Abstract
Background
Bronchopulmonary dysplasia (BPD) is a commonly used outcome for randomized neonatal trials.
Objectives
To determine whether a diagnosis of BPD or respiratory morbidity (RM1 or RM2) at 12 months corrected age better predicted subsequent respiratory morbidity in extremely low gestational age infants (23–28 weeks of gestation).
Methods
Initial analysis was undertaken in a development cohort of 76 infants who underwent pulmonary function tests (PFTs) at 12 months corrected age. Parents completed infant respiratory diaries two weeks pre PFTs. Analysis was then undertaken in a validation cohort of 227 infants whose parents completed a four week respiratory diary when their infant was 12 months corrected age. BPD at 28 days (BPD28d) and 36 weeks post menstrual age (BPD36w), RM1 (≥ three days and/or nights of cough, wheeze, and/or medicine use) and RM2 (≥ four days and/or nights of cough, wheeze and/or respiratory medicine use) each week for two weeks at 12 months corrected age were assessed with regard to prediction of respiratory outcomes at 24 months documented by respiratory health questionnaires.
Results
BPD28d and BPD36w were not significantly associated with any respiratory outcome. Areas under the receiver operator curves were significantly better for either definition of RM than BPD28d or BPD36w for all outcomes.
Conclusions
Respiratory morbidity documented by parental completed diaries at 12 months corrected age better predicted respiratory outcome at 24 months corrected age than BPD regardless of diagnostic criteria.
Keywords: bronchopulmonary dysplasia, pulmonary function, premature infant, respiratory outcome
INTRODUCTION
Infants born at extremely low gestational ages frequently develop bronchopulmonary dysplasia (BPD) and chronic respiratory morbidity [1]. Chronic respiratory morbidity is important as it increases healthcare utilization and the related healthcare costs, as well as adversely impacting on the lives of affected children and their families. Hence, it is essential to determine how chronic respiratory morbidity is best predicted and hence appropriate interventions be most effectively targeted. The incidence of survival without BPD is a commonly used primary outcome in clinical trials, although a diagnosis of BPD may, however, correlate poorly with respiratory morbidity in the first years after birth. For example, Tyson and colleagues studied 807 infants randomised to placebo or Vitamin A and found a small, but significant reduction in the incidence of BPD in infants receiving Vitamin A [2]. Yet, a follow-up study when the infants were one year corrected age revealed no benefits in longer term pulmonary outcome [3]. In contrast, in a randomised trial, recombinant human superoxide dismutase (rhSOD) was not associated with a reduction in the combined outcome of death or BPD at 36 weeks post-menstrual age (PMA). A follow-up study, however, demonstrated significant reductions in episodes of respiratory illness severe enough to require the use of respiratory medications at 12 months corrected age and reductions in hospital admissions and emergency room visits in the highest risk infants who received rhSOD [4].
Parent completed diary cards, to assess respiratory status and respiratory morbidity at follow up have been developed for prematurely born infants [5, 6]. The aim of this study was to determine whether respiratory morbidity as recorded by parental completed diary cards at one year corrected was a better predictor of subsequent respiratory morbidity at follow up, that is at two years of age, than a diagnosis of BPD, whether defined as oxygen dependency at 28 days after birth (BPD28d) or 36 weeks PMA (BPD36w).
METHODS
Study population
The subjects were part of the United Kingdom Oscillation Study (UKOS) [7]. Infants born between 23 and 28 weeks gestational age entered into UKOS were randomised to high frequency oscillation or conventional mechanical ventilation within one hour of birth. There were no statistically significant differences between the two groups in short-term pulmonary outcomes, pulmonary function results at one year [8] or respiratory morbidity up to 24 months corrected age [9], hence the results were pooled for this study. The South Thames Multicentre Research Ethics Committee and the local research-ethics committee at each participating centre approved the studies.
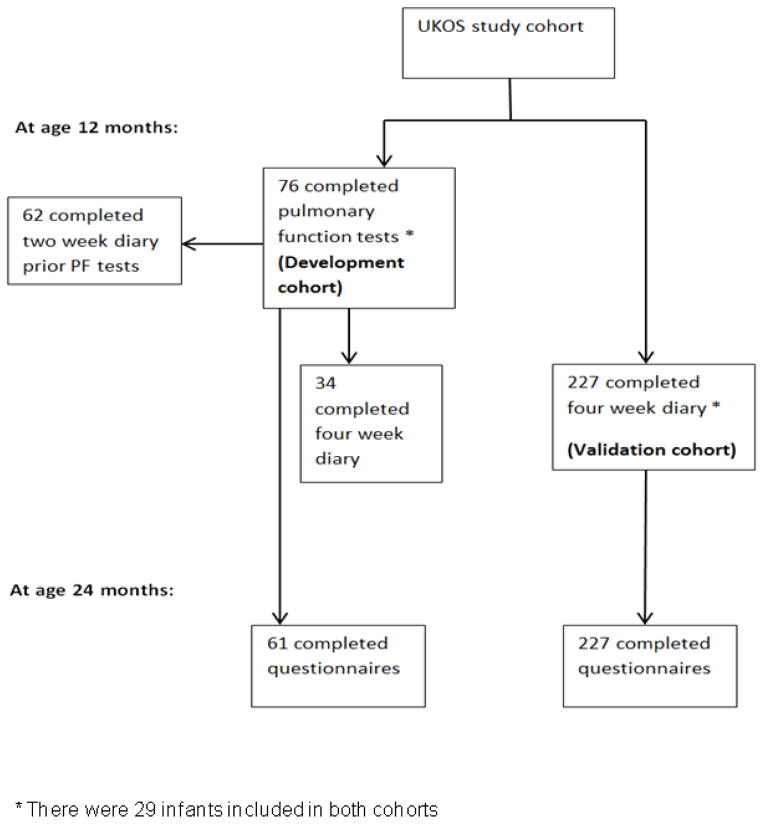
Initial analysis in this study was undertaken in a development cohort who were a subset of 76 infants who participated in detailed pulmonary function assessments at 12 months corrected age (pulmonary function subset) [8]. Subsequent analysis was undertaken in a validation cohort of 227 UKOS infants whose parents completed a four week diary card when their infant was 12 months corrected age (Figure 1).
Figure 1.
Flow diagram of the cohorts
Diary card and respiratory questionnaire
Prior to the PFTs, parents were asked to complete a two week infant respiratory diary card to assess whether or not their infant was too symptomatic to undergo sedation for pulmonary function testing. Parents recorded daily (both day and night) whether their child had coughed, wheezed and/or had taken respiratory medication (inhaled bronchodilators and/or inhaled/systemic corticosteroids).
Paediatricians completed a respiratory questionnaire with parents when their infant was 24 months corrected age during routine follow-up visits. The questionnaires recorded parental reports of whether the child had suffered from cough or wheeze, taken any medication to control or prevent respiratory symptoms or had been admitted to the hospital for respiratory illnesses (all were analysed as yes/no).
Analysis
The definition of respiratory morbidity (RM1) was determined a priori as being at least three days per week of the child having cough, wheeze, and/or use of respiratory medicines (all recorded as yes/no), each week during the two week pre-PFT period (RM1). This definition was chosen as it was felt likely to reflect on going respiratory morbidity rather than short lasting symptoms associated with an acute respiratory tract infection. A sensitivity analysis was then performed using at least four days and/or nights of cough, wheeze, and/or use of respiratory medicines each week for the two week pre PFT period (RM2). For the initial analysis data from the two-week diary was used (n=62 were completed).
The association of BPD28d and BPD36w with the respiratory outcomes at 24 months corrected age were examined. The area under the ROC curve was calculated to compare the strength of association between variables. The associations between respiratory outcomes and RM1 and RM2 were then examined. The strength of associations of BPD28, BPD36 and RM1 and RM2 with respiratory outcomes at 24 months corrected age were compared using the test of equality of ROC areas [10].
A further validation cohort of thirty-four infants who underwent pulmonary function testing and whose parents completed four week diary cards were used to validate the definitions of RM1 and RM2. The sensitivity and specificity, positive and negative predictive values of RM1 and RM2for respiratory outcomes at 24 months corrected age were then calculated using the prior definition and the sensitivity definition described above. A further sensitivity analysis was performed to assess the predictive ability of RM1 and RM2 but using the 227 UKOS infants whose parents completed the four week diary cards at 12 months corrected age. All analyses were performed using Stata v12.1.
RESULTS
Eighty-four percent of the 76 infants in the development cohort were oxygen dependent at 28 days after birth (BPD28d) and 59% were oxygen dependent at 36 weeks PMA (BPD36w) (Table 1). The demographics of the 227 infants in the validation cohort whose results were included in the subsequent analysis were similar (Table 1).
Table 1.
Demographics of the infants who underwent pulmonary function testing (development cohort) and of infants whose parents completed the four week diary card (validation cohort)
| The data are presented as median (range) or n (%) | ||
|---|---|---|
| Development cohort | Validation cohort | |
| N | 76 | 227 |
| Male | 42 (55%) | 113 (50%) |
| Gestational age (week) | 26 [23 to 28] | 27 [23 to 28] |
| Birth weight (g) | 870 [458 to 1335] | 890 [500 to 1459] |
| Birth weight z-score | −0.47 [−3.45 to 1.73] | −0.46 [−3.30 to 2.41] |
| Small for gestational age (birth weight z-score <−1.28) | 13 (17%) | 53 (23%) |
| Multiple birth | 16 (21%) | 59 (26%) |
| Race | ||
| White | 56 (74%) | 208 (92%) |
| black | 13 (17%) | 8 (4%) |
| Other | 7 (9%) | 11 (5%) |
| Maternal smoking in pregnancy | 13 (20%) | 46 (20%) |
| Oxygen dependent at 28 days | 64 (84%) | 190 (84%) |
| Oxygen dependent at 36 wk PMA | 45 (59%) | 142 (63%) |
| Oxygen dependent at discharge | 14 (18%) | 50 (22%) |
| Days on ventilator support | 14 [0 to 112] | 8 [0 to 62] |
| Air leak | 11 (14%) | 28 (12%) |
In the development cohort, neither BPD28d nor BPD36w were significantly related to any respiratory outcome (Table 2). In the validation cohort whose parents completed the four week diary cards, there was no evidence that either definition of BPD was related to any of the respiratory outcomes at 24 months corrected age (Table 2). All respiratory outcomes at 24 months corrected age (except hospital admissions) were significantly related to both RM1 and RM2 (Table 3).
Table 2.
Association of BPD defined as either oxygen dependency at 28 days or at 36 weeks PMA with respiratory outcomes
| (a) Analysis using the data from the development cohort (n=76) | ||||
|---|---|---|---|---|
| i) Oxygen dependency at 28 days (BPD28d) | ||||
| Respiratory outcome at 24 months corrected age | OR (95% CI) | P-value | Area under ROC curve | |
| Respiratory hospital admission | 61 | 3.89 (0.45, 33.6) | 0.22 | 0.55 |
| Cough | 60 | 2.66 (0.50, 14.1) | 0.25 | 0.56 |
| Wheeze | 61 | 1.85 (0.35, 9.86) | 0.47 | 0.54 |
| Respiratory medications (all types) | 60 | 1.52 (0.37, 6.33) | 0.56 | 0.53 |
| Any cough, wheeze and/or use of respiratory medications | 61 | 1.58 (0.38, 6.55) | 0.53 | 0.53 |
| ii) Oxygen dependency at 36 wk PMA (BPD36w) | ||||
| Respiratory outcome at 24 months corrected age | OR (95% CI) | P-value | Area under ROC curve | |
| Respiratory hospital admission | 61 | 1.58 (0.50, 5.00) | 0.43 | 0.55 |
| Cough | 60 | 2.43 (0.81, 7.27) | 0.11 | 0.60 |
| Wheeze | 61 | 2.02 (0.65, 6.28) | 0.23 | 0.58 |
| Respiratory Medications (all types) | 60 | 3.41 (1.16, 9.98) | 0.025 | 0.65 |
| Any cough, wheeze and/or use of respiratory medications | 61 | 2.65 (0.93, 7.58) | 0.069 | 0.62 |
| (b) Analysis using data from the validation cohort with four week diary card results (n=227) | ||||
|---|---|---|---|---|
| i) Oxygen dependency at 28 days (BPD28d) | ||||
| Respiratory outcome at 24 months corrected age | N | OR (95% CI) | P-value | Area under ROC curve |
| Respiratory hospital admission | 225 | 0.94 (0.38, 2.32) | 0.90 | 0.50 |
| Cough | 226 | 1.22 (0.60, 2.48) | 0.59 | 0.51 |
| Wheeze | 217 | 1.72 (0.80, 3.71) | 0.17 | 0.54 |
| Respiratory medications (all types) | 227 | 1.97 (0.97, 4.01) | 0.062 | 0.55 |
| Any cough, wheeze and/or use of respiratory medications | 227 | 1.56 (0.77, 3.18) | 0.22 | 0.53 |
| ii) Oxygen dependency at 36 wk PMA (BPD36w) | ||||
| Respiratory outcome at 24 months corrected age | N | OR (95% CI) | P-value | Area under ROC curve |
| Respiratory hospital admission | 225 | 1.41 (0.69, 2.90) | 0.35 | 0.54 |
| Cough | 226 | 1.10 (0.64, 1.88) | 0.73 | 0.51 |
| Wheeze | 217 | 0.90 (0.51, 1.56) | 0.70 | 0.51 |
| Respiratory Medications (all types) | 227 | 1.55 (0.90, 2.66) | 0.12 | 0.55 |
| Any cough, wheeze and/or use of respiratory medications | 227 | 1.33 (0.76, 2.31) | 0.31 | 0.53 |
Difference is BPD versus no BPD
P-value from t-test
Table 3.
Association of RM1 and RM2 with PFT results and respiratory outcomes at 24 months corrected age
| (a) Analysis using data from the development cohort (n=76) | |||||||
|---|---|---|---|---|---|---|---|
| RM1 | RM2 | ||||||
| Respiratory outcome at 24 months corrected age | OR (95% CI) | P-value | Area under ROC curve | OR (95% CI) | P-value | Area under ROC curve | |
| Respiratory hospital admission | 52 | 3.08 (0.88, 10.7) | 0.077 | 0.64 | 5.72 (1.58, 20.7) | 0.008 | 0.70 |
| Cough | 51 | 11.7 (3.01, 45.4) | < 0.001 | 0.77 | 12.5 (3.25, 48.1) | < 0.001 | 0.77 |
| Wheeze | 52 | 5.5 (1.48, 20.5) | 0.011 | 0.70 | 4.58 (1.33, 15.8) | 0.016 | 0.68 |
| Respiratory medications (all types) | 51 | 7.08 (2.05, 24.5) | 0.002 | 0.73 | 12.0 (2.85, 50.6) | 0.001 | 0.76 |
| Any cough, wheeze and/or use of respiratory medications | 52 | 9.45 (2.62, 34.1) | 0.001 | 0.75 | 20.0 (3.87, 102.9) | < 0.001 | 0.78 |
| (b) Analysis using data from the validation cohort with results from the four week diary card (n=227) | |||||||
|---|---|---|---|---|---|---|---|
| RM1 | RM2 | ||||||
| Respiratory outcome at 24 months corrected age | N | OR (95% CI) | P-value | Area under ROC curve | OR (95% CI) | P-value | Area under ROC curve |
| Respiratory hospital admission | 225 | 5.05 (2.34, 10.9) | < 0.001 | 0.69 | 3.90 (1.92, 7.95) | < 0.001 | 0.66 |
| Cough | 226 | 5.14 (2.91, 9.06) | < 0.001 | 0.69 | 5.54 (3.09, 9.91) | < 0.001 | 0.69 |
| Wheeze | 217 | 5.16 (2.87, 9.28) | < 0.001 | 0.69 | 5.82 (3.21, 10.6) | < 0.001 | 0.70 |
| Respiratory medications (all types) | 227 | 8.02 (4.32, 14.9) | < 0.001 | 0.73 | 10.0 (5.06, 19.8) | < 0.001 | 0.74 |
| Any cough, wheeze and/or use of respiratory medications | 227 | 7.00 (3.69, 13.3) | < 0.001 | 0.72 | 9.95 (4.75, 20.8) | < 0.001 | 0.73 |
The areas under the ROC curves were higher for all respiratory outcomes using either RM1 and RM2 as compared to either BPD28d or BPD36w (Table 4). RM1 and RM2 compared to either BPD28d or BPD36w were statistically significantly more predictive of later outcomes, as judged by comparing the ROC curves, for the combined outcome of cough, wheeze and/or use of respiratory medicines for RM1 compared to BPD28d and for cough for RM1 compared to BPD36w (Table 4). Similar patterns were observed for RM2. In the sensitivity analysis using the larger cohort (n=227), all respiratory outcomes at 24 months were better predicted by RM1 and RM2 than BPD28d and BPD36w.
Table 4.
Comparison of ROC areas of RM1, RM2, BPD28d and BPD36w with respiratory outcomes at 24 months corrected age
| (a) Analysis using data from the development cohort (n=76) | ||||||
|---|---|---|---|---|---|---|
| Respiratory outcome at 24 months | N | Area under ROC curve | ||||
| RM1 | BPD28d * | P-value | BPD36w * | P-value | ||
| Respiratory hospital admission | 52 | 0.64 | 0.55 | 0.33 | 0.52 | 0.26 |
| Cough | 51 | 0.77 | 0.54 | 0.0008 | 0.57 | 0.027 |
| Wheeze | 52 | 0.70 | 0.51 | 0.015 | 0.54 | 0.086 |
| Respiratory medications (all types) | 51 | 0.73 | 0.49 | 0.0017 | 0.60 | 0.18 |
| Any cough, wheeze and/or use of respiratory medications | 52 | 0.75 | 0.50 | 0.0004 | 0.57 | 0.042 |
| Respiratory outcome at 24 months | N | Area under ROC curve | ||||
| RM2 | BPD28d * | P-value | BPD36w * | P-value | ||
| Respiratory hospital admission | 52 | 0.70 | 0.55 | 0.067 | 0.52 | 0.065 |
| Cough | 51 | 0.77 | 0.54 | 0.0008 | 0.57 | 0.030 |
| Wheeze | 52 | 0.68 | 0.51 | 0.036 | 0.54 | 0.16 |
| Respiratory medications (all types) | 51 | 0.76 | 0.49 | 0.0001 | 0.60 | 0.084 |
| Any cough, wheeze and/or use of respiratory medications | 52 | 0.78 | 0.50 | <0.001 | 0.57 | 0.012 |
| (b) Analysis using data from the validation cohort with four week diary card results (n=227) | ||||||
|---|---|---|---|---|---|---|
| Respiratory outcome at 24 months | N | Area under ROC curve | ||||
| RM1 | BPD28d | P-value | BPD36w | P-value | ||
| Respiratory hospital admission | 225 | 0.69 | 0.50 | < 0.001 | 0.54 | 0.003 |
| Cough | 226 | 0.69 | 0.51 | < 0.001 | 0.51 | < 0.001 |
| Wheeze | 217 | 0.69 | 0.54 | < 0.001 | 0.51 | < 0.001 |
| Respiratory medications (all types) | 227 | 0.73 | 0.55 | < 0.001 | 0.55 | < 0.001 |
| Any cough, wheeze and/or use of respiratory medications | 227 | 0.72 | 0.53 | < 0.001 | 0.53 | < 0.001 |
| Respiratory outcome at 24 months | N | Area under ROC curve | ||||
| RM2 | BPD28d | P-value | BPD36w | P-value | ||
| Respiratory hospital admission | 225 | 0.66 | 0.50 | 0.0003 | 0.54 | 0.017 |
| Cough | 226 | 0.69 | 0.51 | < 0.001 | 0.51 | < 0.001 |
| Wheeze | 217 | 0.70 | 0.54 | < 0.001 | 0.51 | < 0.001 |
| Respiratory medications (all types) | 227 | 0.74 | 0.55 | < 0.001 | 0.55 | < 0.001 |
| Any cough, wheeze and/or use of respiratory medications | 227 | 0.73 | 0.53 | < 0.001 | 0.53 | < 0.001 |
Note these values are different from Table 2 since the group of children included in this analysis is smaller
Analysis of the four week diary card data demonstrated that RM1 and RM2 significantly predicted cough, wheeze and/or use of respiratory medications documented by the 24 month corrected age questionnaire (Table 5).
Table 5.
Validation of RM1 and RM2 data using the four week diary card data from the development cohort
| RM1 | RM2 | ||||||
|---|---|---|---|---|---|---|---|
| Respiratory outcome at 24 months corrected age | N | OR (95% CI) | P-value | Area under ROC curve | OR (95% CI) | P-value | Area under ROC curve |
| Respiratory hospital admission | 29 | 6.50 (1.05, 40.1) | 0.044 | 0.71 | 4.67 (0.87, 25.1) | 0.073 | 0.68 |
| Cough | 29 | 16.3 (1.63, 163) | 0.017 | 0.79 | 9.00 (1.35, 59.8) | 0.023 | 0.75 |
| Wheeze | 29 | 8.67 (1.39, 53.8) | 0.020 | 0.74 | 6.50 (1.20, 35.6) | 0.03 | 0.72 |
| Respiratory medications (all types) | 29 | 16.3 (2.46, 107) | 0.004 | 0.80 | 14.0 (2.30, 85.2) | 0.004 | 0.79 |
| Any cough, wheeze and/or use of respiratory medications | 29 | 16.3 (2.46, 107) | 0.004 | 0.80 | 14.0 (2.30, 85.2) | 0.004 | 0.79 |
RM1 and RM2 had specificities ranging 58% to 91% and sensitivity ranging from 64% to 81% for the different follow-up respiratory outcomes at 24 months corrected age in the development cohort. Positive and negative predictive values ranged from 42% to 86% and 67% to 85% respectively. In the validation cohort, the sensitivities and specificities were similar but with narrower confidence intervals (Table 6).
Table 6.
Sensitivity, specificity, positive predictive value and negative predictive value for the RM1 and RM2and the respiratory outcome at 24 months
| (a) Analysis using data from the development cohort (n=76) | ||||||||
|---|---|---|---|---|---|---|---|---|
| RM1 | RM2 | |||||||
| Respiratory outcome at 24 months | Sensitivity (95% CI) | Specificity (95% CI) | Positive Predictive Value (95% CI) | Negative Predictive Value (95%CI) | Sensitivity (95% CI) | Specificity (95% CI) | Positive Predictive Value (95% CI) | Negative Predictive Value (95% CI) |
| Respiratory hospital admission | 69% (41, 89%) | 58% (41, 75%) | 42% (23, 63%) | 81% (61, 93%) | 69% (41, 89%) | 72% (55, 86%) | 52% (30, 74%) | 84% (66, 95%) |
| Cough | 81% (58, 95%) | 73% (54, 88%) | 68% (47, 85%) | 85% (65, 96%) | 71% (48, 89%) | 83% (65, 94%) | 75% (51, 91%) | 81% (63, 93%) |
| Wheeze | 77% (50, 93%) | 63% (45, 79%) | 50% (30, 70%) | 85% (65, 96%) | 65% (38, 86%) | 71% (54, 85%) | 52% (30, 74%) | 81% (63, 93%) |
| Respiratory medications (all types) | 71% (51, 87%) | 74% (52, 90%) | 77% (56, 91%) | 68% (47, 85%) | 64% (44, 81%) | 87% (66, 97%) | 86% (64, 97%) | 67% (47, 83%) |
| Any cough, wheeze and/or use of respiratory medications | 72% (53, 87%) | 78% (56, 93%) | 81% (61, 93%) | 69% (48, 86%) | 66% (46, 82%) | 91% (72, 99%) | 91% (70, 99%) | 68% (49, 83%) |
| (b) Analysis using data from the validation cohort with results from the four week diary card (n=227) | ||||||||
|---|---|---|---|---|---|---|---|---|
| RM1 | RM2 | |||||||
| Respiratory outcome at 24 months | Sensitivity (95% CI) | Specificity (95% CI) | Positive Predictive Value (95% CI) | Negative Predictive Value (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | Positive Predictive Value (95% CI) | Negative Predictive Value (95% CI) |
| Respiratory hospital admission | 76% (61, 88%) | 61% (54, 68%) | 31% (22, 41%) | 92% (85, 96%) | 67% (51, 80%) | 66% (59, 73%) | 31% (22, 42%) | 90% (83, 94%) |
| Cough | 66% (57, 75%) | 72% (63, 80%) | 68% (58, 77%) | 71% (62, 78%) | 61% (51, 70%) | 78% (70, 85%) | 71% (61, 80%) | 69% (60, 77%) |
| Wheeze | 69% (58, 78%) | 70% (62, 78%) | 62% (51, 71%) | 76% (68, 84%) | 64% (53, 74%) | 77% (68, 84%) | 66% (55, 75%) | 75% (67, 83%) |
| Respiratory medications (all types) | 66% (57, 74%) | 80% (71, 88%) | 82% (73, 89%) | 64% (55, 72%) | 61% (52, 69%) | 87% (78, 93%) | 86% (77, 92%) | 62% (54, 70%) |
| Any cough, wheeze and/or use of respiratory medications | 62% (54, 70%) | 81% (71, 89%) | 85% (76, 91%) | 56% (47, 65%) | 57% (49, 66%) | 88% (79, 94%) | 89% (81, 95%) | 55% (46, 63%) |
DISCUSSION
We have demonstrated that respiratory morbidity (RM1 and RM2) as derived from parent completed diary cards completed at one year corrected age was a better predictor of excess respiratory problems at 24 months corrected age than a diagnosis of BPD in extremely low gestational age infants. We undertook the initial analysis on a small subset of ‘UKOS’ infants as we had very detailed information about them including a two week diary card prior to pulmonary function testing (development cohort). Our subsequent analysis, on a larger subset whose parents also completed four week diary cards at 12 months corrected age (validation cohort), regardless of which BPD definition was used, demonstrated that RM was a significantly better predictor of respiratory outcomes at 24 months corrected age.
We compared RM1 and RM2 to two definitions of BPD. Studies have defined BPD as an oxygen requirement at 28 days after birth or 36 weeks PMA. Shennan and colleagues suggested that the need for oxygen supplementation at 36 weeks PMA, rather than 28 days after birth, was a more accurate predictor of longer term outcome [11]. The definition, however, did not correlate well specifically with long term pulmonary outcome [11], whereas we had found in a subsequent study that the 28 day definition to be a better predictor of long term pulmonary outcome [12]. At an NIH consensus conference, the diagnosis of BPD was agreed to be made at 28 days and also included assignment of severity of BPD at 36 weeks PMA in prematurely born infants [13]. Nevertheless, when a cohort of premature infants was followed to 18 to 22 months corrected age, the NIH definition of BPD correctly predicted long-term respiratory morbidity only 35–40% of the time, although the accuracy increased as the severity of BPD worsened [14]. A further major limitation in using BPD as an outcome is that premature infants who do not develop BPD can also suffer chronic respiratory problems.
The definitions of RM1 and RM2 were based on the results of a two week diary card, but we validated the analysis using results from a four week diary in a larger dataset. In that analysis, the infant was required to be symptomatic in any two weeks of a four week period (not necessarily consecutive weeks). We again demonstrated that RM1 and RM2 were significant predictors of respiratory outcomes documented at 24 months corrected age. These results highlight that symptoms of cough and/or wheeze and requirement for respiratory medications are predictive of longer term abnormal respiratory outcome.
We decided a priori to assess a definition of RM as cough, wheeze and/or medication use on three or more days each week for a two week consecutive period. That definition was predictive, but our sensitivity analysis demonstrated that RM2 (at least four days of cough/wheeze/medication use per week for a two week consecutive period) tended to be a stronger predictor of respiratory outcome at 24 months corrected age. Those results suggest, not surprisingly, the more symptomatic the infant is the more likely they will suffer an abnormal respiratory outcome. We did not, however, test this hypothesis further, as using a definition which involved even more days of cough/wheeze/medicine use, although likely to be more specific, would lose sensitivity.
Our study has some potential limitations. The data were prospectively collected to assess respiratory outcome in a high risk population, but were retrospectively analysed. The infants had been entered into a randomised trial (UKOS) [7], but there were no significant differences in the short term outcomes between the two groups, hence we pooled the data for the analysis. Data from only 76 infants (development cohort) were included in the initial analysis as they had had PFTs at 12 months corrected age, but they were representative of the entire UKOS population with regard to their demographics [8]. Hence, we feel these results are generalisable to larger populations of extremely low gestational age infants. At 24 months corrected age, parents completed respiratory questionnaires with their paediatrician who had the hospital records with them so we feel the questionnaires reflected the respiratory outcomes of the infants. Indeed, comparison has shown a good correlation of parental reports with paediatrician records for hospital admissions, asthma and bronchitis [15]. We undertook a subsequent analysis on a larger cohort of infants (validation cohort) and the results confirmed that RM1 and RM2 compared to BPD28 and BPD36 were better predictors of respiratory outcome at 24 months corrected age.
In conclusion, we have demonstrated that respiratory morbidity diagnosed from a two week parent completed diary at one year corrected age better predicted abnormal respiratory outcomes at 24 months corrected age than BPD defined either as oxygen dependency at 28 days or 36 weeks PMA. We, therefore, suggest that data from parent completed diary cards at one year corrected age rather than BPD, may be a better outcome measure when assessing the efficacy of interventions aimed at improving long term respiratory outcome in extremely low gestational age infants.
Acknowledgments
Funding sources: The research was funded/supported by the National Institute of Health (NH56398), National Center for Advancing Translation Sciences (UL1 TR000073) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (ULlRR025752-02). The research was funded/supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Conflict of interest statement: AG has held grants from various ventilator manufacturers and has received honoraria for giving lectures and advising various ventilator manufacturers.
Contributors’ statement: Professors Parad, Davis and Greenough designed the study and approved the final manuscript as submitted. Ms Lo and Professor Peacock undertook the analysis and approved the final manuscript as submitted. All authors were involved in preparation of the manuscript and approved the final manuscript as submitted.
Financial disclosure: NM and AG are NIHR Senior Investigators. AG is an MRC and Asthma UK Centre in Allergic Mechanisms of Asthma Investigator which is supported by an MRC Centre Grant G1000758. Ms Lo was supported by the National Institute for Health Research (NIHR) Biomedical Research at Guy’s and St Thomas’ NHS Foundation Trusts and King’s College London.
References
- 1.Greenough A. Long term respiratory outcomes of extreme prematurity (< 32 weeks) Semin Fetal Neonatal Med. 2012;17:73–76. doi: 10.1016/j.siny.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Tyson JE, Wright LL, Oh W, Kennedy KA, Mele L, Ehrenkranz RA, Stoll BJ, Lemons JA, Stevenson DK, Bauer CR, Korones SB, Fanaroff AA. Vitamin A supplementation for extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med. 1999;340:1962–68. doi: 10.1056/NEJM199906243402505. [DOI] [PubMed] [Google Scholar]
- 3.Ambalavanan N, Tyson JE, Kennedy KA, Hansen NI, Vohr BR, Wright LL, Carlo WA National Institute of Child Health and Human Development Neonatal Research Network. Vitamin A supplementation for extremely low birth weight infants: outcome at 18 to 22 months. Pediatrics. 2005;115:e249–e254. doi: 10.1542/peds.2004-1812. [DOI] [PubMed] [Google Scholar]
- 4.Davis JM, Parad R, Michele T, Allred E, Price A, Rosenfeld W. North American Recombinant Human CuZnSOD Study Group: Pulmonary outcome at 1 year corrected age in premature infants treated at birth with recombinant human CuZn superoxide dismutase. Pediatrics. 2003;111:469–476. doi: 10.1542/peds.111.3.469. [DOI] [PubMed] [Google Scholar]
- 5.Greenough A, Giffin F, Yuksel B, Dimitriou G. Respiratory morbidity in young school children bornprematurely-chronic lung disease is not a risk factor? Eur J Pediatr. 1996;155:823–826. [PubMed] [Google Scholar]
- 6.Broughton S, Thomas MR, Marston L, Calvert SA, Marlow N, Peacock JL, Rafferty GF, Greenough A. Very prematurely born infants wheezing at follow up: lung function and risk factors. Arch Dis Child. 2007;92:776–780. doi: 10.1136/adc.2006.112623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson AH, Peacock JL, Greenough A, Marlow N, Limb ES, Marston L, Calvert SA. United Kingdom Oscillation Study Group: High frequency oscillatory ventilation for the prevention of chronic lung disease of prematurity. New Engl J Med. 2002;347:633–642. doi: 10.1056/NEJMoa020432. [DOI] [PubMed] [Google Scholar]
- 8.Thomas MR, Rafferty GF, Limb ES, Peacock JL, Calvert SA, Marlow N, Milner AD, Greenough A. Pulmonary function at follow-up of very preterm infants from the United Kingdom oscillation study. Am J Respir Crit Care Med. 2004;169:868–872. doi: 10.1164/rccm.200310-1425OC. [DOI] [PubMed] [Google Scholar]
- 9.Marlow N, Greenough A, Peacock JL, Marston L, Limb ES, Johnson AH, Calvert SA. Randomised trial of high frequency oscillatory ventilation or conventional ventilation in babies of gestational age 28 weeks or less: respiratory and neurological outcomes at 2 years. Arch Dis Child Fetal Neonatal Ed. 2006;91:F320–F326. doi: 10.1136/adc.2005.079632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanley JA, McNeil BI. A method of comparing the areas under receiver operating characteristic curves derived from the same case. Radiol. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 11.Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics. 1988;82:527–532. [PubMed] [Google Scholar]
- 12.Kinali M, Greenough A, Dimitriou G, Yuksel B, Hooper R. Chronic respiratory morbidity following premature delivery – prediction by prolonged respiratory support requirement. Eur J Pediatr. 1999;158:493–496. doi: 10.1007/s004310051128. [DOI] [PubMed] [Google Scholar]
- 13.Jobe A, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 14.Ehrenkranz R, Walsh M, Vohr B, Jobe AH, Wright LL, Fanaroff AA, Wrage LA, Poole K. National Institutes of Child Health and Human Development Neonatal Research Network: Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–1360. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 15.Pless CE, Pless IB. How well they remember. The accuracy of parental reports. Arch Pediatr Adolesc Med. 1995;149:553–558. doi: 10.1001/archpedi.1995.02170180083016. [DOI] [PubMed] [Google Scholar]