Abstract
Objective
To estimate the overall risk of cancer in a population-based cohort of patients with inflammatory bowel diseases (IBD), and how IBD-related medications modify this risk.
Methods
We identified all incident cancers (excluding non-melanoma skin cancer) after IBD diagnosis in a cohort of 839 patients diagnosed between January 1, 1940 and December 31, 2004 in Olmsted County, Minnesota, and followed over a median 18 years through December 31, 2011 (122 patients on biologic agents at last follow-up). We calculated standardized incidence ratios (SIR) with 95% confidence intervals (CIs) of all cancers, and compared cancer risk in patients treated with immunomodulators (IMM) and biologics with that of patients not exposed to these medications, using an incidence rate ratio (IRR).
Results
One hundred nine patients developed 135 cancers. The 10-year cumulative probability of cancer was 3.8%. Patients with CD (SIR, 1.6; 95% CI, 1.2–2.1), but not UC (SIR, 1.1; 95% CI, 0.8–1.4), had an increased overall risk of cancer, as compared to the general population. Patients treated with IMM (relative to IMM-naïve patients) had an increased risk of melanoma (IRR 5.3; 95% CI, 1.1–24.8) (and a numerically higher risk of hematological malignancies [IRR, 4.2; 95% CI, 0.9–19.2]), although this risk returned to baseline on discontinuation of IMM. Patients treated with biologics (relative to biologic-naïve patients) had a numerically higher risk of hematological malignancies (IRR, 5.3; 95% CI, 0.7–40.5). There was no significant increase in the risk of gastrointestinal malignancies in IBD patients, as compared to the general population.
Conclusions
We observed an increased risk of melanoma in IMM-treated patients with IBD, and this risk returned to baseline after discontinuation of medications.
Keywords: Cancer, immunomodulators, anti-tumor necrosis factor, inflammatory bowel disease, ulcerative colitis, Crohn’s disease
INTRODUCTION
Chronic gastrointestinal inflammation in inflammatory bowel disease (IBD) has been associated with increased risk of colitis-associated colorectal cancer (CRC).1 Besides CRC, IBD may also be associated with an increased risk of extra-intestinal cancers, in particular hematological malignancies and melanoma.2–6 However, results have been conflicting, in part due to different settings in which these studies have been conducted. Clinic-based studies are prone to selection and detection bias, and may over-estimate cancer risk. On the other hand, population-based studies from unselected cohorts of patients are more representative of the true cancer risk in patients with IBD, and are useful for prognostic information and life insurance estimates.
Predisposing factors for extra-intestinal cancers in patients with IBD are poorly understood. Besides gut-specific changes, IBD is also associated with systemic immune dysregulation leading to impairment of tumor surveillance.7,8 Besides the primary disease process, lifestyle changes and immunosuppressive therapy may modify cancer risk.9 The effect of immunosuppressive medications on cancer risk is of particular interest. Thiopurines have been associated with an increased risk of lymphomas and non-melanoma skin cancers (NMSC);4,10–12 it is unclear whether anti-tumor necrosis factor-α (anti-TNF) agents modify the risk of cancer, with conflicting evidence.13–15
Hence, the aims of this study were: (a) to estimate the cumulative incidence and relative risk of intestinal and extra-intestinal solid organ cancers, hematological malignancies and melanoma by IBD phenotype (UC and Crohn’s disease [CD]), as compared to the general population; and (b) to assess whether the use of medications used to treat IBD (5-aminosalicylates [5-ASA], corticosteroids, immunomodulators [IMM] in particular thiopurines, and anti-TNF agents) modifies the risk of cancer, in a population-based inception cohort of IBD patients from Olmsted County, Minnesota. We hypothesized that patients treated with thiopurines, but not those treated with 5-ASA or anti-TNF agents, would have an increased risk of hematological malignancies.
METHODS
Setting
Olmsted County, in southeastern Minnesota, has a population of 144,260.16 Eighty-three percent of the population is non-Hispanic white, and a substantial proportion is of North European ancestry. Residents of Olmsted County are socioeconomically comparable to the US white population, although a higher proportion are employed in health care services and have a higher level of education.17,18
Healthcare providers in Olmsted County are connected through a unique medical recordlinkage system (Rochester Epidemiology Project [REP]).19 The central diagnostic index of the REP comprises all diagnoses generated from outpatient evaluations, hospitalizations, emergency room evaluations, nursing home visits, surgical procedures, autopsy reports, and death certificates. It is therefore possible to identify all cases of a disease for which patients sought medical attention over a particular period of time.
Evaluation and Medication Use
All potential new cases of CD and UC were identified through the central diagnostic index.20 A diagnosis of CD and UC was confirmed based on standard clinical, endoscopic, radiologic and/or histologic criteria. We abstracted data on medications commonly used to treat IBD, and estimated duration of use using the prescription start and stop dates, through review of individual medical records. Medications were categorized into: sulfasalazine and 5-ASA, corticosteroids, IMM (azathioprine, 6-mercaptopurine, methotrexate, cyclosporine, tacrolimus) and anti-TNF agents (infliximab, adalimumab, certolizumab pegol). We classified patients as: (a) current user, if a patient was on medication at time of cancer diagnosis (or had been on the medication within 2 months prior to cancer diagnosis) and had been on the medication for at least 6 months; (b) former user, if the patient had been exposed to that medication prior to cancer diagnosis (more than 2 months prior to cancer diagnosis), but was not on the medication at time of cancer diagnosis; or (c) non-user, if the patient had never been exposed to that medication (or, in case of corticosteroids, was only given that medication as a pre-medication with administration of infliximab).
Cancer Diagnosis
Using REP, we identified all incident cancers in patients with IBD, with confirmation through medical record review. Cancers were classified into 7 categories based on the primary site involved – intestinal cancer (CRC, small intestinal, biliary tract, hepatocellular, gallbladder, pancreatic, gastric, esophageal and anal cancer), tobacco-related cancers (lung, head and neck including oral, bladder, kidney, and ureter), female reproductive organ cancers (ovarian, endometrial, cervical, vaginal/vulvar and breast), male reproductive organ cancers (testicular and prostate cancer), miscellaneous extra-intestinal cancers (prostate, brain, spinal cord, thyroid, melanoma), hematological cancers (Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, acute and chronic leukemia, multiple myeloma) and melanoma.21 We excluded NMSC from this analysis.
Statistical Analysis
Data were summarized as medians (range, interquartile range [IQR]) for quantitative variables and frequency for categorical variables. We recorded person-years at risk from the date of IBD diagnosis until the date of first cancer diagnosis (except NMSC), death, emigration or end of study period (December 31, 2011) for each individual in the cohort. We included only incident cancers occurring at least 30 days after IBD diagnosis to minimize the risk of detection bias and misclassification; patients with a history of cancer diagnosed prior to IBD diagnosis were still considered at risk of other organ cancers after IBD diagnosis. We estimated the risk of overall and site-specific cancer type, relative to the general population using SIR (observed/expected numbers) with 95% confidence intervals (CI) assuming a Poisson distribution; expected cancer risk was estimated using age- and sex-specific corresponding cancer rates from the Surveillance, Epidemiology, and End Results database (SEER) (white patients from Iowa, 1973–2000). The cumulative probability of any cancer from the diagnosis of CD or UC was estimated using the Kaplan–Meier survival method.
In order to estimate whether IBD-specific medications modify cancer risk, we calculated the incidence rate ratio (IRR) of cancer in current medication users as compared to non-users, by dividing the incidence rate of cancer among current users (number of cancers divided by person-years of follow-up on that medication) by the incidence rate of cancer among never users.4 To assess whether there was any residual effect on cancer risk after discontinuation of medication, we calculated the IRR of cancer in former users as compared to non-users.10 We also evaluated cancer risk among 5-ASA-treated, steroid-treated, IMM-treated and biologic-treated patients with IBD (ever use of medication), relative to the general population, using SIR with expected rates estimated from the SEER population.9
All statistical analyses were conducted using SAS version 9.2 for Windows (SAS Institute Inc., Cary, NC). P-values <.05 were considered statistically significant.
Ethics
This study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards. As per Minnesota state law, we did not include patients who had withdrawn authorization to review their medical records for research purposes.
RESULTS
Baseline Characteristics of the Entire IBD Cohort
We identified 839 patients with IBD (44.9% with CD). Baseline characteristics of these patients are reported in Table 1. Median age at diagnosis of patients with CD was 30.0 years (IQR, 22.2–45.2) and UC was 35.0 years (range, 25.0–48.5). Forty-one patients had a previous history of cancer, prior to IBD diagnosis (or within 30 days of IBD diagnosis). Of 686 patients with documented smoking status, 342 (50%) had ever smoked. One hundred eighty-four patients were treated with IMM (cumulative probability at 1 and 10 years, 7.5% and 17.1%, respectively), while 99 patients were treated with an anti-TNF agent (cumulative probability at 1 and 10 years, 1.6% and 8.3%, respectively).
Table 1.
Baseline demographic and clinical characteristics of patients in 839 patients diagnosed with IBD in Olmsted County, Minnesota, 1940–2011
| Baseline characteristics | Crohn’s Disease (n=377) |
Ulcerative Colitis (n=462) |
|---|---|---|
| Age at time of IBD diagnosis, in years (median, IQR) | 30.0 (22.2–45.2) | 35.0 (25.0–48.5) |
| • <40 years of age | 255 (67.6%) | 268 (58.0%) |
| • ≥40 years of age | 122 (32.4%) | 194 (42.0%) |
| Gender – Males (% total) | 185 (49.1%) | 264 (57.1%) |
| Follow-up, in years (median, IQR) | 16.4 (10.0–28.4) | 19.9 (11.0–29.2) |
| Duration of disease at time of last follow-up | ||
| • <10 years | 94 (24.9%) | 100 (21.6%) |
| • 10–19 years | 126 (33.4%) | 132 (28.6%) |
| • 20–29 years | 70 (18.6%) | 123 (26.6%) |
| • ≥30 years | 87 (23.1%) | 107 (23.2%) |
| Smoking status | ||
| • Current smokers | 42 (13.8%) | 19 (5.0%) |
| • Former smokers | 97 (31.5%) | 126 (33.3%) |
| Treatment at time of last follow-up (ever treated), n (cumulative probability of use at 1 year after diagnosis) | ||
| • 5-aminosalicylates | 279 (57.5%) | 380 (67.7%) |
| • Corticosteroids | 257 (37.1%) | 295 (40.7%) |
| • Immunomodulators | 184 (12.9%) | 60 (3.1%) |
| • Anti-TNF agents | 99 (3.0%) | 23 (0.4%) |
| Number of patients with cancer (excludes NMSC) | 47 (12.5%) | 62 (13.4%) |
| Median age at time of cancer diagnosis, years (IQR) | 61.7(50.5–71.8) | 66.0(51.7–72.3) |
IBD, inflammatory bowel disease; IQR, interquartile range; anti-TNF, anti-tumor necrosis factor; NMSC, non-melanoma skin cancer.
Over a median follow-up of 18.4 years, 109 patients with IBD developed 135 cancers. The cumulative probability of first cancer was 3.8% by 10 years, 9.1% by 20 years, and 16.3% by 30 years.
Overall and Site-Specific Cancer Risk in Crohn’s Disease
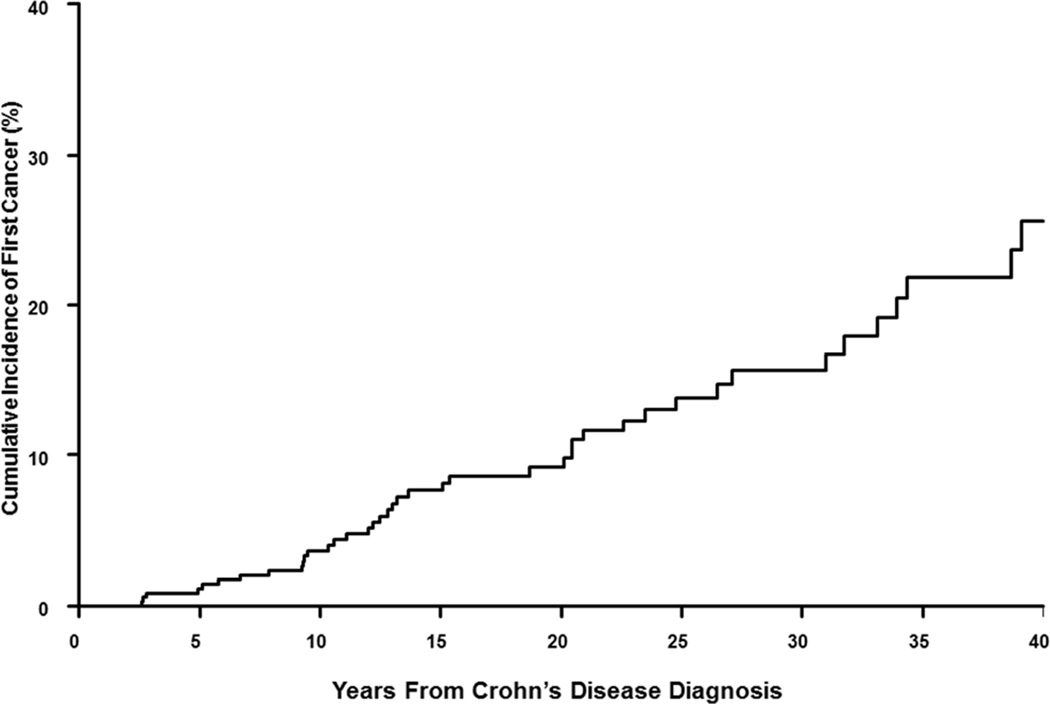
Over a total follow-up of 7487 person-years, 47 patients with CD developed 58 cancers. The 10-year cumulative probability of cancer after the diagnosis of CD was 3.6% (Figure 1A). Overall, patients with CD had a 57% higher risk of cancer as compared to an age- and sex-matched general population (SIR, 1.6; 95% CI, 1.2–2.1). This increased risk was statistically significant in females (SIR, 1.7; 95% CI, 1.1–2.6) but not in males (SIR, 1.4; 95% CI, 0.9–2.2).
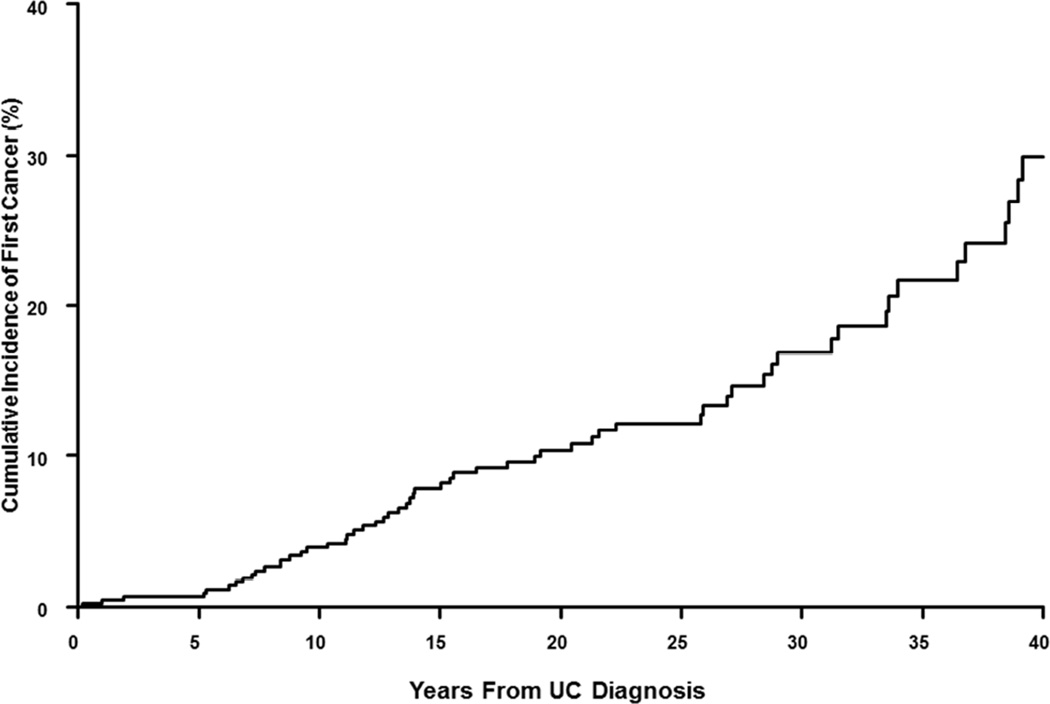
Figure 1.
Cumulative incidence of cancer after diagnosis of (A) Crohn’s disease and (B) ulcerative colitis in a population-based inception cohort of 839 patients with IBD in Olmsted County, Minnesota, 1940–2011
On category-specific analysis, a 3-fold higher risk of hematological malignancies (SIR, 3.0; 95% CI, 1.2–6.9) and a non-significant 2.7-fold higher risk of melanoma (SIR, 2.7; 95% CI, 0.9–6.2) was observed (Table 2A). On site-specific analysis, CD was associated with increased risk of small intestinal cancer (SIR, 9.4; 95% CI, 1.1–33.9), biliary tract cancer (SIR, 21.2; 95% CI, 4.4–61.8), Hodgkin’s lymphoma (SIR, 11.3; 95% CI, 2.3–33.1) and brain cancer (SIR, 4.9; 95% CI, 1.0–14.4); the risk of other solid-organ cancers was not significantly increased, including CRC (SIR, 0.6; 95% CI, 0.1–1.7) (Supplementary Table 1).
Table 2.
Standardized incidence ratios of category-specific cancers in (A) 377 patients with CD and (B) 462 patients with UC, compared with expected numbers in a SEER-based age- and sex-matched general population, in Olmsted County, Minnesota, 1940–2011. Cancers were classified into 7 categories based on the primary site involved – intestinal cancer (including CRC, small intestinal, biliary tract, hepatocellular, gallbladder, pancreatic, gastric, esophageal and anal cancer), tobacco-related cancers (lung, head and neck including oral, bladder, kidney, and ureter), female reproductive organ cancers (ovarian, endometrial, cervical, vaginal/vulvar and breast), male reproductive organ cancers (testicular and prostate cancer), miscellaneous extra-intestinal cancers (prostate, brain, spinal cord, thyroid, melanoma), hematological cancers (Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, acute and chronic leukemia, multiple myeloma) and melanoma. Please note, figures in bold represent statistically significant results.
| (A) | ||||||
|---|---|---|---|---|---|---|
| Cancer Category | Males | Females | Overall | |||
| Observed | Expected | Observed | Expected | SIR | 95% CI | |
| Intestinal cancers | 6 | 4.03 | 5 | 4.01 | 1.37 | 0.68–2.45 |
| Tobacco-related cancers | 7 | 6.06 | 5 | 4.40 | 1.15 | 0.59–2.00 |
| Male reproductive organ cancers | 7 | 6.00 | NA | NA | 1.17 | 0.47–2.40 |
| Female reproductive organ cancers | NA | NA | 12 | 9.65 | 1.24 | 0.64–2.17 |
| Miscellaneous extra-intestinal solid organ cancers | 2 | 0.92 | 3 | 0.97 | 2.64 | 0.86–6.15 |
| Hematological malignancies | 3 | 0.92 | 3 | 0.97 | 3.17 | 1.16–6.89 |
| Melanoma | 4 | 0.89 | 1 | 0.98 | 2.67 | 0.87–6.23 |
| (B) | ||||||
|---|---|---|---|---|---|---|
| Cancer Category | Males | Females | Overall | |||
| Observed | Expected | Observed | Expected | SIR | 95% CI | |
| Intestinal cancers | 6 | 9.08 | 7 | 5.51 | 0.89 | 0.47–1.52 |
| Tobacco-related cancers | 11 | 14.06 | 3 | 5.95 | 0.70 | 0.38–1.17 |
| Male reproductive organ cancers | 14 | 13.14 | NA | NA | 1.07 | 0.58–1.79 |
| Female reproductive organ cancers | NA | NA | 15 | 12.09 | 1.24 | 0.69–2.05 |
| Miscellaneous extra-intestinal solid organ cancers | 2 | 1.86 | 2 | 1.12 | 1.34 | 0.37–3.44 |
| Hematological malignancies | 5 | 1.86 | 3 | 1.12 | 2.69 | 1.16–5.29 |
| Melanoma | 4 | 1.84 | 2 | 1.13 | 2.02 | 0.74–4.40 |
SIR, standardized incidence ratio; 95% CI, 95% confidence interval.
Overall and Site-Specific Cancer Risk in Ulcerative Colitis
Over a total follow-up of 9822 person-years, 62 patients with UC developed 77 cancers. The 10-year cumulative probability of cancer after the diagnosis of UC was 4.9% (Figure 1B). Overall, patients with UC did not have a higher risk of cancer than an age- and sex-matched general population (SIR, 1.1; 95% CI, 0.8–1.4). On sex-specific analysis, females with UC had a 59% higher risk of cancer as compared to females in the general population (SIR, 1.6; 95% CI, 1.1–2.3); there was no increase in the overall risk of cancer in males with UC (SIR, 0.9; 95% CI, 0.6–1.2).
On category-specific analysis, we observed a 2.7-fold higher risk of hematological malignancies (SIR, 2.7; 95% CI, 1.2–5.3) in patients with UC; the risk of melanoma was numerically, but not statistically, higher than expected (SIR, 2.0; 95% CI, 0.7–4.4) (Table 2B). On site-specific analysis, UC was associated with increased risk of biliary tract cancer (SIR, 11.4; 95% CI, 2.4–33.2) and decreased risk of lung cancer (SIR, 0.3; 95% CI, 0.1–0.9); the risk of other solid-organ cancers was not significantly increased, including CRC (SIR, 0.7; 95% CI, 0.2–1.4) (Supplementary Table 2).
Risk of Cancer by Medications Used to Treat IBD
We did not find any increase in the overall risk of cancer in patients treated with 5-ASA (vs. 5-ASA-naïve patients; IRR, 1.0; 95% CI, 0.5–1.8), corticosteroids (vs. corticosteroid-naïve patients; IRR, 1.4; 95% CI, 0.5–3.4), IMM (vs. IMM-naïve patients; IRR, 0.9; 95% CI, 0.3–2.3) or anti-TNF agents (vs. anti-TNF-naïve patients; IRR, 0.6; 95% CI, 0.1–4.5) (Table 3A). On category-specific analysis, we observed an increased risk of melanoma in corticosteroid-treated (IRR, 8.2; 95% CI, 1.4–49.2) or IMM-treated patients (IRR, 5.3; 95% CI, 1.1–24.8); no cases of melanoma were observed in anti-TNF treated patients. There was a numerically, but not statistically, higher risk of hematological malignancies in patients treated with corticosteroids (IRR, 4.9; 95% CI, 0.9 –25.1), IMM (IRR, 4.2; 95% CI, 0.9–19.2) and anti-TNF agents (IRR, 5.3; 95% CI, 0.7–40.5). Hematological malignancies observed in IMM-treated patients were Hodgkin’s (1 patient) and non-Hodgkin’s lymphoma (1 patient); 4 patients had EBV-positive lymphoma.
Table 3.
Incidence rate ratio of category-specific cancer risk in patients with IBD (A) on-treatment (current users v. never users) and (B) off-treatment (former users v. never users). Please note, figures in bold represent statistically significant results
| (A) Cancer risk in patients with IBD on-treatment (current users v. never-users) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 5 – aminosalicylates | Corticosteroids | Immunomodulators | Anti-TNF agents | |||||
| Cancer Category | IRR | 95% CI | IRR | 95% CI | IRR | 95% CI | IRR | 95% CI |
| All | 0.97 | 0.52 – 1.8 | 1.37 | 0.54 – 3.45 | 0.85 | 0.31 – 2.31 | 0.63 | 0.09 – 4.52 |
| Intestinal cancers | 1.20 | 0.37 – 3.94 | 1.22 | 0.16 – 9.50 | 0.0 | - | 0.0 | - |
| Tobacco-related cancers | 1.05 | 0.33 – 3.29 | 2.76 | 0.60 – 12.79 | 0.87 | 0.12 – 6.44 | 0.0 | - |
| Male reproductive organ cancers | 0.73 | 0.13 – 3.98 | 0.0 | - | 1.11 | 0.15 – 8.26 | 0.0 | - |
| Female reproductive organ cancers | 0.82 | 0.24 – 2.82 | 0.0 | - | 0.85 | 0.11 – 6.25 | 0.0 | - |
| Miscellaneous extra-intestinal solid organ cancers | 0.72 | 0.07 – 7.96 | 0.0 | - | 0.0 | - | 0.0 | - |
| Hematological malignancies | 0.48 | 0.05 – 4.63 | 4.88 | 0.95 – 25.13 | 4.21 | 0.92 – 19.20 | 5.29 | 0.69–40.47 |
| Melanoma | 0.0 | - | 8.23 | 1.38 – 49.25 | 5.26 | 1.12 – 24.77 | 0.0 | - |
| (B) Cancer risk in patients with IBD off-treatment (former users v. never-users) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 5 – aminosalicylates | Corticosteroids | Immunomodulators | Anti-TNF agents | |||||
| Cancer Category | IRR | 95% CI | IRR | 95% CI | IRR | 95% CI | IRR | 95% CI |
| All | 1.23 | 0.77 – 1.94 | 1.27 | 0.86 – 1.88 | 1.11 | 0.54 – 2.29 | 1.00 | 0.32 – 3.16 |
| Intestinal cancers | 0.98 | 0.37 – 2.57 | 1.23 | 0.54 – 2.82 | 1.22 | 0.29 – 5.18 | 0.0 | - |
| Tobacco-related cancers | 0.91 | 0.37 – 2.26 | 1.60 | 0.70 – 3.65 | 0.56 | 0.08 – 4.12 | 3.01 | 0.71 – 12.73 |
| Male reproductive organ cancers | 1.71 | 0.57 – 5.14 | 1.55 | 0.64 – 3.74 | 0.70 | 0.09 – 5.26 | 1.78 | 0.24 – 13.30 |
| Female reproductive organ cancers | 1.04 | 0.43 – 2.53 | 1.02 | 0.48 – 2.18 | 0.54 | 0.08 – 3.97 | 0.0 | - |
| Miscellaneous extra-intestinal solid organ cancers | 1.36 | 0.27 – 6.71 | 1.19 | 0.32 – 4.44 | 0.0 | 0.0 | - | |
| Hematological malignancies | 1.51 | 0.42 – 5.48 | 1.33 | 0.42 – 4.2 | 2.74 | 0.60 – 12.52 | 0.0 | - |
| Melanoma | 1.21 | 0.32 – 4.57 | 1.92 | 0.48 – 7.66 | 1.69 | 0.21 – 13.49 | 0.0 | - |
IBD, inflammatory bowel disease; anti-TNF, anti-tumor necrosis factor; IRR, incidence rate ratio.
In order to evaluate whether the increased risk of cancer persisted after discontinuing medications, we compared overall and category-specific cancer risk in patients previously exposed to those medications with IBD patients never exposed to those medications (former users vs. never users). On this analysis, we observed that the overall risk of cancer was not significantly different in former users of 5-ASA, corticosteroids, IMM or anti-TNF agents, compared to patients with IBD who had never been treated with these agents, respectively (Table 3B). The increased risk of melanoma observed in current users of corticosteroids and IMM decreased back to baseline off-treatment. Risk of hematological malignancies in former users of corticosteroids (IRR, 1.3; 95% CI, 0.4–4.2) and IMM (IRR, 2.7; 95% CI, 0.6–12.5) was comparable to the risk observed in patients with IBD never exposed to these patients.
On comparing cancer risk in patients treated with IBD-related medications to the general population, we did not find any significant increase in overall or category-specific cancer risk in 5-ASA, corticosteroid, IMM or anti-TNF treated patients with IBD, except an increase in the risk of hematological malignancies in IMM-treated patients with IBD (Supplementary Table 3).
DISCUSSION
In our population-based cohort study of 839 patients with IBD followed over a median 18 years, we made several key observations. First, we confirmed a modest increase in the overall risk of cancer in patients with CD, but not in UC, as compared with the general population. Second, we observed a 3-fold higher risk of hematological malignancies, both in patients with CD and UC, as compared to the general population, although the overall incidence was low (<0.1% annually); the risk of melanoma was also numerically higher in patients with IBD. Third, we observed an increased risk of melanoma in current users of corticosteroids and IMM; there was a numerically, but not statistically, higher risk of hematological malignancies in corticosteroid-, IMM- and anti-TNF-treated patients. Finally, the relatively increased risk of melanoma and hematological malignancies observed while on specific IBD-related medications returned to baseline after discontinuation of the implicated treatment.
A modestly increased risk of overall cancer incidence3,9,21,22 and mortality23 has been observed in patients with CD, and not in patients with UC. In their recent Danish population-based cohort study, Jess and colleagues observed a 55% higher risk of cancer after CD diagnosis, but no similar increase in cancer risk in patients with UC.9 Systemic immune dysregulation and more prevalent use of immunosuppressive therapy in CD may impair tumor surveillance and predispose these patients to extra-intestinal cancers. We also observed a lower risk of lung cancer in patients with UC compared to the general population, similar to previous observations,22 which may be related to a lower proportion of smokers in patients with UC.
We observed that patients with IBD treated with IMM (primarily thiopurines) had an increased risk of melanoma (and a numerically higher risk of hematological malignancies). Melanoma is an immunogenic tumor;24 systemic IMM may drive the growth of dysplastic nevi, which are strong risk factors for melanoma, by down-regulating tumor surveillance mechanisms, increasing susceptibility to infection with oncogenic viruses such as melanoma-associated retroviruses, or through a direct pharmacologic effects on DNA metabolism.24,25 Although thiopurine analogs have been associated with an increased risk of NMSC, there are only a limited number of studies assessing the risk of melanoma in patients with IBD treated with thiopurines. In a large US administrative database, Long et al did not find an increased risk of melanoma in thiopurine-exposed patients.4 However, follow-up in their cohort was short, and all patients ever-exposed to thiopurines (current or former users) were grouped together. In contrast, in our cohort, the increased risk of melanoma was only seen in current users of thiopurines, and this risk decreased to baseline upon discontinuation of medications. The increased risk of hematological malignancies with thiopurines, particularly EBV-positive lymphoma, has been reported in multiple previous cohort studies, as well as meta-analysis.10–12,26 Importantly, we observed that this increased risk of hematological malignancies decreased back to baseline (comparable to hematological malignancy risk in IMM-naïve patients) after stopping medication, similar to previous findings;10,11 we acknowledge that these observations were not statistically significant.
Patients treated with corticosteroids were also at an increased risk of melanoma (and had a numerically, but not statistically, higher risk of hematological malignancies), as compared to corticosteroid-naïve patients. Corticosteroids have pleiotropic immunosuppressive effects,27 and by impairing tumor surveillance, it is possible that prolonged prednisone use may directly increase the risk of immunogenic tumors. Alternatively, it is possible that this increased malignancy risk observed in corticosteroid-treated patients may not be a direct effect of corticosteroids, but rather represents confounding by severity. In our practice, IMM were used primarily in steroid-dependent patients with IBD, and hence, corticosteroid-treated patients were more likely to have concomitantly received IMM as steroid-sparing agents; the observed malignancy risk may be attributable to increased risk afforded by thiopurine use.
We also observed that patients treated with anti-TNF agents had a numerically, but not statistically, higher risk of hematological malignancies. Prospective cohort studies have reported an increased risk of lymphoproliferative disorders with combination anti-TNF and IMM therapy, but not with anti-TNF monotherapy.12,26,28,29 In our cohort, only one patient (out of 14) was on anti-TNF monotherapy at time of diagnosis of lymphoma. In a recent Danish nationwide cohort study of 56,146 patients with IBD, anti-TNF use (as compared to non-use) was not associated with increased risk of hematological malignancies, after adjusting for age, disease duration, baseline propensity scores and use of IBD-related medications (5-ASA, corticosteroids and IMM) (relative risk, 0.90; 95% CI, 0.42–1.91).30
Our study has several strengths. First, ours represents a well-characterized population-based inception cohort of IBD patients, in a defined geographic area with a stable population, over an extended period of time, and hence is truly representative of the general population. Second, complete and long follow-up over 2 decades allows better assessment of rare outcomes such as cancer. Third, our medical records linkage system allowed pathological confirmation of each cancer case, minimizing the risk of misclassification. Finally, we were able to study the effect of medications on cancer risk in patients with IBD, examining the risk both on-treatment and after discontinuation of treatment, and comparing it to patients with IBD who had never been exposed to these agents.
Our study also has certain limitations that merit discussion. First, our sample size was fixed due to the population-based nature of this study, and hence, may have been underpowered for some detailed pharmacoepidemiological analyses; we did not correct for multiple statistical comparisons. Second, caution must be exercised in interpreting the independent effect of each IBD-related medication on cancer risk. A significant proportion of patients were concomitantly on multiple medications, especially corticosteroids and IMM. Due to the small number of events, multivariate analysis after adjusting for concomitant use of other medications was not possible. Third, duration of follow-up on each medication was variable, with shorter follow-up in patients treated with anti-TNF agents, and this may have influenced the observed cancer risk with these medications. Finally, due to small sample size, we were limited in stratified analyses based on covariates that may modify cancer risk, such as cigarette smoking, duration of IBD, disease location or phenotype and past history of cancer.
Conclusion
In our population-based inception cohort of patients with IBD in Olmsted County from 1940–2011 with median follow-up of 18 years, we observed a modest increase in overall risk of cancer in patients with CD, but not in patients with UC. This observed increase in risk was due to high incidence of hematological malignancies and melanoma, although the overall incidence was low (<0.1% annually). Besides inherent disease-specific processes predisposing to these cancers, immunosuppressive therapy with corticosteroids, IMM or anti-TNF agents may also contribute to this risk. Importantly, this risk reverses to baseline levels after discontinuation of these medications. Future prospective studies evaluating the effect of combination immunosuppressive therapy versus monotherapy are warranted to clarify the impact of dual- or triple-immunosuppression.
Supplementary Material
ACKNOWLEDGEMENTS
Supported in part by the Mayo Foundation for Medical Education & Research, and made possible by the Rochester Epidemiology Project (Grant number R01 AG034676 from the National Institute on Aging).
Dr. Loftus has consulted for AbbVie, UCB, Janssen, Takeda, Immune Pharmaceuticals, Medimmune, Celgene, and Theradiag, and has received research support from AbbVie, UCB, Janssen, Takeda, Shire, GlaxoSmithKline, Bristol-Myers Squibb, Pfizer, Amgen, Genentech, and Robarts Clinical Trials.
ABBREVIATIONS
- 5-ASA
5-aminosalicylate
- Anti-TNF
Anti-tumor necrosis factor-α agents
- CD
Crohn’s disease
- CRC
Colorectal Cancer
- IBD
Inflammatory bowel diseases
- IMM
Immunomodulator
- IRR
Incidence Rate Ratio
- NMSC
Non-melanoma skin cancer
- REP
Rochester Epidemiology Project
- SEER
Surveillance, Epidemiology, and End Results
- SIR
Standardized Incidence Ratio
- UC
Ulcerative colitis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the 77th Annual Scientific Meeting of the American College of Gastroenterology, Las Vegas, NV, October 2012 (Yadav S, Harmsen W, Zinsmeister A, Tremaine W, Loftus EV, Jr.. Cancer risk in a population-based cohort of inflammatory bowel disease. Am J Gastroenterol. 2012; 107:S627-8) and the 78th Annual Scientific Meeting of the American College of Gastroenterology, San Diego, CA, October 2013 (Yadav S, Singh S, Edakkanambeth Varayil J, Tremaine W, Loftus EV, Jr. Cumulative incidence of cancer in a population-based cohort of inflammatory bowel disease. Am J Gastroenterol. 2013;108:S518)
Disclosures: None of the other authors have any financial disclosures.
Conflicts of Interest: None
Author Contributions: SY – Study conception, data collection and interpretation, writing draft of the manuscript; SS – Statistical analysis and interpretation of data, writing draft of the manuscript; WSH – Statistical analysis and interpretation of data, critical revision of the manuscript; JEV – Data collection and interpretation, critical revision of the manuscript; WJT – Critical revision of the manuscript; EVL – Study conception, data collection and interpretation, statistical analysis, critical revision of the manuscript
REFERENCES
- 1.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001 Apr;48(4):526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Askling J, Brandt L, Lapidus A, et al. Risk of haematopoietic cancer in patients with inflammatory bowel disease. Gut. 2005 May;54(5):617–622. doi: 10.1136/gut.2004.051771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001 Feb 15;91(4):854–862. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Long MD, Martin CF, Pipkin CA, Herfarth HH, Sandler RS, Kappelman MD. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012 Aug;143(2):390–399. doi: 10.1053/j.gastro.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh S, Nagpal SJ, Murad MH, et al. Inflammatory Bowel Disease Is Associated With an Increased Risk of Melanoma: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2014 Feb;12(2):210–218. doi: 10.1016/j.cgh.2013.04.033. [DOI] [PubMed] [Google Scholar]
- 6.Sokol H, Beaugerie L, Maynadie M, et al. Excess primary intestinal lymphoproliferative disorders in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2012 Nov;18(11):2063–2071. doi: 10.1002/ibd.22889. [DOI] [PubMed] [Google Scholar]
- 7.Pietersma F, Piriou E, van Baarle D. Immune surveillance of EBV-infected B cells and the development of non-Hodgkin lymphomas in immunocompromised patients. Leuk Lymphoma. 2008 Jun;49(6):1028–1041. doi: 10.1080/10428190801911662. [DOI] [PubMed] [Google Scholar]
- 8.Chow MT, Moller A, Smyth MJ. Inflammation and immune surveillance in cancer. Semin Cancer Biol. 2012 Feb;22(1):23–32. doi: 10.1016/j.semcancer.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Jess T, Horvath-Puho E, Fallingborg J, Rasmussen HH, Jacobsen BA. Cancer risk in inflammatory bowel disease according to patient phenotype and treatment: a danish population-based cohort study. Am J Gastroenterol. 2013 Dec;108(12):1869–1876. doi: 10.1038/ajg.2013.249. [DOI] [PubMed] [Google Scholar]
- 10.Khan N, Abbas AM, Lichtenstein GR, Loftus EV, Jr, Bazzano LA. Risk of lymphoma in patients with ulcerative colitis treated with thiopurines: a nationwide retrospective cohort study. Gastroenterology. 2013 Nov;145(5):1007–1015. doi: 10.1053/j.gastro.2013.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Beaugerie L, Brousse N, Bouvier AM, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009 Nov 7;374(9701):1617–1625. doi: 10.1016/S0140-6736(09)61302-7. [DOI] [PubMed] [Google Scholar]
- 12.Herrinton LJ, Liu L, Weng X, Lewis JD, Hutfless S, Allison JE. Role of thiopurine and anti-TNF therapy in lymphoma in inflammatory bowel disease. Am J Gastroenterol. 2011 Dec;106(12):2146–2153. doi: 10.1038/ajg.2011.283. [DOI] [PubMed] [Google Scholar]
- 13.Hudesman D, Lichtiger S, Sands B. Risk of extraintestinal solid cancer with anti-TNF therapy in adults with inflammatory bowel disease: review of the literature. Inflamm Bowel Dis. 2013 Mar;19(3):644–649. doi: 10.1097/MIB.0b013e318280ebbd. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Olivo MA, Tayar JH, Martinez-Lopez JA, et al. Risk of malignancies in patients with rheumatoid arthritis treated with biologic therapy: a meta-analysis. JAMA. 2012 Sep 5;308(9):898–908. doi: 10.1001/2012.jama.10857. [DOI] [PubMed] [Google Scholar]
- 15.Osterman MT, Sandborn WJ, Colombel JF, et al. Increased Risk of Malignancy with Adalimumab Combination Therapy, Compared to Monotherapy, for Crohn's Disease. Gastroenterology. 2014 Apr;146(4):941–949. doi: 10.1053/j.gastro.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 16.United States Census Bureau, 2010. [Accessed July 4, 2014];2014 Available at: http://quickfacts.census.gov/qfd/states/27/27109.html.
- 17.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012 Dec;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012 Feb;87(2):151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012 Dec;41(6):1614–1624. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ingle SBLE, Tremaine WJ, et al. Increasing incidence and prevalence of inflammatory bowel disease in Olmsted County, Minnesota, 2001–2004. Gastroenterology. 2007;132:A19–A20. [Google Scholar]
- 21.Kappelman MD, Farkas DK, Long MD, et al. Risk of Cancer in Patients With Inflammatory Bowel Diseases: A Nationwide Population-based Cohort Study With 30 Years of Follow-up Evaluation. Clin Gastroenterol Hepatol. 2014 Feb;12(2):265–273. doi: 10.1016/j.cgh.2013.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pedersen N, Duricova D, Elkjaer M, Gamborg M, Munkholm P, Jess T. Risk of extra-intestinal cancer in inflammatory bowel disease: meta-analysis of population-based cohort studies. Am J Gastroenterol. 2010 Jul;105(7):1480–1487. doi: 10.1038/ajg.2009.760. [DOI] [PubMed] [Google Scholar]
- 23.Duricova D, Pedersen N, Elkjaer M, Gamborg M, Munkholm P, Jess T. Overall and cause-specific mortality in Crohn's disease: a meta-analysis of population-based studies. Inflamm Bowel Dis. 2010 Feb;16(2):347–353. doi: 10.1002/ibd.21007. [DOI] [PubMed] [Google Scholar]
- 24.Kubica AW, Brewer JD. Melanoma in immunosuppressed patients. Mayo Clin Proc. 2012 Oct;87(10):991–1003. doi: 10.1016/j.mayocp.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greene MH, Young TI, Clark WH., Jr Malignant melanoma in renal-transplant recipients. Lancet. 1981 May;1(8231):1196–1199. doi: 10.1016/s0140-6736(81)92359-x. [DOI] [PubMed] [Google Scholar]
- 26.Siegel CA, Marden SM, Persing SM, Larson RJ, Sands BE. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn's disease: a meta-analysis. Clin Gastroenterol Hepatol. 2009 Aug;7(8):874–881. doi: 10.1016/j.cgh.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillis S, Crabtree GR, Smith KA. Glucocorticoid-induced inhibition of T cell growth factor production. I. The effect on mitogen-induced lymphocyte proliferation. J Immunol. 1979 Oct;123(4):1624–1631. [PubMed] [Google Scholar]
- 28.Haynes K, Beukelman T, Curtis JR, et al. Tumor necrosis factor alpha inhibitor therapy and cancer risk in chronic immune-mediated diseases. Arthritis Rheum. 2013 Jan;65(1):48–58. doi: 10.1002/art.37740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lichtenstein GR, Rutgeerts P, Sandborn WJ, et al. A pooled analysis of infections, malignancy, and mortality in infliximab- and immunomodulator-treated adult patients with inflammatory bowel disease. Am J Gastroenterol. 2012 Jul;107(7):1051–1063. doi: 10.1038/ajg.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nyboe Andersen N, Pasternak B, Basit S, et al. Association between tumor necrosis factor-alpha antagonists and risk of cancer in patients with inflammatory bowel disease. JAMA. 2014 Jun;311(23):2406–2413. doi: 10.1001/jama.2014.5613. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.