Abstract
Background
Alcohol use disorder commonly occurs in patients with schizophrenia. Most antipsychotic drugs do not lessen alcohol use; although the atypical antipsychotic clozapine has been shown to reduce alcohol use in patients with schizophrenia, its toxicity severely limits its use in patients. With an eye toward creation of a safer clozapine-like drug, we have investigated the pharmacological basis of the clozapine’s effects on alcohol drinking in the Syrian golden hamster. In this animal, as in patients with schizophrenia, clozapine reduces alcohol drinking while the typical antipsychotic haloperidol does not. We have suggested that clozapine decreases alcohol drinking due to its weak dopamine D2 receptor blockade, its potent norepinephrine α-2 receptor antagonism, as well as its ability to elevate plasma norepinephrine.
Methods
We recreated a clozapine-like drug to reduce alcohol drinking in the Syrian golden hamster by combining low dose haloperidol with a norepinephrine α-2 receptor antagonist, idazoxan, and a norepinephrine reuptake inhibitor, desipramine. Hamsters were given free access to water and alcohol (15% v/v) and were treated daily with each drug or with the three-drug combination for 23 days.
Results
The drug combination reduced alcohol drinking and preference significantly as compared to vehicle or to haloperidol, idazoxan or desipramine, while not altering food-intake or bodyweight.
Conclusion
These findings suggest that that haloperidol, which does not reduce alcohol drinking in patients with schizophrenia or the hamster, if combined with idazoxan and desipramine (producing a drug combination that mimics aspects of clozapine’s pharmacology) is able to reduce alcohol drinking in the hamster.
Keywords: Alcoholism, addiction, schizophrenia, antipsychotic, norepinephrine, antipsychotic, norepinephrine reuptake inhibitor
1. INTRODUCTION
Alcohol use disorder (AUD) is 3 times more prevalent in patients with schizophrenia (SCZ) compared to the general population; over 30% of patients with SCZ have an AUD (Regier et al., 1990). Although patients with SCZ tend to consume only moderate amounts of alcohol on a regular basis, even this moderate, but regular, use substantially increases the morbidity of SCZ (Drake and Mueser, 1996). AUD in patients with SCZ is associated with poor treatment response, treatment non-compliance (Owen et al., 1996), relapse and hospitalization (Drake and Mueser, 1996; Gupta et al., 1996), as well as violence (Bartels et al., 1991; Swanson et al., 1990) and suicide (Allebeck et al., 1987; Harkavy-Friedman and Nelson, 1997). There are few treatment options available to control AUD in this difficult-to-treat population; to date, the atypical antipsychotic clozapine (CLOZ) is the only antipsychotic shown to reduce alcohol drinking in patients with SCZ (Drake et al., 2000; Green et al., 2008, 1999; Lee et al., 1998; Zimmet et al., 2000). Unfortunately, CLOZ is only used infrequently because of its toxic side-effect profile.
Our group and others have proposed that patients with SCZ may have a dysregulated brain reward circuit that underpins their alcohol use, and that alcohol may transiently improve the functioning of this circuit (Chambers, 2007; Green et al., 1999). Furthermore, we have also proposed that CLOZ, because of its broad-spectrum effects, including its weak dopamine (DA) D2 receptor antagonism, potent norepinephrine (NE) α-2 antagonism and ability to increase levels of norepinephrine in plasma and brain (through possible NE reuptake inhibition ability [Yoshimura et al., 2000]), may also improve the functioning of this circuitry and thereby limit alcohol/other substance use (Chau et al., 2011, 2004; Green et al., 2008, 1999). We have noted that typical antipsychotics, such as HAL, are not able to decrease alcohol drinking in patients with SCZ (Green et al., 2004), and we have suggested that this may be due, in part, to their potent dopamine D2 receptor antagonism, which may itself produce a reward deficit (Grace, 1991).
We have performed a series of experiments in the alcohol-drinking Syrian golden hamster, deconstructing CLOZ into its pharmacological components, with an eye toward developing medication combinations that, like CLOZ, might limit alcohol drinking in patients with SCZ. The hamster is an appropriate animal with which to assess the potential ability of medications to limit alcohol drinking in patients with schizophrenia, for the following reasons: (a) like patients with schizophrenia who tend to drink regular but moderate amounts of alcohol, the hamster consumes alcohol on a regular basis, achieving moderate blood alcohol levels, and does not develop physiologic withdrawal (Ferris et al., 1998; Harris et al., 1979; Keung et al., 2000); and (b) like patients with schizophrenia, this animal reduces its alcohol consumption when treated with CLOZ, but not when treated with HAL (Green et al., 2004). Data from our hamster studies have strongly suggested that CLOZ’s actions are, indeed, contributed to by its weak dopamine D2 receptor blockade, as well as its modulation of noradrenergic signaling in the brain via both NE reuptake inhibition and α-2 receptor antagonism (Chau et al., 2011; Gulick et al., 2014). Based on these data, we hypothesized that combining a low dose of HAL (to mimic the weak DA D2 receptor blockade of CLOZ) with desipramine (DMI; a NE reuptake inhibitor) and idazoxan (IDAZ; a NE α-2 receptor antagonist) would replicate the effects of CLOZ, and reduce alcohol drinking and preference in the Syrian golden hamster. Findings from this study can further advance our understanding of the mechanisms of action of CLOZ, and moreover, may shed light on the development of new medications that can limit alcohol drinking in patients with SCZ and, potentially, in those with AUD alone.
2. MATERIALS AND METHODS
2.1 Animals
Adult, male Syrian golden hamsters (Mesocricetus Auratus; 100–130g; Harlan Inc., Indianapolis, IN) were individually housed and maintained on a 12 h/12 h light/dark cycle with ad libitum access to food and water. Hamsters were given free access to two drinking bottles (water and 15% alcohol [v/v]); the positions of the two bottles were rotated daily to prevent positional preference), and food. A technician, blinded to the experimental conditions, measured fluid intake every 24 hours, food intake every 48 hours, and body-weight every 4 days. Once the hamsters achieved a steady baseline level of alcohol intake, drug treatment began. All injections were performed 1–2 hours prior to the start of the dark cycle to avoid any immediate locomotor effects of the drugs on alcohol intake. All experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23) revised in 1996 and approved by the Institutional Animal Care and Use Committee of Dartmouth College.
2.2 Procedures
Thirty-four hamsters were given access to separate bottles of water and 15% v/v alcohol for 12 days prior to randomization into 5 groups based on baseline alcohol intake (g/kg; n=7–8 per group); baseline alcohol intake was calculated using the last 4 days of the initial 12-day period of access to alcohol. The groups were subsequently treated daily for 23 days with either: vehicle (VEH); 0.02 mg/kg HAL; 1.5 mg/kg IDAZ; 5 mg/kg DMI; or the combination of all three drugs. All hamsters received free access to food, water, and alcohol during treatment.
2.3 Drugs
HAL, IDAZ and DMI were purchased from Sigma Aldrich (St. Louis, MO). All drugs were dissolved in 0.5 N acetic acid, with the volume adjusted in the 0.5 M sodium acetate vehicle solution (pH 5.5). All drug and VEH solutions were injected subcutaneously (2 ml/kg body-weight).
2.4 Data analysis
Alcohol intake (g/kg), alcohol preference, food-intake (g/kg), and body-weight (g) data were analyzed using a two-way repeated measures analysis of co-variance (RMANCOVA), using time (measured in days) and drug treatment as independent variables and the last four days of alcohol drinking prior to treatment as covariates. The main effects between treatment groups were compared in a pair-wise manner using a Bonferroni confidence interval adjustment. When the analysis indicated that a significant time by treatment interaction was observed, pairwise comparisons between groups were made using the Bonferroni adjustment to help interpret time by treatment interactions from the RMANOVAs; adjustment to p-values was carried out separately at each day. Data are expressed as mean (M) ± standard error of the mean (SEM) and significance was set at p<0.05.
3. RESULTS
3.1 Combining a low dose of haloperidol with idazoxan and desipramine reduces alcohol intake
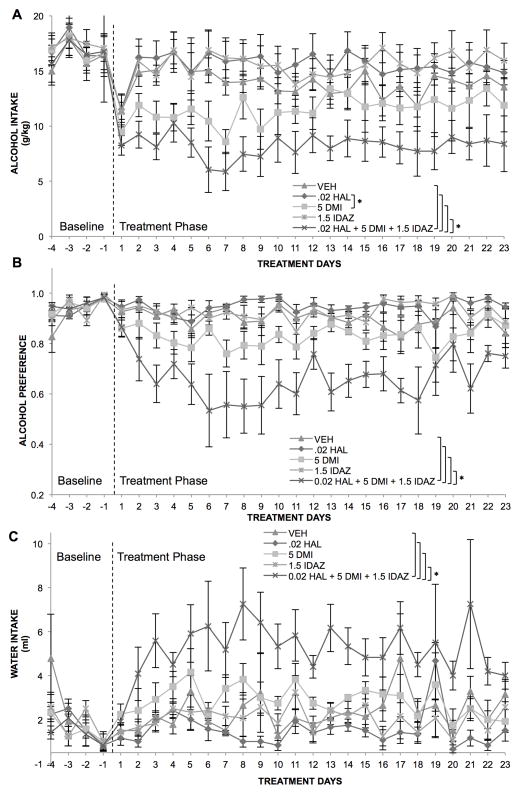
A two-way repeated measures ANOVA (RMANOVA) indicated no effect of time, but a significant effect of treatment F(6,33)=20.237, p<0.001, and a significant time by group interaction, F(132,9726)=1.378, p<0.01, on alcohol intake in the hamster (Figure 1A). Post-hoc pairwise comparisons showed that the combination of HAL, IDAZ and DMI resulted in significantly lower alcohol intake compared to VEH, as well as compared to HAL, IDAZ or DMI alone (p<0.05). In particular, the combination group differed significantly from VEH on days 3–10, 15, 17, 19 and 22; from HAL alone on days 2–11, 13–19 and 22; from DMI alone on days 8, 13 and 22; and from IDAZ alone on days 2–10, 13–17 and 19–22 (p<0.05). Interestingly, while HAL and DMI alone were not significantly different from VEH, they did differ significantly from each other, possibly due to a somewhat transient reduction seen in alcohol drinking with DMI alone combined with a potential increase in alcohol drinking due to HAL alone.
Figure 1.
Combination of haloperidol, idazoxan and desipramine significantly reduces alcohol drinking and preference in the Syrian golden hamster. (A) Alcohol intake and (B) alcohol preference were significantly reduced in the combination group, whereas vehicle-and drug alone-treated animals displayed no such reductions. (C) Water intake increased in the combination-treated group to compensate for decreased alcohol drinking, * p<0.05 compared to vehicle treated animals; n=5–7/group, The dashed line represents start of treatment.
3.2 Combination of low dose haloperidol, idazoxan and desipramine significantly reduces alcohol preference
A two-way repeated measures ANOVA (RMANOVA) indicated no effect of time, but a significant effect of treatment F(6,33)=12.465, p<0.001, and no time by group interaction, on alcohol preference (Figure 1B). Post-hoc pairwise comparisons showed that the combination of HAL, IDAZ and DMI resulted in significantly lower alcohol preference compared to VEH, as well as compared to HAL, IDAZ or DMI alone (p<0.05).
3.3 Combination of low dose haloperidol, idazoxan and desipramine increases water intake, but does not alter food-intake or body-weight
Firstly, a two-way repeated measures ANOVA (RMANOVA) indicated a significant effect of time F(22,660)=1.823, p<0.05, a significant effect of treatment F(6,30)=16.607, p<0.001, but no time by group interaction, on water intake (Figure 1C). Post-hoc pairwise comparisons showed that the combination of HAL, IDAZ and DMI resulted in significantly higher water intake compared to VEH, as well as compared to HAL, IDAZ or DMI alone (p<0.05). These results suggest a compensatory increase in water drinking in response to a decrease in alcohol drinking, consistent with our previous studies in the hamster (Chau et al., 2010; Green et al., 2004).
Second, a two-way repeated measures ANOVA (RMANOVA) indicated a significant effect of time F(132,770)=5.848, p<0.01, but no effect of treatment or time by group interaction, on food-intake (data not shown).
Third, a two-way repeated measures ANOVA (RMANOVA) indicated no effect of time, but a significant effect of treatment F(6,35)=3.264, p<0.05, and a significant time by group interaction F(132,770)=5.848, p<0.01, on body-weight (data not shown). Post-hoc pairwise comparisons showed that the combination of HAL, IDAZ and DMI resulted in significantly lower body-weight compared to the HAL alone group (p<0.05), but did not differ significantly from VEH or the other drugs alone. Lastly, the animals did not appear to be lethargic or to demonstrate any untoward effects of treatment.
4. DISCUSSION
The work presented in this study provides a novel therapeutic avenue toward creating a medication that might reduce alcohol drinking in patients with SCZ. We showed that by replicating aspects of the pharmacological profile of CLOZ, we have been able to make a drug (HAL), which has no ability to reduce alcohol drinking in patients with SCZ or in hamsters, gain the ability to significantly reduce alcohol intake and preference in the hamster by the addition of the NE reuptake inhibitor DMI and the NE α2 receptor antagonist IDAZ. The average magnitude of reduction in alcohol drinking was approximately 65% when compared to vehicle treated animals, comparable to the 60–75% reduction seen in alcohol drinking we have previously reported in CLOZ-treated hamsters (Chau et al., 2010; Green et al., 2004).
In this study, we employed a low dose of HAL, one producing less than 40% DA D2 blockade (Kapur et al., 2003; Nordstrom et al., 1995; Schotte et al., 1989), to emulate the low DA D2 receptor blockade exhibited by CLOZ (Kapur et al., 2003). Our previous studies have shown that higher doses of HAL are no more likely to decrease alcohol drinking in the hamster than the one employed here (Green et al., 2004). As we have noted before (Green et al., 2004), this low dose of HAL did not reduce alcohol drinking by itself, suggesting that weak DA D2 receptor antagonism is not sufficient to reduce alcohol drinking in the hamster. Moreover, neither DMI nor IDAZ, alone, significantly reduced alcohol drinking in the hamster (although DMI produced a modest transient reduction), consistent with our previous findings (Gulick et al., 2014). However, when DMI and IDAZ were added to a low dose of HAL, the combination produced a sustained reduction in alcohol drinking and preference in the hamster.
Recent reviews, as well as older studies, have noted that the addition of agents that modulate NE α-2 receptor activity (e.g., IDAZ or mirtazapine) to typical antipsychotics (e.g., HAL) can increase their clinical antipsychotic efficacy and can reduce their extra-pyramidal side-effects (Brosda et al., 2014; Litman et al., 1996). Moreover, animal studies have noted that the addition of IDAZ to HAL can reduce conditioned avoidance response, suggesting greater efficacy for schizophrenia in a predictively valid model (Wadenberg et al., 2007). Similarly, the addition of IDAZ to raclopride or risperidone (at sub-effective doses), both potent DA D2 antagonists, has also been shown to improve their ability to reduce conditioned avoidance response in rodents (Jacobson and Prus, 2010; Marcus et al., 2005, 2010). In addition, adding the NE reuptake inhibitor, reboxetine, to raclopride has also been shown to enhance the antipsychotic-like effects of raclopride, and increase DA release in the medial prefrontal cortex (Linner et al., 2002). Lastly, a recent study has suggested that by simultaneously blocking NE reuptake and α2 receptors, the autoreceptor-mediated negative feedback on NE activity can be prevented, resulting in a selective enhancement of DA transmission (and increased DA release in the prefrontal cortex) in the mesocorticolimbic circuit (Masana et al., 2011). Taken together, these studies suggest the possibility that the combination of HAL, IDAZ and DMI could potentially increase the efficacy of HAL in decreasing symptoms of schizophrenia.
Some limitations of this study include the use of the hamster and the lack of assessment of the neurochemical effects of the drug combination on neurotransmitter functioning. While the hamster serves as a useful bioassay for assessing the effects of antipsychotics on alcohol drinking in patients with SCZ, it is not an animal model of SCZ. Thus, future studies will need to draw upon such an animal model (e.g., the neonatal ventral hippocampal lesioned rat; Jeanblanc et al., 2014), and on techniques such as magnetic resonance spectroscopy and microdialysis to assess neurotransmitter functioning. Both directions of research are required to better understand the mechanism by which CLOZ or CLOZ-like drugs reduce alcohol drinking in animals, in patients with SCZ, and potentially, in those with AUD alone.
Our findings from this study provide further support for our neurobiologic formulation regarding the actions of CLOZ, which implies increased NE output, via both NE α-2 receptor antagonism and NE re-uptake inhibition, when combined with weak DA D2 receptor antagonism, may modulate brain reward circuitry in a way that effectively decreases alcohol drinking in patients with SCZ (Green et al., 1999). Moreover, data from this study also suggest that we may be able to use this information to recreate a safer CLOZ-like drug using existing drugs with overlapping pharmacologic actions (e.g., quetiapine), in combination with others, to reduce alcohol drinking in these patients. Lastly, we are also currently exploring whether other pharmacological actions of CLOZ (e.g., effects on serotonergic, GABAergic and glutamatergic transmission) may also contribute to its ability to reduce alcohol drinking.
In summary, this study suggests that the typical antipsychotic drug HAL, which does not reduce alcohol drinking in patients with SCZ or in the Syrian golden hamster, if combined with the NE α-2 receptor antagonist IDAZ and the NE re-uptake inhibitor DMI (producing a drug combination that mimics aspects of CLOZ’s pharmacology) is able to reduce alcohol drinking in the hamster. Further research will be needed to assess if this combination will reduce alcohol drinking in patients with SCZ. However, our investigation may be a first step toward the creation of safer CLOZ-like agents for the treatment of patients with SCZ and co-occurring AUD, and possibly in those with AUD alone.
Highlights.
Haloperidol, by itself, does not reduce alcohol drinking in the Syrian golden hamster.
Haloperidol, when combined with idazoxan and desipramine, reduces alcohol drinking in the hamster.
Clozapine’s pharmacology can provide clues toward new treatment development.
Acknowledgments
This work was supported in part by grants from the National Institute of Alcohol Abuse and Alcoholism (AIG; 1R03AA014644 and 1R01AA018151), and from the National Center for Advancing Translational Science (AIG; NCATS UL1TR001086).
Footnotes
Role of Funding Source: Nothing declared
Contributors
Jibran Y. Khokhar: Performed all data analyses and manuscript preparation.
David T. Chau: Primarily involved with study design and data collection.
Ree Dawson: Provided study design and statistical support and contributed to manuscript preparation.
Alan I. Green: Designed the study and oversaw all aspects of the study, including implementation, data analysis and manuscript preparation.
All contributors have read and approved of submission of the paper to Drug and Alcohol Dependence.
Conflict of Interest: In the past three years, Dr. Alan Green has received grants from Novartis and Janssen to support research studies, and has also owned stock in Pfizer, Johnson & Johnson and Mylan. He has served as an (uncompensated) consultant to Otsuka and Alkermes, and as a member (uncompensated) of a Data Monitoring Board for Lilly. Moreover, he is a co-inventor of two patent applications regarding treatment of substance abuse.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allebeck P, Varla A, Kristjansson E, Wistedt B. Risk factors for suicide among patients with schizophrenia. Acta Psychiatr Scand. 1987;76:414–419. doi: 10.1111/j.1600-0447.1987.tb05626.x. [DOI] [PubMed] [Google Scholar]
- Bartels SJ, Drake RE, Wallach MA, Freeman DH. Characteristic hostility in schizophrenic outpatients. Schizophr Bull. 1991;17:163–171. doi: 10.1093/schbul/17.1.163. [DOI] [PubMed] [Google Scholar]
- Brosda J, Jantschak F, Pertz HH. alpha2-Adrenoceptors are targets for antipsychotic drugs. Psychopharmacology (Berl) 2014;231:801–812. doi: 10.1007/s00213-014-3459-8. [DOI] [PubMed] [Google Scholar]
- Chambers RA. Animal modeling and neurocircuitry of dual diagnosis. J Dual Diagn. 2007;3:19–29. doi: 10.1300/J374v03n02_04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau DT, Ahmed J, Wang TT, Xie H, Dawson R, Green AI. Raclopride lessens the ability of clozapine to suppress alcohol drinking in Syrian golden hamsters. Neuropharmacology. 2011;61:646–652. doi: 10.1016/j.neuropharm.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Chau DT, Gulick D, Xie H, Dawson R, Green AI. Clozapine chronically suppresses alcohol drinking in Syrian golden hamsters. Neuropharmacology. 2010;58:351–356. doi: 10.1016/j.neuropharm.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake RE, Mueser KT. Alcohol-use disorder and severe mental illness. Alcohol Health Res World. 1996;40:87–93. [PMC free article] [PubMed] [Google Scholar]
- Drake RE, Xie H, McHugo GJ, Green AI. The effects of clozapine on alcohol and drug use disorders among patients with schizophrenia. Schizophr Bull. 2000;26:441–449. doi: 10.1093/oxfordjournals.schbul.a033464. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Shtiegman K, King JA. Voluntary ethanol consumption in male adolescent hamsters increases testosterone and aggression. Physiol Behav. 1998;63:739–744. doi: 10.1016/s0031-9384(97)00533-7. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Green AI, Chau DT, Keung WM, Dawson R, Mesholam RI, Schildkraut JJ. Clozapine reduces alcohol drinking in Syrian golden hamsters. Psychiatry Res. 2004;128:9–20. doi: 10.1016/j.psychres.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Green AI, Noordsy DL, Brunette MF, O’Keefe C. Substance abuse and schizophrenia: pharmacotherapeutic intervention. J Subst Abuse Treat. 2008;34:61–71. doi: 10.1016/j.jsat.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AI, Zimmet SV, Strous RD, Schildkraut JJ. Clozapine for comorbid substance use disorder and schizophrenia: do patients with schizophrenia have a reward-deficiency syndrome that can be ameliorated by clozapine? Harv Rev Psychiatry. 1999;6:287–296. doi: 10.3109/10673229909017206. [DOI] [PubMed] [Google Scholar]
- Gulick D, Chau DT, Khokhar JY, Dawson R, Green AI. Desipramine enhances the ability of risperidone to decrease alcohol intake in the Syrian golden hamster. Psychiatry Res. 2014;218:329–334. doi: 10.1016/j.psychres.2014.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Hendricks S, Kenkel AM, Bhatia SC, Haffke EA. Relapse in schizophrenia: is there a relationship to substance abuse? Schizophr Res. 1996;20:153–156. doi: 10.1016/0920-9964(95)00108-5. [DOI] [PubMed] [Google Scholar]
- Harkavy-Friedman JM, Nelson E. Management of the suicidal patient with schizophrenia. Psychiatr Clin North Am. 1997;20:625–640. doi: 10.1016/s0193-953x(05)70334-8. [DOI] [PubMed] [Google Scholar]
- Harris RA, Krause W, Goh E, Case J. Behavioral and biochemical effects of chronic consumption of ethanol by hamsters. Pharmacol Biochem Behav. 1979;10:343–347. doi: 10.1016/0091-3057(79)90195-3. [DOI] [PubMed] [Google Scholar]
- Jacobson SM, Prus AJ. Evaluation of the effects of alpha2 adrenoceptor antagonism with the D2 receptor antagonist raclopride on conditioned avoidance responding in rats. Behav Pharmacol. 2010;21:654–659. doi: 10.1097/FBP.0b013e32833e7efd. [DOI] [PubMed] [Google Scholar]
- Jeanblanc J, Balguerie K, Jeanblanc V, Coune F, Legastelois R, Naassila M. Light alcohol intake during adolescence induces alcohol addiction in a neurodevelopmental model of schizophrenia. Addict Biol. 2014 doi: 10.1111/adb.12146. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Kapur S, VanderSpek SC, Brownlee BA, Nobrega JN. Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: a suggested solution based on in vivo occupancy. J Pharmacol Exp Ther. 2003;305:625–631. doi: 10.1124/jpet.102.046987. [DOI] [PubMed] [Google Scholar]
- Keung WM, Kunze L, Li DJ, Lazo O. Volitional ethanol consumption affects overall serotonin metabolism in Syrian golden hamsters (Mesocricetus auratus) Biochem Biophys Res Commun. 2000;271:823–830. doi: 10.1006/bbrc.2000.2718. [DOI] [PubMed] [Google Scholar]
- Lee ML, Dickson RA, Campbell M, Oliphant J, Gretton H, Dalby JT. Clozapine and substance abuse in patients with schizophrenia. Can J Psychiatry. 1998;43:855–856. [PubMed] [Google Scholar]
- Linner L, Wiker C, Wadenberg ML, Schalling M, Svensson TH. Noradrenaline reuptake inhibition enhances the antipsychotic-like effect of raclopride and potentiates D2-blockage-induced dopamine release in the medial prefrontal cortex of the rat. Neuropsychopharmacology. 2002;27:691–698. doi: 10.1016/S0893-133X(02)00350-0. [DOI] [PubMed] [Google Scholar]
- Litman RE, Su TP, Potter WZ, Hong WW, Pickar D. Idazoxan and response to typical neuroleptics in treatment-resistant schizophrenia. Comparison with the atypical neuroleptic, clozapine. Br J Psychiatry. 1996;168:571–579. doi: 10.1192/bjp.168.5.571. [DOI] [PubMed] [Google Scholar]
- Marcus MM, Jardemark KE, Wadenberg ML, Langlois X, Hertel P, Svensson TH. Combined alpha2 and D2/3 receptor blockade enhances cortical glutamatergic transmission and reverses cognitive impairment in the rat. Int J Neuropsychopharmacol. 2005;8:315–327. doi: 10.1017/S1461145705005328. [DOI] [PubMed] [Google Scholar]
- Marcus MM, Wiker C, Franberg O, Konradsson-Geuken A, Langlois X, Jardemark K, Svensson TH. Adjunctive alpha2-adrenoceptor blockade enhances the antipsychotic-like effect of risperidone and facilitates cortical dopaminergic and glutamatergic, NMDA receptor-mediated transmission. Int J Neuropsychopharmacol. 2010;13:891–903. doi: 10.1017/S1461145709990794. [DOI] [PubMed] [Google Scholar]
- Masana M, Bortolozzi A, Artigas F. Selective enhancement of mesocortical dopaminergic transmission by noradrenergic drugs: therapeutic opportunities in schizophrenia. Int J Neuropsychopharmacol. 2011;14:53–68. doi: 10.1017/S1461145710000908. [DOI] [PubMed] [Google Scholar]
- Nordstrom AL, Farde L, Nyberg S, Karlsson P, Halldin C, Sedvall G. D1, D2, and 5-HT2 receptor occupancy in relation to clozapine serum concentration: a PET study of schizophrenic patients. Am J Psychiatry. 1995;152:1444–1449. doi: 10.1176/ajp.152.10.1444. [DOI] [PubMed] [Google Scholar]
- Owen RR, Fischer EP, Booth BM, Cuffel BJ. Medication noncompliance and substance abuse among patients with schizophrenia. Psychiatr Serv. 1996;47:853–858. doi: 10.1176/ps.47.8.853. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Schotte A, de Bruyckere K, Janssen PF, Leysen JE. Receptor occupancy by ritanserin and risperidone measured using ex vivo autoradiography. Brain Res. 1989;500:295–301. doi: 10.1016/0006-8993(89)90325-9. [DOI] [PubMed] [Google Scholar]
- Swanson JW, Holzer CE, 3rd, Ganju VK, Jono RT. Violence and psychiatric disorder in the community: evidence from the Epidemiologic Catchment Area surveys. Hosp Community Psychiatry. 1990;41:761–770. doi: 10.1176/ps.41.7.761. [DOI] [PubMed] [Google Scholar]
- Wadenberg ML, Wiker C, Svensson TH. Enhanced efficacy of both typical and atypical antipsychotic drugs by adjunctive alpha2 adrenoceptor blockade: experimental evidence. Int J Neuropsychopharmacol. 2007;10:191–202. doi: 10.1017/S1461145706006638. [DOI] [PubMed] [Google Scholar]
- Yoshimura R, Yanagihara N, Hara K, Terao T, Nakamura J, Ueno S, Toyohira Y, Uezono Y, Kaneko S, Kawamura M, Abe K, Izumi F. Inhibitory effects of clozapine and other antipsychotic drugs on noradrenaline transporter in cultured bovine adrenal medullary cells. Psychopharmacology (Berl) 2000;149:17–23. doi: 10.1007/s002139900339. [DOI] [PubMed] [Google Scholar]
- Zimmet SV, Strous RD, Burgess ES, Kohnstamm S, Green AI. Effects of clozapine on substance use in patients with schizophrenia and schizoaffective disorder: a retrospective survey. J Clin Psychopharmacol. 2000;20:94–98. doi: 10.1097/00004714-200002000-00016. [DOI] [PubMed] [Google Scholar]