Abstract
The Brazilian Pampa biome is currently under constant threat due to increase of agriculture and improper management of urban effluents. Studies with a focus on the assessment of impacts caused by human activities in this biome are scarce. In the present study, we measured stress-related biomarkers in tadpoles of the leaf frog Phyllomedusa iheringii, an endemic species to the Pampa biome, and tested its suitability as a bioindicator for the assessment of potential aquatic contamination in selected ponds (S1 and S2) nearby agricultural areas in comparison to a reference site. A significant decrease in acetylcholinesterase activity was observed in S2 when compared to S1 and reference. The levels of total-hydroperoxides were increased in S2 site. In parallel, increased activity of the antioxidant enzymes catalase, superoxide dismutase and glutathione S-transferase were observed in S2 when compared to S1 and reference. Further studies are necessary in order to correlate the changes observed here with different chemical stressors in water, as well as to elucidate mechanisms of toxicity induced by pesticides in amphibian species endemic to the Pampa biome. Nevertheless, our study validates Phyllomedusa iheringii as a valuable bioindicator in environmental studies.
Keywords: Aquatic contamination, Anuran larvae, Biomarkers, Oxidative stress, Cholinesterase
Introduction
The Brazilian Pampa biome, located in the southern Brazil, covers a large grassland territory containing a vast number of endemic species (Bencke, 2009). Currently, this biome has been neglected in terms of environmental protection and conservation of its biodiversity (Roesch et al., 2009). The improper management of urban waste and widespread use of pesticides in monocultures, especially soybeans and rice, are major causes of environmental degradation in this biome (Behling & Pillar, 2007). Up to date studies on risk assessment and biomonitoring are scarce, and the actual impacts of human activities to the Pampa’s environmental quality are poorly understood.
The use of biomarkers in aquatic organisms have been pointed out as an effective approach to obtain information about environmental quality and the potential threats caused by pollutants to the aquatic ecosystem (Viarengo et al., 2007). Biomarkers, by definition, consist in a range of biological responses related to exposure to contaminants and may include molecular, cellular, physiological and behavioral responses (Montserrat, Geracitano & Bianchini, 2003). The measurement of biomarkers at the molecular and cellular levels have been proposed as early hallmarks of exposure to chemical pollutants, thus consisting in reliable and sensitive tools for environmental risk assessment studies (Van der Oost, Beyer & Vermeulen, 2003). For instance, measurements of cholinesterase enzymesand cytochrome P450 (CYP) are considered classical biomarkers, whereas oxidative stress-related parameters, such as antioxidant enzymes and glutathione status are widely used as stress responsive biomarkers (Franco et al., 2010). While cholinesterase enzymes are excellent sensors for aquatic contamination with pesticides including organophosphate and carbamates, CYP proteins are strongly induced during exposure episodes to hazardous organic compounds such as aromatic hydrocarbons (Connon, Geist & Werner, 2012).
Amphibians have a life cycle usually dependent on aquatic and terrestrial ecosystems, highly permeable skin, low mobility, high diversity of reproductive modes, and special physiological requirements, and therefore are often vulnerable to human action (Dunson, Wyman & Corbett, 1992; Tocher, Gascon & Zimmerman, 1997). Despite the vast amount of information regarding biomarkers of aquatic contamination in fish species, little is known about the effects of chemical pollutants on amphibians (Collins & Crump, 2009) and tropical countries as Brazil are no exception (see revision in Kopp et al., 2007). Recently, a marked decline in amphibian populations have been observed and the intensification of habitat loss due to agriculture together with uncontrolled effluent discharges are considered major contributors to the impacts caused by human activities to wild amphibian populations (Collins & Crump, 2009; Orton & Tyler, 2014).
Taking into consideration the scarcity of studies on biomarkers of aquatic pollution within the Pampa biome borders and the limited information about the impacts of chemical pollutants to amphibians, in the present study we aimed to validate the suitability of Phyllomedusa iheringii tadpoles for studies on stress-related biomarkers of aquatic pollution. This species is a leaf frog endemic to the Brazilian and Uruguayan Pampa biome whose reproduction is dependent on ponds currently under high agricultural pressure.
Material and Methods
Chemicals
5,5-dithio-bis(2-nitrobenzoic)acid (DTNB), 1-chloro-2,4-dinitrobenzene (CDNB), acetylthiocholine iodide, quercetin, N,N,N′,N′-Tetramethylethylenediamine (TEMED), 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid (HEPES), xylenol orange were purchased from Sigma-Aldrich (Sigma Aldrich, São Paulo, Brazil). All other chemicals were obtained from highest commercial grade available.
Experimental Animals
In the present study, Phyllomedusa iheringii tadpoles were utilized as bioindicators. The subfamily Phyllomedusinae includes 59 species, of which 30 belongs to the Phyllomedusa genus (Frost, 2014). Phyllomedusa utilizes the vegetation for both vocalization and spawning (Haddad & Prado, 2005; Faivovich et al., 2010). Phyllomedusa iheringii Bouleger, 1885 belongs to the P. burmeisteri group (sensu Lutz 1950) and is a leaf frog endemic to the Uruguayan and Brazilian Pampa, inhabiting forest and grassland ecosystems (Duellman, 1999; Maneyro & Carreira, 2012; Frost, 2014). Reproduction of P. iheringii comprises egg deposition into leaves located above the water, where the female folds and glues the leaves, and after about eight days the eggs hatch, releasing exotrophic tadpoles that drop into lentic waters (mode 24 sensu Haddad & Prado, 2005) (Langone, 1994). Thus, we chose P. iheringii as the model organism because: (1) this species has a prolonged breeding season at the study area, with tadpoles occurring from September to March at the monitored waterbodies (T Santos, 2014, unpublished data); (2) its present high abundance in larval phase, and (3) the larvae are easily sampled and identified.
After being captured, animals were gently transferred to tanks containing water from the capture sites and rapidly transported to the laboratory. No signs of stress or injuries were observed in tadpoles after capturing procedures. Animals were allowed to adapt to the laboratory conditions for at least 3 h under constant aeration and controlled temperature (22 ± 1 °C) before sample preparation. A total of 30 weight and size matched individuals with similar developmental stage (stages 34–36 of Gosner, 1960) was divided in three groups (n = 10) and used for the experiments. The experimental groups were: Reference (Ref), Site 1 (S1) and Site 2 (S2). All experimental procedures utilized in this study involving animals were approved by the university’s ethical committee for the use of experimental animals (CEUA Unipampa protocol 043/2013). Field experiments are approved by the Research Council of the Universidade Federal do Pampa (project number: 9.004.13).
Study area
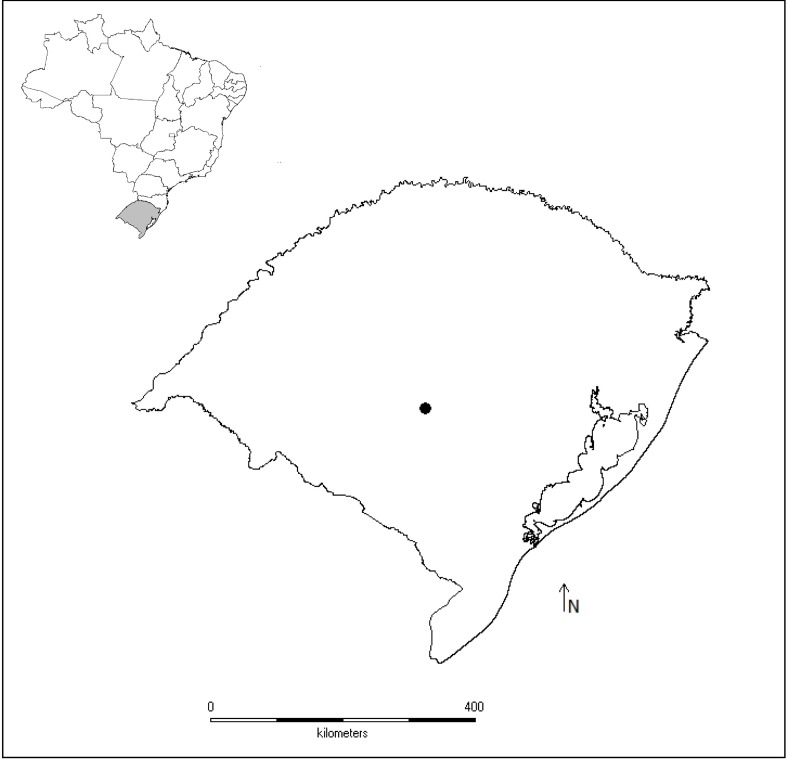
The study was conducted in two ponds of a private area at the municipality of São Sepé, Rio Grande do Sul state, Brazil (Fig. 1). This area belongs to the Planalto Sul-Rio-Grandense (or Serra do Sudeste), a pampean region characterized by rock crystalline shield outcrops covered by a natural mosaic of grassland (Campos) and seasonal forests (IBGE, 2004). Historically, land use in the region was based on cattle raised on natural vegetation, but this economic activity is now being replaced by soybean (summer season) and wheat (winter season) cultivation. The local climate is classified as subtropical wet (Cfaof Köppen–Geiger’ classification) (Peel, Finlayson & McMahon, 2007), with rainfall evenly distributed throughout the year (1200–1600 mm), i.e., with no dry season (Overbeck et al., 2007). Summer temperatures are high (maximum 40 °C), while winter temperatures are low, with median values less than 15 °C during the three months period when frosts are common. Thus, climatic seasonality is mostly determined by variation in temperature and photoperiod (Both et al., 2008).
Figure 1. Map of studied sites.
Map of Brazil highlighting the geopolitical division Rio Grande do Sul state and the municipality of São Sepé (black point), where tadpoles of the leaf frog Phyllomedusa iheringii were studied to access water pollution in the Brazilian Pampa biome by using stress biomarkers.
The studied three ponds (30°15′03.9″S, 53°35′05.1″W, 198 m; 30°15′25.5″S, 53°34′50.6″W, 216 m), one located in a remnant of natural grasslands (reference site), and two ponds surrounded by soybean and wheat along the year (ponds S1 and S2). Ponds S1 and S2 have been historically used to supply tanks of agricultural pesticides with water and in those occasions the chemical reflux is common.
Thiol status and total-hydroperoxides
Muscle tissue was collected, weighed and homogenized in 0.5 M perchloric acid (PCA) and centrifuged at 5,000 g for 5 min at 4 °C, and the supernatant was assayed for glutathione levels in the form of non-protein thiols (NPSH). The pellet was washed 3 times in 0.5 M PCA and re-suspended in 1 ml 0.1 M TRIS/HCl pH 8.0 for determination of protein thiols (PSH). Both NPSH and PSH were measured spectrophotometrically (Cary 60 UV–Vis; Agilent Technologies) at 412 nm (Ellman, 1959). Data were expressed as µmol NPSH or PSH/g wet tissue.
Total-hydroperoxide levels were evaluated through the xylenol orange assay (Gay & Gebicki, 2002), with minor modifications. In short, frog muscle was homogenized in 20 mM HEPES buffer, pH 7.4 and centrifuged at 1,000 g for 10 min at 4 °C. The supernatant was incubated for 30 min in a reaction medium containing 250 mM perchloric acid, 2.5 mM ammonium iron (II) sulfate hexahydrate, and 1 mM xylenol orange. Hydroperoxide levels were determined at 560 nm using hydrogen peroxide as standard.
Enzyme activity
For enzymatic analysis, frog muscle was homogenized in 20 mM HEPES buffer, pH 7.4 and centrifuged at 1,000 g for 10 min at 4 °C and an aliquot of supernatant was used for determination of Acetylcholinesterase (AChE). The remained sample was centrifuged at 20,000 g for 30 min at 4 °C for determination of antioxidant enzymes activity. Acetylcholinesterase activity was assayed by measuring the hydrolysis ratio of acetylthiocholine in the presence of DTNB and formation of thionitrobenzoic acid (Ellman et al., 1961), monitored at 412 nm. The glutathione S-transferase (GST) activity was measured as described by Habig & Jakoby, 1981 using 1-chloro-2,4-dinitrobenzene (CDNB) as a substrate. Activity of catalase (CAT) was measured according to Aebi, 1984 and superoxide dismutase (SOD) was measured following the procedures established by Kostyuk & Potapovich, 1989. Data were expressed as mU/mg total protein. Total protein levels were determined according to Bradford (1976).
Statistical analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Dunnett’s post-hoc test when needed. Results were considered statistically significant when p < 0.05.
Results
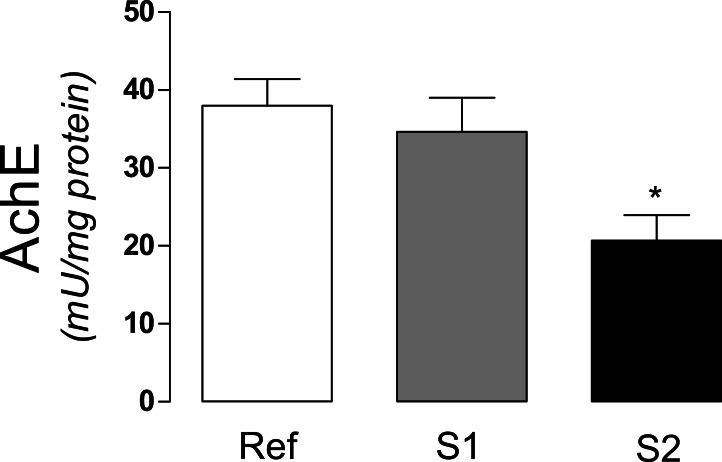
The activity of acetylcholinesterase (AchE), an extensively used biomarker for organophosphate and carbamate pesticides was significantly decreased (p < 0.0163) in S2, when compared to S1 and reference sites (Fig. 2).
Figure 2. Acetylcholinesterase activity in tadpoles.
Acetylcholinesterase activity (AchE) in tadpoles (Phyllomedusa iheringii) captured in the Brazilian Pampa biome sites. Data are expressed as Mean ± SD of enzyme activity (mU/mg of total protein). ∗p < 0.05 when compared to reference site (control).
We measured thiol status, as non-protein (NPSH) and protein (PSH) thiols and lipid hydroperoxides in order to evaluate a potential oxidative stress condition in tadpoles muscle tissue (Fig. 3). According to the data, a significant (p < 0.0044) increase in hydroperoxide levels (Fig. 3A) was observed in S2 site while NPSH and PSH (Figs. 3A and 3B) was not significantly changed. Notwithstanding, a trend (p = 0.0588) to decrease NPSH level in S2 was observed.
Figure 3. Thiol status and hydroperoxide leves in tadpoles.
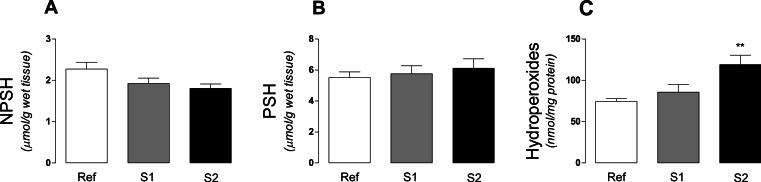
(A) Non-protein thiols, (B) protein thiols and (C) total-hydroperoxide content in tadpoles (Phyllomedusa iheringii) captured in the Brazilian Pampa biome sites. Data are expressed as Mean ± SD of thiol content (µmol/g of wet tissue) and hydroperoxide levels (nmol/mg protein). ∗p < 0.05 when compared to reference site (control).
Regarding the activity of antioxidant enzymes, a significant (p < 0.0028) increase of catalase (CAT) activity was observed at S2 (Fig. 4A). Superoxide dismutase (SOD) activity was also substantially enhanced (p < 0.001) at S2 when compared to S1 and reference (Fig. 4B). The glutathione S-transferase (GST) activity was significantly (p < 0.00239) increased in the muscle of tadpoles captured at S2 site, when compared to both S1 and reference (Fig. 4C).
Figure 4. Antioxidant enzymes in tadpoles.
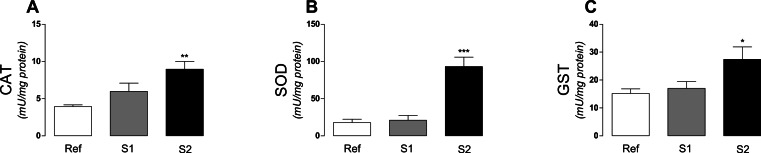
Enzymatic activity of (A) CAT, (B) SOD and (C) GST in tadpoles (Phyllomedusa iheringii) captured in the Brazilian Pampa biome sites. Data are expressed as Mean ± SD of enzyme activity (mU/mg of total protein). ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001 when compared to reference site (control).
Discussion
Up to date, little is known about the adverse influences of human activities on the overall environmental quality of the Pampa biome. Studies on biomarkers of water contamination are limited, and the development of suitable risk assessment protocols have been neglected for decades. In the present study, by measuring changes well established biomarkers of water pollution, we validated the use of Phyllomedusa iheringii tadpoles, an endemic frog species to the Pampa biome, as a valuable tool for the evaluation of harms caused by human activities to aquatic ecosystems and wild life equilibrium. By observing changes in classical biomarkers, such as acetylcholinesterase activity along with xenobiotics/oxidative stress related parameters in tadpoles sampled in ponds with historical records of agricultural use, we drawn attention for the potential presence of pesticides at harmful levels at the studied area.
Pesticides have widespread application and are believed to have relative benign effects on non-target species, but these risks are often not studied at relevant ecological, spatial or temporal scales (Boone & Bridges, 2003). Acetylcholinesterase (AChE) is a classic biomarker for the presence of sublethal concentrations of organophosphorous and carbamate compounds, which are widely used for pest control and can reach water streams through agricultural and urban releases (Viarengo et al., 2007). Several studies using fish species have shown inhibition of cholinesterase activity in the presence of organophosphate compounds in water (Sancho, Ferrando & Andreu, 1997; Dutta & Arends, 2003). In addition to organophosphate and carbamates, other agricultural compounds such as glyphosate are shown to inhibit AchE in fish (Moraes et al., 2007). Regarding organophosphate compounds in frogs, recent studies have shown the accumulation of such compounds in amphibians (Kittusamy et al., 2014). Glyphosate is also shown to inhibit AchE in frogs (Ruamthum et al., 2011). Even though the effects of AchE inhibitors have been demonstrated in amphibian experimental models in vitro and in vivo (Gungordu & Uckun, 2014), few studies have been undertaken in order to address the impacts of such pollutants in situ, especially to wild frog species. In the present study, we found a significant decrease in the activity of AchE in tadpoles captured in ponds utilized for crop activities in the Brazilian Pampa biome. The two ponds (S1 and S2) in which tadpoles were captured are constantly used for farm irrigation purposes, a fact that potentially increases the probability of pesticide releases into the water. The observed decrease in AchE activity in tadpole muscle tissue may reflect the presence of cholinesterase inhibitors at harmful levels, mainly at the S2 site. In line with this hypothesis, Table 1 shows the most utilized agrochemicals (in descending order) used in the Rio Grande do Sul state, according to Brazilian regulatory agencies (Barreto, Herman & Garibotti, 2012). Among them, four compounds are AchE inhibitors, as organophosphates (acephate and methamidophos), glycine analogs (glyphosate) and carbamates (carbofuran). Although measurements of pesticides concentrations in the selected ponds were not undertaken here, the historical records of land use may suggest that one or more of such compounds may be responsible for the inhibitory effect towards AchE in tadpoles of Phyllomedusa iheringii.
Table 1. Agrochemicals.
List of most commonly used agrochemicals in Rio Grande do Sul State, Brazil (Adapted from Barreto, Herman & Garibotti, 2012).
| Agrochemical | Chemical group |
|---|---|
| Glyphosate | Glycine analogue |
| Acephate | Organophosphate |
| Difenoconazole | Triazole |
| Methamidophos | Organophosphate |
| Metalaxyl | Phenylamide |
| Cypermethrin | Pyrethroid |
| Diflubenzuron | Benzamide |
| Carbofuran | Carbamate |
Oxidative stress, which is defined as an unbalance between pro- and antioxidants in organisms (Halliwell & Gutteridge, 2007) has been shown as an important mechanism of toxicity of environmental contaminants, including AchE inhibitors (Karami-Mohajeri & Abdollahi, 2011; Lushchak, 2011; Hellou, Ross & Moon, 2012). The presence of persistent organic pollutants in water may result in induction of reactive oxygen species (ROS) and consequently, oxidative stress in aquatic organisms (Lushchak, 2011; Hellou, Ross & Moon, 2012). Then, observing changes in oxidative stress-related parameters and use them as biomarkers of exposure to contaminants in aquatic of semi-aquatic animals may represent a valuable tool for the assessment of environmental quality.
Under oxidative stress conditions, a cellular adaptive response may take place in order to counteract the deleterious effects of oxidative stress (Lushchak, 2011; Schulke et al., 2012). The adaptive response to oxidative challenges is mediated by the transcription factor Nrf2 through the antioxidant response element (ARE). Once oxidative stress signals are generated, Nrf2 triggers the transcription of endogenous antioxidant enzymes as glutathione S-transferase (GST), glutathione peroxidase (GPx), glutathione reductase (GR), superoxide dismutase (SOD), catalase (CAT), NAD(P)H oxidase, NADPH quinine oxidoreductase (NQO-1), glutamate-cysteine ligase (GCL) and thioredoxin system through its binding to the DNA sites known as the antioxidant responsive element “ARE” (Lushchak, 2011; Schulke et al., 2012). We observed a significant increase in the activity of CAT, SOD and GST in tadpoles captured at S2 site (Fig. 4). GST activity is related to detoxification of xenobiotic compounds being widely used as a biomarker for environmental exposures to exogenous compounds (Stegeman & Lech, 1991; Hellou, Ross & Moon, 2012). The increase in GST activity can be interpreted as an adaptive response in order to eliminate toxic xenobiotics present in water and potentially accumulating in tadpoles muscle tissue. The increase in CAT and SOD, which are primary enzymes in the control of intracellular ROS levels (Halliwell & Gutteridge, 2007) may also be interpreted as an adaptive response in tadpoles to survive under the presence of oxidative stressors, corroborating previous literature reports (Clasen et al., 2012).
The activity of antioxidant enzymes, the amount of thiol groups and markers of ROS-induced damage to biomolecules are frequently used for monitoring the presence of pro-oxidant compounds in aquatic environments (Trevisan et al., 2013). The levels of thiols, both in proteins (PSH) or low molecular weight compounds (NPSH), is an indicative of the antioxidant capacity of the organism (Reischl et al., 2007). Markers of oxidative damage to biomolecules, such as lipid peroxidation by-products can be environmentally induced in aquatic organisms (Lushchak, 2011). We observed a significant increase in lipid hydroperoxides and a trend to decrease NPSH levels. NPSH represents an index of cellular levels of glutathione (GSH), a key low-molecular ROS scavenger in living organisms (Ellman, 1959; Lushchak, 2011). Together, these results are indicative of a pro-oxidative condition in which tadpoles are exposed in the studied sites. Pesticides that inhibit AchE can influence amphibian by direct and/or indirect effects, such as paralysis, reduction of foraging, increase predation rates, as well as prohibit or delay metamorphosis and cause death (see review in Boone & Bridges, 2003). In this way, we suggest that future studies should address how chemical stressors interact with abiotic (temperature, pH, ultraviolet light) and biotic (competition and predation) environmental factors, and to measure possible effects of these contaminants on population juveniles recruitment.
Overall, by using tadpoles as bioindicators we observed changes in biomarkers related to the presence of cholinesterase inhibitors and oxidative stress inducers in the studied sites. Since amphibians are especially prone to the adverse effects of water contaminants, further studies are necessary in order to correlate the changes observed here with different chemical stressors in water, as well as to elucidate mechanisms of toxicity induced by pesticides in amphibian species endemic to the Pampa biome. Nevertheless, our study validates Phyllomedusa iheringii as a valuable bioindicator in environmental studies.
Supplemental Information
Funding Statement
CNPq, FAPERGS and Unipampa provided financial support. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
TG Santos conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, reviewed drafts of the paper.
R Melo, DG Costa-Silva and MEM Nunes performed the experiments.
NR Rodrigues reviewed drafts of the paper, revised the manuscript.
JL Franco conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
CEUA Unipampa protocol 043/2013.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
Field experiments are approved by the Research Council of the Universidade Federal do Pampa (project number: 9.004.13).
References
- Aebi (1984).Aebi H. Catalase in vitro. Methods in Enzymology. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Barreto, Herman & Garibotti (2012).Barreto S, Herman L, Garibotti V. Levantamentos dos agrotóxicos usados no Rio Grande do Sul por Bacia Hidrográfica. Boletim Epidemológico. 2012;14:3–6. [Google Scholar]
- Behling & Pillar (2007).Behling H, Pillar VD. Late Quaternary vegetation, biodiversity and fire dynamics on the southern Brazilian highland and their implication for conservation and management of modern Araucaria forest and grassland ecosystems. Philosophical Transactions of the Royal Society of London Biological Sciences. 2007;362:243–251. doi: 10.1098/rstb.2006.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencke (2009).Bencke GA. Diversidade e conservação da fauna dos campos do Sul do Brasil. In: Pillar VP, Müller SC, Castilhos ZMS, Jacques AVA, editors. Campos Sulinos, conservação e uso sustentável da biodiversidade. Brasília: MMA; 2009. pp. 101–121. [Google Scholar]
- Boone & Bridges (2003).Boone MD, Bridges CM. Effects of pesticides on amphibian populations. In: Smlitsch RD, editor. Amphibian conservation. Washington, D.C.: Smithsonian Institution; 2003. pp. 152–167. [Google Scholar]
- Both et al. (2008).Both C, Kaefer IL, Santos TG, Cechin SZ. An austral anuran assemblage in the Neotropics: seasonal occurrence correlated with photoperiod. Journal of Natural History. 2008;42(3–4):205–222. doi: 10.1080/00222930701847923. [DOI] [Google Scholar]
- Bradford (1976).Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clasen et al. (2012).Clasen B, Loro VL, Cattaneo R, Moraes B, Lopes T, de Avila LA, Zanella R, Reimche GB, Baldisserotto B. Effects of the commercial formulation containing fipronil on the non-target organism Cyprinus carpio: implications for rice-fish cultivation. Ecotoxicology and Environmental Safety. 2012;77:45–51. doi: 10.1016/j.ecoenv.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Collins & Crump (2009).Collins JP, Crump ML. Extinction in our times. New York: Oxford University Press; 2009. [Google Scholar]
- Connon, Geist & Werner (2012).Connon RE, Geist J, Werner I. Effect-based tools for monitoring and predicting the ecotoxicological effects of chemicals in the aquatic environment. Sensors (Basel) 2012;12:12741–12771. doi: 10.3390/s120912741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duellman (1999).Duellman WE. Distribution patterns of amphibians in South America. In: Duellman WE, editor. Patterns of distribution of amphibians: a global perspective. Baltimore and London: Johns Hopkins University Press; 1999. pp. 255–328. [Google Scholar]
- Dunson, Wyman & Corbett (1992).Dunson WA, Wyman RL, Corbett ES. A symposium on the amphibians declines and habitat acidification. Journal of Herpetology. 1992;26(4):349–352. doi: 10.2307/1565110. [DOI] [Google Scholar]
- Dutta & Arends (2003).Dutta HM, Arends DA. Effects of endosulfan on brain acetylcholinesterase activity in juvenile bluegill sunfish. Environmental Research. 2003;91:157–162. doi: 10.1016/S0013-9351(02)00062-2. [DOI] [PubMed] [Google Scholar]
- Ellman (1959).Ellman GL. Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Ellman et al. (1961).Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Faivovich et al. (2010).Faivovich J, Haddad CFB, Baeta D, Jungfer KH, Alvares GFR, Brandao RA, Bario-Amoros CL, Cruz CG, Wheeler WC. The phylogenetics relationships of Phyllomedusinae (Anura, Hylidae): a group of poster frogs. Cladistics. 2010;26(2010):227–261. doi: 10.1111/j.1096-0031.2009.00287.x. [DOI] [PubMed] [Google Scholar]
- Franco et al. (2010).Franco JL, Trevisan R, Posser T, Trivella DBB, Hoppe R, Rosa JM, Dinslaken DF, Decker H, Tasca CI, Leal RB, Marques MRF, Bainy ACD, Dafre AL. Biochemical alterations in caged Nile tilapia Oreochromis niloticus. Ecotoxicology and Environmental Safety. 2010;73:864–872. doi: 10.1016/j.ecoenv.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Frost (2014).Frost DR. Amphibian Species of the World [online] 2014. Available at http://research.amnh.org/herpetology/amphibia/index.html (accessed 28 March 2014)
- Gay & Gebicki (2002).Gay CA, Gebicki JM. Perchloric acid enhances sensitivity and reproducibility of the ferric-xylenol orange peroxide assay. Analytical Biochemistry. 2002;304:42–46. doi: 10.1006/abio.2001.5566. [DOI] [PubMed] [Google Scholar]
- Gosner (1960).Gosner KL. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica. 1960;16:183–190. [Google Scholar]
- Gungordu & Uckun (2014).Gungordu A, Uckun M. Comparative assessment of in vitro and in vivo toxicity of azinphos methyl and its commercial formulation. Environ Toxicol. 2014 doi: 10.1002/tox.21982. Epub ahead of print Mar 11 2014. [DOI] [PubMed] [Google Scholar]
- Habig & Jakoby (1981).Habig WH, Jakoby WB. Assays for differentiation of glutathione S-transferases. Methods in Enzymology. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Haddad & Prado (2005).Haddad CFB, Prado CPA. Reproductive modes in frogs and their unexpected diversity in the Atlantic Forest of Brazil. BioScience. 2005;55(3):207–217. doi: 10.1641/0006-3568(2005)055[0207:RMIFAT]2.0.CO;2. [DOI] [Google Scholar]
- Halliwell & Gutteridge (2007).Halliwell B, Gutteridge J. Free radicals in biology and medicine. 4th edition. Oxford: Oxford University Press; 2007. p. 851. [Google Scholar]
- Hellou, Ross & Moon (2012).Hellou J, Ross NW, Moon TW. Glutathione, glutathione S-transferase, and glutathione conjugates, complementary markers of oxidative stress in aquatic biota. Environmental Science and Pollution Research International. 2012;19:2007–2023. doi: 10.1007/s11356-012-0909-x. [DOI] [PubMed] [Google Scholar]
- IBGE (2004).IBGE Mapa de Biomas do Brasil. Primeira aproximação [online] 2004. Available at http://www2.ibge.gov.br/download/mapas_murais/biomas_pdf.zip (accessed 16 March 2014)
- Karami-Mohajeri & Abdollahi (2011).Karami-Mohajeri S, Abdollahi M. Toxic influence of organophosphate, carbamate, and organochlorine pesticides on cellular metabolism of lipids, proteins, and carbohydrates: a systematic review. Human and Experimental Toxicology. 2011;30:1119–1140. doi: 10.1177/0960327110388959. [DOI] [PubMed] [Google Scholar]
- Kittusamy et al. (2014).Kittusamy G, Kandaswamy C, Kandan N, Subramanian M. Pesticide residues in two frog species in a paddy agroecosystem in Palakkad district, Kerala, India. Bulletin of Environmental Contamination and Toxicology. 2014;93:728–734. doi: 10.1007/s00128-014-1351-1. [DOI] [PubMed] [Google Scholar]
- Kopp et al. (2007).Kopp K, Antoniosi Filho NR, Alves MIR, Bastos RP. Publicações sobre efeitos de pesticidas em anfíbios no período de 1980 a 2007. Multiciência. 2007;8:173–186. [Google Scholar]
- Kostyuk & Potapovich (1989).Kostyuk VA, Potapovich AI. Superoxide–driven oxidation of quercetin and a simple sensitive assay for determination of superoxide dismutase. Biochemistry International. 1989;19:1117–1124. [PubMed] [Google Scholar]
- Langone (1994).Langone JA. Ranas y sapos del Uruguay (reconocimiento y aspectos biológicos) (Serie Divulgacion).Museo Damaso Antonio Larrañaga. 1994;5:1–123. Available at http://www.academia.edu/626066/Ranas_y_sapos_del_Uruguay_reconocimiento_y_aspectos_biol%C3%B3gicos . [Google Scholar]
- Lushchak (2011).Lushchak VI. Environmentallyinducedoxidative stress in aquaticanimals. Aquatic Toxicology. 2011;101:13–30. doi: 10.1016/j.aquatox.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Maneyro & Carreira (2012).Maneyro R, Carreira S. Guía de anfíbios del Uruguay. Montevideo, Uy: Ediciones de la Fuga; 2012. [Google Scholar]
- Montserrat, Geracitano & Bianchini (2003).Montserrat JM, Geracitano LA, Bianchini A. Current and future perspectives using biomarkers to assess pollution in aquatic ecosystems. Comments on Toxicology. 2003;9:255–269. doi: 10.1080/08865140390450359. [DOI] [Google Scholar]
- Moraes et al. (2007).Moraes BS, Loro VL, Glusczak L, Pretto A, Menezes C, Marchezan E, de Oliveira Machado S. Effects of four rice herbicides on some metabolic and toxicology parameters of teleost fish (Leporinusobtusidens) Chemosphere. 2007;68:1597–1601. doi: 10.1016/j.chemosphere.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Orton & Tyler (2014).Orton F, Tyler CR. Do hormone-modulating chemicals impact on reproduction and development of wild amphibians? Biological Reviews. 2014 doi: 10.1111/brv.12147. Epub ahead of print October 22 2014. [DOI] [PubMed] [Google Scholar]
- Overbeck et al. (2007).Overbeck GE, Müller SC, Fidelis A, Pfadenhauer J, Pillar VD, Blanco CC, Boldrini II, Both R, Forneck ED. Brazil’s neglected biome: the South Brazilian Campos. 2007;9:101–116. [Google Scholar]
- Peel, Finlayson & McMahon (2007).Peel MC, Finlayson BL, McMahon TA. Updated world map of the Köppen–Geiger climate classification. Hydrology and Earth System Sciences. 2007;11:1633–1644. doi: 10.5194/hess-11-1633-2007. [DOI] [Google Scholar]
- Reischl et al. (2007).Reischl E, Dafre AL, Franco JL, Wilhelm Filho D. Distribution, adaptation and physiological meaning of thiols from vertebrate hemoglobins. Comparative Biochemistry and Physiology—Part C: Toxicology & Pharmacology. 2007;146:22–53. doi: 10.1016/j.cbpc.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Roesch et al. (2009).Roesch LF, Vieira F, Pereira V, Schünemann AL, Teixeira I, Senna AJ, Stefenon VM. The Brazilian pampa: a fragile biome. Diversity. 2009;1:182–198. doi: 10.3390/d1020182. [DOI] [Google Scholar]
- Ruamthum et al. (2011).Ruamthum W, Visetson S, Milne JR, Bullangpoti V. Effect of glyphosate-based herbicide on acetylcholinesterase activity in tadpoles. Hoplobatrachusrugulosus. Communication in Agricultural and Applied Biological Sciences. 2011;76:923–930. [PubMed] [Google Scholar]
- Sancho, Ferrando & Andreu (1997).Sancho E, Ferrando MD, Andreu E. Response and recovery of brain acetylcholinesterase activity in the European eel, Anguilla anguilla, exposed to fenitrothion. Ecotoxicology and Environmental Safety. 1997;38:205–209. doi: 10.1006/eesa.1997.1579. [DOI] [PubMed] [Google Scholar]
- Schulke et al. (2012).Schulke S, Dreidax D, Malik A, Burmester T, Nevo E, Band M, Avivi A, Hankeln T. Living with stress: regulation of antioxidant defense genes in the subterranean, hypoxia-tolerant mole rat. Spalax. Gene. 2012;500:199–206. doi: 10.1016/j.gene.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Stegeman & Lech (1991).Stegeman JJ, Lech JJ. Cytochrome P-450 monooxygenase systems in aquatic species: carcinogen metabolism and biomarkers for carcinogen and pollutant exposure. Environmental Health Perspectives. 1991;90:101–109. doi: 10.2307/3430851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocher, Gascon & Zimmerman (1997).Tocher MD, Gascon C, Zimmerman BL. Fragmentation effects on a central Amazonian frog community: a ten-year study. In: Lawrence WF, Bierregaard JRRO, editors. Tropical forests remnants: ecology, management and conservation of fragmented communities. 1st edition. Chicago: University of Chicago Press; 1997. pp. 124–137. [Google Scholar]
- Trevisan et al. (2013).Trevisan R, Uliano-Silva M, Franco JL, Posser T, Hoppe R, Farina M, Bainy AC, Dafre AL. Confinement during field studies may jeopardize antioxidant and physiological responses of Nile tilapia to contaminants. Marine Environmental Research. 2013;91:97–103. doi: 10.1016/j.marenvres.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Van der Oost, Beyer & Vermeulen (2003).Van der Oost R, Beyer J, Vermeulen NP. Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environmental Toxicology and Pharmacology. 2003;13:57–149. doi: 10.1016/S1382-6689(02)00126-6. [DOI] [PubMed] [Google Scholar]
- Viarengo et al. (2007).Viarengo A, Lowe D, Bolognesi C, Fabbri E, Koehler A. The use of biomarkers in biomonitoring: a 2-tier approach assessing the level of pollutant-induced stress syndrome in sentinel organisms. Comparative Biochemistry and Physiology—Part C: Toxicology & Pharmacology. 2007;146(3):281–300. doi: 10.1016/j.cbpc.2007.04.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.