed of varying amounts of animal, algal, diatomaceous, and detrital material. Herbaceous taxa had longer digestive tracts and higher activity of the carbohydrases amylase and laminarinase in their guts, whereas insectivorous species had higher chitinase activities. The latter pattern had not been apparent in previous surveys of fish species, but those surveys did not focus on closely related species that lack large differences in gut size and predigestive mechanical processing that can confound the analysis (179).
Phylogenetically informed analyses of digestive enzymes in birds have revealed both dietary and phylogenetic influences. American robins, and other closely related species such as European starlings and gray catbirds, all members of the large (≈ 600 species) and monophyletic sturnid-muscicapid lineage lack intestinal sucrase activity (310). Among other passerine birds that do express sucrase-isomaltase, sucrase activity is ten times higher in the hummingbird lineage (Trochilidae), even when compared with other nectar-consuming passerine birds (393). But, hummingbirds are unremarkable in regards to other enzyme activities such as maltase and aminopeptidase-N. Maltase activity appears to be strongly correlated with diet among bird species. Nectarivorous and omnivorous species have higher maltase activities compared to insectivorous species (309), and, in phylogenetically informed analyses, maltase activity was positively correlated with dietary level of starch (262) or seeds (373). Pancreatic amylase was also significantly correlated with dietary starch level in a phylogentically informed comparison among six passerine species that consume diets with differing amounts of starch (262).
Molecular mechanisms for differences in enzyme activities between populations/species
Improvements in molecular information have allowed better characterization of the changes in particular genes and proteins responsible for differences in digestive capacity. These advances have been especially marked in studies of changes in carbohydrases coincident with inclusion of starchy foods and milk products in the human diet. In the case of starchy foods, the focus has been on salivary amylase. The salivary amylase gene Amy1 is closely related to the pancreatic amylase gene Amy2 from which it originated by duplication (8). Its function may be to (i) augment pancreatic amylase activity (salivary amylase persists in the stomach after swallowing), or initiate starch breakdown in the mouth and thus either (ii) speed glucose absorption or (iii) release sugars for tasting and thus help in the identification of nutritious (starchy) foods (8, 363). Among humans sampled by Perry et al. (363), there was a positive correlation between AMY1 copy gene number (range 2 – 14 copies) and mg AMY1 protein/mg saliva (range <0.2 up to ca. 6). They compared copy number between three “high starch” populations and four “low starch” populations and found that the copy number was significantly higher in the high starch populations. The populations were geographically widely distributed and the interpopulation variation in copy number was related most strongly to diet and not geographic proximity. Furthermore, AMY1 copy number and salivary amylase protein levels in humans generally are at least three times higher than in chimpanzees and bonobos, whose diets are composed predominantly of fruit and leaves that contain much less starch than the diets of most human populations. The picture that emerges is one of correlated evolution of diet and amylase coincident with the dietary shift early in hominin evolutionary history toward starch-rich plant underground storage organs such as bulbs, corms and tubers and later to grains.
Single-nucleotide polymorphisms (SNPs) seem to explain differences among human populations in the capacity to digest lactose in milk. Milk is produced only by mammals, and its primary carbohydrate is lactose in most species. Lactose is hydrolyzed by the membrane-bound intestinal enzyme lactase-phlorizin hydrolase (or lactase, for simplicity), which is coded by the lactase gene (LCT). In most mammals lactase activity is high at birth and declines sharply around weaning. Ingestion of large amounts of lactose post-weaning normally results in escape of undigested lactose to the distal GI tract where it is fermented, leading to production of gases (CO2, H2, and methane) and sometimes osmotic diarrhea. The majority of humans are lactose intolerant, but members of a small number of populations that have been associated historically with domestic ungulates (cows, sheep, and goats) are lactose tolerant. The first evidence for SNPs as causative factors in lactose intolerance came from a study of Finnish families where a DNA variant (C/T-13910) located in the enhancer element upstream of LCT associated with lactose intolerance (140). The allele that carries the T-13910 variant was subsequently found to correlate with many global populations with lactose tolerance, and a variety of functional studies have revealed some of the molecular steps by which the allele controls the expression of lactase in intestinal cells (138). But, there was more to the story because some populations (e.g., in sub-Saharan Africa and Saudi Arabia) that lacked the variant T-13910 nonetheless had a high prevalence of lactose tolerance. Subsequently, other SNPs were identified that correlated with lactose tolerance, and analyses seem to indicate that convergent evolution of the phenotype occurred a number of times at different locations (138). Based on genetic patterns and analysis of Neolithic human skeletons, it seems that the ancestral human condition is lactose intolerance, but in a number of locations (i.e., cultures) humans’ consumption of dairy products created a strong selection pressure for evolution of genes that support digestion of lactose (8).
Genetic variants of amylase have been described in some invertebrates such as molluscs (221, 369) and several insect species (12, 105, 325). Research on these systems indicates that the enzyme gene polymorphisms may be non-neutral and can give important advantages processing diets and in turn beneficial rewards for growth and/or reproduction to individuals carrying certain genotypes, although the details of these scenarios are not as well established as in the aforementioned examples based on research in humans. There are practically no selection experiments (169) designed to test for adaptation of digestive enzymes. Flour beetles (Tribolium castaneum) that were raised on a variety of diets, whose carbohydrate contents likely varied but were not measured, showed some significant variation in amylase activity along with significant differences in growth rates and survival (25).
Catalytic enzymes and the microbiota
Some of the food substrates listed in Table 2 are degraded mainly or entirely by enzymes from the GI microbiota, but the host’s intrinsic catalytic enzymes may nonetheless play a critical role in managing this symbiotic relationship and in harvesting useful products from it. Recent findings about intestinal alkaline phosphate (IAP) have provided new insights about the former function, and intestinal lysozyme and pancreatic ribonuclease are key components of the latter function.
Alkaline phosphatase is found broadly across vertebrate and invertebrate taxa and in many organs within mammals, including intestine (276). It is a brush border enzyme that hydrolyzes monophosphate esters, but its physiological role in digestion has not been well understood. For example, IAP-deficient mice have no apparent digestion deficits (337). For many years its natural substrate(s) were not known, but its presence was widely used in intestinal studies as a marker of the apical brush border and as a marker for crypt-villus differentiation (276). In 1997, Poelstra et al. (366) showed that lipopolysaccharide (LPS), a major component of the bacterial outer membrane, acts as a substrate at physiologically relevant pH. By dephosphorylating bacterial LPS, IAP reduces its toxicity. In subsequent studies, IAP-deficient (knockout) mice (190) and zebrafish (19) have been found to be hypersensitive to LPS toxicity compared with wild-type animals. Dephosphorylation of LPS appears to inhibit its binding to receptors that initiate upregulation of inflammation-related genes that lead to inflammation and increased bacterial transmucosal passage (173, 276). Thus, IAP helps keep in check the intestine’s defensive mechanism(s) against bacteria, and in this way, it participates in intestinal tolerance of commensal bacteria. Interestingly, bacterial colonization induces synthesis of IAP, whereas IAP levels are low in germ-free animals (19).
Lysozyme is another antimicrobial enzyme found broadly across vertebrate and invertebrate taxa in many kinds of tissues including the vertebrate intestine. In that tissue, lysozyme and other bactericidal proteins called defensins are secreted by Paneth cells located at the base of intestinal crypts (367). Lysozyme hydrolyzes the bacterial cell walls and the defensins insert into membranes where they interact with one another to form pores that disrupt membrane function and lead to the death of the bacterial cell (268). But, another fascinating aspect of lysozymes is that they have been recruited as digestive enzymes over evolutionary time in several vertebrate and invertebrate taxa including foregut fermenting mammals and birds (248), insects (64, 166, 167, 375) and arachnids (Acari) (141).
Digesting microbes requires first breaking the bacterial cell walls and then hydrolyzing and absorbing the contents of the bacterial cell. Bacterial cell walls are made primarily of peptidoglycan, which is hydrolyzed by the enzyme lysozyme. Most animals that assimilate their gut microbes have a compartment of the gut to culture the microbes and another one to digest them. In at least two mammalian lineages and one avian species, the latter can be a site of lysozyme secretion.
Ruminants, colobine monkeys, and hoatzins have evolved independently a lysozyme that functions as a digestive enzyme [reviewed in reference (248)]. This digestive lysozyme has many characteristics that distinguish it from the bacteriostatic lysozyme that is expressed in tears, milk, the Paneth cells of the small intestine, and in the whites of bird eggs. The digestive lysozyme is expressed in the acidic compartment of the foregut, has an acidic pH optimum, and is relatively resistant to breakdown by pepsin [reviewed by reference (303)]. Colobine and ruminant lysozymes converged in the amino acid sequences that confer these enzymes their unique pH optima and pepsin resistance. The digestive lysozyme of hoatzins has a different genetic origin from that found in colobine monkeys and ruminants. The primate and ruminant digestive lysozyme evolved from a “conventional” lysozyme, whereas that in the hoatzin evolved from a calcium-binding lysozyme that is expressed in the egg white (248).
Most mammals and birds have a single gene copy that codes for lysozyme. Ruminants, in contrast, have many copies (467). In ruminants, large-scale production of digestive lysozyme entailed both gene duplication and changes in the molecular structure of the protein. A common explanation for the origin of multiple gene copies is that these allow making more protein product (see Section “Molecular mechanisms for differences in enzyme activities between populations/species”). Indeed, lysozyme accounts for 10% of the total gastric mucosal protein and messenger RNA in ruminants. The activity of lysozyme in the stomach of the foregut fermenters is over three orders of magnitude higher than that found in animals with no foregut fermentation.
Ribonucleases, secreted by the exocrine pancreas into the lumen of the small intestine, digest the abundant RNAs of rapidly growing bacteria. Although there has not been a good phylogenetically informed analysis, available evidence suggests that the ribonuclease content of the pancreas is higher in foregut fermenters and in some cecal fermenters that practice coprophagy than in omnivores and noncoprophagous herbivores [reviewed in reference (248)]. In addition, in ruminants and colobine monkeys the gene for ribonuclease duplicated, and one of the copies became specialized for the efficient digestion of bacterial RNA in the small intestine (23, 491).
Hindgut fermenting animals may also digest bacteria when they reingest their feces (coprophagy/cecotrophy). In this regard, it is interesting that rabbits secrete lysozyme in the distal colon under a circadian schedule that follows tightly that of the production of cecotrophs, which are the special pellets excreted from the cecum (62). Thus, the cecotrophs that reach the stomach contain large amounts of lysozyme and, presumably, of bacteria with partially hydrolyzed cell walls ready to be digested. A curious feature of the colonic rabbit lysozyme is that its pH optimum is very different from that of other lysozymes expressed in rabbits. It is acidic rather than neutral (230). This observation suggests that in rabbits one of the lysozymes has been coopted from its original antibacterial role into the role of a digestive enzyme.
The assimilation of bacterial protein by herbivorous birds is perplexing because birds do not seem to have spatial separation of culturing and digestion of microbes. Also, to our knowledge no one has yet measured the activity of lysozyme in the GI tract of birds. Much remains to be learned about the mechanisms that vertebrate hindgut fermenters use to take advantage of their GIT microbes.
The Gut Microbiota and Fermentative Digestion
Overview of the animal gut microbiota
The GI tract of healthy animals is colonized by resident populations of microorganisms. In some animals, the gut microbiota contributes directly to nutrition by the fermentative degradation of plant cell-wall polysaccharides. Recent advances in sequencing technologies are transforming our capacity to study the diversity and function of the gut microbiota, and we consider these general issues first.
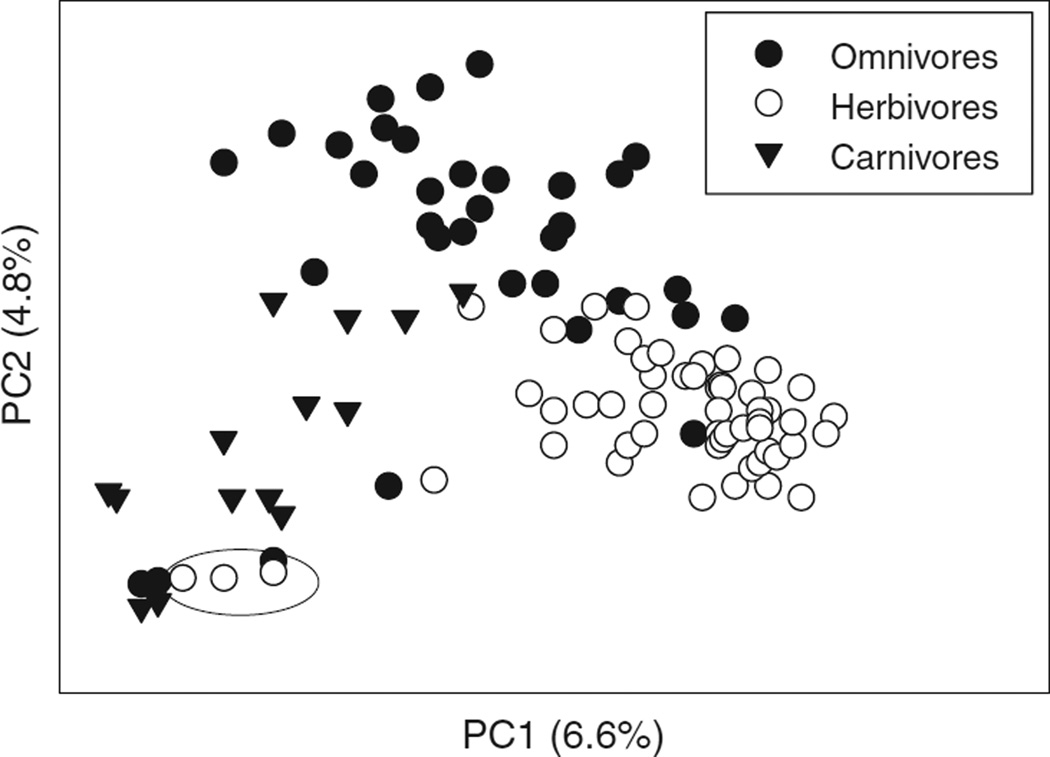
The taxonomic composition of the microbiota in the animal GI tract varies with phylogenetic position and diet of the animal, and with location in the GI tract (116, 334, 372). Recent research on the diversity of the microbiota in the GI tract has been dominated by molecular analyses of bacterial diversity in the feces of humans and model rodent species, based on the assumption that fecal diversity is representative of the microbial community in situ. The bacterial complement in mammals is dominated by two phyla, the Bacteroidetes and Firmicutes, each of which is represented by tens-to-hundreds of taxa, as identified by 16S rRNA gene sequence data (486). Among humans, the composition varies widely among individuals, and is influenced by age (87, 259), diet (334), and medical condition (161), including history of orally administered antibiotic treatment (232, 305). Fecal analyses of a range of mammals reveal diet as an important determinant of taxonomic composition (290) and genetic capacity for metabolism (334), such that the microbiota of mammals cluster according to whether the host is a carnivore, omnivore or herbivore, largely independent of the phylogenetic position of the mammal (Fig. 6). Interesting outliers in this dataset are the pandas which, although folivores, have a microbiota that clusters with carnivores. This result is a likely consequence of the recent evolutionary transition from carnivory to herbivory in these species, and is correlated with their anatomically simple, “carnivore-like” gut. In humans and other mammals, all regions of the GI tract are colonized, including the highly acidic stomach, which bears a diverse community of bacteria and some fungi (30).
Figure 6.
Variation in bacterial communities of mammals with diet, analyzed by principal components analysis. The analysis was conducted on 106 individuals of 60 species from 13 orders of mammals. The three herbivores circled are individuals of red and giant panda, which are members of the order Carnivora. [Data from reference (290)].
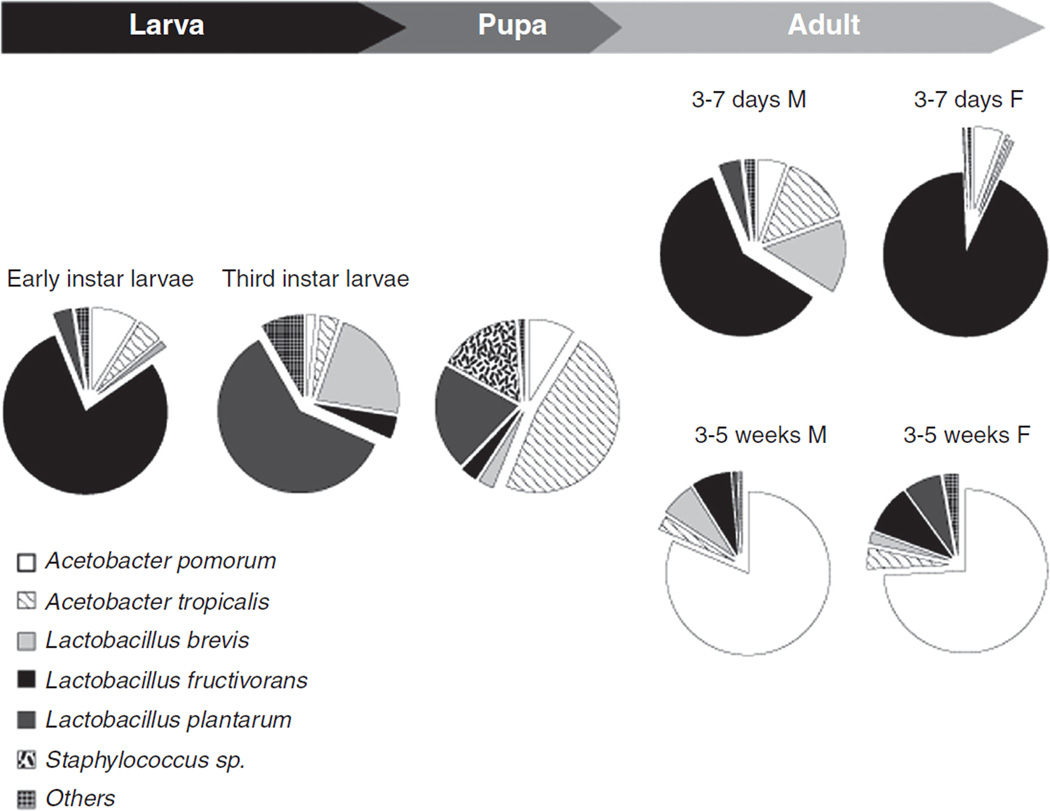
The species richness of the microbiota in the GI tract of many invertebrate animals is apparently an order of magnitude lower than in mammals, commonly with just 10 to 20 taxa per individual (7, 22, 123, 131, 285, 381, 475). Nevertheless, the global diversity of microorganisms associated with the GI tract of invertebrates is substantial with different dominant species, phyla or even kingdoms in different animal taxa. For example, the bacteria in the GI tract of Drosophila fruit-flies with a natural diet of rotting fruit are dominated by Acetobacter and Lactobacillus species (98, 101), while the related tephritid Med fly, Ceratitis capitata, feeding on unripe fruits is colonized principally by Enterobacteriaceae, including Klebsiella, Pantoea, and Enterobacter species (21). Analysis of the gut microbiota in Drosophila has revealed considerable variation in the dominant bacterial taxon with developmental age, even under uniform rearing conditions (Fig. 7). The incidence of eukaryotic microorganisms (e.g., protists and yeasts) in the GI tract of invertebrates is not well studied, although the Cryptocercidae woodroaches and “lower” termites are renowned for their possession of taxonomically unique groups of Oxymonadid and Hypermastigid flagellated protists (91, 349).
Figure 7.
Composition of bacterial species at different life stages of Drosophila melanogaster. “F” represents females and “M” represents males. [Data from reference (475)].
Microbial transformation of digestively intractable food constituents to compounds that are readily used by the animal
Microorganisms in the GI tract of many animals have a great diversity of glucohydrolases active against complex plant polysaccharides. For example, metagenomic analyses have identified more than 700 candidate glucohydrolase genes of bacterial origin in the hindgut paunch of Nasutitermes termites, most of which have predicted capacity to degrade cellulose and xylans (462), and a remarkable 27,755 putative carbohydrate-active genes have been detected in the metagenome of the cow rumen contents, most of which are bacterial in origin, have less than 75% sequence identity with previously described genes, and many of which are likely active against cellulose (210). Resident bacteria in the GI tract of humans also have considerable capacity to utilize carbohydrates, including complex plant polysaccharides. The genome of one common human gut symbiont Bacteroides thetaiotaomicron contains a total of 261 glycoside hydrolases and polysaccharide lyases (479). A metagenome analysis of fecal samples from 18 human individuals revealed a very diverse array of bacterial genes active against carbohydrates, collectively accounting for 2.6% of the sequences; the particularly high interindividual variation in the complement of glucoside hydrolase genes, even among members of the same family, was attributed to dietary factors (441). The relationship between the degradative capabilities of the bacteria in the GI tract and diet is further vividly illustrated by the discovery of genes for porphyranases and agarases in the gut bacterium Bacteroides plebeius isolated from Japanese but not North American individuals (207). These enzymes are active against the sulfated polysaccharides in Porphyra seaweeds that form a regular part of the typical Japanese, but not North American, diet. Furthermore, there is phylogenetic evidence that the genes for these glucohydrolase activities have been transferred horizontally from marine bacteria associated with Porphyra to the gut bacteria of humans. The GI tracts of animals, including herbivorous mammals and wood-feeding insects, are recognized as cellulose-rich environments that are currently being targeted in gene discovery projects for biofuels development and other industrial purposes (130).
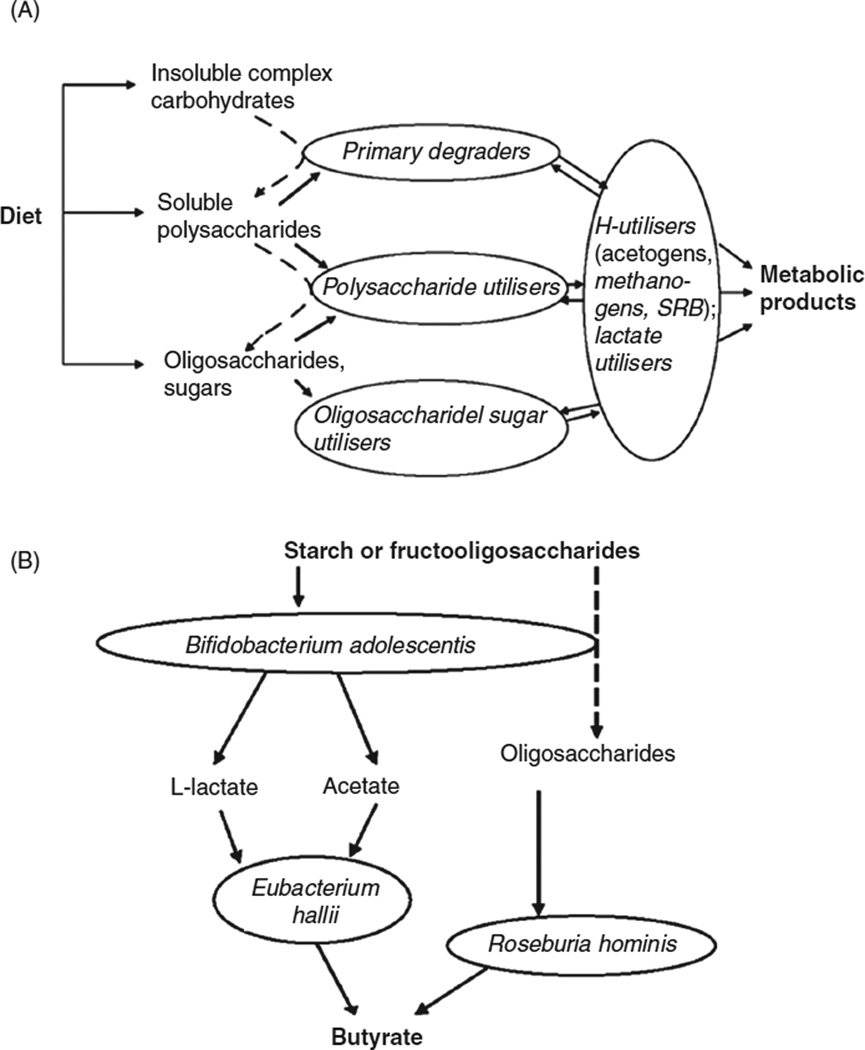
Microbial breakdown of complex carbohydrates can be nutritionally significant to the animal host, where the gut habitat is oxygen deficient, such that the microbial metabolism is strictly fermentative, and not aerobic. Specifically, the complex polysaccharides are hydrolyzed to simple sugars, and then subjected to bacterial fermentation, with the net release of fermentation waste products, typically SCFAs, including acetate, butyrate, and propionate (420). These final products diffuse across the animal gut wall, and are used as substrates for aerobic respiration, gluconeogenesis, and lipogenesis in the animal. The suite of reactions responsible for the transformation of complex carbohydrates to SCFAs is mediated by consortia of multiple bacteria with complementary capabilities (156), with cross-feeding of intermediate metabolites among bacteria with different capabilities (Fig. 8A). For example, in the human colon, Bacteroides species degrade complex polysaccharides to sugars; the sugars are respired by Bifidobacterium and other anaerobic bacteria to lactate; and the lactate is fermented by bacteria such as Eubacterium hallii and Roseburia hominis, producing butyrate (Fig. 8B). Butyrate, which is a waste product of the microbial community metabolism, is the principal respiratory substrate used by the gut epithelial cells (124). In this way, at least 50% of ingested cellulose and 80% of noncellulosic polysaccharides are degraded by microorganisms in the human colon, contributing at least 10% of the human energetic needs (103).
Figure 8.
Fermentative degradation of complex carbohydrates by consortia of bacteria in the human colon. (A) Functional groups of bacteria (SRBs, sulfate-reducing bacteria). (B) Major bacterial taxa responsible for degradation of starch and fructan-carbohydrates. [Redrawn from reference (156)], with permission.
Multiple factors beyond the biochemical capabilities of the microbiota determine the nutritional significance of microbial fermentation for an animal. Of particular importance are: (a) the intrinsic capacity of the animal to degrade complex polysaccharides and (b) diet composition. All vertebrates apparently lack the capacity to degrade cellulose and related complex polysaccharides of plant cell walls. Consequently, the amount of breakdown in the vertebrate GI tract is dictated by the scale of microbial fermentation, which varies from trivial, for example, in pandas (Ailurus fulgens, A. melanoleuca) (121, 465), grazing goose species (48), and wood-feeding catfish (176), to extensive, for example, in ungulates and many rodents. Some invertebrate animals have enzymes capable of degrading plant cell-wall components. The phylogenetic distribution of intrinsic cellulases is not fully understood, but genome analyses indicate that members of at least five phyla have cellulases of glucose hydrolase family 9: the mollusks, annelids, arthropods, echinoderms, and nonvertebrate chordates (specifically tunicates) (112). The relative importance of intrinsic and microbial cellulolysis has been investigated, especially in insects (464), revealing considerable variation. For example, 75% of the cellulase activity in the GI tract of the termite Mastotermes darwiniensis can be assigned to microbial fermentation by protist symbionts in the hind gut, with the remainder accounted for by intrinsic enzymes in the midgut and salivary glands (453); but the cellulase activity in the GI tract of the beetle Tenebrio molitor was unaffected by elimination of the microbiota (174), indicating that the observed microbial fermentation does not make a necessary contribution to cellulose digestion. The capacity of some insects to degrade plant cell-wall components is further illustrated by the identification of 167 enzymes from eight enzyme families capable to degrading plant cell-wall polysaccharides in a recent sequence analysis of seven species of phytophagous beetles (358).
Turning to the relationship between diet and microbial fermentation, various studies suggest that the taxonomic composition and metabolic traits of the gut microbiota can be influenced by diet, potentially with effects on the digestive function of the GI tract. For example, the rumen microbiota differed significantly between cattle reared on bermudagrass hay (68% fiber) and wheat pasture (44% fiber) (365); and the microbiota in the GI tract of the house cricket Acheta domesticus differed between insects reared on high protein and high carbohydrate diets, with correlated differences in the amount and composition of SCFA produced (387). Indications that the microbial changes can be very rapid come from an analysis of laboratory mice with GI tract colonized by the microbiota from human fecal samples. Remarkably, the composition of the microbiota and gene expression profile was altered within a single day of transferring the mice from a low-fat diet with high plant polysaccharide content to a high-fat, high-sugar diet (441).
Although the entire length of the GI tract is colonized by microorganisms in most animals, the highest microbial densities and abundance tend to be in postgastric regions, for example, the large intestine of mammals, hind gut of insects, and this is the usual site of microbial fermentation chambers. From the perspective of the animal, the key benefit of a postgastric fermentation chamber is that the substrates available to the microorganisms are those that are intractable to digestive action in the gastric region. This design minimizes the competition between animal and resident microorganisms for ingested nutrients that can be processed readily by the animal. Pregastric fermentation chambers have evolved rarely, and are apparently restricted principally to mammals, with five independent evolutionary origins [in the Artiodactyls (in the ruminants, camels, and hippos), in the colobine monkeys, and the Macropodidae (kangaroos)]; the remarkable S American bird, the hoatzin, also has a pregastric fermentation chamber (188, 476). The relative merits of pre- and postgastric fermentation have been discussed extensively (421, 450). The key disadvantage of pregastric fermentation for the animal is that ingested food is available for microbial metabolism before digestion by the animal. This can result in reduced nutritional gain from high-quality foods. For example, an animal derives more energy from simple sugars by gastric digestion and assimilation than by microbial fermentation; and more nitrogen from protein by gastric processing than microbial metabolism. The adaptive advantage of pregastric fermentation for very efficient breakdown of the plant polysaccharides is enhanced by rumination (i.e., regurgitation of partially fermented ingesta to the mouth, where it is chewed, and then reswallowed) because this behavior allows the plant material to be subjected to multiple, repeated cycles of mechanical disruption and fermentation, resulting in very efficient breakdown of the plant polysaccharides. Rumination has evolved independently in the ruminants and camels; kangaroos display more irregular cycles of regurgitation/swallowing that is known as merycism. It has been argued that pregastric fermentation chambers may have evolved in relation to functions other than cellulose degradation, for example to facilitate microbial detoxification of allelochemicals in ingested plant foods, and only subsequently became important in digestion of plant material (233).
Some animals possess a substantial fermentative microbiota that produces SCFAs without a morphologically distinct fermentation chamber. This is particularly evident among herbivorous fish, including various tropical perciforms (89). In one detailed analysis of three temperate fish species feeding on seaweed, the rate of production of one SCFA, acetate, was similar to those in the guts of herbivorous reptiles and mammals, even though the fish lacked coherent fermentation chambers (333). Further research is required to determine the mechanisms underlying fermentation in these fish, and the nutritional significance of the SCFAs produced.
Absorption
General principles
Absorption refers to the transfer of compounds from the gut lumen across the gut wall to the body tissues, including the lymph or blood of vertebrates and hemolymph of arthropods. At the cellular level, organic compounds can be absorbed from the gut lumen by paracellular and transcellular routes. Paracellular transport refers to movement between cells of the gut epithelium, while the transcellular route involves transport across the apical cell membrane of gut epithelial cells, transit across the cell (for some molecules with metabolic transformations in the cell), and then export at the basolateral membrane. We distinguish the term “absorption” (transport from gut lumen to body tissues by either the paracellular or transcellular route) from “uptake,” which refers to the transport from the gut lumen across the apical membrane of the gut epithelial cell (one step in transcellular transport).
This section considers absorption of organic compounds, particularly products of digestion: monosaccharides, the digestive breakdown products of complex carbohydrates; peptide and amino acid products of protein digestion; and lipids, SCFAs (generated by hydrolysis of triglycerides), and SCFAs (products of fermentative breakdown of complex carbohydrates by gut microbes). With the exception of SCFAs, these products are absorbed principally distal to the gastric region of the alimentary tract, for example, small intestine of vertebrates and midgut of insects. The absorptive cells are columnar epithelial cells called enterocytes. Exceptionally, SCFAs produced by the microbiota in the hindgut (e.g., mammalian colon and cecum) are absorbed across the hindgut wall by cells that are variously known as enterocytes, colonic enterocytes, or colonocytes.
In this section, two aspects of nutrient absorption are addressed: the modes of transport of the major classes of organic solutes and variation in nutrient absorption among animal taxa, in relation to nutritional habits and phylogeny and its mechanistic basis. Diet-related determinants of absorption in individual animals are addressed in Section “Matches of GI system biochemistry (enzymes, transporters) to changes in diet composition.”
Transcellular transport of organic solutes
Carrier-mediated transport
Most organic compounds absorbed across animal guts are polar, and their transport is predominantly or exclusively carrier-mediated, that is, mediated by membrane-bound transporters and displaying the twin characteristics of saturation kinetics and competitive inhibition. Two forms of carrier-mediated transport are recognized: facilitated diffusion, which is energy-independent and mediates transport down the electrochemical potential gradient; and active transport, which is concentrative and dependent, directly or indirectly, on cellular energy. Simple diffusion, that is, down the concentration gradient and involving neither a carrier nor cellular energy, is an additional mode of absorption that is especially important for small, nonpolar molecules.
Absorption of carbohydrates
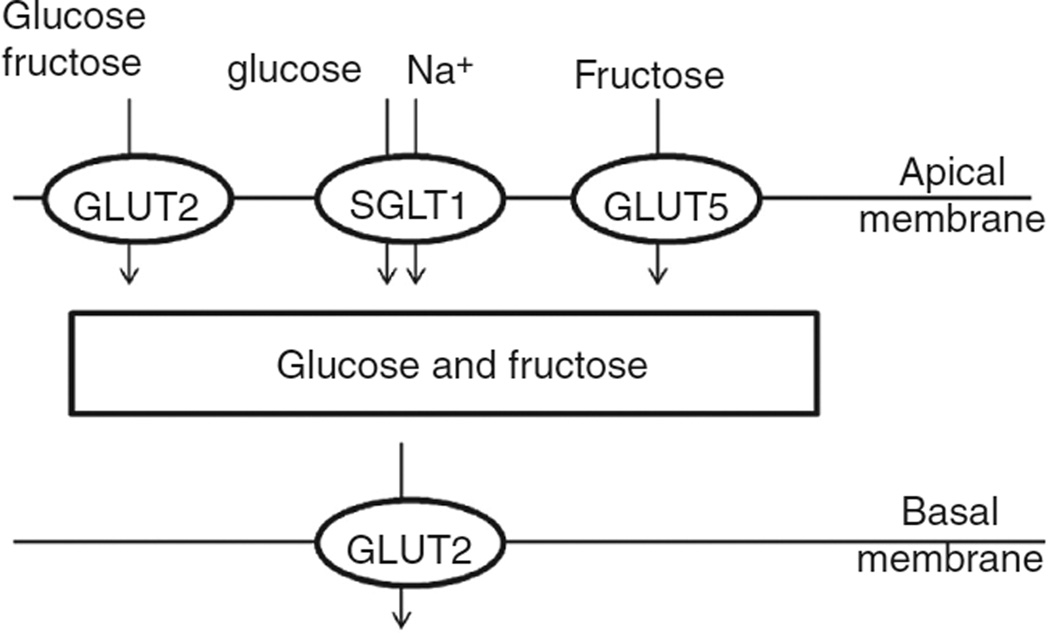
Monosaccharides cross the apical and basolateral membranes of gut epithelial cells by carrier-mediated mechanisms. The key glucose transporters in mammals and birds (184) are a Na+/glucose cotransporter SGLT1 (a member of the Na+/solute symporter family) and the facilitative transporter GLUT2, which transports glucose, fructose, mannose, and galactose with low affinity and N-acetyl-glucosamine with high affinity (444). Fructose is transported principally via the facilitative transporter GLUT5 (126). These transporters are expressed predominantly in the small intestine.
The expression of SGLT1 in the intestine is restricted to the apical membrane of enterocytes. Its capacity to take up glucose from very low concentrations in the intestinal lumen is driven by the downhill gradient of Na+ ions maintained by the Na+/K+-ATPase on the basolateral membrane (Fig. 9) (206). Once in the cell, the glucose is widely accepted to be transported down its concentration gradient across the basolateral membrane into the circulation by GLUT2. Under conditions of high luminal glucose content, however, GLUT2 in rodents is inserted into the apical membrane, where it mediates the high flux of glucose into the enterocyte (254). Some data suggest that sugar-induced translocation of GLUT2 may not occur universally in mammals (18, 330), and further research is required to establish the distribution of this effect with respect to phylogeny and diet.
Figure 9.
Transport of glucose and fructose across the mammalian enterocyte by SGLT1, GLUT2, and GLUT5. The insertion of GLUT2 into the apical membrane is mediated by the detection of luminal glucose by the TIR2/3 receptors and Ca2+ signaling, as described in text.
The mechanism by which GLUT2 is inserted into the apical enterocyte membrane is understood in outline (253). Under high glucose conditions, the inward flux of Na+ ions via SGLT1 results in depolarization of the membrane and Ca2+ influx, which, in turn, causes a large-scale reorganization of the cytoskeleton, facilitating access of proteins to the apical membrane. In parallel, high concentrations of luminal glucose and fructose activate the TIR2/3 receptor on the apical membrane, resulting in trafficking of phospholipase (PLC)β2 and protein kinase C (PKC)βII to the apical membrane. Diacylglycerol generated by PLCβ2, together with the high Ca2+, activates PKCβII, permitting the insertion of GLUT2 into the apical membrane and the resultant high capacity uptake of glucose and fructose. This process occurs very rapidly.
In the mouse, the responsiveness of GLUT2 insertion to luminal sugars varies among sugars, being triggered much less efficiently by glucose and complex sugars than by fructose, sucrose, and a mixture of glucose and fructose (193); mice fed on a high-fructose diet have been reported to bear GLUT2 permanently on the apical membrane of enterocytes (434). Artificial sweeteners, such as sucralose, dramatically increase GLUT2 insertion and the resultant uptake of glucose, such that the sugar is absorbed efficiently from lower concentrations in the presence of the artificial sweetener than in its absence (302). The implications of these rodent studies for human nutrition are not yet fully resolved.
Phylogenetic analysis assigns the mammalian GLUT2 to a clade that includes three further mammalian GLUTs (GLUT1, 3, and 4) and invertebrate, but no nonmetazoan, GLUTs, suggesting that this group of transporters may have evolved in the basal metazoans or immediate ancestors of animals (472). There is also evidence that SGLT1 and GLUT transporters contribute to intestinal glucose absorption in nonmammalian vertebrates, including fish (72, 269). The molecular basis of sugar uptake across the gut wall has not, however, been investigated widely in the invertebrates. Among insects, glucose transport across the midgut of the hymenopteran parasite Aphidius ervi is mediated by a SGLT1-like transporter on the apical membrane, together with a GLUT2-like transporter on both the apical and basolateral membranes of the enterocytes; and a second passive transporter similar to GLUT-5 is implicated in fructose uptake (58). There is also persuasive molecular and physiological evidence for the involvement of SGLT and GLUT transporters in glucose absorption from the midgut of the pyrrochorid bug Dysdercus peruvianus, with K+, not Na+, as the likely counterion of SGLT (28). This condition is not, however, universal among insects. For example, genome annotation of the pea aphid Acyrthosiphon pisum revealed no Na+/solute symporter with plausible specificity for sugars, but 29 candidate sugar transporters in the MFS family, equivalent to GLUT (368). These included an abundantly expressed gene ApSt3, a hexose uniporter with specificity for glucose and fructose in the distal midgut. Aphids may not, however, be typical of insects because their diet of plant phloem sap is sugar rich, and a concentration gradient from gut lumen to epithelial cell and hemocoel is maintained by the excess sugar in the gut lumen (127).
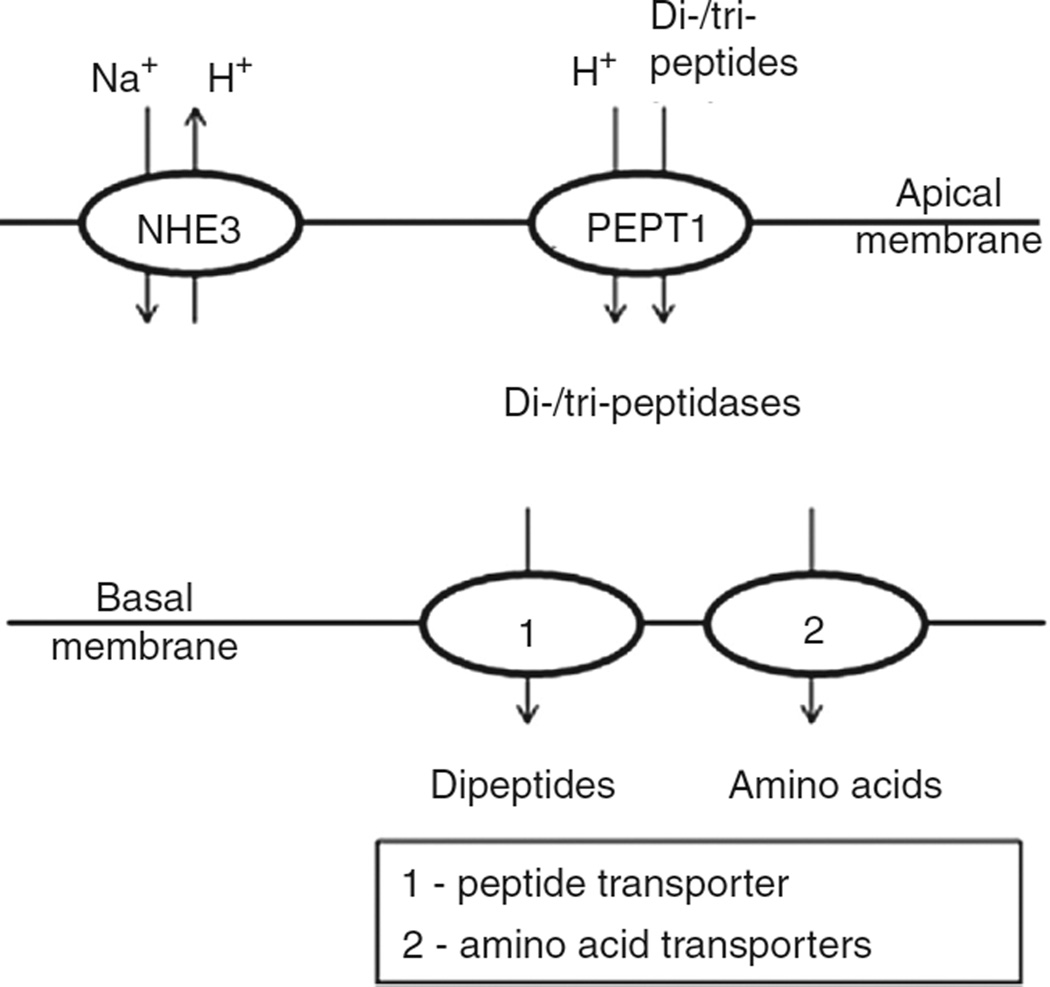
Pathways for amino acid and peptide absorption
The products of protein digestion taken up by enterocytes of the mammalian intestine are free amino acids, dipeptides, and tripeptides. Free amino acids are taken up from the small intestine of mammals by multiple carriers with overlapping specificities, with the result that most individual amino acids are transported by more than one transporter. By contrast, peptides are taken up by a single transporter with very low selectivity, as considered at the end of this section.
The amino acid transporters are classified by their activity (specificity and kinetics) into multiple systems, and by sequence homology into solute carrier (SLC) families. The SLC nomenclature was devised by the Human Genome Organization for transporters in the human genome (with all members of each family having >20%–25% amino acid sequence homology), and is widely used for other animals. The principal transporters mediating amino acid transport in the human intestine are summarized in Table 3.
Table 3.
| Systema | Solute carrier (SLC) group |
Amino acids transportedc |
Transport properties | Location | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutral amino acids (AA0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| B0 | B0AT1 | SLC6A19 | All AA0 | AA0/Na+ symport | Apical | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ASC | ASCT2 | SLC1A5 | A, S, C, T, Q | AA0 antiporter (no net AA0 uptake of | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| L | 4F2hc/LAT22 | SLC3A2/SLC7A8 | All AA0 except P | Na+ independent transport | Basolateral | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T | TATI | SLC16A19 | F, Y, W | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cationic amino acids (AA+) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| b0,+ | rBAT/b0,+ATb | SLC3A1/SLC7A9 | R, K, O, cystine | AA+ or cystine (uptake)/AA0 (efflux) antiport | Apical | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| y+L | 4F2hc/y+LAT1b | SLC3A2/SLC7A7 | K, R | AA+ (efflux)/AA0 antiport | Basolateral | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4F2hc/y+LAT2b | SLC3A2/SLC7A6 | K, R, C | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Anionic amino acids (AA−) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| X−AG | EAAT3 | SLC1A1 | E, D | AA−/3Na+ symport | Apical | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Proline and glycine | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IMINO | IMINO | SLC6A20 | P, HO-P | P/Na+ symport | Apical | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PAT | PAT1 | SLC36A1 | P, G, A | P or G/H+ symportd | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The systems of Christensen (left), originally developed for amino acid transporters in non-epithelial cells; and alternative systems names developed for epithelial cells.
Heterodimeric amino acid transporters, comprising two subunits: heavy chain (rBAT or 4F2hc) and light chain. The light chain confers transport activity, and heavy chain mediates localization of the complex to the cell membrane.
Standard single-letter abbreviations for amino acids (O: ornithine; HO-P: hydroxyproline).
Also reported to transport dipeptides gly-gly and gly-gly mimetics (164).
Studies on human, rodent and rabbit suggest that the amino acid transporters in the mammalian small intestine can be assigned to four groups, mediating the transport of neutral, cationic, anionic, and imino acids, respectively (41). Uptake across the apical membrane is mediated by: Na+-coupled transporters, for example, the B0 transporter with broad specificity for neutral amino acids and found in all parts of the small intestine; proton-motive force, as in the uptake of proline and glycine by the transporter PAT; and amino acid exchange, for example, uptake of cationic amino acids and cystine linked to efflux of neutral amino acids by b0,+ system. Transport across the basolateral membrane is also mediated by amino acid exchange, for example, y+L for efflux of cationic amino acids, or by facilitative diffusion, for example, transporters of the L and T system for efflux of neutral and aromatic amino acids, respectively.
The rich classical literature on the kinetics of amino acid transport across the intestinal epithelium of various nonmammalian vertebrates and invertebrates is summarized by (246) and (341), and there is increasing interest in analysis from a molecular perspective [e.g., for birds, see reference (184)]. The midgut amino acid transporters that have been studied in insects belong principally to the Na+-coupled symporter family SLC6. As in mammals, multiple transporters are expressed, with overlapping specificities for amino acids. Some are very specific, for example, NAT6 and NAT8 in the distal midgut of mosquito Anopheles gambiae transport just aromatic amino acids (318, 319). Other SLC6 transporters have a very broad range. Notably, the neutral amino acid transporter in Drosophila (DmNAT6) can mediate the transport of most amino acids apart from lysine, arginine, aspartate, and glutamate; and, remarkably, it can also take up D-isomers of several amino acids (321). This capability can be linked to the abundance of D-amino acids in the cell walls of bacteria, which are an important component of the natural diet of Drosophila species. DmNAT6 is an active transporter, capable of mediating uptake against the concentration gradient.
Exceptionally, amino acid transport in the midgut of larval Lepidoptera is coupled to K+ ions, and not Na+ ions (158, 340). This trait is believed to be linked to the high K+/low Na+ conditions in the gut of these insects, which eat plants with high ratios of K+/Na+. Multiple transporters are involved with a range of specificities, including two neutral amino acid transporters in Manduca sexta (KAAT1 and CAATCH1), both members of the SL6 family (71, 145) with distinctive amino acid selectivities (322). The cotransport of the K+ ions and amino acid into enterocytes is coupled to the ATPase-dependent extrusion of K+ ions from adjacent goblet cells. The coupled functions of electrogenic K+ transport and K+/amino acid uptake are mediated by different cells, presumably because the high emf generated by the goblet cells could compromise the function of the SL6 and other transporters.
Amino acid transporters are also expressed in the apical membrane of the insect hindgut epithelium, where they mediate the uptake of amino acids in the primary urine produced in the Malpighian tubules. For example, glycine, serine, alanine, and threonine are actively resorbed into the cells of the rectal pads of the locust by a Na+ cotransporter of the SLC6 family (430). Proline is also taken up, and is a major respiratory substrate of rectal cells (76).
Returning to mammals, a single proton-oligopeptide transporter, PEPT1 (member of SLC15A family) mediates the uptake of peptides across the apical membrane (Fig. 10). It can transport thousands of di- and tripeptides with low affinity and high capacity, but neither free amino acids nor tetrapeptides (106). This property is intelligible from the structural features of the binding pocket of the protein, which can accommodate compounds with oppositely charged head groups (carboxyl and amino groups) separated by a carbon backbone of 0.55 to 0.63 nm (compatible with di-/tripeptides) and a capacity to accommodate a great variety of size and charge in the side groups (125). The acid load of the enterocyte imposed by H+ influx associated with PEPT1-mediated peptide/H+ symport is relieved by Na+/H+ exchange at the apical membrane (170). (Early reports that peptide transport is Na+-linked are erroneous.) Neutral and most cationic peptides are cotransported with one proton, while anionic peptides require two protons (228). Peptides taken up into the enterocyte are hydrolyzed by a diversity of cytoplasmic peptidases (Fig. 10), and the resultant amino acids are exported via transporters on the basolateral membrane (Table 3).
Figure 10.
Peptide absorption. Uptake of di- and tripeptides across the apical membrane of enterocytes is mediated by PEPT1/H+ symport, with the H+ transport coupled to the Na+/H+ antiporter NHE3. The peptides are hydrolyzed by multiple cytosolic hydrolases, and the resultant amino acids are exported via the basolateral membrane by multiple transporters (see Table 3). The efflux of unhydrolyzed peptides across the basolateral membrane is mediated by peptide transporters that have not been identified at molecular level.
Low-affinity/high-capacity peptide transporters expressed in the alimentary tract have been characterized functionally in nonmammalian vertebrates, notably the chicken (184), zebrafish (454), and other fish (455), and in Caenorhabditis elegans (317) and Drosophila (382). The peptide transporter family to which the mammalian PEPT1 protein belongs is ancient, with the defining peptide transporter motif (PTR) motif evident in proteins of bacteria, fungi, plants, and animals (107). Analysis of basal animal groups is required to establish the evolutionary origin(s) of gut-borne peptide transporter(s) in metazoans.
Of central importance is the relative importance of peptide and amino acid uptake in the protein nutrition of the animal. Humans with mutational defects in amino acid uptake systems do not suffer from essential amino acid deficiencies, for example, abolition of cystine uptake caused by defect in b0,+ system (condition known as cystinuria), and aromatic amino acid uptake by defect in B0 system (Hartnup disease); and this suggests that PEPT1-mediated uptake of peptides can be substantial, sufficient to meet the dietary requirements for these essential amino acids (106). The significance of PEPT1 for the protein nutrition of other animals remains to be established.
Transcellular pathways for lipid absorption
In vertebrates, the absorption of lipid hydrolysis products and sterols is dependent on their incorporation into micelles formed in the lumen of the small intestine. Micelles are 4 to 8 nm diameter aggregations of the hydrophobic lipid products with bile acids, which act as amphipathic detergents and mediate the passage of the lipid products across the aqueous boundary layer to the apical membrane of intestinal enterocytes. A proportion of the micelle-associated molecules pass across the apical membrane by simple diffusion, according to the concentration and permeability coefficient of each compound, but carrier-mediated transport is also involved.
The dominant lipids in most diets are triacylglycerols (TAGs), accompanied by small amounts of various polar and nonpolar lipids, including phospholipids, sterols, and the fat-soluble vitamins A and E. The products of lipid digestion include free FAs, glycerol, monoglycerides, and lysophospholipids. Following uptake by diffusion and via transporters, these products are transported to the endoplasmic reticulum, where they are used to synthesize diacylglycerols (DAGs), TAGs, phospholipids, cholesterol esters, etc. They are then packaged with lipoproteins to form chylomicrons, which are passed through the Golgi apparatus for exocytosis. In mammals, the chylomicrons are delivered to the lymphatic vessels. The mechanism of chylomicron assembly is reviewed by reference (227).
Of particular note are the transporters mediating sterol flux across the apical membrane of enterocytes. In mammals, a steep diffusion gradient across the apical membrane is generated by acyl-CoA:cholesterol acyltransferase (ACAT2)-mediated esterification of cholesterol in the enterocyte (Fig. 11), and it used to be assumed—erroneously—that cholesterol is taken up exclusively by simple diffusion. There is now overwhelming physiological and molecular evidence for carrier-mediated uptake and also efflux across the apical membrane (Fig. 11). The key transporter mediating cholesterol uptake is Niemann Pick C1-like 1 (NPC1L1) protein, identified initially as the transporter sensitive to ezetimibe, a highly specific and potent inhibitor of intestinal cholesterol absorption (6, 111, 234). However, overexpression of NPC1L1 in nonenterocyte cells has not yielded cholesterol transport activity, suggesting that additional proteins may be required to reconstitute a fully functional cholesterol transporter. NPC1L1 has 50% amino acid homology to the NPC1 protein, which functions in intracellular cholesterol trafficking and is defective in the Niemann Pick type C cholesterol storage disease (70). Importantly, cholesterol is also exported across the apical membrane, via the ATP-binding cassette (ABC) transporters ABCG5 and ABCG8 (24). ABC transporters generally have 12 transmembrane domains, but each of ABCG5 and ABCG8 has just six transmembrane domains; transport activity is mediated by the heterodimer, comprising a 12-transmembrane protein complex (194). Cholesterol molecules that are not esterified in the endoplasmic reticulum are eliminated from the enterocyte to the intestinal lumen and voided via the feces.
Figure 11.
Absorption of cholesterol in mammalian intestine. Cholesterol presented in micelles to the apical membranes of enterocytes is taken up by Niemann-Pick C1-like-1 (NPC1L1) transporter, and esterified by acyl-CoA:cholesterol acyltransferase (ACAT2), an enzyme in the endoplasmic reticulum membrane. These esterified products are incorporated into apolipoprotein (apo)B48-containing chylomicrons in a microsomal triglyceride transport protein-dependent manner. After further processing, the chylomicrons are released from the basolateral membrane by exocytosis. Nonesterified sterol is eliminated into the gut lumen via ATP-binding cassette (ABC) transporters ABCG5 and ABCG8.
Nevertheless, ABCG5/G8 does not function exclusively in relation to cholesterol. Mammals feeding on fungal or plant material need to process the dominant sterols in these foods: ergosterol and phytosterols, respectively. These sterols have the tetracyclic ring structure and side chain at C17, as in cholesterol, but the side chain in phytosterols is alkylated at C-24 (e.g., with ethyl substituent in sitosterol), and some phytosterols (e.g., stigmasterol) also have double bonds in the side chain. They are taken up by NCP1L1 into enterocytes, but they are not esterified by ACAT2 and are eliminated via ABCG5/G8. Wang (2007) has described ABCG5/G8 as “the gatekeeper to avoid high plant sterols in plasma." This role is illustrated vividly by patients with mutations in ABCG5/G8, resulting in elevated absorption and plasma levels of sitosterol, a condition known as sitosterolemia. In healthy individuals, dietary phytosterols reduce serum cholesterol levels, probably through their more efficient incorporation than cholesterol into micelles, resulting in reduced cholesterol uptake (223); this is why sitosterol is sold as a functional food. A dietary supply of cholesterol is not required by mammals, which can synthesize sterols de novo.
Among invertebrates, most research on lipid absorption has concerned insects. The products of insect lipid digestion are absorbed principally across the midgut epithelium, although absorption in the foregut, e.g. the crop of the cockroach Periplaneta americana, can also occur (63, 447). Lipid absorption in insects differs from vertebrates in several important respects. (i) Although, as in vertebrates, the products of lipid hydrolysis are packaged into micelles, the amphipathic molecules of insect micelles are fatty acid-amino acid, lysophospholipid, and glycolipid complexes (442), and not bile acids (which insects lack). (ii) The lipids synthesized in all insect enterocytes studied to date are dominated by DAGs, not TAGs; and sterols appear to be absorbed without esterification in the enterocyte (442). (iii) The functional equivalent to chylomicrons in insects is the high-density lipoprotein, lipophorin, which mediates the transport of DAGs exported from enterocytes (9). Unlike chylomicrons, lipophorin is not synthesized in enterocytes; it is localized in the hemolymph (blood), where it acts as a shuttle delivering lipids to the fat body and other organs. Lipophorin has been implicated in the transport of hydrocarbons, carotenoids, sterols, and phosopholipids, as well as DAGs. (iv) The role of transporters in the absorption of lipidic compounds in insects is poorly studied, although a NPC-like transporter, NPC1b, has been demonstrated to mediate sterol uptake from the midgut of Drosophila (456), and a fatty acid transporter on the apical membrane has been invoked (63).
The products of lipid digestion in the gut of the spider Polybetes phythagoricus are taken up by cells of the midgut diverticulum, where they are processed to TAGs and phospholipids and exported via two distinct carriers: a high-density lipoprotein (equivalent to the insect lipophorin) and a very high density lipoprotein that also contains hemocyanin (275).
Pathways for absorption of short chain fatty acids
This class of lipid-related molecules is distinctive from other lipids in two important respects. First, they have lower hydrophobicity than long-chain fatty acids. Consequently, SCFAs permeate membranes more slowly by simple diffusion, and cellular transport mechanisms are especially important for SCFA absorption. Second, they are waste products of fermentative respiration of resident bacteria in nongastric, anoxic regions of the alimentary tract (not products of animal digestion), with the implication that they are produced and absorbed across the hindgut (and pregastric fermentation chambers of some animals, see Section “Basic designs of digestive tracts”), not midgut, small intestine etc. For example, in humans, acetate, propionate, and butyrate are produced in the ratio 3:1:1; and contribute up to 10% of respiratory fuel; butyrate is particularly important, as the primary carbon source for colonocytes (156). Topics not considered here are the role of SCFAs in the regulation of fluid and electrolyte movement of the vertebrate gut, reviewed by reference (32), and importance of butyrate in the regulation of colonic cell proliferation and differentiation [see review of reference (198)].
SCFAs are transported across the colon wall of mammals by a combination of simple diffusion and carrier-mediated processes. The SCFA transporter(s) have yet to be identified definitively. Studies with colonic epithelial tissue and luminal perfusion experiments point to SCFA/HCO3− exchangers, with evidence for saturation kinetics and competitive inhibition by acetate, butyrate, and propionate, but not lactate (203, 204, 312, 378). However, the transport proteins responsible for SCFA/HCO3− exchange have yet to be identified, raising the possibility that SCFA is coupled to HCO3− via multiple transporters, for example, SCFA/H+ cotransport and Cl−/HCO3− exchange (99). SCFAs are transported by the H+/monocarboxylate transporter MCT1 in several colonic cancer cell lines, including Caco-2 cells, (282) and by a Na+-dependent SCFA transporter, SLCA8, cloned from the human intestine (324), but the relevance of these transporters to SCFA transport in the colon and cecum of healthy mammals in vivo is uncertain.
The fate of SCFAs in the gut epithelium has been studied particularly in the rumen. A proportion of the SCFAs taken up is metabolized to lactate and ketonic acids (including acetoacetate and 3-hydroxybutyrate); these products are transported from the basolateral membrane of epithelial cells, probably via MCT1, to the blood. The intraepithelial metabolism of SCFAs contributes to the high-energy demands of these cells. Additional advantages are the maintenance of the concentration gradient between the lumen of the rumen and epithelial cell contents, so promoting sustained SCFA uptake, and the greater solubility of the products (lactate etc.) than SCFAs, and therefore, facilitating transport in the blood to other organs.
Paracellular transport of organic molecules
Paracellular transport across the gut is constrained by tight junctions at the apical end of the lateral membrane of all cells in the epithelium. Tight junctions have selective permeability, discriminating among solutes by charge and size. Two pathways across the tight junction have been identified in various epithelial cell types, including gut epithelia: a high-capacity pore pathway, permeable to small uncharged molecules and ions (<0.8 nm diam.); and a leak pathway mediating low capacity flux of larger, uncharged molecules. Caco-2 cells display a third pathway that allows the passage of molecules up to 0.13 nm diameter, suggesting an additional route in the mammalian gut intestine (448). Although the contribution of the various tight junction proteins to the restriction of movement between epithelial cells is not fully understood, there is growing evidence that: (i) the claudins (a family of membrane proteins spanning the tight junction) play a crucial role in the pore pathway, with individual family members forming cation- or anion-selective pores; (ii) two further tight junction proteins, occludin and zona occludens-1, are important in the leak pathway; and (iii) various intracellular and extracellular signals mediate cross-talk between the two pathways, resulting in dynamic regulation of flux of different classes of compounds by the paracellular route. For an excellent review on the molecular determinants of the function and plasticity of tight junctions, the reader is referred to (398).
For humans and biomedical rodent models, the paracellular pathway makes a negligible contribution to absorption of many solutes. Despite the growing evidence for dynamic selective permeability of tight junctions, the predominance of transcellular transport has been attributed to the superior selectivity of transcellular transport via carrier-mediated transporters on the apical membrane of enterocytes, thereby protecting the animal from many toxins or otherwise deleterious compounds breaching the gut wall.
Nevertheless, there is substantial evidence for extensive paracellular transport of solutes in flying birds and fruit bats. Particular insight into the mode of sugar transport comes from parallel analysis of absorption of L-glucose (the stereoisomer that does not interact with the glucose transporters and is transported exclusively by paracellular route), and D-glucose or 3-O-methyl-d-glucose (3OMD-glucose), a nonmetabolizable analogue of D-glucose that can be transported into cells. Karasov and colleagues measured total absorption (mediated and passive) of D-glucose or 3OMD-glucose and passive absorption of L-glucose in intact animals by a standard pharmacokinetic methodology, for example, references (78, 244, 278, 280). In experiments conducted on avian species, the fractional absorption of D-glucose and 3OMD-glucose did not differ significantly; and L-glucose was found to account for the majority (range 50 to > 90%) of glucose absorption (79, 238, 316) (Fig. 12). In analogous studies in rats (443), dogs (277), and humans (154) L-glucose, and hence passive absorption, is quantitatively much less important, confirming the likely phylogenetic difference between birds and mammals in the importance of paracellular transport.
Figure 12.
Paracellular absorption of glucose in the American robin (Turdus migratorius) investigated by pharmacokinetic methodology, using D-glucose, L-glucose (the glucose stereoisomer that is not be transported across the intestinal membrane), and 3-O-methyl-d-glucose (3OMD-glucose, a nonmetabolizable but actively transported analogue of D-glucose). (A) The dose-corrected plasma concentration of [3H]L-glucose as a function of time since American robins were injected (unfilled symbols) or gavaged (filled symbols) with the probe solution containing L-glucose. The areas under the curves (AUCs) are used to calculate fractional absorption, f, which averaged 87 ± 3%. (B) Time course of absorption of [3H]L-glucose, and [14C]D-glucose and 3OMD-glucose. Over early time points, the amounts of L-glucose absorbed was 50% to 70% of the amounts of D-glucose absorbed, which was interpreted to mean that the majority of glucose was absorbed by the paracellular pathway. Adapted from Figures 1 and 2 from reference (316), with permission.
Intestinal paracellular absorption in nonflying mammals and birds appears to be qualitatively similar in regards to molecular size selectivity, as characterized using a series of nonelectrolyte water-soluble probes that differ in molecular dimension (80, 199) and in charge selectivity as characterized using relatively inert charged peptides (81, 205). Quantitatively, paracellular absorption is at least twice greater in small birds (< 400g) than in nonflying mammals (Fig. 13A), with the difference declining with increasing body size (278).
Figure 13.
(A) Fractional absorption of water soluble carbohydrates by intact birds (triangles, solid line) and nonflying eutherian mammals (circles, dashed line). Arabinose, rhamnose, cellobiose, and lactulose are inert, nonactively transported compounds whereas 3-O-methyl-d-glucose is not metabolized but is transported actively as well as passively absorbed. Fractional absorption of the passively absorbed probes declined with increasing molecule size and differed significantly between the two taxa, although the difference diminished with increasing molecule size. In contrast, absorption of 3-Omethyl-d-glucose did not differ significantly between the taxa. The interpretation is that species in both groups absorb most glucose, but that birds relied more on the passive, paracellular route. Figure 4A adapted, with permission, from reference (243). (B) Small intestine nominal (smoothbore tube) surface area in omnivorous birds and mammals (same symbols and lines as in A). There was no significant difference in slope between birds and nonflying mammals (n = 46 species and 41 species in birds and mammals, respectively). When the lines were fit to the common slope of 0.73, the calculated proportionality coefficients (intercept at unity) were significantly lower for birds than for mammals. Hence, small intestine nominal surface area in birds is 36% lower than that in nonflying mammals. Figure 4B adapted from reference (75).
The difference in paracellular absorption between birds and nonflying mammals is not simply explained by mediated absorption in birds of the carbohydrate probes that are presumed to be absorbed passively. In studies using radiolabeled L-glucose and L-arabinose, their uptake by intestine in vitro was not significantly inhibited by high concentrations (50–100 mmol/L) of unlabeled L-glucose, L-arabinose, L-rhamnose, or D-glucose (280), which makes it unlikely that their absorption is carrier mediated. Nor is the difference in paracellular absorption between birds and nonflying mammals explained by longer retention of digesta in the gut of the former relative to the latter. Avian species typically have shorter mean retention time of digesta than do similar sized nonflying mammalian species (315). Because birds typically achieve higher paracellular absorption with less intestinal length and surface area than do similar sized nonflying mammals, there apparently are differences in intestinal permeability per unit intestinal tissue. This was confirmed in a comparison of pigeons and laboratory rats. Under similar recirculating duodenal perfusion conditions, anesthetized rats, and pigeons absorbed D-glucose at a comparable rate but pigeons had significantly greater (>2× higher) absorption of inert carbohydrate probes (280). The difference in paracellular solute absorption between mammals and birds cannot be linked to differences in solvent drag because it is so difficult (155) to distinguish between water absorbed by the paracellular route versus aquaporins, which occur in intestine of both mammals and birds (229).
Enhanced paracellular absorption may have evolved as a compensation for smaller intestinal size in birds compared with nonflying mammals (Fig. 13B). In a phylogenetically informed allometric analysis, flying birds had shorter intestines and about 36% less nominal small intestine surface area (area of a smooth bore tube) as compared with nonflying mammals (279). Small intestine volume, a direct function of tube length and area, and consequently the potential mass of digesta carried, was relatively smaller in birds, by 32%. The difference in intestinal surface area between birds and nonflying mammals did not depend on diet in the analysis. (Diet did have a significant effect on gut size, but the effect was on cecal and large intestine size.) Another advantage of paracellular absorption is that it is an energetically cheap way to match absorption rate to substrate concentration in the diet and lumen.
If there has indeed been natural selection for smaller intestinal size in fliers, and increased paracellular absorption as a compensation, then one might expect to find the same patterns found in flying birds versus nonflying mammals in a comparison within mammals between fliers (i.e., bats) and nonfliers. Preliminary evidence suggests that this is the case (75), but more extensive sampling is necessary.
Dietary and phylogenetic correlates of transporter activity
Generally, in vertebrates, the more carnivorous the species, the lower its rate of intestinal mediated glucose absorption (246). This pattern, first described in a survey of more than 40 species drawn from the major vertebrate classes (245), is apparent also in comparative studies within fish (51) and birds (247). Based on phlorizin-binding studies in a limited number of species, it appeared that species differences in tissue-specific glucose uptake may largely reflect species differences in the number of copies of the main apical membrane glucose transporter SGLT1, although it is possible that differences in turnover time of the transporter can also contribute (150).
There was no marked pattern of higher intestinal transport activity for amino acids among the more carnivorous vertebrate species (245, 246). Likewise for digestive enzymes, it seems typical to find significant positive relationships between carbohydrases and dietary carbohydrate but not between proteases/peptidases and dietary protein, at least for fish (179), and in birds (261). This is perhaps expected because all animals, regardless of diet, need protein and so there should not be strong selection for very low protein processing capability in animals. In addition, it has been argued (214) that it would be advantageous for herbivores with relatively rapid gut throughput to have compensatorily higher biochemical capacity to process proteins and recover them rather than excrete them.
Matches of GI System Biochemistry (Enzymes and Transporters) to Changes in Diet Composition
General principles
There is overwhelming evidence that the digestive and absorptive function of the GI tract of animals can vary with diet composition. This flexibility is exhibited at two levels: anatomical, including the overall size and architecture of the GI tract (Section “Models help in understanding the diversity of digestive systems and guide mechanistic, integrative research”); and biochemical, especially the activity of digestive enzymes and transporters. The biochemical flexibility is generally considered to maximize the acquisition of carbon for energy production and essential nutrients for maintenance and growth, while protecting against the acquisition of excessive, potentially toxic, amounts of certain dietary constituents (e.g., iron). Any nutritional imbalance that might arise from this strategy is widely considered to be corrected postabsorption, so that the retention and use of certain nutrients are optimized, while surplus metabolites can be eliminated (249, 416). In this section, the relationship between diet composition and digestive enzyme activity is addressed first, followed by consideration of transporters in the GI tract.
Flexible adjustment of digestive enzymes to diet change
Many studies on vertebrates have demonstrated that the production of digestive enzymes increases with availability of substrate in the gut lumen. For example, this effect has been confirmed in rodents for all of the major pancreatic enzymes (amylase, lipase, and proteases) and enzymes of the intestinal brush border (sucrase-isomaltase, maltase-glucoamylase, and aminopeptidase-N) (246). Other data relate to a variety of mammals, birds, reptiles and fish, as well as a number of invertebrates [reviewed in reference (249)]. This mode of regulation both maximizes the digestibility of substrates and minimizes the cost of synthesizing excess enzyme when the substrate is at low levels. The mechanistic basis of the impact of diet on digestive enzyme activity has not been investigated in most species but, where studied, there is persuasive evidence that differential enzyme activity is underpinned by changes in gene expression. For example, the elevated expression of intestinal sucrase-isomaltase gene in the intestine of rats and mice fed on high-carbohydrate diets is controlled by the transcription factors Cdx-2 and HNF-1 (36); and the recruitment of these transcription factors to the promoter region is correlated with the acetylation of histones H3 and H4 associated with this gene (215). In Drosophila, the activity of amylase in the midgut is significantly higher in larvae feeding on starch diets than sugar diets, and the 5’ cis-regulatory region that regulates gene expression of the amylase genes has been identified (226).
Adaptive variation in digestive enzyme activity with diet composition is crucial to the lifestyle of many animals. For example, female Aedes aegypti mosquitoes feed on both sugar-rich nectar and protein-rich vertebrate blood. The gut protease activity is undetectable in individuals feeding on a sugar meal but, within hours of taking a bloodmeal, the digestive protease activity in the midgut increases rapidly, reaching a maximum after about 2 days. A. aegypti has three trypsin genes expressed in the midgut. The synthesis of two trypsins, known as the late trypsins, is regulated by dietary protein content. Initial production (within 3 h of feeding) is from a preformed mRNA, in response to protein in the blood; and subsequent production (8–10 h after feeding) comes from de novo trypsin gene expression, induced by amino acid products of trypsin-mediated digestion of blood proteins (146). The other midgut trypsin, called early trypsin, is synthesized constitutively.
Nevertheless, some studies have found that the secretion of digestive enzymes does not vary in a simple fashion with substrate concentration. For some insects feeding on a nutritionally unbalanced diet, such that one dietary component is in excess, the enzymes mediating the degradation of that dietary component can be downregulated. For example, locusts Locusta migratoria feeding on diets with excess protein or carbohydrate display reduced activity of digestive α-chymotrypsin and α-amylase, respectively (93) (Fig. 14). These data suggest that an insect has the capacity to regulate digestive enzymes homeostatically, such that enzymes yielding nutrients in excess are secreted at lower rates than enzymes that generate nutrients in deficit. The production of some digestive enzymes appears to be regulated by integrated sensing of both the nutrients available in the gut and the nutritional requirements of the animal. This complexity may not be revealed in the nutritionally sufficient diets that are commonly used for laboratory maintenance of animals, but could be important for animals in the field with access diets of variable and often suboptimal composition.
Figure 14.
The activity of α-chymotrypsin and α-amylase in the gastrointestinal tract of the locust L. migratoria fed on diets of different composition: PC (21% protein:21% carbohydrate), pc (10.5% protein: 10.5% carbohydrate), Pc (35% protein: 7% carbohydrate), and pC (7% protein: 35% carbohydrate). The enzyme activities were downregulated in insects on diets containing an excess of the substrate. [Data from Fig. 1 C and D of Clissold et al. (93).]
Flexible adjustments of transporters to diet change
Current understanding of the matching of transporter function to diet composition derives largely from the classic work of Diamond and colleagues (120, 149) conducted on isolated intestine preparations of mice. The transport of nutrients that are metabolized for energy production increase with increasing dietary supply, while those mediating the uptake of essential but non energy-yielding nutrients tend to decrease with increasing dietary supply. Thus, transporter activity for sugars (e.g., glucose and fructose) and nonessential and essential amino acids and peptides increase with their content in the diet, but transport of most vitamins and minerals decrease with dietary content. Interestingly, the uptake of dietary essential amino acids, such as histidine, lysine, leucine, and methionine, tends to increase slightly at low dietary levels (the reverse of the response to nonessential amino acids), indicating the central role of dietary essential amino acids for protein synthesis and use of nonessential amino acids as a respiratory substrate. How this differential response to essential versus nonessential amino acids is achieved despite the overlapping substrate specificities of the various amino acid transporters (Table 3) is not fully understood.
Kinetic analyses of nutrient uptake indicate that the diet-dependent variation in sugar and amino acids transporter activity is mediated predominantly by changes in the density of transporters on the apical membrane (149). Two processes can mediate increased transporter function: recruitment of preexisting transporter protein in the cytoplasm to the membrane (as occurs for GLUT2 in response to dietary glucose, see Section “Absorption of carbohydrates”), and elevated gene expression. Most research has focused on expression response to dietary nutrients. For example, in response to high dietary supply of sugars, the expression of genes encoding the transporters SGLT1 (for glucose) and GLUT5 (for fructose) is increased. GLUT5 expression is elevated in isolated rat intestine preparations perfused with fructose (425); horses fed on diets with high levels of digestible carbohydrate display elevated expression of SGLT1 in both the duodenum and ileum (133); and piglets raised on isoenergetic diets with different concentrations of digestible carbohydrate exhibit elevated expression of SGLT1 when fed on diets with more than 50% digestible carbohydrate (330) (Fig. 15).
Figure 15.
The effect of dietary soluble carbohydrate on the transcript abundance of the glucose transporter gene SGLT1 in (A) the mid-intestine of 28-day-old piglets and (B) the duodenum of horses fed sequentially on different diets including hay (essentially starch-free) and grain (containing 0.3% starch). [Data from Fig. 1A of reference (330) and Fig. 3A of reference (133).]
The activity of the Pept-1 peptide transporter in the intestine is elevated by high dietary protein. In the rat intestine, the Pept-1 mRNA is elevated twofold in the intestine of rats fed on high-protein diet (50% protein), relative to low-protein diet (4%), and this effect of high dietary protein can be replicated by a dietary supplement of a single dipeptide Gly-Phe (142, 400).
The expression of various transporter genes is regulated in anticipation of food. Adult rats exhibit diurnal variation in expression of sugar transporters in the intestine, with induction of GLUT2 (glucose transporter), GLUT5 (fructose transporter), and Pept-1 expression 3 to 4 h before the onset of peak feeding by the animal (100, 371, 402). Diurnal variation of GLUT2 and Pept-1 is regulated by the vagus nerve, and GLUT5 by paracrine and endocrine signals in the intestine (371, 427). In addition, preexisting pools of transporter proteins, probably localized in the cytosol, are likely localized to the membrane; this can achieve more rapid changes in transporter activity than changes in gene expression.
The central role of transporters in the modulation of absorption with diet raises important questions about the capacity of an animal to regulate uptake of nutrients with significant levels of passive absorption. For these nutrients, uptake is predicted to increase monotonically with concentration in the gut lumen. The uptake of the vitamins pantothenic acid, ascorbic acid, and choline conforms to this expectation. Absorption of these vitamins is predominantly passive and, unlike other essential nutrients, it is not upregulated in response to low dietary supply (418). Nutrients that are taken up by the paracellular route are also predicted not to be tightly regulated. This effect is important, for example, for the uptake of various solutes by passerine birds, for which paracellular absorption is significant (Section “Paracellular transport of organic molecules”).
Dietary and Phylogenetic Correlates of Biochemical Flexibility
The biochemical flexibility of the GI tract in a given animal is the product of its evolutionary history, with taxa that have diets of variable composition predicted to display greater phenotypic flexibility than those with relatively uniform diets. This issue has been explored particularly in relation to variation in the capacity of animal species with different diets to modulate their transporter activity. For example, glucose transporter function in vertebrates tends to be higher and more flexible to diet in herbivores and omnivores than in carnivores (246). These differences reflect evolutionary adaption to diet, with a lower and more uniform carbohydrate: protein content in the diet of carnivores than omnivores and herbivores.
Two specific comparisons illustrate the relationship between diet and the phenotypic flexibility in the biochemistry of nutrient acquisition in the GI tract. The first comparison relates to sugar transport in domestic dogs and cats. Domestic dogs and other canids are opportunistic carnivores (carnoomnivores) that utilize a varied diet, occasionally including vegetable material; and felids, including the domestic cat, are specialized carnivores adapted to a high-protein/fat diet containing very little carbohydrate. The glucose transporter SGLT1 is expressed in the intestine of both the domestic dog and cat, but its expression level is twofold greater and is more responsive to dietary carbohydrate in the dog than the cat (18, 52). Despite the poor capacity of the domestic cat to utilize diets with significant levels of carbohydrate, many commercial cat diets contain relatively high levels of carbohydrate. For cats maintained on these diets, it is likely that rodents, small birds, etc. are an absolute dietary requirement (135, 211).
The second example of interspecies differences in nutritional flexibility concerns two passerine birds, the house sparrow P. domesticus, which can use a range of diets including protein-rich insects and starchy seeds, and the zebra finch, Teniopygia gutta, which has a relatively fixed diet dominated by seeds. In the field, the initial diet of nestling house sparrows is dominated by insects, but switches subsequently to seeds. In nestling sparrows fed on a diet containing starch, the gut maltase activity of the birds increased by more than twofold (Fig. 16 A), the effect was specific because aminopeptidase-N activity was unaltered (Fig. 16B) (43), and this effect could be reversed by transfer back to starch-free diet (44). Furthermore, this effect was correlated with changes in transcript abundance of the maltase gene, indicating the central role of gene expression in regulating digestive function (242, 243). In contrast to the house sparrow, the intestinal maltase activity of zebra finch was not responsive to variation in dietary starch content (45). As the comparison of house sparrow and zebra finch illustrates, interspecific difference in dietary flexibility is underpinned by a parallel difference in biochemical and genetic flexibility.
Figure 16.
Diet-induced changes in the activity of digestive enzymes in 12-day-old nestling house sparrows (2–3 days before fledging). The birds were hand-fed on either 0-starch diet (mimicking insect food), comprising 20% corn oil and 59.63% casein; or +starch containing 25.4% corn starch, 8% corn oil, and 46.23% casein designed to mimic a mixture of insects and plant (seed) material. (A) Maltase activity. (B) Amino-peptidase N activity [Data from Fig. 5 of reference (43).]
Matches of GI System to Nutritional Changes during Ontogeny
Major changes in GI enzymes and transporters occur during development in many animals. In some groups such as ruminant mammals, insects, amphibians, and fish, these are also accompanied also by dramatic changes in GI structure. The reviews by Buddington and colleagues in the early 1990s (49, 50, 54) summarized results for about 12 vertebrate species, and additional work in the past 15 years has resulted in many more studies of developmental changes in digestion and features of digestive physiology, as well as an expanded list of species including more than a dozen fish species (see below), six amphibian species, a turtle (35), five avian species, and a dozen mammals. The latter class has been most intensively studied, and reviews of work in that group (148, 208, 354, 461) provide some major themes that apply as well to other groups.
Patterns in mammals
Henning (208) provides a good overview of GI development in mammals, especially in the laboratory rat, the most studied of about a dozen mammalian species that have been surveyed to date (3, 17, 49, 56, 57, 65, 134, 196, 219, 238, 263, 294, 323, 347, 362, 390, 394, 397, 433, 471, 483, 489, 490, 492). During the gestational phase, organs undergo morphological maturation [see also reference (354)] and many proteins required for digestion and absorption of components of milk are expressed (e.g., amino acid transporters and the glucose transporter SLGT1). The second major phase of changes occurs at the onset of weaning (day 15 in the rat), when the GI tract acquires proteins required for digestion and absorption of solid food that contains substrates not contained in milk, such as fructose and starch. Until weaning, the stomach of the neonate is not acidic and substantial amounts of gastric and pancreatic proteases are not expressed. Accordingly, the small intestine has a high capacity for pinocytotic absorption of intact protein and intracellular breakdown by lysosomal proteinases. The pinocytotic uptake capacity declines at weaning, although molecular details of this have not been elucidated. Large changes occur in proteins important in processing of carbohydrate, which is the diet component that changes most dramatically (e.g., from lactose to sucrose and starch). Activity increases markedly for sucrase-isomaltase, maltase-glucoamylase, trehalase, and GLUT-5, the fructose transporter, in most cases accompanied by increases in the expression of their genes. Activity of lactase-phlorizin hydrolase declines at this time, associated with changes in transcription, translation, and protein turnover (see discussion about lactase, above). If a young mammal is allowed to prolong suckling, or is fed on a lactose-containing diet after weaning, the onset of the decline in lactase is delayed, but only slightly. Many of these patterns are apparent in at least a dozen other species of mammals that have been studied, although in species such as carnivorous marine mammals and ruminants sucrase activity remains low (246), and in ruminants dramatic changes occur in GI tract structure postnatally [i.e., development of multichambered foregut (257, 258)] coordinated with changes in gene expression (97). Also, in some species (e.g., pigs and humans) the patterns of postnatal enzyme development occur earlier than in the rat (246). Studies using rat, mouse, and human fetal intestine grafted into adult hosts, or using altered diets, have shown that many of these changes occur in the absence of specific ontogenetic signals from either the lumen or circulation. In addition to this intrinsic timing, circulating levels of hormones such as glucocorticoid and epidermal growth factor are involved in maturation and growth.
An interesting illustration of some of the variability in patterns of development comes from a comparison of patterns for two major sugar transporters, SGLT1 and GLUT5 (148). In rats, SGLT1 (primary D-glucose transporter) is expressed before birth whereas GLUT5 (fructose transporter) is first expressed only during or after weaning. The increased fructose transport activity coincides with increased abundance of mRNA and GLUT5 protein. This is not necessarily the case for increased glucose-transport activity, which may occur without a coinciding large increase in SGLT1 mRNA in rats and in lamb intestine [though see reference (294)]. The large ontogenetic increases in glucose and fructose transport in rats can occur in the absence of any dietary signal (and are thus sometimes called “hard wired”), but early introduction of fructose during weaning in rats will induce earlier expression of GLUT5 mRNA, protein, and fructose transport. In neonate rats, luminal or dietary carbohydrate does not induce glucose transport (which is contrary to the situation in adult rats), whereas in lambs dietary glucose is required for induction of glucose transport activity. A final notable difference is that luminal fructose is specifically required for induction of GLUT5, whereas glucose transport activity can be induced with glucose and a number of other sugars and even nonmetabolizable sugar analogues.
As growth continues after weaning, tissue-specific intestinal enzyme activities and transport rates tend to be relatively constant or decrease, but total capacity increases due to the increase in intestinal mass (50, 53, 55, 56, 347, 354, 370, 435, 490). Studies in cats and rats yielded some evidence for particular changes in transporter-specific activity or intestinal mass coinciding with whole-organism growth rate peaks (53, 435). But, for the most part, growth of the intestine matches the mammal’s increase in body mass or metabolic mass (body mass3/4) and the growing animal maintains a digestive and absorptive capacity that matches or slightly exceeds the demands set by increases in food intake.
Patterns in birds
Developmental changes in GI function during the pre- and postnatal periods also occur in birds, as chicks accommodate the transition from a lipid-rich yolk diet inside the egg to a carbohydrate- and protein-based diet post hatch. At least six avian species have been studied: chicken [citations below, plus references (83, 163, 185, 332)], jungle fowl and duck (citations below), turkey (114, 144, 160, 270, 396, 449), and house sparrow [below, plus reference (42)], and zebra finch (45). Some species (e.g., poultry and ducks) are precocial in development, possessing advanced locomotory, and thermoregulatory features at hatch compared with other species that are altricial (e.g., perching or passerine birds). Based on expression profiling and measures of activity, species in both groups have at hatch the full suite of enzymatic, pancreatic, and intestinal activities to digest fat, carbohydrate, and protein [e.g., references (74, 184, 186, 292, 407, 480)].
Sklan and colleagues (404–406, 445, 446) and Planas and colleagues (16, 413) have studied the molecular basis for ontogenetic changes in carbohydrate digestion and absorption in chickens during the week before and after hatching. The sucrase-isomaltase (SI) gene was expressed 6 days before hatch, but expression of SGLT1 mRNA was not detected until 2 days before hatch (Fig. 17 A and B). Increases in both SI activity and glucose transport occurred 2 days before hatch and at hatch day. The cdxA protein, which was shown in an electrophoretic mobility shift assay to bind to the promoter region of SI in chicks (as it does in mammals—see Section “Flexible adjustment of digestive enzymes to diet change”), also rose during these few days prehatch (Fig. 17 C), suggesting that it may have initiated transcription of SI (405). [SGLT1 expression has not been found to be influenced by cdx in mammals (405)]. Posthatch changes in SI activity also seemed correlated with changes in SI mRNA, suggesting that SI expression is transcriptionally controlled (446). Some regulation of glucose transport activity by posttranscriptional mechanisms is suggested by the fact that transport did not change significantly during the week posthatch (348, 446, 452) whereas SGLT1 mRNA significantly increased (405). Likewise, when hexose transport in jejunal brush border membrane vesicles declined with age in older chicks, the site density of SGLT1 declined in parallel but SGLT1 mRNA did not change significantly (16).
Figure 17.
Ontogenetic changes related to carbohydrate digestion and absorption in chicks. (A) Changes related to glucose absorption: activity was measured in jejunal homogenates prehatch (446), and posthatch in everted jejunal sleeves (348) [see also measures in vesicles (452)]. SGLT1 mRNA from references (405, 446). (B) Changes related to carbohydrate breakdown: sucrase isomaltase activity was measured in jejunal homogenates prehatch (446) and post hatch (445). SI mRNA from reference (405). (C) Changes related to homeobox gene of the caudal family (cdxA): protein and mRNA from reference (405).
In both young chickens and house sparrows, the posthatch increases in maltase activity are controlled by intrinsic regulatory mechanisms, but maltase activity can also be doubled by increased dietary carbohydrate (33, 43), and this is correlated with a doubling in maltase-glucoamylase mRNA transcription in the house sparrows (242).
Large changes occur posthatch in intestine size and digestive capacity as birds grow. For example, in altricial house sparrows digestive biochemistry was dynamic over their 2-week period from hatching to fledging from the nest. Tissue-specific activities of some intestinal enzymes increased by more than 10 times (e.g., sucrase and maltase), and total pancreatic amylase activity increased 100 times between hatch and fledging through a combination of increases in tissue specific activity and pancreas mass (74). In three precocial species [chickens (33, 348), wild jungle fowl (231), ducks (256)] tissue-specific enzyme, and transport rates were constant or declined with age but overall digestive and absorptive capacity increased, along with intestine mass, in direct proportion to metabolic body mass, which was the pattern described for mammals.
Patterns in fish and amphibians
A large number of studies of GI development in at least a dozen fish species have been published in the past decade (59, 67, 96, 104, 187, 191, 200, 213, 224, 225, 240, 260, 264, 269, 273, 281, 327–329, 359, 481, 484, 485) due to their importance in aquaculture, and many studies include newer molecular and gene expression approaches (109, 272). As in birds, a major ontogenetic change in fish is that the source of nutrients and energy necessary to continue larval development changes from the yolk reserves to the ingested food, which is mainly protein and fat in carnivores but higher in carbohydrates in omnivores and herbivores. Initially, a functional gastric region may be absent [e.g., references (335)] and, as described for mammals, pinocytosis and intracellular digestion may function as a major mechanism of nutrient absorption (246, 352, 481) followed later by expression of gastric proton pump and pepsinogen for protein digestion (108). Saele et al. (386) describe developmental changes in expression and activity of lipases in a carnivore, the Atlantic cod (Gadus morhua). The major pancreatic neutral lipase is bile activated lipase, and cod also have a nonfunctional pancreatic lipase related protein, but the expression of only the former increases during development. The activity of neutral lipase did not increase in parallel to gene expression. The mismatch between activity and gene expression measurements was partly explained by a nonspecific analytical method, because the whole body is analyzed (the gut of very small larvae is not isolated) and some fish tissues outside the GI tract could have lipase activity. Also, in cod and some other fish (213) neutral lipase activity in prey (i.e., in digesta) may be considerable. Interestingly, the Atlantic cod genome does not seem to contain colipase (386) that typically is essential for pancreatic lipase activity.
Fish amylases and glucose transporters appear to be molecularly closely related to those in mammals and to have comparable characteristics (165, 269). Maltase activity is found even in the intestine of carnivorous fish such as trout, and apparently can be induced, perhaps permanently, by feeding high dextrin (25%–60%) diet early in life (182). Prickleback fishes, which include species that shift during development from carnivory to herbivory as well as species that remain carnivores, have provided examples of intrinsic vs. dietary induced changes in GI structure and function (51, 177, 178), but the picture is a complicated one in which intrinsic changes, diet, and phylogeny all play a role in determining developmental patterns. For example, even when maintained on a carnivore type diet (55% protein, 10% lipid, and <4% carbohydrate), two species that naturally shift diet during development (Cebidichthys violaceus and Xiphister mucosus) increased α-amylase and maltase activity as they grew, which indicates an intrinsic genetic developmental program matched well to their natural diet shift (178). But, one of the congeneric but obligate carnivores (Xiphister atropurpureus) also increased α-amylase without a diet shift, which suggested that phylogeny plays a role. The diet shifter C. violaceus increased mediated glucose transport activity even as it grew but without an accompanying shift to a higher carbohydrate diet (51), providing another example of an apparent genetically programmed developmental change.
An important life-cycle digestive/nutritional change in some amphibians occurs at metamorphosis, when the digestive tract may be restructured and the diet may change (217, 283). Many frogs [e.g., references (436, 470)] shift from primarily herbivory to insectivory/carnivory coincident with a large decrease in length of the gut and the number of gut coils. Some new proteolytic enzymes are produced, such as pancreatic trypsin and stomach pepsin and chitinase(s) (217), which increase the capacity to digest animal matter. Surprisingly, the ratio of intestinal glucose uptake to proline uptake, which is an index for the relative capacity for glucose and proline absorption, did not change between bullfrog tadpoles and adults and was characteristic of vertebrate carnivores (436). There is some digestive plasticity evident during frog development, because the glucose/proline ratio was nearly doubled in bullfrog tadpoles raised on lettuce compared with those raised on beef (437).
Patterns in invertebrates
Shifts during development in feeding versus nonfeeding or in dietary habits occur in diverse invertebrates, including lobsters (235) and insects (301), and digestive enzyme levels may change in correlation with changes in the major dietary substrates. Insects provide some of the best researched examples of developmental changes in digestive biochemistry. Common cutworms (Spodoptera litura; Lepidoptera), a highly polyphagous pest of subtropical and tropical crops, can be used to illustrate a pattern that is probably common (488). As in many insects, chymotrypsin-like SPs are major midgut digestive enzymes. Two have been identified in cutworms, Slctlp 1 and 2, and expression of the latter gene was analyzed in sixth instar larvae following molting from the fifth instar until pupation a week later (Fig. 18). Slctlp 2 was expressed on feeding days and downregulated on nonfeeding days and stages (such as pupa) (Fig. 18A), and the investigators showed using Western blotting that protein changed in parallel. No transcripts were found at the adult stage, perhaps because the adult moths do not feed on protein. Apparent transcription control of SP activity was also demonstrated in the scarabaeid beetle Costelytra zealandica (306). Food appears to act as a proximate signal for expression, based on up-and-down expression in cutworm larvae according to feeding regime (488) (Fig. 18B). It seems reasonable that digestive SPswould be downregulated during nonfeeding stages or during fasting within a stage given the energy required to produce these proteins and to ensure that pupating larva are protected from spurious self digestion (306).
Figure 18.
Expression of serine protease Slctlp2 in common cutworm larvae (S. litura; Lepidoptera). (A) mRNA from midguts of sixth instar larvae at days 0 to 7. Day 0 is the day the larvae just molted. At days 6 and 7 of the sixth larval stadium, the larvae stopped feeding and entered the prepupal stage. Data are transcript abundance normalized to actin transcript. Each bar represents the mean of three independent repeats of the experiment. (B) Induced expression of Slctlp2 mRNA by starvation and refeeding in sixth instar larvae. “F” represents larvae that just molted into the sixth instar and fed for 6, 24, 48, and 72 h post sixth instar molt. “S” represents those starved for 6, 24, 48, and 72 h. “RF” represents larvae starved for half the time period indicated and then fed the latter half of the time period indicated. Each bar represents the mean of three independent repeats of the experiment. Bars (i.e., means) within a discrete time period (i.e., at 6, 24, 48, or 72 h) that share a common letter did not differ significantly, whereas different letters indicate significant differences at P < 0.05. Both figures based on data from reference (488).
Other interesting comparisons are provided by social insects, where the division of labor may include individuals in castes that collect and digestively process plant and animal foods and then feed other material to individuals in the colony. In the wood eating termite Reticulitermes speratus, for example, intrinsic cellulase gene expression is much reduced in reproductives compared with workers (399), and protease levels are much reduced in colony members of ants, wasps, and honeybees that are fed amino-acid-rich excretions of other colony members (159, 218).
Interactions between Naturally Occurring Toxins and Digestive Physiology
Secondary metabolites (SMs) are compounds produced and/or sequestered by plants and animals that do not appear to play a major role in their primary nutritional or regulatory metabolism. Their functions include communication, attraction, or in defense against herbivores, predators, pathogens, and competitors (202). SMs are so pervasive that it is almost a certainty that any thorough analysis of a plant food, and maybe even many animal foods, will identify some SMs. Some are thought to play an important role in human health, variously acting as antioxidants or antimicrobials, modifying hormone titers, and interfering with DNA synthesis. Other SMs directly damage GIT mucosa, such as lectins (451), proanthocyanidins (2), and hydrolysable tannins (251). Protease inhibitors can permeabilize the peritrophic membrane of caterpillars (326). In the following sections, we highlight numerous examples of key digestive processes being influenced by compounds from many of the major groups of SMs (Table 4). But, also, considering the structural and functional diversity of digestive tracts among animals, it should not surprise that impacts of SMs are not necessarily general but depend on digestive features and perhaps even adaptive counterresponses of consumers.
Table 4.
Examples of Impacts of Plant Secondary Metabolites on Digestive Processes
| Process | Compound(s) | Source | Effect | Species | References(s) |
|---|---|---|---|---|---|
| Digesta transit | Ricinoleic acid | Castor plant (Ricinus communis) | Faster digesta transit | Human | (27) |
| Digesta transit | Senna anthraquinone glycosides | Genus Senna | Faster digesta transit | Human | (414) |
| Digesta transit | Trihydroxyanthraquinone (emodin/cascara) | Buckthorn Rhamnus alaternus | Increased time between defecations (slower transit?) | Yellow-vented Bulbul (Pycnonotus xanthopygos) | (440) |
| Digesta transit | Pyridine alkaloids—nicotine, anabasine | Tree Tobacco (Nicotiana glauca) | Faster digesta transit | Palestine Sunbird (Nectarinia osea) | (426) |
| Carbohydrate Hydrolysis | Alkaloids (sugar mimics in latex sap) | Mulberry (Mortis spp.) | Inhibition of sucrase and trehalase | Silkworm S. ricini | (212) |
| Carbohydrate Hydrolysis | 27 kDa protein | Mungbean (Vigna radiata) seeds | Inhibition of α-amylase | Callosobruchus maculates (insect) | (473) |
| Carbohydrate Hydrolysis | Extracts | Morus alba | Inhibition of sucrase, maltase | Humans, rat | (350) |
| Proteolysis | Nonprotein compound | Castor bean (Ricninus communis) | Trypsin inhibition | Insect-coffee leaf miner (L. coffeella) | (383) |
| Proteolysis | Proteinase inhibitor | Soybean | Trypsin inhibition | Pig | (293) |
| Proteolysis | Ovorubin (protein) | Snail (Pomacea canaliculata) egg | Trypsin inhibition | Bovine | (129) |
| Proteolysis | Proteinase inhibitor | N. alata | Chymotrypsin Inhibition | Helicoverpa larvae | (132) |
| Lipolysis | Diterpene carnosic acid | Sage (Salvia officinalis) | Lipase inhibition | Mice | (344) |
| Lipolysis | Phenolics or other extracts | Tea | Inhibition of lipid emulsification | Humans | (320,401) |
| Lipolysis | Phenols, tannin-strictinin | Tea | Inhibition of lipase | Pigs | (192) |
| Lipolysis | Triterpenoid saponin(s) | Acanthopanax senticosus fruit | Inhibition of lipase | Pig | (291) |
| Fiber digestion | Polyphenolics (anthraquinone, proanthocyanidins and other tannins | Inhibition | Ruminants | (271,314) | |
| Fiber digestion | Terpenoids (essential oils, saponins) | Inhibition | Ruminants | (2,357) |
Secondary metabolites and transit time
There is a long history of use by humans of natural products as laxatives (31). Some SMs that alter digesta transit in humans and wild animals are listed in Table 4. An important consequence of rapid digesta transit can be malabsorption, as occurs even for animals with rapid transit time ingesting passively absorbed compounds. For example, digestion time (and glucose absorption) was reduced when sunbirds ingested nectar from tobacco plants that contain particular alkaloids (426). In contrast, the anthraquinone, emodin, which tends to speed digesta through the gut of humans (137), appears to have the opposite effect on the frugivorous bird the Yellow-vented bulblul, and increases the bird’s apparent digestive efficiency on emodin-containing fruit (440). The few examples in Table 4 show how the compounds that influence transit time are chemically heterogeneous, and they also could act through a variety of mechanisms. These might include osmotically based mechanisms, which might draw water into the lumen by acting as introduced osmolytes or by receptor-mediated increase in secretion of ions, or by a nonosmotic mechanism such as direct action on motility patterns via receptor-mediated changes in neuromuscular activity [e.g., reference (27)].
Secondary metabolites and endogenous enzyme activity
Chemicals from many of the major groupings of SMs (e.g., alkaloids, phenolics, and terpenoids) inhibit animals’ intrinsic mechanisms of breakdown of carbohydrates, fats, and proteins (Table 4). In many cases, the compounds have been shown to inhibit enzymatic breakdown in vitro, and effects are also manifest at the whole animal level in reduced nutrient digestibility and/or growth rate [e.g., references (212, 344, 473)]. Mechanisms vary, including competitive (350) and noncompetitive (473) enzyme inhibition as well as disruptions of the emulsification process important in digestion of fat (401).
One of the best studied chemical groups are protease or proteinase inhibitors (PIs), which bind to digestive proteins and reduce digestive efficiency and hence growth rate (237, 385). Many insects, mammals, and birds respond by increasing secretion of proteolytic enzymes and, in the vertebrates, by increasing the size of the pancreas, which synthesizes many of the enzymes, often with the net effect of restoring digestive efficiency and growth rate. A competing hypothesis about the animals’ response is that overproduction of digestive proteins is to the detriment of other essential proteins in the body, and that growth rate thus does not recover (237). Research suggests antagonistic coevolution between plants and herbivores in which the plants produce a variety of PIs with specific action against different kinds of proteases and the animals produce digestive enzyme variants that are fairly insensitive to the PIs (237). Trypsin inhibitor in castor bean leaf extract inhibited trypsin-like activity in the coffee leaf miner (Leucoptera coffeella; Table 4) but not bovine trypsin (383). Helicoverpa larvae have been identified whose chymotrypsin activity is resistant to a serine PI from Nicotiana alata, whereas other Helicoverpa larvae have an enzyme variant that is susceptible (132). Their respective cDNAs were isolated and critical residues that conferred resistance were identified. Helicoverpa larvae were also found to produce midgut proteases (85) or trypsin isoforms (313) that were either sensitive or insensitive to inhibition by soybean trypsin inhibitor (STI). The STI-senstive trypsim isoforms were produced constitutively, but production of the induced STI-insensitive forms was regulated transcriptionally following ingestion of STI (313).
A somewhat analogous scenario is emerging from studies of inhibitors of carbohydrases. Mulberry leaves produce sugar-mimic alkaloids that inhibit sucrase and trehalase activity (Table 4). However, activities in domesticated silkworms (Bombyx mori), which are mulberry specialists, are not affected whereas activities in Eri silkworms (Samia ricini), which are generalist insect herbivores, were inhibited by very low concentrations of the alkaloids (212). In another example, when larvae of bean weavils (Zabrotes subfasciatus) were fed seeds of Phaseolus vulgaris they secreted inducible isoforms of alpha-amylases that were insensitive to the alpha-amylase inhibitor that is found in the plant, whereas their constitutively produced alpha-amylase was inhibited by SMs in the plant [reference (29); see also references (29, 403)]. The entire topic of coevolution of digestive enzymes and plants SMs is not only interesting but also very important, because plant biologists are now experimentally manipulating in crop plants the genes that regulate inhibitory SMs to enhance resistance to crop pests.
Tannins are water-soluble polyphenolic compounds with a molecular weight between 300 and 3000 Da, and have the putative function as possible digestibility reducers (248). They can interact with proteins and other macromolecules in vitro through hydrogen bonding and hydrophobic bonds, and thus bind enzymes and their nutrient substrates. Several studies document their inhibition of many enzyme activities in vitro: proteases, lipase, alpha-amylase, maltase, sucrase, and lactase [e.g., references (69, 304)]. These data lead to an expectation that they will reduce diet digestibility (26).
Even if digestive enzymes are inhibited in vitro, the effects can, in principle, be prevented or reversed in vivo by change in pH or by surfactants (detergents) such as bile acids or other tannin-binding material in the gut such as mucus (26). Some mammals that commonly consume tannins secrete proline-rich (20%–40% proline) proteins in their saliva that are thought to preferentially bind tannins (197). The complexed tannins may escape both enzymatic and microbial degradation, and may be excreted in the feces, thus protecting the animal from either damage to the gut epithelium, true digestibility reduction, or toxicity (11). But, this response leads to increased fecal loss of the energy and nitrogen in the tannin-protein complex and thus to a decline in apparent digestive efficiency, though not true digestive efficiency per se (409). In some species, the relationship between dietary tannin content and reduction in apparent digestibility can be used to increase the accuracy of predictive equations of food digestibility based on food chemical composition (201). Thus, with tannins, the effects on animals are not general but depend on the particular tannin structure, concentration, and on particularities of the consumer. Because of this, it has been argued that they are not typically disruptors of intrinsic breakdown processes in either insects (26) or monogastric mammals (409).
Intestinal enzymes can activate certain toxins. Some SMs are synthesized and stored in plants as glycosides, that is, essentially bound to a glucose molecule, which can provide the plant a measure of self-protection from the more toxic aglycone (202). These SMs are thus stored in an inactive form until activated by a glycohydrolase enzyme (e.g., β-glucosidase). The enzyme may be stored in the plant, in which case maceration by a consumer causes release of the aglycone toxin, or the enzyme might be a component of the consumer’s physiological processes such as intrinsic digestive enzymes or microbial enzymes (202).
β-glucosidases are an important group of glucohydrolases found in the small intestine tissue of mammals, with apical membrane-bound lactase phlorizin hydrolase and broad-specificity cytosolic β-glucosidase being the most widely studied, including in humans, rats, and guinea pigs (95, 113, 342). β-glucosidase activity has also been measured in guts of numerous invertebrates (5, 143, 151, 157, 183, 374, 391). β-glucosidase activity is reduced in some insects that have either been selected for tolerance to plant glycosides (114) or habituated to diets with higher levels of glycosides (152, 355). It is not known whether such genetic or phenotypic adaptive response to dietary glycosides occurs in a vertebrate species. Although birds may have a homolog of the lactase gene (162), it is uncertain whether birds are capable of hydrolyzing plant glycosides, which might make them relatively immune to these plant toxins compared to other animals. Levels of lactase activity are trace or immeasurably low in chickens (84) and in house sparrows (P. domesticus) and common bulbuls (Pycnonotus bartatus) (K. M. Lessner andW. H. Karasov, unpublished data). Also, in a study with cedar waxwings (Bombycilla cedrorum), the birds were not affected by the toxic glycoside, amygdalin, when administered orally, excreting it intact (422).
Secondary metabolites and the microbiota
SMs from major groups such as phenolics and terpenoids are known to have antimicrobial activity (460). Terpenoid compounds, including essential oils and saponins (glycosides of terpenes and steroids), appear to have the largest negative effects, based on a meta-analysis of 185 treatments in ruminants in 36 studies (357). For example, the magnitude of inhibition of plant cell-wall digestibility was 23% for essential oils, 11% for saponins, and 3% for tannins (all relative to controls). Scores of specific essential oils have been tested and found to be inhibitory against many bacterial genera (2), and in the meta-analysis, they and saponins also appeared to inhibit protozoal growth (357). Besides inhibiting fermentation, essential oils can decrease the rate of bacterial deamination of protein in the lumen (2).
The complexing ability of proanthocyanidins and other tannins makes them reactive with bacterial cell walls and extracellular enzymes (311, 314). This could be the basis for how they can reduce microbial fermentation (39, 181, 300, 432) and growth, alter microflora populations, and reduce attachment of fungi and bacteria to substrates (2). Another phenolic SM, usnic acid found in some lichens, had a potent antimicrobial effect against 25 of 26 anaerobic rumen bacterial isolates from reindeer (Rangifer tarandus) (424), but one isolate was resistant.
The usnic acid-resistant microbe is one of at least three fairly well-documented examples of ruminal microorganisms that can apparently tolerate some SMs. Sundset et al. (423, 424) showed that usnic acid was apparently degraded in the rumen, and characterized a resistant bacterium that they proposed be named Eubacterium rangiferina. Their findings help explain earlier findings that rumenal microbiota from reindeer performed better at in vitro digestion when usnic acid was added, whereas addition of usnic acid to sheep rumenal microbiota depressed digestion (355). Another famous example is the bacterium Synergistes jonesii, which is capable of degrading mimosine metabolites and imparts mimosine resistance in the host ruminant, allowing it to eat Leucaena spp. (248). Finally, some GI microorganisms can apparently tolerate high concentrations of tannins, and tannin-tolerant or tannin-degrading bacterial species (189, 388) have been isolated from a variety of wild mammals worldwide, especially those that consume diets high in tannin content (314). Some of the features that may impart microbial tolerance to tannins are secretion of extracellular polysaccharide that separates the microbial cell wall from the tannin (314) and microbial enzymes such as gallate decarboxylase and tannin acyl hydrolase (2).
Secondary metabolites and absorption
Most reports of impacts of SMs on absorption refer to polyphenolic compounds, of which there are at least ten classes of compounds characterized by possessing several hydroxyl groups on aromatic rings. Martel et al. (307) provide a recent review of impacts of polyphenolics on intestinal absorption of organic cations, thiamin, folic acid, and glucose.
Many studies indicate that a variety of polyphenolics (mainly flavonoids) inhibit mediated glucose uptake by SGLT1 and/or GLUT2, based on experiments using intestine in situ, isolated tissue and cells, brush border membrane vesicles, and Xenopus laevis oocytes expressing the transporter proteins (307), and one study found that polyphenols depressed SGLT1 gene expression (351). These compounds occur in plant foods typically as glycosides. Phloretin (an aglycone) and phloridzin (its glycoside), members of the flavonoid subclass chalcones, are used as inhibitors of GLUT-2 and SGLT-1 respectively, in glucose absorption studies. But, studies have shown that a variety of flavonoids from multiple subclasses inhibit glucose transport (82, 255, 267, 274, 307, 408, 411). Proposed mechanisms for flavonoids inhibiting glucose absorption include competitive and noncompetitive inhibition. Only the mechanism for phloridzin’s inhibition of SGLT-1 has been rigorously proven to be competitive inhibition by phloridzin binding to SGLT-1 directly (346, 477, 478).
Skopec and Karasov (408) predicted that phloridzin would inhibit glucose absorption at the whole animal level when administered at ecological concentrations (they used 10 mmol/L), and that the effects would be more pronounced in nonflying mammals that rely on mediated pathway(s) for glucose absorption than birds that rely more on a nonmediated, paracellular pathway. They found that phloridzin inhibited whole-animal glucose absorption efficiency by more than 36% in laboratory rats, whereas it did not significantly decrease glucose absorption in American robins (408). Another flavonoid, isoquercetrin, also significantly decreased glucose absorption in rats but not in robins. They did not ascribe the difference to any major difference between rat and robin in the types of intestinal glucose transporters, because birds and mammals appear to share the similar suite of intestinal sugar transporters (292, 332). Instead, they ascribed the difference in the inhibition by these plant SMs of glucose absorption to the rats’ much greater reliance on glucose transporters for intestinal glucose absorption than is the case for robins. Because plant toxins mediate so many interactions between mammals and birds and their plant resources (e.g., leaf, fruit and seed diet selection, and seed and pollen dispersal), physiological differences between mammals and birds in their responses to toxins should have many ecological ramifications (86).
There is evidence that some flavonoid glycosides may be transported by SGLT-1 (10, 82, 274, 459), which could potentially lead to competitive inhibition of glucose transport. However, Kottra and Daniel (267) used Xenopus oocytes expressing SGLT1 in a two-electrode voltage clamp technique to test 27 flavonoids carrying glucose residues at different positions as well as their aglycones. None of them generated significant transport currents, which seems to be good direct evidence for lack of Na+-coupled transport via SGLT1. But, as has been demonstrated many times, some glycosylated and nonglycosylated flavonoids did show structure-dependent inhibition of glucose transport. Competitive inhibition by flavonoid transport does not seem to be the mechanism.
Reports of impacts of SMs on absorption of other substrates are scanty. The phenolic, tannic acid, nonspecifically inhibited D-glucose and L-proline uptake by isolated mouse intestine, possibly by reduction in the Na+ gradient for Na+-coupled nutrient uptake across the apical membrane (251). Another set of phenolics, catechins, which are monomeric flavanols, are reported to inhibit cholesterol absorption, perhaps by reducing micellar solubility and precipitating cholesterol (222), and they are reported to interact with lipid bilayers (336), which could lead to alterations in transport.
It is to be expected that water-soluble toxins that are not too large in molecular size will also have access to the paracellular pathway (238b). Some of the major classes of naturally occurring toxins in plants, such as alkaloids and phenolics (202), include many water-soluble compounds in the molecular size range that could access the paracellular space (243). Nicotine, for example, has a MW of 162 Da, its cationic forms are water soluble, and it was found to be absorbed by the paracellular pathway in cell culture (TR146 cells) (343).
Lipophilic toxins are also anticipated to permeate membranes passively at rates positively related to their octanol or oil:water partition coefficients, which was found to be the case in a survey of 36 flavonoids using Caco-2 cell monolayers (431). In this experimental model, rates can be decreased by the presence of salivary proteins that form complexes with polyphenols (60, 61). Other physical barriers proposed to limit passive diffusions of SMs are the peritrophic envelope of insects and surfactants (14, 15, 284). The discovery of efflux transporters over the past 2 to 3 decades across many animal phyla revealed another process by which passive absorption of lipophillic SMs might be limited. These include the ABC transporters such as multidrug resistance proteins and permeability glycoprotein, or P-glycoprotein. Several reviews are available regarding their interactions with SMs (299, 331, 412).
Conclusion and future directions
This review has uncovered numerous areas for future research focused on important gaps in the comparative physiology of the GI tract. Thus, we end with a short list of some of the potential areas for future research.
Technical advances. Understanding of the physiology of the GI tract is being transformed by the advent of increasingly sophisticated molecular and postgenomic tools to study the expression of digestive enzymes and transporters. We can anticipate tremendous strides in our understanding of the mechanistic basis of digestive function and absorption. Application of these tools has already advanced knowledge about molecular steps in dietary and developmental regulation of gene expression of carbohydrases in rodents (Section “Flexible adjustment of digestive enzymes to diet change”) and chickens (Section “Patterns in birds”). The new tools will also illuminate mechanistic details about differences between species, as has been done for differences between human populations for carbohydrases related to starch and lactose digestion (Section “Molecular mechanisms for differences in enzyme activities between populations/species”).
The importance of research on invertebrates. The literature on the function of the animal GI tract is dominated by research on vertebrates, especially mammals. Although this is not surprising, given the biomedical importance of much of this work, the field of comparative digestive physiology is constrained by our ignorance of most invertebrate groups. Molecular and physiological research on sugar and amino acid transport in a few invertebrates, for example, has revealed that it may occur on familiar transporters, such as SGLT1 for glucose, but with unfamiliar counterions (K+, not Na+), or even on transporters unfamiliar to vertebrate biologists (Sections “Absorption of carbohydrates” and “Pathways for amino acid and peptide absorption”). Furthermore, the digestive physiology of the key invertebrate model species, Drosophila melanogaster and C. elegans, is very little studied. Although this aspect of the biology of these species includes many features adapted to their specific diet (both species are predominantly microbivores), there is tremendous opportunity to use both these model species to investigate the fundamentals of GI tract function. As an illustration, recent work on Drosophila has revealed the central importance of Janus kinase/signal transducers and activators of transcription signaling and the resident microbiota as determinants of the turnover of midgut epithelial cells and gut homeostasis in this insect (47).
Integration of mechanisms with whole organism processes and performance. There is opportunity to integrate molecular events with whole organism processes, including response to diet composition and the nutritional requirements of the animal (that can change with age, developmental stage, environmental circumstance, etc.). House sparrows that have the ability as nestlings to upregulate carbohydrase activity on high-carbohydrate diet (Section “Flexible adjustments of transporters to diet change”) lose the ability in adulthood (73), and the molecular details of such regulatory differences during development remain to be described in this and other species. The finding of apparent homeostatic regulation of digestive enzymes in Locusta (Section “Flexible adjustment of digestive enzymes to diet change”) is novel and should be further investigated in this and other species. There is an increasing appreciation of the need to study responses to complex diets and diets in which two or several different classes of nutrients may not vary independently. Emphasizing this point, it is becoming increasingly evident that the physiological response of animals to diets can be complex, and may not necessarily serve to maximize energy gain or even fitness under some circumstances. Disentangling these complexities represents a major challenge for the coming years.
The significance of the microbiota. The taxonomic diversity of gut microbiota remains to be described in many major animal taxa and compared across metabolic groupings (e.g., vertebrate homeotherms vs. poikilotherms). Besides differences among animal species, microbial biodiversity can vary among populations within species and even among individuals within populations. The genetic, developmental, and environmental (e.g., diet) determinants of all this microbial biodiversity in guts remain to be determined as well as its functional significance to the host [but see reference (334)]. Our understanding of how hosts recover important nutrients from their microbiota is incomplete, especially for those that do not engage in coprophagy such as pigs, humans, herbivorous birds, and fish. Questions include (i) the location and magnitude of lysozyme and other digestive enzyme capacity and the pathways for absorption of microbially produced essential nutrients; and (ii) whether the associated fluxes are great enough to affect nutritional requirements.
Importance of an evolutionary perspective. Overlaying these important questions is the central role of evolutionary processes in any explanation of the diversity of digestive physiology across the animal kingdom. Although phylogenetically informed methods have been used in biology for many years, there remain great opportunities to apply these approaches to comparative physiology of digestion and absorption. These methods will lead to new hypotheses that will require testing by the full range of molecular and organismal physiological techniques and will encourage much-needed research on the physiology of various animal groups. Without doubt, there is much functional diversity and mechanistic novelty in the digestive systems of animals still awaiting discovery.
Acknowledgements
William Karasov has appreciated support from the U.S. National Science Foundation (IOS-1025886), U.S. Department of Agriculture (Hatch), and the U.S.-Israel Binational Science Foundation. Angela Douglas has appreciated support from U.S. National Science Foundation (IOS-0919765), National Institutes of Health (1R01GM095372), U.S. Department of Agriculture (2009-02179), and the Sarkaria Institute of Insect Physiology and Toxicology.
References
- 1.Abe T, Higashi M. Cellulose centered perspective on community structure. Oikos. 1991;60:127–133. [Google Scholar]
- 2.Acamovic T, Brooker JD. Biochemistry of plant secondary metabolites and their effects in animals. Proc Nutr Soc. 2005;64:403–412. doi: 10.1079/pns2005449. [DOI] [PubMed] [Google Scholar]
- 3.Adeola O, King DE. Developmental changes in morphometry of the small intestine and jejunal sucrase activity during the first nine weeks of postnatal growth in pigs. J Anim Sci. 2006;84:112–118. doi: 10.2527/2006.841112x. [DOI] [PubMed] [Google Scholar]
- 4.Afik D, Karasov WH. The trade-offs between digestion rate and efficiency in warblers and their ecological implications. Ecology. 1995;76:2247–2257. [Google Scholar]
- 5.Allardyce BJ, Linton SM, Saborowski R. The last piece in the cellulase puzzle: The characterisation of beta-glucosidase from the herbivorous gecarcinid land crab Gecarcoidea natalis . J Exp Biol. 2010;213:2950–2957. doi: 10.1242/jeb.041582. [DOI] [PubMed] [Google Scholar]
- 6.Altmann SW, Davis HR, Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N, Graziano MP. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 7.Andert J, Marten A, Brandl R, Brune A. Inter- and intraspecific comparison of the bacterial assemblages in the hindgut of humivorous scarab beetle larvae (Pachnoda spp.) FEMS Microbiol Ecol. 2010;74:439–449. doi: 10.1111/j.1574-6941.2010.00950.x. [DOI] [PubMed] [Google Scholar]
- 8.Arjamaa O, Vuorisalo T. Gene-culture coevolution and human diet. Am Sci. 2010;98:140–147. [Google Scholar]
- 9.Arrese EL, Soulages JL. Insect fat body: Energy, metabolism, and regulation. Annu Rev Entomol. 2010;55:207–225. doi: 10.1146/annurev-ento-112408-085356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arts ICW, Sesink ALA, Hollman PCH. Quercetin-3-glucoside is transported by the glucose carrier SGLT1 across the brush border membrane of rat small intestine. J Nutr. 2002;132:2823–2823. doi: 10.1093/jn/132.9.2823. [DOI] [PubMed] [Google Scholar]
- 11.Austin PJ, Suchar LA, Robbins CT, Hagerman AE. Tannin-binding proteins in saliva of deer and their absence in saliva of sheep and cattle. J Chem ecol. 1989;15:1335–1347. doi: 10.1007/BF01014834. [DOI] [PubMed] [Google Scholar]
- 12.Baker JE, Lum PTM, Halliday WR. Phenotypic variants and total [alpha]-amylase activity in the maize weevil (Coleoptera: Curculionidae) J Kansas Entomol Soc. 1989;62:430–434. [Google Scholar]
- 13.Bale JS, Masters GJ, Hodkinson ID, Awmack C, Bezemer TM, Brown VK, Butterfield J, Buse A, Coulson JC, Farrar J, Good JEG, Harrington R, Hartley S, Jones TH, Lindroth RL, Press MC, Symrnioudis I, Watt AD, Whittaker JB. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob Change Biol. 2002;8:1–16. [Google Scholar]
- 14.Barbehenn R. Role of transport proteins in drug absorption, distribution and excretion. Xenobiotica. 2001;31:469–497. doi: 10.1080/00498250110060969. [DOI] [PubMed] [Google Scholar]
- 15.Barbehenn RV. Formation of insoluble and colloidally dispersed tannic acid complexes in the midgut fluid of Manduca sexta (Lepidoptera: Sphingidae): An explanation for the failure of tannic acid to cross the peritrophic envelopes of lepidopteran larvae. Arch Insect Biochem Physiol. 1998;39:109–117. [Google Scholar]
- 16.Barfull A, Garriga C, Montserrat M, Planas JM. Ontogenetic expression and regulation of Na-D-glucose cotransporter in jejunum of domestic chicken. Am J Physiol. 2002;282:G559–G564. doi: 10.1152/ajpgi.00262.2001. [DOI] [PubMed] [Google Scholar]
- 17.Barszcz M, Skomial J. The development of the small intestine of piglets - chosen aspects. J Anim Feed Sci. 2011;20:3–15. [Google Scholar]
- 18.Batchelor DJ, Al-Rammahi M, Moran AW, Brand JG, Li X, Haskins M, German AJ, Shirazi-Beechey SP. Sodium/glucose cotransporter-1, sweet receptor, and disaccharidase expression in the intestine of the domestic dog and cat: Two species of different dietary habit. Am J Physiol Regul Integr Comp Physiol. 2011;300:R67–R75. doi: 10.1152/ajpregu.00262.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bates JM, Akerlund J, Mittge E, Guillemin K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe. 2007;2:371–382. doi: 10.1016/j.chom.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Batzli GO, Broussard Ad, Oliver RJ. The integrated processing response in herbivorous small mammals. In: Chivers DJ, Langer P, editors. The Digestive System in Mammal: Food, Form and Function. Cambridge: Cambridge University Press; 1994. pp. 324–336. [Google Scholar]
- 21.Behar A, Yuval B, Jurkevitch E. Enterobacteria-mediated nitrogen fixation in natural populations of the fruit fly Ceratitis capitata. Mol Ecol. 2005;14:2637–2643. doi: 10.1111/j.1365-294X.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- 22.Behar A, Yuval B, Jurkevitch E. Gut bacterial communities in the Mediterranean fruit fly (Ceratitis capitata) and their impact on host longevity. J Insect Physiol. 2008;54:1377–1383. doi: 10.1016/j.jinsphys.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Beintema JJ. Ribonucleases in ruminants. Science. 2002;297:1121–1122. doi: 10.1126/science.297.5584.1121b. [DOI] [PubMed] [Google Scholar]
- 24.Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Kwiterovich P, Shan B, Barnes R, Hobbs HH. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–1775. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- 25.Bergerson O, Wool D. The process of adaptation of flour beetles to new environments. Genetica (Dordrecht) 1988;77:3–13. [Google Scholar]
- 26.Bernays EA, Driver GC, Bilgener M. Herbivores and plant tannins. Adv Ecol Res. 1989;19:263–302. [Google Scholar]
- 27.Beubler E, Juan H. Effect of ricinoleic acid and other laxatives on net water flux and prostaglandin E release by the rat colon. J Pharm Pharmacol. 1979;10:681–685. doi: 10.1111/j.2042-7158.1979.tb13628.x. [DOI] [PubMed] [Google Scholar]
- 28.Bifano TD, Alegria TG, Terra WR. Transporters involved in glucose and water absorption in the Dysdercus peruvianus (Hemiptera: Pyrrhocoridae) anterior midgut. Comp Biochem Physiol B Biochem Mol Biol. 2010;157:1–9. doi: 10.1016/j.cbpb.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Bifano TD, Samuels RI, Alexandre D, Silva CP. Host-mediated induction of alpha-amylase by larvae of the Mexican bean weevil Zabrotes subfasciatus (Coleoptera: Chrysomelidae:Bruchinae) is irreversible and observed from the initiation of the feeding period. Arch Insect Biochem Physiol. 2010;74:247–260. doi: 10.1002/arch.20375. [DOI] [PubMed] [Google Scholar]
- 30.Bik EM, Eckburg PB, Gill SR, Nelson KE, Purdom EA, Francois F, Perez-Perez G, Blaser MJ, Relman DA. Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci U S A. 2006;103:732–737. doi: 10.1073/pnas.0506655103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Binder HJ. Pharmacology of laxatives. Annu Rev Pharmacol Toxicol. 1977;17:355–367. doi: 10.1146/annurev.pa.17.040177.002035. [DOI] [PubMed] [Google Scholar]
- 32.Binder HJ. Role of colonic short-chain fatty acid transport in diarrhea. Annu Rev Physiol. 2010;72:297–313. doi: 10.1146/annurev-physiol-021909-135817. [DOI] [PubMed] [Google Scholar]
- 33.Biviano AB, Del Rio CM, Phillips DL. Ontogenesis of intestine morphology and intestinal disaccharidases in chickens (Gallus gallus) fed contrasting purified diets. J Comp Physiol B. 1993;163:508–518. doi: 10.1007/BF00346936. [DOI] [PubMed] [Google Scholar]
- 34.Bolognesi R, Terra WR, Ferreira C. Peritrophic membrane role in enhancing digestive efficiency: Theoretical and experimental models. J Insect Physiol. 2008;54:1413–1422. doi: 10.1016/j.jinsphys.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Bouchard SS, Bjorndal KA. Ontogenetic diet shifts and digestive constraints in the omnivorous freshwater turtle Trachemys scripta . Physiol Biochem Zool. 2006;79:150–158. doi: 10.1086/498190. [DOI] [PubMed] [Google Scholar]
- 36.Boudreau F, Rings EH, van Wering HM, Kim RK, Swain GP, Krasinski SD, Moffett J, Grand RJ, Suh ER, Traber PG. Hepatocyte nuclear factor-1 alpha, GATA-4, and caudal related homeodomain protein Cdx2 interact functionally to modulate intestinal gene transcription. Implication for the developmental regulation of the sucrase-isomaltase gene. J Biol Chem. 2002;277:31909–31917. doi: 10.1074/jbc.M204622200. [DOI] [PubMed] [Google Scholar]
- 37.Bowen SH, Lutz EV, Ahlgren MO. Dietary protein and energy as determinants of food quality: Trophic strategies compared. Ecology. 1995;76:899–907. [Google Scholar]
- 38.Bowen SH. Composition and nutritional value of detritus. In: Moriarty DJW, Pullin RSV, editors. Proceedings of the 14th ICLARM Conference on Detritus and Microbial Ecology in Aquaculture. Manila, Philippines: International Center for Living Aquatic Resources Management; 1987. pp. 192–216. [Google Scholar]
- 39.Bravo L, Abia R, Eastwood MA, Saura-Calixto F. Degradation of polyphenols (catechin and tannic acid) in the rat intestinal tract. Effect on colonic fermentation and faecal output. British J Nutr. 1994;71:933–946. doi: 10.1079/bjn19940197. [DOI] [PubMed] [Google Scholar]
- 40.Brett JR, Groves TDD. Physiological energetics. In: Hoar WS, Randall DJ, Brett JR, editors. Fish Physiology. London: Academic Press; 1979. pp. 279–352. [Google Scholar]
- 41.Broer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev. 2008;88:249–286. doi: 10.1152/physrev.00018.2006. [DOI] [PubMed] [Google Scholar]
- 42.Brzek P, Caviedes-Vidal E, Hoefer K, Karasov WH. Effect of age and diet on total and paracellular glucose absorption in nestling house sparrows. Physiol Biochem Zool. 2010;83:501–511. doi: 10.1086/651098. [DOI] [PubMed] [Google Scholar]
- 43.Brzek P, Kohl K, Caviedes-Vidal E, Karasov WH. Developmental adjustments of house sparrow (Passer domesticus) nestlings to diet composition. J Exp Biol. 2009;212:1284–1293. doi: 10.1242/jeb.023911. [DOI] [PubMed] [Google Scholar]
- 44.Brzek P, Kohl KD, Caviedes-Vidal E, Karasov WH. Fully reversible phenotypic plasticity of digestive physiology in young house sparrows: Lack of long-term effect of diet composition. J Exp Biol. 2011;214:2755–2760. doi: 10.1242/jeb.058727. [DOI] [PubMed] [Google Scholar]
- 45.Brzek P, Lessner KM, Caviedes-Vidal E, Karasov WH. Low plasticity in digestive physiology constrains feeding ecology in diet specialist, Zebra finch (Taeniopygia guttata) J Exp Biol. 2010;213:798–807. doi: 10.1242/jeb.037259. [DOI] [PubMed] [Google Scholar]
- 46.Bucher EH, Tamburini D, Abril A, Torres P. Folivory in the white-tipped plantcutter Phytotoma rutila: Seasonal variations in diet composition and quality. J Avian Biol. 2003;34:211–216. [Google Scholar]
- 47.Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009;23:2333–2344. doi: 10.1101/gad.1827009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buchsbaum R, Wilson J, Valiela I. Digestibility of plant constituents by Canada geese and Atlantic brant. Ecology. 1986;67:386–393. [Google Scholar]
- 49.Buddington RK. Intestinal nutrient transport during ontogeny of vertebrates. Am J Physiol. 1992;263:R503–R509. doi: 10.1152/ajpregu.1992.263.3.R503. [DOI] [PubMed] [Google Scholar]
- 50.Buddington RK. Nutrition and ontogenetic development of the intestine. Can J Physiol Pharmacol. 1994;72:251–259. doi: 10.1139/y94-039. [DOI] [PubMed] [Google Scholar]
- 51.Buddington RK, Chen JW, Diamond JM. Genetic and phenotypic adaptation of intestinal nutrient transport to diet in fish. J Physiol. 1987;393:261–281. doi: 10.1113/jphysiol.1987.sp016823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buddington RK, Chen JW, Diamond JM. Dietary regulation of intestinal brush-border sugar and amino acid transport in carnivores. Am J Physiol. 1991;261:R793–R801. doi: 10.1152/ajpregu.1991.261.4.R793. [DOI] [PubMed] [Google Scholar]
- 53.Buddington RK, Diamond J. Ontogenetic development of nutrient transporters in cat intestine. Am J Physiol. 1992;263:G605–G616. doi: 10.1152/ajpgi.1992.263.5.G605. [DOI] [PubMed] [Google Scholar]
- 54.Buddington RK, Diamond JM. Ontogenetic development of intestinal nutrient transporters. Annu Rev Physiol. 1989;51:602–619. doi: 10.1146/annurev.ph.51.030189.003125. [DOI] [PubMed] [Google Scholar]
- 55.Buddington RK, Diamond JM. Ontogenetic development of monosaccharide and amino acid transporters in rabbit intestine. Am J Physiol. 1990;259:G544–G555. doi: 10.1152/ajpgi.1990.259.4.G544. [DOI] [PubMed] [Google Scholar]
- 56.Buddington RK, Malo C. Postnatal development of nutrient transport in the intestine of dogs. Am J Vet Res. 2003;64:635–645. doi: 10.2460/ajvr.2003.64.635. [DOI] [PubMed] [Google Scholar]
- 57.Buddington RK, Malo C, Sangild PT, Elnif J. Intestinal transport of monosaccharides and amino acids during postnatal development of mink. Am J Physiol Regul Integr Comp Physiol. 2000;279:R2287–R2296. doi: 10.1152/ajpregu.2000.279.6.R2287. [DOI] [PubMed] [Google Scholar]
- 58.Caccia S, Casartelli M, Grimaldi A, Losa E, de Eguileor M, Pennacchio F, Giordana B. Unexpected similarity of intestinal sugar absorption by SGLT1 and apical GLUT2 in an insect (Aphidius ervi, Hymenoptera) and mammals. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2284–R2291. doi: 10.1152/ajpregu.00847.2006. [DOI] [PubMed] [Google Scholar]
- 59.Cahu C, Infante JZ. Ontogenesis of digestive functions and nutritional requirements in marine fish larvae. Cybium. 2007;31:217–226. [Google Scholar]
- 60.Cai KH, Bennick A. Effect of salivary proteins on the transport of tannin and quercetin across intestinal epithelial cells in culture. Biochem Pharmacol. 2006;72:974–980. doi: 10.1016/j.bcp.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 61.Cai KH, Hagerman AE, Minto RE, Bennick A. Decreased polyphenol transport across cultured intestinal cells by a salivary proline-rich protein. Biochem Pharmacol. 2006;71:1570–1580. doi: 10.1016/j.bcp.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 62.Camara VM, Prieur DJ. Secretion of colonic isozyme of lisozyme in association with cecotrophy in rabbits. Am J Physiol. 1984;247:G19–G23. doi: 10.1152/ajpgi.1984.247.1.G19. [DOI] [PubMed] [Google Scholar]
- 63.Canavoso LE, Jouni ZE, Karnas KJ, Pennington JE, Wells MA. Fat metabolism in insects. Annu Rev Nutr. 2001;21:23–46. doi: 10.1146/annurev.nutr.21.1.23. [DOI] [PubMed] [Google Scholar]
- 64.Cancado FC, Valerio AA, Marana SR, Barbosa J. The crystal structure of a lysozyme c from housefly Musca domestica, the first structure of a digestive lysozyme. J Struct Biol. 2007;160:83–92. doi: 10.1016/j.jsb.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 65.Cano M, Ilundain AA. Ontogeny of D-mannose transport and metabolism in rat small intestine. J Membr Biol. 2010;235:101–108. doi: 10.1007/s00232-010-9259-0. [DOI] [PubMed] [Google Scholar]
- 66.Cant JP, McBride BW, Croom WJ., Jr The regulation of intestinal metabolism and its impact on whole animal energetics. J Anim Sci. 1996;74:2541–2553. doi: 10.2527/1996.74102541x. [DOI] [PubMed] [Google Scholar]
- 67.Cara JB, Moyano FJ, Cardenas S, Fernandez-Diaz C, Yufera M. Assessment of digestive enzyme activities during larval development of white bream. J Fish Biol. 2003;63:48–58. [Google Scholar]
- 68.Carey HV. Gastrointestinal responses to fasting in mammals: Lessons from hibernators. In: Starck JM, Wang T, editors. Physiological and Ecological Adaptations to Feeding in Vertebrates. Enfield, New Hampshire: Science Publishers, Inc; 2005. pp. 229–254. [Google Scholar]
- 69.Carmona A, Borgudd L, Borges G, LevyBenshimol A. Effect of black bean tannins on in vitro carbohydrate digestion and absorption. J Nutr Biochem. 1996;7:445–450. [Google Scholar]
- 70.Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB, Nagle J, Polymeropoulos MH, Sturley SL, Ioannou YA, Higgins ME, Comly M, Cooney A, Brown A, Kaneski CR, Blanchette-Mackie EJ, Dwyer NK, Neufeld EB, Chang TY, Liscum L, Strauss JF, III, Ohno K, Zeigler M, Carmi R, Sokol J, Markie D, O’Neill RR, van Diggelen OP, Elleder M, Patterson MC, Brady RO, Vanier MT, Pentchev PG, Tagle DA. Niemann-Pick C1 disease gene: Homology to mediators of cholesterol homeostasis. Science. 1997;277:228–231. doi: 10.1126/science.277.5323.228. [DOI] [PubMed] [Google Scholar]
- 71.Castagna M, Shayakul C, Trotti D, Sacchi VF, Harvey WR, Hediger MA. Cloning and characterization of a potassium-coupled amino acid transporter. Proc Natl Acad Sci U S A. 1998;95:5395–5400. doi: 10.1073/pnas.95.9.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Castillo J, Crespo D, Capilla E, Diaz M, Chauvigne F, Cerda J, Planas JV. Evolutionary structural and functional conservation of an ortholog of the GLUT2 glucose transporter gene (SLC2A2) in zebrafish. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1570–R1581. doi: 10.1152/ajpregu.00430.2009. [DOI] [PubMed] [Google Scholar]
- 73.Caviedes-Vidal E, Afik D, Martinez del Rio C, Karasov WH. Dietary modulation of intestinal enzymes of the house sparrow (Passer domesticus): Testing an adaptive hypothesis. Comp Biochem Physiol A. 2000;125:11–24. doi: 10.1016/s1095-6433(99)00163-4. [DOI] [PubMed] [Google Scholar]
- 74.Caviedes-Vidal E, Karasov WH. Developmental changes in digestive physiology of nestling house sparrows Passer domesticus . Physiol Biochem Zool. 2001;74:769–782. doi: 10.1086/322966. [DOI] [PubMed] [Google Scholar]
- 75.Caviedes-Vidal E, McWhorter TJ, Lavin SR, Chediack JG, Tracy CR, Karasov WH. The digestive adaptation of flying vertebrates: High intestinal paracellular absorption compensates for smaller guts. Proc Natl Acad Sci U S A. 2007;104:19132–19136. doi: 10.1073/pnas.0703159104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chamberlain ME, Phillips JE. Oxidative metabolism in the locust rectum. J Comp Physiol B. 1983;151:191–198. [Google Scholar]
- 77.Chan AS, Horn MH, Dickson KA, Gawlicka A. Digestive enzyme activity in carnivores and herbivores: Comparisons among four closely related prickleback fishes (Teleostei: Stichaeidae) from a California rocky intertidal habitat. J Fish Biol. 2004;65:848–858. [Google Scholar]
- 78.Chang MH, Chediack JG, Caviedes-Vidal E, Karasov WH. L-glucose absorption in house sparrows (Passer domesticus) is nonmediated. J Comp Physiol B. 2004;174:181–188. doi: 10.1007/s00360-003-0403-3. [DOI] [PubMed] [Google Scholar]
- 79.Chang MH, Karasov WH. How the house sparrow, Passer domesticus, absorbs glucose. J Exp Biol. 2004;207:3109–3121. doi: 10.1242/jeb.01154. [DOI] [PubMed] [Google Scholar]
- 80.Chediack JG, Caviedes-Vidal E, Fasulo V, Yamin LJ, Karasov WH. Intestinal passive absorption of water-soluble compounds by sparrows: Effect of molecular size and luminal nutrients. J Comp Physiol B. 2003;173:187–197. doi: 10.1007/s00360-002-0314-8. [DOI] [PubMed] [Google Scholar]
- 81.Chediack JG, Caviedes-Vidal E, Karasov WH. Electroaffinity in para-cellular absorption of hydrophilic D-dipeptides by sparrow intestine. J Comp Physiol B. 2006;176:303–309. doi: 10.1007/s00360-005-0052-9. [DOI] [PubMed] [Google Scholar]
- 82.Chen CH, Hsu HJ, Huang YJ, Lin CJ. Interaction of flavonoids and intestinal facilitated glucose transporters. Planta Medica. 2007;73:348–354. doi: 10.1055/s-2007-967172. [DOI] [PubMed] [Google Scholar]
- 83.Chen H, Pan YX, Wong EA, Webb KE. Dietary protein level and stage of development affect expression of an intestinal peptide transporter (cPepT1) in chickens. J Nutr. 2005;135:193–198. doi: 10.1093/jn/135.2.193. [DOI] [PubMed] [Google Scholar]
- 84.Chotinsky D, Toncheva E, Profirov Y. Development of disaccharidase activity in the small intestine of broiler chickens. Br Poult Sci. 2001;42:389–393. doi: 10.1080/00071660120055386. [DOI] [PubMed] [Google Scholar]
- 85.Chougule NP, Giri AP, Sainani MN, Gupta VS. Gene expression patterns of Helicoverpa armigera gut proteases. Insect Biochem Mol Biol. 2005;35:355–367. doi: 10.1016/j.ibmb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 86.Cipollini ML. Secondary metabolites of vertebrate-dispersed fruits: Evidence for adaptive functions. Revista Chilena De Historia Natural. 2000;73:421–440. [Google Scholar]
- 87.Claesson MJ, Cusack S, O’Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, Marchesi JR, Falush D, Dinan T, Fitzgerald G, Stanton C, van Sinderen D, O’Connor M, Harnedy N, O’Connor K, Henry C, O’Mahony D, Fitzgerald AP, Shanahan F, Twomey C, Hill C, Ross RP, O’Toole PW. Microbes and Health Sackler Colloquium: Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A. 2010;108:4586–4591. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Clark TM. Evolution and adaptive significance of larval midgut alkalinization in the insect superorder Mecopterida. J Chem ecol. 1999;25:1945–1960. [Google Scholar]
- 89.Clements KD. Fermentation and gstrointestinal microorganisms in fishes. In: Mackie RI, White BA, editors. Gastrointestinal Microbiology. New York: Chapman and Hall; 1997. pp. 156–198. [Google Scholar]
- 90.Clements KD, Raubenheimer D, Choat JH. Nutritional ecology of marine herbivorous fishes: Ten years on. Funct Ecol. 2009;23:79–92. [Google Scholar]
- 91.Cleveland LR, Hall SR, Sanders EP, Collier J. The wood-feeding roach Cryptocercus, its protozoa and the symbiosis between protozoa and roach. Mem Amer Acad Arts Sci. 1934;17:185–342. [Google Scholar]
- 92.Clissold FJ, Sanson GD, Read J. Indigestibility of plant cell wall by the Australian plague locust Chortoicetes terminifera . Entomol Exp Appl. 2004;112:159–168. [Google Scholar]
- 93.Clissold FJ, Tedder BJ, Conigrave AD, Simpson SJ. The gastrointestinal tract as a nutrient balancing organ. Proc R Soc B. 2010;277:1751–1759. doi: 10.1098/rspb.2009.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Coll M, Guershon M. Omnivory in terrestrial arthropods: Mixing plant and prey diets. Annu Rev Entomol. 2002;47:267–297. doi: 10.1146/annurev.ento.47.091201.145209. [DOI] [PubMed] [Google Scholar]
- 95.Colombo V, Lorenz-Meyer H, Semenza G. Small intestinal phlorizin hydrolase: The beta-glycosidase complex. Biochim Biophys Acta. 1973;327:412–424. doi: 10.1016/0005-2744(73)90425-7. [DOI] [PubMed] [Google Scholar]
- 96.Comabella Y, Mendoza R, Aguilera C, Carrillo O, Hurtado A, Garcia-Galano T. Digestive enzyme activity during early larval development of the Cuban gar Atractosteus tristoechus . Fish Physiol Biochem. 2006;32:147–157. [Google Scholar]
- 97.Connor EE, Li RW, Baldwin RL, Li C. Gene expression in the digestive tissues of ruminants and their relationships with feeding and digestive processes. Animal. 2010;4:993–1007. doi: 10.1017/S1751731109991285. [DOI] [PubMed] [Google Scholar]
- 98.Corby-Harris V, Pontaroli AC, Shimkets LJ, Bennetzen JL, Habel KE, Promislow DE. Geographical distribution and diversity of bacteria associated with natural populations of Drosophila melanogaster. Appl Environ Microbiol. 2007;73:3470–3479. doi: 10.1128/AEM.02120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cordat E, Casey JR. Bicarbonate transport in cell physiology and disease. Biochem J. 2009;417:423–439. doi: 10.1042/BJ20081634. [DOI] [PubMed] [Google Scholar]
- 100.Corpe CP, Burant CF. Hexose transporter expression in rat small intestine: Effect of diet on diurnal variations. Am J Physiol. 1996;271:G211–G216. doi: 10.1152/ajpgi.1996.271.1.G211. [DOI] [PubMed] [Google Scholar]
- 101.Cox CR, Gilmore MS. Native microbial colonization of Drosophila melanogaster and its use as a model of Enterococcus faecalis pathogenesis. Infect Immun. 2007;75:1565–1576. doi: 10.1128/IAI.01496-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Crava CM, Bel Y, Lee SF, Manachini B, Heckel DG, Escriche B. Study of the aminopeptidase N gene family in the lepidopterans Ostrinia nubilalis (Hubner) and Bombyx mori (L.): Sequences, mapping and expression. Insect Biochem Mol Biol. 2010;40:506–515. doi: 10.1016/j.ibmb.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 103.Cummings JH, Macfarlane GT. The control and consequences of bacterial fermentation in the human colon. J Appl Bacteriol. 1991;70:443–459. doi: 10.1111/j.1365-2672.1991.tb02739.x. [DOI] [PubMed] [Google Scholar]
- 104.Cuvier-Peres A, Kestemont P. Development of some digestive enzymes in Eurasian perch larvae Perca fluviatilis . Fish Physiol Biochem. 2001;24:279–285. [Google Scholar]
- 105.da Lage JL, Cariou ML, David JR. Geographical polymorphism of amylase in Drosophila ananassae and its relatives. Heredity. 1989;63:67–72. doi: 10.1038/hdy.1989.76. [DOI] [PubMed] [Google Scholar]
- 106.Daniel H. Molecular and integrative physiology of intestinal peptide transport. Annu Rev Physiol. 2004;66:361–384. doi: 10.1146/annurev.physiol.66.032102.144149. [DOI] [PubMed] [Google Scholar]
- 107.Daniel H, Spanier B, Kottra G, Weitz D. From bacteria to man: Archaic proton-dependent peptide transporters at work. Physiology (Bethesda) 2006;21:93–102. doi: 10.1152/physiol.00054.2005. [DOI] [PubMed] [Google Scholar]
- 108.Darias MJ, Murray HM, Gallant JW, Douglas SE, Yufera M, Martinez-Rodriguez G. Ontogeny of pepsinogen and gastric proton pump expression in red porgy (Pagrus pagrus): Determination of stomach functionality. Aquaculture. 2007;270:369–378. [Google Scholar]
- 109.Darias MJ, Zambonino-Infante JL, Hugot K, Cahu CL, Mazurais D. Gene expression patterns during the larval development of European sea bass (Dicentrarchus labrax) by microarray analysis. Mar Biotechnol (NY) 2008;10:416–428. doi: 10.1007/s10126-007-9078-1. [DOI] [PubMed] [Google Scholar]
- 110.Dash MC, Nanda B, Mishra PC. Digestive enzymes in three species of Enchytraeidae (Oligochaeta) Oikos. 1981;36:316–318. [Google Scholar]
- 111.Davis HR, Jr, Zhu LJ, Hoos LM, Tetzloff G, Maguire M, Liu J, Yao X, Iyer SP, Lam MH, Lund EG, Detmers PA, Graziano MP, Altmann SW. Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J Biol Chem. 2004;279:33586–33592. doi: 10.1074/jbc.M405817200. [DOI] [PubMed] [Google Scholar]
- 112.Davison A, Blaxter M. Ancient origin of glycosyl hydrolase family 9 cellulase genes. Mol Biol Evol. 2005;22:1273–1284. doi: 10.1093/molbev/msi107. [DOI] [PubMed] [Google Scholar]
- 113.Day AJ, Canada FJ, Diaz JC, Kroon PA, McLauchlan R, Faulds CB, Plumb GW, Morgan RA, Williamson G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS letters. 2000;468:166–170. doi: 10.1016/s0014-5793(00)01211-4. [DOI] [PubMed] [Google Scholar]
- 114.de Oliveira JE, Druyan S, Uni Z, Ashwell CM, Ferket PR. Prehatch intestinal maturation of turkey embryos demonstrated through gene expression patterns. Poult Sci. 2009;88:2600–2609. doi: 10.3382/ps.2008-00548. [DOI] [PubMed] [Google Scholar]
- 115.Desroches P, Mandon N, Baehr JC, Huignard J. Mediation of host-plant use by a glucoside in Callosobruchus maculatus F (Coleoptera: Bruchidae) J Insect Physiol. 1997;43:439–446. [Google Scholar]
- 116.Dethlefsen L, McFall-Ngai M, Relman DA. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature. 2007;449:811–818. doi: 10.1038/nature06245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Diamond J. Evolutionary design of intestinal nutrient absorption: Enough but not too much. News Physiol Sci. 1991;6:92–96. [Google Scholar]
- 118.Diamond J, Hammond K. The matches, achieved by natural selection, between biological capacities and their natural loads. Experientia. 1992;48:551–557. doi: 10.1007/BF01920238. [DOI] [PubMed] [Google Scholar]
- 119.Diamond JM. Evolutionary physiology. In: Boyd CAR, Noble D, editors. The Logic of Life: The Challenge of Integrative Physiology. NewYork: Oxford University Press; 1993. pp. 89–111. [Google Scholar]
- 120.Diamond JM, Karasov WH. Adaptive regulation of intestinal nutrient transporters. Proc Natl Acad Sci U S A. 1987;84:2242–2245. doi: 10.1073/pnas.84.8.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dierenfeld E, Hintz H, Robertson J, Van Soest P, Oftedal O. Utilization of bamboo by the giant panda. J Nutr. 1982;112:636–641. doi: 10.1093/jn/112.4.636. [DOI] [PubMed] [Google Scholar]
- 122.Dierenfeld ES, Hintz HF, Robertson JB, Van Soest PJ, Oftedal OT. Utilization of bamboo by the giant panda. J Nutr. 1992;112:636–641. doi: 10.1093/jn/112.4.636. [DOI] [PubMed] [Google Scholar]
- 123.Dillon RJ, Dillon VM. The gut bacteria of insects: Nonpathogenic interactions. Annu Rev Entomol. 2004;49:71–92. doi: 10.1146/annurev.ento.49.061802.123416. [DOI] [PubMed] [Google Scholar]
- 124.Donohoe DR, Garge N, Zhang X, Sun W, O’Connell TM, Bunger MK, Bultman SJ. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Doring F, Will J, Amasheh S, Clauss W, Ahlbrecht H, Daniel H. Minimal molecular determinants of substrates for recognition by the intestinal peptide transporter. J Biol Chem. 1998;273:23211–23218. doi: 10.1074/jbc.273.36.23211. [DOI] [PubMed] [Google Scholar]
- 126.Douard V, Ferraris RP. Regulation of the fructose transporter GLUT5 in health and disease. Am J Physiol Endocrinol Metab. 2008;295:E227–E237. doi: 10.1152/ajpendo.90245.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Douglas AE. Phloem-sap feeding by animals: Problems and solutions. J Exp Bot. 2006;57:747–754. doi: 10.1093/jxb/erj067. [DOI] [PubMed] [Google Scholar]
- 128.Douglas AE. The microbial dimension in insect nutritional ecoogy. Funct Ecol. 2009;23:38–47. [Google Scholar]
- 129.Dreon MS, Ituarte S, Heras H. The role of the proteinase inhibitor ovorubin in apple snail eggs resembles plant embryo defense against predation. Plos One. 2010;5:e15059. doi: 10.1371/journal.pone.0015059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Duan CJ, Feng JX. Mining metagenomes for novel cellulase genes. Biotechnol Lett. 2010;32:1765–1775. doi: 10.1007/s10529-010-0356-z. [DOI] [PubMed] [Google Scholar]
- 131.Dunn AK, Stabb EV. Culture-independent characterization of the microbiota of the ant lion Myrmeleon mobilis (Neuroptera: Myrmeleontidae) Appl Environ Microbiol. 2005;71:8784–8794. doi: 10.1128/AEM.71.12.8784-8794.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dunse KM, Kaas Q, Guarino RF, Barton PA, Craik DJ, Anderson MA. Molecular basis for the resistance of an insect chymotrypsin to a potato type II proteinase inhibitor. Proc Natl Acad Sci U S A. 2010;107:15016–15021. doi: 10.1073/pnas.1009327107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dyer J, Al-Rammahi M, Waterfall L, Salmon KS, Geor RJ, Boure L, Edwards GB, Proudman CJ, Shirazi-Beechey SP. Adaptive response of equine intestinal Na+/glucose co-transporter (SGLT1) to an increase in dietary soluble carbohydrate. Pflugers Arch. 2009;458:419–430. doi: 10.1007/s00424-008-0620-4. [DOI] [PubMed] [Google Scholar]
- 134.Egorova V, Nikitina A, Timofeeva N. Effect of weaning terms and protein deficit in rat pup nutrition on activities of digestive enzymes. J Evol Biochem Physiol. 2008;44:591–598. [PubMed] [Google Scholar]
- 135.Eisert R. Hypercarnivory and the brain: Protein requirements of cats reconsidered. J Comp Physiol B. 2010;181:1–17. doi: 10.1007/s00360-010-0528-0. [DOI] [PubMed] [Google Scholar]
- 136.Elliott J, Bellwood D. Alimentary tract morphology and diet in three coral reef fish familes. J Fish Biol. 2003;63:1598–1609. [Google Scholar]
- 137.Elvin-Lewis M. Should we be concerned about herbal remedies. J Ethnopharmacol. 2001;75:141–164. doi: 10.1016/s0378-8741(00)00394-9. [DOI] [PubMed] [Google Scholar]
- 138.Enattah NS, Jensen TGK, Nielsen M, Lewinski R, Kuokkanen M, Rasinpera H, El-Shanti H, Seo JK, Alifrangis M, Khalil IF, Natah A, Ali A, Natah S, Comas D, Mehdi SQ, Groop L, Vestergaard EM, Imtiaz F, Rashed MS, Meyer B, Troelsen J, Peltonen L. Independent introduction of two lactase-persistence alleles into human populations reflects different history of adaptation to milk culture. Am J Hum Genet. 2008;82:57–72. doi: 10.1016/j.ajhg.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Englyst HN, Dingman SM, Cummings JH. Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr. 1992;46:S33–S50. [PubMed] [Google Scholar]
- 140.Enattah NS, Sahi T, Savilahti E, Terwilliger JD, Peltonen L, Äûrvela I. Identification of a variant associated with adult-type hypolactasia. Nature Genetics. 2002;30:233–237. doi: 10.1038/ng826. [DOI] [PubMed] [Google Scholar]
- 141.Erban T, Hubert J. Digestive function of lysozyme in synanthropic acaridid mites enables utilization of bacteria as a food source. Exp Appl Acarol. 2008;44:199–212. doi: 10.1007/s10493-008-9138-x. [DOI] [PubMed] [Google Scholar]
- 142.Erickson RH, Gum JR, Jr, Lindstrom MM, McKean D, Kim YS. Regional expression and dietary regulation of rat small intestinal peptide and amino acid transporter mRNAs. Biochem Biophys Res Commun. 1995;216:249–257. doi: 10.1006/bbrc.1995.2617. [DOI] [PubMed] [Google Scholar]
- 143.Erthal M, Silva CP, Samuels RI. Digestive enzymes in larvae of the leaf cutting ant Acromyrmex subterraneus (Hymenoptera: Formicidae: Attini) J Insect Physiol. 2007;53:1101–1111. doi: 10.1016/j.jinsphys.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 144.Escribano F, Rahn BI, Sell JL. Development of lipase activity in yolk membrane and pancreas of young turkeys. Poult Sci. 1988;67:1089–1097. doi: 10.3382/ps.0671089. [DOI] [PubMed] [Google Scholar]
- 145.Feldman DH, Harvey WR, Stevens BR. A novel electrogenic amino acid transporter is activated by K+ or Na+, is alkaline pH-dependent, and is Cl–independent. J Biol Chem. 2000;275:24518–24526. doi: 10.1074/jbc.M907582199. [DOI] [PubMed] [Google Scholar]
- 146.Felix CR, Betschart B, Billingsley PF, Freyvogal TA. Post-feeding induction of trypsin in the midgut of Aedes aegypti L. (Diptera: Culicidae) is separatable into two cellular phases. Insect Biochem. 1991;21:197–203. [Google Scholar]
- 147.Felsenstein J. Phylogenies and the comparative method. Am Nat. 1985;125:1–15. [Google Scholar]
- 148.Ferraris RP. Dietary and developmental regulation of intestinal sugar transport. Biochem J. 2001;360:265–276. doi: 10.1042/0264-6021:3600265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ferraris RP, Diamond JM. Specific regulation of intestinal nutrient transporters by their dietary substrates. Annu Rev Physiol. 1989;51:125–141. doi: 10.1146/annurev.ph.51.030189.001013. [DOI] [PubMed] [Google Scholar]
- 150.Ferraris RP, Lee PP, Diamond JM. Origin of regional and species differences in intestinal glucose uptake. Am J Physiol. 1989;257:G689–G697. doi: 10.1152/ajpgi.1989.257.5.G689. [DOI] [PubMed] [Google Scholar]
- 151.Ferreira AHP, Ribeiro AF, Terra WR, Ferreira C. Secretion of beta-glycosidase by middle midgut cells and its recycling in the midgut of Tenebrio molitor larvae. J Insect Physiol. 2002;48:113–118. doi: 10.1016/s0022-1910(01)00151-2. [DOI] [PubMed] [Google Scholar]
- 152.Ferreira C, Marana SR, Terra WR. Consumption of sugars, hemicellulose, starch, pectin and cellulose by the grasshopper. Aracris flavol Entomol Exp Appl. 1992;65:113–117. [Google Scholar]
- 153.Ferreira C, Parra JRP, Terra WR. The effect of dietary plant glycosides on larval midgut beta-glucosidases from Spodoptera frugiperda and Diatraea saccharalis. Insect Biochem Mol Biol. 1997;27:55–59. [Google Scholar]
- 154.Fine KD, Santa Ana CA, Porter JL, Fordtran JS. Effect of D-glucose on intestinal permeability and its passive absorption in human small intestine in vivo. Gastroenterology. 1993;105:1117–1125. doi: 10.1016/0016-5085(93)90957-e. [DOI] [PubMed] [Google Scholar]
- 155.Fischbarg J. Fluid transport across leaky epithelia: Central role of the tight junction and supporting role of aquaporins. Physiol Rev. 2010;90:1271–1290. doi: 10.1152/physrev.00025.2009. [DOI] [PubMed] [Google Scholar]
- 156.Flint HJ, Duncan SH, Scott KP, Louis P. Interactions and competition within the microbial community of the human colon: Links between diet and health. Environ Microbiol. 2007;9:1101–1111. doi: 10.1111/j.1462-2920.2007.01281.x. [DOI] [PubMed] [Google Scholar]
- 157.Fonseca FV, Silva JR, Samuels RI, DaMatta RA, Terra WR, Silva CP. Purification and partial characterization of a midgut membrane-bound alpha-glucosidase from Quesada gigas (Hemiptera: Cicadidae) Comp Biochem Physiol B Biochem Mol Biol. 2010;155:20–25. doi: 10.1016/j.cbpb.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 158.Forcella M, Berra E, Giacchini R, Parenti P. Leucine transport in brush border membrane vesicles from freshwater insect larvae. Arch Insect Biochem Physiol. 2006;63:110–122. doi: 10.1002/arch.20147. [DOI] [PubMed] [Google Scholar]
- 159.Fowler HG, Forti LC, Brandao CRF, Delabie JHC, Vasconcelos HL. Ecologia nutritonal de formigas. In: Panizzi AR, Parra JRP, editors. Ecologia Nutricional de Insetos e Suas Implicacoes no Manejo de Pragas. Sao Palo, Brazil: Editora Manole LTDA; 1991. [Google Scholar]
- 160.Foye OT, Black BL. Intestinal adaptation to diet in the young domestic and wild turkey (Meleagris gallopavo) Comp Biochem Physiol A Mol Integr Physiol. 2006;143:184–192. doi: 10.1016/j.cbpa.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 161.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Freund J-N, Jost B, Lorentz O, Duluc I. Identification of homologues of the mammalian intestinal lactase gene in non-mammals (birds and molluscs) Biochem J. 1997;322:491–498. doi: 10.1042/bj3220491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Friedman A, Bar-Shira E, Sklan D. Ontogeny of gut associated immune competence in the chick. Worlds Poult Sci J. 2003;59:209–219. [Google Scholar]
- 164.Frolund S, Holm R, Brodin B, Nielsen CU. The proton-coupled amino acid transporter, SLC36A1 (hPAT1), transports Gly-Gly, Gly-Sar and other Gly-Gly mimetics. Br J Pharmacol. 2010;161:589–600. doi: 10.1111/j.1476-5381.2010.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Froystad MK, Lilleeng E, Sundby A, Krogdahl A. Cloning and characterization of alpha-amylase from Atlantic salmon (Salmo salar L.) Comp Biochem Physiol A Mol Integr Physiol. 2006;145:479–492. doi: 10.1016/j.cbpa.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 166.Fujita A, Shimizu I, Abe T. Distribution of lysozyme and protease, and amino acid concentration in the guts of a wood-feeding termite Reticulitermes speratus (Kolbe): Possible digestion of symbiont bacteria transferred by trophallaxis. Physiol Entomol. 2001;26:116–123. [Google Scholar]
- 167.Fujita AI. Lysozymes in insects: What role do they play in nitrogen metabolism? Physiol Entomol. 2004;29:305–310. [Google Scholar]
- 168.Fuller MF, Reeds PJ. Nitrogen cycling in the gut. Annu Rev Nutr. 1998;18:385–411. doi: 10.1146/annurev.nutr.18.1.385. [DOI] [PubMed] [Google Scholar]
- 169.Fuller RC, Baer CF, Travis J. How and when selection experiments might actually be useful. Integr Comp Biol. 2005;45:391–404. doi: 10.1093/icb/45.3.391. [DOI] [PubMed] [Google Scholar]
- 170.Ganapathy, Leibach FH. Is intestinal peptide transport energized by a proton gradient? Am J Physiol. 1985;249:G153–G160. doi: 10.1152/ajpgi.1985.249.2.G153. [DOI] [PubMed] [Google Scholar]
- 171.Gao F, Yang HS, Xu Q, Wang FY, Liu GB, German DP. Phenotypic plasticity of gut structure and function during periods of inactivity in Apostichopus japonicus . Comp Biochem Physiol B Biochem Mol Biol. 2008;150:255–262. doi: 10.1016/j.cbpb.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 172.Garland T, Jr, Adolph SC. Why not to do two-species comparative studies: Limitations on infering adaptation. Physiol Zool. 1994;67:797–828. [Google Scholar]
- 173.Geddes K, Philpott DJ. A new role for intestinal alkaline phosphatase in gut barrier maintenance. Gastroenterology. 2008;135:8–12. doi: 10.1053/j.gastro.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 174.Genta FA, Dillon RJ, Terra WR, Ferreira C. Potential role for gut microbiota in cell wall digestion and glucoside detoxification in Tenebrio molitor larvae. J Insect Physiol. 2006;52:593–601. doi: 10.1016/j.jinsphys.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 175.German DP. Do herbivorous minnows have “plug-flow reactor” guts? Evidence from digestive enzyme activities, gastrointestinal fermentation, and luminal nutrient concentrations. J Comp Physiol B. 2009;179:759–771. doi: 10.1007/s00360-009-0359-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.German DP, Bittong RA. Digestive enzyme activities and gastroin-testinal fermentation in wood-eating catfishes. J Comp Physiol B. 2009;179:1025–1042. doi: 10.1007/s00360-009-0383-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.German DP, Horn MH. Gut length and mass in herbivorous and carnivorous prickleback fishes (Teleostei: Stichaeidae): Ontogenetic, dietary, and phylogenetic effects. Mar Biol. 2006;148:1123–1134. doi: 10.1086/422228. [DOI] [PubMed] [Google Scholar]
- 178.German DP, Horn MH, Gawlicka A. Digestive enzyme activities in herbivorous and carnivorous prickleback fishes (teleostei: Stichaeidae): Ontogenetic, dietary, and phylogenetic effects. Physiol Biochem Zool. 2004;77:789–804. doi: 10.1086/422228. [DOI] [PubMed] [Google Scholar]
- 179.German DP, Nagle BC, Villeda JM, Ruiz AM, Thomson AW, Contreras Balderas S, Evans DH. Evolution of herbivory in a carnivorous clade of minnows (Teleostei: Cyprinidae): Effects on gut size and digestive physiology. Physiol Biochem Zool. 2010;83:1–18. doi: 10.1086/648510. [DOI] [PubMed] [Google Scholar]
- 180.German DP, Neuberger DT, Callahan MN, Lizardo NR, Evans DH. Feast to famine: The effects of food quality and quantity on the gut structure and function of a detritivorous catfish (Teleostei: Loricariidae) Comp Biochem Physiol A Mol Integr Physiol. 2010;155:281–293. doi: 10.1016/j.cbpa.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 181.Getachew G, Pittroff W, Putnam DH, Dandekar A, Goyal S, DePeters EJ. The influence of addition of gallic acid, tannic acid, or quebracho tannins to alfalfa hay on in vitro rumen fermentation and microbial protein synthesis. Anim Feed Sci Technol. 2008;140:444–461. [Google Scholar]
- 182.Geurden I, Aramendi M, Zambonino-Infante J, Panserat S. Early feeding of carnivorous rainbow trout (Oncorhynchus mykiss) with a hyper-glucidic diet during a short period: Effect on dietary glucose utilization in juveniles. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2275–R2283. doi: 10.1152/ajpregu.00444.2006. [DOI] [PubMed] [Google Scholar]
- 183.Ghadamyari M, Hosseininaveh V, Sharifi M. Partial biochemical characterization of alpha- and beta-glucosidases of lesser mulberry pyralid, Glyphodes pyloalis Walker (Lep.: Pyralidae) Comptes Rendus Biologies. 2010;333:197–204. doi: 10.1016/j.crvi.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 184.Gilbert ER, Li H, Ernmersonj DA, Webb KE, Wong EA. Developmental regulation of nutrient transporter and enzyme mRNA abundance in the small intestine of broilers. Poult Sci. 2007;86:1739–1753. doi: 10.1093/ps/86.8.1739. [DOI] [PubMed] [Google Scholar]
- 185.Gilbert ER, Li HF, Emmerson DA, Webb KE, Wong EA. Dietary protein quality and feed restriction influence abundance of nutrient transporter mRNA in the small intestine of broiler chicks. J Nutr. 2008;138:262–271. doi: 10.1093/jn/138.2.262. [DOI] [PubMed] [Google Scholar]
- 186.Gilbert ER, Williams PM, Ray WK, Li HF, Emmerson DA, Wong EA, Webb KE. Proteomic evaluation of chicken brush-border membrane during the early posthatch period. J Proteome Res. 2010;9:4628–4639. doi: 10.1021/pr1003533. [DOI] [PubMed] [Google Scholar]
- 187.Gisbert E, Gimenez G, Fernandez I, Kotzamanis Y, Estevez A. Development of digestive enzymes in common dentex Dentex dentex during early ontogeny. Aquaculture. 2009;287:381–387. [Google Scholar]
- 188.Godoy-Vitorino F, Ley RE, Gao Z, Pei Z, Ortiz-Zuazaga H, Pericchi LR, Garcia-Amado MA, Michelangeli F, Blaser MJ, Gordon JI, Dominguez-Bello MG. Bacterial community in the crop of the hoatzin, a neotropical folivorous flying bird. Appl Environ Microbiol. 2008;74:5905–5912. doi: 10.1128/AEM.00574-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Goel G, Puniya AK, Aguilar CN, Singh K. Interaction of gut microflora with tannins in feeds. Naturwissenschaften. 2005;92:497–503. doi: 10.1007/s00114-005-0040-7. [DOI] [PubMed] [Google Scholar]
- 190.Goldberg RF, Austen WG, Zhang XB, Munene G, Mostafa G, Biswas S, McCormack M, Eberlin KR, Nguyen JT, Tatlidede HS, Warren HS, Narisawa S, Millan JL, Hodin RA. Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. Proc Natl Acad Sci U S A. 2008;105:3551–3556. doi: 10.1073/pnas.0712140105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gomes MLM, Matta SLP, Araujo VA, Silva GMF, Zacaro AA. Larval ontogeny and morphology of giant trahira Hoplias lacerdae. J Fish Biol. 2010;76:852–861. [Google Scholar]
- 192.Gondoin A, Grussu D, Stewart D, McDougall GJ. White and green tea polyphenols inhibit pancreatic lipase in vitro. Food Res Int. 2010;43:1537–1544. [Google Scholar]
- 193.Gouyon F, Caillaud L, Carriere V, Klein C, Dalet V, Citadelle D, Kellett GL, Thorens B, Leturque A, Brot-Laroche E. Simple-sugar meals target GLUT2 at enterocyte apical membranes to improve sugar absorption: A study in GLUT2-null mice. J Physiol. 2003;552:823–832. doi: 10.1113/jphysiol.2003.049247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Graf GA, Li WP, Gerard RD, Gelissen I, White A, Cohen JC, Hobbs HH. Coexpression of ATP-binding cassette proteins ABCG5 and ABCG8 permits their transport to the apical surface. J Clin Invest. 2002;110:659–669. doi: 10.1172/JCI16000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Grajal A, Strahl SD, Parra R, Dominguez MG, Neher A. Foregut fermentation in the hoatzin, a neotropical leaf-eating bird. Science. 1989;245:1236–1238. doi: 10.1126/science.245.4923.1236. [DOI] [PubMed] [Google Scholar]
- 196.Guilloteau P, Zabielski R, Hammon HM, Metges CC. Nutritional programming of gastrointestinal tract development. Is the pig a good model for man? Nutr Res Rev. 2010;23:4–22. doi: 10.1017/S0954422410000077. [DOI] [PubMed] [Google Scholar]
- 197.Hagerman AE, Butler LG. Specificity of proantho-cyanidin-protein interactions. J Biol Chem. 1981;256:4494–4497. [PubMed] [Google Scholar]
- 198.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: The role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 199.Hamilton I, Rothwell J, Archer D, Axon TR. Permeability of the rat small intestine to carbohydrate probe molecules. Clin Sci. 1987;73:189–196. doi: 10.1042/cs0730189. [DOI] [PubMed] [Google Scholar]
- 200.Hamza N, Mhetli M, Kestemont P. Effects of weaning age and diets on ontogeny of digestive activities and structures of pikeperch (Sander lucioperca) larvae. Fish Physiol Biochem. 2007;33:121–133. [Google Scholar]
- 201.Hanley TA, Robbins CT, Hagerman AE, McArthur C. Predicting digestible protein and digestible dry matter in tannin-containing forages consumed by ruminants. Ecology. 1992;73:537–541. [Google Scholar]
- 202.Harborne JB. Introduction to Ecological Biochemistry. New York, NY: Academic Press; 1993. [Google Scholar]
- 203.Harig JM, Ng EK, Dudeja PK, Brasitus TA, Ramaswamy K. Transport of n-butyrate into human colonic luminal membrane vesicles. Am J Physiol. 1996;271:G415–G422. doi: 10.1152/ajpgi.1996.271.3.G415. [DOI] [PubMed] [Google Scholar]
- 204.Harig JM, Soergel KH, Barry JA, Ramaswamy K. Transport of propionate by human ileal brush-border membrane vesicles. Am J Physiol. 1991;260:G776–G782. doi: 10.1152/ajpgi.1991.260.5.G776. [DOI] [PubMed] [Google Scholar]
- 205.He YL, Murby S, Warhurst G, Gifford L, Walker D, Ayrton J, Eastmond R, Rowland M. Species differences in size discrimination in the paracellular pathway reflected by oral bioavailability of poly(ethylene glycol) and D-peptides. J Pharm Sci. 1998;87:626–633. doi: 10.1021/js970120d. [DOI] [PubMed] [Google Scholar]
- 206.Hediger MA, Coady MJ, Ikeda TS, Wright EM. Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. Nature. 1987;330:379–381. doi: 10.1038/330379a0. [DOI] [PubMed] [Google Scholar]
- 207.Hehemann JH, Correc G, Barbeyron T, Helbert W, Czjzek M, Michel G. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature. 2010;464:908–912. doi: 10.1038/nature08937. [DOI] [PubMed] [Google Scholar]
- 208.Henning SJ. Gastrointestinal development: An overview. In: Halter F, Winton D, Wright NA, editors. The Gut as a Model in Cell and Molecular Biology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1997. pp. 49–60. [Google Scholar]
- 209.Herrel A, Huyghe K, Vanhooydonck B, Backeljau T, Breugelmans K, Grbac I, Van Damme R, Irschick DJ. Rapid large-scale evolutionary divergence in morphology and performance associated with exploitation of a different dietary resource. Proc Natl Acad Sci U S A. 2008;105:4792–4795. doi: 10.1073/pnas.0711998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hess M, Sczyrba A, Egan R, Kim TW, Chokhawala H, Schroth G, Luo S, Clark DS, Chen F, Zhang T, Mackie RI, Pennacchio LA, Tringe SG, Visel A, Woyke T, Wang Z, Rubin EM. Metagenomic discovery of biomass-degrading genes and genomes from cow rumen. Science. 2011;331:463–467. doi: 10.1126/science.1200387. [DOI] [PubMed] [Google Scholar]
- 211.Hewson-Hughes AK, Hewson-Hughes VL, Miller AT, Hall SR, Simpson SJ, Raubenheimer D. Geometric analysis of macronutrient selection in the adult domestic cat Felis catus . J Exp Biol. 2011;214:1039–1051. doi: 10.1242/jeb.049429. [DOI] [PubMed] [Google Scholar]
- 212.Hirayama C, Konno K, Wasano N, Nakamura M. Differential effects of sugar-mimic alkaloids in mulberry latex on sugar metabolism and disaccharidases of Eri and domesticated silkworms: Enzymatic adaptation of Bombyx mori to mulberry defense. Insect Biochem Mol Biol. 2007;37:1348–1358. doi: 10.1016/j.ibmb.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 213.Hoehne-Reitan K, Kjorsvik E, Reitan KI. Lipolytic activities in developing turbot larvae as influenced by diet. Aquacult Int. 2003;11:477–489. [Google Scholar]
- 214.Hofer R, Schiemer F. Proteolytic activity in the digestive tract of several species of fish with different feeding habits. Oecologia. 1981;48:342–345. doi: 10.1007/BF00346492. [DOI] [PubMed] [Google Scholar]
- 215.Honma K, Mochizuki K, Goda T. Carbohydrate/fat ratio in the diet alters histone acetylation on the sucrase-isomaltase gene and its expression in mouse small intestine. Biochem Biophys Res Commun. 2007;357:1124–1129. doi: 10.1016/j.bbrc.2007.04.070. [DOI] [PubMed] [Google Scholar]
- 216.Horn MH, Messer KS. Fish guts as chemical reactors: A model of the alimentary canals of marine herbivorous fishes. Mar Biol. 1992;113:527–535. [Google Scholar]
- 217.Hourdry J, Lhermite A, Ferrand R. Changes in the digestive tract and feeding behavior of anuran amphibians during metamorphosis. Physiol Zool. 1996;69:219–251. [Google Scholar]
- 218.Hrassnigg N, Crailsheim K. Differences in drone and worker physiology in honeybees (Apis mellifera) Apidologie. 2005;36:255–277. [Google Scholar]
- 219.Huber K, Roesler U, Muscher A, Hansen K, Widiyono I, Pfeffer E, Breves G. Ontogenesis of epithelial phosphate transport systems in goats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R413–R421. doi: 10.1152/ajpregu.00357.2002. [DOI] [PubMed] [Google Scholar]
- 220.Hummel J, Sudekum KH, Streich WJ, Clauss M. Forage fermentation patterns and their implications for herbivore ingesta retention times. Funct Ecol. 2006;20:989–1002. [Google Scholar]
- 221.Huvet A, Jeffroy F, Fabioux C, Daniel JY, Quillien V, Van Wormhoudt A, Moal J, Samain JF, Boudry P, Pouvreau S. Association among growth, food consumption-related traits and amylase gene polymorphism in the Pacific oyster Crassostrea gigas. Anim Genet. 2008;39:662–665. doi: 10.1111/j.1365-2052.2008.01776.x. [DOI] [PubMed] [Google Scholar]
- 222.Ikeda I, Kobayashi M, Hamada T, Tsuda K, Goto H, Imaizumi K, Nozawa A, Sugimoto A, Kakuda T. Heat-epimerized tea catechins rich in gallocatechin gallate and catechin gallate are more effective to inhibit cholesterol absorption than tea catechins rich in epigallocatechin gallate and epicatechin gallate. J Agric Food Chem. 2003;51:7303–7307. doi: 10.1021/jf034728l. [DOI] [PubMed] [Google Scholar]
- 223.Ikeda I, Tanaka K, Sugano M, Vahouny GV, Gallo LL. Discrimination between cholesterol and sitosterol for absorption in rats. J Lipid Res. 1988;29:1583–1591. [PubMed] [Google Scholar]
- 224.Infante JLZ, Cahu CL. Ontogeny of the gastrointestinal tract of marine fish larvae. Comp Biochem Physiol C. 2001;130:477–487. doi: 10.1016/s1532-0456(01)00274-5. [DOI] [PubMed] [Google Scholar]
- 225.Infante JLZ, Cahu CL. Dietary modulation of some digestive enzymes and Metabolic processes in developing marine fish: Applications to diet formulation. Aquaculture. 2007;268:98–105. [Google Scholar]
- 226.Inomata N, Nakashima S. Short 5’-flanking regions of the Amy gene of Drosophila kikkawai affect amylase gene expression and respond to food environments. Gene. 2008;412:102–109. doi: 10.1016/j.gene.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 227.Iqbal J, Hussain MM. Intestinal lipid absorption. Am J Physiol Endocrinol Metab. 2009;296:E1183–E1194. doi: 10.1152/ajpendo.90899.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Irie M, Terada T, Katsura T, Matsuoka S, Inui K. Computational modelling of H+-coupled peptide transport via human PEPT1. J Physiol. 2005;565:429–439. doi: 10.1113/jphysiol.2005.084582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Isokpehi RD, Rajnarayanan RV, Jeffries CD, Oyeleye TO, Cohly HH. Integrative sequence and tissue expression profiling of chicken and mammalian aquaporins. Bmc Genomics. 2009;10:S7. doi: 10.1186/1471-2164-10-S2-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ito Y, Hirashima M, Yamada H, Imoto T. Colonic lysozymes of rabbit (Japanese white) - recent divergence and functional conversion. J Biochem. 1994;116:1346–1353. doi: 10.1093/oxfordjournals.jbchem.a124686. [DOI] [PubMed] [Google Scholar]
- 231.Jackson S, Diamond J. Ontogenetic development of gut function, growth, and metabolism in a wild bird, the Red Jungle Fowl. Am J Physiol. 1995;269:R1163–R1173. doi: 10.1152/ajpregu.1995.269.5.R1163. [DOI] [PubMed] [Google Scholar]
- 232.Jakobsson HE, Jernberg C, Andersson AF, Sjolund-Karlsson M, Jansson JK, Engstrand L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS One. 2010;5:e9836. doi: 10.1371/journal.pone.0009836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Janis C. The evolutionary strategy of the Equidae and the origins of rumen and cecal digestion. Evolution. 1976;30:757–774. doi: 10.1111/j.1558-5646.1976.tb00957.x. [DOI] [PubMed] [Google Scholar]
- 234.Jia L, Betters JL, Yu L. Niemann-pick C1-like 1 (NPC1L1) protein in intestinal and hepatic cholesterol transport. Annu Rev Physiol. 2011;73:239–259. doi: 10.1146/annurev-physiol-012110-142233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Johnston DJ. Ontogenetic changes in digestive enzyme activity of the spiny lobster Jasus edwardsii (Decapoda; Palinuridae) Mar Biol. 2003;143:1071–1082. [Google Scholar]
- 236.Johnston M, Johnston D, Richardson A. Digestive capabilities reflect the major food sources in three species of talitrid amphipods. Comp Biochem Physiol B Biochem Mol Biol. 2005;140:251–257. doi: 10.1016/j.cbpc.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 237.Jongsma MA, Bolter C. The adaptation of insects to plant protease inhibitors. J Insect Physiol. 1997;43:885–895. doi: 10.1016/s0022-1910(97)00040-1. [DOI] [PubMed] [Google Scholar]
- 238.Juan ME, Turmo MC, Planas JM. Ontogenetic and regional changes in alpha-methyl-D-glucoside and L-proline intestinal transport in guinea pig. Am J Physiol Regul Integr Comp Physiol. 1998;275:R897–R904. doi: 10.1152/ajpregu.1998.275.3.R897. [DOI] [PubMed] [Google Scholar]
- 239.Jumars PA, Martinez del Rio C. The tau of continuous feeding on simple foods. Physiol Biochem Zool. 1999;72:633–641. doi: 10.1086/316695. [DOI] [PubMed] [Google Scholar]
- 240.Jutfelt F, Olsen RE, Bjornsson BT, Sundell K. Parr-smolt transformation and dietary vegetable lipids affect intestinal nutrient uptake, barrier function and plasma cortisol levels in Atlantic salmon. Aquaculture. 2007;273:298–311. [Google Scholar]
- 241.Karasov WH, Caviedes-Vidal E, Hartman Bakken B, Izhaki I, Samuni-Blank M, Arad Z. Capacity for absorption of watear-soluble secondary metabolites greater in birds than in rodents. PLoS ONE. 2012;7(2):e32417. doi: 10.1371/journal.pone.0032417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Karasov WH, Gatica-Sosa C, Brzek P, Caviedes-Vidal E. Gene expression basis for flexibility of intestinal maltase activity in young house sparrows. FASEB J. 2010:lb617. [Google Scholar]
- 243.Karasov WH. Digestive physiology: A view from molecules to ecosystem. Am J Physiol Regul Integr Comp Physiol. 2011;301:R276–R284. doi: 10.1152/ajpregu.00600.2010. [DOI] [PubMed] [Google Scholar]
- 244.Karasov WH, Cork SJ. Glucose absorption by a nectarivorous bird: The passive pathway is paramount. Am J Physiol. 1994;267:G18–G26. doi: 10.1152/ajpgi.1994.267.1.G18. [DOI] [PubMed] [Google Scholar]
- 245.Karasov WH, Diamond JM. Interplay between physiology and ecology in digestion. Bioscience. 1988;38:602–611. [Google Scholar]
- 246.Karasov WH, Hume ID. Vertebrate gastrointestinal system. In: Dantzler W, editor. Handbook of Comparative Physiology. Bethesda, MD: American Physiological Society; 1997. pp. 409–480. [Google Scholar]
- 247.Karasov WH, Levey DJ. Digestive system trade-offs and adaptations of frugivorous passerine birds. Physiol Zool. 1990;63:1248–1270. [Google Scholar]
- 248.Karasov WH, Martínez del Rio C. Physiological Ecology: How Animals Process Energy, Nutrients, and Toxins. New Jersey: Princeton University Press; 2007. [Google Scholar]
- 249.Karasov WH, Martinez del Rio C, Caviedes-Vidal E. Ecological physiology of diet and digestive systems. Annu Rev Physiol. 2011;73:69–93. doi: 10.1146/annurev-physiol-012110-142152. [DOI] [PubMed] [Google Scholar]
- 250.Karasov WH, McWilliams SR. Digestive constraint in mammalian and avian ecology. In: Starck JM, Wang T, editors. Physiological and Ecological Adaptations to Feeding In Vertebrates. Enfield, New Hampshire: Science Publishers, Inc; 2005. pp. 87–112. [Google Scholar]
- 251.Karasov WH, Meyer MW, Darken BW. Tannic acid inhibition of amino acid and sugar absorption by mouse and vole intestine - tests following acute and subchronic exposure. J Chem ecol. 1992;18:719–736. doi: 10.1007/BF00994610. [DOI] [PubMed] [Google Scholar]
- 252.Karasov WH, Pinshow B, Starck JM, Afik D. Anatomical and histological changes in the alimentary tract of migrating blackcaps (Sylvia atricapilla): A comparison among fed, fasted, food-restricted and refed birds. Physiol Biochem Zool. 2004;77:149–160. doi: 10.1086/381465. [DOI] [PubMed] [Google Scholar]
- 253.Kellett GL, Brot-Laroche E, Mace OJ, Leturque A. Sugar absorption in the intestine: The role of GLUT2. Annu Rev Nutr. 2008;28:35–54. doi: 10.1146/annurev.nutr.28.061807.155518. [DOI] [PubMed] [Google Scholar]
- 254.Kellett GL, Helliwell PA. The diffusive component of intestinal glucose absorption is mediated by the glucose-induced recruitment of GLUT2 to the brush-border membrane. Biochem J. 2000;350(Pt 1):155–162. [PMC free article] [PubMed] [Google Scholar]
- 255.Kimmich GA, Randles J. Phloretin-like action of bioflavonoids on sugar accumulation capability of isolated intestinal cells. Membr Biochem. 1978;1:221–237. doi: 10.3109/09687687809063849. [DOI] [PubMed] [Google Scholar]
- 256.King DE, Asem EK, Adeola O. Ontogenetic development of intestinal digestive functions in white Pekin ducks. J Nutr. 2000;130:57–62. doi: 10.1093/jn/130.1.57. [DOI] [PubMed] [Google Scholar]
- 257.Knott KK, Barboza PS, Bowyer RT. Postnatal development and organ maturation in Rangifer tarandus and Ovibos moschatus . J Mammal. 2005;86:121–130. [Google Scholar]
- 258.Knott KK, Barboza PS, Bowyer RT, Blake JE. Nutritional development of feeding strategies in arctic ruminants: Digestive morphometry of reindeer Rangifer tarandus, and muskoxen Ovibos moschatus . Zoology. 2004;107:315–333. doi: 10.1016/j.zool.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 259.Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE. Microbes and Health Sackler Colloquium: Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kofuji PYM, Akimoto A, Hosokawa H, Masumoto T. Seasonal changes in proteolytic enzymes of yellowtail Seriola quinqueradiata (Temminck & Schlegel; Carangidae) fed extruded diets containing different protein and energy levels. Aquac Res. 2005;36:696–703. [Google Scholar]
- 261.Kohl K, Brzek P, Caviedes-Vidal E, Karasov WH. Matching between dietary preferences and digestive capacity in passerine birds. Integr Comp Biol. 2010;50:e92. [Google Scholar]
- 262.Kohl KD, Brzek P, Caviedes-Vidal E, Karasov WH. Pancreatic and intestinal carbohydrases are matched to dietary starch level in wild Passerine birds. Physiol Biochem Zool. 2011;84:195–203. doi: 10.1086/658146. [DOI] [PubMed] [Google Scholar]
- 263.Kojima T, Nishimura M, Yajima T, Kuwata T, Suzuki Y, Goda T, Takase S, Harada E. Developmental changes in the regional Na+/glucose transporter mRNA along the small intestine of suckling rats. Comp Biochem Physiol B Biochem Mol Biol. 1999;122:89–95. doi: 10.1016/s0305-0491(98)10159-1. [DOI] [PubMed] [Google Scholar]
- 264.Kolkovski S. Digestive enzymes in fish larvae and juveniles - implications and applications to formulated diets. Aquaculture. 2001;200:181–201. [Google Scholar]
- 265.Konarzewski M, Diamond J. Evolution of basal metabolic rate and organ masses in laboratory mice. Evolution. 1995;49:1239–1248. doi: 10.1111/j.1558-5646.1995.tb04450.x. [DOI] [PubMed] [Google Scholar]
- 266.Konarzewski M, Koyama S, Swierubska T, Lewonczuk B. Effect of short-term feed restriction, realimentation and overfeeding on growth of Song Thrush (Turdus philomelos) nestlings. Funct Ecol. 1996;10:97–105. [Google Scholar]
- 267.Kottra G, Daniel H. Flavonoid glycosides are not transported by the human Na+/glucose transporter when expressed in Xenopus laevis oocytes, but effectively inhibit electrogenic glucose uptake. J Pharmacol Exp Ther. 2007;322:829–835. doi: 10.1124/jpet.107.124040. [DOI] [PubMed] [Google Scholar]
- 268.Kourie JI, Shorthouse AA. Properties of cytotoxic peptide-formed ion channels. Am J Physiol Cell Physiol. 2000;278:C1063–C1087. doi: 10.1152/ajpcell.2000.278.6.C1063. [DOI] [PubMed] [Google Scholar]
- 269.Krogdahl A, Hemre GI, Mommsen TP. Carbohydrates in fish nutrition: Digestion and absorption in postlarval stages. Aquacult Nutr. 2005;11:103–122. [Google Scholar]
- 270.Krogdahl A, Sell JL. Influence of age on lipase, amylase, and protease activities in pancreatic tissue and intestinal contents of young turkeys. Poult Sci. 1989;68:1561–1568. doi: 10.3382/ps.0681561. [DOI] [PubMed] [Google Scholar]
- 271.Kung L, Smith KA, Smagala AM, Endres KM, Bessett CA, Ranjit NK, Yaissle J. Effects of 9,10 anthraquinone on ruminal fermentation, totaltract digestion, and blood metabolite concentrations in sheep. J Anim Sci. 2003;81:323–328. doi: 10.2527/2003.811323x. [DOI] [PubMed] [Google Scholar]
- 272.Kurokawa T, Suzuki T. Development of intestinal brush border aminopeptidase in the larval Japanese flounder Paralichthys olivaceus. Aquaculture. 1998;162:113–124. [Google Scholar]
- 273.Kvale A, Mangor-Jensen A, Moren M, Espe M, Hamre K. Development and characterisation of some intestinal enzymes in Atlantic cod (Gadus morhua L.) and Atlantic halibut (Hippoglossus hippoglossus L.) larvae. Aquaculture. 2007;264:457–468. [Google Scholar]
- 274.Kwon O, Eck P, Chen SL, Corpe CP, Lee JH, Kruhlak M, Levine M. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J. 2007;21:366–377. doi: 10.1096/fj.06-6620com. [DOI] [PubMed] [Google Scholar]
- 275.Laino A, Cunningham ML, Garcia F, Heras H. First insight into the lipid uptake, storage and mobilization in arachnids: Role of midgut diverticula and lipoproteins. J Insect Physiol. 2009;55:1118–1124. doi: 10.1016/j.jinsphys.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 276.Lalles JP. Intestinal alkaline phosphatase: Multiple biological roles in maintenance of intestinal homeostasis and modulation by diet. Nutr Rev. 2010;68:323–332. doi: 10.1111/j.1753-4887.2010.00292.x. [DOI] [PubMed] [Google Scholar]
- 277.Lane JS, Whang EE, Rigberg DA, Hines OJ, Kwan D, Zinner MJ, McFadden DW, Diamond J, Ashley SW. Paracellular glucose transport plays a minor role in the unanesthetized dog. Am J Physiol. 1999;276:G789–G794. doi: 10.1152/ajpgi.1999.276.3.G789. [DOI] [PubMed] [Google Scholar]
- 278.Lavin SR, Karasov WH. Allometry of paracellular absorption in birds. Physiol Biochem Zool. 2008;81:551–560. doi: 10.1086/588176. [DOI] [PubMed] [Google Scholar]
- 279.Lavin SR, Karasov WH, Ives AR, Middleton KM, Garland TJ. Morphometrics of the avian small intestine compared with that of nonflying mammals: A phylogenetic approach. Physiol Biochem Zool. 2008;81:526–550. doi: 10.1086/590395. [DOI] [PubMed] [Google Scholar]
- 280.Lavin SR, McWhorter TJ, Karasov WH. Mechanistic bases for differences in passive absorption. J Exp Biol. 2007;210:2754–2764. doi: 10.1242/jeb.006114. [DOI] [PubMed] [Google Scholar]
- 281.Lazo JP, Holt GJ, Arnold CR. Ontogeny of pancreatic enzymes in larval red drum Sciaenops ocellatus. Aquacult Nutr. 2000;6:183–192. [Google Scholar]
- 282.Lecona E, Olmo N, Turnay J, Santiago-Gomez A, Lopez de Silanes I, Gorospe M, Lizarbe MA. Kinetic analysis of butyrate transport in human colon adenocarcinoma cells reveals two different carrier-mediated mechanisms. Biochem J. 2008;409:311–320. doi: 10.1042/BJ20070374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Ledon-Rettig CC, Pfennig DW, Nascone-Yoder N. Ancestral variation and the potential for genetic accommodation in larval amphibians: Implications for the evolution of novel feeding strategies. Evol Dev. 2008;10:316–325. doi: 10.1111/j.1525-142X.2008.00240.x. [DOI] [PubMed] [Google Scholar]
- 284.Lehane M. Peritrophic matrix structure and function. Annu Rev Entomol. 1997;42:525–550. doi: 10.1146/annurev.ento.42.1.525. [DOI] [PubMed] [Google Scholar]
- 285.Lehman RM, Lundgren JG, Petzke LM. Bacterial communities associated with the digestive tract of the predatory ground beetle, Poecilus chalcites, and their modification by laboratory rearing and antibiotic treatment. Microb Ecol. 2009;57:349–358. doi: 10.1007/s00248-008-9415-6. [DOI] [PubMed] [Google Scholar]
- 286.Lepczyk CA, Caviedes-Vidal E, Karasov WH. Digestive responses during food restriction and realimentation in nestling house sparrows (Passer domesticus) Physiol Zool. 1998;71:561–573. doi: 10.1086/515965. [DOI] [PubMed] [Google Scholar]
- 287.Levey DJ, Karasov WH. Digestive responses of temperate birds switched to fruit or insect diets. Auk. 1989;106:675–686. [Google Scholar]
- 288.Levey DJ, Karasov WH. Digestive modulation in a seasonal frugivore, the American robin (Turdus migratorius) Am J Physiol. 1992;262:G711–G718. doi: 10.1152/ajpgi.1992.262.4.G711. [DOI] [PubMed] [Google Scholar]
- 289.Levey DJ, Place AR, Rey PJ, Martinez del Rio C. An experimental test of dietary enzyme modulation in pine warblers Dendroica pinus . Physiol Biochem Zool. 1999;72(5):576–587. doi: 10.1086/316689. [DOI] [PubMed] [Google Scholar]
- 290.Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS, Schlegel ML, Tucker TA, Schrenzel MD, Knight R, Gordon JI. Evolution of mammals and their gut microbes. Science. 2008;320:1647–1651. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Li F, Li W, Fu HW, Koike K. Pancreatic lipase-inhibiting triterpenoid saponins from fruits of Acanthopanax senticosus. Chem Pharm Bull. 2007;55:1087–1089. doi: 10.1248/cpb.55.1087. [DOI] [PubMed] [Google Scholar]
- 292.Li H, Gilbert ER, Zhang Y, Crasta O, Emmerson D, Webb KE, Wong EA. Expression profiling of the solute carrier gene family in chicken intestine from the late embryonic to early post-hatch stages. Animal Genetics. 2008;39:407–424. doi: 10.1111/j.1365-2052.2008.01744.x. [DOI] [PubMed] [Google Scholar]
- 293.Li S, Sauer WC, Caine WR. Response of nutrient digestibilities to feeding diets with low and high levels of soybean trypsin inhibitors in growing pigs. J Sci Food Agric. 1998;76:357–363. [Google Scholar]
- 294.Liao SF, Harmon DL, Vanzant ES, McLeod KR, Boling JA, Matthews JC. The small intestinal epithelia of beef steers differentially express sugar transporter messenger ribonucleic acid in response to abomasal versus ruminal infusion of starch hydrolysate. J Anim Sci. 2010;88:306–314. doi: 10.2527/jas.2009-1992. [DOI] [PubMed] [Google Scholar]
- 295.Linton SM, Greenaway P. A review of feeding and nutrition of herbivorous land crabs: Adaptations to low quality plant diets. J Comp Physiol B. 2007;177:269–286. doi: 10.1007/s00360-006-0138-z. [DOI] [PubMed] [Google Scholar]
- 296.Liu QS, Wang DH. Effects of diet quality on phenotypic flexibility of organ size and digestive function in Mongolian gerbils (Meriones unguiculatus) J Comp Physiol B. 2007;177:509–518. doi: 10.1007/s00360-007-0149-4. [DOI] [PubMed] [Google Scholar]
- 297.Logan JD, Joern A, Wolesensky W. Location, time, and temperature dependence of digestion in simple animal tracts. J Theor Biol. 2002;216:5–18. doi: 10.1006/jtbi.2002.2541. [DOI] [PubMed] [Google Scholar]
- 298.Logan JD, Joern A, Wolesensky W. Chemical reactor models of optimal digestion efficiency with constant foraging costs. Ecol Modell. 2003;168:25–38. [Google Scholar]
- 299.Lohner K, Schnabele K, Daniel H, Oesterle D, Rechkemmer G, Gottlicher M, Wenzel U. Flavonoids alter P-gp expression in intestinal epithelial cells in vitro and in vivo. Mol Nutr Food Res. 2007;51:293–300. doi: 10.1002/mnfr.200600225. [DOI] [PubMed] [Google Scholar]
- 300.Lowry JB, McSweeney CS, Palmer B. Changing perceptions of the effect of plant phenolics on nutrient supply in the ruminant. Aust J Agric Res. 1996;47:829–842. [Google Scholar]
- 301.Lundgren JG, Weber DC. Changes in digestive rate of a predatory beetle over its larval stage: Implications for dietary breadth. J Insect Physiol. 2010;56:431–437. doi: 10.1016/j.jinsphys.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 302.Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol. 2007;582:379–392. doi: 10.1113/jphysiol.2007.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Mackie RI. Mutualistic fermentative digestion in the gastrointestinal tract: Diversity and evolution. Integr Comp Biol. 2002;42:319–326. doi: 10.1093/icb/42.2.319. [DOI] [PubMed] [Google Scholar]
- 304.Mandal S, Ghosh K. Inhibitory effect of Pistia tannin on digestive enzymes of Indian major carps: An in vitro study. Fish Physiol Biochem. 2010;36:1171–1180. doi: 10.1007/s10695-010-9395-6. [DOI] [PubMed] [Google Scholar]
- 305.Manichanh C, Reeder J, Gibert P, Varela E, Llopis M, Antolin M, Guigo R, Knight R, Guarner F. Reshaping the gut microbiome with bacterial transplantation and antibiotic intake. Genome Res. 2010;20:1411–1419. doi: 10.1101/gr.107987.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Marshall SDG, Gatehouse LN, Becher SA, Christeller JT, Gatehouse HS, Hurst MRH, Boucias DG, Jackson TA. Serine proteases identified from a Costelytra zealandica (White) (Coleoptera: Scarabaeidae) midgut EST library and their expression through insect development. Insect Mol Biol. 2008;17:247–259. doi: 10.1111/j.1365-2583.2008.00798.x. [DOI] [PubMed] [Google Scholar]
- 307.Martel F, Monteiro R, Calhau C. Effect of polyphenols on the intestinal and placental transport of some bioactive compounds. Nutr Res Rev. 2010;23:47–64. doi: 10.1017/S0954422410000053. [DOI] [PubMed] [Google Scholar]
- 308.Martin AW, Fuhrman FA. The relationship between summated tissue respiration and metabolic rate in the mouse and dog. Physiol Zool. 1955;28:18–34. [Google Scholar]
- 309.Martinez del Rio C. Dietary, phylogenetic, and ecological correlates of intestinal sucrase and maltase activity in birds. Physiol Zool. 1990;63:987–1011. [Google Scholar]
- 310.Martinez del Rio C. Sugar preferences in hummingbirds: The influence of subtle chemical differences on food choice. Condor. 1990;92:1022–1030. [Google Scholar]
- 311.Martinez TF, McAllister TA, Wang YX, Reuter T. Effects of tannic acid and quebracho tannins on in vitro ruminal fermentation of wheat and corn grain. J Sci Food Agric. 2006;86:1244–1256. [Google Scholar]
- 312.Mascolo N, Rajendran VM, Binder HJ. Mechanism of short-chain fatty acid uptake by apical membrane vesicles of rat distal colon. Gastroenterology. 1991;101:331–338. doi: 10.1016/0016-5085(91)90008-9. [DOI] [PubMed] [Google Scholar]
- 313.Mazumdar-Leighton S, Broadway RM. Transcriptional induction of diverse midgut trypsins in larval Agrotis ipsilon and Helicoverpa zea feeding on the soybean trypsin inhibitor. Insect Biochem Mol Biol. 2001;31:645–657. doi: 10.1016/s0965-1748(00)00169-7. [DOI] [PubMed] [Google Scholar]
- 314.McSweeney CS, Palmer B, McNeill DM, Krause DO. Microbial interactions with tannins: Nutritional consequences for ruminants. Anim Feed Sci Technol. 2001;91:83–93. [Google Scholar]
- 315.McWhorter TJ, Caviedes-Vidal E, Karasov WH. The integration of digestion and osmoregulation in the avian gut. Biol Rev. 2009;84:553–565. doi: 10.1111/j.1469-185X.2009.00086.x. [DOI] [PubMed] [Google Scholar]
- 316.McWhorter TJ, Green AK, Karasov WH. Assessment of radiolabeled D-glucose and the nonmetabolizable analog 3-O-methyl-D-glucose as tools for in vivo absorption studies. Physiol Biochem Zool. 2010;83:376–384. doi: 10.1086/597524. [DOI] [PubMed] [Google Scholar]
- 317.Meissner B, Boll M, Daniel H, Baumeister R. Deletion of the intestinal peptide transporter affects insulin and TOR signaling in Caenorhabditis elegans. J Biol Chem. 2004;279:36739–36745. doi: 10.1074/jbc.M403415200. [DOI] [PubMed] [Google Scholar]
- 318.Meleshkevitch EA, Assis-Nascimento P, Popova LB, Miller MM, Kohn AB, Phung EN, Mandal A, Harvey WR, Boudko DY. Molecular characterization of the first aromatic nutrient transporter from the sodium neurotransmitter symporter family. J Exp Biol. 2006;209:3183–3198. doi: 10.1242/jeb.02374. [DOI] [PubMed] [Google Scholar]
- 319.Meleshkevitch EA, Robinson M, Popova LB, Miller MM, Harvey WR, Boudko DY. Cloning and functional expression of the first eukaryotic Na+-tryptophan symporter, AgNAT6. J Exp Biol. 2009;212:1559–1567. doi: 10.1242/jeb.027383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Mika M, Wikiera A, Zyla K. Effects of non-fermented tea extracts on in vitro digestive hydrolysis of lipids and on cholesterol precipitation. Eur Food Res Technol. 2008;226:731–736. [Google Scholar]
- 321.Miller MM, Popova LB, Meleshkevitch EA, Tran PV, Boudko DY. The invertebrate B(0) system transporter, D, melanogaster NAT1, has unique d-amino acid affinity and mediates gut and brain functions. Insect Biochem Mol Biol. 2008;38:923–931. doi: 10.1016/j.ibmb.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Miszner A, Peres A, Castagna M, Bette S, Giovannardi S, Cherubino F, Bossi E. Structural and functional basis of amino acid specificity in the invertebrate cotransporter KAAT1. J Physiol. 2007;581:899–913. doi: 10.1113/jphysiol.2007.132555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Mitjans M, Ferrer R. Morphometric study of the guinea pig small intestine during development. Microsc Res Tech. 2004;63:206–214. doi: 10.1002/jemt.20030. [DOI] [PubMed] [Google Scholar]
- 324.Miyauchi S, Gopal E, Fei YJ, Ganapathy V. Functional identification of SLC5A8, a tumor suppressor down-regulated in colon cancer, as a Na(+)-coupled transporter for short-chain fatty acids. J Biol Chem. 2004;279:13293–13296. doi: 10.1074/jbc.C400059200. [DOI] [PubMed] [Google Scholar]
- 325.Moens PB, Kolodziejczyk S. Isozymes of amylase, alcohol dehydrogenase, malic enzyme, malate dehydrogenase, and superoxide dimutase in Chloealtis conspersa (Orthoptera) Genome. 1989;32:596–600. [Google Scholar]
- 326.Mohan S, Ma PWK, Williams WP, Luthe DS. A naturally occurring plant cysteine protease possesses remarkable toxicity against insect pests and synergizes Bacillus thuringiensis toxin. Plos One. 2008;3:e1786. doi: 10.1371/journal.pone.0001786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Morais S, Conceicao LEC, Ronnestad I, Koven W, Cahu C, Infante JLZ, Dinis MT. Dietary neutral lipid level and source in marine fish larvae: Effects on digestive physiology and food intake. Aquaculture. 2007;268:106–122. [Google Scholar]
- 328.Morais S, Koven W, Ronnestad I, Dinis MT, Conceicao LEC. Dietary protein: Lipid ratio and lipid nature affects fatty acid absorption and metabolism in a teleost larva. British J Nutr. 2005;93:813–820. doi: 10.1079/bjn20051378. [DOI] [PubMed] [Google Scholar]
- 329.Morais S, Lacuisse M, Conceicao LEC, Dinis MT, Ronnestad I. Ontogeny of the digestive capacity of (Solea senegalensis), with respect to and metabolism of amino acids from Senegalese sole digestion, absorption Artemia. Mar Biol. 2004;145:243–250. [Google Scholar]
- 330.Moran AW, Al-Rammahi MA, Arora DK, Batchelor DJ, Coulter EA, Ionescu C, Bravo D, Shirazi-Beechey SP. Expression of Na+/glucose co-transporter 1 (SGLT1) in the intestine of piglets weaned to different concentrations of dietary carbohydrate. Br J Nutr. 2010;104:647–655. doi: 10.1017/S0007114510000954. [DOI] [PubMed] [Google Scholar]
- 331.Morris ME, Zhang SZ. Flavonoid-drug interactions: Effects of flavonoids on ABC transporters. Life Sciences. 2006;78:2116–2130. doi: 10.1016/j.lfs.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 332.Mott CR, Siegel PB, Webb KE, Wong EA. Gene expression of nutrient transporters in the small intestine of chickens from lines divergently selected for high or low juvenile body weight. Poult Sci. 2008;87:2215–2224. doi: 10.3382/ps.2008-00101. [DOI] [PubMed] [Google Scholar]
- 333.Mountfort DO, Campbell J, Clements KD. Hindgut fermentation in three species of marine herbivorous fish. Appl Environ Microbiol. 2002;68:1374–1380. doi: 10.1128/AEM.68.3.1374-1380.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Muegge BD, Kuczynski J, Knights D, Clemente JC, Gonzalez A, Fontana L, Henrissat B, Knight R, Gordon JI. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science. 2011;332:970–974. doi: 10.1126/science.1198719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Murray HM, Gallant JW, Johnson SC, Douglas SE. Cloning and expression analysis of three digestive enzymes from Atlantic halibut (Hippoglossus hippoglossus) during early development: Predicting gastrointestinal functionality. Aquaculture. 2006;252:394–408. [Google Scholar]
- 336.Nakayama T, Hashimoto T, Kajiya K, Kumazawa S. Affinity of polyphenols for lipid bilayers. Biofactors. 2000;13:147–151. doi: 10.1002/biof.5520130124. [DOI] [PubMed] [Google Scholar]
- 337.Narisawa S, Huang L, Iwasaki A, Hasegawa H, Alpers DH, Millan JL. Accelerated fat absorption in intestinal alkaline phosphatase knockout mice. Mol Cell Biol. 2003;23:7525–7530. doi: 10.1128/MCB.23.21.7525-7530.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Navarro E, Mendez S, Ibarrola I, Urrutia MB. Comparative utilization of phytoplankton and vascular plant detritus by the cockle Cerastoderma edule: Digestive responses during diet acclimation. Aqua Biol. 2009;6:247–262. [Google Scholar]
- 339.Naya DE, Karasov WH, Bozinovic F. Phenotypic plasticity in laboratory mice and rats: A meta-analysis of current ideas on gut size flexibility. Evol Ecol Res. 2007;9:1–12. [Google Scholar]
- 340.Neal JJ. Brush border membrane and amino acid transport. Arch Insect Biochem Physiol. 1996;32:55–64. [Google Scholar]
- 341.Nedergaard S. Amino acid transport. In: Gupta BL, Moreton RB, Oschman JL, Wall BJ, editors. Transport of Ions and Water in Animals. London: Academic Press; 1977. pp. 381–401. [Google Scholar]
- 342.Nemeth K, Plumb GW, Berrin JG, Juge N, Jacob R, Naim HY, Williamson G, Swallow DM, Kroon PA. Deglycosylation by small intestinal epithelial cell beta-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur J Nutr. 2003;42:29–42. doi: 10.1007/s00394-003-0397-3. [DOI] [PubMed] [Google Scholar]
- 343.Nielsen HM, Rassing MR. Nicotine permeability across the buccal TR146 cell culture model and porcine buccal mucosa in vitro: Effect of pH and concentration. Eur J Pharm Sci. 2002;16:151–157. doi: 10.1016/s0928-0987(02)00083-0. [DOI] [PubMed] [Google Scholar]
- 344.Ninomiya K, Matsuda H, Shimoda H, Norihisa N, Kasajima N, Yoshino T, Morikawa T, Yoshikawa M. Carnosic acid, a new class of lipid absorption inhibitor from sage. Bioorg Med Chem Lett. 2004;14:1943–1946. doi: 10.1016/j.bmcl.2004.01.091. [DOI] [PubMed] [Google Scholar]
- 345.Nisbet AJ, Billingsley PF. A comparative survey of the hydrolytic enzymes of ectoparasitic and free-living mites. Int J Parasitol. 2000;30:19–27. doi: 10.1016/s0020-7519(99)00169-1. [DOI] [PubMed] [Google Scholar]
- 346.Novakova R, Homerova D, Kinne RKH, Kinne-Saffran E, Lin JT. Identification of a region critically involved in the interaction of phlorizin with the rabbit sodium-D-glucose cotransporter SGLT1. J Membr Biol. 2001;184:55–60. doi: 10.1007/s00232-001-0073-6. [DOI] [PubMed] [Google Scholar]
- 347.O’Connor TP, Diamond J. Ontogeny of intestinal safety factors: Lactase capacities and lactose loads. Am J Physiol Regul Integr Comp Physiol. 1999;276:R753–R765. doi: 10.1152/ajpregu.1999.276.3.R753. [DOI] [PubMed] [Google Scholar]
- 348.Obst BS, Diamond J. Ontogeny of intestinal nutrient transport in domestic chickens (Gallus gallus) and its relation to growth. Auk. 1992;109:451–464. [Google Scholar]
- 349.Ohkuma M, Noda S, Hongoh Y, Nalepa CA, Inoue T. Inheritance and diversification of symbiotic trichonymphid flagellates from a common ancestor of termites and the cockroach Cryptocercus. Proc Biol Sci. 2009;276:239–245. doi: 10.1098/rspb.2008.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Oku T, Yamada M, Nakamura M, Sadamori N, Nakamura S. Inhibitory effects of extractives from leaves of Morus alba on human and rat small intestinal disaccharidase activity. Br J Nutr. 2006;95:933–938. doi: 10.1079/bjn20061746. [DOI] [PubMed] [Google Scholar]
- 351.Oliveira DM, Freitas HS, Souza MFF, Arcari DP, Ribeiro ML, Carvalho PO, Bastos DHM. Yerba mate (Ilex paraguariensis) aqueous extract decreases intestinal SGLT1 gene expression but does not affect other biochemical parameters in Alloxan-Diabetic Wistar Rats. J Agric Food Chem. 2008;56:10527–10532. doi: 10.1021/jf8021404. [DOI] [PubMed] [Google Scholar]
- 352.Onal U, Langdon C, Celik I. Ontogeny of the digestive tract of larval percula clownfish, Amphiprion percula (Lacepede 1802): A histological perspective. Aquac Res. 2008;39:1077–1086. [Google Scholar]
- 353.Orlando PA, Brown JS, Whelan CJ. Co-adaptations of feeding behaviours and gut modulation as a mechanism of co-existence. Evol Ecol Res. 2009;11:541–560. [Google Scholar]
- 354.Pacha J. Development of intestinal transport function in mammals. Physiol Rev. 2000;80(4):1633–1667. doi: 10.1152/physrev.2000.80.4.1633. [DOI] [PubMed] [Google Scholar]
- 355.Palo RT. Usnic acid, a secondary metabolite of lichens and its effect on in vitro digestibility in reindeer. Rangifer. 1993;13:39–43. [Google Scholar]
- 356.Pankoke H, Bowers MD, Dobler S. Influence of iridoid glycoside containing host plants on midgut beta-glucosidase activity in a polyphagous caterpillar, Spilosoma virginica Fabricius (Arctiidae) J Insect Physiol. 2010;56:1907–1912. doi: 10.1016/j.jinsphys.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 357.Patra AK. Meta-analyses of effects of phytochemicals on digestibility and rumen fermentation characteristics associated with methanogenesis. J Sci Food Agric. 2010;90:2700–2708. doi: 10.1002/jsfa.4143. [DOI] [PubMed] [Google Scholar]
- 358.Pauchet Y, Wilkinson P, Chauhan R, Ffrench-Constant RH. Diversity of beetle genes encoding novel plant cell wall degrading enzymes. PLoS One. 2010;5:e15635. doi: 10.1371/journal.pone.0015635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Pena R, Dumas S, Villalejo-Fuerte M, Ortiz-Galindo JL. Ontogenetic development of the digestive tract in reared spotted sand bass Paralabrax maculatofasciatus larvae. Aquaculture. 2003;219:633–644. [Google Scholar]
- 360.Penry DL. Digestive kinematics of suspension-feeding bivalves: Modeling and measuring particle-processing in the gut of Potamocorbula amurensis. Mar Ecol Prog Ser. 2000;197:181–192. [Google Scholar]
- 361.Penry DL, Jumars PA. Modeling animal guts as chemical reactors. Am Nat. 1987;129:69–96. [Google Scholar]
- 362.Peral MJ, Galvez M, Soria ML, Ilundain AA. Developmental decrease in rat small intestinal creatine uptake. Mech Ageing Dev. 2005;126:523–530. doi: 10.1016/j.mad.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 363.Perry GH, Dominy NJ, Claw KG, Lee AS, Fiegler H, Redon R, Werner J, Villanea FA, Mountain JL, Misra R, Carter NP, Lee C, Stone AC. Diet and the evolution of human amylase gene copy number variation. Nat Genet. 2007;39:1256–1260. doi: 10.1038/ng2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Piersma T. Energetic bottlenecks and other design constraints in avian annual cycles. Integr Comp Biol. 2002;42:51–67. doi: 10.1093/icb/42.1.51. [DOI] [PubMed] [Google Scholar]
- 365.Pitta DW, Pinchak E, Dowd SE, Osterstock J, Gontcharova V, Youn E, Dorton K, Yoon I, Min BR, Fulford JD, Wickersham TA, Malinowski DP. Rumen bacterial diversity dynamics associated with changing from bermudagrass hay to grazed winter wheat diets. Microb Ecol. 2010;59:511–522. doi: 10.1007/s00248-009-9609-6. [DOI] [PubMed] [Google Scholar]
- 366.Poelstra K, Bakker WW, Klok PA, Hardonk MJ, Meijer DKF. A physiologic function for alkaline phosphatase: Endotoxin detoxification. Lab Invest. 1997;76:319–327. [PubMed] [Google Scholar]
- 367.Porter EM, Bevins CL, Ghosh D, Ganz T. The multifaceted Paneth cell. Cell Mol Life Sci. 2002;59:156–170. doi: 10.1007/s00018-002-8412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Price DR, Tibbles K, Shigenobu S, Smertenko A, Russell CW, Douglas AE, Fitches E, Gatehouse AM, Gatehouse JA. Sugar transporters of the major facilitator superfamily in aphids; from gene prediction to functional characterization. Insect Mol Biol. 2010;19(Suppl 2):97–112. doi: 10.1111/j.1365-2583.2009.00918.x. [DOI] [PubMed] [Google Scholar]
- 369.Prudence M, Moal J, Boudry P, Daniel JY, Quéré C, Jeffroy F, Mingant C, Ropert M, Bédier E, Van Wormhoudt A, Samain JF, Huvet A. An amylase gene polymorphism is associated with growth differences in the Pacific cupped oyster Crassostrea gigas . Anim Genet. 2006;37:348–351. doi: 10.1111/j.1365-2052.2006.01481.x. [DOI] [PubMed] [Google Scholar]
- 370.Puchal AA, Buddington RK. Postnatal development of monosaccharide transport in pig intestine. Am J Physiol. 1992;262:G902–G902. doi: 10.1152/ajpgi.1992.262.5.G895. [DOI] [PubMed] [Google Scholar]
- 371.Qandeel HG, Duenes JA, Zheng Y, Sarr MG. Diurnal expression and function of peptide transporter 1 (PEPT1) J Surg Res. 2009;156:123–128. doi: 10.1016/j.jss.2009.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Jian M, Zhou Y, Li Y, Zhang X, Qin N, Yang H, Wang J, Brunak S, Dore J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, Bork P, Ehrlich SD. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Ramirez-Otarola N, Narvaez C, Sabat P. Membrane-bound intestinal enzymes of passerine birds: Dietary and phylogenetic correlates. J Comp Physiol B. 2011;181:817–827. doi: 10.1007/s00360-011-0557-3. [DOI] [PubMed] [Google Scholar]
- 374.Ramzi S, Hosseininaveh V. Biochemical characterization of digestive alpha-amylase, alpha-glucosidase and beta-glucosidase in pistachio green stink bug Brachynema germari Kolenati (Hemiptera: Pentatomidae) J Asia-Pacific Entomol. 2010;13:215–219. [Google Scholar]
- 375.Regel R, Matioli SR, Terra WR. Molecular adaptation of Drosophila melanogaster lysozymes to a digestive function. Insect Biochem Mol Biol. 1998;28:309–319. doi: 10.1016/s0965-1748(97)00108-2. [DOI] [PubMed] [Google Scholar]
- 376.Reid GK, Liutkus M, Bennett A, Robinson SMC, MacDonald B, Page F. Absorption efficiency of blue mussels (Mytilus edulis and M. trossulus) feeding on Atlantic salmon (Salmo salar) feed and fecal particulates: Implications for integrated multi-trophic aquaculture. Aquaculture. 2010;299:165–169. [Google Scholar]
- 377.Remis MJ, Dierenfeld ES. Digesta passage, digestibility and behavior in captive gorillas under two dietary regimens. Int J Primatol. 2004;25:825–845. [Google Scholar]
- 378.Reynolds DA, Rajendran VM, Binder HJ. Bicarbonate-stimulated [14C]butyrate uptake in basolateral membrane vesicles of rat distal colon. Gastroenterology. 1993;105:725–732. doi: 10.1016/0016-5085(93)90889-k. [DOI] [PubMed] [Google Scholar]
- 379.Ribble D, Smith M. Relative intestine length and feeding ecology of freshwater fishes. Growth. 1983;47:292–300. [PubMed] [Google Scholar]
- 380.Rivest J, Bernier JF, Pomar C. A dynamic model of protein digestion in the small intestine of pigs. J Anim Sci. 2000;78:328–340. doi: 10.2527/2000.782328x. [DOI] [PubMed] [Google Scholar]
- 381.Robinson CJ, Schloss P, Ramos Y, Raffa K, Handelsman J. Robustness of the bacterial community in the cabbage white butterfly larval midgut. Microb Ecol. 2010;59:199–211. doi: 10.1007/s00248-009-9595-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Roman G, Meller V, Wu KH, Davis RL. The opt1 gene of Drosophila melanogaster encodes a proton-dependent dipeptide transporter. Am J Physiol. 1998;275:C857–C869. doi: 10.1152/ajpcell.1998.275.3.C857. [DOI] [PubMed] [Google Scholar]
- 383.Rossi GD, dos Santos CD, Cardoso MD, Correa AD, de Abreu CMP, Paiva LV. Coffee leaf miner trypsin inhibition with castor bean leaf extracts mediated by a non-protein agent. Ciencia E Agrotecnologia. 2010;34:361–366. [Google Scholar]
- 384.Rougiere N, Carre B. Comparison of gastrointestinal transit times between chickens from D+ and D- genetic lines selected for divergent digestion efficiency. Animal. 2010;4:1861–1872. doi: 10.1017/S1751731110001266. [DOI] [PubMed] [Google Scholar]
- 385.Ryan CA. Proteinase inhibitors. In: Rosenthal GA, Janzen DH, editors. Herbivores: Their Interaction with Secondary Plant Metabolites. New York: Academic Press; 1979. pp. 559–618. [Google Scholar]
- 386.Saele O, Nordgreen A, Olsvik PA, Hamre K. Characterization and expression of digestive neutral lipases during ontogeny of Atlantic cod (Gadus morhua) Comp Biochem Physiol A Mol Integr Physiol. 2010;157:252–259. doi: 10.1016/j.cbpa.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 387.Santo Domingo JW, Kaufman MG, Klug MJ, Holben WE, Harris D, Tiedge JM. Influence of diet on the structure and function of the bacterial hindgut community of crickets. Mol Ecol. 1998;7:761–767. [Google Scholar]
- 388.Sasaki E, Shimada T, Osawa R, Nishitani Y, Spring S, Lang E. Isolation of tannin-degrading bacteria isolated from feces of the Japanese large wood mouse Apodemus speciosus, feeding on tannin-rich acorns. Syst Appl Microbiol. 2005;28:358–365. doi: 10.1016/j.syapm.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 389.Sather BT. A comparative study of amylases and proteinases in some decapod Crustacea. Comp Biochem Physiol. 1969;28:371–379. doi: 10.1016/0010-406x(69)91350-4. [DOI] [PubMed] [Google Scholar]
- 390.Sauter SN, Roffler B, Philipona C, Morel C, Rome V, Guilloteau P, Blum JW, Hammon HM. Intestinal development in neonatal calves: Effects of glucocorticoids and dependence on colostrum feeding. Biol Neonate. 2004;85:94–104. doi: 10.1159/000074965. [DOI] [PubMed] [Google Scholar]
- 391.Scharf ME, Kovaleva ES, Jadhao S, Campbell JH, Buchman GW, Boucias DG. Functional and translational analyses of a beta-glucosidase gene (glycosyl hydrolase family 1) isolated from the gut of the lower termite Reticulitermes flavipes . Insect Biochem Mol Biol. 2010;40:611–620. doi: 10.1016/j.ibmb.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 392.Schondube JE, Herrera LG, Martinez del Rio C. Diet and evolution of digestion and renal function in phyllostomid bats. Zoology. 2001;104:59–73. doi: 10.1078/0944-2006-00007. [DOI] [PubMed] [Google Scholar]
- 393.Schondube JE, Martinez del Rio C. Sugar and protein digestion in flowerpiercers and hummingbirds: A comparative test of adaptive convergence. J Comp Physiol B. 2004;174:263–273. doi: 10.1007/s00360-003-0411-3. [DOI] [PubMed] [Google Scholar]
- 394.Schroder B, Dahl MR, Nurnus U, Breves G. Development of the intestinal calcium and phosphate absorption in piglets and calves during early postnatal life. Tierarztliche Praxis Ausgabe Grobtiere Nutztiere. 1999;27:351–355. [Google Scholar]
- 395.Secor SM, Diamond JM. Evolution of regulatory responses to feeding in snakes. Physiol Biochem Zool. 2000;73:123–141. doi: 10.1086/316734. [DOI] [PubMed] [Google Scholar]
- 396.Sell JL, Koldovsky O, Reid BL. Intestinal disaccharidases of young turkeys: Temporal development and influence of diet composition. Poult Sci. 1989;68:265–277. doi: 10.3382/ps.0680265. [DOI] [PubMed] [Google Scholar]
- 397.Shafizadeh TB, Halsted CH. Postnatal ontogeny of intestinal GCPII and the RFC in pig. Am J Physiol Gastrointest Liver Physiol. 2009;296:G476–G481. doi: 10.1152/ajpgi.00446.2007. [DOI] [PubMed] [Google Scholar]
- 398.Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: A dynamic duo. Annu Rev Physiol. 2011;73:283–309. doi: 10.1146/annurev-physiol-012110-142150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Shimada K, Maekawa K. Changes in endogenous cellulase gene expression levels and reproductive characteristics of primary and secondary reproductives with colony development of the termite Reticulitermes speratus (Isoptera: Rhinotermitidae) J Insect Physiol. 2010;56:1118–1124. doi: 10.1016/j.jinsphys.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 400.Shiraga T, Miyamoto K, Tanaka H, Yamamoto H, Taketani Y, Morita K, Tamai I, Tsuji A, Takeda E. Cellular and molecular mechanisms of dietary regulation on rat intestinal H+/Peptide transporter PepT1. Gastroenterology. 1999;116:354–362. doi: 10.1016/s0016-5085(99)70132-0. [DOI] [PubMed] [Google Scholar]
- 401.Shishikura Y, Khokhar S, Murray BS. Effects of tea polyphenols on emulsification of olive oil in a small intestine model system. J Agric Food Chem. 2006;54:1906–1913. doi: 10.1021/jf051988p. [DOI] [PubMed] [Google Scholar]
- 402.Shu R, David ES, Ferraris RP. Luminal fructose modulates fructose transport and GLUT-5 expression in small intestine of weaning rats. Am J Physiol. 1998;274:G232–G239. doi: 10.1152/ajpgi.1998.274.2.G232. [DOI] [PubMed] [Google Scholar]
- 403.Silva CP, Terra WR, de Sa MFG, Samuels RI, Isejima EM, Bifano TD, Almeida JS. Induction of digestive alpha-amylases in larvae of Zabrotes subfasciatus (Coleoptera : Bruchidae) in response to ingestion of common bean alpha-amylase inhibitor 1. J Insect Physiol. 2001;47:1283–1290. doi: 10.1016/s0022-1910(01)00115-9. [DOI] [PubMed] [Google Scholar]
- 404.Sklan D. Development of the digestive tract of poultry. Worlds Poult Sci J. 2001;57:415–428. [Google Scholar]
- 405.Sklan D, Geyra A, Tako E, Gal-Gerber O, Uni Z. Ontogeny of brush border carbohydrate digestion and uptake in the chick. Br J Nutr. 2003;89(6):747–753. doi: 10.1079/BJN2003853. [DOI] [PubMed] [Google Scholar]
- 406.Sklan D, Noy Y. Hydrolysis and absorption in the small intestines of posthatch chicks. Poult Sci. 2000;79:1306–1310. doi: 10.1093/ps/79.9.1306. [DOI] [PubMed] [Google Scholar]
- 407.Sklan D, Noy Y. Functional development and intestinal absorption in the young poult. Br Poult Sci. 2003;44:651–658. doi: 10.1080/00071660310001618325. [DOI] [PubMed] [Google Scholar]
- 408.Skopec MM, Green AK, Karasov WH. Flavonoids have differential dffects on glucose absorption in rats (Rattus norvegicus) and American robins (Turdis migratorius) J Chem ecol. 2010;36:236–243. doi: 10.1007/s10886-010-9747-9. [DOI] [PubMed] [Google Scholar]
- 409.Skopec MM, Hagerman AE, Karasov WH. Do salivary proline-rich proteins counteract dietary hydrolysable tannin in laboratory rats? J Chem ecol. 2004;30:1679–1692. doi: 10.1023/b:joec.0000042395.31307.be. [DOI] [PubMed] [Google Scholar]
- 410.Slansky F, Scriber JM. Food consumption and utilization. In: Kerkut GA, Gilbert LI, editors. Comprehensive Insect Physiology, Biochemistry and Pharmacology. Vol. 4. Oxford: Pergammon Press; 1985. pp. 87–163. [Google Scholar]
- 411.Song J, Kwon O, Chen SL, Daruwala R, Eck P, Park JB, Levine M. Flavonoid inhibition of sodium-dependent vitamin C transporter 1 (SVCT1) and glucose transporter isoform 2 (GLUT2), intestinal transporters for vitamin C and glucose. J Biol Chem. 2002;277:15252–15260. doi: 10.1074/jbc.M110496200. [DOI] [PubMed] [Google Scholar]
- 412.Sorensen JS, Dearing MD. Efflux transporters as a novel herbivore countermechanism to plant chemical defenses. J Chem Ecol. 2006;32:1181–1196. doi: 10.1007/s10886-006-9079-y. [DOI] [PubMed] [Google Scholar]
- 413.Soriano ME, Planas JM. Developmental study of a-methyl-D-glucoside and L-proline uptake in the small intestine of the White Leghorn chicken. Poult Sci. 1998;77:1347–1353. doi: 10.1093/ps/77.9.1347. [DOI] [PubMed] [Google Scholar]
- 414.Spiller HA, Winter MI, Weber JA, Krenzelok EP, Anderson II, Ryan MI. Skin breakdown and blisters from senna-containing laxatives in young children. Ann Pharmacother. 2004;37:636–639. doi: 10.1345/aph.1C439. [DOI] [PubMed] [Google Scholar]
- 415.Srinivasan A, Giri AP, Gupta VS. Structural and functional diversities in Lepidopteran serine proteases. Cell Mol Biol Lett. 2006;11:132–154. doi: 10.2478/s11658-006-0012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Starck JM. Structural flexibility of the digestive system of tetrapods - patterns and processes at the cellular and tissue level. In: Starck JM, Wang T, editors. Physiological and Ecological Adaptations to Feeding in Vertebrates. Enfield, UK: Science Publishers; 2005. pp. 175–200. [Google Scholar]
- 417.Starck JM, Beese K. Structural flexibility of the intestine of Burmese python in response to feeding. J Exp Biol. 2001;204:325–335. doi: 10.1242/jeb.204.2.325. [DOI] [PubMed] [Google Scholar]
- 418.Stein ED, Diamond JM. Do dietary levels of pantothenic acid regulate its intestinal uptake in mice? J Nutr. 1989;119:1973–1983. doi: 10.1093/jn/119.12.1973. [DOI] [PubMed] [Google Scholar]
- 419.Stevens CE, Hume ID. Comparative Physiology of the Vertebrate Digestive System. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- 420.Stevens CE, Hume ID. Contributions of microbes in vertebrate gastrointestinal tract to production and conservation of nutrients. Physiol Rev. 1998;78:393–427. doi: 10.1152/physrev.1998.78.2.393. [DOI] [PubMed] [Google Scholar]
- 421.Stevens HE, Hume ID. Comparative Physiology of the Vertebrate Digestive System. Cambridge: Cambridge University Press; 2004. [Google Scholar]
- 422.Struempf HM, Schondube JE, Martinez del Rio C. The cyanogenic glycoside amygdalin does not deter consumption of ripe fruit by cedar waxwings. Auk. 1999;116:749–758. [Google Scholar]
- 423.Sundset MA, Barboza PS, Green TK, Folkow LP, Blix AS, Mathiesen SD. Microbial degradation of usnic acid in the reindeer rumen. Naturwissenschaften. 2010;97:273–278. doi: 10.1007/s00114-009-0639-1. [DOI] [PubMed] [Google Scholar]
- 424.Sundset MA, Kohn A, Mathiesen SD, Praesteng Ke. Eubacterium rangiferina, a novel usnic acid-resistant bacterium from the reindeer rumen. Naturwissenschaften. 2008;95:741–749. doi: 10.1007/s00114-008-0381-0. [DOI] [PubMed] [Google Scholar]
- 425.Suzuki T, Douard V, Mochizuki K, Goda T, Ferraris RP. Diet-induced epigenetic regulation in vivo of the intestinal fructose transporter Glut5 during development of rat small intestine. Biochem J. 2011;435:43–53. doi: 10.1042/BJ20101987. [DOI] [PubMed] [Google Scholar]
- 426.Tadmor-Melamed H, Markman S, Arieli A, Distl M, Wink M, Izhaki I. Limited ability of Palestine Sunbirds Nectarinia osea to cope with pyridine alkaloids in nectar of Tree Tobacco Nicotiana glauca . Funct Ecol. 2004;18:844–850. [Google Scholar]
- 427.Tavakkolizadeh A, Ramsanahie A, Levitsky LL, Zinner MJ, Whang EE, Ashley SW, Rhoads DB. Differential role of vagus nerve in maintaining diurnal gene expression rhythms in the proximal small intestine. J Surg Res. 2005;129:73–78. doi: 10.1016/j.jss.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 428.Terra WR, Ferreira C. Insect digestive enzymes - properties, compartmentalization and function. Comp Biochem Physiol B Biochem Mol Biol. 1994;109:1–62. [Google Scholar]
- 429.Terra WR, Ferreira C. Biochemistry of digestion. In: Lawrence IG, Kostas I, Sarjeet SG, editors. Comprehensive Molecular Insect Science. Amsterdam: Elsevier; 2005. pp. 171–224. [Google Scholar]
- 430.Thomas KK, Nation JL. Absorption of glucose, glycine and palmitic acid by isolated midgut and hindgut from crickets. Comp Biochem Physiol A Physiol. 1984;79:289–295. [Google Scholar]
- 431.Tian XJ, Yang XW, Yang XD, Wang K. Studies of intestinal permeability of 36 flavonoids using Caco-2 cell monolayer model. Int J Pharm. 2009;367:58–64. doi: 10.1016/j.ijpharm.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 432.Tiemann TT, Avila P, Ramirez G, Lascano CE, Kreuzer M, Hess HD. In vitro ruminal fermentation of tanniniferous tropical plants: Plant-specific tannin effects and counteracting efficiency of PEG. Anim Feed Sci Technol. 2008;146:222–241. [Google Scholar]
- 433.Timofeeva NM, Egorova VV, Nikitina AA, Dmitrieva JV. Delayed effects of the terms of separation of rat pups from lactating females and low-protein diet on enzyme activity in digestive and non-digestive organs. Bull Exp Biol Med. 2008;145:676–679. doi: 10.1007/s10517-008-0179-2. [DOI] [PubMed] [Google Scholar]
- 434.Tobin V, Le Gall M, Fioramonti X, Stolarczyk E, Blazquez AG, Klein C, Prigent M, Serradas P, Cuif MH, Magnan C, Leturque A, Brot-Laroche E. Insulin internalizes GLUT2 in the enterocytes of healthy but not insulin-resistant mice. Diabetes. 2008;57:555–562. doi: 10.2337/db07-0928. [DOI] [PubMed] [Google Scholar]
- 435.Toloza EM, Diamond J. Ontogenetic development of nutrient transporters in rat intestine. Am J Physiol. 1992;263:G593–G604. doi: 10.1152/ajpgi.1992.263.5.G593. [DOI] [PubMed] [Google Scholar]
- 436.Toloza EM, Diamond JM. Ontogenetic development of nutrient transporters in bullfrog intestine. Am J Physiol. 1990;258:G760–G769. doi: 10.1152/ajpgi.1990.258.5.G760. [DOI] [PubMed] [Google Scholar]
- 437.Toloza EM, Diamond JM. Ontogenetic development of transporter regulation in bullfrog intestine. Am J Physiol. 1990;258:G770–G773. doi: 10.1152/ajpgi.1990.258.5.G770. [DOI] [PubMed] [Google Scholar]
- 438.Torrallardona D, Harris CI, Fuller MF. Lysine synthesized by the gastrointestinal microflora of pigs is absorbed, mostly in the small intestine. Am J Physiol. 2003;284:E1177–E1180. doi: 10.1152/ajpendo.00465.2002. [DOI] [PubMed] [Google Scholar]
- 439.Tracy CR, Diamond J. Regulation of gut function varies with life-history traits in chuckwallas (Sauromalus obesus: Iguanidae) Physiol Biochem Zool. 2005;78:469–481. doi: 10.1086/430232. [DOI] [PubMed] [Google Scholar]
- 440.Tsahar E, Friedman J, Izhaki I. Secondary metabolite emodin increases food assimilation efficiency of Yellow-vented Bulbuls (Pycnonotus xanthopygos) Auk. 2003;120:411–417. [Google Scholar]
- 441.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Turunen S, Crailsheim K. Lipid and sugar absorption. In: Lehane MJ, Billingsley PF, editors. Biology of the Insect Midgut. London: Chapman and Hall; 1996. pp. 293–320. [Google Scholar]
- 443.Uhing MR, Kimura RE. Active transport of 3-O-methyl-glucose by the small intestine in chronically catheterized rats. J Clin Invest. 1995;95:2799–2805. doi: 10.1172/JCI117984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Uldry M, Ibberson M, Hosokawa M, Thorens B. GLUT2 is a high affinity glucosamine transporter. FEBS Lett. 2002;524:199–203. doi: 10.1016/s0014-5793(02)03058-2. [DOI] [PubMed] [Google Scholar]
- 445.Uni Z, Noy Y, Sklan D. Posthatch development of small intestinal function in the poult. Poult Sci. 1999;78(2):215–222. doi: 10.1093/ps/78.2.215. [DOI] [PubMed] [Google Scholar]
- 446.Uni Z, Tako E, Gal-Garber O, Sklan D. Morphological, molecular, and functional changes in the chicken small intestine of the late-term embryo. Poult Sci. 2003;82:1747–1754. doi: 10.1093/ps/82.11.1747. [DOI] [PubMed] [Google Scholar]
- 447.Van der Horst DJ, Roosendaal SD, Rodenburg KW. Circulatory lipid transport: Lipoprotein assembly and function from an evolutionary perspective. Mol Cell Biochem. 2009;326:105–119. doi: 10.1007/s11010-008-0011-3. [DOI] [PubMed] [Google Scholar]
- 448.Van Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, Proctor W, Colegio OR, Anderson JM. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci. 2008;121:298–305. doi: 10.1242/jcs.021485. [DOI] [PubMed] [Google Scholar]
- 449.Van L, Pan YX, Bloomquist JR, Webb KE, Wong EA. Developmental regulation of a turkey intestinal peptide transporter (PepT1) Poult Sci. 2005;84:75–82. doi: 10.1093/ps/84.1.75. [DOI] [PubMed] [Google Scholar]
- 450.van Soest PJ. Allometry and ecology of feeding behavior and digestive capacity in herbivores: A review. Zoo Biology. 1997;15:455–479. [Google Scholar]
- 451.Vasconcelos IM, Oliveira JTA. Antinutritional properties of plant lectins. Toxicon. 2004;44:385–403. doi: 10.1016/j.toxicon.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 452.Vasquez CM, Rovira M, Ruiz-Gutierrez V, Planas JM. Developmental changes in glucose transport, lipid composition, and fluidity of jejunal BBM. Am J Physiol. 1997;273:R1086–R1093. doi: 10.1152/ajpregu.1997.273.3.R1086. [DOI] [PubMed] [Google Scholar]
- 453.Veivers PC, Musca NY, O’Brien RW, Slayter M. Digestive enzymes of the salivary glands and gut of Mastotermes darwiniensis . Insect Biochem. 1982;12:35–40. [Google Scholar]
- 454.Verri T, Kottra G, Romano A, Tiso N, Peric M, Maffia M, Boll M, Argenton F, Daniel H, Storelli C. Molecular and functional characterisation of the zebrafish (Danio rerio) PEPT1-type peptide transporter. FEBS Lett. 2003;549:115–122. doi: 10.1016/s0014-5793(03)00759-2. [DOI] [PubMed] [Google Scholar]
- 455.Verri T, Romano A, Barca A, Kottra G, Daniel H, Storelli C. Transport of di- and tripeptides in teleost fish intestine. Aquac Res. 2010;41:641–653. [Google Scholar]
- 456.Voght SP, Fluegel ML, Andrews LA, Pallanck LJ. Drosophila NPC1b promotes an early step in sterol absorption from the midgut epithelium. Cell Metab. 2007;5:195–205. doi: 10.1016/j.cmet.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 457.Vonk HJ, Western RH. Comparative Biochemistry and Physiology of Enzymatic Digestion. London: Academic Press; 1984. [Google Scholar]
- 458.Wagner CE, McIntyre PB, Buels KS, Gilbert DM, Michel E. Diet predicts intestine length in Lake Tanganyika’s cichlid fishes. Funct Ecol. 2009;23:1122–1131. [Google Scholar]
- 459.Walgren RA, Lin JT, Kinne RKH, Walle T. Cellular uptake of dietary flavonoid quercetin 4’-beta-glucoside by sodium-dependent glucose transporter SGLT1. J Pharmacol Exp Ther. 2000;294:837–843. [PubMed] [Google Scholar]
- 460.Wallace RJ. Antimicrobial properties of plant secondary metabolites. Proc Nutr Soc. 2004;63:621–629. doi: 10.1079/pns2004393. [DOI] [PubMed] [Google Scholar]
- 461.Walthall K, Cappon GD, Hurtt ME, Zoetis T. Postnatal development of the gastrointestinal system: A species comparison. Birth Defects Res B Dev Reprod Toxicol. 2005;74:132–156. doi: 10.1002/bdrb.20040. [DOI] [PubMed] [Google Scholar]
- 462.Warnecke F, Luginbuhl P, Ivanova N, Ghassemian M, Richardson TH, Stege JT, Cayouette M, McHardy AC, Djordjevic G, Aboushadi N, Sorek R, Tringe SG, Podar M, Martin HG, Kunin V, Dalevi D, Madejska J, Kirton E, Platt D, Szeto E, Salamov A, Barry K, Mikhailova N, Kyrpides NC, Matson EG, Ottesen EA, Zhang X, Hernandez M, Murillo C, Acosta LG, Rigoutsos I, Tamayo G, Green BD, Chang C, Rubin EM, Mathur EJ, Robertson DE, Hugenholtz P, Leadbetter JR. Metagenomic and functional analysis of hindgut microbiota of a wood-feeding higher termite. Nature. 2007;450:560–565. doi: 10.1038/nature06269. [DOI] [PubMed] [Google Scholar]
- 463.Watanabe H, Todkuda G. Animal cellulases. Cell Mol Life Sci. 2001;58:1167–1178. doi: 10.1007/PL00000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Watanabe H, Tokuda G. Cellulolytic systems in insects. Annu Rev Entomol. 2010;55:609–632. doi: 10.1146/annurev-ento-112408-085319. [DOI] [PubMed] [Google Scholar]
- 465.Wei FW, Feng ZJ, Wang ZW, Zhou A, Hou JC. Use of the nutrients in bamboo by the red panda (Ailurus fulgens) J Zool. 1999;248:535–541. [Google Scholar]
- 466.Weiss SL, Lee EA, Diamond J. Evolutionary matches of enzyme and transporter capacities to dietary substrate loads in the intestinal brush border. Proc Natl Acad Sci U S A. 1998;95:2117–2121. doi: 10.1073/pnas.95.5.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Wen Y, Irwin DM. Mosaic evolution of ruminant stomach lysozyme genes. Mol Phylogenet Evol. 1999;13:474–482. doi: 10.1006/mpev.1999.0651. [DOI] [PubMed] [Google Scholar]
- 468.Whelan CJ, Brown JS. Optimal foraging and gut constraints: Reconciling two schools of thought. Oikos. 2005;110:481–496. [Google Scholar]
- 469.Whelan CJ, Brown JS, Schmidt KA, Steele BB, Willson MF. Linking consumer-resource theory and digestive physiology: Application to diet shifts. Evol Ecol Res. 2000;2:911–934. [Google Scholar]
- 470.Wickramasinghe DD, Oseen KL, Wassersug RJ. Ontogenetic changes in diet and intestinal morphology in semi-terrestrial tadpoles of Nannophrys ceylonensis (Dicroglossidae) Copeia. 2007:1012–1018. [Google Scholar]
- 471.Wijtten PJA, van der Meulen J, Verstegen MWA. Intestinal barrier function and absorption in pigs after weaning: A review. Br J Nutr. 2011;105:967–981. doi: 10.1017/S0007114510005660. [DOI] [PubMed] [Google Scholar]
- 472.Wilson-O’Brien AL, Patron N, Rogers S. Evolutionary ancestry and novel functions of the mammalian glucose transporter (GLUT) family. BMC Evol Biol. 2010;10:152. doi: 10.1186/1471-2148-10-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Wisessing A, Engkagul A, Wongpiyasatid A, Choowongkomon K. Biochemical characterization of the alpha-amylase inhibitor in mungbeans and Its application in inhibiting the growth of Callosobruchus maculatus . J Agric Food Chem. 2010;58:2131–2137. doi: 10.1021/jf903411x. [DOI] [PubMed] [Google Scholar]
- 474.Wolesensky W, Joern A, Logan JD. A model of digestion modulation in grasshoppers. Ecol Modell. 2005;188:358–373. [Google Scholar]
- 475.Wong CN, Ng P, Douglas AE. Low-diversity bacterial community in the gut of the fruitfly Drosophila melanogaster. Environ Microbiol. 2011;13:1889–1900. doi: 10.1111/j.1462-2920.2011.02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Wright AD, Northwood KS, Obispo NE. Rumen-like methanogens identified from the crop of the folivorous South American bird, the hoatzin (Opisthocomus hoazin) ISME J. 2009;3:1120–1126. doi: 10.1038/ismej.2009.41. [DOI] [PubMed] [Google Scholar]
- 477.Xia XB, Lin CT, Wang G, Fang HQ. Binding of phlorizin to the C-terminal loop 13 of the Na+/glucose cotransporter does not depend on the 560–608 disulfide bond. Arch Biochem Biophys. 2004;425:58–64. doi: 10.1016/j.abb.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 478.Xia XB, Lin JT, Kinne RKH. Binding of phlorizin to the isolated C-terminal extramembranous loop of the Na+/glucose cotransporter assessed by intrinsic tryptophan fluorescence. Biochemistry. 2003;42:6115–6120. doi: 10.1021/bi020695b. [DOI] [PubMed] [Google Scholar]
- 479.Xu J, Bjursell MK, Himrod J, Deng S, Carmichael LK, Chiang HC, Hooper LV, Gordon JI. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science. 2003;299:2074–2076. doi: 10.1126/science.1080029. [DOI] [PubMed] [Google Scholar]
- 480.Yadgary L, Yair R, Uni Z. The chick embryo yolk sac membrane expresses nutrient transporter and digestive enzyme genes. Poult Sci. 2011;90:410–416. doi: 10.3382/ps.2010-01075. [DOI] [PubMed] [Google Scholar]
- 481.Yang RB, Xie CX, Fan QX, Gao C, Fang LB. Ontogeny of the digestive tract in yellow catfish Pelteobagrus fulvidraco larvae. Aquaculture. 2010;302:112–123. [Google Scholar]
- 482.Yang Y, Joern A. Gut size changes in relation to variable food quality and body size in grasshoppers. Funct Ecol. 1994;8:36–45. [Google Scholar]
- 483.Yoneshige A, Sasaki A, Miyazaki M, Kojirna N, Suzuki A, Matsuda J. Developmental changes in glycolipids and synchronized expression of nutrient transporters in the mouse small intestine. J Nutr Biochem. 2010;21:214–226. doi: 10.1016/j.jnutbio.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 484.Yu DD, Xiao ZZ, Liu QH, Xu SH, Ma DY, Li J, Webb KA. Ontogeny of gastrointestinal tract in hybrid flounder jasum Paralichthys olivaceus × P-dentatus . J World Aquacult Soc. 2010;41:344–357. [Google Scholar]
- 485.Yufera M, Darias MJ. The onset of exogenous feeding in marine fish larvae. Aquaculture. 2007;268:53–63. [Google Scholar]
- 486.Zaneveld JR, Lozupone C, Gordon JI, Knight R. Ribosomal RNA diversity predicts genome diversity in gut bacteria and their relatives. Nucleic Acids Res. 2010;38:3869–3879. doi: 10.1093/nar/gkq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Zhan QL, Zheng SC, Feng QL, Liu L. A midgut-specific chymotrypsin cDNA (SLCTP1) from Spodoptera litura: Cloning, characterization, localization and expression analysis. Arch Insect Biochem Physiol. 2011;76:130–143. doi: 10.1002/arch.20353. [DOI] [PubMed] [Google Scholar]
- 488.Zhang C, Zhou DH, Zheng SC, Liu L, Tao S, Yang L, Hu SN, Feng QL. A chymotrypsin-like serine protease cDNA involved in food protein digestion in the common cutworm Spodoptera litura: Cloning, characterization, developmental and induced expression patterns, and localization. J Insect Physiol. 2010;56:788–799. doi: 10.1016/j.jinsphys.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 489.Zhang HZ, Malo C, Boyle CR, Buddington RK. Diet influences development of the pig (Sus scrofa) intestine during the first 6 hours after birth. J Nutr. 1998;128:1302–1310. doi: 10.1093/jn/128.8.1302. [DOI] [PubMed] [Google Scholar]
- 490.Zhang HZ, Malo C, Buddington RK. Suckling induces rapid intestinal growth and changes in brush border digestive functions of newborn pigs. J Nutr. 1997;127:418–426. doi: 10.1093/jn/127.3.418. [DOI] [PubMed] [Google Scholar]
- 491.Zhang JZ, Zhang YP, Rosenberg HF. Adaptive evolution of a duplicated pancreatic ribonuclease gene in a leaf-eating monkey. Nat Genet. 2002;30:411–415. doi: 10.1038/ng852. [DOI] [PubMed] [Google Scholar]
- 492.Zhao JM, Qiu LH, Ning XX, Chen AQ, Wu HF, Li CH. Cloning and characterization of an invertebrate type lysozyme from Venerupis philippinarum. Comp Biochem Physiol B Biochem Mol Biol. 2010;156:56–60. doi: 10.1016/j.cbpb.2010.02.001. [DOI] [PubMed] [Google Scholar]