Abstract
Background
Activity of xanthine oxidase is induced in cancer cachexia, and its inhibition by allopurinol or oxypurinol improves survival and reduces wasting in the Yoshida hepatoma cancer cachexia model. Here, we tested the effects of the second-generation xanthine oxidase inhibitor febuxostat compared with placebo in the same model as used previously by our group.
Methods
Wistar rats (∽200 g) were treated daily with febuxostat at 5 mg/kg/day or placebo via gavage for a maximum of 17 days. Weight change, quality of life, and body composition were analysed. After sacrifice, proteasome activity in the gastrocnemius muscle was measured. Muscle-specific proteins involved in metabolism were analysed by western blotting.
Results
Treatment of the tumour-bearing rats with febuxostat led to a significantly improved survival compared with placebo (hazard ratio: 0.45, 95% confidence interval: 0.22–0.93, P = 0.03). Loss of body weight was reduced (−26.3 ± 12.4 g) compared with placebo (−50.2 ± 2.1 g, P < 0.01). Wasting of lean mass was attenuated (−12.7 ± 10.8 g) vs. placebo (−31.9 ± 2.1 g, P < 0.05). While we did not see an effect of febuxostat on proteasome activity at the end of the study, the pAkt/Akt ratio was improved by febuxostat (0.94 ± 0.09) vs. placebo (0.41 ± 0.05, P < 0.01), suggesting an increase in protein synthesis.
Conclusions
Febuxostat attenuated cachexia progression and improved survival of tumour-bearing rats.
Keywords: Cancer cachexia, Yoshida hepatoma animal model, Weight loss, Wasting, Survival
Introduction
Cachexia is frequently observed in chronic illnesses such as cancer, heart failure, and chronic obstructive pulmonary disease1,2 and is characterized by weight loss of at least 5% as a consequence of hypercatabolism with or without impaired feeding. Cachexia is a common complication in patients with advanced cancer and accounts for up to 30% of cancer deaths.2 Taken together, effective treatment for cancer cachexia is considered a great unmet medical need.3
Hyperuricaemia, which is often observed in cancer patients regardless of tumour lysis syndrome,4 has been reported as a negative prognostic factor in terminally ill cancer patients.5 The increased uric acid levels reflect higher xanthine oxidase (XO) enzymatic activity, which also results in an increased generation of reactive oxygen species (ROS).6 ROS in turn plays an important role in wasting by activation of calcium-activated protease, caspase, and proteasome system.7 We have previously shown that the inhibition of XO by allopurinol reduced wasting and improved outcome in a rat model of cancer cachexia.8
Although allopurinol is a commonly used XO inhibitor for the treatment of hyperuricaemia and has generally acceptable efficacy and safety profiles, rare but severe adverse reactions have been also reported.9 Febuxostat is a novel non-purine selective XO inhibitor10 and could be a better alternative to allopurinol.11 The purpose of this study is to assess the effect of febuxostat on survival, body weight, and body composition using the Yoshida hepatoma model.
Materials and methods
Study design
Juvenile Wistar Han rats aged 7 weeks (weight ∽200 g) were housed in our Specific Pathogen Free (SPF)-animal facility at a constant temperature of 22°C and exposed to a 12 h light cycle. On Day 1, rats were inoculated with 108 growing Yoshida hepatoma AH-130 cells12 into the peritoneum. Before tumour inoculation, baseline weight and body composition were assessed. Rats were randomized to placebo (n = 56) or 5 mg/kg/day febuxostat (n = 23). Treatment with febuxostat or placebo was given via gavage daily. Febuxostat was obtained from Tocris Bioscience (Wiesbaden, Germany). Eleven rats on placebo and seven on febuxostat were scheduled to be sacrificed on Day 11 for the early assessment; the remaining 45 animals on placebo and 16 on febuxostat were analysed for survival, defined as reaching ethical endpoints for euthanasia (apathy, reduced body temperature, and disturbed blood flow).8 Additionally, 10 rats were injected with saline (=sham), treated by placebo, and used as a reference. Body composition and weight were recorded after removal of the tumour on Day 11, Day 17, or the respective days of killing, if rats had to be euthanized earlier owing to reaching ethical endpoints. Weight of tissue and organs was recorded, and tumour cells were counted using a Neubauer chamber.
Body composition
Body composition (fat and lean body mass) was analysed with a nuclear magnetic resonance spectroscopy device EchoMRI-700TM (Echo Medical Systems, Houston, TX, USA) as described before.13
Quality-of-life indicators
Spontaneous movement and food intake were recorded as indicators of quality of life. Animals were housed individually, and their activity was measured by an infrared monitoring system (Supermex, Muromachi, Tokyo, Japan) over a 24 h period as described previously.14
Echocardiography
Echocardiography using the high-resolution Veno770 system (Visual Sonics, Toronto, Canada) was performed as described previously.15 Briefly, rats were anaesthetized with 1.5% isoflurane and laid in a supine position on a heated surface to maintain body temperature, and hair was removed from the left chest. Recordings were made in B-mode and M-mode to calculate functional parameters and measure cardiac function and dimensions.
Proteasome activity
Proteasome activity was analysed as described previously.16 Briefly, the gastrocnemius muscle was homogenized in an ice-cold buffer. Protein was incubated with fluorogenic substrates (benzyloxycarbonyl-Leu-Leu-Glu-7-amido-4-methylcoumarin for trypsin-like activity, succinyl-Leu-Leu-Val-Try-7-amido-4-methylcoumarin for chymotrypsin-like activity and benzoyl-Val-Gly-Arg-7-amidocoumarin for peptidyl-glutamyl protein-hydrolysing activity, Biomol, Hamburg, Germany). The fluorescence intensity was measured with a fluorometer (Twinkle LB 970, Berthold, Bad Wildbad, Germany) at 360 and 460 nm emission. The activity, expressed as nanomole per milligramme per minute, was calculated by using free amidomethylcoumarin as a working standard.
Caspase activity
Caspase-3 and caspase-6 activities were analysed as described previously.16 Briefly, the gastrocnemius muscle was homogenized in ice-cold buffer. The homogenate was frozen on dry ice/ethanol and heated to 37°C for three cycles. After centrifugation (20 000 g for 30 min), 100 µg protein was pre-incubated in assay buffer (100 mM 4-(2-hydroxyethyl)-1-piperanzineethanesulfonic acid (HEPES) pH 7.5, 10% sucrose, 0.1% 3-(3-cholamidepropyl)dimethylammonio-1-propanesulphonate (CHAPS), 2% dimethyl sulphoxide (DMSO), and 10 mM dithiothreitol (DTT) with or without 50 μM caspase-3 or caspase-6 inhibitor Ac-DEVD-CHO or Ac-VEID-CHO) at 37°C for 30 min. The fluorogenic substrate (50 μM) Ac-DEVD-AMC or Ac-VEID-AMC was added for caspase-3 and caspase-6. Assay conditions were identical to proteasome assay.
Western blotting
Protein lysates were prepared from the gastrocnemius muscle according to standard protocols. We used primary antibodies against Akt (9272), pAkt (Ser473; 4051), pAkt (Thr308; 9275), p-p70S6K (Thr389; 9025), 4E-BP1 (53H11; 9644), p4E-BP1 (Ser65; 9451), and p4E-BP1 (Thr 37/47; 9459), all from Cell Signaling, and p70S6K (sc-230; Santa Cruz), as well as appropriate secondary antibodies.
Statistics
Data were analysed with GraphPad PRISM 5.0 (GraphPad Software, Inc., La Jolla, CA, USA).17 Results are shown as mean ± standard error of mean. For the comparisons among groups, data were analysed with analysis of variance followed by post hoc comparisons using Tukey's test. Survival was assessed by Cox proportional hazard analysis. A P-value of <0.05 was considered significant.
Results
No treatment effect was observed on tumour growth. On Day 11, total tumour cell numbers were 4.63 ± 0.39 × 109 in the placebo-treated rats and 4.04 ± 0.28 × 109 cells in rats with febuxostat (P > 0.4). At the end of the study, total tumour cell numbers were 4.20 ± 0.29 × 109 cells in the placebo-treated rats and 4.19 ± 0.40 × 109 cells in rats with febuxostat (P > 0.9). There were no observations of other malignancies during necropsy.
Body weight and body composition
Average loss of body weight from baseline to the end of study was higher in the placebo group than in treated rats (−50.2 ± 2.1 g vs. −26.3 ± 12.4 g, P < 0.01; Figure1A). The loss of lean and fat mass was also higher in the placebo group than in treated rats (−31.0 ± 2.4 g vs. −12.7 ± 10.8 g, P < 0.05 for lean mass and −12.8 ± 0.5 g vs. −9.1 ± 1.8 g, P < 0.05 for fat mass, Figure1B and C). No significant difference was observed between the two groups on Day 11.
Figure 1.
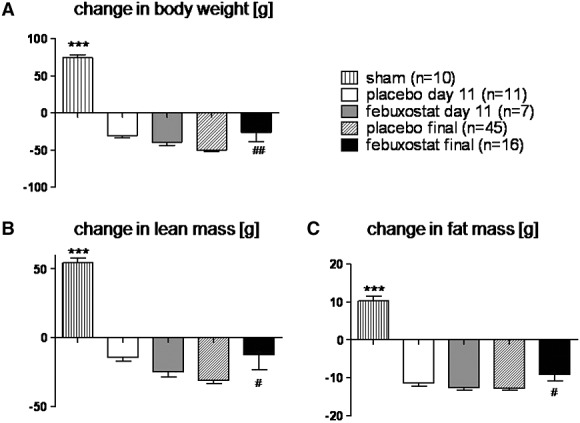
Change in body weight (A), lean body mass (B), and fat mass (C): average loss of body weight, lean mass, and fat mass were higher in the placebo group than in treated rats at the end of study (P < 0.01 for body weight, P < 0.05 for lean mass, and P < 0.05 for fat mass). No significant difference was observed between both groups on Day 11. ***P < 0.001 vs. placebo Day 11. #P < 0.05, ##P < 0.01 vs. placebo final.
Organ weights
Organ weights on Day 11 and the end of study are shown in Table 1. The weights of the heart, liver, kidney, gastrocnemius, and tibialis were comparable among tumour-bearing rats regardless of their treatment and study length. At the end of study, the weights of the spleen, white adipose tissue, extensor digitalis longus, and soleus were higher in febuxostat than in placebo group.
Table 1.
Organ and tissue weights at Day 11 and the end of study
| Organ weight (mg) | Sham (n = 10) | Placebo Day 11 (n = 11) | Febuxostat Day 11 (n = 7) | Placebo final (n = 45) | Febuxostat final (n = 16) |
|---|---|---|---|---|---|
| Heart | 893 ± 41** | 636 ± 17 | 543 ± 15 | 553 ± 15 | 587 ± 29 |
| Liver | 11 292 ± 511** | 7 480 ± 232 | 6 775 ± 359 | 5 640 ± 151 | 6 378 ± 675 |
| Spleen | 632 ± 32** | 278 ± 29 | 302 ± 39 | 159 ± 8* | 253 ± 44*** |
| Kidney left | 1 036 ± 26** | 728 ± 18 | 692 ± 37 | 705 ± 18 | 752 ± 36 |
| White adipose tissue | 1 282 ± 173** | 203 ± 85 | 136 ± 70 | 48 ± 28 | 501 ± 221*** |
| Brown adipose tissue | 311 ± 43** | 100 ± 19 | 116 ± 15 | 95 ± 5 | 139 ± 16 |
| Musculus gastrocnemius | 1 426 ± 27** | 953 ± 30 | 874 ± 51 | 795 ± 20 | 911 ± 83 |
| Musculus tibialis | 501 ± 7** | 315 ± 14 | 318 ± 19 | 280 ± 7 | 312 ± 32 |
| Musculus extensor digitalis longus | 115 ± 6** | 80 ± 4 | 77 ± 4 | 68 ± 1 | 83 ± 8*** |
| Musculus soleus | 121 ± 6* | 91 ± 4 | 85 ± 5 | 76 ± 2 | 91 ± 7*** |
P < 0.01 vs. placebo Day 11;
P < 0.001 vs. placebo Day 11;
P < 0.05 vs. placebo final.
Quality of life
Change in food intake was 5.1 ± 0.9 g/day in sham and reduced in both the placebo (−14.0 ± 0.8, P < 0.001 vs. sham) and febuxostat (−10.0 ± 2.1, P < 0.001 vs. sham) groups. There was no significant difference between the placebo and febuxostat groups. Change in locomotor activity was −2619 ± 3453 counts/day in sham and also reduced in both the placebo (−39 054 ± 2119, P < 0.001 vs. sham) and febuxostat (−30 693 ± 5432, P < 0.01 vs. sham) groups. There was no significant difference between the placebo and febuxostat groups.
Echocardiography
Echocardiographic parameters are shown in Figure2. The declines in left ventricular mass in 11 days were more pronounced in placebo-treated rats than febuxostat-treated rats (−81.2 ± 10.3 vs. −3.4 ± 27.8 mg, respectively, P < 0.01, Figure2A). Changes to baseline in left ventricular contraction, diameter, wall thickness, and stroke volume were comparable between the two groups (Figure2B–H).
Figure 2.
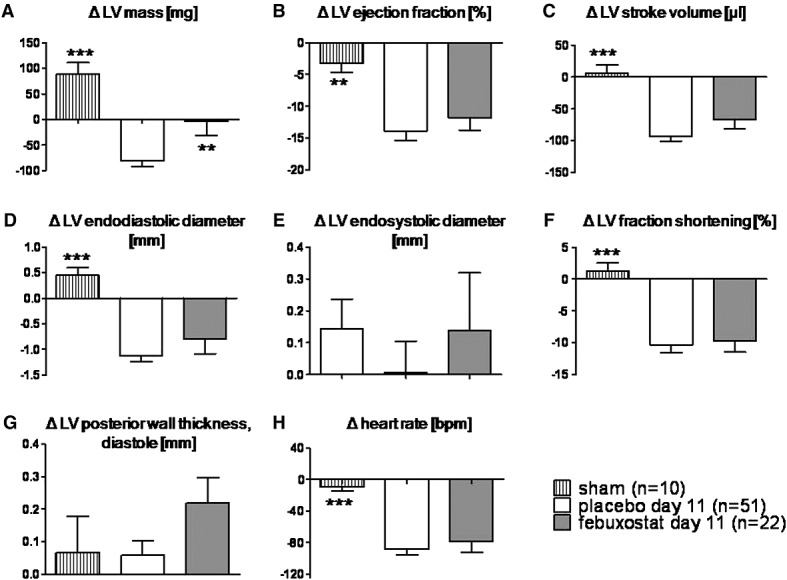
Echocardiography: the declines in left ventricular (LV) mass (A) in 11 days were more remarkable in placebo-treated rats than in febuxostat-treated rats (P < 0.01). Changes in left ventricular contraction (B, C, and F), diameter (D and E), wall thickness (G), and heart rate (H) were comparable between both groups. **P < 0.01, ***P < 0.001 vs. placebo Day 11.
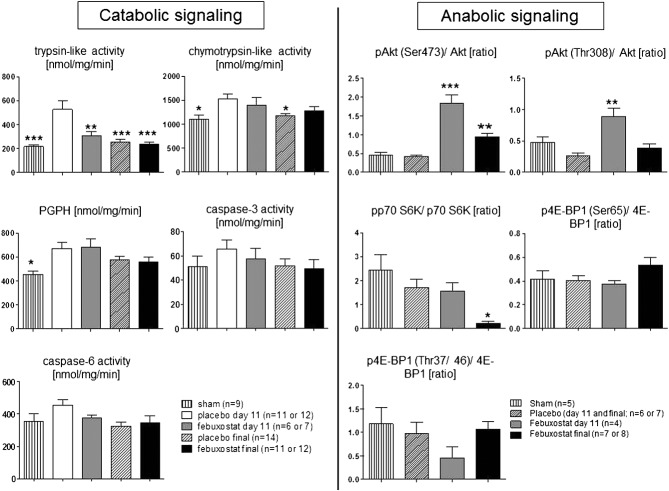
Catabolic and anabolic signalling
Catabolic and anabolic signalling are shown in Figure3. On Day 11, trypsin-like proteasome activity was significantly lower in febuxostat-treated rats than in placebo-treated rats (305.7 ± 35.6 vs. 527.2 ± 70.7 nmol/mg/min, respectively, P < 0.01). Chymotrypsin-like activity, peptidyl-glutamyl protein-hydrolyzing activity, and the activities of caspase-3 and caspase-6 were comparable between both groups on Day 11 and at the end of study. The phosphorylation of the Ser 473 site in Akt was increased both on Day 11 (the pAkt/Akt ratio; 1.84 ± 0.23 in febuxostat vs. 0.41 ± 0.05 in placebo, P < 0.001) and at the end of study (0.94 ± 0.09 in febuxostat, P < 0.01). The phosphorylation of the Thr 308 site in Akt was increased on Day 11 (0.89 ± 0.14 in febuxostat vs. 0.26 ± 0.05 in placebo, P < 0.01). However, its downstream target p70 S6K and 4E-BP1 phosphorylation were comparable between both groups.
Figure 3.
Catabolic and anabolic signalling in the gastrocnemius muscle: on Day 11, trypsin-like proteasome activity was significantly lower in febuxostat-treated rats than in placebo-treated rats (P < 0.01). Chymotrypsin-like, peptidyl-glutamyl protein-hydrolyzing (PGPH), caspase-3, and caspase-6 activities were comparable between both groups both on Day 11 and at the end of study. The phosphorylation of the Ser 473 site in Akt was increased both on Day 11 (P < 0.001 vs. placebo) and at the end of study (P < 0.01 vs. placebo). The phosphorylation of the Thr 308 site in Akt was increased on Day 11 (P < 0.01 vs. placebo). However, its downstream target p70 S6K and 4E-BP1 phosphorylation were comparable between both groups. **P < 0.01, ***P < 0.001 vs. placebo day 11.
Survival
On Day 17, the overall survival was 20% in the placebo group and 59% in the febuxostat group (Figure4). The median survival was 14 days in the placebo group and 17 days in the febuxostat group. Treatment with febuxostat significantly reduced mortality (hazard ratio: 0.45; 95% confidence interval: 0.22–0.93; P = 0.03).
Figure 4.
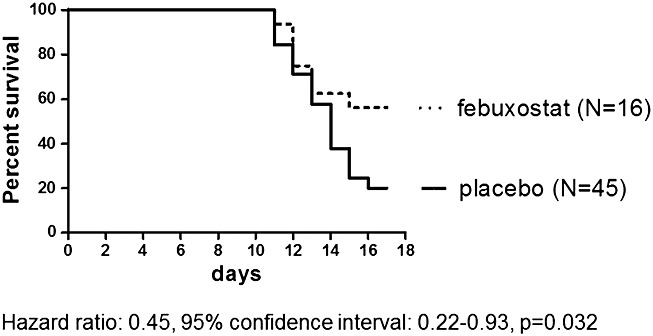
Kaplan–Meier survival curves: febuxostat reduced mortality compared with placebo.
Discussion
In the present study, treatment of tumour-bearing rats with febuxostat led to a significantly improved survival compared with placebo. Loss of body weight and wasting of lean/fat mass were attenuated in treated animals vs. placebo. No effect on food intake and locomotor activity was observed. While we did not see an effect of febuxostat on the proteasome activity at the end of the study, the pAkt/Akt ratio was improved by febuxostat vs. placebo, suggesting an increase in protein synthesis.
The main finding of the present study is attenuated weight loss and the improved survival in animals treated with febuxostat. The primary underlying mechanism would be XO inhibition. We have previously shown the increased XO-dependent ROS production in the same rat model as the present study and that XO inhibition by allopurinol and oxypurinol drastically reduced ROS.8 In addition, ROS reduction may lead to reduced catabolic signalling such as the ubiquitin–proteasome pathway through inflammatory transcription factors and attenuate wasting.8,18 Also in the present study, reduced proteasome activity was indicated by the fact that trypsin-like activity in gastrocnemius muscle was significantly reduced on Day 11 in febuxostat-treated rats. On the other hand, enhanced anabolic signalling was also shown by significantly increased Akt phosphorylation in febuxostat-treated rats. It may be explained by the hypothesis that ROS-induced reduction in Akt protein19,20 would be reversed by febuxostat. In a previous study, however, febuxostat did not affect myocardial Akt phosphorylation in a heart failure model.21
Although allopurinol is known to be a safe and effective XO inhibitor, serious adverse reactions such as allopurinol hypersensitivity syndromes have also been reported.9 Given that accumulation or metabolic actions of allopurinol and its metabolites might account for such adverse reactions especially in patients with renal dysfunction, it might be of importance to note that febuxostat is mainly metabolized in the liver.10 In addition, febuxostat might be able to attenuate ROS more effectively than allopurinol because allopurinol, but not febuxostat, might induce a resistance to inhibition in XO and undesirable production of superoxide.22 As a result, the mortality of febuxostat-treated animals was significantly lower than placebo-treated animals in the present study. To the best of our knowledge, there is only one study referring to the effect of febuxostat on survival, which indicated no survival benefit in a left ventricular hypertrophy mice model.21 There are also some experimental studies about non-hypouricaemic effect by febuxostat, which includes improved endothelial function23 or left ventricular performance,24 reduced intracellular ROS production,25 and no effect on blood pressure.26 In some clinical trial using febuxostat for hyperuricaemia, reduction in inflammatory signal (tumour necrosis factor-α) or oxidative stress was observed.27,28 Together with our previous reports, febuxostat as well as allopurinol or oxypurinol would be candidates for the treatment drugs for cachexia.
We also assessed the effect of febuxostat on cardiac function by echocardiography. On Day 11, loss of left ventricular mass was significantly reduced in febuxostat, indicating attenuated cardiac wasting in cancer cachexia, which was also demonstrated in our previous study by oxypurinol.29 However, the effect by febuxostat in left ventricular ejection fraction did not reach statistical significance despite previously reported improvement in ejection fraction by oxypurinol.29 Although little is known about the consequences of wasting on cardiac function30 and whether it leads to heart failure or impaired survival, some of the drugs used in heart failure treatment would be effective in our previous study.16 As febuxostat was also reported to be effective in mouse and dog models of heart failure,21,24 the survival effect in the present study could be influenced by the effect on the heart to some extent.
The Yoshida hepatoma model is a well-established cancer cachexia model and known to have a high mortality rate without treatment. Its high mortality was also shown in our previous studies,14,31 which are in accordance with the results of this study. The dose of febuxostat (5 mg/kg/day) was as appropriate as that used in diabetic rats model23 or mice models of heart failure.21
There are also some limitations in the present study. We did not measure inflammation and ROS, although we had found a significant reduction in interleukin-6 and tumour necrosis factor-α expression and reduced ROS production by allopurinol and oxypurinol.8 The mechanisms under up-regulated anabolic signal, especially those in the downstream of Akt, were not elucidated precisely in this study, where some of such signals (p70 S6K and 4E-BP1s phosphorylation32) were not up-regulated significantly. The lack of information about catabolic pathways other than proteasome/caspase activity is also a limitation.
In conclusion, the present study demonstrated that treatment with febuxostat improved survival and attenuated the loss of lean and fat mass in the Yoshida hepatoma rat model of cancer cachexia. The improvement in protein synthesis suggested by enhanced phosphorylation in Akt was also observed and might underlie the beneficial effect of febuxostat. Our data suggest that febuxostat might be a candidate for the treatment drug for cancer cachexia, and clinical studies are warranted.
Acknowledgments
M.K. was supported by the Sumitomo Life Welfare and Culture Foundation operating grant as a long-term overseas research fellow. The authors have read and certified that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia, and Muscle.
Conflict of Interest
None declared.
References
- Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Farkas J, von Haehling S, Kalantar-Zadeh K, Morley JE, Anker SD, Lainscak M. Cachexia as a major public health problem: frequent, costly, and deadly. J Cachexia Sarcopenia Muscle. 2013;4:173–178. doi: 10.1007/s13539-013-0105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Haehling S, Anker SD. Cachexia vs obesity: where is the real unmet clinical need? J Cachexia Sarcopenia Muscle. 2013;4:245–246. doi: 10.1007/s13539-013-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarsten J, Hogstedt B. Clinical, haemodynamic, anthropometric, metabolic and insulin profile of men with high-stage and high-grade clinical prostate cancer. Blood Press. 2004;13:47–55. doi: 10.1080/08037050310025735. [DOI] [PubMed] [Google Scholar]
- Shin HS, Lee HR, Lee DC, Shim JY, Cho KH, Suh SY. Uric acid as a prognostic factor for survival time: a prospective cohort study of terminally ill cancer patients. J Pain Symptom Manage. 2006;31:493–501. doi: 10.1016/j.jpainsymman.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Terada LS, Guidot DM, Leff JA, Willingham IR, Hanley ME, Piermattei D, et al. Hypoxia injures endothelial cells by increasing endogenous xanthine oxidase activity. Proc Natl Acad Sci U S A. 1992;89:3362–3366. doi: 10.1073/pnas.89.8.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2005;288:R337–R344. doi: 10.1152/ajpregu.00469.2004. [DOI] [PubMed] [Google Scholar]
- Springer J, Tschirner A, Hartman K, Palus S, Wirth EK, Ruis SB, et al. Inhibition of xanthine oxidase reduces wasting and improves outcome in a rat model of cancer cachexia. Int J Cancer. 2012;131:2187–2196. doi: 10.1002/ijc.27494. [DOI] [PubMed] [Google Scholar]
- Arellano F, Sacristan JA. Allopurinol hypersensitivity syndrome: a review. Ann Pharmacother. 1993;27:337–343. doi: 10.1177/106002809302700317. [DOI] [PubMed] [Google Scholar]
- Takano Y, Hase-Aoki K, Horiuchi H, Zhao L, Kasahara Y, Kondo S, et al. Selectivity of febuxostat, a novel non-purine inhibitor of xanthine oxidase/xanthine dehydrogenase. Life Sci. 2005;76:1835–1847. doi: 10.1016/j.lfs.2004.10.031. [DOI] [PubMed] [Google Scholar]
- Becker MA, Schumacher HR, Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353:2450–2461. doi: 10.1056/NEJMoa050373. [DOI] [PubMed] [Google Scholar]
- Costelli P, Carbo N, Tessitore L, Bagby GJ, Lopez-Soriano FJ, Argiles JM, et al. Tumor necrosis factor-alpha mediates changes in tissue protein turnover in a rat cancer cachexia model. J Clin Invest. 1993;92:2783–2789. doi: 10.1172/JCI116897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimpson SA, Leonard MS, Clifton LG, Poole JC, Turner SM, Shearer TW, et al. Longitudinal changes in total body creatine pool size and skeletal muscle mass using the D-creatine dilution method. J Cachexia Sarcopenia Muscle. 2013;4:217–223. doi: 10.1007/s13539-013-0110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschirner A, von Haehling S, Palus S, Doehner W, Anker SD, Springer J. Ursodeoxycholic acid treatment in a rat model of cancer cachexia. J Cachexia Sarcopenia Muscle. 2012;3:31–36. doi: 10.1007/s13539-011-0044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akashi YJ, Palus S, Datta R, Halem H, Taylor JE, Thoene-Reineke C, et al. No effects of human ghrelin on cardiac function despite profound effects on body composition in a rat model of heart failure. Int J Cardiol. 2009;137:267–275. doi: 10.1016/j.ijcard.2008.06.094. [DOI] [PubMed] [Google Scholar]
- Springer J, Tschirner A, Haghikia A, von Haehling S, Lal H, Grzesiak A, et al. Prevention of liver cancer cachexia-induced cardiac wasting and heart failure. Eur Heart J. 2014;35:932–941. doi: 10.1093/eurheartj/eht302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenk K, Palus S, Schur R, Datta R, Dong J, Culler MD, et al. Effect of ghrelin and its analogues, BIM-28131 and BIM-28125, on the expression of myostatin in a rat heart failure model. J Cachexia Sarcopenia Muscle. 2013;4:63–69. doi: 10.1007/s13539-012-0085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanzani A, Conraads VM, Penna F, Martinet W. Molecular and cellular mechanisms of skeletal muscle atrophy: an update. J Cachexia Sarcopenia Muscle. 2012;3:163–179. doi: 10.1007/s13539-012-0074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Falasca M, Blough ER. Akt/protein kinase B in skeletal muscle physiology and pathology. J Cell Physiol. 2011;226:29–36. doi: 10.1002/jcp.22353. [DOI] [PubMed] [Google Scholar]
- Martin D, Salinas M, Fujita N, Tsuruo T, Cuadrado A. Ceramide and reactive oxygen species generated by H2O2 induce caspase-3-independent degradation of Akt/protein kinase B. J Biol Chem. 2002;277:42943–42952. doi: 10.1074/jbc.M201070200. [DOI] [PubMed] [Google Scholar]
- Xu X, Hu X, Lu Z, Zhang P, Zhao L, Wessale JL, et al. Xanthine oxidase inhibition with febuxostat attenuates systolic overload-induced left ventricular hypertrophy and dysfunction in mice. J Card Fail. 2008;14:746–753. doi: 10.1016/j.cardfail.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik UZ, Hundley NJ, Romero G, Radi R, Freeman BA, Tarpey MM, et al. Febuxostat inhibition of endothelial-bound XO: implications for targeting vascular ROS production. Free Radic Biol Med. 2011;51:179–184. doi: 10.1016/j.freeradbiomed.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SJ, Lee KH, Jang HH, Lee SR, Woo JS, Lee HJ, et al. Febuxostat contributes to improvement of endothelial dysfunction in an experimental model of streptozocin-induced diabetic rats. Int J Cardiol. 2014;171:e110–e112. doi: 10.1016/j.ijcard.2013.12.023. [DOI] [PubMed] [Google Scholar]
- Hou M, Hu Q, Chen Y, Zhao L, Zhang J, Bache RJ. Acute effects of febuxostat, a nonpurine selective inhibitor of xanthine oxidase, in pacing induced heart failure. J Cardiovasc Pharmacol. 2006;48:255–263. doi: 10.1097/01.fjc.0000249961.61451.da. [DOI] [PubMed] [Google Scholar]
- Nomura J, Busso N, Ives A, Tsujimoto S, Tamura M, So A, et al. Febuxostat, an inhibitor of xanthine oxidase, suppresses lipopolysaccharide-induced MCP-1 production via MAPK phosphatase-1-mediated inactivation of JNK. PLoS One. 2013;8:e75527. doi: 10.1371/journal.pone.0075527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szasz T, Davis RP, Garver HS, Burnett RJ, Fink GD, Watts SW. Long-term inhibition of xanthine oxidase by febuxostat does not decrease blood pressure in deoxycorticosterone acetate (DOCA)-salt hypertensive rats. PLoS One. 2013;8:e56046. doi: 10.1371/journal.pone.0056046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sezai A, Soma M, Nakata K, Hata M, Yoshitake I, Wakui S, et al. Comparison of febuxostat and allopurinol for hyperuricemia in cardiac surgery patients (NU-FLASH Trial) Circ J. 2013;77:2043–2049. doi: 10.1253/circj.cj-13-0082. [DOI] [PubMed] [Google Scholar]
- Tausche AK, Christoph M, Forkmann M, Richter U, Kopprasch S, Bielitz C, et al. As compared to allopurinol, urate-lowering therapy with febuxostat has superior effects on oxidative stress and pulse wave velocity in patients with severe chronic tophaceous gout. Rheumatol Int. 2014;34:101–109. doi: 10.1007/s00296-013-2857-2. [DOI] [PubMed] [Google Scholar]
- Springer J, Tschirner A, Hartman K, von Haehling S, Anker SD, Doehner W. The xanthine oxidase inhibitor oxypurinol reduces cancer cachexia-induced cardiomyopathy. Int J Cardiol. 2013;168:3527–3531. doi: 10.1016/j.ijcard.2013.05.063. [DOI] [PubMed] [Google Scholar]
- Coats AJ. Research on cachexia, sarcopenia and skeletal muscle in cardiology. J Cachexia Sarcopenia Muscle. 2012;3:219–223. doi: 10.1007/s13539-012-0090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K, von Haehling S, Doehner W, Palus S, Anker SD, Springer J. IGF-1 treatment reduces weight loss and improves outcome in a rat model of cancer cachexia. J Cachexia Sarcopenia Muscle. 2011;2:105–109. doi: 10.1007/s13539-011-0029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma K, Yamaguchi A. Sarcopenia and cachexia: the adaptations of negative regulators of skeletal muscle mass. J Cachexia Sarcopenia Muscle. 2012;3:77–94. doi: 10.1007/s13539-011-0052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]