Abstract
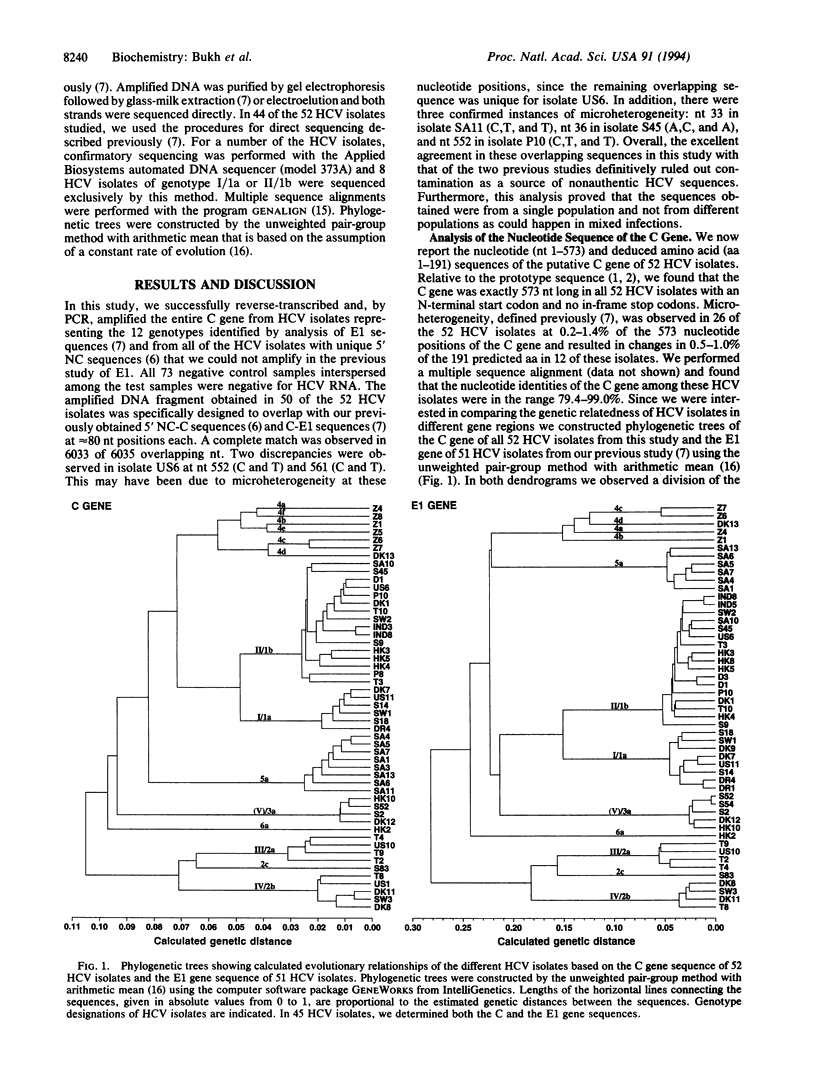
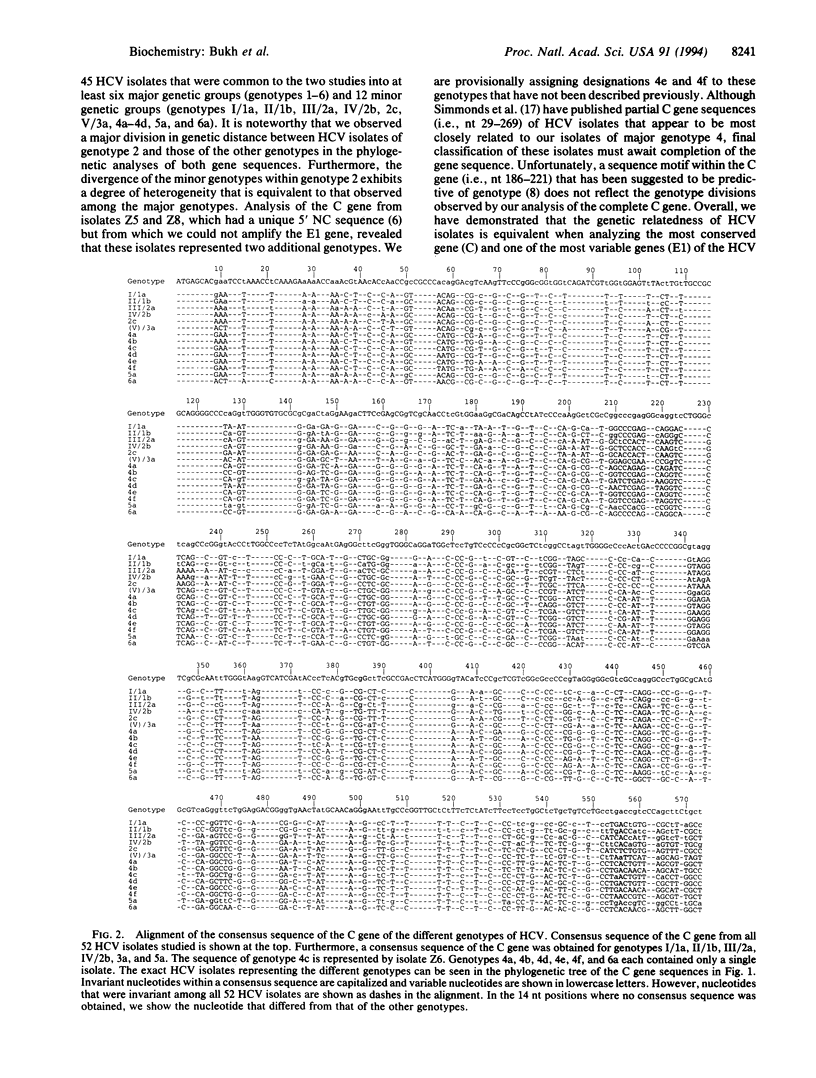
We previously sequenced the 5' noncoding region of 44 isolates of hepatitis C virus (HCV), as well as the envelope 1 (E1) gene of 51 HCV isolates, and provided evidence for the existence of at least 6 major genetic groups consisting of at least 12 minor genotypes of HCV (i.e., genotypes I/1a, II/1b, III/2a, IV/2b, 2c, V/3a, 4a-4d, 5a, and 6a). We now report the complete nucleotide sequence of the putative core (C) gene of 52 HCV isolates that represent all of these 12 genotypes as well as two additional genotypes provisionally designated 4e and 4f that we identified in this study. The phylogenetic analysis of the C gene sequences was in agreement with that of the E1 gene sequences. A major division in the genetic distance was observed between HCV isolates of genotype 2 and those of the other genotypes in analysis of both the E1 and C genes. The C gene sequences of 9 genotypes have not been reported previously (i.e., genotypes 2c, 4a-4f, 5a, and 6a). Our analysis indicates that the C gene-based methods currently used to determine the HCV genotype, such as PCR with genotype-specific primers, should be revised in light of these data. We found that the predicted C gene was exactly 573 nt long in all 52 HCV isolates, with an N-terminal start codon and no in-frame stop codons. The nucleotide and predicted amino acid identities of the C gene sequences were in the range of 79.4-99.0% and 85.3-100%, respectively. Furthermore, we mapped universally conserved, as well as genotype-specific, nucleotide and deduced amino acid sequences of the C gene. The predicted C proteins of the different HCV genotypes shared the following features: (i) high content of proline residues, (ii) high content of arginine and lysine residues located primarily in three domains with 10 such residues invariant at positions 39-62, (iii) a cluster of 5 conserved tryptophan residues, (iv) two nuclear localization signals and a DNA-binding motif, (v) a potential phosphorylation site with a serine-proline motif, and (vi) three conserved hydrophilic domains that have been shown by others to contain immunogenic epitopes. Thus, we have extended analysis of the predicted C protein of HCV to all of the recognized genotypes, confirmed the existence of highly conserved regions of this important structural protein, and demonstrated that the genetic relatedness of HCV isolates is equivalent when analyzing the most conserved (i.e., C) and the most variable (i.e., E1) genes of the HCV genome.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bukh J., Purcell R. H., Miller R. H. At least 12 genotypes of hepatitis C virus predicted by sequence analysis of the putative E1 gene of isolates collected worldwide. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8234–8238. doi: 10.1073/pnas.90.17.8234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukh J., Purcell R. H., Miller R. H. Importance of primer selection for the detection of hepatitis C virus RNA with the polymerase chain reaction assay. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):187–191. doi: 10.1073/pnas.89.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukh J., Purcell R. H., Miller R. H. Sequence analysis of the 5' noncoding region of hepatitis C virus. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4942–4946. doi: 10.1073/pnas.89.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha T. A., Beall E., Irvine B., Kolberg J., Chien D., Kuo G., Urdea M. S. At least five related, but distinct, hepatitis C viral genotypes exist. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7144–7148. doi: 10.1073/pnas.89.15.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. W., McOmish F., Holmes E. C., Dow B., Peutherer J. F., Follett E., Yap P. L., Simmonds P. Analysis of a new hepatitis C virus type and its phylogenetic relationship to existing variants. J Gen Virol. 1992 May;73(Pt 5):1131–1141. doi: 10.1099/0022-1317-73-5-1131. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Richman K. H., Han J. H., Berger K., Lee C., Dong C., Gallegos C., Coit D., Medina-Selby R., Barr P. J. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Larson R., Belzer S. K., Retzel E. Proteins encoded by bovine viral diarrhea virus: the genomic organization of a pestivirus. Virology. 1988 Jul;165(1):200–208. doi: 10.1016/0042-6822(88)90673-3. [DOI] [PubMed] [Google Scholar]
- Hijikata M., Kato N., Ootsuyama Y., Nakagawa M., Shimotohno K. Gene mapping of the putative structural region of the hepatitis C virus genome by in vitro processing analysis. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5547–5551. doi: 10.1073/pnas.88.13.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N., Hijikata M., Ootsuyama Y., Nakagawa M., Ohkoshi S., Sugimura T., Shimotohno K. Molecular cloning of the human hepatitis C virus genome from Japanese patients with non-A, non-B hepatitis. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9524–9528. doi: 10.1073/pnas.87.24.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanford R. E., Notvall L., Chavez D., White R., Frenzel G., Simonsen C., Kim J. Analysis of hepatitis C virus capsid, E1, and E2/NS1 proteins expressed in insect cells. Virology. 1993 Nov;197(1):225–235. doi: 10.1006/viro.1993.1583. [DOI] [PubMed] [Google Scholar]
- Machida A., Ohnuma H., Tsuda F., Munekata E., Tanaka T., Akahane Y., Okamoto H., Mishiro S. Two distinct subtypes of hepatitis C virus defined by antibodies directed to the putative core protein. Hepatology. 1992 Oct;16(4):886–891. doi: 10.1002/hep.1840160406. [DOI] [PubMed] [Google Scholar]
- Miller R. H., Purcell R. H. Hepatitis C virus shares amino acid sequence similarity with pestiviruses and flaviviruses as well as members of two plant virus supergroups. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2057–2061. doi: 10.1073/pnas.87.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S., Kato N., Yagyu A., Tanaka T., Ikeda Y., Petchclai B., Chiewsilp P., Kurimura T., Shimotohno K. A new type of hepatitis C virus in patients in Thailand. Biochem Biophys Res Commun. 1992 Feb 28;183(1):334–342. doi: 10.1016/0006-291x(92)91648-a. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Kurai K., Okada S., Yamamoto K., Lizuka H., Tanaka T., Fukuda S., Tsuda F., Mishiro S. Full-length sequence of a hepatitis C virus genome having poor homology to reported isolates: comparative study of four distinct genotypes. Virology. 1992 May;188(1):331–341. doi: 10.1016/0042-6822(92)90762-e. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Okada S., Sugiyama Y., Kurai K., Iizuka H., Machida A., Miyakawa Y., Mayumi M. Nucleotide sequence of the genomic RNA of hepatitis C virus isolated from a human carrier: comparison with reported isolates for conserved and divergent regions. J Gen Virol. 1991 Nov;72(Pt 11):2697–2704. doi: 10.1099/0022-1317-72-11-2697. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Sugiyama Y., Okada S., Kurai K., Akahane Y., Sugai Y., Tanaka T., Sato K., Tsuda F., Miyakawa Y. Typing hepatitis C virus by polymerase chain reaction with type-specific primers: application to clinical surveys and tracing infectious sources. J Gen Virol. 1992 Mar;73(Pt 3):673–679. doi: 10.1099/0022-1317-73-3-673. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Tokita H., Sakamoto M., Horikita M., Kojima M., Iizuka H., Mishiro S. Characterization of the genomic sequence of type V (or 3a) hepatitis C virus isolates and PCR primers for specific detection. J Gen Virol. 1993 Nov;74(Pt 11):2385–2390. doi: 10.1099/0022-1317-74-11-2385. [DOI] [PubMed] [Google Scholar]
- Shih C. M., Lo S. J., Miyamura T., Chen S. Y., Lee Y. H. Suppression of hepatitis B virus expression and replication by hepatitis C virus core protein in HuH-7 cells. J Virol. 1993 Oct;67(10):5823–5832. doi: 10.1128/jvi.67.10.5823-5832.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds P., Holmes E. C., Cha T. A., Chan S. W., McOmish F., Irvine B., Beall E., Yap P. L., Kolberg J., Urdea M. S. Classification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS-5 region. J Gen Virol. 1993 Nov;74(Pt 11):2391–2399. doi: 10.1099/0022-1317-74-11-2391. [DOI] [PubMed] [Google Scholar]
- Simmonds P., McOmish F., Yap P. L., Chan S. W., Lin C. K., Dusheiko G., Saeed A. A., Holmes E. C. Sequence variability in the 5' non-coding region of hepatitis C virus: identification of a new virus type and restrictions on sequence diversity. J Gen Virol. 1993 Apr;74(Pt 4):661–668. doi: 10.1099/0022-1317-74-4-661. [DOI] [PubMed] [Google Scholar]
- Stuyver L., Rossau R., Wyseur A., Duhamel M., Vanderborght B., Van Heuverswyn H., Maertens G. Typing of hepatitis C virus isolates and characterization of new subtypes using a line probe assay. J Gen Virol. 1993 Jun;74(Pt 6):1093–1102. doi: 10.1099/0022-1317-74-6-1093. [DOI] [PubMed] [Google Scholar]
- Stuyver L., Van Arnhem W., Wyseur A., DeLeys R., Maertens G. Analysis of the putative E1 envelope and NS4a epitope regions of HCV type 3. Biochem Biophys Res Commun. 1993 Apr 30;192(2):635–641. doi: 10.1006/bbrc.1993.1462. [DOI] [PubMed] [Google Scholar]
- Sällberg M., Rudén U., Wahren B., Magnius L. O. Immunodominant regions within the hepatitis C virus core and putative matrix proteins. J Clin Microbiol. 1992 Aug;30(8):1989–1994. doi: 10.1128/jcm.30.8.1989-1994.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M., Summers J. Phosphorylation of the duck hepatitis B virus capsid protein associated with conformational changes in the C terminus. J Virol. 1994 May;68(5):2965–2969. doi: 10.1128/jvi.68.5.2965-2969.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]



