Abstract
The field of exercise-oncology has increased dramatically over the past two decades, with close to 100 published studies investigating the efficacy of structured exercise training interventions in patients with cancer. Of interest, despite considerable differences in study population and primary study end point, the vast majority of studies have tested the efficacy of an exercise prescription that adhered to traditional guidelines consisting of either supervised or home-based endurance (aerobic) training or endurance training combined with resistance training, prescribed at a moderate intensity (50–75% of a predetermined physiological parameter, typically age-predicted heart rate maximum or reserve), for two to three sessions per week, for 10 to 60 min per exercise session, for 12 to 15 weeks. The use of generic exercise prescriptions may, however, be masking the full therapeutic potential of exercise treatment in the oncology setting. Against this background, this opinion paper provides an overview of the fundamental tenets of human exercise physiology known as the principles of training, with specific application of these principles in the design and conduct of clinical trials in exercise-oncology research. We contend that the application of these guidelines will ensure continued progress in the field while optimizing the safety and efficacy of exercise treatment following a cancer diagnosis.
Introduction
The field of ‘Exercise-Oncology’ has burgeoned dramatically in scope and impact since publication of the first scientific papers in the mid to late 1980s.1 Several systematic reviews and meta-analyses have evaluated the efficacy of structured exercise treatment in cancer.2–6 For example, Speck et al.7 identified a total of 66 studies reported as ‘high-quality’ that examined the impact of exercise treatment in a broad array of oncology scenarios (e.g. differences in histological primary site, stage and treatment) on a total of 60 different primary end points in adults with cancer. Intriguingly, despite the degree of heterogeneity in how exercise was being utilized to manipulate physiological adaptation, the nature of the exercise prescription in the vast majority of studies was similar. More specifically, almost all prescriptions followed traditional guidelines consisting of either supervised or home-based endurance (aerobic) training or endurance training combined with resistance training, prescribed at a moderate-intensity (50–75% of a predetermined physiological parameter, typically age-predicted heart rate maximum or reserve), for two to three sessions per week, for 10 to 60 min per exercise session, for 12 to 15 weeks. Despite the adoption of a relatively homogeneous prescription approach, exercise training was, for the most part, associated with benefit across a diverse range of end points, largely irrespective of the oncology setting.7
On this evidence, it could be argued that a standardized, largely homogeneous exercise prescription that adopts a conventional approach is safe, efficacious, and therefore sufficient. This has resulted in a limited perceived need to elucidate the optimal dose, sequencing, or combination of different training stresses to specifically alter a desired physiological end point in any clinical population, including oncology. However, the significant benefit of generically dosed exercise treatment on heterogeneous end points rather reflects the remarkable pleiotropic physiologic impact of exercise. Moreover, the use of generic exercise prescriptions (irrespective of clinical population or primary end point) is masking the full therapeutic promise of exercise treatment. Indeed, for more than half a century, exercise training has been continually refined, precisely dosed, and scheduled to minimize injury and optimize human/athletic performance; the basis of all training prescriptions in this arena adheres to fundamental tenets of human exercise physiology known as the principles of training. These tenets are rarely applied or even considered when designing exercise trials in clinical populations.8,9 Accordingly, the purpose of this Opinion paper is to provide an overview of the application of these principles in the design and conduct of clinical trials in exercise-oncology research. This paper will focus primarily on application of these principles to aerobic-based training, although the concepts also apply to resistance training or combination training programs.
Exercise intervention design considerations
Stage 1 Objective assessment of response to exercise: An essential prerequisite in the design of all exercise training trials is the objective assessment of baseline physiological functioning of the systems that will be primarily targeted. This generally refers to either the cardiopulmonary responses to exercise (in the context of aerobic training) or the assessment of maximal muscular strength or endurance (in the context of resistance training). The relative strengths, weaknesses, and conduct of the various different assessment tools available to researchers have been reviewed previously.10–12 In the context of aerobic training studies, the preferred assessment tool is a cardiopulmonary exercise test (CPET). The use of a CPET offers a number of distinct advantages compared with other assessments. Of these, arguably the most important is that CPET provides specific information on patients' peak rate of oxygen consumption (VO2peak) as well as their submaximal cardiopulmonary responses to exercise, permitting precise tailoring of training to the individual patient. In settings in which CPET is not available, investigators can also use a maximal incremental exercise tolerance test (ETT) (without direct VO2 measurement) to determine peak workload and peak exercise heart rate.10,13 In addition, both CPETs and ETTs are performed with 12-lead ECG and blood pressure monitoring, thus, they also provide important pre-exercise training clearance information on the detection of any exercise-induced impairments (e.g. ischemia) or symptom limitations at both submaximal and maximal exercise tolerance.14 The latter information is of critical importance when considering the inclusion of high-intensity (≥75% of VO2peak) aerobic training sessions in the prescription.
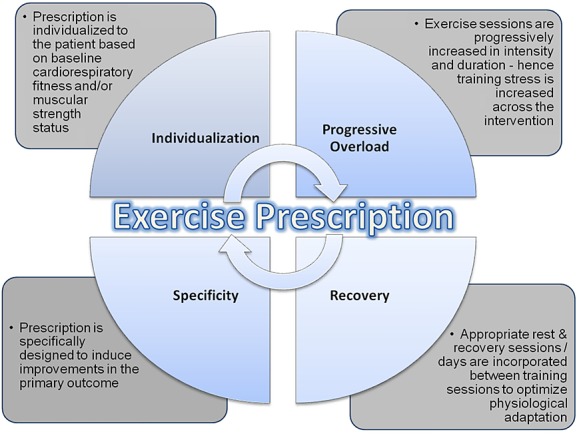
Stage 2 Application of the principles of training: Perhaps the most essential principles of training are individualization, specificity, progressive overload, and rest/recovery (Figure 1). A brief overview of each of these principles is provided herein.
Individualization: The concept of individualization is defined as the customized application of training towards the physiological status of the patient/ subject.15,16 Clearly, even within carefully selected homogenous clinical trial cohorts, considerable heterogeneity likely still exists in cardiopulmonary function, lifestyle behaviour, age, prior treatment, concomitant comorbidities, and, of course, genetic predisposition. Thus, application of a generic prescription that fails to consider such parameters will most often result in either an under-dosing or over-dosing of exercise treatment. Fortunately, there is a broad array of parameters available on which to individualize training. The preferred approach is individualization on the basis of workloads (e.g. treadmill speed and power output) corresponding to a specific percent of VO2peak or peak workload (e.g. 55%, 65%) elicited during each individual patient's pre-randomization CPET or ETT. The corresponding heart rate, blood pressure, and rating of perceived exertion (RPE) directly measured at each percent workload is used to ensure that subjects are training at, or close to, the prescribed intensity during subsequent exercise sessions (Figure 2).17 It is important to note that although the absolute training intensity may be different between individual patients, the relative intensity of training is similar, ensuring standardization across patients—a critical aspect of clinical trial conduct.
Specificity: It is widely acknowledged that the physiological adaptations elicited by aerobic training are unique from those caused by resistance training. Lesser appreciated, however, is that even within the same exercise modality, there is enormous capacity to manipulate the nature and configuration of the elements of exercise prescription (i.e. frequency, intensity, duration, and time) to confer remarkably distinct physiological adaptations.18,19 The concept of specificity addresses the notion that the selected exercise stress must be specific and targeted to the primary underlying system(s) or pathway(s) known or postulated to underpin the primary end point of interest. For example, in a trial designed to examine the impact of aerobic training on VO2peak in a particular cancer cohort, a critical prerequisite for the design of the optimal exercise prescription is determination of the primary limitation (determinant) to VO2peak; in other words, is there a cardiovascular, ventilatory, and/or skeletal muscle limitation to exercise?20 For example, if the primary limitation is cardiovascular exercise training emphasizing moderate intensity, aerobic training (i.e. 50 to 70% of VO2peak) for longer duration (≥45 min) is indicated to initially enhance plasma volume and structural changes in the heart and blood vessels.21 In contrast, if more general deconditioning is observed and skeletal muscle adaptation is the desired outcome, aerobic training at a higher relative intensity (i.e. > 70% of VO2peak) that induces enzymatic adaptation and increased capillarization and mitochondrial biogenesis may be emphasized (after a period of more general training that acclimatizes the patient to exercise).22–24
Progressive overload: Aerobic training provides a potent physiological stimulus that perturbs the equilibrium of multiple organ systems.18 Perturbations in the cellular and systemic environments create biological stress, which challenges homeostasis. Chronic (repeated) disturbance of homeostasis triggers a highly preserved, multi-organ stress-response leading to physiologic adaptation (i.e. the concept of hormesis25 or supercompensation), wherein the host can withstand greater future system perturbations, meaning that a greater stress stimulus is required to further perturb homeostasis. By definition therefore, a requirement of effective exercise prescriptions is optimization and progressive increase in stress to confer continued physiological adaptation.26 Of equal importance is recognition that increasing exercise stress pushed beyond the homeostasis will result in chronic overreaching or overtraining, leading to fatigue, maladaptation, or even illness and injury.27 Accordingly, optimization of progressive overload requires appropriate increase in training stress across the training prescription and monitoring of the individual's physiological response or ‘readiness’ to receive the increased exercise load.
Rest and recovery: The tenet that is most frequently underemphasized or commonly neglected in the design of exercise prescriptions is the principle of rest and recovery. Central to this principle is the availability of nutrients and rest (or reduced training load) in order to permit necessary biological resynthesis to replace the required constituents of the impacted system(s). Quantifying optimal recovery is challenging28 because of the multidimensional application of the exercise stress.29 Conversely, an extended period without adequate stress will result in a loss of adaptation and a down-regulation of the entire system.30 Clearly, a balance between the principles of progressive overload and recovery are necessary to elicit optimal physiological adaptations.31 A training stress must perturb homeostasis with enough impulse to lead to chronic supercompensatory adaptations; however, the appropriate volume of recovery must be prescribed in concert with this perturbation in order to optimize adaptation. There are many techniques in the prescription of an exercise training program that ensure adequate and optimal rest and recovery; this is most commonly achieved by altering the frequency and duration of training (while maintaining intensity) or sequencing the training stress across each week in order to reduce the accumulated fatigue that may amass through repeated, consecutive high-intensity bouts of training.32,33 This is especially critical in clinical populations in which demographic and medical characteristics (e.g. cardiopulmonary function, immune status, comorbidities, and current therapies) further complicate optimal adaptation. With appropriate balance, positive adaptations will continue, permitting increases in exercise stresses to be applied and further physiologic conditioning towards the targeted end point.
Figure 1.
The principles of training.
Figure 2.
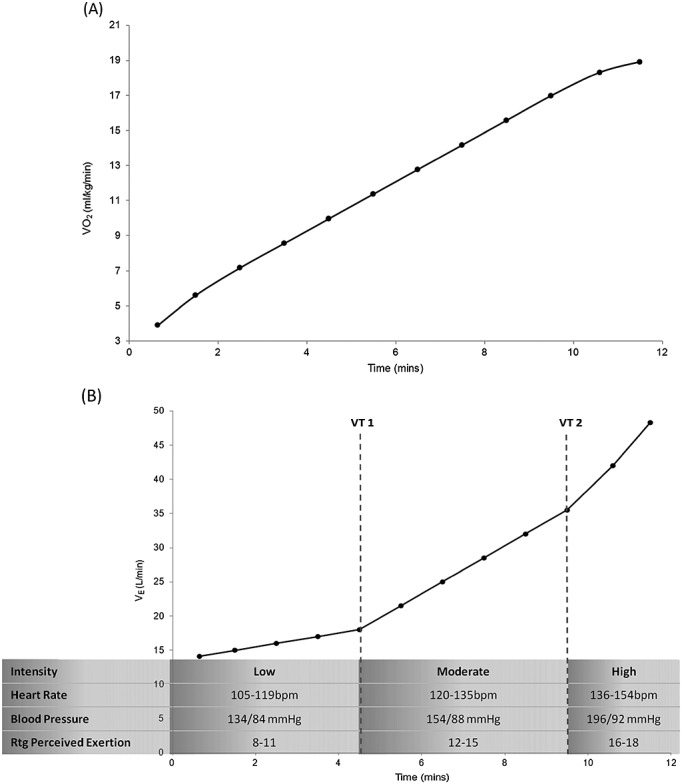
Oxygen consumption and ventilatory responses to incremental treadmill exercise in a 65 year-old woman with early-stage breast cancer. (A) Increasing workloads during the cardiopulmonary exercise test causes linear increases in oxygen consumption (VO2 in mL/kg/min) to the point of volitional fatigue at a VO2peak of 18.9 mL/kg/min. (B) A graphical representation of alveolar ventilation (VE in L/min) demonstrate two exponential ‘breakpoints’ in ventilation corresponding to Ventilatory Threshold 1 (VT1) and Ventilatory Threshold 2 (VT2). These thresholds demarcate the transition of low, medium, and high exercise intensity, and correspond to specific parameters that may be used for identification of relative intensity for exercise prescription and monitoring. These intensities and the corresponding ranges of physiological identification thereof (heart rate, blood pressure, and rating (Rtg) of perceived exertion on a 6–20 scale) provide an appropriate tool for indirect assessment of training stress and intensity.
Stage 3 Design of the exercise prescription: Exercise prescriptions are most often operationalized using the following parameters: Frequency (sessions per week), Intensity (how hard per session), Time (session duration), and Type (modality) or F.I.T.T.34 In the next section, we will outline the design of an exercise prescription (using F.I.T.T) that adopts either a conventional approach (Figure 3A) or an alternative approach that systematically adheres to the principles of training (Figure 3B). To facilitate practical understanding, the utility of both prescription approaches is presented in the context of the following ‘mock’ clinical trial vignette: A randomized trial to determine the efficacy of supervised exercise training on cardiac function in older (≥65 years) women following completion of anthracycline-containing adjuvant therapy for early-stage breast cancer.
Figure 3.
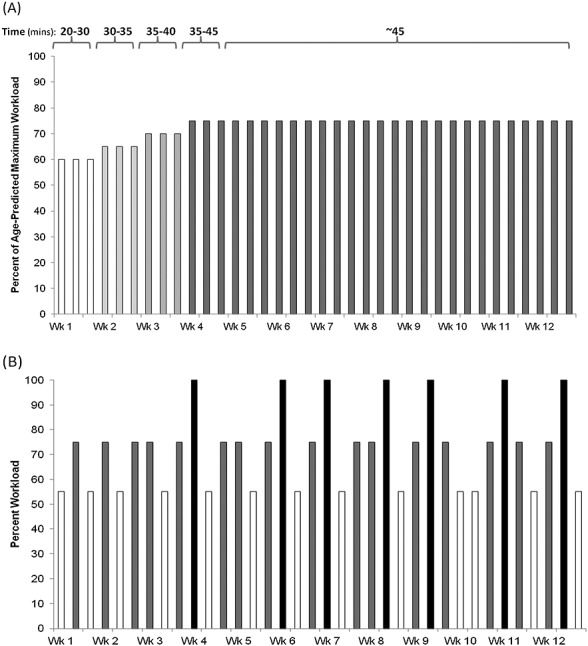
Comparison of linear and nonlinear exercise prescriptions. Each bar represents a training session at prescribed workloads based on the absolute or relative intensity. (A) The conventional (linear) approach utilizes standard intensity, frequency, and duration parameters after an initial lead in period, with static increases in session duration (i.e. 20 to 45 min). (B) The alternative non-linear approach considers the principles of exercise training in order to optimize the adaptations to the exercise stimulus. Sessions are tailored to an individual's relative intensity, based on cardiopulmonary exercise testing or exercise tolerance testing, and specified to address a particular endpoint. Sessions and weeks progress over the course of the prescription and vary between low intensity (e.g. 55% VO2peak; white bars) and moderate (e.g. 75%; grey bars) and high intensity (e.g. 100% VO2peak; black bars) training in order to target various physiological systems involved in the cardiopulmonary response to exercise. Session intensity is inversely related to session duration, that is, sessions involving high relative intensity workloads are conducted in shorter bouts through short duration training sessions and are less frequent to ensure recovery between sessions. VO2peak, peak rate of oxygen consumption.
In the conventional approach (Figure 3A), exercise training intensity is prescribed using the heart rate reserve technique (a method based on chronological age and resting heart rate) without consideration of the patients' functional limitations or the primary study end point. This is the most common method of individualizing training in the oncology setting.7 Such an approach is problematic, however, given the 10 to 12–beat-per-minute variation in maximal heart rate in normal subjects.35,36 There may be even greater variation in cancer patients, given the documented impact of current or previous systemic therapy on autonomic function.37 This aerobic training intensity prescribed at 75% heart rate reserve (for example) may elicit very different physiologic adaptations between patients (because of the inaccuracy of age-predicted maximum heart rate and the decreased heart rate reserve due to elevated resting heart rate). Further, the majority of sessions within this prescription are performed at the same intensity and duration, as determined by their initial CPET or ETT; the workload prescribed to correspond with their percent heart rate reserve will no longer be appropriate after an initial adaptation period. In this instance, training volume remains constant and does not progress across the entire intervention. This is problematic considering that as cardiorespiratory fitness improves, the adaptation from an identical exercise stimulus diminishes; therefore, there is an insufficient stimulus to induce further physiologic adaptation. As such, this prescription fails to consider three important principles of training, namely individualization, specificity, and progressive overload.
Contrastingly, in the alternative exercise prescription approach, the use of appropriate baseline testing (i.e. CPET) permits aerobic training to be tailored to a patients’ baseline VO2peak (Figure 2), and thereby adhering to the principle of individualization. Second, the intensity, duration, and occasionally, the frequency of training sessions are sequenced in such a fashion that training volume is continually increased across the entire program (i.e. the principles of specificity and progressive overload). This approach also adds important variety to the prescription that not only continually alters the exercise ‘stress’ (to optimize adaptation) but can also stimulate patient interest and motivation. Third, training intensity is sequenced, such that higher intensity or higher volume training is followed by lower intensity (recovery) training and rest days to optimize adaptation (i.e. the principle of rest and recovery).38 Finally, although not specifically outlined in Figure 3B, the principle of reversibility may be a particularly important consideration in aerobic training trials conducted in oncology populations because patients may be forced to temporarily discontinue training because of therapy-induced toxicity and/or disease progression.39 Detraining effects can occur rapidly (within days to weeks), thus, subsequent training sessions may need to be resumed at reduced duration and intensity than initially planned. Such dose reductions will also impact training efficacy.40–42
Efficacy of exercise prescriptions adhering to the principles of training in oncology
Despite the proven efficacy in the arena of sports/athletic performance, consideration of the principles of training has not been translated into the design of exercise prescriptions in clinical populations. Indeed, to our knowledge, only one trial to date has compared the efficacy of an exercise prescription following a non-linear approach vs. a traditional linear approach in any clinical population. Specifically, Klijn et al. compared the efficacy of non-linear periodized training with that of traditionally-prescribed linear combined aerobic and resistance training in 110 patients with severe chronic obstructive pulmonary disease.43 Exercise training in both arms was performed three times a week for 10 weeks. Results indicated that non-linear exercise training was associated with superior improvements in cycling endurance and health-related quality of life compared with linearly prescribed training. It is important to state that a common perception is that studies examining the efficacy of high-intensity interval training (HITT) also adhere to the principles of training / non-linear approach. However, if studies exclusively test HITT (i.e. all exercise sessions are HITT) and proceed without appropriate progression, then these programs are also linear in design and do not adhere to the principles of training. In the oncology setting, approximately six studies to date have examined the safety, tolerability, and preliminary efficacy of non-linear aerobic training, compared with a usual care (no exercise training) control group. As presented in Table 1, exercise prescriptions adhering to a non-linear approach appear to be safe (low adverse event rate), tolerable (mean adherence ≥75% of prescribed sessions both during and after primary adjuvant therapy), and efficacious, conferring favourable improvements in VO2peak, quality of life, and other physiological outcomes. On the basis of this data, our group is comparing the efficacy of either non-linear periodized training or traditionally prescribed linear aerobic training with an attention control group (i.e. supervised progressive stretching) in 174 women completing primary therapy for early-stage breast cancer. Aerobic training in both arms is being performed four times a week for 16 weeks. The primary end point is VO2peak.44
Table 1.
Exercise training studies adopting a non-linear approach in exercise-oncology research (chronological order)
| Authors | Population/setting/design/N | Non-linear aerobic exercise training intervention | Major findings |
|---|---|---|---|
| Jones et al. 200745 | Operable lung cancer/pre-operative/prospective single-group/25 | Duration: 4–6 weeks | Adverse events: Abnormal decline in systolic blood pressure >20 mmHg, which normalized after exercise determination (n = 2) |
| Frequency: five times a week | |||
| Modality: cycle ergometry | Adherence: 72% (number of sessions attended divided by the total number of sessions prescribed) | ||
| Week 1: five sessions: 20 min at 60%; VO2peak up to 30 min at 65% VO2peak | |||
| Weeks 2–3: four sessions: 25–30 min at 60–65% VO2peak; one session: 20–25 min at VTa | Outcomes: ITT: VO2peak increased 2.4 mL/kg/min (P = 0.002) and 6 MWD increased 40 m from baseline to pre-surgery; No significant change in any pulmonary function outcome was observed from baseline to pre-surgery (P > 0.05). PPA: Adherence > 80% (n = 12): VO2peak increased 3.3 mL/kg/min (P = 0.006) and 6MWD increased 49 m (P = 0.013) | ||
| Week 4+: three sessions: 25–30 min at 60–65% VO2peak; one session: 20–25 min at VT; one session: 10–15 times (30–60 s at VO2peak, followed by 60 s of active recovery) | |||
| Jones et al. 200846 | Non-small cell lung cancer (Stage I–IIIb)/post-surgical /prospective single-group/20 | Duration: 14 weeks | Adverse events: none |
| Frequency: three times a week | |||
| Modality: cycle ergometry | |||
| Week 1: three sessions: 15–20 min at 60% Wmax | Adherence: 85% (number of sessions attended divided by the total number of sessions prescribed) | ||
| Weeks 2–4: three sessions: increased progressively to 30 min at 65% Wmax | |||
| Week 5–6: two sessions: 30–45 min at 60–65% Wmax; one session: 20–25 min at VT | Outcomes: ITT: VO2peak increased 1.1 mL/kg/min (P = 0.11) and Wmax increased 9 W (P = 0.003). Significant favourable changes were also observed for functional well being (P = 0.007) and fatigue (P < 0.03). PPA: Patients not receiving adjuvant chemotherapy (n = 11): VO2peak increased 1.7 mL/kg/min (P = 0.008). Peak heart rate (P = 0.05), Wmax (P < 0.001), and workload @ VT (P = 0.05) also increased. Patients receiving adjuvant chemotherapy (n = 8): VO2peak at VT decreased (P = 0.03) | ||
| Week 7–10: two sessions: 30–45 min at 60–70% Wmax; one session: 20–30 min at VT | |||
| Week 10–14: two sessions: 30–45 min at 60–70% Wmax; one session: 10–15 times (30 s at VO2peak, followed by 60 s of active recovery) | |||
| Courneya et al. 200847 | Mild-to-moderately anaemic patients with solid tumours/during or post-cancer therapy/single-centre, two-armed randomized controlled trial comparing darbepoetin alfa (an erythropoiesis-stimulating agent) alone vs. darbepoetin alfa plus aerobic training/55 | Duration: 12 weeks | Adverse events: none |
| Frequency: three times a week | Adherence: 85% (number of sessions attended divided by the total number of sessions prescribed) | ||
| Modality: cycle ergometry | Outcomes: VO2peak was significantly greater in the exercise group (+3.0 mL/kg/min; P < 0.001), as were Wmax (P = 0.028) and VT (P = 0.001). The exercise group showed trends towards a more rapid hemoglobin response with less drug administration compared with the non-exercise group (P = 0.07–0.12) | ||
| Intensity: 60–100% of baseline Wmax | |||
| Courneya et al. 200948 | Lymphoma/during chemotherapy or following treatment / single-centre, two-armed randomized control trial comparing usual care vs. usual care plus aerobic exercise training/122 | Duration: 12 weeks | Adverse events: none |
| Frequency: three times a week | |||
| Modality: cycle ergometry | |||
| Week 1–4: three sessions: 15–20 min at 60% Wmax, increasing 5% per week to 75% by Week 4 | Adherence: 78% (duration and intensity criteria were met during 99.0% and 90.7% of sessions) | ||
| Week 5–7: three sessions: 15–20 min at 75% Wmax, increasing 5 min per week to 25–30 min by Week 9 | |||
| Week 7–8: two sessions: 30–35 min at 75% Wmax, increasing 5 min to 35–40 min in Week 8; one session: exercise at VT (time N/R) | Outcomes: Aerobic training was superior to usual care on all indicators of cardiovascular fitness, including VO2peak (+5.2 mL/kg/min), Wmax, (+28 W) and VT (+0.33 L/min) (P < 0.001). Aerobic training was superior to usual care for patient related physical functioning, quality of life, fatigue, happiness and depression (P < 0.05). Aerobic training did not interfere with treatment completion or response. | ||
| Week 9–12: two sessions: 30–35 min at 75% Wmax, increasing 5 min per week to 40–45 min by Week 9 to Week 12; one session: interval training at VO2peak (time N/R) | |||
| Hornsby et al. 201349 | Operable breast cancer (stage IIb–IIIc)/receiving neo-adjuvant chemotherapy/single-centre, two-armed randomized control trial comparing neoadjuvant AC alone vs. AC plus aerobic exercise training/20 | Duration: 12 weeks | Adverse events: During baseline exercise testing: exercise-induced oxygen desaturation (SpO2 < 84%) (n = 1), anxiety attack (n = 1), and dizziness (n = 1). All symptoms/signs resolved promptly upon cessation of exercise and did not preclude study participation; During training: Unexplained leg pain that resolved following exercise cessation (n = 1). |
| Frequency: three times a week | |||
| Modality: cycle ergometry | |||
| Week 1: three sessions: 15–20 min at 60% Wmax | Attendance: 82% (number of sessions attended divided by the total number of sessions prescribed) | ||
| Weeks 2–4: three sessions: increased progressively to 30 min at 65% Wmax | |||
| Week 5–6: two sessions: 30–45 min at 60–65% Wmax; one session: 20–25 min at VT | Adherence: 66% (number of sessions successfully completed divided by the number of planned sessions attended. Non-adherence was defined as any exercise session requiring exercise dose modification of either the planned exercise duration or intensity. | ||
| Week 7–10: two sessions: 30–45 min at 60–70% Wmax; one session: 20–30 min at VT | Outcomes: ITT: VO2peak increased by 2.6 mL/kg/min (+13.3%) in the aerobic training group and decreased by 1.5 mL/kg/min (−8.6%) in the AC alone group (mean difference +4.1 mL/kg/min, P = 0.001). Differences between groups were also observed for Wmax and oxygen pulse (P < 0.05). | ||
| Week 10–12: two sessions: 30–45 min at 60–70% Wmax; one session: 10–15 times (30 s at 100% Wmax, followed by 60 s of active recovery) | |||
| Jones et al. 201450 | Localized (stage I–II) prostate adenocarcinoma/post bilateral nerve sparing radical prostatectomy / single-centre, two-armed randomized control trial comparing usual care alone vs. usual care plus aerobic exercise training / 50 | Duration: 6 months | Adverse Events: Ischemic ECG changes reflected by significant ST segment depression were observed in three patients during baseline CPET. 129 independent non-serious adverse events occurred that required modification or early cessation of the exercise training prescription. The majority of events were training-induced leg cramps (55%) or back pain (26%). |
| Frequency: five times a week (minimum three supervised, then two supervised or home setting) | Attendance (supervised): 83% (number of sessions attended divided by the total number of sessions prescribed) | ||
| Modality: treadmill walking | Compliance (supervised): 79% (number of sessions successfully completed divided by the number of planned sessions attended. Non- adherence was defined as any exercise session requiring exercise dose modification of either the planned exercise duration or intensity. | ||
| Duration and intensity: non-linear prescription aimed to meet 30–45 min/session at 55–100% of VO2peak. Intensity based on treadmill speed/grade corresponding %VO2peak elicited during the pre-randomization or mid-point CPET. | Outcomes: VO2peak increased 1.9 mL/kg/min (P = 0.016) and brachial artery flow mediated dilation increased 1.4% (P = 0.03) in the aerobic training group compared with usual care. There were no significant between-group differences in any erectile function subscale. There were significant correlations between exercise training adherence and change in FMD (r = 0.38; P = 0.081) and VO2peak (r = 0.57; P = 0.003) but not between exercise training adherence and any erectile function end points. |
ITT, intention to treat analysis; PPA, per protocol analysis; Wmax, maximal work rate; VO2peak, peak oxygen consumption; AC, doxorubicin plus cyclophosphamide; CPET, cardiopulmonary exercise test; VT, ventilatory threshold; MWD, minute walk distance.
VT determined by a systematic increase in the VE/VO2 ratio, whereas VE/VCO2 remained constant.
Conclusion
The purpose of this commentary was to provide an overview of the application of the fundamental principles of training in the design and conduct of clinical trials in exercise-oncology research. It is hoped that attention to these issues will provide the platform for constructive dialogue with the view towards the development of best practice guidelines to optimize exercise training in the oncology setting. Application of these guidelines will ensure continued progress in the field by producing the high-quality evidence base necessary to convince oncology professionals that exercise training is an integral aspect of the therapeutic armamentarium in the treatment and control of cancer.
Acknowledgments
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle (von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7–8.)
Funding
J.F.C. is supported by Research Grants from TRYGFONDEN, the Novo Nordic Foundation, the Danish Cancer Society, and the Beckett Foundation. L.W.J. is supported in part by research grants from the NCI and AKTIV Against Cancer.
Conflict of interest
L.W.J. is a founder of Exercise by Science, Inc.
References
- Jones LW, Alfano CM. Exercise-oncology research: past, present, and future. Acta Oncol. 2013;52:195–215. doi: 10.3109/0284186X.2012.742564. [DOI] [PubMed] [Google Scholar]
- Fong DY, Ho JW, Hui BP, Lee AM, Macfarlane DJ, Leung SS, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ. 2012;344:e70. doi: 10.1136/bmj.e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft LL, Vaniterson EH, Helenowski IB, Rademaker AW, Courneya KS. Exercise effects on depressive symptoms in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol, Biomarkers Prev: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21:3–19. doi: 10.1158/1055-9965.EPI-11-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ: Can Med Assoc J = Journal de l'Association medicale canadienne. 2006;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G. Physical exercise in cancer patients during and after medical treatment: a systematic review of randomized and controlled clinical trials. J Clin Oncol: official journal of the American Society of Clinical Oncology. 2005;23:3830–3842. doi: 10.1200/JCO.2005.02.148. [DOI] [PubMed] [Google Scholar]
- Jones LW, Demark-Wahnefried W. Diet, exercise, and complementary therapies after primary treatment for cancer. Lancet Oncol. 2006;7:1017–1026. doi: 10.1016/S1470-2045(06)70976-7. [DOI] [PubMed] [Google Scholar]
- Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv: research Practice. 2010;4:87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- Winters-Stone KM, Neil SE, Campbell KL. Attention to principles of exercise training: a review of exercise studies for survivors of cancers other than breast. Br J Sports Med. 2014;48:987–995. doi: 10.1136/bjsports-2012-091732. [DOI] [PubMed] [Google Scholar]
- Campbell KL, Neil SE, Winters-Stone KM. Review of exercise studies in breast cancer survivors: attention to principles of exercise training. Br J Sports Med. 2012;46:909–916. doi: 10.1136/bjsports-2010-082719. [DOI] [PubMed] [Google Scholar]
- Jones LW, Eves ND, Haykowsky M, Joy AA, Douglas PS. Cardiorespiratory exercise testing in clinical oncology research: systematic review and practice recommendations. Lancet Oncol. 2008;9:757–765. doi: 10.1016/S1470-2045(08)70195-5. [DOI] [PubMed] [Google Scholar]
- American College of Sports M: American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41:687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- Abernethy P, Wilson G, Logan P. Strength and power assessment. Issues, controversies and challenges. Sports Med. 1995;19:401–417. doi: 10.2165/00007256-199519060-00004. [DOI] [PubMed] [Google Scholar]
- American Thoracic S. American College of Chest P: ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- Kenjale AA, Hornsby WE, Crowgey T, Thomas S, Herndon JE, 2nd, Khouri MG, et al. Pre-exercise participation cardiovascular screening in a heterogeneous cohort of adult cancer patients. Oncologist. 2014;19:999–1005. doi: 10.1634/theoncologist.2014-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varray AL, Mercier JG, Terral CM, Prefaut CG. Individualized aerobic and high intensity training for asthmatic children in an exercise readaptation program. Is training always helpful for better adaptation to exercise? Chest. 1991;99:579–586. doi: 10.1378/chest.99.3.579. [DOI] [PubMed] [Google Scholar]
- Vallet G, Ahmaidi S, Serres I, Fabre C, Bourgouin D, Desplan J, et al. Comparison of two training programmes in chronic airway limitation patients: standardized versus individualized protocols. Eur Respir J. 1997;10:114–122. doi: 10.1183/09031936.97.10010114. [DOI] [PubMed] [Google Scholar]
- Davis JA. Anaerobic threshold: review of the concept and directions for future research. Med Sci Sports Exerc. 1985;17:6–21. [PubMed] [Google Scholar]
- Hawley JA, Hargreaves M, Joyner MJ, Zierath JR. Integrative biology of exercise. Cell. 2014;159:738–749. doi: 10.1016/j.cell.2014.10.029. [DOI] [PubMed] [Google Scholar]
- Hawley JA. Adaptations of skeletal muscle to prolonged, intense endurance training. Clin Exp Pharmacol Physiol. 2002;29:218–222. doi: 10.1046/j.1440-1681.2002.03623.x. [DOI] [PubMed] [Google Scholar]
- Lakoski SG, Eves ND, Douglas PS, Jones LW. Exercise rehabilitation in patients with cancer. Nat Rev Clin Oncol. 2012;9:288–296. doi: 10.1038/nrclinonc.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convertino VA. Blood volume response to physical activity and inactivity. Am J Med Sci. 2007;334:72–79. doi: 10.1097/MAJ.0b013e318063c6e4. [DOI] [PubMed] [Google Scholar]
- Bishop DJ, Granata C, Eynon N. Can we optimise the exercise training prescription to maximise improvements in mitochondria function and content? Biochim Biophys Acta. 2014;1840:1266–1275. doi: 10.1016/j.bbagen.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Roberts AD, Billeter R, Howald H. Anaerobic muscle enzyme changes after interval training. Int J Sports Med. 1982;3:18–21. doi: 10.1055/s-2008-1026055. [DOI] [PubMed] [Google Scholar]
- MacDougall JD, Hicks AL, MacDonald JR, McKelvie RS, Green HJ, Smith KM. Muscle performance and enzymatic adaptations to sprint interval training. J Appl Physiol. 1985;84:2138–2142. doi: 10.1152/jappl.1998.84.6.2138. [DOI] [PubMed] [Google Scholar]
- Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson RC, Hagberg JM, Ehsani AA, Holloszy JO. Time course of the adaptive responses of aerobic power and heart rate to training. Med Sci Sports Exerc. 1981;13:17–20. [PubMed] [Google Scholar]
- Kreher JB, Schwartz JB. Overtraining syndrome: a practical guide. Sports Health. 2012;4:128–138. doi: 10.1177/1941738111434406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saboul D, Balducci P, Millet G, Pialoux V, Hautier C. A pilot study on quantification of training load: The use of HRV in training practice. Eur J Sport Sci. 2015:1–10. doi: 10.1080/17461391.2015.1004373. [DOI] [PubMed] [Google Scholar]
- Urhausen A, Kindermann W. Diagnosis of overtraining: what tools do we have? Sports Med. 2002;32:95–102. doi: 10.2165/00007256-200232020-00002. [DOI] [PubMed] [Google Scholar]
- Hsia CC. Coordinated adaptation of oxygen transport in cardiopulmonary disease. Circulation. 2001;104:963–969. doi: 10.1161/hc3401.094928. [DOI] [PubMed] [Google Scholar]
- Fry RW, Morton AR, Keast D. Periodisation of training stress--a review. Can J Sport Sci. 1992;17:234–240. [PubMed] [Google Scholar]
- Fry RW, Morton AR, Keast D. Periodisation and the prevention of overtraining. Can J Sport Sci. 1992;17:241–248. [PubMed] [Google Scholar]
- Hellard P, Avalos M, Lacoste L, Barale F, Chatard JC, Millet GP. Assessing the limitations of the Banister model in monitoring training. J Sports Sci. 2006;24:509–520. doi: 10.1080/02640410500244697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffart LM, Galvao DA, Brug J, Chinapaw MJ, Newton RU. Evidence-based physical activity guidelines for cancer survivors: current guidelines, knowledge gaps and future research directions. Cancer Treat Rev. 2014;40:327–340. doi: 10.1016/j.ctrv.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Fairbarn MS, Blackie SP, McElvaney NG, Wiggs BR, Pare PD, Pardy RL. Prediction of heart rate and oxygen uptake during incremental and maximal exercise in healthy adults. Chest. 1994;105:1365–1369. doi: 10.1378/chest.105.5.1365. [DOI] [PubMed] [Google Scholar]
- Panton LB, Graves JE, Pollock ML, Garzarella L, Carroll JF, Leggett SH, et al. Relative heart rate, heart rate reserve, and VO2 during submaximal exercise in the elderly. J Gerontol A Biol Sci Med Sci. 1996;51:M165–M171. doi: 10.1093/gerona/51a.4.m165. [DOI] [PubMed] [Google Scholar]
- Jones LW, Eves ND, Haykowsky M, Freedland SJ, Mackey JR. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol. 2009;10:598–605. doi: 10.1016/S1470-2045(09)70031-2. [DOI] [PubMed] [Google Scholar]
- Seiler KS, Kjerland GO. Quantifying training intensity distribution in elite endurance athletes: is there evidence for an ‘optimal’ distribution? Scand J Med Sci Sports. 2006;16:49–56. doi: 10.1111/j.1600-0838.2004.00418.x. [DOI] [PubMed] [Google Scholar]
- Orio F, Giallauria F, Palomba S, Manguso F, Orio M, Tafuri D, et al. Metabolic and cardiopulmonary effects of detraining after a structured exercise training programme in young PCOS women. Clin Endocrinol (Oxf) 2008;68:976–981. doi: 10.1111/j.1365-2265.2007.03117.x. [DOI] [PubMed] [Google Scholar]
- Saltin B, Blomqvist G, Mitchell JH, Johnson RL, Jr, Wildenthal K, Chapman CB. Response to exercise after bed rest and after training. Circulation. 1968;38:VII1–VII78. [PubMed] [Google Scholar]
- Rodon J, Saura C, Dienstmann R, Vivancos A, Ramon y Cajal S, Baselga J, et al. Molecular prescreening to select patient population in early clinical trials. Nat Rev Clin Oncol. 2012;9:359–366. doi: 10.1038/nrclinonc.2012.48. [DOI] [PubMed] [Google Scholar]
- Carden CP, Sarker D, Postel-Vinay S, Yap TA, Attard G, Banerji U, et al. Can molecular biomarker-based patient selection in Phase I trials accelerate anticancer drug development? Drug Discov Today. 2010;15:88–97. doi: 10.1016/j.drudis.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Klijn P, van Keimpema A, Legemaat M, Gosselink R, van Stel H. Nonlinear exercise training in advanced chronic obstructive pulmonary disease is superior to traditional exercise training. A randomized trial. Am J Respir Crit Care Med. 2013;188:193–200. doi: 10.1164/rccm.201210-1829OC. [DOI] [PubMed] [Google Scholar]
- Jones LW, Douglas PS, Eves ND, Marcom PK, Kraus WE, Herndon JE, 2nd, et al. Rationale and design of the Exercise Intensity Trial (EXCITE): a randomized trial comparing the effects of moderate versus moderate to high-intensity aerobic training in women with operable breast cancer. BMC Cancer. 2010;10:531. doi: 10.1186/1471-2407-10-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LW, Peddle CJ, Eves ND, Haykowsky MJ, Courneya KS, Mackey JR, et al. Effects of presurgical exercise training on cardiorespiratory fitness among patients undergoing thoracic surgery for malignant lung lesions. Cancer. 2007;110:590–598. doi: 10.1002/cncr.22830. [DOI] [PubMed] [Google Scholar]
- Jones LW, Eves ND, Peterson BL, Garst J, Crawford J, West MJ, et al. Safety and feasibility of aerobic training on cardiopulmonary function and quality of life in postsurgical nonsmall cell lung cancer patients: a pilot study. Cancer. 2008;113:3430–3439. doi: 10.1002/cncr.23967. [DOI] [PubMed] [Google Scholar]
- Courneya KS, Jones LW, Peddle CJ, Sellar CM, Reiman T, Joy AA, et al. Effects of aerobic exercise training in anemic cancer patients receiving darbepoetin alfa: a randomized controlled trial. Oncologist. 2008;13:1012–1020. doi: 10.1634/theoncologist.2008-0017. [DOI] [PubMed] [Google Scholar]
- Courneya KS, Sellar CM, Stevinson C, McNeely ML, Peddle CJ, Friedenreich CM, et al. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J Clin Oncol. 2009;27:4605–4612. doi: 10.1200/JCO.2008.20.0634. [DOI] [PubMed] [Google Scholar]
- Hornsby WE, Douglas PS, West MJ, Kenjale AA, Lane AR, Schwitzer ER, et al. Safety and efficacy of aerobic training in operable breast cancer patients receiving neoadjuvant chemotherapy: a phase II randomized trial. Acta Oncol. 2014;53:65–74. doi: 10.3109/0284186X.2013.781673. [DOI] [PubMed] [Google Scholar]
- Jones LW, Hornsby WE, Freedland SJ, Lane A, West MJ, Moul JW, et al. Effects of nonlinear aerobic training on erectile dysfunction and cardiovascular function following radical prostatectomy for clinically localized prostate cancer. Eur Urol. 2014;65:852–855. doi: 10.1016/j.eururo.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


