Abstract
Background
The diversity of definitions proposed for sarcopenia has been rarely tested in the same population, and so far, their clinical utilities for predicting physical difficulties could not be clearly understood. Our objective is to report the prevalence of sarcopenia and the characteristics of sarcopenic community-dwelling older women according to the different definitions of sarcopenia currently proposed. We also assessed these definitions for their incremental predictive value over currently standard predictors for some self-reported difficulties in physical function and knee extension strength.
Methods
Cross-sectional analysis included data from 3025 non-disabled women aged 75 years or older without previous history of hip fracture from the inclusion visit of the EPIDémiologie de l'OStéoporose study. A total body composition evaluation was available for 2725 women. Sarcopenia was defined using six different definitions of sarcopenia based on different muscle mass, gait speed, and grip strength cut-offs. Self-reported difficulties in physical function and knee extension strength were collected. Logistic regression and multiple linear regression models were built for each physical dysfunction, and the predictive capacity of sarcopenia (one model for each definition) was studied using the C-statistic, the net reclassification index, or adjusted R2.
Results
The estimated prevalence of sarcopenia ranged from 3.3–20.0%. Only 85 participants (3.1%) were identified having sarcopenia according to all definitions. All definitions were, to some degree, associated with self-reported difficulties in physical function and knee extension strength, but none improved the predictive ability of the self-reported difficulties in physical function. Conversely, all definitions accounted for a small but significant amount of explained variation for predicting knee extension strength.
Conclusions
Prevalence of sarcopenia varies widely depending on the definition adopted. Based on this research, the current definitions for sarcopenia does not substantially increment the predictive value of clinical characteristics of patients to predict self-reported physical difficulties and knee extension strength.
Keywords: Sarcopenia, Muscle mass, Definition, Physical function
Introduction
Sarcopenia is a growing area of research and a great opportunity for the development of new drugs because it is an important determinant of physical function in older people1 and a potential pharmaceutical target in the prevention of mobility disability in older people. Researchers agree that sarcopenia is defined by a loss of muscle mass, muscle strength, and muscle quality, but a unique consensual operational definition of sarcopenia is lacking. In fact, no less than six clinical approaches have been proposed since 1998 to establish the diagnosis of sarcopenia in practice.2–7 To our knowledge, no definition has clearly proven its superiority over previous definitions. The first approach2 to define sarcopenia has been used by a number of investigators and relies on an arbitrary cut-off of appendicular muscle mass assessed by dual X-ray absorptiometry (DXA). A different statistical approach4 and a different cut-off of muscle mass have also been proposed few years later to reduce the limitation of this first approach in obese and thin subjects and to improve the rationale of the cut-point.5 However, these first proposals are not completely satisfactory because the influence of muscle mass on mobility is complex. Muscle mass is definitely not the only determinant of mobility, and physical performances measures have appeared as prominent determinants of lower functional capacities. Then, the last three definitions of sarcopenia3,6,7 are characterized by different easy-to-measure functional tool (gait speed and/or handgrip strength with a different cut-off) in addition to muscle mass (with a different cut-off).
This controversy in the definition of sarcopenia results in inconsistent conclusions across cohorts and impossible comparison and represents an important limitation for drug agencies such as the Federal Drug Administration or the European Medicines Agency to consider sarcopenia as a treatable condition. A cohesive view of sarcopenia is urgently needed.
The aims of this study were to report the prevalence of sarcopenia and the characteristics of the sarcopenic older people according to six current different definitions in a large cohort study and also to quantify the improvement in self-reported difficulties in physical function and knee extension strength (KES) prediction offered by these definitions.
Materials and methods
EPIDémiologie de l'OStéoporose cohort
We studied data from the Epidemiologie de l'Osteoporose (EPIDOS) study, a prospective cohort study whose primary purpose was to evaluate risk factors for hip fracture in a healthy community-dwelling population of elderly women. The sampling and data collection procedures have been previously described in detail.8 Briefly, between January 1992 and January 1994, 7598 women recruited from the electoral lists, in five French cities, volunteered to participate. Women disabled for walking (walking aids allowed), with a history of femoral neck fracture, hip replacement or institutionalization, or subjects unable to understand or answer the questionnaire were excluded. The present study was limited to the 1563 participant included in Lyon and the 1462 participant included in Toulouse. These participants completed a total body composition assessment [bone mineral density, lean mass (LM), and fat mass (FM)] by DXA and a physical exam including anthropometric measures (height, weight, and calf circumference). A total body composition assessment was available for 2725 women. Cognition was evaluated using the short portable mental status questionnaire (SPMSQ, 10). The SPMSQ, a 10-item questionnaire, was developed to detect the presence of cognitive decline in community-dwelling older adults. The validated cut-off value for normal cognitive functioning is a score of 8 or above.9 The local ethics committee of the participating centres approved the study, and each woman signed informed consent.
Sarcopenia definitions
Six different definitions were used to define sarcopenia (coded I to VI). Three of them rely on muscle mass only (2, 4, and 5), two rely on muscle mass and gait speed,6,7 and one on muscle mass, gait speed, and/or handgrip strength.3
Assessment of body composition
The body composition of all participants was measured using DXA. The DXA apparatus (Hologic QDR 4500 W, Hologic, Waltham, MA, USA) was regularly calibrated, and the DXA protocol was performed by a trained technician. We used an accurate method to quantify appendicular skeletal muscle mass (ASM),10,11 which corresponds to the sum of the muscle mass (in kilogrammes) of the four limbs.
Assessment of gait speed
A standardized assessment of gait speed was performed at baseline. Participants were asked to perform a 6 m walk at their usual pace; walking aids were allowed.12,13 Timing began when the command was given, and time in seconds needed to complete the 6 m walk was recorded. The faster of two walks was retained for the present analysis. Gait speed was calculated in metres (m) per second (s).
Assessment of handgrip strength
Handgrip strength was measured for the dominant hand with a hydraulic hand dynamometer (Martin Vigorimeter, Medizin Tecnik, Tuttlingen, Germany). The size of the grip was adjusted so that the participant felt comfortable. The participant stood upright with the arm vertical and the dynamometer close to the body. The maximal peak pressure expressed in Newton per square metre (Nm−2) was recorded for a set of three contractions and was used for the present analyses. Handgrip strength was analysed in quartiles. This following overview summarizes chronologically the six diagnostic criteria used in this study. They have been previously described in detail2–6,14.
Definition
Sarcopenia definition proposed by Baumgartner et al.2 Baumgartner defined sarcopenia using the ASM/height2 ratio establishing the threshold at two standard deviations lower than the average of a young reference population defined sarcopenia. The cut-off that defines sarcopenia in women is ASM/height2 ≤ 5.45 kg/m2.
Definition II
Sarcopenia definition using the residuals method proposed by Newman et al.4 A measure of relative LM (LM, kilogrammes, kg) was derived by adjusting for fat mass (FM, kg) in addition to height (metres, m). The residuals of the regression were used to identify those whose LM was much lower or higher than the predicted value. A positive residual would indicate a relatively muscular individual, whereas negative values would indicate relatively sarcopenic individuals. The 20th percentile of the distribution of residuals was used as the cut-off for sarcopenia.
Definition III
Sarcopenia definition using the ASM cut-off proposed by Delmonico et al.5 Instead of comparing ASM/height2 with a cut-off from younger population, participants were classified as sarcopenic if their ASM/height2 fell into the sex-specific lowest 20% of the health ageing and body composition study distribution of the index (ASM/height2 ≤ 5.67 kg/m2).
Definition IV
Sarcopenia definition proposed by the European Working Group on Sarcopenia in Older People3 This definition is based on the presence of a low ASM using the Baumgartner's criteria (ASM/height2 ≤ 5.45 kg/m2) combined with a low gait speed with a threshold established at ≤0.8 m/s (s) or the presence of low handgrip strength with a cut-off of 20 kg using the dynamometer. In our population, as handgrip strength was measured in Newton per square metre, a pressure unit that cannot be translated into kilogramme, we used an approximation of this definition with a cut-off based on the lower quartile of the distribution as was done in the cardiovascular health study.
Definition V
Sarcopenia using the ASM and gait speed defined by Muscaretoli et al.14 This definition used Baumgartner's cut-off (ASM/height2 ≤ 5.45 kg/m2) and a gait speed ≤0.8 m/s.
Definition VI
Sarcopenia definition proposed by the International Working Group on Sarcopenia6,7 The definition combined Delmonico's definition (ASM/h2 ≤ 5.67 kg/m2) with a poor performance at the gait speed test. The threshold of gait speed was established at ≤1 m/s.
In our study, we used gait speed only because a 6 m walk test was not assessed. This definition is quite similar than the Definition V, but the cut-off of ASM and gait speed is different.
Self-reported difficulty in physical function
Participants were asked by a trained nurse whether they had difficulties (no/some/serious difficulty) performing various physical movements such as walking, climbing stairs, descending stairs, rising from a chair, picking up an object from the floor, and lifting heavy objects. The categories some and serious difficulties were grouped all together. Women with three or more difficulties (labelled as ‘moving difficulties’) were grouped together.15,16
Assessment of knee extension strength
To measure maximum isometric KES, participants were seated in an adjustable straight back chair with the pelvis fixed by a strap and a strength gauge attached by a strap just above the ankle [ADCRO (Association pour le Développement de la Chirurgie Réparatrice et Orthopédique) electronic statometers, Valenton, France].17 For analysis, we used the mean of the highest score of three attempts of each leg, recorded in Newton (N). Each time, verbal encouragement was given to obtain the maximal score.
Assessment of health and disability
Trained nurses performed the assessment of health and disability.
A physical examination and a health status questionnaire were used to record age and co-morbid disease [hypertension, diabetes, dyslipidemia, coronary heart disease, peripheral vascular disease, cancer, stroke, Parkinson's disease, depression, and pain (pain of the back, hip, knee, ankle, or feet)]. Obesity was defined as a body mass index (weight/height2) above 30. Impaired vision was assessed using a visual acuity test. It was measured at a distance of 5 m with a Snellen letter test chart. Smoking (previous or current) and alcohol intake were noted. Monthly income was classified into three groups. Educational level was assessed as a dichotomous variable indicating receipt of the French certificate of elementary school education. Participants were also asked whether they had had a previous job. Dressing, toileting, and mobility were items assessed for basic activities of daily living and categorized as a dichotomous variable, independent or not, for all of the three items. Participants were considered disabled if they had limitation in at least one of the three items.18 Women were also asked whether they had taken hormone replacement therapy or corticosteroids during the last 3 months. Participants reported in a structured questionnaire whether they practised recreational activities such as walking, gymnastics, cycling, swimming, or gardening. Type, frequency, and duration of each activity were recorded. As the previous paper, the ‘physically active’ variable was constructed to obtain the fittest 20% of the study sample; this was equivalent to a participant practising at least one activity for at least 1 h a week for the past month or more. This approach has been used previously.19
Statistical analysis
The prevalence of sarcopenia was calculated according to the six different definitions. Characteristics of the sarcopenic participants were expressed as means and standard deviations or median and inter quartile range for quantitative variables and as frequencies and percentage for qualitative variables.
A reference model was built for each of the five specific self-reported difficulties in physical function including relevant predictive factors (age, obesity, income, previous job, education, physical activity, cognitive status, hypertension, diabetes mellitus, dyslipidemia, coronary heart disease, peripheral vascular disease, cancer, stroke, Parkinson's disease, depression, pain, anger, visual impairment, hormone replacement therapy, corticosteroid treatment, smoking, alcohol intake, and living alone). The goodness-of-fit of each model was estimated using Akaike's information criteria (AIC). These criteria help for identifying an optimal model from a class of competing models taking model complexity into account. The chosen model is the one that minimizes the AIC. The concordance (C)-statistic was calculated to evaluate the discriminatory ability of the reference models. This statistic represents the capacity to distinguish high from low risk subjects and is analogous to the area under the receiver-operating characteristic curve. We performed an internal validation of the model using a bootstrapping method (5000 random bootstrap samplings) for correcting the C-statistic for optimism.20 To assess the calibration of our models, the Hosmer–Lemeshow statistic was calculated.21 To test the relevance of sarcopenia as a predictive factor, we inserted into the reference model sarcopenia using the six published definitions. First order interaction between sarcopenia and the other predictors was tested. In these final models, the value of the AIC, the C-statistic, and the calibration were re-estimated. Finally, we assessed the incremental value of the variable defining sarcopenia calculating the net reclassification index (NRI, 95% confidence interval, CI). The NRI is the net fraction of reclassifications in the right direction by making decisions based on predictions with the model including sarcopenia compared with a decision without this information.22
The same approach was performed for KES. A reference model was built for, except that the analysis was performed using multiple linear regression models and that gain in prediction was measured comparing adjusted R2, a measure of the proportion of the total variability explained by the model, using bootstrapping.
Tests were two-sided, and P-values lower than 0.05 were considered significant. Data analysis was performed using STATA 11.2 software (Stata Corporation, College Station, TX, USA).
Results
Baseline characteristics and prevalence of sarcopenia according to the six different definitions are presented in Table 1. The prevalence extended from 3.3(95% CI, 2.6–4, Definition V)–20.0% (95% CI, 17.3–20.3, Definition III).
Table 1.
Sample characteristics by sarcopenia definitions
| Entire population | Sarcopenic patients | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline characteristics | Definition I Baumgartner 1998 | Definition II Newman 2003 | Definition III Delmonico 2007 | Definition IV Cruz-Jentoft 2010 EWGSOP | Definition V Muscaretoli 2010 | Definition VI Fielding 2011 IWGSMorley 2011 | ||
| Variables, n (%) | n = 3025 | ASM/h2b ≤5.45 kg/m2 | ASM <20th percentile of residual distribution | ASM/h2 ≤5.67 kg/m2 | ASM/h2 ≤5.45 kg/m2 + GSc ≤0.8 m/s and/or lowest quartile HGd | ASM/h2 ≤5.45 kg/m2 + GS ≤0.8 m/s | ASM/h2 ≤5.67 kg/m2 + GS ≤1 m/s | |
| n = 283 (10.4%)e | n = 541 (20.0%)e | n = 511 (18.8%)e | n = 142 (5.2%)e | n = 89 (3.3%)e | n = 390 (14.3)e | |||
| Age (years), mean ± SD | 80.5 ± 3.9 | 80.3 ± 3.9 | 80.1 ± 3.0 | 80.4 ± 3.8 | 81.4 ± 4.1 | 81.9 ± 4.0 | 80.8 ± 3.9 | |
| Living alone, n (%) | 1684 (55.7) | 150 (53.0) | 291 (53.4) | 277 (54.2) | 73 (51.4) | 42 (47.2) | 206 (52.8) | |
| Certificate of elementary education, n (%) | yes | 2430 (80.4) | 244 (86.2) | 482 (83.7) | 442 (86.7) | 115 (81.0) | 70 (78.6) | 335 (86.1) |
| Disability, n (%) | yes | 844 (27.9) | 88 (31.9) | 161 (30.5) | 149 (29.7) | 43 (31.6) | 23 (27.1) | 111 (29.1) |
| Physically active, n (%) | Yes | 825 (27.3) | 101 (35.7) | 1254 (28.5) | 156 (30.5) | 34 (23.9) | 19 (21.4) | 99 (25.4) |
| SPMSQ, n (%) | ≥8 | 2533 (83.7) | 240 (84.8) | 459 (84.8) | 438 (85.7) | 114 (80.3) | 67 (75.3) | 326 (83.6) |
| <8 | 492 (16.3) | 43 (15.2) | 82 (15.2) | 73 (14.3) | 28 (19.7) | 22 (24.7) | 64 (16.4) | |
| Cancer, n (%) | 398 (14.8) | 41 (10.3) | 89 (22.4) | 85 (16.9) | 17 (4.3) | 11 (2.8) | 64 (16.1) | |
| Depression, n (%) | 445 (16.5) | 49 (17.4) | 98 (18.2) | 96 (19.0) | 31 (21.8) | 22 (24.7) | 83 (21.3) | |
| Hypertension, n (%) | 1280 (47.5) | 122 (9.5) | 262 (20.5) | 219 (17.1) | 64 (5.0) | 46 (3.6) | 174 (13.6) | |
| Pain, n (%) | 2063 (76.4) | 205 (9.9) | 411 (19.9) | 374 (18.1) | 98 (4.8) | 65 (3.2) | 288 (14.0) | |
| Visual impairment, n (%) | 1583 (58.9) | 168 (10.6) | 323 (20.4) | 303 (60.2) | 92 (65.7) | 61 (3.9) | 242 (15.3) | |
| Fat mass (kg), mean ± SD | 31.5 ± 7.4 | 17.1 ± 5.7 | 21.5 ± 6.4 | 18.18 ± 6.2 | 16.8 ± 5.8 | 17.3 ± 5.7 | 18.4 ± 6.5 | |
| Lean mass (kg), mean ± SD | 34.7 ± 2.6 | 30.6 ± 2.8 | 31.0 ± 2.8 | 31.1 ± 2.7 | 30.2 ± 2.3 | 30.4 ± 2.2 | 31.0 ± 2.6 | |
| Bone mineral density (g/cm2), mean ± SD | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.0 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | |
| Calf circumference (cm), mean ± SD | 34.6 ± 3.1 | 31.6 ± 2.3 | 33.0 ± 2.7 | 32.2 ± 2.4 | 31.3 ± 2.3 | 31.3 ± 2.4 | 32.1 ± 2.5 | |
| Gait speed (m/s), mean ± SD | 0.8 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.8 ± 0.2 | 0.7 ± 0.2 | 0.8 ± 0.2 | |
| ASM/h2 (kg/m2), mean ± SD | 6.3 ± 0.7 | 5.2 ± 0.2 | 5.5 ± 0.3 | 5.4 ± 0.2 | 5.2 ± 0.2 | 5.2 ± 0.2 | 5.4 ± 0.3 | |
| Handgrip strength, n (%) | ||||||||
| Lowest quartile | 758 (25.1) | 98 (34.9) | 160 (29.5) | 159 (31.4) | 98 (69.5) | 45 (51.1) | 133 (34.5) | |
| Highest quartile | 738 (2.4) | 37 (13.2) | 92 (17.0) | 79 (15.6) | 6 (4.3) | 6 (6.8) | 50 (13.0) | |
| Knee extension strength (N), mean ± SD | 161.7 ± 48.9 | 141.8 ± 43.1 | 149.2 ± 43.6 | 144.9 ± 42.7 | 131.8 ± 39.6 | 126.3 ± 37.5 | 139.6 ± 41.4 | |
SD, standard deviation; EWGOP, European Working Group on Sarcopenia in Older People, IWGS, International Working Group on Sarcopenia
ASM, appendicular skeletal muscle mass.
h, height.
GS, gait speed in metre per seconds.
HG, handgrip strength.
Prevalence of sarcopenia calculated on 2725 available data of total body composition evaluation.
Disability in dressing and toileting and mobility.
SPMSQ, short portable mental status questionnaire, a 10-item questionnaire, to detect the presence of cognitive decline in community-dwelling older adults. The validated cut-off value for normal cognitive functioning is a score of 8 or above.
Figure 1 (a Venn diagram build by STATA) shows the respective distribution of participants identified as sarcopenic according to the different definitions. A large overlapping exists between the six definitions but only 85 participants (3.1%) matched with all six definitions of sarcopenia.
Figure 1.
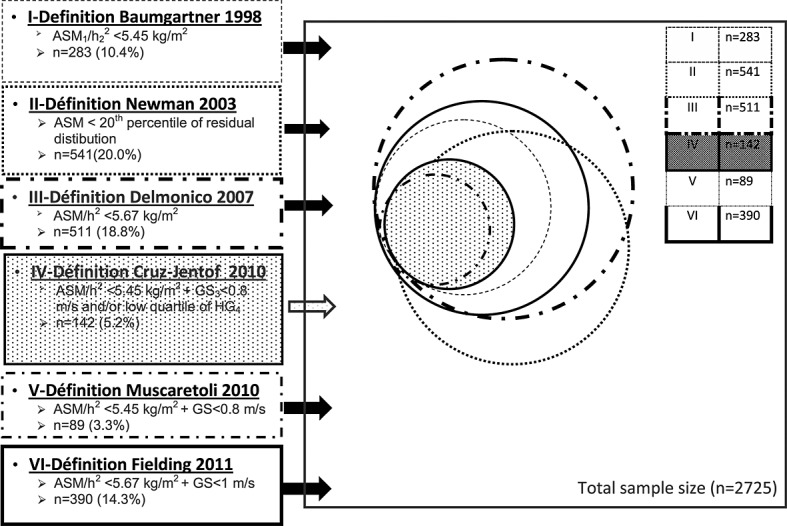
Distribution of a participant identified as sarcopenic according to the different definitions. ASM, appendicular skeletal muscle mass; h, height; GS, gait speed, HG, handgrip strength.
Table 2 shows the goodness of fit and the discriminatory ability for each reference model that include all clinically relevant predictors for the different self-reported difficulties in physical function. For each reference model of relevant predictors for the difficulties in physical function, we added a different definition of sarcopenia. Concerning the different self-reported difficulties in physical function, 3.7% have walking difficulties, 40.9% have climbing stairs difficulties, 51.2% have descending stairs difficulties, 39.5% have rising a chair difficulties, and 48.5% have more than three difficulties. The predictive power of each reference model varied between 68% and 81%.
Table 2.
Association between self-reported physical dysfunction and each sarcopenia definitions
| Difficulty for physical function | ||||||
|---|---|---|---|---|---|---|
| n (%)/Na | Walking difficultiesb 95 (3.7)/2661 | Climbing stairsc 1100 (40.9)/2688 | Descending stairsd 1371 (51.2)/2677 | Rising from a chaire 1061 (39.5)/2685 | Moving difficultiesf (≥3 difficulties)g 1301 (48.5)/2680 | |
| Initial model | AIC | 792 | 2896 | 3156 | 3029 | 2879 |
| AUC %g | 68 | 79 | 76 | 77 | 81 | |
| Definition I (n = 283) Baumgartner 1998, sarcopenia defined by ASM/h2 <5.45 kg/m2 | ORa | 1.5 | 1.1 | 1.4 | 1.3 | 1.6 |
| 95% CI | 0.8, 2.7 | 0.8–1.6 | 1.0, 1.8 | 0.9, 1.1 | 1.2, 2.2 | |
| AIC | 793 | 2897 | 3153 | 3029 | 2871 | |
| AUC %g | 68 | 80 | 76 | 77 | 81 | |
| Definition II (n = 541) Newman 2003, sarcopenia defined by linear regression | ORa | 1.6 | 1.3 | 1.5 | 1.4 | 1.5 |
| 95% CI | 0.9, 2.6 | 1.0, 1.7 | 1.2, 1.9 | 1.1, 1.7 | 1.2, 1.9 | |
| AIC | 792 | 2893 | 3148 | 3025 | 2862 | |
| AUC %g | 67 | 80 | 76 | 77 | 81 | |
| Definition III (n = 511) Delmonico 2007, sarcopenia defined by ASM/h2 <5.67 kg/m2 | ORa | 1.8 | 1.3 | 1.4 | 1.3 | 1.5 |
| 95% CI | 1.1, 2.9 | 1.0, 1.7 | 1.1, 1.7 | 1.0, 1.6 | 1.2, 1.9 | |
| AIC | 787 | 2894 | 3151 | 3028 | 2870 | |
| AUC %g | 68 | 80 | 76 | 77 | 81 | |
| Definition IV (n = 142) Cruz-Jentoft 2010, sarcopenia defined by ASM/h2 <5.45 kg/m2 + GS <0.8 m/s or lowest quartile HG | ORa | 1.7 | 1.5 | 2.2 | 1.4 | 2.1 |
| 95% CI | 0.7, 3.7 | 1.0, 2.2 | 1.5, 3.2 | 1.0, 2.2 | 1.4, 3.2 | |
| AIC | 793 | 2895 | 3142 | 3028 | 2867 | |
| AUC %g | 67 | 80 | 76 | 77 | 81 | |
| Definition V (n = 89) Muscaretoli 2010, sarcopenia defined by ASM/h2 <5.45 kg/m2 + GS <0.8 m/s | ORa | 1.4 | 2.3 | 2.2 | 2.4 | 2.7 |
| 95% CI | 0.5, 3.8 | 1.4, 3.9 | 1.3, 3.6 | 1.5, 3.9 | 1.6, 4.6 | |
| AIC | 794 | 2887 | 3148 | 3018 | 2860 | |
| AUC %g | 67 | 80 | 76 | 77 | 81 | |
| Definition VI (n = 390) Fielding and Morley 2011, sarcopenia defined by ASM/h2 <5.67 kg/m2 + GS <1 m/s | ORa | 2.0 | 1.6 | 1.6 | 1.4 | 1.6 |
| 95% CI | 1.2, 3.3 | 1.2, 2.1 | 1.2, 2.0 | 1.1, 1.8 | 1.3, 2.1 | |
| AIC | 787 | 2886 | 3145 | 3024 | 2867 | |
| AUC %g | 68 | 80 | 76 | 77 | 81 | |
AIC, Akaike information criteria; ASM, appendicular skeletal muscle mass; AUC, area under curve; CI, confidence interval; GS, gait speed in metre per second (m/s); h, height; HG, handgrip strength; ORa, adjusted odds ratio.
Number and percentage of patients reporting the specified physical difficulty/number of patients in each model.
Adjusted for age, hypertension, pain, visual impairment, and previous job.
Adjusted for age, obesity, peripheral vascular disease, cancer, depression, pain, visual impairment, physical activity, and cognitive status.
Adjusted for age, obesity, hypertension, cancer, depression, pain, visual impairment, coronary heart disease, and physical activity.
Adjusted for age, obesity, peripheral vascular disease, depression, pain, visual impairment, and physical activity.
Moving difficulties: walking, climbing stairs, rising from a chair or bed, picking an object from the floor, lifting heavy objects, and reaching an object.
Adjusted for age, obesity, cancer, depression, pain, visual impairment, coronary heart disease, physical activity, and cognitive status.
Optimist was corrected by bootstrap procedure.
All the definitions of sarcopenia were significantly associated with these items ‘descending stairs’ and ‘moving difficulties’. They provided a better fit for the data than the reference model with AIC systematically lower in models including sarcopenia definition. Regarding the item ‘moving difficulties’, the adjusted odd ratio ranged from (ORa) = 1.5 (95% CI, 1.2 and 1.9, Definition III) to 2.7 (95% CI, 1.6 and 4.6, Definition V).
But in spite of significant association and improvement of model fit, no definitions significantly improved the predictive capacity of the reference model. In fact, the area under curve remained unchanged in the different models explored. Moreover, for all definitions, the NRIs were zero or almost nil. By looking at physical difficulties one by one, Definition I added complexity to the reference model without improving model fit (except for descending stairs and moving difficulties) without any improvement of predictive power.
Definitions III and VI were the only ones that clearly improved the modelling of all self-reported difficulties in physical function but, once more, without improvement of predictive power.21 The conclusions are the same for models picking and lifting an object from the floor (data not shown).
Table 3 shows the adjusted mean difference in KES between sarcopenic and non-sarcopenic women and the proportion of the total variability explained using the six definitions of sarcopenia. Whatever the definitions used, all sarcopenic women had a significantly lower KES than non-sarcopenic women. The initial model fit was always improved by adding one of the definitions of sarcopenia and all definitions accounted for a small but significant amount of explained variation (compared with the initial model, difference in adjusted R2 varied between 1 and 2%).
Table 3.
Association between maximum knee extension strength and each sarcopenia definitions
| n = 2419 | Maximum knee extension strength (Newton) | |
|---|---|---|
| Initial modela | AIC | 25 469 |
| Adjusted R2% | 8.3 | |
| Definition I (n = 283) Baumgartner 1998, sarcopenia defined by ASM/h2 <5.45 kg/m2 | Coef | −19.7 |
| 95% CI | −26.0, −13.2 | |
| AIC | 25 434 | |
| Adjusted R2% | 9.6b | |
| Definition II (n = 541) Newman 2003, sarcopenia defined by linear regression | Coef | −14.1 |
| 95% CI | −18.8, −9.4 | |
| AIC | 25 436 | |
| Adjusted R2% | 9.5b | |
| Definition III (n = 511) Delmonico 2007, sarcopenia defined by ASM/h2 <5.67 kg/m2 | Coef | −18.4 |
| 95% CI | −23.5, −13.3 | |
| AIC | 25 420 | |
| Adjusted R2% | 10.1b | |
| Definition IV (n = 142) Cruz-Jentoft 2010, sarcopenia defined by ASM/h2 <5.45 kg/m2 + GS <0.8 m/s or lowest quartile HG | Coef | −26.5 |
| 95% CI | −32.5, −17.8 | |
| AIC | 25 435 | |
| Adjusted R2% | 9.6 | |
| Definition V (n = 89) Muscaretoli 2010, sarcopenia defined by ASM/h2 <5.45 kg/m2 + GS <0.8 m/s | Coef | −29.4 |
| 95% CI | −40.4, −18.3 | |
| AIC | 25 443 | |
| Adjusted R2% | 9.3b | |
| Definition VI (n = 390) Fielding and Morley 2011, sarcopenia defined by ASM/h2 <5.67 kg/m2 + GS <1 m/s | Coef | −21.3 |
| 95% CI | −26.8, −15.7 | |
| AIC | 25 414 | |
| Adjusted R2% | 10.4b |
AIC, Akaike information criteria; ASM, appendicular skeletal muscle mass; CI, confidence interval; GS, gait speed; h, height; HG, handgrip strength; Coef, mean difference in kes between sarcoepnic and non sarcoepnic.
Adjusted for age, obesity, depression, visual impairment, pain, physical activity, corticosteroid treatment, and income.
Significant difference between adjusted R2 in the model with sarcopenia and the initial model (tested by bootstrap).
Discussion
This study examines different aspects of six definitions of sarcopenia in the same and large cohort of elderly women. Five of these definitions were exactly the same than the ones previously described; one was approximated using a different cut-off for handgrip strength than the one initially used because there is no possible unit conversion between Newton per square metre and kilogramme. Our study confirms the large range of prevalence (3.3–20.0%) depending on the definition adopted. These results have been highlighted by previous reviews of different cohorts.23 A tiny difference in the cut-off of the muscle mass ratio (i.e. 0.22 kg/m2 between the cut-offs) or gait speed (i.e. 0.2 m/s between the cut-offs) results in large differences in the prevalence.
It should be noted that a clear overlapping exists between these different definitions of sarcopenia (Figure 1).
Based on our statistical approach, none of the definitions clearly added predictive value comparatively to the other clinical predictors may be because clinical factors included in the reference model were sufficient to reach the threshold of 70% required for a useful predictive model.21
Definitions that combine mass and physical performance measures seem not more relevant than the definition based on muscle mass only in predicting physical difficulties. None of the six definitions of sarcopenia explored seemed superior to the other. In other words, sarcopenia, whatever the definition used, did not significantly improve the prediction for the self-reported physical difficulties. However, Definitions I and IV clearly added complexity in the different statistical modelling. A tiny statistical advantage can be reported for Definitions III and VI, probably because its large thresholds select more participants with the poorer muscle mass index and the slower gait speed compared with the other definitions. However, assessment of different physical tasks could have resulted in different trends. Definition V results in the smallest prevalence of sarcopenia (only 3.3%). This definition may exclude participants who could benefit from intervention against sarcopenia.
In this work, the different definitions of sarcopenia did not improve the predictive capacity to report the self-reported difficulties in physical function. No significant improvement of predictive value was found with any of the six definitions, compared to the model based only on the clinical characteristics of the participants. The different definitions of sarcopenia were significantly associated with some of the reported difficulties in physical function, but the clinical characteristics of the participant provided statistically similar predictive values. The six definitions of sarcopenia explored in this work were statistically and significantly inversely associated with maximum KES. Compared to the initial model that only includes patients' clinical characteristics, all definitions slightly improved KES prediction. However, additional information provided by these definitions was small and not clinically relevant.
Our results support that the cut-offs used in the different definitions of sarcopenia were not appropriate. This hypothesis is supported by several studies showing no or tiny significant relationship between muscle mass and incidence of clinical adverse outcomes.24–26 Currently, the relationship between muscle mass, physical performances, and physical function remains unclear. However, growing evidence suggests that muscle quality, a marker of muscle strength developed by the amount of muscle mass, should be investigated.27 Muscle power, the strength multiplied by speed, is a key component of muscle quality. These domains involved large number of component of mobility (the peripheral nervous system, muscle metabolism, balance, and cognitive function) other than muscle mass. The loss of muscle mass that includes the kinetic of the muscle lost may also be more predictive of future functional decline than the punctual muscle mass. In the field of nutrition in geriatrics, the predictive value of weight loss is stronger than the weight at one time.28
Low muscle strength and poor physical performance measures have been repeatedly reported to predict functional decline, while conflicting results are reported for the predicting value of low muscle mass.29–31 While physical performance measures can capture an overall neuromuscular function, they are nevertheless not specific to the muscle function.30 Whether physical performance measures alone would have results in a similar association with difficulties in physical function as the composite definitions of sarcopenia are off topic in this study, as no definition of sarcopenia relies on physical performances measures alone. Some functional difficulties are more or less sensitive to loss of lean body mass.32–36 The assessment of other physical tasks may also have result in other results. These results seem to be related to the different cut-offs chosen; therefore, we think continuous measures of the muscle mass and handgrip have probable other benefits. Dichotomization is artificial and often unnecessary. Further, it discards potentially important quantitative information, thus reducing the power to detect a real association.37 To our knowledge, this is one of the few studies that examine the current definitions of sarcopenia in the same population.38,39 One study38 recently explores the degree of agreement between different diagnostic criteria for sarcopenia, but this study involved 329 women and 325 men older than 60 years, body composition assessment by bio-impedance analysis, and gait speed was not assessed. Another recent studies39 are attempting to identify existing associations between sarcopenia and the risk of falling. Indeed, Scott et al.39 noted a positive association with the increases in fall risks over 5 years in community-dwelling middle-age and older adults. However, they also supported that the significant associations could not be explained by muscle strength only and suggested that other physiological criteria that contributed to falls. This finding may be reflective of our study and may indicate that the criteria of definition of sarcopenia should be use with differential weightings for muscle mass and functional performance. Indeed, in this study, gait speed was not assessed; therefore, they would not be able to use the latest consensual definitions of sarcopenia (European Working Group on Sarcopenia in Older People or International Working Group on Sarcopenia). Using respectively eight and two different definitions of sarcopenia, Batsis et al.40 and Dam et al.41 previously reported the large range of prevalence of sarcopenia depending on the research definitions adopted. Our study confirms these works and reinforced the need for consensus reliable criteria.
Our study is however a cross-sectional analysis and does not allow conclusion about cause and effect relationships. Our population was composed only of elderly women; consequently, our conclusions cannot be extrapolated to men, and we must the possibility that our results may not be valid for younger groups in whom sarcopenia could help to predict functional limitation occurring in later years. Another important limitation of our study is that the difficulties in physical function were self-reported. Self-reported measures are known to be affected by environmental,42,43 cultural, and socio-economic differences.44,45 However, reliability of self-reported physical function in older adults is about 85%,46 and it has been shown that self-reported measures may complement performance measures in providing useful information about functional status and health outcome.47,48 Moreover, our results on self-reported difficulties in physical function were concordant with our results on KES, which are an objective measure of functional limitation. Lastly, some potential confounding variables were not addressed in this study such as low motivation and environmental factor.
The originality of this work was being supported by the use of different definitions of sarcopenia into one cohort.
Deciding the most appropriated criteria and cut-off to define sarcopenia has been a complicated work during the past 20 years because sarcopenia escapes from the traditional definitions of diseases. What constitutes an indication for a treatment remains unclear. At this stage, and for this newly described condition, our point of view is that a first consensual start, inevitably open to criticism, is needed. In future studies, thresholds will have to be determined on the basis of the probability that an intervention can avoid adverse outcomes such as fracture, difficulty for major mobility tasks, or disability that risks occurring in the coming years (i.e. 10 years). In this highly prevalent condition, cost-effectiveness considerations will be warranted. The same process can be observed in the field of osteoporosis. The National Osteoporosis Foundation currently warrants a cost-effective treatment intervention threshold49 after years of definition based on arbitrary bone mineral density cut-point. However, important differences exist between sarcopenia and osteoporosis. In osteoporosis, a clear and unique clinical outcome is defined as the optimal combination of sensitivity and specificity to determine the risk of fractures.50,51
In conclusion, the different definitions explored were, to some degree, associated with the self-reported difficulty in physical function and with the KES. Based on our results, the never-ending discussion on the criteria to define sarcopenia remains open. Prevalence of sarcopenia varies widely depending on the definition adopted. The clinical and pathological characteristics of patients have sufficient ability to predict self-reported physical difficulties.
Acknowledgments
The EPIDOS study participants included co-ordinators (G. Breart, P. Dargent-Molina, P. J. Meunier, A. M. Schott, D. Hans, and P. D. Delmas) and principal investigators [C. Baudoin and J. L. Sebert (Amiens), M. C. Chapuy and A. S. M. Schott (Lyon), F. Favier and C. Marcelli (Montpellier), C. J. Menkes, C. Cormier, and E. Hausherr (Paris), and H. Grandjean and C. Ribot (Toulouse)]. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia, and Muscle 2010, 1:7–8 (von Haehling, S.; Morley, J. E.; Coats, A. J.; and Anker, S. D.).
Funding
Charlotte Dupuy was supported by a CIFRE PhD studentship (No. 2010/1072) which was jointly funded by Nutricia Nutrition Clinique and the French National Association of Technical Research (ANRT).
Conflict of interest
None declared.
References
- Sowers MR, Crutchfield M, Richards K, Wilkin MK, Furniss A, Jannausch M, Zhang D, Gross M. Sarcopenia is related to physical functioning and leg strength in middle-aged women. J Gerontol A Biol Sci Med Sci. 2005;60:486–490. doi: 10.1093/gerona/60.4.486. [DOI] [PubMed] [Google Scholar]
- Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB Health ABC Study Investigators. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–1609. doi: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- Delmonico MJ, Harris TB, Lee JS, Visser M, Nevitt M, Kritchevsky SB, Tylavsky FA, Newman AB Health, Aging and Body Composition Study. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc. 2007;55:769–774. doi: 10.1111/j.1532-5415.2007.01140.x. [DOI] [PubMed] [Google Scholar]
- Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G, Boirie Y, Bosaeus I, Cederholm T, Costelli P, Fearon KC, Laviano A, Maggio M, Rossi Fanelli F, Schneider SM, Schols A, Sieber CC. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) ‘cachexia-anorexia in chronic wasting diseases’ and ‘nutrition in geriatrics’. Clin Nutr. 2010;29:154–159. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dargent-Molina P, Favier F, Grandjean H, Baudoin C, Schott AM, Hausherr E, Meunier PJ, Bréart G EPIDOS Group. Fall-related factors and risk of hip fracture: the EPIDOS prospective study. Lancet. 1996;348:145–149. doi: 10.1016/s0140-6736(96)01440-7. [DOI] [PubMed] [Google Scholar]
- Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- Heymsfield SB, Smith R, Aulet M, Bensen B, Lichtman S, Wang J, Pierson RN., Jr Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nut. 1990;52:214–218. doi: 10.1093/ajcn/52.2.214. [DOI] [PubMed] [Google Scholar]
- Gillette-Guyonnet S, Nourhashemi F, Andrieu S, Cantet C, Albarède JL, Vellas B, Grandjean H. Body composition in French women 75+ years of age: the EPIDOS study. Mech Ageing Dev. 2003;124:311–316. doi: 10.1016/s0047-6374(02)00198-7. [DOI] [PubMed] [Google Scholar]
- Annweiler C, Schott AM, Montero-Odasso M, Berrut G, Fantino B, Herrmann FR, Beauchet O. Cross-sectional association between serum vitamin D concentration and walking speed measured at usual and fast pace among older women: the EPIDOS study. J Bone Miner Res. 2010;25:1858–1866. doi: 10.1002/jbmr.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, Cesari M, Donini LM, Gillette Guyonnet S, Inzitari M, Nourhashemi F, Onder G, Ritz P, Salva A, Visser M, Vellas B. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13:881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, Cederholm T, Stewart Coats AJ, Cummings SR, Evans WJ, Fearon K, Ferrucci L, Fielding RA, Guralnik JM, Harris TB, Inui A, Kalantar-Zadeh K, Kirwan B-A, Mantovani G, Muscaritoli M, Newman AB. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12:403–409. doi: 10.1016/j.jamda.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland Y, Lauwers-Cances V, Cournot M, Nourhashémi F, Reynish W, Rivière D, Vellas B, Grandjean H. Sarcopenia, calf circumference, and physical function of elderly women: a cross-sectional study. J Am Geriatr Soc. 2003;51:1120–1124. doi: 10.1046/j.1532-5415.2003.51362.x. [DOI] [PubMed] [Google Scholar]
- Rolland Y, Lauwers-Cances V, Cristini C, Grandjean H, Banks WA, Morley JE, Vellas B. Disability in obese elderly women: lower limb strength and recreational physical activity. Obes Res Clin Pract. 2007;1:1–78. doi: 10.1016/j.orcp.2006.10.001. , doi: 10.1016/j.orcp.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Edwards RH, Young A, Hosking GP, Jones DA. Human skeletal muscle function: description of tests and normal values. Clin Sci Mol Med. 1977;52:283–290. doi: 10.1042/cs0520283. [DOI] [PubMed] [Google Scholar]
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- Rolland Y, Lauwers-Cances V, Cesari M, Vellas B, Pahor M, Grandjean H. Physical performance measures as predictors of mortality in a cohort of community-dwelling older French women. Eur J Epidemiol. 2006;21:113–122. doi: 10.1007/s10654-005-5458-x. [DOI] [PubMed] [Google Scholar]
- Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed. John Wiley & Sons, Inc: New York NY; 2000. [Google Scholar]
- Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- Melton LJ, 3rd, Khosla S, Crowson CS, O'Connor MK, O'Fallon WM, Riggs BL. Epidemiology of sarcopenia. J Am Geriatr Soc. 2000;48:625–630. [PubMed] [Google Scholar]
- Barbat-Artigas S, Dupontgand S, Fex A, Karelis AD, Aubertin-Leheudre M. Relationship between dynapenia and cardiorespiratory functions in healthy postmenopausal women: novel clinical criteria. Menopause. 2011;18:400–405. doi: 10.1097/gme.0b013e3181f7a596. [DOI] [PubMed] [Google Scholar]
- Visser M, Newman AB, Nevitt MC, Kritchevsky SB, Stamm EB, Goodpaster BH, Harris TB. Reexamining the sarcopenia hypothesis. Muscle mass versus muscle strength. Health, Aging, and Body Composition Study Research Group. Ann N Y Acad Sci. 2000;904:456–461. [PubMed] [Google Scholar]
- Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- Barbat-Artigas S, Rolland Y, Zamboni M, Aubertin-Leheudre M. How to assess functional status: a new muscle quality index. J Nutr Health Aging. 2012;16:67–77. doi: 10.1007/s12603-012-0004-5. [DOI] [PubMed] [Google Scholar]
- Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- Schaap LA, Koster A, Visser M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol Rev. 2012 doi: 10.1093/epirev/mxs006. Dec 4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Cesari M, Kritchevsky SB, Newman AB, Simonsick EM, Harris TB, Penninx BW, Brach JS, Tylavsky FA, Satterfield S, Bauer DC, Rubin SM, Visser M, Pahor M Health ABC study. Added value of physical performance measures in predicting adverse health-related events: results from the health, aging and body composition study. J Am Geriatr Soc. 2009;57:251–259. doi: 10.1111/j.1532-5415.2008.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland Y, Czerwinski S, Abellan Van Kan G, Morley JE, Cesari M, Onder G, Onder G, Woo J, Baumgartner R, Pillard F, Boirie Y, Chumlea WMC, Vellas B. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging. 2008;12:433–450. doi: 10.1007/BF02982704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, Fiatarone Singh MA. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci. 2001;56:B209–217. doi: 10.1093/gerona/56.5.b209. [DOI] [PubMed] [Google Scholar]
- Hairi NN, Cumming RG, Naganathan V, Handelsman DJ, Le Couteur DG, Creasey H, Waite LM, Seibel MJ, Sambrook PN. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: the Concord Health and Ageing in Men Project. J Am Geriatr Soc. 2010;58:2055–2062. doi: 10.1111/j.1532-5415.2010.03145.x. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25:127–141. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- Yu R, Leung J, Woo J. Incremental predictive value of sarcopenia for incident fracture in an elderly Chinese cohort: results from the osteoporotic fractures in men (MrOs) study. J Am Med Dir Assoc. 2014;15:551–558. doi: 10.1016/j.jamda.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Bijlsma AY, Meskers CG, Ling CH, Narici M, Kurrle SE, Cameron ID, Westendorp RG, Maier AB. Defining sarcopenia: the impact of different diagnostic criteria on the prevalence of sarcopenia in a large middle aged cohort. Age (Dordr) 2013;35:871–881. doi: 10.1007/s11357-012-9384-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D, Hayes A, Sanders KM, Aitken D, Ebeling PR, Jones G. Operational definitions of sarcopenia and their associations with 5-year changes in falls risk in community-dwelling middle-aged and older adults. Osteoporos Int. 2014;25:187–193. doi: 10.1007/s00198-013-2431-5. [DOI] [PubMed] [Google Scholar]
- Batsis JA, Barre LK, Mackenzie TA, Pratt SI, Lopez-Jimenez F, Bartels SJ. Variation in the prevalence of sarcopenia and sarcopenic obesity in older adults associated with different research definitions: dual-energy x-ray absorptiometry data from the National Health and Nutrition Examination Survey 1999–2004. J Am Geriatr Soc. 2013;61:974–980. doi: 10.1111/jgs.12260. [DOI] [PubMed] [Google Scholar]
- Dam TT, Peters KW, Fragala M, Cawthon PM, Harris TB, McLean R, Shardell M, Alley DE, Kenny A, Ferrucci L, Guralnik J, Kiel DP, Kritchevsky S, Vassileva MT, Studenski S. An evidence-based comparison of operational criteria for the presence of sarcopenia. J Gerontol A Biol Sci Med Sci. 2014;69:584–590. doi: 10.1093/gerona/glu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke P, Ailshire JA, Bader M, Morenoff JD, House JS. Mobility disability and the urban built environment. Am J Epidemiol. 2008;168:506–513. doi: 10.1093/aje/kwn185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman VA, Grafova IB, Schoeni RF, Rogowski J. Neighborhoods and disability in later life. Soc Sci Med. 2008;66:2253–2267. doi: 10.1016/j.socscimed.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpierre C, Lauwers-Cances V, Datta GD, Lang T, Berkman L. Using self-rated health in epidemiological studies: a risk for underestimating the gap between social classes? J Epidemiol Community Health. 2009;63:426–432. doi: 10.1136/jech.2008.080085. [DOI] [PubMed] [Google Scholar]
- Banks J, Marmot M, Oldfield Z, Smith J. Disease and disadvantage in the United States and in England. JAMA. 2006;295:2037–2045. doi: 10.1001/jama.295.17.2037. [DOI] [PubMed] [Google Scholar]
- Smith LA. Branch LG. Scherr PA. Wetle T, Evans DA, Hebert L, Taylor JO. Short-term variability of measures of physical function in older people. J Am Geriatr Soc. 1990;38:993–998. doi: 10.1111/j.1532-5415.1990.tb04422.x. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J of Gerontol. 1994;48:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- Reuben DB, Siu AL, Kimpau S. The predictive validity of self-report and performance-based measures of function and health. J Gerontol. 1992;47:M106–M110. doi: 10.1093/geronj/47.4.m106. [DOI] [PubMed] [Google Scholar]
- Tosteson AN, Melton LJ, 3rd, Dawson-Hughes B, Baim S, Favus MJ, Khosla S, Lindsay RL. National Osteoporosis Foundation Guide Committee. Cost-effective osteoporosis treatment thresholds: the United States perspective. Osteoporos Int. 2008;19:437–447. doi: 10.1007/s00198-007-0550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijlsma AY, Meskers CG, Westendorp RG, Maier AB. Chronology of age-related disease definitions: osteoporosis and sarcopenia. Ageing Res Rev. 2012;11:320–324. doi: 10.1016/j.arr.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Altman DG, Lausen B, Sauerbrei W, Schumache M. Dangers of using ‘optimal’ cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst. 1994;86:829–835. doi: 10.1093/jnci/86.11.829. [DOI] [PubMed] [Google Scholar]


