Abstract
Objectives
To study the longitudinal course of sleep timing and circadian preferences in individuals with bipolar disorder (BP) compared to individuals with non-BP psychopathology and healthy controls.
Methods
Individuals with bipolar I and bipolar II disorder (n = 257), non-BP psychopathology (n = 105), and healthy controls (n = 55) (mean age 40.2 years, 21.3% male, 85.1% Caucasian) were followed on average every 27 months for a mean of four years. Sleep timing parameters and circadian preference were reported using the Sleep Timing Questionnaire and The Composite Scale for Morningness. Group comparisons were adjusted for multiple comparisons and between-group differences in demographic variables and psychopharmacological treatment.
Results
Regardless of their current mood state, individuals with BP showed more sleep onset latency (SOL), awakening after sleep onset (WASO), and evening preference in comparison to both individuals with non-BP psychopathology and healthy controls. Individuals with BP also showed less stability of bed and awakening times in comparison to the other two groups, though these results were dependent on mood state. Non-BP individuals only showed more WASO and less stability in bed and awakening times before work/school days than healthy controls. Adjusting for comorbid disorders yielded similar results. Within-group analyses found little to no effect of time and BP subtype on sleep timing and circadian preference.
Conclusions
Disturbances of sleep timing are prominent in individuals with BP. These disturbances are worse during mood episodes, but still apparent during euthymic periods. Evening preference was not associated with polarity type, or mood state in BP, suggesting that this characteristic may be a trait marker.
Keywords: bipolar disorder, circadian, eveningness, morningness, sleep
Dysregulation of circadian rhythms, along with various types of sleep disturbances, are common features of both major depressive disorder (MDD) and bipolar disorder (BP) (1–3). Although not required for diagnosis, most individuals with BP do experience sleep disturbances, mainly in the form of decreased need for sleep during manic episodes and insomnia or hypersomnia during depressive episodes (2). Sleep disturbances are considered common prodromes for mania and depression (4, 5) and are associated with poor quality of life, functional impairment, and suicidality in individuals with MDD and BP (6, 7). Furthermore, subjective sleep problems appear to be a risk marker for mood disturbances among relatives of individuals with mood disorders (7).
There is evidence that sleep disturbances are not only confined to acute mood episodes in BP. In a cross-sectional comparison between euthymic individuals with BP, individuals with primary insomnia only, and healthy controls (n = 20 per group), Harvey and colleagues (8) reported that 70% of the euthymic individuals with BP exhibited clinically significant sleep disturbances. An evaluation of 19 euthymic adult individuals with BP over five consecutive nights using mood diaries and actigraphy also showed that they slept longer, had longer sleep onset latencies, and displayed greater variability across nights in comparison with healthy controls (9). A non-controlled study that retrospectively investigated the sleep disturbances of 106 euthymic individuals with BP during a period of two months reported high prevalence of initial (25%) and middle insomnia (81%) (10). These findings were further confirmed by a recent Brazilian cross-sectional study that included 105 euthymic individuals with BP and 104 healthy controls (11). Recently, the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) reported sleep disturbances in 15% of individuals with bipolar I disorder (BP-I) and bipolar II disorder (BP-II) who had at least eight weeks of euthymia (5). Finally, two small studies using actigraphy and sleep diaries reported significantly more sleep difficulties in euthymic individuals with BP when compared to healthy controls (12, 13).
Very few studies have evaluated the longitudinal course of sleep disturbances and their association with different mood states in individuals with BP. Bauer and colleagues (14) analyzed self-reported mood, sleep, and bed rest in 59 individuals with BP for approximately 160 days and reported a significant inverse correlation between sleep and/or bed rest duration and changes in mood, regardless of polarity. There was a latency of one day between the change of sleep and the change in mood. Perlman and colleagues (15) showed that shorter sleep duration predicted greater depressive, but not manic, symptoms over six months in 54 individuals with BP-I. Finally, 468 individuals with BP who participated in the STEP-BD study were followed over a 12-month period to examine the predictive value of sleep duration and variability for mood episodes (16). Shorter sleep duration was associated with increased severity of mania, while greater sleep variability (i.e., maximum minus minimum sleep duration) was associated with increased mania and depression. However, the effects of psychotropic medications were not evaluated. While the above-noted studies suggest persistent sleep disturbances in BP and a possible association between the longitudinal course of sleep disturbances and mood changes, the findings are inconsistent. Furthermore, except for the STEP-BD study, most studies included small samples, included no controls or only healthy controls, and only followed individuals for short periods of time.
In addition to the sleep disturbances, the circadian preference of individuals with BP has been studied. In general, individuals can be distributed on a scale of circadian preference that ranges from morning to evening preferences. These preferences are thought to affect the phase position of the endogenous circadian physiological rhythms, including: plasma cortisol, core body temperature, and behavioral rhythms related to eating, exercise and performance (17–19). One of the most consistent findings of circadian rhythmicity in symptomatic (20–22) and euthymic (23) individuals with BP is their evening preference. However, it is not yet clear whether this circadian preference remains stable across the longitudinal course of illness as, to our knowledge, all existing studies are cross sectional. Studies of circadian rhythmicity in individuals with BP have reported clear differences between individuals with BP and healthy controls, even during periods of clinical recovery from mood episodes. One study, using actigraphy to monitor circadian rhythmicity, reported sustained phase advance and lower average daily activity in euthymic individuals with BP as compared to healthy controls (24). These findings, however, were limited by a small sample (n = 37) and short duration of follow-up (72 hours).
In a previous report, we found that individuals with BP (n = 128) preferred the evening more than controls (n = 128) (21). These results appeared to be stable during two years of follow-up in a subgroup of participants (n = 52). In this report, we sought to extend our prior results by including a larger sample and extending the follow-up time. To our knowledge, this is the first study to evaluate the longitudinal course of sleep timing and circadian preference in a large sample of individuals with BP in comparison to individuals with non-BP psychopathology and healthy controls. We hypothesized that after adjusting for confounding variables, individuals with BP will show significantly more sleep disturbances, especially sleep onset latency (SOL), and wake after sleep onset (WASO), i.e., the amount of sleep time lost trying to fall asleep and awakening in the night in addition to evening preference when compared to both individuals with non-BP psychopathology and healthy controls. We also hypothesized that sleep disturbances and circadian preference will persist throughout the follow-up time during episodic and euthymic periods of the illness.
Methods
Subjects
Adults with and without BP were recruited through the Pittsburgh Bipolar Offspring Study (BIOS) (21), which aims to evaluate the lifetime prevalence of psychiatric disorders in offspring of parents with BP. Briefly, individuals with BP were recruited through advertisement (67.0%), BP studies (31.5%), and outpatient clinics (1.5%). Individuals were required to fulfill DSM-IV criteria for BP-I or BP-II (25). Exclusion criteria were current or lifetime diagnoses of schizophrenia, mental retardation, mood disorders secondary to substance abuse, medical conditions that interfered with the evaluation, and living more than 200 miles away from the hospital. Controls, group matched for demographic variables, were recruited at random from the community at a ratio of one healthy control to two individuals with BP. The exclusion criteria were the same as those for the individuals with BP, with an additional exclusion criterion of having any lifetime or current BP and/or history of BP in first-degree relatives and current use of any psychoactive medications.
Assessments
After Institutional Review Board approval and informed consent were obtained, individuals were assessed for psychopathology, family history of psychiatric disorders, treatment, psychosocial functioning, family environment, and exposure to negative life events. Only instruments relevant to this article are discussed. Axis I disorders and severity of current mood episode were evaluated using the DSM-IV Structured Clinical Interview (SCID) (26). Euthymia was defined as lack of current mood (manic, hypomanic, depressive) episodes within previous two months as ascertained through the SCID. Overall functioning was evaluated using the DSM-IV Global Assessment of Functioning (GAF) (25). Current and past pharmacological treatments (mood stabilizers, antipsychotics, stimulants, and antidepressants) were ascertained using the Adult Health Medical Screening Interview developed for BIOS. Socioeconomic status (SES) was evaluated using the Four-factor Hollingshead Scale (27). All assessments were completed by bachelors- or masters-level interviewers with at least two years of experience and were carried out in the participants’ homes. All assessments were presented to a psychiatrist who was blind to the psychiatric status of the individuals. Inter-rater reliabilities for the SCID diagnoses were good (kappa ≥ 0.8).
Sleep timing parameters and circadian preferences were assessed using the Sleep Timing Questionnaire (STQ) (28) and the Composite Scale for Morningness (CSM), respectively (29, 30). The STQ is a reliable valid measure of sleep timing that provides equivalent measurements of sleep to those obtained from a formal sleep diary. It also yields measures of sleep timing regularity and sleep quality (31). Outcome variables analyzed from the STQ included the sleep onset latency (SOL), and wake after sleep onset (WASO) calculated in minutes. The STQ also recorded stability of bedtimes before work/school, bedtimes before days off, awakening times before work/school days, and awakening times before days off. The stability variables were recorded as ordinal scales using the following distinctions: 1: 0–15 min, 2: 16–30 min, 3: 31–45 min, 4: 46–60 min, 5: 61–75 min, 6: 76–90 min, 7: 91–105 min, 8: 106–120 min, 9: 2–3 hours, 10: 3–4 hours, and 11: > 4 hours (note that these intervals are not of equal length, thus precluding the use of linear modeling). The STQ also includes questions about actual bedtimes and waking up times that were not included in the analysis. The CSM is a validated adaptation of the Horne–Ostberg scale (32). It is composed of 13 multiple-choice sentences used to evaluate to what extent an individual prefers the morning or the evening. The morningness/eveningness CSM scale ranges from 13–55 and is calculated by adding the scores of the individual questions. Lower scores indicate more evening preference while higher scores indicate greater morning preference. A recent study confirmed the validity of CSM against actigraphy in individuals with bipolar depression (33).
Statistical analyses
Between-group demographic and clinical characteristics were compared using chi-square, ANOVA, and non-parametric tests as appropriate. Pairwise comparisons were adjusted using Bonferroni corrections. The STQ and CSM variables were prospectively ascertained over the course of follow-up, so mixed models were used to account for within-patient correlation. The composite score, SOL, and WASO measures are continuous and were thus modeled using linear mixed models (Satterthwaite approximation was used since standard deviations differed across groups). The bedtime and awakening time stability variables were measured on ordinal scales of unequal length and could not be normalized; thus ordered logistic mixed modeling was used for the analyses. Demographic and pharmacological variables in the univariate analyses with p-values < 0.2 were entered into each model as controls/covariates. Given that polychoric correlations between comorbidities and group distinction ranged from 0.5 to 0.8, clinical diagnoses were excluded as controls to prevent multi-collinearity. Each model was only run with variables for group and time because the ‘group-by-time’ interactions were non-significant. For the linear mixed models in the analysis, least square mean group contrasts were estimated at each follow-up time. For the ordered logistic mixed models, odds ratios with 95% confidence intervals were estimated at each follow-up time to compare the stability of bedtimes and awakening times among the groups.
To adjust for the effects of exposure to major groups of psychoactive medications, we employed three different methods of parameterizing medication usage: a variable indicating usage of certain groups of medications at any time in the study, a variable indicating usage of each group of medications at each specific follow-up time, and a variable indicating the percentage of visits during which individuals were on each group of medications. Each of the three methods showed good explanatory power. However, in this report we only included the ‘by follow-up time’ variable, as it yielded the best model fit and the most clinical information. All p-values were based on two-tailed tests with α = 0.05. All analyses were performed using the Statistical Analysis System (SAS) versions 9.3 and 9.4.
Results
As shown in Table 1, 257 individuals with BP, 105 with non-BP psychopathology, and 55 healthy controls were included in the analyses. Ages of the participants ranged from 21–62 years (mean = 40.2 years). Individuals were followed for a mean of four years (median = 4.0 years; standard deviation = 1.2) and were assessed at intake (Time 1), approximately at two years (Time 2) (BP = 226, non-BP = 99, healthy controls = 55), and four years (Time 3) (BP = 180, non-BP = 81, healthy controls = 44). There were no significant between-group differences in length of follow-up. Individuals who dropped out or who have yet to return for follow-up assessment differed from those who completed the follow-ups in that dropouts were more likely to be unmarried, non-white, and older, and they had lower SES and more history of disruptive behavior disorders (DBD) (all p ≤ 0.02).
Table 1.
Demographic characteristics
| BP subjects (n = 257) |
Non-BP subjects (n = 105) |
Healthy controls (n = 55) |
Statistics | p-value | |
|---|---|---|---|---|---|
| Age, years, mean (SD) | 39.9 (8.2) | 40.4 (8.8) | 40.8 (7.3) | F = 0.30 | 0.7 |
| SES, mean (SD) | 34.2 (14.5)a | 35.9 (12.9)a | 43.6 (13.3)b | F = 10.36 | < 0.0001 |
| Gender, male, % | 79.4 | 77.1 | 78.2 | 0.23 | 0.9 |
| Race, White, % | 88.7a | 78.1b | 81.8a,b | 7.19 | 0.03 |
| Marital status, married, % | 50.2a | 61a | 89.1b | 28.63 | < 0.0001 |
| GAF score, mean (SD) | 63.1 (13.3)a | 80.8 (10.5)b | 89.7 (5.6)c | 160.53 | < 0.0001 |
Differing superscripts denote significant between-group differences with p-values ≤ 0.05 after Bonferroni correction. BP = bipolar disorder; SD = standard deviation; SES = socioeconomic status; GAF = Global Assessment of Functioning.
Demographic and clinical characteristics
At intake (Time 1), individuals with BP and non-BP psychopathology were less likely to be married and had lower SES than healthy controls. Mean GAF was significantly lower in individuals with BP followed by individuals with non-BP psychopathology and highest for healthy controls. Subjects with BP were more likely to be white than non-BP subjects, but neither group differed from healthy controls in race frequency (for all above noted comparisons p-values ≤ 0.03). The rate of BP-I was 69% and BP-II was 31% (Table 2). Regarding the polarity of the episodes, at Time 1 there were 80 subjects with depressive polarity and 61 with manic/hypomanic polarity; at Time 2, 47 with depression and 43 with mania/hypomania; and at Time 3, 48 with depression and 25 with mania/hypomania. As shown in Table 2, at intake, individuals with BP showed significantly higher lifetime prevalence of all psychiatric disorders when compared to the non-BP group with the exception of social anxiety disorder (all p < 0.004). Also, with the exception of non-stimulant attention-deficit hyperactivity disorder (ADHD) medications, antihistamines, opiates, and muscle relaxants, individuals with BP were more likely to be taking psychoactive medications at assessment than non-BP individuals.
Table 2.
Lifetime Axis I psychiatric disorders and pharmacological treatment
| BP subjects (n = 257) |
Non-BP subjects (n = 105) |
χ2 statistic | p-value | |
|---|---|---|---|---|
| Lifetime Axis I psychiatric disorders, % | ||||
| BP-I BP-II BP-NOS |
69.1 30.5 0.4 |
– – – |
– – – |
– – – |
| MDD | – | 37.1 | – | – |
| DD | – | 9.5 | – | – |
| Psychosis | 13.2 | 1.9 | 10.68 | 0.001 |
| ADHD | 25.3 | 5.7 | 18.12 | < 0.0001 |
| DBD ODD CD |
36.2 26.9 21.8 |
8.6 4.8 3.8 |
28.09 22.36 17.43 |
< 0.0001 < 0.0001 < 0.0001 |
| SUD | 64.6 | 39.1 | 19.87 | < 0.0001 |
| Any anxiety Panic SAD GAD PTSD OCD |
71.2 42.0 8.6 30.7 38.1 14.4 |
25.7 6.7 6.7 3.8 13.3 3.8 |
63.33 42.99 0.36 30.59 21.46 8.32 |
< 0.0001 < 0.0001 0.50 < 0.0001 < 0.0001 0.004 |
| Eating disorder | 17.5 | 5.7 | 8.57 | 0.003 |
| Pharmacological treatments, % | ||||
| Antipsychotics | 57.2 | 4.8 | 84.15 | < 0.0001 |
| Antidepressants | 69.7 | 31.4 | 44.88 | < 0.0001 |
| Stimulants | 9.3 | 1.0 | 8.15 | 0.004 |
| Lithium | 32.7 | 0.0 | 44.69 | < 0.0001 |
| Antimanic anticonvulsants | 33.5 | 2.9 | 37.66 | < 0.0001 |
| Other anticonvulsants | 54.9 | 5.7 | 74.66 | < 0.0001 |
| ADHD medications | 2.0 | 1.0 | Fisher's | 0.70 |
| Sedatives/hypnotics | 51.4 | 15.2 | 40.25 | < 0.0001 |
| Antihistamines | 15.2 | 8.6 | 2.83 | 0.09 |
| Opiates | 12.5 | 10.5 | 0.28 | 0.60 |
| Muscle relaxants | 3.9 | 4.8 | Fisher's | 0.80 |
| Other psychotropic medications | 9.3 | 7.6 | 0.27 | 0.60 |
BP = bipolar disorder; BP-I = bipolar I disorder; BP-II = bipolar II disorder; BP-NOS = bipolar disorder not otherwise specified; MDD = major depressive disorder; DD = dysthymic disorder; ADHD = attention-deficit hyperactivity disorder; DBD = disruptive behavior disorder; ODD = oppositional defiant disorder; CD = conduct disorder; SUD = substance use disorder; SAD = separation anxiety disorder; GAD = generalized anxiety disorder; PTSD = posttraumatic stress disorder; OCD = obsessive compulsive disorder.
Sleep timing and circadian preferences
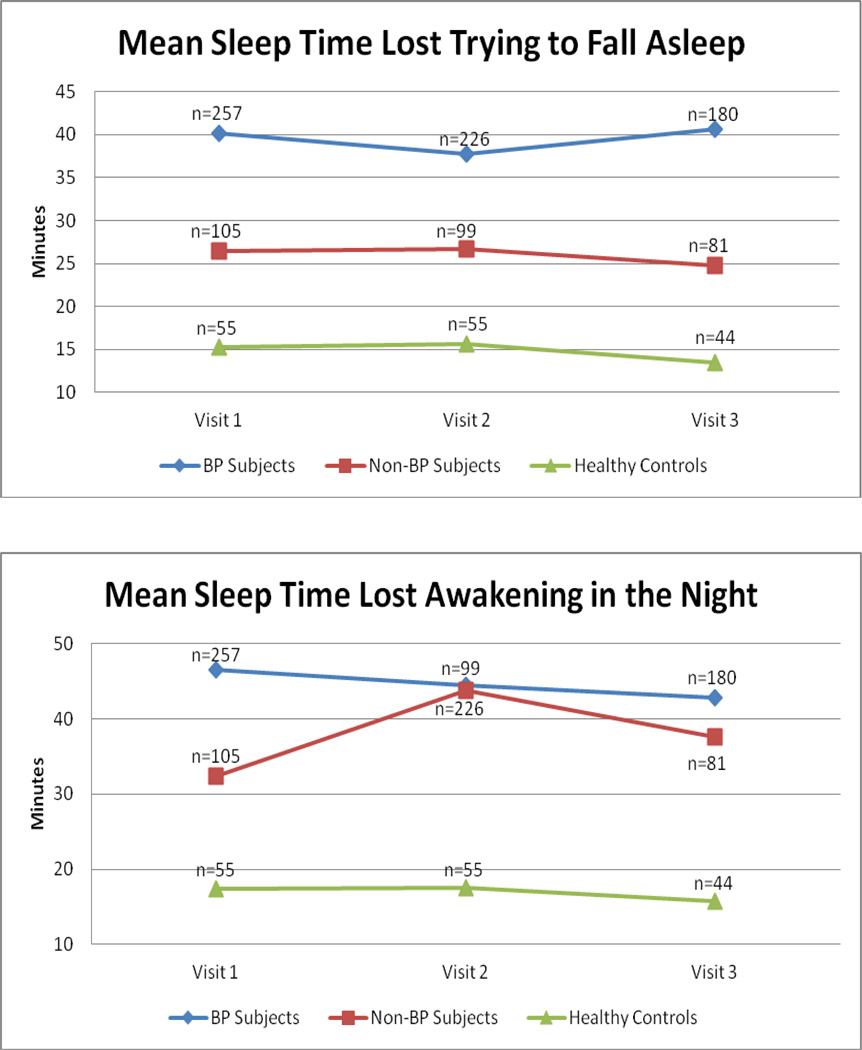
After adjusting for the significant demographic and pharmacological differences noted above, there were significant between-group differences in the longitudinal sleep-timing parameters (all p < 0.0001) (Tables 3 and 4). Specifically, Bonferroni-adjusted pairwise comparisons showed that individuals with BP reported significantly more SOL and WASO and less stability in bedtimes and awakening times before work/school days, and days off than non-BP subjects and healthy controls at all follow-up time points. Non-BP individuals reported more WASO and less bedtime and awakening time stability before work/school days than healthy controls at all time points, but they did not differ in SOL or bedtime and awakening time stability before days off (see Fig. 1 for insomnia variables). For all above comparisons, there was no significant group-by-time interaction.
Table 3.
Morningness/eveningness and sleep-time lost
| Time 1 | Time 2 | Time 3 | Statistic | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Time | Group × Time | |||||||
| F | p-value | F | p-value | F | p-value | ||||
| Composite score (morningness/eveningness), mean ± SD | |||||||||
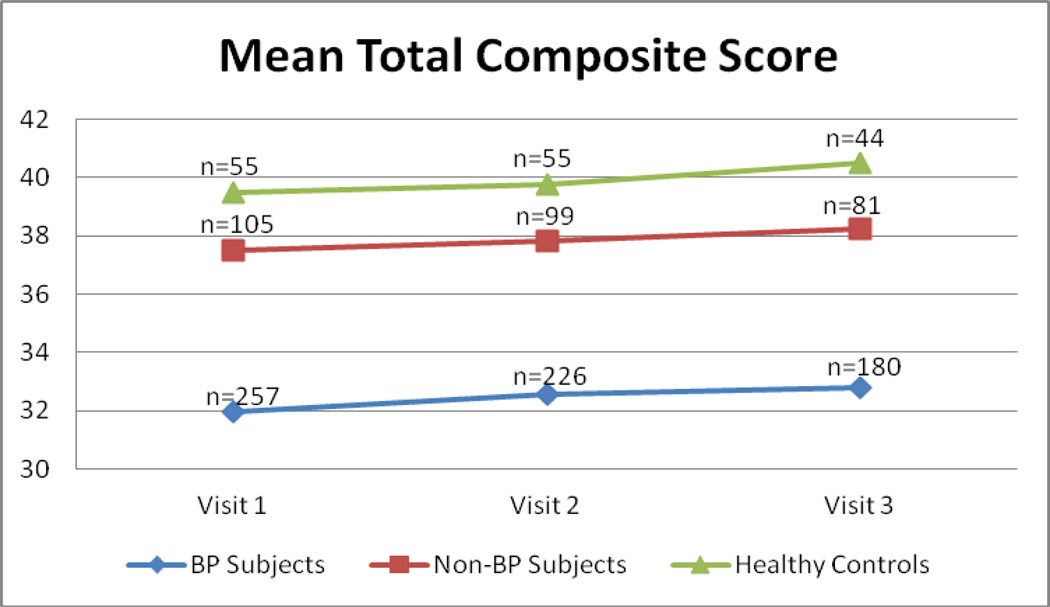
| BP | 32.0a ± 8.8 | 32.6a ± 8.7 | 32.8a ± 9.0 | 19.78 | < 0.0001 | 3.85 | 0.02 | 0.22 | 0.90 |
| Non-BP | 37.5b ± 7.3 | 37.8b ± 7.4 | 38.2b ± 7.4 | ||||||
| HC | 39.5b ± 6.8 | 39.8b ± 6.5 | 40.5b ± 6.5 | ||||||
| Sleep onset latency, mean ± SD | |||||||||
| BP | 40.2a ± 35.2 | 37.7a ± 36.1 | 40.6a ± 49.1 | 22.81 | < 0.0001 | 0.92 | 0.40 | 0.25 | 0.90 |
| Non-BP | 26.4b ± 28.1 | 26.7b ± 27.9 | 24.8b ± 27.3 | ||||||
| HC | 15.2b ± 10.1 | 15.6b ± 9.7 | 13.5b ± 7.0 | ||||||
| Wake after sleep onset, mean ± SD | |||||||||
| BP | 46.5a ± 51.8 | 44.5a ± 49.9 | 42.8a ± 46.5 | 29.97 | < 0.0001 | 0.85 | 0.40 | 1.50 | 0.20 |
| Non-BP | 32.4b ± 47.3 | 43.8b ± 50.9 | 37.6b ± 46.7 | ||||||
| HC | 17.4c ± 25.4 | 17.6c ± 23.9 | 15.8c ± 36.9 | ||||||
Different superscripts denote significant between-group differences with p-values ≤ 0.05 after Bonferroni correction. BP = bipolar disorder; HC = healthy control; SD = standard deviation.
Table 4.
Bedtime and awakening time stability
| Time 1 | Time 2 | Time 3 | Statistic | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Time | Group × Time | |||||||
| F | p-value | F | p-value | F | p-value | ||||
| Bedtime stability before work/school days, OR (CI) | |||||||||
| BP vs. Non-BP | 1.90a (1.33–2.73) | 1.90a (1.33–2.73) | 1.90a (1.33–2.73) | 16.09 | <0.0001 | 0.14 | 0.90 | 0.37 | 0.80 |
| BP vs. HC | 3.58a (2.23–5.77) | 3.58a (2.23–5.77) | 3.58a (2.23–5.77) | ||||||
| Non-BP vs. HC | 1.89a (1.13–3.14) | 1.89a (1.13–3.14) | 1.89a (1.13–3.14) | ||||||
| Bedtime stability before days off, OR (CI) | |||||||||
| BP vs. Non-BP | 1.72a (1.19–2.51) | 1.72a (1.19–2.51) | 1.72a (1.19–2.50) | 9.47 | <0.0001 | 1.05 | 0.30 | 0.73 | 0.60 |
| BP vs. HC | 2.42a (1.51–3.89) | 2.43a (1.51–3.90) | 2.43a (1.51–3.90) | ||||||
| Non-BP vs. HC | 1.41 (0.86–2.29) | 1.41 (0.86–2.30) | 1.41 (0.86–2.30) | ||||||
| Awakening time stability before work/school days, OR (CI) | |||||||||
| BP vs. Non-BP | 2.49a (1.69–3.66) | 2.49a (1.69–3.66) | 2.49a (1.69–3.66) | 23.38 | <0.0001 | 0.03 | 0.97 | 0.69 | 0.60 |
| BP vs. HC | 5.09a (3.06–8.46) | 5.09a (3.06–8.46) | 5.09a (3.06–8.46) | ||||||
| Non-BP vs. HC | 2.04a (1.21–3.47) | 2.04a (1.21–3.46) | 2.04a (1.21–3.47) | ||||||
| Awakening time stability before days off, OR (CI) | |||||||||
| BP vs. Non-BP | 1.71a (1.16–2.51) | 1.71a (1.16–2.51) | 1.71a (1.16–2.51) | 11.91 | <0.0001 | 0.33 | 0.70 | 2.24 | 0.06 |
| BP vs. HC | 3.26a (1.98–5.38) | 3.26a (1.98–5.38) | 3.26a (1.98–5.38) | ||||||
| Non-BP vs. HC | 1.91 (1.11–3.28) | 1.91 (1.11–3.28) | 1.91 (1.11–3.28) | ||||||
BP = bipolar disorder; HC = healthy control; OR = odds ratio; CI = confidence interval.
Indicates significant between-group differences with p-values ≤ 0.05 after Bonferroni adjustment.
Fig. 1.
Sleep onset latency and wake after sleep onset. BP = bipolar disorder.
With regard to circadian preference, subjects with BP reported significantly more evening preference than the other two groups at all time points (Fig. 2). There were no differences in circadian preference between individuals with non-BP psychopathology and the healthy controls (Table 3). For the above comparisons, there were no significant group-by-time interactions.
Fig. 2.
Circadian preference. The morningness/eveningness composite score scale ranges from 13 to 55 and is calculated by adding up the scores of the individual questions. Lower scores mean more evening preference and vice-versa. BP = bipolar disorder.
Within-group longitudinal analyses found that none of the sleep timing or circadian preference parameters differed by time point within the subjects with BP and the healthy control groups. Within the non-BP subjects group, sleep timing parameters did not differ by time point with the exception of WASO (p = 0.03) and awakening time stability before days off (p = 0.04).
Since being euthymic or having a mood episode had a significant effect on sleep and circadian parameters, analyses presented in Tables 3 and 4 were rerun subdividing the BP group into individuals experiencing a current mood episode and euthymic individuals (see Supplementary Tables S1 and S2). Within the BP group, individuals experiencing a mood episode at the time of assessment (Time 1 = 141, Time 2 = 90, Time 3 = 73) reported significantly more SOL during time points 1 and 3 and WASO at time points 2 and 3 than euthymic individuals. There were no significant differences in circadian preference between individuals with and without current mood episodes. Additionally, no significant differences in sleep timing or circadian preference were found between individuals with manic versus major depressive episodes. Both the current mood and euthymic individuals with BP continued to differ significantly from healthy controls in all sleep variables at all time points. Individuals with current mood episodes significantly differed from non-BP individuals in all sleep variables at all time points, while euthymic individuals with BP differed from non-BP individuals only in SOL, WASO, evening preference, and awakening time stability before work/school days.
Analyses comparing individuals with BP-I versus those with BP-II showed no significant differences in sleep timing parameters. After evaluating the effects of comorbid disorders, results found that psychosis, ADHD, DBD, substance use disorder (SUD), and anxiety disorders were associated with sleep timing and circadian preference (all p ≤ 0.04). A sensitivity analysis was performed excluding healthy controls since they had no comorbidities. Results found that ADHD was associated with evening preference, anxiety and psychosis were associated with WASO, SUD was associated with awakening time stability before work/school days and days off, and DBD was associated with all sleep timing parameters except awakening time stability before work/school days and days off. An additional sensitivity analysis was run within the BP group to separate the effect of BP from the effects of comorbidities, and results were identical except SUD had no significant association with any sleep or circadian parameter.
Finally, to evaluate whether sleep timing and circadian preference as reported by the individuals with BP were associated with the recruitment source, analyses were also conducted comparing individuals with BP recruited through clinical settings and those recruited through advertisement. Those recruited through clinical settings had more evening preference, less bedtime stability before work/school days, and less awakening time stability before work/school days and days off (p ≤ 0.03).
Discussion
In general, sleep-timing preferences were more disturbed in individuals with BP in a current mood episode followed by euthymic individuals with BP, non-BP individuals, and healthy controls. More specifically, after adjusting for between group differences (e.g., SES, pharmacological treatment) and multiple comparisons, individuals with BP showed more SOL and WASO, and less stability of bedtimes and awakening times at intake and across follow-up in comparison to the healthy controls. These results were more evident in individuals with BP with current mood episodes of any polarity. However, differences were also evident in euthymic individuals with BP. During mood episodes, and to a lesser extent during euthymic periods, individuals with BP showed significantly more disturbed sleep-timing parameters than individuals with non-BP disorders. Finally, non-BP individuals only showed more WASO and less bedtime and awakening time stability before work/school days than the healthy controls. Regarding circadian preference, individuals with BP showed significantly more evening preference at intake and during the follow-up in comparison with the non-BP and healthy controls. In contrast with the sleep disturbances that were affected by the mood state, evening preference was independent of these factors. For all above comparisons, there was no effect of BP subtype, no significant group-by-time interaction, and little to no within-group effect of time on sleep timing parameters or circadian preference analyzed in this study.
Before further discussing the results, it is important to highlight the limitations of this study. First, the sample was recruited through a high-risk study for BP (21). As a consequence, the results may not be generalizable to other populations. However, taking into account the age and sex of the control individuals, the lifetime prevalence of psychiatric disorders in the whole sample was similar to that reported in the National Comorbidity Survey Replication study (34). Also, the rates of comorbid psychiatric disorders in individuals with BP in our sample were similar to those reported in the adult BP literature (34–36). Second, the information collected in this study was obtained only from self-reports; objective measures to evaluate sleep (e.g., actigraphy) were not included. Thus, inaccurate reporting regarding sleep preference/disturbances may have occurred and may have in turn contributed to between-group differences. Third, employment was included as part of SES and not specifically. Fourth, although the effects of using the common groups of psychoactive medications were adjusted for, any influence of any given individual medication on the results cannot be ruled out. Finally, individuals with non-BP psychopathology included several disorders that could have differentially affected sleep timing disturbances and circadian preferences of this group. The individual parameters of sleep timing and circadian preference in each one of these psychiatric disorders could not be evaluated using the current design.
Consistent with the literature (1–3), individuals with BP who were in a current mood episode showed greater severity of sleep timing disturbances than the healthy controls. Also, extending the existing literature (8–11), we found that despite being euthymic, individuals with BP continued to have sleep timing difficulties. The continuation of sleep timing disturbances during periods of euthymia has been attributed to subsyndromal mood symptomatology, comorbid psychiatric disorders, and medication side effects (9, 10). We did not evaluate subsyndromal symptomatology, but in our study, there were no effects of medications and comorbid disorders. Alterations in sleep duration and timing in individuals with BP have also been attributed to a possible shared genetic mechanism (2).
To our knowledge, no previous studies have compared sleep timing disturbances between BP and non-BP individuals, except for a study comparing individuals with BP and individuals with primary insomnia (8). During mood episodes, and to a lesser extent during periods of euthymia, individuals with BP showed less stability in awakening times at all time points and, less consistently, insomnia, than non-BP individuals. Moreover, there were not meaningful differences in sleep timing parameters between individuals with non-BP psychopathology and healthy controls, suggesting that the above findings may be specific for BP.
The persistent evening preference in individuals with BP reported in the current study replicates and extends previous studies using different methodologies (23) and in different cultures (22). Contradicting these findings, one study using actigraphy reported circadian phase advance in both manic and remitted BP (24). However, this study included a small sample of individuals and a short period of follow-up. Unlike the above noted results in sleep timing parameters, evening circadian preferences seems to be a stable characteristic of BP regardless of mood status. Together with the literature (21–24, 37, 38), our findings support continued investigation of evening preference as a putative trait marker of BP, including examining the extent to which there is co-transmission of evening preference along with liability for BP. However, since the follow-up of our individuals started after the onset of BP, we do not know whether evening preference is a cause or consequence of BP.
We did not find differences in circadian preference between individuals with non-BP psychopathology and healthy controls, further supporting evening preference as a marker in individuals with BP. However, since different alterations of circadian preference, including delayed and advanced sleep phase, have been reported in individuals with non-BP psychopathology (39–44) and the non-BP group consisted of individuals with heterogeneous psychopathology, our findings need to be taken with caution.
In conclusion, individuals with BP showed more sleep timing disturbances at intake and across follow-up in comparison to the non-BP and healthy control groups. These disturbances were independent of BP subtype but more evident in individuals with BP with current mood episodes. Sleep disturbances by themselves have been associated with psychosocial difficulties and they can increase the risk for further mood episodes warranting their early identification and management (4, 45). In addition, independent of BP subtype, mood state and treatment, BP was specifically associated with evening preference providing further evidence that this construct may be a putative biological marker for BP. The specific relationship between BP and circadian rhythm has already fostered the investigation of a possible association between the so-called clock genes, which control the mammalian circadian system, and BP, with promising results (46–52). In fact, several genes, including TIMELESS, CLOCK, and BMal1, known to be involved in the regulation of sleep and circadian preferences, appear to be associated with the pathophysiology of BP (20, 46, 47, 53, 54). The above findings also emphasize the importance of management of sleep and circadian disturbances in individuals with BP. In fact, sleep deprivation, bright light, sleep phase advance, cognitive behavioral therapy for insomnia, and Interpersonal and Social Rhythm Therapy appear to accelerate and sustain the response of depressed individuals with BP to treatment (55–61).
Supplementary Material
Acknowledgements
This work was supported by Grant #MH-060952 from the National Institute of Mental Health (BB). The content of this article does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health. Thanks to the families for their participation, Melissa Cade, the BIOS research staff, and Shelli Avenevoli, Ph.D., from the National Institute of Mental Health for their support.
TRG receives research support from the National Institute of Mental Health (NIMH), NIDA, NICHD, The Pittsburgh Foundation, The Ryan Licht Sang Foundation, and The Fine Foundation; and receives royalties from Guilford Press. BIG has received research support from Pfizer; is a consultant for Bristol-Myers Squibb; and has received speakers honoraria from Purdue Pharma. BB has received research support from NIMH; and receives royalties from Random House, Inc., Lippincott Williams & Wilkins, and UpToDate.
Footnotes
Disclosures
MS, JM, DAA, DAB, VLN, RSD, DS, and DJK report no biomedical financial interests or potential conflicts of interest.
References
- 1.Wehr TA, Sack DA, Rosenthal NE. Sleep reduction as a final common pathway in the genesis of mania. Am J Psychiatry. 1987;144:201–214. doi: 10.1176/ajp.144.2.201. [DOI] [PubMed] [Google Scholar]
- 2.Harvey AG. Sleep and circadian rhythms in bipolar disorder: seeking synchrony, harmony, and regulation. Am J Psychiatry. 2008;165:820–829. doi: 10.1176/appi.ajp.2008.08010098. [DOI] [PubMed] [Google Scholar]
- 3.Dallaspezia S, Benedetti F. Melatonin, circadian rhythms, and the clock genes in bipolar disorder. Curr Psychiatry Rep. 2009;11:488–493. doi: 10.1007/s11920-009-0074-1. [DOI] [PubMed] [Google Scholar]
- 4.Jackson A, Cavanagh J, Scott J. A systematic review of manic and depressive prodromes. J Affect Disord. 2003;74:209–217. doi: 10.1016/s0165-0327(02)00266-5. [DOI] [PubMed] [Google Scholar]
- 5.Sylvia LG, Dupuy JM, Ostacher MJ, et al. Sleep disturbance in euthymic bipolar patients. J Psychopharmacol. 2012;26:1108–1112. doi: 10.1177/0269881111421973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giglio LM, Andreazza AC, Andersen M, et al. Sleep in bipolar patients. Sleep Breath. 2009;13:169–173. doi: 10.1007/s11325-008-0215-5. [DOI] [PubMed] [Google Scholar]
- 7.Lai YC, Huang MC, Chen HC, et al. Familiality and clinical outcomes of sleep disturbances in major depressive and bipolar disorders. J Psychosom Res. 2014;76:61–67. doi: 10.1016/j.jpsychores.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 8.Harvey AG, Schmidt DA, Scarna A, Semler CN, Goodwin GM. Sleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problems. Am J Psychiatry. 2005;162:50–57. doi: 10.1176/appi.ajp.162.1.50. [DOI] [PubMed] [Google Scholar]
- 9.Millar A, Espie CA, Scott J. The sleep of remitted bipolar outpatients: a controlled naturalistic study using actigraphy. J Affect Disord. 2004;80:145–153. doi: 10.1016/S0165-0327(03)00055-7. [DOI] [PubMed] [Google Scholar]
- 10.Brill S, Penagaluri P, Roberts RJ, Gao Y, El-Mallakh RS. Sleep disturbances in euthymic bipolar patients. Ann Clin Psychiatry. 2011;23:113–116. [PubMed] [Google Scholar]
- 11.Rocha PMB, Neves FS, Corrêa H. Significant sleep disturbances in euthymic bipolar patients. Comprehensive Psychiatry. 2013;54:1003–1008. doi: 10.1016/j.comppsych.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Geoffroy PA, Boudebesse C, Bellivier F, et al. Sleep in remitted bipolar disorder: a naturalistic case-control study using actigraphy. J Affect Disord. 2014;158:1–7. doi: 10.1016/j.jad.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 13.St-Amand J, Provencher MD, Belanger L, Morin CM. Sleep disturbances in bipolar disorder during remission. J Affect Disord. 2013;146:112–119. doi: 10.1016/j.jad.2012.05.057. [DOI] [PubMed] [Google Scholar]
- 14.Bauer M, Grof P, Rasgon N, Bschor T, Glenn T, Whybrow PC. Temporal relation between sleep and mood in patients with bipolar disorder. Bipolar Disord. 2006;8:160–167. doi: 10.1111/j.1399-5618.2006.00294.x. [DOI] [PubMed] [Google Scholar]
- 15.Perlman CA, Johnson SL, Mellman TA. The prospective impact of sleep duration on depression and mania. Bipolar Disord. 2006;8:271–274. doi: 10.1111/j.1399-5618.2006.00330.x. [DOI] [PubMed] [Google Scholar]
- 16.Gruber J, Miklowitz DJ, Harvey AG, et al. Sleep matters: sleep functioning and course of illness in bipolar disorder. J Affect Disord. 2011;134:416–420. doi: 10.1016/j.jad.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kerkhof GA, Van Dongen HP. Morning-type and evening-type individuals differ in the phase position of their endogenous circadian oscillator. Neurosci Lett. 1996;218:153–156. doi: 10.1016/s0304-3940(96)13140-2. [DOI] [PubMed] [Google Scholar]
- 18.Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav Neurosci. 2001;115:895–899. doi: 10.1037//0735-7044.115.4.895. [DOI] [PubMed] [Google Scholar]
- 19.Baehr EK, Revelle W, Eastman CI. Individual differences in the phase and amplitude of the human circadian temperature rhythm: with an emphasis on morningness-eveningness. J Sleep Res. 2000;9:117–127. doi: 10.1046/j.1365-2869.2000.00196.x. [DOI] [PubMed] [Google Scholar]
- 20.Mansour HA, Monk TH, Nimgaonkar VL. Circadian genes and bipolar disorder. Ann Med. 2005;37:196–205. doi: 10.1080/07853890510007377. [DOI] [PubMed] [Google Scholar]
- 21.Wood J, Birmaher B, Axelson D, et al. Replicable differences in preferred circadian phase between bipolar disorder patients and control individuals. Psychiatry Res. 2009;166:201–209. doi: 10.1016/j.psychres.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahn YM, Chang J, Joo YH, Kim SC, Lee KY, Kim YS. Chronotype distribution in bipolar I disorder and schizophrenia in a Korean sample. Bipolar Disord. 2008;10:271–275. doi: 10.1111/j.1399-5618.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 23.Giglio LM, Magalhaes PV, Andersen ML, Walz JC, Jakobson L, Kapczinski F. Circadian preference in bipolar disorder. Sleep Breath. 2010;14:153–155. doi: 10.1007/s11325-009-0301-3. [DOI] [PubMed] [Google Scholar]
- 24.Salvatore P, Ghidini S, Zita G, et al. Circadian activity rhythm abnormalities in ill and recovered bipolar I disorder patients. Bipolar Disord. 2008;10:256–265. doi: 10.1111/j.1399-5618.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- 25.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 26.First MB, Spitzer RL, Gibbon M, Williams JBW. User's Guide for the Structured Clinical Interview for DSM-IV Axis-I Disorders Research Version (SCID-I, Version 2.0) New York: Biometrics Research, New York State Psychiatric Institute; 1996. [Google Scholar]
- 27.Hollingshead AB. Four-factor index of social status. New Haven, CT: Yale University Sociology Department; 1975. [Google Scholar]
- 28.Monk TH, Frank E, Potts JM, Kupfer DJ. A simple way to measure daily lifestyle regularity. J Sleep Res. 2002;11:183–190. doi: 10.1046/j.1365-2869.2002.00300.x. [DOI] [PubMed] [Google Scholar]
- 29.Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol. 1989;74:728–738. doi: 10.1037/0021-9010.74.5.728. [DOI] [PubMed] [Google Scholar]
- 30.Monk TH, Buysse DJ, Potts JM, DeGrazia JM, Kupfer DJ. Morningness-eveningness and lifestyle regularity. Chronobiol Int. 2004;21:435–443. doi: 10.1081/cbi-120038614. [DOI] [PubMed] [Google Scholar]
- 31.Monk TH, Buysse DJ, Kennedy KS, Pods JM, DeGrazia JM, Miewald JM. Measuring sleep habits without using a diary: the sleep timing questionnaire. Sleep. 2003;26:208–212. doi: 10.1093/sleep/26.2.208. [DOI] [PubMed] [Google Scholar]
- 32.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 33.Boudebesse C, Geoffroy PA, Bellivier F, et al. Correlations between objective and subjective sleep and circadian markers in remitted patients with bipolar disorder. Chronobiol Int. 2014;31:698–704. doi: 10.3109/07420528.2014.895742. [DOI] [PubMed] [Google Scholar]
- 34.Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corrigan PW, Watson AC. Findings from the National Comorbidity Survey on the frequency of violent behavior in individuals with psychiatric disorders. Psychiatry Res. 2005;136:153–162. doi: 10.1016/j.psychres.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 37.Mansour HA, Wood J, Chowdari KV, et al. Circadian phase variation in bipolar I disorder. Chronobiol Int. 2005;22:571–584. doi: 10.1081/CBI-200062413. [DOI] [PubMed] [Google Scholar]
- 38.Milhiet V, Boudebesse C, Bellivier F, et al. Circadian abnormalities as markers of susceptibility in bipolar disorders. Front Biosci (Schol Ed) 2014;6:120–137. doi: 10.2741/s419. [DOI] [PubMed] [Google Scholar]
- 39.Bromundt V, Koster M, Georgiev-Kill A, et al. Sleep-wake cycles and cognitive functioning in schizophrenia. Br J Psychiatry. 2011;198:269–276. doi: 10.1192/bjp.bp.110.078022. [DOI] [PubMed] [Google Scholar]
- 40.Wulff K, Dijk DJ, Middleton B, Foster RG, Joyce EM. Sleep and circadian rhythm disruption in schizophrenia. Br J Psychiatry. 2012;200:308–316. doi: 10.1192/bjp.bp.111.096321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wulff K, Joyce E, Middleton B, Dijk DJ, Foster RG. The suitability of actigraphy, diary data, and urinary melatonin profiles for quantitative assessment of sleep disturbances in schizophrenia: a case report. Chronobiol Int. 2006;23:485–495. doi: 10.1080/07420520500545987. [DOI] [PubMed] [Google Scholar]
- 42.Wirz-Justice A, Haug HJ, Cajochen C. Disturbed circadian rest-activity cycles in schizophrenia patients: an effect of drugs? Schizophr Bull. 2001;27:497–502. doi: 10.1093/oxfordjournals.schbul.a006890. [DOI] [PubMed] [Google Scholar]
- 43.Falcon E, McClung CA. A role for the circadian genes in drug addiction. Neuropharmacology. 2009;56(Suppl. 1):91–106. doi: 10.1016/j.neuropharm.2008.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wasielewski JA, Holloway FA. Alcohol's interactions with circadian rhythms. A focus on body temperature. Alcohol Res Health. 2001;25:94–100. [PMC free article] [PubMed] [Google Scholar]
- 45.Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165:830–843. doi: 10.1176/appi.ajp.2008.08010077. [DOI] [PubMed] [Google Scholar]
- 46.Kripke DF, Nievergelt CM, Joo E, Shekhtman T, Kelsoe JR. Circadian polymorphisms associated with affective disorders. J Circadian Rhythms. 2009;7:2. doi: 10.1186/1740-3391-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mansour HA, Wood J, Logue T, et al. Association study of eight circadian genes with bipolar I disorder, schizoaffective disorder and schizophrenia. Genes Brain Behav. 2006;5:150–157. doi: 10.1111/j.1601-183X.2005.00147.x. [DOI] [PubMed] [Google Scholar]
- 48.Nievergelt CM, Kripke DF, Barrett TB, et al. Suggestive evidence for association of the circadian genes PERIOD3 and ARNTL with bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:234–241. doi: 10.1002/ajmg.b.30252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Artioli P, Lorenzi C, Pirovano A, et al. How do genes exert their role? Period 3 gene variants and possible influences on mood disorder phenotypes. Eur Neuropsychopharmacol. 2007;17:587–594. doi: 10.1016/j.euroneuro.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 50.Benedetti F, Dallaspezia S, Colombo C, Pirovano A, Marino E, Smeraldi E. A length polymorphism in the circadian clock gene Per3 influences age at onset of bipolar disorder. Neurosci Lett. 2008;445:184–187. doi: 10.1016/j.neulet.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 51.Shi J, Wittke-Thompson JK, Badner JA, et al. Clock genes may influence bipolar disorder susceptibility and dysfunctional circadian rhythm. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1047–1055. doi: 10.1002/ajmg.b.30714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McGrath CL, Glatt SJ, Sklar P, et al. Evidence for genetic association of RORB with bipolar disorder. BMC Psychiatry. 2009;9:70. doi: 10.1186/1471-244X-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee HJ, Paik JW, Kang SG, Lim SW, Kim L. Allelic variants interaction of CLOCK gene and G-protein beta3 subunit gene with diurnal preference. Chronobiol Int. 2007;24:589–597. doi: 10.1080/07420520701534632. [DOI] [PubMed] [Google Scholar]
- 54.Lamont EW, Legault-Coutu D, Cermakian N, Boivin DB. The role of circadian clock genes in mental disorders. Dialogues Clin Neurosci. 2007;9:333–342. doi: 10.31887/DCNS.2007.9.3/elamont. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sit D, Wisner KL, Hanusa BH, Stull S, Terman M. Light therapy for bipolar disorder: a case series in women. Bipolar Disord. 2007;9:918–927. doi: 10.1111/j.1399-5618.2007.00451.x. [DOI] [PubMed] [Google Scholar]
- 56.Benedetti F, Dallaspezia S, Fulgosi MC, Barbini B, Colombo C, Smeraldi E. Phase advance is an actimetric correlate of antidepressant response to sleep deprivation and light therapy in bipolar depression. Chronobiol Int. 2007;24:921–937. doi: 10.1080/07420520701649455. [DOI] [PubMed] [Google Scholar]
- 57.Wu JC, Kelsoe JR, Schachat C, et al. Rapid and sustained antidepressant response with sleep deprivation and chronotherapy in bipolar disorder. Biol Psychiatry. 2009;66:298–301. doi: 10.1016/j.biopsych.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 58.Wehr TA, Goodwin FK, Wirz-Justice A, Breitmaier J, Craig C. 48-hour sleep-wake cycles in manic-depressive illness: naturalistic observations and sleep deprivation experiments. Arch Gen Psychiatry. 1982;39:559–565. doi: 10.1001/archpsyc.1982.04290050037008. [DOI] [PubMed] [Google Scholar]
- 59.Frank E, Kupfer DJ, Thase ME, et al. Two-year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Arch Gen Psychiatry. 2005;62:996–1004. doi: 10.1001/archpsyc.62.9.996. [DOI] [PubMed] [Google Scholar]
- 60.Goldstein TR, Fersch-Podrat R, Axelson DA, et al. Early intervention for adolescents at high risk for the development of bipolar disorder: pilot study of Interpersonal and Social Rhythm Therapy (IPSRT) Psychotherapy (Chic) 2014;51:180–189. doi: 10.1037/a0034396. [DOI] [PubMed] [Google Scholar]
- 61.Steinan MK, Krane-Gartiser K, Langsrud K, Sand T, Kallestad H, Morken G. Cognitive behavioral therapy for insomnia in euthymic bipolar disorder: study protocol for a randomized controlled trial. Trials. 2014;15:24. doi: 10.1186/1745-6215-15-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.