Abstract
Objective
During normal pregnancy, studies have shown increased activity of the renin-angiotensin-aldosterone system (RAAS) and a dissociation of plasma renin activity (PRA) and aldosterone (Aldo) evidenced by a greater increase in Aldo relative to PRA. The aims of this study were to examine the RAAS response to stimulation by upright posture and suppression by saline infusion and to investigate the PRA-Aldo dissociation under these two conditions.
Methods
We studied 24 healthy normotensive women (mean ± standard error of mean, ages 29 ± 1 yrs) in sodium (Na) balance in the second and third trimesters and postpartum. Subjects underwent a 24-hour urine collection which was analyzed for Na, norepinephrine (NE), epinephrine (Epi), and dopamine (DA); a posture study with analysis of blood pressure (BP), PRA, Aldo, NE, Epi, DA, and cortisol; and a 0.9% NaCl infusion study (500 mL/hr for 3 hrs) with analysis of BP, PRA, Aldo, cortisol, and digitalis-like factor (DLF). Analyses included paired t tests to compare posture and saline responses, repeated measures to compare across periods, and percent change to evaluate the PRA-Aldo dissociation.
Results
During pregnancy, PRA, Aldo, BP, catecholamines, and cortisol levels were significantly greater in upright than left lateral decubitus (LLD) posture, and the percent change in Aldo was significantly greater than the percent change in PRA. During pregnancy in response to saline infusion, BP did not change; the PRA and Aldo significantly decreased; the percent change in Aldo was significantly greater than the percent change in PRA in the second trimester; and serum DLF and cortisol levels significantly decreased.
Conclusions
In longitudinally studied normal pregnancy, PRA and Aldo levels were dissociated at baseline, with stimulation and, to a lesser degree, with suppression. Norepinephrine, adrenocorticotrophic hormone, and DLF may contribute to this dissociation, and clarification of these interactions may provide insight into the regulation of aldosterone during normal and hypertensive pregnancy.
Keywords: Renin-angiotensin-aldosterone system, Pregnancy, Catecholamines, Digitalis-like factor, Cortisol
INTRODUCTION
Normal pregnancy is characterized by increased activity of the renin-angiotensin-aldosterone system [RAAS; (1–4)]. Prior-studies demonstrated that, not only are levels of the RAAS hormones increased, but that these hormones maintain responsiveness to changes in posture (5–7), oral sodium intake (8, 9), and saline infusion (7, 10–12). Studies have shown that the plasma renin activity (PRA) and plasma aldosterone (Aldo) relationship is dissociated during pregnancy, such that Aldo is increased to a greater degree than PRA during both the second (13, 14) and third (13–15) trimesters. This dissociation has also been observed with upright posture, a stimulator of the RAAS (16), and saline infusion, a suppressor of the RAAS (17). The dissociation suggests that either pregnancy confers greater sensitivity of the adrenal aldosterone response to changes in PRA (mediated by angiotensin II) or factors other than the RAAS hormones contribute to aldosterone secretion in pregnancy. Factors that have been implicated in affecting aldosterone release outside of pregnancy include potassium (18, 19), catecholamines (20–24), adrenocorticotrophic hormone [ACTH; (25)], and digitalis-like factor [DLF; (26–28)]. Dopamine may also play a role in the modulation of aldosterone levels, independently of angiotensin II (AngII) and potassium (29). The roles these factors play in regulating aldosterone release during normal pregnancy, as well as any potential interaction with each other, have not been elucidated.
Because there has not been a comparison of both RAAS stimulation and suppression in the same pregnant women in sodium balance, we conducted a systematic study of the baseline and dynamic responses of the RAAS during normal pregnancy. To help clarify the potential differences in PRA and Aldo response, we simultaneously investigated potassium, catecholamines, and cortisol (as a proxy for ACTH activity) in response to acute changes in volume both during stimulation by upright posture and suppression by saline infusion. DLF response to saline infusion was also measured. To test the hypothesis that the RAAS system is functionally modified during pregnancy in the setting of posture and volume manipulation, we measured the levels of these factors, as well as PRA and Aldo, simultaneously in the same women during the course of one uncomplicated pregnancy and postpartum period. In addition, given the wide range of normal values for PRA during pregnancy, each woman was brought into sodium balance on a diet standardized for sodium and potassium intake and served as her own control to reduce variability and optimize data reliability.
METHODS
Study Population
Twenty-five normotensive women were recruited at random from the antenatal clinical offices at the Brigham and Women’s Hospital. They were enrolled in the study prior to 20 weeks gestational age (GA) and fulfilled the entry criteria of an uncomplicated pregnancy at the initiation of study, no medication use except prenatal vitamins, and no current medical illness, including hypertension, diabetes, or nephropathy. One woman who developed pregnancy-induced hypertension was excluded from the analyses. The remaining 24 women had uncomplicated pregnancies.
The Human Research Committee at the Brigham and Women’s Hospital approved the study protocol, and all women provided informed written consent prior to participation. Pre-pregnancy weight, height, and normal blood pressure measurements before enrollment were confirmed by investigator review of prenatal medical records. The subjects underwent the study protocol between 19 and 23 weeks GA in the second trimester and between 32 and 37 weeks GA in the third trimester. The postpartum studies were performed between 6 weeks and one year after delivery, and none of the subjects was pregnant at the time of the postpartum study. One subject declined participation in the saline infusion study protocol and a second subject was unable to return for the postpartum studies. In addition, three subjects had begun oral contraceptive use at the time of the postpartum study and their data were examined separately to identify any potential impact on the statistical analyses.
Posture Study
Prior to the study, women were placed on a standardized diet for 2 days containing 100 mEq/day of sodium and 100 mEq/day of potassium. On the second of these days, a 24-hour urine collection was obtained and analyzed for volume, sodium, potassium, creatinine, epinephrine, norepinephrine, and dopamine. On the morning of the third day, the subjects arrived at the General Clinical Research Center (GCRC) and were provided a breakfast standardized for sodium and potassium. An intravenous catheter was placed in each arm: one for blood drawing and the second for infusion, and the subject was instructed to remain upright (sitting) for 30 minutes. At the end of this time, blood was drawn for PRA, aldosterone, epinephrine, norepinephrine, dopamine, and cortisol. The subject then assumed the left lateral decubitus (LLD) position and, after 60 minutes, blood was re-drawn for these same hormones. Blood pressure was measured using a Dinamap™ automated blood pressure monitor (Critikon, Inc., Tampa, FL) in the upright position and the end of the 30 minutes (in the LLD position). The LLD time point served as the “baseline” time point both for the posture and saline studies.
Saline Study
While remaining in the LLD position, 0.9% NaCl was infused at a rate of 500 mL/hour for 3 hours. Blood was drawn and assayed for sodium, creatinine, potassium, PRA, aldosterone, cortisol, and DLF at time 0 and 180 minutes. Blood pressure was measured at baseline and end of infusion using a Dinamap™ automated blood pressure monitor (Critikon, Inc., Tampa, FL).
Laboratory Assays
Blood samples were placed immediately on ice and centrifuged in a refrigerated centrifuge at 2000 RPM for 15 minutes (4°). The plasma was then frozen at −70°C until assays were performed. Autoanalyzers were used to measure urine and plasma sodium, potassium (Nova 1; Nova Biomedical, Waltham, MA) and creatinine (Beckman Creatinine Analyzer II, Beckman Instruments, Fullerton, CA). The PRA was determined by the GammaCoat® [I125] Plasma Renin Activity Radioimmunoassay (RIA) Kit following the manufacturer’s protocol (Diasorin, Inc., Stillwater, MN). The Aldo was determined by the Coat-a-Count® Aldosterone RIA following the manufacturer’s protocol (Diagnostic Products Corporation, Los Angeles, CA). Cortisol assessment was performed according to the manufacturer’s protocol using the GammaCoat® [I125] Cortisol RIA Kit (Incstar Corporation, Stillwater, MN).
The blood for catecholamines was collected in 5 mM solution of EGTA/reduced glutathione. The plasma catecholamines were assayed using a modification of the method described by Peuler and Johnson (30). The urine catecholamines were assayed using high-performance liquid chromatography analysis. DLF was measured as the percent inhibition of [Na, K] ATPase hydrolytic activity as described by Graves et al. (27).
Statistical Analysis
The SPSS version 9.0.0 for Windows (1989, Chicago, IL) statistical package was used to perform these analyses. Data are expressed as the mean ± standard error of the mean (SEM). A p value < 0.05 was considered statistically significant. The data were tested for normal distribution using the Shapiro-Wilk Test.
The mean differences in posture study parameters (change from the LLD to the upright position) or saline study parameters (baseline to end of infusion) were compared using the Student’s paired samples t test for normally distributed data; otherwise, the Wilcoxon Rank Sum Test was used. Comparisons of second trimester, third trimester, and postpartum data were made using the General Linear Model repeated measures analysis with post hoc pairwise comparisons tested using Fisher’s protected least square difference when Mauchley’s W p value was insignificant; otherwise, Bonferroni adjustments were made when Mauchley’s Test of Sphericity was violated. The multiparous versus nulliparous groups and the lactating versus non-lactating groups were compared using the Student’s independent samples t test. Two methods of comparing the data were used to address the potential confounding of comparing data with different baselines: the absolute value was used when comparing the numerical data; and the percent change was used to examine the PRA and Aldo relationship, and it was calculated as the baseline value minus the endpoint value then this difference was divided by the baseline value.
RESULTS
Subject Demographics
The demographic data of the 24 subjects are summarized in Table 1. The majority of the subjects were Caucasian and all had singleton pregnancies. Of the 24 women who were studied postpartum, 9 were breastfeeding, 9 had resumed menses, and 3 were using oral contraceptives. The nulliparous (n = 15) and multiparous (n = 9) subjects were compared and the baseline demographic and clinical characteristics between the groups did not differ (data not shown). There were also no statistically significant differences among the hormonal parameters based on parity, so the nulliparous and multiparous subjects’ data were combined and analyzed as one group. In addition, the women who were lactating postpartum (n = 9) were compared against those who were not lactating (n = 15) and no significant differences were observed on RAAS measurements between the two groups (data not shown).
Table 1.
Subject demographics.
| All subjects (n = 24) | |
|---|---|
| Age (years) | 29 ± 1 |
| Nulliparous subjects (n) | 15 |
| Caucasian (n) | 22 |
| Pre-pregnancy weight (kg) | 64.1 ± 3.2 |
| Pre-pregnancy body mass index (kg/m2) | 24.0 ± 1.2 |
| Study date (weeks) | |
| Second trimester | 21.3 ± 0.2 |
| Third trimester | 34.0 ± 0.2 |
| Postpartum | 15.2 ± 1.7 |
Data are mean ± SEM.
The women who were using oral contraceptives at the time of the postpartum study (n = 3) had completed one cycle of pills or less at the time of study. The distribution of these subjects’ RAAS hormone data was examined and was found to overlap the data obtained from the other subjects. The RAAS hormone analyses were also performed with and without these women included in the analyses and the results were not significantly different. Subsequently, these women’s data were maintained in the postpartum analyses to preserve uniformity of the comparisons across the three study periods.
24-Hour Urine Collection
The sodium content was evaluated in the context of the uniform dietary intake of 100 mEq of sodium for the 2 days prior to the study. All of the subjects were determined to be in sodium balance because the mean content of urinary sodium in each study period did not differ significantly from the 100 mEq of sodium ingested: second trimester (2T), 97 ± 7; third trimester (3T), 101 ± 7; postpartum (PP), 91 ± 6 mEq (Table 2). Urine potassium and creatinine were both greater during pregnancy than postpartum, with third trimester values greater than second trimester values in each instance. Norepinephrine values were significantly greater in pregnancy (2T, 44 ± 6 ρg/mL, p < 0.05; 3T, 35 ± 4 ρg/mL, p = 0.05) than postpartum (PP, 28 ± 4 ρg/mL), while epinephrine and dopamine values were not significantly different across study periods.
Table 2.
Baseline data–24-hour urine.
| Second trimester (2T) | Third trimester (3T) | Postpartum (PP) | |
|---|---|---|---|
| Volume (mL) | 1657 ± 106* | 1766 ± 142* | 1352 ± 145 |
| Sodium (mEq) | 97 ± 7 | 101 ± 7 | 91 ± 6 |
| Potassium (mEq) | 67 ± 3* | 70 ± 4* | 57 ± 4 |
| Creatinine (mg) | 1125 ± 39* | 1150 ± 40* | 1031 ± 46 |
| Norepinephrine (ρg/mL) | 44 ± 6* | 35 ± 4 | 28 ± 4 |
| Epinephrine (ρg/mL) | 8 ± 1 | 5 ± 0 | 5 ± 0 |
| Dopamine (ρg/mL) | 293 ± 41 | 240 ± 21 | 251 ± 36 |
Data are mean ± SEM.
p < 0.05 pregnancy vs. PP.
Posture Study
LLD data are shown in Table 3 and the data after upright posture are shown in Table 4. There were no significant differences in the systolic blood pressure (SBP) across pregnancy and postpartum periods, and the SBP and diastolic blood pressures (DBP) both significantly decreased when the subjects changed from the upright position to LLD within each study period (p < 0.001).
Table 3.
Left lateral decubitus data-blood pressure/serum.
| Second trimester (2T) | Third trimester (3T) | Postpartum (PP) | |
|---|---|---|---|
| SBP (mmHg) | 94 ± 2 | 94 ± 2 | 93 ± 2 |
| DBP (mmHg) | 50 ± 2† | 53 ± 2 | 52 ± 2 |
| PRA (ng/mL/hr) | 9.2 ± 1.1* | 14.2 ± 2.3* | 2.0 ± 0.3 |
| Aldosterone (ng/dL) | 55.1 ± 5.0*,† | 81.0 ± 6.8* | 14.7 ± 1.9 |
| Norepinephrine (ρg/mL) | 239.3 ± 31.2* | 172.2 ± 21.9 | 147.7 ± 18.8 |
| Epinephrine (ρg/mL) | 13.7 ± 1.7 | 9.6 ± 1.5 | 12.1 ± 2.4 |
| Dopamine (ρg/mL) | 21.6 ± 3.8† | 34.8 ± 6.9 | 30.1 ± 6.8 |
| Cortisol (μg/dL) | 24.7 ± 1.5* | 27.5 ± 1.0* | 12.6 ± 1.6 |
| DLF† % inhibition [Na, K]-ATPase | 4.5 ± 0.4 | 4.8 ± 0.5 | 4.1 ± 0.4 |
| Na (mEq) | 138 ± 0* | 139 ± 0* | 141 ± 0 |
| K (mEq) | 4.1 ± 0.1* | 4.0 ± 0* | 4.4 ± 0.1 |
| Cr (mg) | 0.7 ± 0* | 0.7 ± 0* | 0.8 ± 0 |
Data are mean ± SEM.
p < 0.05 2T vs. 3T.
p < 0.05 pregnancy vs. PP.
Table 4.
Data after upright posture (stimulation).
| Second trimester (2T)
|
Third trimester (3T)
|
Postpartum (PP)
|
|
|---|---|---|---|
| Upright | Upright | Upright | |
| SBP (mmHg) | 109 ± 2 | 108 ± 3 | 102 ± 3 |
| DBP (mmHg) | 63 ± 2 | 62 ± 1 | 63 ± 3 |
| PRA (ng/mL/hr) | 11.9 ± 1.6** | 15.0 ± 2.2** | 2.8 ± 0.4 |
| Aldosterone (ng/dL) | 73.2 ± 6.3** | 112.7 ± 8.6**,† | 19.4 ± 2.6 |
| Cortisol (μg/dL) | 28.6 ± 1.5** | 32.1 ± 1.2** | 16.0 ± 1.8 |
Data are mean ± SEM.
p < 0.001 pregnancy vs. PP.
p < 0.05 2T vs. 3T.
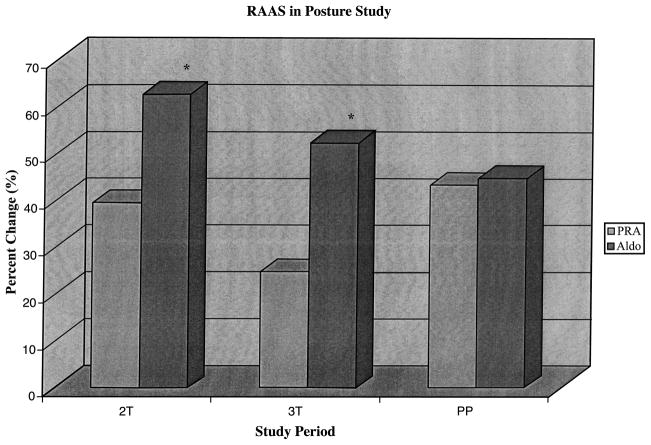
PRA and Aldo were higher in the LLD position during pregnancy (2T: PRA, 9.2 ± 1.1; Aldo, 55.1 ± 5.0 ng/mL; and 3T: PRA, 14.2 ± 2.3; Aldo, 81.0 ± 6.8 ng/mL) compared to postpartum (PRA, 2.0 ± 0.3; Aldo, 14.7 ± 1.9 ng/mL; p < 0.05 for pregnancy vs. postpartum). Both PRA and Aldo were higher in the upright than the LLD positions during pregnancy (2T: PRA, 11.9 ± 1.6; Aldo, 73.2 ± 6.3 ng/mL; and 3T: PRA, 15.0 ± 2.2; Aldo, 112.7 ± 8.6 ng/mL) and postpartum (PRA, 2.8 ± 0.4; Aldo, 19.4 ± 2.6 ng/mL; p < 0.001 for pregnancy vs. postpartum). Because PRA and Aldo basal values differed dramatically in pregnancy compared with the postpartum period, we calculated the percent change for both PRA and Aldo to compare across study periods (Fig. 1). The percent change was calculated as the baseline value (LLD) minus the endpoint value (upright), then this difference divided by the baseline value (LLD). The percent change in PRA was greatest in the postpartum state, reaching statistical significance when compared to the third trimester (2T, 39.4 ± 5.5; 3T, 24.7 ± 5.1; PP, 43.3 ± 5.8%; 3T vs. PP, p < 0.05). The percent change in Aldo was greatest in the second trimester (62.6 ± 3.1%) compared with the third trimester (52.3 ± 3.4%, p < 0.05) or postpartum (44.8 ± 9.0%, p = 0.15). Furthermore, the magnitude of the percent change in Aldo was significantly greater than the magnitude of the percent change in PRA (p < (0.001) during both second and third trimesters. During the postpartum period, the percent change in Aldo was similar to that in PRA (p = 0.89).
Figure 1.
RAAS in posture study. Percent increase when changing from LLD to upright posture among the RAAS hormones. *Aldo v PRA, p < 0.0001. Across study periods, the % change in PRA was greater postpartum than during pregnancy (3T v PP, p < 0.05) but Aldo was greater during pregnancy compared to postpartum (2T v 3T, p < 0.05; 2T v PP, p = 0.15). Within study periods, the % change in Aldo was greater than the % change in PRA during both trimesters ( p < 0.0001). Postpartum the % change in Aldo was similar to the % change in PRA.
The plasma catecholamine data demonstrated that, at baseline in LLD position, norepinephrine values were higher during pregnancy (2T, 239.3 ± 31.2; 3T, 172.2 ± 21.9 ρg/mL) than postpartum (147.7 ± 18.8 ρg/mL; p < 0.05 for both comparisons) with 2T significantly greater than 3T (p < 0.05). Epinephrine values at baseline were similar during pregnancy (2T, 13.7 ± 1.7; 3T, 9.6 ± 1.5 ρg/mL) and postpartum (PP, 12.1 ± 2.4 ρg/mL) but were significantly lower 3T than 2T in both LLD and upright positions (p < 0.01 for both comparisons). The dopamine LLD position values were also similar during pregnancy and postpartum (2T, 21.6 ± 3.8; 3T, 34.8 ± 6.9; PP, 30.1 ± 6.8 ρg/mL), with the 3T difference significantly greater than 2T (p < 0.05).
Norepinephrine was higher in upright than in the LLD position in each study period, significantly during 3T and PP (2T, 264.4 ± 26.2, p = 0.27; 3T, 269.9 ± 31.1, p < 0.0001; PP, 193.9 ± 11.5 ρg/mL, p < 0.05). Epinephrine was significantly higher in the upright than in the LLD position in each study period (2T, 24.7 ± 2.7; 3T, 16.6 ± 1.8 ρg/mL; p < 0.0001), and the postpartum period (PP, 25.0 ± 6.6 ρg/mL; p = 0.015). Although the dopamine levels were greater in the upright (2T, 25.3 ± 3.5; 3T, 44.7 ± 10.7; PP, 47.1 ± 14.1 ρg/mL) than in the LLD position (2T, 21.6 ± 3.8; 3T, 34.8 ± 6.9; PP, 30.1 ± 6.8 ρg/mL) in each study period, these differences were not statistically significant.
The cortisol values during pregnancy were significantly greater compared to those postpartum (2T vs. PP and 3T vs. PP, p < 0.0001), and they were all significantly higher in the upright (2T, 28.6 ± 1.5; 3T, 32.1 ± 1.2; PP, 16.0 ± 1.8 μg/dL) than in the LLD position sampled one hour later (2T, 24.7 ± 1.5; 3T, 27.5 ± 1.0; PP, 12.6 ± 1.6 μg/dL; p < 0.0001 for all comparisons).
Saline Study
There were no significant differences in either SBP or DBP from baseline to the end of the saline infusion during any of the three study periods (Table 5). Both PRA and Aldo demonstrated significant reductions in response to the saline infusion, with the greatest decreases seen in pregnancy, notably the third trimester. When comparing across study periods, the PRA level was greater during the 3T (4.6 ± 1.2 ng/mL) compared to the 2T (2.8 ± 0.5 ng/mL, p = 0.10) or PP (0.6 ± 0.1 ng/mL, p < 0.0001). The Aldo level in response to saline was significantly greater during pregnancy: 3T (25.5 ± 2.1 ng/mL) greater than either the 2T (10.7 ± 0.8 ng/mL, p = 0.001) or PP (3.3 ± 0.3 ng/mL, p < 0.0001).
Table 5.
Data after saline infusion (suppression).
| Second trimester (2T) | Third trimester (3T) | Postpartum (PP) | |
|---|---|---|---|
| SBP (mmHg) | 91 ± 2 | 97 ± 3 | 95 ± 3 |
| DBP (mmHg) | 47 ± 2 | 52 ± 1 | 53 ± 2 |
| PRA (ng/mL/hr) | 4.3 ± 0.6* | 5.4 ± 0.7* | 0.7 ± 0.1 |
| Aldosterone (ng/dL) | 10.7 ± 0.8*,† | 25.5 ± 2.1* | 3.3 ± 0.3 |
| Cortisol (μg/dL) | 13.0 ± 0.9*,† | 16.7 ± 0.8* | 5.6 ± 0.5 |
| DLF % inhibition [Na, K]-ATPase | 3.3 ± 0.5*,† | 4.1 ± 0.4 | 4.4 ± 0.6 |
| Na (mEq) | 139 ± 0* | 139 ± 0* | 143 ± 0 |
| K (mEq) | 4.1 ± 0.1 | 4.0 ± 0.1* | 4.2 ± 0.1 |
| Cr (mg) | 0.6 ± 0* | 0.6 ± 0* | 0.7 ± 0 |
Data are mean ± SEM.
p < 0.05 pregnancy vs. PP.
p < 0.05 2T vs. 3T.
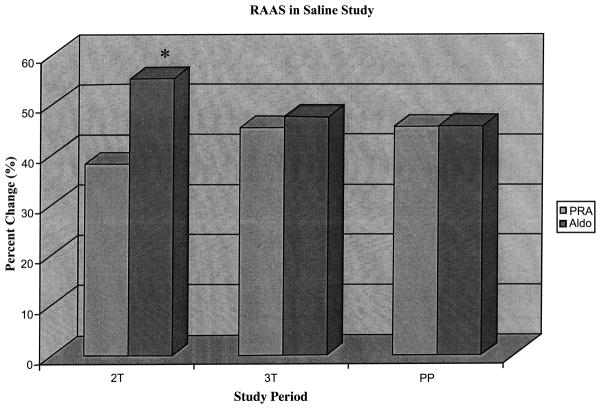
The percent change during saline infusion was calculated as the baseline value (LLD) minus the endpoint value (end of infusion), then this difference was divided by the baseline value (LLD). When comparing the percent changes in PRA in response to the saline infusion, no significant differences across study periods were observed (2T, 38.1 ± 5.5; 3T, 45.3 ± 3.0; PP, 45.5 ± 5.1%; Fig. 2). The percent change in Aldo was greater during pregnancy compared to postpartum, reaching statistical significance in the second trimester (2T, 55.1 ± 2.3 vs. 3T, 47.5 ± 2.8%, p < 0.05) compared to postpartum (45.7 ± 5.8%).
Figure 2.
RAAS in saline study. Percent decrease when changing from baseline to end of saline infusion among the RAAS hormones. *Aldo v PRA, p < 0.001. Across study periods, the % change was similar for PRA. Aldo % change was greatest second trimester reaching statistical significance compared to the third trimester ( p < 0.05). Within study periods, the % change in Aldo was greater than the % change in PRA during both trimesters but only reached statistical significance during the second trimester ( p < 0.01). Aldo was similar to PRA during postpartum.
Serum DLF levels were slightly but insignificantly higher during pregnancy than postpartum in the LLD position (2T, 4.5 ± 0.4; 3T, 4.8 ± 0.5; PP, 4.1 ± 0.4% inhibition [Na, K] ATPase). In response to saline infusion, the serum DLF values decreased during pregnancy and were minimally increased in the PP period. The post-infusion values were significantly lower in the 2T (3.3 ± 0.5%) than the 3T (4.1 ± 0.4%, p < 0.05) and the PP value (4.4 ± 0.6%, p < 0.05).
Cortisol levels were greater during pregnancy (2T, 13.0 ± 0.9; 3T, 16.7 ± 0.8 μg/dL) compared to postpartum (5.6 ± 0.5 μg/dL, both p < 0.001) after saline infusion. Cortisol decreased significantly from baseline to the end of the saline infusion 3 hours later during each study period (p < 0.001 for all periods).
DISCUSSION
Each evaluation of the RAAS was conducted with women in sodium balance on an intermediate sodium intake as low or high sodium intake would confound interpretation because it would stimulate or suppress the RAAS, respectively. In addition, this study was longitudinal, where each of the subjects served as her own control during pregnancy and the postpartum period. We also assessed the RAAS simultaneously under both stimulation and suppression. Furthermore, in an effort to explain the PRA-Aldo dissociation, we have included the measurement of potassium, catecholamines, DLF, and cortisol (as a proxy for ACTH activity). These factors have been shown to affect Aldo independently of the RAAS.
This study demonstrates that PRA and Aldo were significantly greater during pregnancy than postpartum. Moreover, the absolute and relative increases in Aldo were significantly greater than those increases in PRA during pregnancy. As has been previously described (13–15), we observed the PRA-Aldo dissociation with change from LLD to upright posture during pregnancy evidenced by a greater change in Aldo relative to the change in PRA. Our data demonstrated a significantly greater Aldo response in the third trimester compared to the second trimester. This Aldo response contrasts the PRA postural responses during pregnancy that were similar in both trimesters. In response to saline infusion, the concentrations of PRA and Aldo, although higher in pregnancy than postpartum, decreased significantly during each of the three study periods, demonstrating that these components of the RAAS respond to suppression in both the pregnant and nonpregnant states. The magnitude of the fall in these RAAS hormones was greater during pregnancy, particularly during the third trimester, than during the postpartum period.
The PRA-Aldo dissociation of pregnancy was also evidenced during suppression, but we observed statistical significance only during the second trimester. The percent change in PRA did not differ among the three study periods. However, the percent change in Aldo was significantly greater second trimester compared with third trimester and postpartum. In addition, the second trimester PRA and Aldo percent changes during suppression were not significantly different from the second trimester PRA and Aldo percent changes observed with the stimulation studies.
This study does not provide a definite physiological explanation for the greater Aldo response for a given change in PRA during pregnancy, but several possibilities are raised. A change in adrenal sensitivity to endogenous angiotensin II (AngII) could explain the greater Aldo response during pregnancy. For the vasculature, pregnancy has been shown to be a state of relative AngII insensitivity (17, 31, 32). However, a dissociation of vascular and adrenal responsiveness to AngII has been observed to occur in non-pregnant subjects on a low-salt diet (33). A low-salt diet increases AngII levels resulting in a decrease in AngII receptors in both the vasculature and adrenal gland. This down-regulation explains the blunted pressor responsiveness to AngII on a low-salt diet. Conversely, a low-salt diet stimulates aldosterone synthase leading to higher levels of Aldo. These effects lead to a blunted pressor but a heightened Aldo response to AngII on a low-salt diet. However, in this study, both pregnant and nonpregnant women were in 100 mEq sodium balance. Nonetheless, other factors that are elevated in pregnancy may increase aldosterone synthase activity, such as estradiol (34).
When analyzing other potential non-RAAS factors to explain the PRA-Aldo dissociation, we observed that DLF demonstrated a pattern that paralleled the Aldo levels in that it was higher during pregnancy than during the postpartum period. DLF appears to have the ability to increase Aldo (35, 36); however, the precise mechanism through which DLF exerts its effect is unclear.
In addition, cortisol paralleled Aldo levels: increased during pregnancy, increased to a greater degree with upright posture, and decreased with saline infusion. During the posture study, the cortisol decrease is likely a consequence of its diurnal fall over the morning; however, the levels were still higher during pregnancy than postpartum at comparable time points. Aldo is stimulated by ACTH (25, 37), albeit a secondary influence compared to AngII and potassium regulation. The increased cortisol levels we observed during pregnancy, although in part due to increased level of cortisol binding globulin characteristic of pregnancy, likely also reflect increased unbound cortisol. Data demonstrate that the increase in ACTH during pregnancy reflects increased placental production of cortisol releasing hormone (38). This increase in ACTH may result in augmented Aldo responsiveness to changes in AngII and contribute to the PRA-Aldo dissociation observed at baseline, with stimulation and, to a lesser degree, with suppression of the RAAS. Given the limits of this study, we were not able to explore this possibility further.
The remaining non-RAAS factors measured did not explain the PRA-Aldo dissociation. Prior studies on plasma catecholamines have been conflicting: observations have reported unchanged (24, 39), increased (40), or decreased (22, 23) catecholamine levels during pregnancy compared to nonpregnancy. We observed an increased plasma norepinephrine during the second trimester of pregnancy at baseline and during posture study, which was most pronounced during the third trimester. However, higher norepinephrine would be expected to have a suppressive effect on Aldo given evidence that alpha-adrenergic activity suppresses the RAAS (41). Although epinephrine and dopamine increased when moving from LLD to upright position, the values were not significantly different comparing pregnancy to postpartum. Serum potassium levels were lower during pregnancy and did not differ between trimesters despite the increased Aldo being markedly greater in the third trimester, consistent with prior data (42). Consequently, serum potassium does not explain the dissociation.
During the saline suppression study, plasma sodium and potassium concentration did not change during any of the study periods. Cortisol decreased during the saline infusion, likely reflecting diurnal changes. DLF decreased in response to saline infusion during pregnancy, similar to PRA and Aldo. However, the DLF was significantly lower post-infusion in the second trimester compared with the third trimester. Therefore, although the change in Aldo that occurs with suppression in pregnancy could be entirely secondary to changes in PRA, there is the possibility that DLF may play a role in the PRA-Aldo dissociation observed in the second trimester.
In conclusion, PRA and Aldo increase with upright posture and decrease with saline infusion during pregnancy in women in sodium balance. These responses are markedly greater for Aldo and therefore not completely explained by changes in PRA. The exaggerated Aldo response is likely due to non-RAAS regulatory factors, and ACTH and DLF may be implicated. Additional examination of the PRA-Aldo dissociation may prove beneficial in advancing our understanding of cardiovascular health and blood pressure regulation in normal and hypertensive pregnancy.
Acknowledgments
The authors would like to thank the staff of the GCRC for their support during this study and they thank their subjects for their participation. This study was supported by NIH grants including GCRC (5 MO1 RR02635), SCOR in Hypertension (2 P50 HL 55000-06), RO1 (HL67332-01), and K24 (RR018613-01).
References
- 1.Weir RJ, Doji A, Fraser R, Monton JJ, Parboosingh J, Robertson JIS, Wilson A. Studies of the renin-angiotensin-aldosterone system, cortisol, DOC and ADH in normal and hypertensive pregnancy. In: Lindheimer MD, Katz AL, Zuspan FR, editors. Hypertension in Pregnancy. New York: John Wiley; 1976. pp. 251–261. [PubMed] [Google Scholar]
- 2.Wilson M, Morganti AA, Zervoudakis I, Letcher RL, Romney BM, Von Oeyon P, Papera S, Sealey JE, Laragh JH. Blood pressure, the renin-aldosterone system, and sex steroids throughout normal pregnancy. Am J Med. 1980;68:97–104. doi: 10.1016/0002-9343(80)90178-3. [DOI] [PubMed] [Google Scholar]
- 3.Pipkin FB. The renin-angiotensin system in normal and hypertensive pregnancies. In: Rubin PC, editor. Handbook of Hypertension: Hypertension in Pregnancy. Vol. 10. Amsterdam: Elsevier; 1988. pp. 118–141. [Google Scholar]
- 4.Brown MA, Nicholson E, Ross MR, Gallery EDM. Progressive resetting of sodium-renin-aldosterone relationships in human pregnancy. Clin Exp Hypertens. 1987;B5:349–374. [Google Scholar]
- 5.Brown MA, Zammit VC, Adsett D. Stimulation of active renin release in normal and hypertensive pregnancy. Clin Sci. 1990;79:505–511. doi: 10.1042/cs0790505. [DOI] [PubMed] [Google Scholar]
- 6.Lindheimer MD, DelGreco F, Ehrlich EN. Postural effects on sodium and steroid excretion, and serum renin activity during pregnancy. J Appl Physiol. 1973;35:343–348. doi: 10.1152/jappl.1973.35.3.343. [DOI] [PubMed] [Google Scholar]
- 7.Weinberger MH, Kramer NJ, Grim CE, Peterson LP. The effect of posture and saline loading on plasma renin activity and aldosterone concentration in pregnant, non-pregnant and estrogen-treated women. J Clin Endocrinol Metab. 1977;44:69–77. doi: 10.1210/jcem-44-1-69. [DOI] [PubMed] [Google Scholar]
- 8.Bay WH, Ferris TF. Factors controlling plasma renin and aldosterone during pregnancy. Hypertens. 1979;1(4):410–415. doi: 10.1161/01.hyp.1.4.410. [DOI] [PubMed] [Google Scholar]
- 9.Brown MA. Sodium and plasma volume regulation in normal and hypertensive pregnancy: a review of physiology and clinical implications. Clin Exp Hypertens. 1988;B7:265–282. [Google Scholar]
- 10.Brown MA, Gallery EDM, Ross MR, Esber RP. Sodium excretion in normal and hypertensive pregnancy: a prospective study. Am J Obstet Gynecol. 1988;159:297–307. doi: 10.1016/s0002-9378(88)80071-1. [DOI] [PubMed] [Google Scholar]
- 11.Vaklotton MB, Davison JM, Riondel AM, Lindheimer MD. Response of the renin-aldosterone system and antidiuretic hormone to oral water loading and hypertonic saline infusion during and after pregnancy. Clinical and Experimental Hypertension. 1982;B:385–400. doi: 10.3109/10641958209139861. [DOI] [PubMed] [Google Scholar]
- 12.Brown MA, Nicholson E, Gallery ED. Sodium-renin-aldosterone relations in normal and hypertensive pregnancy. Br J Obstet Gynecol. 1988;95(12):1237–1246. doi: 10.1111/j.1471-0528.1988.tb06812.x. [DOI] [PubMed] [Google Scholar]
- 13.Wu SJ, Tsai YL, Hsieh BS. Plasma renin activity, aldosterone level, serum and urinary electrolytes in normal pregnant women aged 35 and older. J Formos Med Assoc. 1992;91(3):366–369. [PubMed] [Google Scholar]
- 14.Wu SJ, Tsai YL, Hsieh BS. Sequential changes in plasma renin activity and aldosterone level during pregnancy. J Formos Med Assoc. 1991;9(10):932–935. [PubMed] [Google Scholar]
- 15.Brown MA, Zammit VC, Mitar DA, Whitworth JA. Renin-aldosterone relationships in pregnancy-induced hypertension. Am J Hypertens. 1992;5:366–371. doi: 10.1093/ajh/5.6.366. [DOI] [PubMed] [Google Scholar]
- 16.Fagundes VG, Lamas CC, Francischetti EA. Renin-angiotensin-aldosterone system in normal and hypertensive pregnancy: response to postural stimuli. Hypertens. 1992;19(2 suppl):II74–II78. doi: 10.1161/01.hyp.19.2_suppl.ii74. [DOI] [PubMed] [Google Scholar]
- 17.Brown MA, Pipkin FB, Symonds EM. The effects of intravenous angiotensin II upon blood pressure and sodium and urate excretion in human pregnancy. J Hypertens. 1988;6(6):457–464. doi: 10.1097/00004872-198806000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Lindheimer MD, Richardson DA, Ehrlich EN, Katz AI. Potassium homeostasis in pregnancy. J Reprod Med. 1987;32(7):517–522. [PubMed] [Google Scholar]
- 19.Weir RJ, Brown JJ, Fraser R, Lever AF, Logan RW, Mcilwaine GM, Morton JJ, Robertson JI, Tree M. Relationship between plasma renin, renin-substrate, angiotensin II, aldosterone and electrolytes in normal pregnancy. J Clin Endocrinol Metab. 1975;40(1):108–115. doi: 10.1210/jcem-40-1-108. [DOI] [PubMed] [Google Scholar]
- 20.Sowers JR, Glub MS, Eggena PH, Catania RA. Influence of sodium homeostasis on dopaminergic modulation of aldosterone, renin, and prolactin secretion in man. J Clin Endocrinol Metab. 1982;54(1):121–126. doi: 10.1210/jcem-54-1-121. [DOI] [PubMed] [Google Scholar]
- 21.Mueller RA, Fishburne JI, Jr, Brenner WE, Braaksma JT, Staurovsky LG, Hoffer JL, Hendricks CH. Changes in human plasma catecholamines and dopamine-beta-hydroxylase produced by prostaglandin F2 alpha. Prostaglandins. 1972;2(3):219–226. doi: 10.1016/s0090-6980(72)80027-3. [DOI] [PubMed] [Google Scholar]
- 22.Natrajan PG, Mcgarrigle HH, Lawrence DM, Lachelin GC. Plasma noradrenaline and adrenaline levels in normal pregnancy and in pregnancy-induced hypertension. Br J Obstet Gynecol. 1982;89(12):1041–1045. doi: 10.1111/j.1471-0528.1982.tb04661.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Zhang W, Zhao Y. The study of maternal and fetal plasma catecholamines levels during pregnancy and delivery. J Perinat Med. 1999;27(3):195–198. doi: 10.1515/JPM.1999.027. [DOI] [PubMed] [Google Scholar]
- 24.Pedersen EB, Rasmussen AB, Christensen NJ, Johannesen P, Lauritsen JG, Kristensen S, Wohlert M. Plasma noradrenaline and adrenaline in pre-eclampsia, essential hypertension in pregnancy and normotensive pregnant control subjects. Acta Endocrinol (Copenh) 1982;99(4):594–600. doi: 10.1530/acta.0.0990594. [DOI] [PubMed] [Google Scholar]
- 25.Brown MA, Thou ST, Whitworth JA. Stimulation of aldosterone by ACTH in normal and hypertensive pregnancy. Am J Hypertens. 1995;8(3):260–267. doi: 10.1016/0895-7061(94)00213-U. [DOI] [PubMed] [Google Scholar]
- 26.Gregoire I, el Esper N, Gondry J, Boitte F, Fievet P, Makdassi R, Westeel PF, Lalau JD, Favre H, de Bold A, Fournier A. Plasma atrial natriuretic factor and urinary excretion of a ouabain displacing factor and dopamine in normotensive pregnant women before and after delivery. Am J Obstet Gynecol. 1990;162(1):71–76. doi: 10.1016/0002-9378(90)90823-p. [DOI] [PubMed] [Google Scholar]
- 27.Graves SW, Lincoln K, Cook SL, Seely EW. Digitalis-like factor and digoxin-like immunoreactive factor in diabetic women with preeclampsia, transient hypertension of pregnancy, and normotensive pregnancy. Am J Hypertens. 1995;8(1):5–11. doi: 10.1016/0895-7061(94)00167-A. [DOI] [PubMed] [Google Scholar]
- 28.Graves SW, Eder JP, Schryber SM, Sharma K, Brena A, Antman KH, Peters WP. Endogenous digoxin-like immunoreactive factor and digitalis-like factor associated with the hypertension of patients receiving multiple alkylating agents as part of autologous bone marrow transplantation. Clin Sci. 1989;77:501–507. doi: 10.1042/cs0770501. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Robles R, Ruilope L, Mancheno E, Hurtado A, Alcazar JM, Varela C, De La Calle H, Rodicio JL, Sancho J. Dopaminergic modulation of aldosterone secretion: effect of sodium balance and postural changes. Rev Esp Fisiol. 1984;40(1):63–68. [PubMed] [Google Scholar]
- 30.Peuler JD, Johnson G. Simultaneous single isotope radioenzymatic assay of plasma norepinephrine, epinephrine, and dopamine. Life Sci. 1977;21:625. doi: 10.1016/0024-3205(77)90070-4. [DOI] [PubMed] [Google Scholar]
- 31.Broughton PF, Morrison R, O’Brien PM. Prostacyclin attenuates both the pressor and adrenocortical response to angiotensin II in human pregnancy. Clin Sci. 1989;76(5):529–534. doi: 10.1042/cs0760529. [DOI] [PubMed] [Google Scholar]
- 32.Gant NF, Whalley PJ, Everett RB, Borley RJ, MacDonald PC. Control of vascular reactivity in pregnancy. Am J Kidney Dis. 1987;9(4):303–307. doi: 10.1016/s0272-6386(87)80126-9. [DOI] [PubMed] [Google Scholar]
- 33.Williams GH, Hollenberg NK, Braley LM. Influence of sodium intake on vascular and adrenal angiotensin II receptors. Endocrinology. 1976;98:1343–1350. doi: 10.1210/endo-98-6-1343. [DOI] [PubMed] [Google Scholar]
- 34.Kau M-M, Lo M-J, Tsai S-C, Chen J-J, Lu C-C, Lin H, Wang S-W, Wang PS. Effect of estradiol on aldosterone secretion in ovariectomized rate. J Cell Biochem. 1999;73:137–144. doi: 10.1002/(sici)1097-4644(19990401)73:1<137::aid-jcb15>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 35.Tamura M, Piston DW, Tani M, Naruse M, Landon EJ, Inagami T. Ouabain increases aldosterone release from bovine adrenal glomerulosa cells: role of renin-angiotensin system. Am J Physiol. 1996;270:E27–E35. doi: 10.1152/ajpendo.1996.270.1.E27. [DOI] [PubMed] [Google Scholar]
- 36.Antonipillai I, Hong H, Horton R. Magnesium modulates ouabain action on angiotensin II-induced aldosterone synthesis in vitro. Magn Res. 1997;10:307–313. [PubMed] [Google Scholar]
- 37.Nolten WE, Lindheimer MD, Oparil S, Ehrlich EN. Desoxycorticosterone in normal pregnancy. I. Sequential studies of the secretory patterns of desoxycorticosterone, aldosterone, and cortisol. Am J Obstet Gynecol. 1978;132(4):414–420. [PubMed] [Google Scholar]
- 38.Goland RS, Jozak S, Conwell I. Placental corticotropin-releasing hormone and the hypercortisolism of pregnancy. Am J Obstet Gynecol. 1994;171(5):1287–1291. doi: 10.1016/0002-9378(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 39.Barron WM, Mujais SK, Zinaman M, Bravo EL, Lindheimer MD. Plasma catecholamine responses to physiologic stimuli in normal human pregnancy. Am J Obstet Gynecol. 1986;154(1):80–84. doi: 10.1016/0002-9378(86)90397-2. [DOI] [PubMed] [Google Scholar]
- 40.Hobel CJ, Castro L, Rosen D, Greenspoon JS, Nessim S. The effect of thigh-length support stockings on the hemodynamic response to ambulation in pregnancy. Am J Obstet Gynecol. 1996;174(6):1734–1740. doi: 10.1016/s0002-9378(96)70204-1. [DOI] [PubMed] [Google Scholar]
- 41.Trovati M, Massucco P, Cavalot F, Anfossi G, Mularoni E, Busca G, Orecchia C, Emanuelli G. Alpha-adrenergic blockade with phentolamine increases the response of the renin-angiotensin-aldosterone system to insulin-induced hypoglycemia in man. Horm Metab Res. 1989;21(5):290–291. doi: 10.1055/s-2007-1009217. [DOI] [PubMed] [Google Scholar]
- 42.Brown MA, Sinosich MJ, Saunders DM, Gallery EDM. Potassium regulation and progesterone-aldosterone interrelationships in human pregnancy: a prospective study. Am J Obstet Gynecol. 1986;155(2):349–353. doi: 10.1016/0002-9378(86)90824-0. [DOI] [PubMed] [Google Scholar]