Abstract
Background
Cachexia augments cancer-related mortality and has devastating effects on quality of life. Pre-clinical studies indicate that systemic inflammation-induced loss of muscle oxidative phenotype (OXPHEN) stimulates cancer-induced muscle wasting. The aim of the current proof of concept study is to validate the presence of muscle OXPHEN loss in newly diagnosed patients with lung cancer, especially in those with cachexia.
Methods
Quadriceps muscle biopsies of comprehensively phenotyped pre-cachectic (n = 10) and cachectic (n = 16) patients with non-small cell lung cancer prior to treatment were compared with healthy age-matched controls (n = 22). OXPHEN was determined by assessing muscle fibre type distribution (immunohistochemistry), enzyme activity (spectrophotometry), and protein expression levels of mitochondrial complexes (western blot) as well as transcript levels of (regulatory) oxidative genes (quantitative real-time PCR). Additionally, muscle fibre cross-sectional area (immunohistochemistry) and systemic inflammation (multiplex analysis) were assessed.
Results
Muscle fibre cross-sectional area was smaller, and plasma levels of interleukin 6 were significantly higher in cachectic patients compared with non-cachectic patients and healthy controls. No differences in muscle fibre type distribution or oxidative and glycolytic enzyme activities were observed between the groups. Mitochondrial protein expression and gene expression levels of their regulators were also not different.
Conclusion
Muscle OXPHEN is preserved in newly diagnosed non-small cell lung cancer and therefore not a primary trigger of cachexia in these patients.
Keywords: Cancer cachexia, Skeletal muscle, Oxidative phenotype, Systemic inflammation, Non-small cell lung cancer
Introduction
Cachexia is a devastating syndrome that affects a substantial proportion of patients with non-small cell lung cancer (NSCLC)1 and that leads to substantial weight loss primarily from loss of skeletal muscle and body fat. It has been well established that weight loss and muscle weakness adversely affect tumour therapy responsiveness, quality of life, and survival.2,3 For tailored implementation of currently available and future intervention strategies to combat cachexia throughout the cancer trajectory, a cachexia staging system was introduced, distinguishing pre-cachexia from cachexia. It is yet unclear if these stages are indeed successive in individual patients or merely reflect different disease manifestations among patients, but the classification of newly diagnosed patients in pre-cachexia and cachexia is clearly relevant in terms of clinical implications.2 Skeletal muscle weakness may not only be due to wasting of muscle mass but also due to loss of muscle oxidative phenotype (OXPHEN) that is characterized by a decreased proportion of oxidative slow-twitch type I fibres as well as loss of mitochondrial function and capacity. Furthermore, mitochondrial pathways recently have emerged as central players in muscle mass maintenance.4–6 For example, in patients with Chronic Obstructive Pulmonary Disease (COPD) loss of muscle OXPHEN and mitochondrial dysfunction was more pronounced in cachectic patients as compared with non-cachectic COPD patients.7–9 No data are available yet in NSCLC except for indirect evidence demonstrating an impaired cycle exercise capacity in newly diagnosed patients even in those with pre-cachexia.10 This could be contributed to a loss of OXPHEN as this is a well-established determinant of exercise capacity.11,12 In numerous experimental models of cancer cachexia, mitochondrial impairments have clearly been related to muscle atrophy and activation of muscle proteolytic pathways.13–20 Moreover, mice suffering from cancer cachexia also demonstrated a smaller proportion of oxidative type I muscle fibres and a larger proportion of glycolytic type II fibres in soleus muscle.21,22 Because type II fibres are more susceptible to catabolic stimuli,21,23 this fibre type shift may further enhance or maybe even initiate muscle wasting in cancer cachexia. Therefore, our first hypothesis was that NSCLC is characterized by a loss of skeletal muscle OXPHEN, especially in cachectic patients.
Increased systemic pro-inflammatory signalling has been causally related to the loss of OXPHEN observed in skeletal muscle in an experimental model of intestinal cancer cachexia.15,24 Further experimental in vitro research indeed confirms that the pro-inflammatory mediators interleukin 6 (IL-6) and tumour necrosis factor alpha (TNF-α) can induce alterations in oxidative metabolism by decreasing muscular activation of the master regulator of oxidative metabolism, peroxisome proliferator-activated receptor gamma co-activator 1-alpha (PGC-1α).8 Elevated levels of these pro-inflammatory cytokines are well established in patients with NSCLC cachexia2,10 and considered responsible for the activation of muscular proteolytic pathways,25–27 which further strengthen the notion that energy metabolism and protein turnover are intertwined in cancer cachexia.28 Hence, our second hypothesis was that the anticipated gradual loss of muscle OXPHEN is associated with increasing systemic inflammation in NSCLC.
The aim of this study is therefore to determine if muscle OXPHEN loss indeed occurs in patients with NSCLC and is associated with cachexia and systemic inflammation.
Materials and methods
Study population
Twenty-six patients with newly diagnosed stage III or IV NSCLC and 22 age-matched healthy control subjects were enrolled in this cross-sectional study. Patients were recruited at the Department of Respiratory Medicine, Maastricht University Medical Centre +, between July 2007 and July 2010. NSCLC was confirmed by pathology and was classified according to the 6th tumour–node–metastasis (TNM) classification for lung cancer.29 Only patients with TNM stages III and IV were included to minimize confounding on the studied markers by malignant disease characteristics. Patients suffering from COPD GOLD III–IV, cardiac failure NYHA IV, severe endocrine, hepatic or renal disorders, other malignancies in the last 3 years, and chronic inflammatory diseases, or acute infection were excluded because of potential interference with muscle energy metabolism in these conditions. For this reason, also patients who underwent recent surgery (<3 months) or received treatment with corticosteroids or hormonal therapy were excluded. The weight loss prior to study entry was assessed by asking the patient for their usual weight 6 months prior to the diagnosis of lung cancer. Patients were subsequently assigned to the pre-cachexia or cachexia subgroup according to the international cachexia consensus.2
Healthy controls were recruited via local newspaper advertisements and were matched to the NSCLC patients with respect to age and sex. Written informed consent was obtained from all subjects, and the ethical review board of the Maastricht University Medical Centre + approved the study (reference number 08-2-059). Because public trial registration was not implemented in clinical practice (WHO guideline indicates January 2009) at the start of enrollment (July 2007), the study was not registered in a public trial registry.
Assessment of the parameters described in the succeeding paragraphs was performed in the morning after 8 h fasting.
Body composition
Dual energy X-ray absorptiometry (DEXA; DPX-L, Lunar Radiation Corp., Madison, WI, USA) was used to determine whole body fat-free mass as well as the composition of arms and legs together (appendicular) and legs only. Body mass index and fat-free mass indices were calculated by dividing the respective weights through the squared body height. DEXA measurements were performed in the fasted state. Sarcopenia was defined as an appendicular lean mass index ≤2 SD below the mean of a young reference group, being 7.26 kg/m2 for men and 5.45 kg/m2 for women.30
Spirometry
Forced expiratory volume in one second and forced vital capacity (FVC) were assessed by spirometry to assess airflow obstruction as a potential result of the smoking history of patients. As metabolic derangements in skeletal muscle occur in COPD and could therefore influence outcome parameters, the relations between lung function and muscular metabolic characteristics were assessed.
Physical activity and quadriceps muscle function
The Medical Studies Study Short Form-20 (SF-20) questionnaire was used to assess physical activity, and the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-C30 (QLQ-C30) questionnaire was used to assess the quality of life, which includes physical performance. Isometric and isokinetic strength of quadriceps and hamstrings muscles was measured by Biodex dynamometer (Biodex system version 3.3, Biodex Medical Systems, Inc. Shirley, NY, USA). During this test, subjects were seated on the dynamometer chair with belts attached at the level of the thigh and ankle for stability. Isometric muscle strength was assessed by three maximal voluntary contractions (MVCs) at an angle of 60°. Isokinetic muscle strength was assessed at angular velocities of 60°/s (set of five MVCs) and 180°/s (set of 10 MVCs). Muscle strength was defined as the highest muscular force output (peak torque) in Newton metres (Nm).
Plasma inflammatory markers
Plasma inflammatory mediators were assessed using a Human Multiplex Antibody assay (Luminex® System, Life Technologies Ltd, Paisley, UK) to determine plasma TNF-α, soluble TNF-receptor 1 and interleukin 6 (IL-6) (lower detection limit 5–28 pg/mL). All samples were analyzed at Invitrogen Luminex Testing Services (Paisley, UK).
Muscle biopsies collection and processing
Muscle biopsies of vastus lateralis muscle (part of quadriceps muscle) were obtained by needle biopsies using a technique described by Bergström.31 For (immuno)histochemical analysis, a part of each muscle biopsy was embedded in Tissue-Tek® OCT™ (Sakura Finetek Europe B.V. Alphen aan den Rijn, The Netherlands) and frozen in melting isopentane (which was pre-cooled in liquid nitrogen). Serial cross-sections (5 µm) were cut on a cryostat microtome at −20°C and mounted on SuperFrost microscope slides (Menzel-Gläser, Gerhard Menzel GmbH Braunschweig, Germany), which were kept at −80°C until analysis. The remaining muscle tissue was snap-frozen and crushed to powder in liquid nitrogen and stored at −80°C until further biochemical analyses, including western blotting, enzyme activity measurements, and gene expression levels.
Muscle fibre type distribution and size
Immunohistochemical staining of laminin was used to determine muscle fibre cross-sectional area. Subsequently, a combination of immunohistochemical staining and myosin adenosine 5′-triphosphatase (mATPase) staining was used (as described before32) to identify fibres expressing different myosin heavy chain (MyHC) subtypes, that is, oxidative type I, hybrid I/II, and glycolytic II fibres. Slides were incubated with primary: anti-laminin (dilution 1:50; #L-9393, Sigma, St. Louis, MO, USA); anti-type I MyHC (dilution 1:40; #A4840, Developmental Studies Hybridoma Bank (DSHB), Iowa City, IA, USA); and anti-type II MyHC (dilution 1:40; #N2261, Santa Cruz, CA, USA), and secondary antibodies: Alexa Fluor 350 (dilution 1:100; #A-11069); Alexa Fluor 488 (dilution 1:1000; #A-21121) and Alexa Fluor 555 (dilution 1:1000; #A-21426, Invitrogen, Madison, Wisconsin, USA). Images for analysis were obtained with fluorescent microscopy. Computer image analysis was performed using Lucia Software version 4.81 (Laboratory Imaging, s.r.o. Prague Czech Republic). For mATPase staining, sections were processed using acidic pre-incubation (NaAc 7.8 g/L, KCl 7.56 g/L) at pH 4.40, followed by acidic incubation (glycine 3.75 g/L, CaCl2•2H2O 4.26 g/L, NaCl 38 g/L, ATP 1.7 g/L) at pH 9.4. Sections were dehydrated in ethanol [50–70–96–100%-ultraclear (Fisher Scientific Emergo)]. Images for analysis were obtained by light microscopy. Per biopsy, 200 fibres were analyzed on average (minimum of 100 fibres). Damaged and detached fibres were excluded from analysis.
Muscle protein expression analysis
Approximately 50 mg of powdered muscle tissue was dissolved in lysis buffer (400 µl) consisting of Tris pH 7.4 (50 mM), NaCl (150 mM), glycerol (10%), NP-40 (0.5%), EDTA (1 mM), Na3VO4 (1 mM), NaF (5 mM), β-glycerophosphate (10 mM), Na-pyro-PO4 (1 mM), DTT (1 mM), leupeptin (10 µg/ml), aprotenin (1%), and PMSF (1 mM) and homogenized using a Polytron PT (Polytron PT 1600 E, Kinematica AG). Following an incubation step of 30 min, muscle samples were sonicated and centrifuged at 4°C (16 000 rcf) for 30 min. Protein concentration was assessed using BCA Protein Assay Kit (Pierce, Thermo Fisher). Next, sample buffer (dilution 1:4; 4× Stacking buffer: 0.250 M Tris–HCl, 8% SDS, 40% glycerol, 0.4 M DTT, 0.02% bromphenol blue) was added, and samples were incubated for 5 min at 95°C. Electrophoresis was performed on an Electrophoresis Cell system (Bio-Rad), where equal amounts of protein were loaded per lane of a 26 Wells Criterion XT 4–12% Bis-Tris precast gel (Bio-Rad). Two standard samples were included in every blot in order to correct for blot-to-blot variation. The gels were transferred to nitrocellulose membranes (Whatman, GE Healthcare), followed by incubation for 60 min in 2% BSA or 5% milk in TBS Tween 20 (v/v 0.05%) before incubation with primary antibodies. Primary antibodies for Oxphos ATP synthase and I–IV complexes were used (1:1000; #MS604, Mito Sciences) and glyceraldehyde 3-phosphate dehydrogenase (1:1000; #2118, Cell Signalling) was used as loading control. The membranes were incubated with primary antibodies overnight at 4°C. Then, a successive incubation step with secondary antibodies (dilution 1:5000) of anti-mouse IgG peroxidase (#A85PI-1000.S1, Bio-Connect) and anti-rabbit IgG peroxidase (#A85PI-2000.S1, Bio-Connect) was performed. Detection of protein signals was performed using SuperSignal West Pico Chemiluminescent substrate (Thermo Scientific). Densitometry was used to quantify signals using Quantitiy One (version 4.6.2, Bio-Rad).
Muscle enzyme activity analysis
Crushed muscle tissue was dissolved in 5% (w/v) SET buffer [containing sucrose (250 mM), EDTA (2.5 mM), and Tris (10 mM)] and subsequently homogenized using a Polytron PT (Polytron PT 1600 E, Kinematica AG). Enzyme activities of citrate synthase (CS), β-hydroxyacyl-CoA dehydrogenase (HAD), and phosphofructokinase (PFK) were analyzed spectrophotometrically (Multiskan Spectrum; Thermo Labsystems, Breda, the Netherlands), based on the conversion rate of their respective substrates as described previously.32
Muscle gene expression analysis
To extract RNA, ToTALLY RNA™ Kit (Ambion Ltd.) was used according to the guidelines of the manufacturer. In short, powdered tissue (10–30 mg) was homogenized by a Polytron PT 1600 E (Kinematica AG), and total RNA was extracted. Then, contaminating genomic DNA was removed using RNeasy Mini Kit with RNase-free DNase (Qiagen). Concentration of total RNA in the respective samples was assessed using spectrophotometry (NanoDrop ND-1000, Isogen Lifescience). Total RNA (400 ng) was reverse transcribed to cDNA with anchored oligo(dT) primers following manufacturer's guidelines (Transcriptor First Strand cDNA Synthesis kit, Roche Diagnostics). Primers (Sigma Genosys) were designed for the following: PGC-1α, mitochondrial transcription factor A, CS, HAD, hexokinase II, PFK, mitochondrial-encoded cytochrome c oxidase III, cytochrome c oxidase subunit IV isoform 1, MyHC I, MyHC IIA, and MyHC IIx (for primer sequences, see the online supplemental document, Table 1). Primers were used in quantitative real-time PCR analysis (reaction contained 1× SensiMix SYBR & Fluorescein Kit (Bioline) with 300 nM primers), and Hard-Shell 96-well Semi-skirted PCR plates (Bio-Rad) were used on a MyiQ thermocycler (Bio-Rad). The assay programme consisted of an initial 15 min incubation step at 95°C, followed by 40 cycles of 95°C for 15 s and 60°C for 45 s of thermal cycling. To correct for variance within each reaction, gene expression was corrected for a sample specific geNorm factor calculated from CYCLOPHILIN, BETA-ACTIN (β-ACTIN) and Large Ribosomal Protein (RPLPO) reference gene expression. Standard curves from pooled cDNA and melt curves were analyzed to verify efficiency and specificity of amplification.
Table 1.
Subject characteristics
| Healthy controls | NSCLC | NSCLC pre-cachexia | NSCLC cachexia | |
|---|---|---|---|---|
| N (m/f) | 22 (13/9) | 26 (17/9) | 10 (8/2) | 16 (9/7) |
| Age (years) | 61.4 ± 7.0 | 60.8 ± 9.0 | 62.4 ± 10.4 | 59.8 ± 8.2 |
| Disease stagea: IIIB (%)/IV (%) | — | 38/62 | 60/40 | 25/75 |
| Histology: adenocarcinoma (%)/squamous cell (%) | — | 61/39 | 70/30 | 56/44 |
| Smoking: (current %/former %/never %) | 5/54/22 | 38/58/4* | 20/80/0* | 50/44/6* |
| FEV1f (% predicted) | 114.7 ± 19.3 | 66.7 ± 18.6* | 77.0 ± 18.4* | 61.9 ± 17.2* |
| FVCg (% predicted) | 125.4 ± 13.8 | 83.2 ± 22.1* | 100.0 ± 9.9* | 75.5 ± 22.0*† |
| Tiffeneau index | 0.74 ± 0.08 | 0.64 ± 0.13* | 0.60 ± 0.12* | 0.65 ± 0.13* |
| Mean weight loss in 6 months prior to diagnosis (%)b | 0 ± 0 | 8.0 ± 6.7* | 1.7 ± 1.4 | 12.0 ± 5.5*† |
| Body mass index (BMI) (kg/m2) | 24.1 ± 3.3 | 24.0 ± 4.5 | 25.7 ± 3.4 | 23.0 ± 4.8 |
| Fat-free mass index (FFMI) (kg/m2) | 18.4 ± 2.2 | 17.2 ± 2.5 | 18.5 ± 1.6 | 16.5 ± 2.7* |
| Appendicular fat-free mass index (kg/m2) | 8.1 ± 1.1 | 7.0 ± 1.1* | 7.7 ± 0.8 | 6.6 ± 1.0*† |
| Leg fat-free mass index (kg/m2) | 6.1 ± 0.8 | 5.2 ± 0.8* | 5.7 ± 0.6 | 4.9 ± 0.7*† |
| Sarcopenia (N) | 0 | 12* | 2 | 10*† |
| IL-6 (pg/ml)d | 56.7 ± 33.2 | 120.4 ± 107.7* | 70.1 ± 50.8 | 151.8 ± 122.6*† |
| TNF-α (pg/ml)d | 105.7 ± 43.8 | 117.0 ± 93.1 | 131.6 ± 129.9 | 106.6 ± 58.1 |
| Soluble TNF receptor 1 (pg/ml)e | 2404 ± 728 | 3712 ± 1449* | 3482 ± 1747* | 3855 ± 1270* |
| Peak torque flexion 180°/s (Nm) | 64.3 ± 25.5 | 34.7 ± 15.4* | 37.6 ± 14.2* | 32.9 ± 16.3* |
| Peak torque extension 180°/s (Nm) | 75.8 ± 27.6 | 40.8 ± 17.9* | 51.2 ± 17.5* | 34.2 ± 15.0* |
| Peak torque flexion 60° (Nm) | 77.4 ± 19.8 | 60.2 ± 22.4* | 71.0 ± 25.3 | 53.4 ± 18.1*† |
| Peak torque extension 60° (Nm) | 137.1 ± 35.5 | 109.8 ± 39.8* | 133.0 ± 40.2 | 95.3 ± 32.8*† |
NSCLC, non-small cell lung cancer.
Stage of non-small cell lung cancer according to the 6th tumour–node–metastasis classification system.
Mean percentage of within patient weight loss in the 6 months prior to diagnosis.
Interleukin-6.
Tumour necrosis factor alpha.
Soluble tumour necrosis factor alpha receptor 1.
Quality of Life Questionnaire C30.
Medical Studies Study Short Form-20 (physical performance questionnaire).
Forced expiratory volume in one second.
Forced vital capacity.
Statistically significant difference compared with healthy controls (P < 0.05).
Statistically significant difference compared to pre-cachexia (P < 0.05).
Data represent mean ± SD.
Statistics
For the sample size calculation, please see the online supplemental document. Data were analyzed using Statistical Package for the Social Sciences (SPSS version 15 for Windows, SPSS Inc.). Except for baseline body weight loss, which represents weight loss within individual patients in the 6 months prior to diagnosis, all data represent comparisons between healthy controls, pre-cachectic, and cachectic patient groups. Continuous variables were compared using one-way ANOVA with least significant difference (LSD) post hoc analysis. Pearson chi-squared test was used for comparison of categorical variables. Correlations were evaluated using Pearson correlations. Data in tables are represented as mean ± standard deviation. Error bars in figures represent the standard error of mean. Significance was set at P < 0.05.
Results
Subject characteristics
The basic characteristics of healthy controls, NSCLC patients, and pre-cachectic and cachectic NSCLC patients are shown in Table 1. All except one patient had a history of cigarette smoking, and as a result, lung function was worse in patients with lung cancer. Pre-cachectic and cachectic patients with NSCLC showed no significant differences in tumour stage or histological subtype. Pre-cachectic patients showed mild within patient body weight loss (1.7%) in 6 months prior to diagnosis, whereas patients with cachexia showed significant weight loss in this period (12%). Body mass index was comparable between all groups. Appendicular and leg fat-free mass indices were lower in NSCLC as compared with healthy subjects, predominantly in the cachectic patients [including whole body fat-free mass index (FFMI)]. Muscle strength was also lower in NSCLC patients, again being more pronounced in the cachectic patients. Reported physical functioning indices from the questionnaires were also significantly lower in NSCLC, especially in the cachectic patients (∽70% and ∽62% of that of controls, respectively; data not shown).
Increased interleukin 6 and soluble tumour necrosis factor receptor 1 levels in patients with cancer cachexia
Expression of the pro-inflammatory cytokine considered the most potent in inducing loss of OXPHEN in experimental cancer cachexia. IL-6 was elevated in NSCLC, predominantly in the cachectic patients, compared with healthy controls (Table 1). TNF-α, another putative mediator of OXPHEN regulation, was not differentially expressed between groups. However, circulating levels of its receptor, soluble TNF receptor 1, were significantly elevated in the plasma of NSCLC patients when compared with healthy controls (Table 1).
Preserved muscle OXPHEN in patients with lung cancer pre-cachexia and cachexia
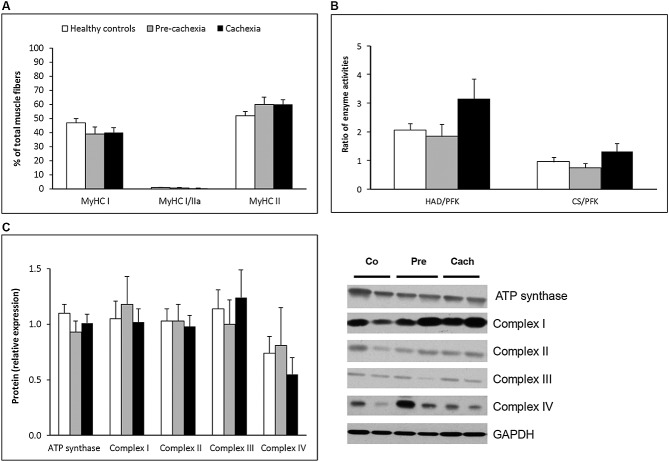
As can be observed in Figure 1, patients with lung cancer showed no differences from healthy controls concerning the proportion of type I, hybrid I/II or II muscle fibres, although trends towards a somewhat lower type I and higher type II fibre proportion (P = 0.06) could be appreciated. Also, no indications for a fibre type shift during early development of cancer cachexia were found. That is, no differences were observed between the cachectic and the pre-cachectic group or pre-cachectic patients (Figure 1A). Similarly, ratios of oxidative (HAD and CS) to glycolytic (PFK) enzyme activity and protein expression levels of ATP synthase and I–IV Oxphos protein complexes were comparable in all groups (Figure 1B and C). To exclude gender effects, these analyses were also performed in men only, which indeed did not affect the outcome. Moreover, partial correlations, controlling for gender, between muscle OXPHEN parameters and the indices of muscle mass revealed no positive associations within the cancer patients.
Figure 1.
Normal OXPHEN in patients with lung cancer pre-cachexia and cachexia. Quadriceps muscle biopsies were processed for analysis of muscle fibre subtypes, enzyme activity, and protein expression. (A) Distribution of oxidative type I and glycolytic type II muscle fibre types in quadriceps muscle. Assessment of fibres expressing different myosin heavy chain isoforms was performed using immunohistochemistry and myosin adenosine 5′-triphosphatase staining. (B) Muscle oxidative and glycolytic enzyme activity. Activity of oxidative (β-hydroxyacyl-CoA dehydrogenase and citrate synthase) and glycolytic (phosphofructokinase) enzymes was assessed. Ratios of oxidative to glycolytic were calculated for the different enzymes. (C) Protein expression of Oxphos proteins. Expression of ATP synthase and I–IV Oxphos protein complexes was assessed using western blot analysis. Glyceraldehyde 3-phosphate dehydrogenase was used as a loading control. Co, healthy controls; Pre, pre-cachectic patients, Cach, cachectic patients. * Significant difference between indicated groups, P < 0.05.
Preserved muscular gene expression of mediators regulating and representing oxidative metabolism in lung cancer cachexia
Muscle gene expression levels are shown in Table 2. PGC-1α and mitochondrial transcription factor A, key regulators of oxidative metabolism, were not differentially expressed in lung cancer patients, nor in the pre-cachexia or cachexia subgroups. Likewise, gene expression levels of their downstream oxidative markers HAD and CS were comparable, as well as of the glycolytic markers PFK and hexokinase II (Table 2). mRNA transcripts of mitochondrial-encoded cytochrome c oxidase III and cytochrome c oxidase subunit IV isoform 1 were however significantly lower in cancer patients than in healthy controls, but there was no difference between pre-cachexia and cachexia.
Table 2.
Gene expression profiles
| Healthy controls | Pre-cachexia | Cachexia | |
|---|---|---|---|
| PGC-1α (AU) | 0.20 ± 0.19 | 0.13 ± 0.05 | 0.13 ± 0.06 |
| TFAM (AU) | 0.16 ± 0.04 | 0.14 ± 0.04 | 0.16 ± 0.04 |
| CS (AU) | 0.21 ± 0.10 | 0.15 ± 0.06 | 0.17 ± 0.05 |
| HAD (AU) | 0.19 ± 0.04 | 0.19 ± 0.04 | 0.21 ± 0.04 |
| HKII (AU) | 0.18 ± 0.12 | 0.12 ± 0.13 | 0.21 ± 0.27 |
| PFK (AU) | 0.19 ± 0.10 | 0.17 ± 0.09 | 0.18 ± 0.05 |
| COX III (AU) | 0.25 ± 0.08 | 0.19 ± 0.06* | 0.15 ± 0.05* |
| COX IV (AU) | 0.22 ± 0.07 | 0.18 ± 0.06* | 0.17 ± 0.05* |
| MyHC I (AU) | 0.20 ± 0.09 | 0.18 ± 0.09 | 0.17 ± 0.09 |
| MyHC IIA (AU) | 0.22 ± 0.10 | 0.24 ± 0.11 | 0.16 ± 0.05 |
| MyHC IIx (AU) | 0.15 ± 0.07 | 0.32 ± 0.25* | 0.17 ± 0.11† |
AU, arbitrary units; PGC-1α, peroxisome proliferator-activated receptor gamma co-activator 1-alpha; TFAM, mitochondrial transcription factor A; CS, citrate synthase; HAD, β-hydroxyacyl-CoA dehydrogenase; HKII, hexokinase II; PFK, phosphofructokinase; COX III, mitochondrial-encoded cytochrome c oxidase III; COX IV, cytochrome c oxidase subunit IV isoform 1; MyHC, myosin heavy chain.
Statistically significant difference compared with healthy controls (P < 0.05).
Statistically significant difference compared with pre-cachexia (P < 0.05).
Data represent mean ± standard deviation.
Corresponding to the absence of histological alterations in the proportion of type I fibres, no differences in gene expression levels of oxidative MyHC I were observed between the groups (Table 2). Patients with pre-cachexia showed a relative increase in glycolytic MyHC IIx transcript levels when compared with cachectic patients and healthy controls subjects (Table 2), albeit variation was rather large.
Muscle fibre atrophy is independent of fibre type distribution in lung cancer cachexia
Compared with the healthy subjects, muscle fibre cross-sectional area was smaller in NSCLC regardless of the fibre type (P < 0.05), indicative of muscle fibre atrophy (Figure 2). In accordance, selective fibre type II muscle atrophy predominated in the cachectic cancer patients. No significant differences were observed in the sizes of hybrid type I/II fibres in any of the study groups.
Figure 2.
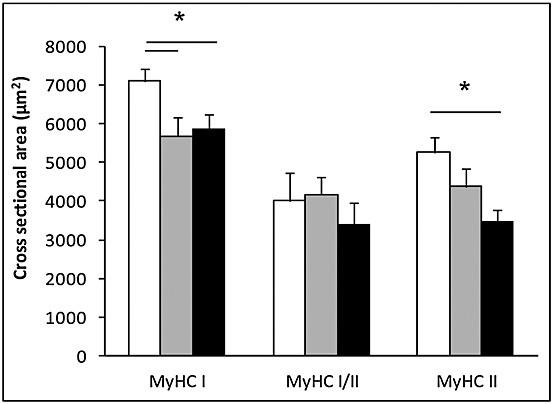
Muscle fibre atrophy is independent of fibre type in lung cancer cachexia. Cross-sectional of individual muscle fibres was assessed using immunohistochemical staining of laminin. Assessment of fibres expressing different myosin heavy chain isoforms was performed using immunohistochemistry and myosin adenosine 5′-triphosphatase staining.
There were no significant associations between FFMI as marker of muscle mass and the following metabolic markers: fibre type distribution, oxidative–glycolytic enzyme activity ratios or transcript levels of (regulatory) oxidative markers in any of the patient groups (data not shown). Conversely, FFMI was significantly and negatively associated with the expression of several of the Oxphos proteins in pre-cachectic patients (complex II and complex III, P < 0.05) and cachectic patients (ATP synthase and complex III, P < 0.05), whereas weight loss was positively associated with the expression of Oxphos proteins in cachectic patients (ATP synthase, complex II, and complex III, P < 0.05) (Figure 3).
Figure 3.
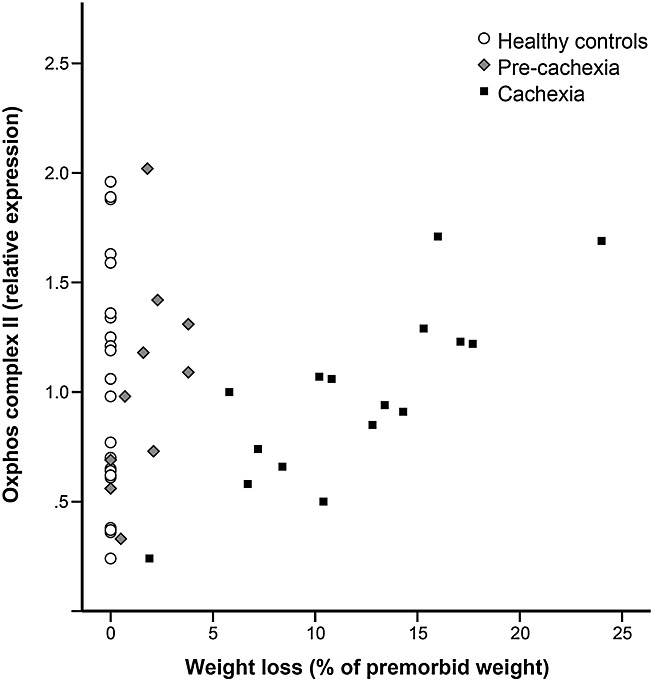
Correlation between weight loss and protein expression of Oxphos complex II in healthy controls, pre-cachectic, and cachectic patients. A significant correlation between weight loss and Oxphos complex II protein expression was found in cachectic patients (R = 0.826, P < 0.05) but not in healthy control subjects or pre-cachectic patients.
Discussion
In contrast to the findings in experimental cancer cachexia, this clinical study demonstrates that skeletal muscle OXPHEN is preserved in newly diagnosed patients with lung cancer, ncluding the subgroup of patients with cachexia, despite significantly elevated levels of circulating pro-inflammatory mediators (i.e. IL-6) as putative triggers. This is illustrated by the absence of alterations in fibre type distribution, mRNA transcript levels of regulators of oxidative signalling, protein expression of mitochondrial complexes, and oxidative enzyme activity.
To the best of our knowledge, this is the first study that addresses the question whether loss of muscle OXPHEN is involved in patients with cancer cachexia. Although we did see a lower gene expression of cytochrome c oxidase in the cancer patients, this was not associated with cachexia because there were no differences between the patient subgroups. More importantly, there were no differences at the protein level. This is the first study in its kind, and although the current design may not be ideal for subgroup analyses, our findings suggest that these clinical findings do not confirm the observations in most experimental models of cancer cachexia. This is in line with a previous clinical study in patients with gastrointestinal cancer; no alterations in microcirculation, capillary density, or muscular energy metabolites were found33, and although these measures do not reflect muscle OXPHEN per se, these data are in line with the current findings. Also in contrast to these experimental models, a hepatoma cancer cachexia model recently showed elevated transcript levels of the OXPHEN regulator PGC-1α and its downstream mediators.34 These findings are consistent with the positive and negative correlations between Oxphos complexes and respectively weight and FFMI in the current population of NSCLC patients. Together, this implicates that the loss of OXPHEN observed in the majority of experimental cancer cachexia models is not merely a result of the presence of malignant disease but is likely dependent on (a combination of) yet to be determined additional host metabolic alterations that could be related to a more aggressive and active nature of tumour development. However, it must be noted that none of the aforementioned experimental models were real lung cancer models (i.e. a tumour in the lungs), and loss of OXPHEN associated with cachexia in such a real model of lung cancer could help interpret the current clinical findings.
The unaffected OXPHEN in the current patient population with lung cancer cachexia are dissimilar to the observations in COPD, a chronic lung disease in which cachexia and loss of skeletal muscle OXPHEN are frequently observed.6,35,36 An important difference between cachexia in lung cancer and COPD is the time frame in which cachexia progresses. In COPD, cachexia generally develops gradually over a long time period, that is, months or years. The loss of OXPHEN might indeed play a role as accelerator of muscle wasting in this gradually developing type of cachexia.6 However, in lung cancer, cachexia develops relatively rapidly, that is, in weeks to months, because of the aggressive nature of the disease, and the current findings in newly diagnosed patients indicate that loss of OXPHEN does not precede or accompany this rapid developing cachexia, which suggests that the molecular mechanisms of muscle wasting are different in both conditions. An additional indication that different wasting mechanisms indeed occur in these respective diseases is the generalized muscle fibre atrophy of type I as well as type II fibres in the current population with lung cancer cachexia, whereas patients with COPD typically exhibit selective type II fibre atrophy.35 A potential reason for the differences in muscle OXPHEN in lung cancer cachexia and COPD could be that specific triggers, such as hypoxia or slow adaptation to sedentary lifestyle, which might not be as predominant in malignant disease,10 and contribute significantly to intrinsic metabolic alterations in skeletal muscle of COPD patients.35 Independent of the cause of the differences, the currently observed phenotype seems specific for lung cancer and not influenced by the presence of impaired lung function, as the proportion and diameter of glycolytic muscle fibres did not correlate with the lung function parameters in any of the current patient groups (data not shown).
Although muscle OXPHEN is not altered in lung cancer patients, the question remains whether stimulation of oxidative metabolism through interventions like exercise training or pharmaceutical stimulation could still have beneficial effects on muscle mass preservation and functional performance during cancer cachexia via the potential increase in contractile efficiency, reduced muscle proteolysis and increased muscle performance. Moreover, patients in the current study were included at diagnosis, prior to anti-tumour treatment; it cannot be excluded that muscle OXPHEN could be affected by therapy-induced inflammation.37,38 Studies performed in humans and rodents show that endurance exercise increases mRNA and protein expression of the master OXPHEN regulator PGC-1α39,40 and muscle-specific overexpression of PGC-1α resulted in improved muscle performance in mice.41,42However, pharmacological stimulation or genetic overexpression of PGC-1α could not rescue muscle mass consistently in all experimental models of cachexia,43,44 and extreme caution should be taken into account when testing strategies like systemic stimulation of PGC-1α expression or activity, as overexpression of PGC-1α has been associated with increased tumour growth in a model of cancer cachexia.44 Therefore, exercise programmes and/or (the less demanding) neuromuscular electrical stimulation might be a safer alternative at this point.45,46
In summary, findings demonstrate that despite evident pro-inflammatory signalling, muscle OXPHEN is preserved in (pre-)cachexia associated with NSCLC, which implies that OXPHEN loss is not a primary trigger of cancer cachexia-related muscle wasting prior to tumour treatment.
Acknowledgments
We thank all the study subjects and their families for participation. This study was supported by grants of the Dutch Cancer Institution (grant UM 2006-3703) and The Netherlands Organisation for Health Research and Development (grant 40-00703-98-10594). The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle (von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7–8.)
Conflict of interest
None declared.
Supporting Information
Appendix S1. Sample size calculation and interpretation.
Table S1. Primer sequences.
Supporting info item
Supporting info item
Supporting info item
References
- Baracos VE, Reiman T, Mourtzakis M, Gioulbasanis I, Antoun S. Body composition in patients with non-small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr. 2010;91:1133S–1137S. doi: 10.3945/ajcn.2010.28608C. [DOI] [PubMed] [Google Scholar]
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, Mackey JR, Koski S, Pituskin E, Sawyer MB. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009;15:2920–2926. doi: 10.1158/1078-0432.CCR-08-2242. [DOI] [PubMed] [Google Scholar]
- Calvani R, Joseph AM, Adhihetty PJ, Miccheli A, Bossola M, Leeuwenburgh C, Bernabei R, Marzetti E. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol Chem. 2013;394:393–414. doi: 10.1515/hsz-2012-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C, Li JiL. Role of PGC-1alpha signaling in skeletal muscle health and disease. Ann N Y Acad Sci. 2012;1271:110–117. doi: 10.1111/j.1749-6632.2012.06738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remels AH, Gosker HR, Langen RC, Schols AM. The mechanisms of cachexia underlying muscle dysfunction in COPD. J Appl Physiol. 2013;114:1253–1262. doi: 10.1152/japplphysiol.00790.2012. [DOI] [PubMed] [Google Scholar]
- Rabinovich RA, Bastos R, Ardite E, Llinas L, Orozco-Levi M, Gea J, Vilaró J, Barberà JA, Rodríguez-Roisin R, Fernández-Checa JC, Roca J. Mitochondrial dysfunction in COPD patients with low body mass index. Eur Respir J. 2007;29:643–650. doi: 10.1183/09031936.00086306. [DOI] [PubMed] [Google Scholar]
- Remels AH, Gosker HR, Schrauwen P, Hommelberg PP, Sliwinski P, Polkey M, Galdiz J, Wouters EF, Langen RC, Schols AM. TNF-alpha impairs regulation of muscle oxidative phenotype: implications for cachexia? FASEB J. 2010;24:5052–5062. doi: 10.1096/fj.09-150714. [DOI] [PubMed] [Google Scholar]
- Remels AH, Schrauwen P, Broekhuizen R, Willems J, Kersten S, Gosker HR, Schols AM. Peroxisome proliferator-activated receptor expression is reduced in skeletal muscle in COPD. Eur Respir J. 2007;30:245–252. doi: 10.1183/09031936.00144106. [DOI] [PubMed] [Google Scholar]
- Op den Kamp CM, Langen RC, Minnaard R, Kelders MC, Snepvangers FJ, Hesselink MK, Dingemans AC, Schols AM. Pre-cachexia in patients with stages I-III non-small cell lung cancer: systemic inflammation and functional impairment without activation of skeletal muscle ubiquitin proteasome system. Lung Cancer. 2012;76:112–117. doi: 10.1016/j.lungcan.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Hoppeler H. The different relationship of VO2max to muscle mitochondria in humans and quadrupedal animals. Respir Physiol. 1990;80:137–145. doi: 10.1016/0034-5687(90)90077-c. [DOI] [PubMed] [Google Scholar]
- Ivy JL, Costill DL, Maxwell BD. Skeletal muscle determinants of maximum aerobic power in man. Eur J Appl Physiol Occup Physiol. 1980;44:1–8. doi: 10.1007/BF00421757. [DOI] [PubMed] [Google Scholar]
- Fontes-Oliveira CC, Busquets S, Toledo M, Penna F, Paz Aylwin M, Sirisi S, Silva AP, Orpí M, García A, Sette A, Inês Genovese M, Olivan M, López-Soriano FJ, Argilés JM. Mitochondrial and sarcoplasmic reticulum abnormalities in cancer cachexia: altered energetic efficiency? Biochim Biophys Acta. 2013;1830:2770–2778. doi: 10.1016/j.bbagen.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Julienne CM, Dumas JF, Goupille C, Pinault M, Berri C, Collin A, Tesseraud S, Couet C, Servais S. Cancer cachexia is associated with a decrease in skeletal muscle mitochondrial oxidative capacities without alteration of ATP production efficiency. J Cachexia Sarcopenia Muscle. 2012;3:265–275. doi: 10.1007/s13539-012-0071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JP, Baltgalvis KA, Puppa MJ, Sato S, Baynes JW, Carson JA. Muscle oxidative capacity during IL-6-dependent cancer cachexia. Am J Physiol Regul Integr Comp Physiol. 2011;300:R201–R211. doi: 10.1152/ajpregu.00300.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padrao AI, Oliveira P, Vitorino R, Colaco B, Pires MJ, Marquez M, Castellanos E, Neuparth MJ, Teixeira C, Costa C, Moreira-Gonçalves D, Cabral S, Duarte JA, Santos LL, Amado F, Ferreira R. Bladder cancer-induced skeletal muscle wasting: disclosing the role of mitochondria plasticity. Int J Biochem Cell Biol. 2013;45:1399–1409. doi: 10.1016/j.biocel.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Fermoselle C, Garcia-Arumi E, Puig-Vilanova E, Andreu AL, Urtreger AJ, de Kier Joffe ED, Tejedor A, Puente-Maestu L, Barreiro E. Mitochondrial dysfunction and therapeutic approaches in respiratory and limb muscles of cancer cachectic mice. Exp Physiol. 2013;98:1349–1365. doi: 10.1113/expphysiol.2013.072496. [DOI] [PubMed] [Google Scholar]
- Tzika AA, Fontes-Oliveira CC, Shestov AA, Constantinou C, Psychogios N, Righi V, Mintzopoulos D, Busquets S, Lopez-Soriano FJ, Milot S, Lepine F, Mindrinos MN, Rahme LG, Argiles JM. Skeletal muscle mitochondrial uncoupling in a murine cancer cachexia model. Int J Oncol. 2013;43:886–894. doi: 10.3892/ijo.2013.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinou C, Fontes de Oliveira CC, Mintzopoulos D, Busquets S, He J, Kesarwani M, Mindrinos M, Rahme LG, Argíles JM, Tzika AA. Nuclear magnetic resonance in conjunction with functional genomics suggests mitochondrial dysfunction in a murine model of cancer cachexia. Int J Mol Med. 2011;27:15–24. doi: 10.3892/ijmm.2010.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamoui AV, Kim JS. Candidate mechanisms underlying effects of contractile activity on muscle morphology and energetics in cancer cachexia. Eur J Cancer Care (Engl) 2012;21:143–157. doi: 10.1111/j.1365-2354.2011.01287.x. [DOI] [PubMed] [Google Scholar]
- Acharyya S, Ladner KJ, Nelsen LL, Damrauer J, Reiser PJ, Swoap S, Guttridge DC. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest. 2004;114:370–378. doi: 10.1172/JCI20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffee GM, Kalfas K, Al-Majid S, McCarthy DO. Altered expression of skeletal muscle myosin isoforms in cancer cachexia. Am J Physiol Cell Physiol. 2002;283:C1376–C1382. doi: 10.1152/ajpcell.00154.2002. [DOI] [PubMed] [Google Scholar]
- Baracos VE, DeVivo C, Hoyle DH, Goldberg AL. Activation of the ATP-ubiquitin-proteasome pathway in skeletal muscle of cachectic rats bearing a hepatoma. Am J Physiol. 1995;268:E996–E1006. doi: 10.1152/ajpendo.1995.268.5.E996. [DOI] [PubMed] [Google Scholar]
- White JP, Puppa MJ, Sato S, Gao S, Price RL, Baynes JW, Kostek MC, Matesic LE, Carson JA. IL-6 regulation on skeletal muscle mitochondrial remodeling during cancer cachexia in the ApcMin/+ mouse. Skelet Muscle. 2012;2:14. doi: 10.1186/2044-5040-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argiles JM, Busquets S, Lopez-Soriano FJ. The pivotal role of cytokines in muscle wasting during cancer. Int J Biochem Cell Biol. 2005;37:2036–2046. doi: 10.1016/j.biocel.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Carson JA, Baltgalvis KA. Interleukin 6 as a key regulator of muscle mass during cachexia. Exerc Sport Sci Rev. 2010;38:168–176. doi: 10.1097/JES.0b013e3181f44f11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Kamp CM Op, Langen RC, Snepvangers FJ, de Theije CC, Schellekens JM, Laugs F, Dingemans AM, Schols AM. Nuclear transcription factor kappa B activation and protein turnover adaptations in skeletal muscle of patients with progressive stages of lung cancer cachexia. Am J Clin Nutr. 2013;98:738–748. doi: 10.3945/ajcn.113.058388. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Rhee J, Lin J, Wu Z, Yoon JC, Zhang CY, Krauss S, Mootha VK, Lowell BB, Spiegelman BM. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol Cell. 2001;8:971–982. doi: 10.1016/s1097-2765(01)00390-2. [DOI] [PubMed] [Google Scholar]
- Greene FL. AJCC Cancer Staging Manual. 6th ed. New York: Springer-Verlag; 2002. [Google Scholar]
- Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35:609–616. [PubMed] [Google Scholar]
- van den Borst B, Slot IG, Hellwig VA, Vosse BA, Kelders MC, Barreiro E, Schols AM, Gosker HR. Loss of quadriceps muscle oxidative phenotype and decreased endurance in patients with mild-to-moderate COPD. J Appl Physiol. 2013;114:1319–1328. doi: 10.1152/japplphysiol.00508.2012. [DOI] [PubMed] [Google Scholar]
- Weber MA, Krakowski-Roosen H, Schroder L, Kinscherf R, Krix M, Kopp-Schneider A, Essig M, Bachert P, Kauczor HU, Hildebrandt W. Morphology, metabolism, microcirculation, and strength of skeletal muscles in cancer-related cachexia. Acta Oncol. 2009;48:116–124. doi: 10.1080/02841860802130001. [DOI] [PubMed] [Google Scholar]
- Fuster G, Busquets S, Ametller E, Olivan M, Almendro V, de Oliveira CC, Figueras M, López-Soriano FJ, Argilés JM. Are peroxisome proliferator-activated receptors involved in skeletal muscle wasting during experimental cancer cachexia? Role of beta2-adrenergic agonists. Cancer Res. 2007;67:6512–6519. doi: 10.1158/0008-5472.CAN-07-0231. [DOI] [PubMed] [Google Scholar]
- Gosker HR, Wouters EF, van der Vusse GJ, Schols AM. Skeletal muscle dysfunction in chronic obstructive pulmonary disease and chronic heart failure: underlying mechanisms and therapy perspectives. Am J Clin Nutr. 2000;71:1033–1047. doi: 10.1093/ajcn/71.5.1033. [DOI] [PubMed] [Google Scholar]
- Puente-Maestu L, Lazaro A, Humanes B. Metabolic derangements in COPD muscle dysfunction. J Appl Physiol. 2013;114:1282–1290. doi: 10.1152/japplphysiol.00815.2012. [DOI] [PubMed] [Google Scholar]
- De Ruysscher D, Dehing C, Bremer RH, Bentzen SM, Koppe F, Pijls-Johannesma M, Harzée L, Minken A, Wanders R, Hochstenbag M, Dingemans AM, Boersma L, van Haren E, Geraedts W, Pitz C, Simons J, Wouters B, Rosier JF, Lambin P. Maximal neutropenia during chemotherapy and radiotherapy is significantly associated with the development of acute radiation-induced dysphagia in lung cancer patients. Ann Oncol. 2007;18:909–916. doi: 10.1093/annonc/mdm005. [DOI] [PubMed] [Google Scholar]
- Ramnath N, Dilling TJ, Harris LJ, Kim AW, Michaud GC, Balekian AA, Diekemper R, Detterbeck FC, Arenberg DA. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e314S–e340S. doi: 10.1378/chest.12-2360. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol. 2003;546:851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puppa MJ, White JP, Velazquez KT, Baltgalvis KA, Sato S, Baynes JW, Carson JA. The effect of exercise on IL-6-induced cachexia in the Apc (Min/+) mouse. J Cachexia Sarcopenia Muscle. 2012;3:117–137. doi: 10.1007/s13539-011-0047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo JA, Daniels TG, Wang X, Paul A, Lin J, Spiegelman BM, Stevenson SC, Rangwala SM. Muscle-specific expression of PPARgamma coactivator-1alpha improves exercise performance and increases peak oxygen uptake. J Appl Physiol. 2008;104:1304–1312. doi: 10.1152/japplphysiol.01231.2007. [DOI] [PubMed] [Google Scholar]
- Tadaishi M, Miura S, Kai Y, Kano Y, Oishi Y, Ezaki O. Skeletal muscle-specific expression of PGC-1alpha-b, an exercise-responsive isoform, increases exercise capacity and peak oxygen uptake. PLoS One. 2011;6:e28290. doi: 10.1371/journal.pone.0028290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault JJ, Jespersen JG, Goldberg AL. Peroxisome proliferator-activated receptor gamma coactivator 1alpha or 1beta overexpression inhibits muscle protein degradation, induction of ubiquitin ligases, and disuse atrophy. J Biol Chem. 2010;285:19460–19471. doi: 10.1074/jbc.M110.113092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Pickrell AM, Zimmers TA, Moraes CT. Increase in muscle mitochondrial biogenesis does not prevent muscle loss but increased tumor size in a mouse model of acute cancer-induced cachexia. PLoS One. 2012;7:e33426. doi: 10.1371/journal.pone.0033426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddocks M, Gao W, Higginson IJ, Wilcock A. Neuromuscular electrical stimulation for muscle weakness in adults with advanced disease. Cochrane Database Syst Rev. 2013;1 doi: 10.1002/14651858.CD009419.pub2. : CD009419. [DOI] [PubMed] [Google Scholar]
- Phillips SM. Resistance exercise: good for more than just grandma and grandpa's muscles. Appl Physiol Nutr Metab. 2007;32:1198–1205. doi: 10.1139/H07-129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item
Supporting info item
Supporting info item