Abstract
Background
Anabolic-androgenic steroid (AAS) use is associated with psychiatric symptoms including increased aggression as well as with cognitive dysfunction. The brain effects of long-term AAS use have not been assessed in humans.
Methods
This multimodal magnetic resonance imaging study of the brain compared 10 male weightlifters reporting long-term AAS use with 10 age-matched weightlifters reporting no AAS exposure. Participants were administered visuospatial memory tests and underwent neuroimaging. Brain volumetric analyses were performed; resting-state fMRI functional connectivity (rsFC) was evaluated using a region-of-interest analysis focused on the amygdala; and dorsal anterior cingulate cortex (dACC) metabolites were quantified by proton magnetic resonance spectroscopy (MRS).
Results
AAS users had larger right amygdala volumes than nonusers (P=0.002) and reduced rsFC between right amygdala and frontal, striatal, limbic, hippocampal, and visual cortical areas. Left amygdala volumes were slightly larger in AAS users (P=0.061) but few group differences were detected in left amygdala rsFC. AAS users also had lower dACC scyllo-inositol levels (P=0.004) and higher glutamine/glutamate ratios (P=0.028), possibly reflecting increased glutamate turnover. On a visuospatial cognitive task, AAS users performed more poorly than nonusers, with the difference approaching significance (P=0.053).
Conclusions
Long-term AAS use is associated with right amygdala enlargement and reduced right amygdala rsFC with brain areas involved in cognitive control and spatial memory, which could contribute to the psychiatric effects and cognitive dysfunction associated with AAS use. The MRS abnormalities we detected could reflect enhanced glutamate turnover and increased vulnerability to neurotoxic or neurodegenerative processes, which could contribute to AAS-associated cognitive dysfunction.
Keywords: Anabolic-androgenic steroids, magnetic resonance imaging, magnetic resonance spectroscopy, amygdala, scyllo-inositol, glutamate
1. INTRODUCTION
Illicit anabolic-androgenic steroids (AAS) use poses a growing public health problem worldwide, with 2.9–4.0 million individuals in the US alone having used these drugs (Pope et al., 2014). Virtually all of these users are male (Kanayama et al., 2007). Although used by elite athletes since the 1950s, AAS did not spread widely to the general population until the 1980s (Kanayama et al., 2008). Thus, even the oldest AAS users, who initiated AAS use as youths in the 1980s, are mostly under age 50 today. As the leading edge of this AAS-user population passes through middle age, adverse general health effects of long-term AAS exposure are becoming increasingly apparent (Pope et al., 2014).
AAS are known to cause acute psychiatric effects such as aggression (Copeland et al., 2000; Perry et al., 2003; Pope et al., 2014), violence, including increased partner violence (Beaver et al., 2008; Choi and Pope, 1994; Middleman et al., 1995; Pope and Katz, 1990; Skarberg et al., 2010; Thiblin and Parlklo, 2002), and impulsive behaviors including risky sexual and other behaviors (Hildebrandt et al., 2014; Middleman et al., 1995; Midgley et al., 2000). We (Kouri et al., 1995; Pope et al., 2000) and others (Su et al., 1993; Yates et al., 1999) have documented such effects in controlled human studies. AAS also increase aggressive behaviors in adolescent and adult rodents (Kalinine et al., 2014; Melloni and Ferris, 1996), which may be associated with reduced glutamate uptake and increased N-methyl-D-aspartate (NMDA) receptor activity (Kalinine et al., 2014).
AAS also may cause chronic cognitive effects. Recently, we reported (Kanayama et al., 2013) that long-term AAS users exhibited deficits on two tests of visuospatial memory from the widely used CANTAB battery (Cambridge Cognition, 2007), and the severity of these deficits was associated with lifetime dose of AAS used. One of these tests, Paired Associates Learning, has previously been shown to predict the development of dementia (Swainson et al., 2001). Consistent with our human findings, rodent studies have shown that AAS exposure can impair performance on the Morris water maze test of spatial learning and memory (Magnusson et al., 2009; Novaes Gomes et al., 2014; Pieretti et al., 2012; Tanehkar et al., 2013). Impaired inhibitory control and attention also were recently reported in men actively taking AAS, with greater impairment found in adolescent- than adult-onset AAS users (Hildebrandt et al., 2014).
While the human brain substrates for these AAS effects have yet to be elucidated, the findings reviewed above suggest that attentional regions of the brain associated with threat reactivity and regulation, such as the amygdala, the hippocampus, and the dorsal anterior cingulate cortex (dACC), may be particularly vulnerable to chronic AAS use.. The amygdala is involved in threat processing and aggression (Siever, 2008). The rat amygdala is androgen-sensitive (Cooke et al., 1999; Lynch and Story, 2000) and androgen administration to male rats induces amygdala neurogenesis and neuronal soma and astrocyte volume and complexity increases (Fowler et al., 2003; Cooke et al., 1999; Johnson et al., 2008, 2012). Functional MRI (fMRI) studies in healthy men report positive associations between amygdala reactivity to angry or fearful faces and levels of the endogenous AAS testosterone (Derntl et al., 2009). Similarly, testosterone administration to healthy men acutely increased amygdala reactivity to angry faces (Goetz et al., 2014). Further, amygdala volume increases have been associated with aggressive behavior among substance users (Schiffer et al., 2011). Collectively these findings suggest that AAS could increase amygdala volume and possibly catalyze or enable aggression behaviors. The hippocampus is involved in spatial memory processes (Squire, 1992). In rats, AAS induce hippocampal apoptosis (Ma and Liu, 2015; Tugyan et al., 2013), and inhibit hippocampal neurogenesis (Brannvall et al., 2005; Novaes Gomes et al., 2014), suggesting that AAS could reduce hippocampal volume, which could be a basis for the AAS-associated spatial memory impairments observed in human and animal studies. The dACC is a cognitive control region involved in attentional processes (Bush and Shin, 2006), which as noted above are abnormal in human AAS users (Hildebrandt et al., 2014). Abnormal dACC activation has been documented in alcohol-dependent subjects performing a spatial working memory fMRI task (Vollstadt-Klein et al., 2010), suggesting that visuospatial dysfunction among AAS users could be related to dACC dysfunction.
Although case reports have documented cerebrovascular problems associated with human AAS use (Akhter et al., 1994; Shimada et al., 2012), no systematic neuroimaging studies have yet assessed human brain effects of long-term AAS use. Accordingly, we acquired from long-term AAS users and nonusers 3 Tesla structural magnetic resonance imaging (MRI), resting state functional connectivity (rsFC) MRI (which maps brain regions thought to be functionally coupled and inherently organized at rest (Greicius, 2008)), and proton magnetic resonance spectroscopy (MRS, which evaluates neurochemistry). We also administered the two computerized tests of visuospatial memory revealing deficits in AAS users in our prior study (Kanayama et al., 2013). Because it is technically challenging to acquire high-quality 3 Tesla MRS spectra from the small, irregularly-shaped amygdala and hippocampus, due in part to partial volume effects (contamination by adjacent structures), we acquired MRS spectra from the dACC, from which MRS data be more reliably acquired. Also, as discussed above, this region may contribute to deficits associated with AAS use. We used MRI to determine whether long-term AAS use is associated with abnormal amygdala and hippocampal volumes and connectivities. Also, given rodent studies suggesting that AAS may enhance the effects of glutamate neurotransmission (Kalinine et al., 2014; Orlando et al., 2007), we used MRS to determine whether long-term AAS use is associated with dACC glutamate abnormalities.
2. METHODS AND MATERIALS
2.1 Participants
Study participants were drawn from a pool of about 150 experienced male weightlifters aged 35–55 who had been evaluated in 2011–2014 in a large ongoing study of the cardiac effects of long-term AAS use (Weiner et al., 2013). Since virtually all AAS users are weightlifters, we initially recruited these men by advertising in gymnasiums frequented by AAS users and nonusers (Kanayama et al., 2003; Pope et al., 2012). Participants received a comprehensive interview covering athletic, medical, and psychiatric histories, and alcohol and drug use history, with detailed questions about AAS use (types of drugs used, doses, and durations), and questions about use of other appearance- and performance-enhancing drugs. Individuals reporting either a) long-term AAS use (≥2 years of cumulative lifetime AAS exposure) or b) no AAS use were selected for further study. To assess the validity of participants’ self-reports, we tested hair and urine samples for AAS and other drugs of abuse, and performed measures of body muscularity to detect possible surreptitious AAS users. Individuals with inconsistencies between these measures and their self-reports were excluded from study participation. We have detailed these procedures previously (Pope et al., 2012). Participants provided informed consent both for the prior cardiac and present imaging studies, which were approved by the McLean Hospital Institutional Review Board.
2.2 Study Procedures
We selected 10 long-term AAS users and 10 nonusers from our pool described above to return for a single three-hour midday visit, without specific control for time or dietary factors. We first reviewed participants’ interim history since their initial evaluation, and then screened them for recent alcohol (Alco-Sensor IV; Intoximeters, Saint Louis, Missouri) and standard drugs of abuse (AmediCheck 12-Panel Drug Test Cups; Amedica Biotech, Hayward, CA). They then received the Pattern Recognition Memory (PRM) and Paired Associates Learning (PAL) tests from the widely-used computerized (touch-screen) CANTAB battery (Cambridge Cognition, 2007). The PRM tests visual memory (Sahakian et al., 1988) by presenting two sets of 12 visual patterns, each followed by a recognition phase during which participants are shown two patterns and asked to touch the one they have previously seen. The PAL tests visuospatial memory and new learning (Robbins et al., 1997) by presenting several white boxes that serially “open” to reveal underlying patterns. Participants are shown each pattern and asked to touch the box covering that pattern. We selected these tests because they revealed deficits in AAS users in our previous study (Kanayama et al., 2013). We also administered the short (35-word) version of the North American Adult Reading Test (NAART35; Uttl, 2002) to estimate intellectual ability.
Following cognitive testing, participants underwent neuroimaging using a Siemens TIM Trio 3 Tesla scanner (Erlangen, Germany) and a 32-channel head coil. A clinical brain scan including a high-resolution T1-weighted, dual echo MPRAGE3D anatomical whole-brain image (repetition time (TR)=2.1s, echo time (TE1)=3.3ms, (TE2)=6.99ms, slices=128, matrix=256x256, resolution=1x1x1.33mm) was collected. Then, a whole-brain resting state echo-planar functional MRI scan was acquired with the following parameters: TR=2.5s, TE=30ms, flip angle=90°, slices=42, voxel size=3.5mm isotropic, scan duration=6 minutes. Subjects were instructed to relax with their eyes open and to think of nothing in particular during the scan.
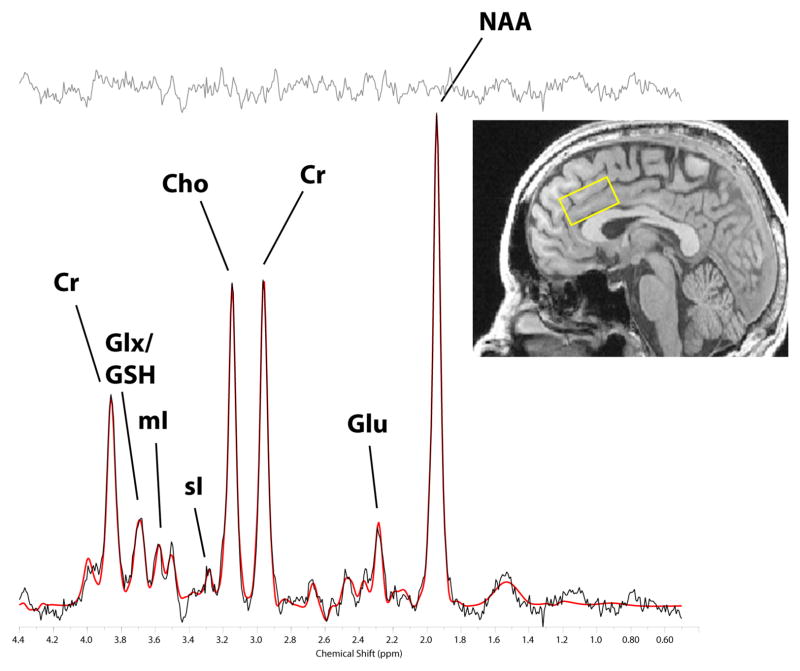
MRS data were acquired from a 2.0cm (I-S) x 2.5cm (L-R) x 3.5cm (A-P) voxel placed medially in the dACC and obliqued such that the voxel inferior face was tangent to the corpus callosum superior surface (Figure 1, inset). We used a modified J-resolved PRESS protocol (2D-JPRESS) which steps the 2nd echo-time to sample J-coupling of coupled metabolites, providing increased spectral resolution and metabolite detection reliability. The voxel magnetic field was optimized by automated shimming followed by manual shimming to minimize water linewidth. Following additional automated optimization of water suppression power, carrier-frequency, tip angles and coil tuning, we collected 22 TE-stepped spectra (TEs ranged from 30–350ms in 15ms increments). Acquisition parameters were: TR=2s, f1 acquisition bandwidth=67Hz, spectral bandwidth=2kHz, readout duration=512ms, excitations (NEX)=16/TE-step, total scan duration=12min.
Figure 1.
LCModel spectrum fit of a dorsal anterior cingulate cortex J = 0.0 Hz spectrum. The voxel position (inset, yellow box), the source spectrum (black trace), the LCModel-fitted spectrum (red trace), and the residual (gray trace at top of figure) are shown. Individual metabolite peaks are labeled (Cr: total creatine; Glx: glutamate + glutamine; GSH: glutathione; mI: myo-inositol; sI: scyllo-inositol; Cho: choline-containing metabolites; Glu: glutamate; NAA: N-acetylaspartate).
2.3 Data Analysis
2.3.1. Demographic and cognitive measures
Comparisons between AAS users and nonusers on demographic variables were performed using Fisher’s exact test, two-tailed, for ordinal variables and linear regression (a technique mathematically identical to analysis of variance) for continuous variables, including NAART35 scores. Cognitive testing results were assessed by linear regression with adjustment for NAART35 scores, as intellectual ability is known to affect cognitive processes. Notably, the cognitive measures had apparently skewed distributions, and given the sparseness of the data, we could not check assumptions about the distributions of these measures. Therefore we analyzed these measures using ranked data.
Analyses of these and all subsequent numerical data were performed using Stata 9.2 (Stata Corporation, College Station, Texas), with α=0.05, 2-tailed. Note that the study generated multiple comparisons for structural MRI and MRS measures, increasing the likelihood of a type I error. However, Bonferroni or similar corrections would likely be too conservative and inflate type II error rates, which would obscure potential signals arising from this small study with limited power. Thus, we present results without correction. However, when evaluating significance tests reported below, one must consider the greater possibility of chance associations.
2.3.2. Brain volumetric analysis
MPRAGE3D images were processed using Freesurfer (version 5.3.0) default parameters to evaluate total gray matter, white matter, and cerebrospinal fluid volumes, as well as volumes of subcortical regions. Group differences on volumetric measures were analyzed by linear regression, again using ranked data.
2.3.3 Resting state fMRI analysis
The resting state fMRI data analysis was conducted using FSL 4.1.9 (FMRIB Software Library, Oxford UK). Data from 3 AAS users currently taking prescribed opioids were excluded from this analysis for reasons noted below in section 3.2. Functional data were preprocessed using motion correction with MCFLIRT, brain extraction using BET, slice timing correction, spatial smoothing with a Gaussian kernel of full-width half-maximum of 6mm and high-pass temporal filtering with a Gaussian-weighted least-square straight-line fitting with σ=100 s. Images were registered using FLIRT and refined with FNIRT, and were warped to the Montreal Neurological Institute (MNI) atlas space and resampled to 2 mm isotropic voxels. To prevent motion and other sources of noise from influencing resting state analyses, each subject’s data was denoised using FSL’s MELODIC (Multivariate Exploratory Linear Decomposition into Independent Components). First, MELODIC was used to identify all independent components (ICs) on an individual subject basis. The spatial maps and associated time courses for each of these ICs were then visually inspected and those ICs representing noise were identified (Kelly et al., 2010). The noise components, which could be due to participant motion or scanner artifact, were then regressed out of the fMRI data using FSL’s “fsl_regfilt” utility. After identifying an amygdala volume effect of AAS use (see Results), time courses from the whole right and left amygdala, as defined by Freesurfer, were then individually extracted for each participant and regressed against the resting-state data. At the group level, resting-state coupling between the right or left amygdala and all voxels in the brain was compared between individuals who did or did not use AAS.
2.3.4 MRS Data Analysis
Data processing and analyses were undertaken on a LINUX workstation. To quantify in vivo metabolites with JPRESS data, the 22 free-induction decays first were zero-filled to 64 points, Gaussian-filtered, and Fourier transformed. Every J-resolved spectral extraction within a bandwidth of 67Hz was fitted with LCModel (Provencher, 1993) and its theoretically-correct template, using an optimized GAMMA-simulated J-resolved basis set modeled for 2.89 T (Jensen et al., 2009). The integrated area under the entire surface for each metabolite was calculated by summing the raw peak areas across all 64 J-resolved metabolite extractions. Metabolite areas were normalized to creatine and expressed as creatine ratios. A sample LCModel-fitted MRS spectrum is shown in Figure 1. Group differences on MRS metabolite levels were assessed by linear regression. Within the AAS-user group, the association between MRS measures and lifetime cumulative AAS dose was also assessed by linear regression. We used ranked data for the reasons described above.
3. RESULTS
3.1 Demographic Measures and Cognitive Test Findings
The groups were well matched on demographic variables (Table 1). AAS users reported usage patterns similar to those of long-term AAS users evaluated in our prior studies (Kanayama et al., 2003; Pope et al., 2012; Pope and Katz, 1994; see Table 1), involving about 80% injectable AAS (e.g., testosterone, nandrolone, boldenone) and 20% oral preparations (e.g., methandienone, methenolone, stanozolol). Some participants reported current use of prescription psychoactive medications, including 3 AAS users taking prescription opioids (two taking buprenorphine and one taking oxycodone). One AAS user had taken cocaine and one nonuser had taken marijuana on the day prior to evaluation. Three other individuals (two AAS users and one nonuser) showed detectable cannabinoids on their urine samples but reported no marijuana use during the 24 hours prior to evaluation. Overall, 5 AAS users and 3 nonusers had taken at least one licit or illicit psychoactive substance within the past 24 hours (Table 1).
Table 1.
Attributes and measures of AAS Users versus Non-AAS-Using Weightlifters
| Attribute/measurea | AAS users N = 10 |
AAS Nonusers N = 10 |
Pb |
|---|---|---|---|
| Age, years | 42.4 (1.3) | 42.6 (2.1) | 0.94 |
| Race/ethnicity | |||
| Non-Hispanic White | 9 (90) | 10 (10) | 1.0 |
| Hispanic White | 1 (10) | ||
| Four-year college graduate | 4 (40) | 8 (80) | 0.17 |
| Annual income > $50,000 | 4 (40) | 5 (50) | 1.00 |
| Lifetime years of regular weightlifting | 20.2 (6.8) | 22.4 (9.8) | 0.57 |
| Age at first AAS use, yrs | 23.4 (5.1) | - | |
| Cumulative lifetime AAS use, weeks | 482 (582) | - | |
| Cumulative lifetime AAS dose, mgc | 704,000 (537,000) | - | |
| Average weekly AAS dose, mgc | 1460 (620) | ||
| Maximum weekly AAS dose, mgc | 2300 (1230) | ||
| Use of injectable AAS, as percent of total use | 80 (18) | ||
| Time since last AAS use: | |||
| Current use | 4 (40) | - | |
| 2–4 months | 2 (20) | - | |
| > 20 months | 4 (40) | - | |
| Current psychoactive medication use:d | |||
| Opioids | 3 | 0 | |
| Selective serotonin reuptake inhibitors | 2 | 2 | |
| Other psychoactive medications | 4 | 1 | |
| Illicit drug use in past 24 hours: | |||
| Cocaine | 1 | 0 | |
| Cannabis | 0 | 1 | |
| Current use of any psychoactive substance | 5 | 3 | |
|
| |||
| Cognitive test results: | |||
| NAART35 score | 15.7 (7.6)e | 17.1 (7.7) | 0.69 |
| Pattern Recognition Memory, total errors | 1.5 (0, 4.25) | 2.5 (2.0, 4.25) | 0.38 |
| Paired Associates Learning, 6-box errors | 5.0 (2.0, 9.5) | 2.0 (1.5, 4.75) | 0.053 |
| Paired Associates Learning, 8-box errors | 29.5 (4.75, 34.25) | 10 (17, 22.25) | 0.14 |
Demographic attributes and NAART35 scores shown as mean (SD) for continuous variables and as N (%) for ordinal variables; cognitive test results are shown as median (interquartile range).
See text for details of statistical methods.
Expressed as mg of testosterone equivalent, calculated as in previous studies (Pope & Katz, 1994).
Note that participants could be counted in more than one category (e.g. a participant could be using both opioids and selective serotonin reuptake inhibitors).
Excludes one AAS user who was not a native speaker of English.
On visuospatial memory tests, we found no significant group difference in PRM. However, AAS users produced more errors on the 6-level analysis of PAL, with the difference approaching statistical significance (P=0.053; Table 1). A similar but weaker trend was observed with the 8-level analysis of PAL (P=0.14; Table 1), with both groups having broader performance distributions as task difficulty increased.
3.2 Volumetric Findings
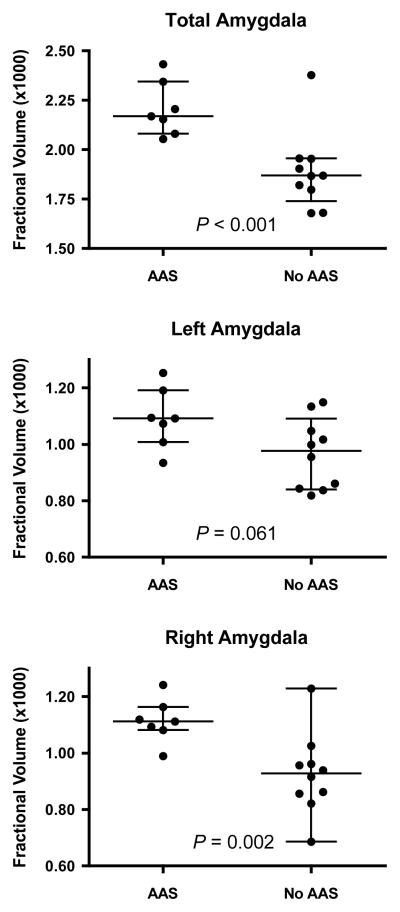
No gross brain structural abnormalities were detected on clinical scans of AAS subjects. Freesurfer volumetric analysis revealed larger bilateral amygdala volumes in AAS users than nonusers at a trend significance level (P=0.056; Table 2). However, as current opioid use is associated with reduced amygdala volumes (Upadhyay et al., 2010; Younger et al., 2011), we reanalyzed the amygdala data after excluding the 3 AAS users on prescribed opioids. This analysis yielded a strongly significant group difference in total amygdala volume (P<0.001; Figure 2, Table 2). Notably, the AAS users showed only modestly increased left amygdala volumes versus nonusers (P=0.061; Figure 2, Table 2), but strikingly increased right amygdala volumes (P=0.002; Figure 2, Table 2). We then repeated these comparisons excluding the 5 AAS users and 3 nonusers currently taking any psychotropic agent (Table 1). Even in this reduced sample (5 AAS users, 7 nonusers), group differences in total amygdala and right amygdala volumes remained significant (P=0.020 and 0.032, respectively).
Table 2.
Brain Volume and MRS Metabolite Comparisons Between AAS and Control Subjects
| Measurea | AAS users N = 10 |
AAS Non-users N = 10 |
P |
|---|---|---|---|
| Freesurfer brain volume measures | |||
| Total Intracranial Volume (TIV), cm3 | 1726 (1508, 1796) | 1697 (1550, 1800) | 0.83 |
| Supratentorial volume (%TIV) | 62.1 (60.2, 65.1) | 62.1 (59.0, 63.4) | 0.66 |
| Total gray matter (%TIV) | 38.3 (35.4, 40.9) | 37.3 (34.8, 39.1) | 0.66 |
| Total white matter (%TIV) | 30.7 (29.8, 32.2) | 31.0 (29.8, 32.0) | 0.89 |
| Cortical gray matter (%TIV) | 28.2 (26.0, 29.8) | 27.3 (24.9, 29.0) | 0.56 |
| Cerebellum (%TIV) | 8.5 (7.9, 8.9) | 8.4 (7.9, 9.0) | 0.83 |
| Subcortical gray (%TIV) | 3.5 (3.4, 3.7) | 3.5 (3.4, 3.8) | 0.77 |
| Ventricles (%TIV) | 1.0 (0.8, 1.6) | 1.1 (0.8, 1.4) | 0.83 |
| Thalamus (%TIV) | 0.93 (0.85, 0.99) | 0.97 (0.96, 1.01) | 0.11 |
| Putamen (%TIV) | 0.65 (0.63, 0.70) | 0.65 (0.57, 0.69) | 0.72 |
| Hippocampus (%TIV) | 0.47 (0.45, 0.53) | 0.46 (0.44, 0.50) | 0.51 |
| Caudate nucleus (%TIV) | 0.47 (0.45, 0.49) | 0.44 (0.41, 0.47) | 0.21 |
| Amygdala (%TIV) | 0.21 (0.19, 0.22) | 0.19 (0.18, 0.20) | 0.056 |
| -Amygdala (%TIV) minus opioid usersb | 0.22 (0.21, 0.23) | 0.19 (0.18, 0.20) | <0.001 |
| -Left Amygdala (%TIV) minus opioid usersb | 0.11 (0.10, 0.12) | 0.10 (0.08, 0.11) | 0.061 |
| -Right Amygdala (%TIV) minus opioid usersb | 0.11 (0.11, 0.12) | 0.09 (0.08, 0.10) | 0.002 |
| Corpus callosum (%TIV) | 0.19 (0.18, 0.21) | 0.19 (0.18, 0.20) | 0.51 |
| Globus Pallidus (%TIV) | 0.18 (0.17, 0.20) | 0.19 (0.15, 0.21) | 0.89 |
| White matter hypointensities (%TIV) | 0.089 (0.079, 0.104) | 0.104 (0.067, 0.139 | 0.38 |
| Nucleus accumbens (%TIV) | 0.059 (0.053, 0.075) | 0.062 (0.050, 0.077) | 0.89 |
| Dorsal anterior cingulate cortex MRS metabolite levels (creatine-normalized)c | |||
| Aspartate | 0.43 (0.39, 0.46) | 0.41 (0.33, 0.44) | 0.19 |
| Choline | 0.83 (0.73, 0.91) | 0.84 (0.71, 0.91) | 0.93 |
| Gamma-amino butyric acid (GABA) | 0.044 (0.028, 0.055) | 0.051 (0.037, 0.075) | 0.34 |
| Glutamate | 0.88 (0.81, 0.90) | 0.85 (0.79, 0.97) | 0.87 |
| Glutamine | 0.53 (0.42, 0.57) | 0.38 (0.31, 0.46) | 0.059 |
| Glutathione (GSH) | 0.31 (0.29, 0.34) | 0.28 (0.25, 0.32) | 0.092 |
| Glutamine/glutamate ratio (Gln/Glu) | 0.63 (0.49, 0.67) | 0.41 (0.37, 0.54) | 0.028 |
| Myo-inositol | 0.69 (0.65, 0.70) | 0.64 (0.59, 0.68) | 0.092 |
| N-acetylaspartate (NAA) | 1.03 (0.89, 1.10) | 1.06 (0.99, 1.16) | 0.19 |
| N-acetylaspartylglutamate | 0.14 (0.11, 0.18) | 0.13 (0.10, 0.17) | 0.67 |
| Scyllo-inositol | 0.018 (0.016, 0.022) | 0.028 (0.25, 0.37) | 0.004 |
| Taurine | 0.087 (0.071, 0.11) | 0.059 (0.050, 0.095) | 0.092 |
| Lactate | 0.096 (0.067, 0.13) | 0.11 (0.076, 0.14) | 0.55 |
Measures shown as median (interquartile range)
Three AAS users who were currently taking prescribed opioids were excluded from these analyses
N = 8 for AAS users because of insufficient data quality in two participants
Figure 2.
Amygdala fractional volume (expressed as percent of total intracranial volume) in AAS users and AAS non-users (No AAS). Note that 3 subjects taking prescription opioids are excluded from these comparisons because of evidence that opioid use substantially influences amygdala volume (see text). Top: total amygdala volumes; Middle: left amygdala volumes; Bottom: right amygdala volumes. Also shown are medians and interquartile ranges.
3.3 Resting-State fMRI Findings
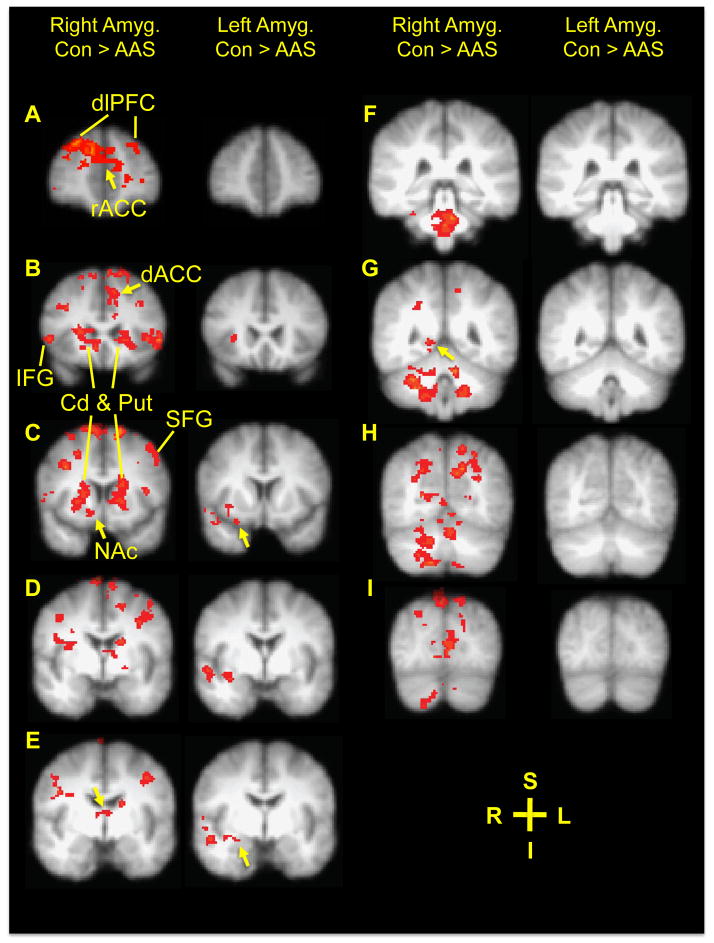
Given these selective amygdala abnormalities, we conducted a seed-region analysis of resting state fMRI data to determine whether right or left whole amygdala functional coupling differed between AAS users (N=7, excluding men on opioids) and AAS nonusers (N=9, one unusable fMRI data set). Resting state coupling between right amygdala and numerous brain regions was significantly decreased in AAS users versus nonusers (Figure 3). These regions included the left dACC, right nucleus accumbens, and right posterior hippocampus, as well as bilateral caudate nucleus and putamen; anterior insula; dorsomedial thalamus; superior, middle and inferior frontal gyri; postcentral gyri; precuneus; striate cortex; parieto-occipital transition area; cerebellar hemispheres. AAS users also showed reduced connectivity between the right amygdala and several medial structures, including pregenual cingulate cortex, cerebellar vermis, and midbrain and brainstem areas. By contrast, in AAS users, left amygdala coupling was reduced with respect to only a few regions, including the right anterior and posterior insula, right piriform cortex, right amygdala and extended amygdala, and right superior temporal gyrus.
Figure 3.
Amygdala resting state fMRI functional connectivity differences. Shown (Panels A–H) are right (left columns) and left (right columns) amygdala resting state functional connectivity difference maps showing areas of greater connectivity in AAS nonusers (No AAS, N = 9) than in AAS users (N = 7), all overlaid on composite coronal brain slices. Panel A (y = 50), arrow: pregenual cingulate cortex and lines: dorsolateral prefrontal cortex (dlPFC); Panel B: (y = 24), arrow: left dACC and lines: caudate nucleus (Cd), putamen (Put), and inferior frontal gyrus (IFG); Panel C: (y = 12), left arrow: right nucleus accumbens, right arrow: right piriform cortex, and lines: Cd, Put, and superior frontal gyrus (SFG); Panel D: (y = 0); Panel E: (y = −4), left arrow: dorsomedial thalamus, right arrow: right amygdala and extended amygdala; Panel F: (y = −34); Panel G: (y = −48), arrow: right posterior hippocampus; Panel H: (y = −64); Panel I: (y = −80). Right amygdala connectivity also was greater in AAS nonusers versus AAS users bilaterally in the caudate nucleus and putamen (Panels B–E), anterior insula (Panels B–D), superior, middle and inferior frontal gyri (Panels A–E), postcentral gyri (Panel E), precuneus (Panels G–I), striate cortex (Panels H and I), parieto-occipital transition area (Panel I), and cerebellar hemispheres (Panels F–I). Left amygdala connectivity was reduced in AAS users relative to nonusers in the right anterior and posterior insula (Panels B–E), right piriform cortex (Panel C), right amygdala and extended amygdala (Panels D–E), and right superior temporal gyrus (Panels C–E). Legend: S = superior; I = inferior; R = right; L = Left.
3.4 MRS Findings
We found higher dACC glutamine/glutamate ratios in AAS users (P=0.028; Table 2) and markedly lower scyllo-inositol levels (P=0.004; Table 2). Moreover, among AAS users, higher total lifetime AAS dose was associated with lower scyllo-inositol at the trend level (P=0.070). When we repeated these analyses excluding the 5 users and 3 nonusers currently taking any psychotropic agent, group differences for scyllo-inositol and gln/glu remained significant despite the diminished sample sizes (P=0.020 and 0.017, respectively).
4. DISCUSSION
In this brain imaging study, long-term AAS users showed 1) markedly increased right amygdala volumes; 2) markedly decreased right amygdala rsFC, and 3) reduced dACC gln/glu and scyllo-inositol levels compared to nonusers. We also found group differences approaching statistical significance on a visuospatial memory test. To our knowledge, these data represent the first report of brain structural and functional effects of long-term human AAS use.
4.1 Structural and Resting-State FMRI Findings
AAS use was associated with a striking right amygdala enlargement in non-opioid-using AAS users compared to nonusers. The apparently selective anatomical effect of AAS use on the amygdala is consistent with reports documenting that the amygdala contains among the highest androgen receptor mRNA densities in the brain (Menard and Harlan, 1993; Michael et al., 1995) and is androgen-sensitive even in adults (see Introduction). The apparently lateralized effect we detected also is consistent with preclinical findings, as in adult male rats, androgens exert right-lateralized effects on the posterodorsal medial (MePD) amygdala, increasing astrocyte numbers and volumes (Johnson et al., 2008; Morris et al., 2008).
Given the role of the hippocampus in spatial memory (Squire, 1992), together with our findings of visuospatial cognitive deficits in AAS users presently, in our prior study (Kanayama et al., 2013), and in animal studies (see Introduction), we anticipated that we would detect hippocampal volume abnormalities in AAS users. Our negative finding could have resulted if AAS-induced abnormalities are limited to dentate gyrus granule cells, the neurogenesis of which is inhibited by AAS (Brannvall et al., 2005; Novaes Gomes et al., 2014). Since dentate gyrus volume averages less than 5% of total hippocampal volume (Boldrini et al., 2013; McKinnon et al., 2009), even large dentate gyrus volume changes might have gone undetected with the present methods, which had a variability (standard deviation) of about 8% for whole hippocampus measurement.
We also found among non-opioid-using AAS users that resting-state functional coupling between the amygdala and cortical, limbic, cerebellar, and brainstem areas was reduced versus AAS nonusers. These regions regulate a number of behaviors and are involved in a wide range of cognitive processes. In AAS users, right amygdala connectivity was substantially more abnormal than left amygdala connectivity in terms of overall numbers of voxels exhibiting reduced connectivity, consistent with our structural finding of a greater right versus left amygdala enlargement in AAS users. Reduced right amygdala connectivity with the striate cortex (primary visual cortex, Brodmann area 17) and posterior hippocampus in AAS subjects could be related to the abnormal visuospatial memory detected in our AAS subjects, given that these regions all are involved in spatial information processing (Hampstead et al., 2010; Libby et al., 2014; Pegna et al., 2002).
Rodent studies suggest that left MePD amygdala astrocytes are more developed and have more complicated three-dimensional structures than right amygdala astrocytes (Johnson et al., 2008). If this pattern extends to human amygdala, then lateralized differences in tetrapartite synaptic structure could constrain left amygdala synaptic plasticity when AAS are present. By contrast, AAS could induce greater right amygdala plasticity, which could reorganize or disrupt connectivity patterns.
4.2 MRS Findings
On MRS measures, AAS users had higher dACC gln/glu ratios and markedly lower scyllo-inositol levels. Elevated gln/glu ratios could reflect glutamate-glutamine cycle upregulation via enhanced neuronal glutamate release and conversion to glutamine in glia (Brennan et al., 2010; Pereira et al., 2008). This seems consistent with findings that AAS exposure increased extracellular glu levels in rats, an effect accompanied by increased aggressive behavior mediated by NMDA receptors (Kalinine et al., 2014). Increased glutamatergic stimulation also may produce excitotoxic neuronal effects (Danbolt, 2001) that could compromise cognitive function. Indeed, elevated glutamine levels or glutamine/glutamate ratios have been observed with other abused drugs such as ketamine (Rowland et al., 2005), phencyclidine (Iltis et al., 2009), and amphetamine (Pereira et al., 2008), which can induce neurotoxicity in animals (Green and Cote, 2009; Wang and Johnson, 2005) and humans (Jeong et al., 2013).
The scyllo-inositol reduction we found in AAS users is intriguing given its large effect size, the trend negative association between lifetime AAS dose and scyllo-inositol levels, and reports that scyllo-inositol can reduce the effects of neurotoxic proteins including β-amyloid, α-synuclein, and huntingtin (Lai et al., 2014; McLaurin et al., 2000; Vekrellis et al., 2009). Given observations that the commonly used AAS 17β-trenbolone and methandienone increase β-amyloid levels in male rats (Ma and Liu, 2015) and enhance β-amyloid toxicity in neuronal cultures (Caraci et al., 2011), it follows that human AAS users may be at increased risk for β-amyloid toxicity, and that this risk could be compounded by scyllo-inositol depletion.
Moreover, hypogonadism, which is often prolonged in “off-cycle” AAS users (Coward et al., 2013; Kanayama et al., In press; Pope et al., 2014), can also elevate plasma β-amyloid levels (Almeida et al., 2004), and has been associated with Alzheimer’s, Parkinson’s, and Huntington’s diseases (Hogervorst et al., 2004; Markianos et al., 2005; Okun et al., 2002). Hypogonadism could lead not only to persistently reduced systemic testosterone and estrogen levels, but also to reduced aromatase activity in some brain areas (Resko et al., 2000). In turn, brain aromatase activity reductions could reduce brain-derived estrogen levels, compromise neuroprotection (Liu et al., 2014; Veiga et al., 2005), and enhance AAS-amplified neurotoxicity (Orlando et al., 2007). The combination of hypogonadism with scyllo-inositol depletion in long-term AAS users might further increase vulnerability to β-amyloid and other neurotoxic stimuli. If subsequent studies confirm that AAS use depletes scyllo-inositol, then scyllo-inositol supplementation, currently being studied as a treatment for Alzheimer’s disease (Salloway et al., 2011), could be tested in AAS users.
4.3 Limitations
This study has several limitations, including small sample sizes and the possible influence of unmeasured confounders, such as current or past psychoactive substance use. Another limitation is that human amygdala coupling can be evaluated in greater detail than presently by anatomically parsing it into subcomponents, which have different resting state functional connectivities (Roy et al., 2009). With our small sample size, we limited fMRI analyses to left/right amygdala comparisons. However, since we found reduced resting state coupling with in AAS users brain areas corresponding to connectivities of all three amygdala zones assessed by Roy et al. (2009), it appears that AAS use may affect connectivities of all amygdala zones.
Finally, we cannot assume that our findings of amygdala, dACC, and cognitive abnormalities in AAS users are all attributable to a common mechanism. Instead, chronic supraphysiologic levels of AAS might induce deleterious effects and possible neurotoxicity by multiple pathways. By analogy, AAS compromise cardiac function by causing cardiomyopathy (Weiner et al., 2013) and atherosclerotic disease (Pursani et al., 2013), independent pathways of cardiac toxicity.
4.4 Conclusions
Collectively, our findings indicate for the first time in humans that long-term AAS use alters amygdala structure, decreases resting state amygdala coupling with a number of brain regions, and induces dACC neurochemical abnormalities, and further augments the evidence that AAS use impairs visuospatial memory. While it is premature to estimate the clinical consequences of these phenomena, since the majority of illicit AAS users are presently under age 50, they may still be too young to exhibit other brain structural abnormalities or gross cognitive dysfunction, but these deficits may be evolving. Larger studies clearly are needed to better characterize these and other potential adverse brain effects of long-term AAS use.
Highlights.
Anabolic-androgenic steroids (AAS) cause psychiatric and cognitive abnormalities
We conducted the first systematic brain imaging study of human long-term AAS users
AAS users had larger right amygdalas and reduced right amygdala fMRI connectivity
AAS users also had dorsal anterior cingulate cortex neurochemical abnormalities
AAS use causes brain changes that may underlie psychiatric and cognitive changes
Acknowledgments
Role of funding source: The sponsors did not have any role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Funding for this study was provided in part by NIDA grants R01 DA-29141 (to Drs. Hudson, Kanayama, and Pope) and K01 DA-029645 (to Dr. Janes), and by NIMH grant K23 MH092397 (to Dr. Brennan).
Footnotes
Contributors
All authors made: (1) substantial contributions to the conception and analysis and interpretation of data; (2) drafting the article and revising it critically for important intellectual content; and (3) gave approval of the version to be published.
Drs. Pope and Kanayama and Mr. Kerrigan oversaw subject recruitment and screening. Drs. Pope, Kanayama, Kaufman, Jensen, and Mr. Kerrigan oversaw data acquisition and management. Drs. Hudson, Pope, and Janes oversaw statistical analyses. Drs. Kaufman, Pope, Janes, Jensen, and Brennan drafted the manuscript. All co-authors reviewed and approved the manuscript and revisions.
Conflict of interest: Dr. Pope has testified approximately once per year in legal cases involving anabolic-androgenic steroids. Dr. Hudson has received grant support from Genentech and Shire; and has received consulting fees from Genentech, Roche, and Shire. All other authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akhter J, Hyder S, Ahmed M. Cerebrovascular accident associated with anabolic steroid use in a young man. Neurology. 1994;44:2405–2406. doi: 10.1212/wnl.44.12.2405. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Waterreus A, Spry N, Flicker L, Martins RN. One year follow-up study of the association between chemical castration, sex hormones, beta-amyloid, memory and depression in men. Psychoneuroendocrinol. 2004;29:1071–1081. doi: 10.1016/j.psyneuen.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Beaver KM, Vaughn MG, Delisi M, Wright JP. Anabolic-androgenic steroid use and involvement in violent behavior in a nationally representative sample of young adult males in the United States. Am J Public Health. 2008;98:2185–2187. doi: 10.2105/AJPH.2008.137018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Santiago AN, Hen R, Dwork AJ, Rosoklija GB, Tamir H, Arango V, John Mann J. Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacol. 2013;38:1068–1077. doi: 10.1038/npp.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannvall K, Bogdanovic N, Korhonen L, Lindholm D. 19-Nortestosterone influences neural stem cell proliferation and neurogenesis in the rat brain. Eur J Neurosci. 2005;21:871–878. doi: 10.1111/j.1460-9568.2005.03942.x. [DOI] [PubMed] [Google Scholar]
- Brennan BP, Hudson JI, Jensen JE, McCarthy J, Roberts JL, Prescot AP, Cohen BM, Pope HG, Jr, Renshaw PF, Ongur D. Rapid enhancement of glutamatergic neurotransmission in bipolar depression following treatment with riluzole. Neuropsychopharmacol. 2010;35:834–846. doi: 10.1038/npp.2009.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Shin LM. The Multi-Source Interference Task: an fMRI task that reliably activates the cingulo-frontal-parietal cognitive/attention network. Nat Protoc. 2006;1:308–313. doi: 10.1038/nprot.2006.48. [DOI] [PubMed] [Google Scholar]
- Cambridge Cognition. [Accessed April 1, 2015];CANTAB Bibliography. 2015 Available online at: http://www.cambridgecognition.com/bibliography/log-in.
- Caraci F, Pistara V, Corsaro A, Tomasello F, Giuffrida ML, Sortino MA, Nicoletti F, Copani A. Neurotoxic properties of the anabolic androgenic steroids nandrolone and methandrostenolone in primary neuronal cultures. J Neurosci Res. 2011;89:592–600. doi: 10.1002/jnr.22578. [DOI] [PubMed] [Google Scholar]
- Choi PY, Pope HG., Jr Violence toward women and illicit androgenic-anabolic steroid use. Ann Clin Psychiatry. 1994;6:21–25. doi: 10.3109/10401239409148835. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Tabibnia G, Breedlove SM. A brain sexual dimorphism controlled by adult circulating androgens. Proc Nat Acad Sci USA. 1999;96:7538–7540. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland J, Peters R, Dillon P. Anabolic-androgenic steroid use disorders among a sample of Australian competitive and recreational users. Drug Alcohol Depend. 2000;60:91–96. doi: 10.1016/s0376-8716(99)00141-6. [DOI] [PubMed] [Google Scholar]
- Coward RM, Rajanahally S, Kovac JR, Smith RP, Pastuszak AW, Lipshultz LI. Anabolic steroid induced hypogonadism in young men. J Urol. 2013;190:2200–2205. doi: 10.1016/j.juro.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Derntl B, Windischberger C, Robinson S, Kryspin-Exner I, Gur RC, Moser E, Habel U. Amygdala activity to fear and anger in healthy young males is associated with testosterone. Psychoneuroendocrinol. 2009;34:687–693. doi: 10.1016/j.psyneuen.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Freeman ME, Wang Z. Newly proliferated cells in the adult male amygdala are affected by gonadal steroid hormones. J Neurobiol. 2003;57:257–269. doi: 10.1002/neu.10273. [DOI] [PubMed] [Google Scholar]
- Goetz SM, Tang L, Thomason ME, Diamond MP, Hariri AR, Carre JM. Testosterone rapidly increases neural reactivity to threat in healthy men: a novel two-step pharmacological challenge paradigm. Biol Psychiatry. 2014;76:324–331. doi: 10.1016/j.biopsych.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SM, Cote CJ. Ketamine and neurotoxicity: clinical perspectives and implications for emergency medicine. Ann Emerg Med. 2009;54:181–190. doi: 10.1016/j.annemergmed.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Hampstead BM, Lacey S, Ali S, Phillips PA, Stringer AY, Sathian K. Use of complex three-dimensional objects to assess visuospatial memory in healthy individuals and patients with unilateral amygdalohippocampectomy. Epilepsy Behav. 2010;18:54–60. doi: 10.1016/j.yebeh.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Hildebrandt T, Langenbucher JW, Flores A, Harty S, Berlin HA. The influence of age of onset and acute anabolic steroid exposure on cognitive performance, impulsivity, and aggression in men. Psychol Addict Behav. 2014;28:1096–1104. doi: 10.1037/a0036482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogervorst E, Bandelow S, Combrinck M, Smith AD. Low free testosterone is an independent risk factor for Alzheimer’s disease. Exper Gerontol. 2004;39:1633–1639. doi: 10.1016/j.exger.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Iltis I, Koski DM, Eberly LE, Nelson CD, Deelchand DK, Valette J, Ugurbil K, Lim KO, Henry PG. Neurochemical changes in the rat prefrontal cortex following acute phencyclidine treatment: an in vivo localized (1)H MRS study. NMR Biomed. 2009;22:737–744. doi: 10.1002/nbm.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JE, Licata SC, Ongur D, Friedman SD, Prescot AP, Henry ME, Renshaw PF. Quantification of J-resolved proton spectra in two-dimensions with LCModel using GAMMA-simulated basis sets at 4 Tesla. NMR Biomed. 2009;22:762–769. doi: 10.1002/nbm.1390. [DOI] [PubMed] [Google Scholar]
- Jeong HS, Lee S, Yoon S, Jung JJ, Cho HB, Kim BN, Ma J, Ko E, Im JJ, Ban S, Renshaw PF, Lyoo IK. Morphometric abnormalities of the lateral ventricles in methamphetamine-dependent subjects. Drug Alcohol Depend. 2013;131:222–229. doi: 10.1016/j.drugalcdep.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RT, Breedlove SM, Jordan CL. Sex differences and laterality in astrocyte number and complexity in the adult rat medial amygdala. J Compar Neurol. 2008;511:599–609. doi: 10.1002/cne.21859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RT, Schneider A, DonCarlos LL, Breedlove SM, Jordan CL. Astrocytes in the rat medial amygdala are responsive to adult androgens. J Compar Neurol. 2012;520:2531–2544. doi: 10.1002/cne.23061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinine E, Zimmer ER, Zenki KC, Kalinine I, Kazlauckas V, Haas CB, Hansel G, Zimmer AR, Souza DO, Muller AP, Portela LV. Nandrolone-induced aggressive behavior is associated with alterations in extracellular glutamate homeostasis in mice. Horm Behav. 2014;66:383–392. doi: 10.1016/j.yhbeh.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Kanayama G, Boynes M, Hudson JI, Field AE, Pope HG., Jr Anabolic steroid abuse among teenage girls: an illusory problem? Drug Alcohol Depend. 2007;88:156–162. doi: 10.1016/j.drugalcdep.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Hudson J, DeLuca J, Isaacs S, Baggish A, Weiner R, Bhasin S, Pope H. Prolonged hypogonadism in males following withdrawal from anabolic-androgenic steroids: an underrecognized problem. Addiction. 2015;11:823–831. doi: 10.1111/add.12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Hudson JI, Pope HG., Jr Long-term psychiatric and medical consequences of anabolic-androgenic steroid abuse: a looming public health concern? Drug Alcohol Depend. 2008;98:1–12. doi: 10.1016/j.drugalcdep.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Kean J, Hudson JI, Pope HG. Cognitive deficits in long-term anabolic-androgenic steroid users. Drug Alcohol Depend. 2013;130:208–214. doi: 10.1016/j.drugalcdep.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Pope HG, Cohane G, Hudson JI. Risk factors for anabolic-androgenic steroid use among weightlifters: a case-control study. Drug Alcohol Depend. 2003;71:77–86. doi: 10.1016/s0376-8716(03)00069-3. [DOI] [PubMed] [Google Scholar]
- Kelly RE, Jr, Alexopoulos GS, Wang Z, Gunning FM, Murphy CF, Morimoto SS, Kanellopoulos D, Jia Z, Lim KO, Hoptman MJ. Visual inspection of independent components: defining a procedure for artifact removal from fMRI data. J Neurosci Method. 2010;189:233–245. doi: 10.1016/j.jneumeth.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouri EM, Lukas SE, Pope HG, Jr, Oliva PS. Increased aggressive responding in male volunteers following the administration of gradually increasing doses of testosterone cypionate. Drug Alcohol Depend. 1995;40:73–79. doi: 10.1016/0376-8716(95)01192-7. [DOI] [PubMed] [Google Scholar]
- Lai AY, Lan CP, Hasan S, Brown ME, McLaurin J. Scyllo-Inositol promotes robust mutant Huntingtin protein degradation. J Biol Chem. 2014;289:3666–3676. doi: 10.1074/jbc.M113.501635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby LA, Hannula DE, Ranganath C. Medial temporal lobe coding of item and spatial information during relational binding in working memory. J Neurosci. 2014;34:14233–14242. doi: 10.1523/JNEUROSCI.0655-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Kelley MH, Herson PS, Hurn PD. Neuroprotection of sex steroids. Minerva Endocrinol. 2014;35:127–143. [PMC free article] [PubMed] [Google Scholar]
- Lynch CS, Story AJ. Dihydrotestosterone and estrogen regulation of rat brain androgen-receptor immunoreactivity. Physiol Behav. 2000;69:445–453. doi: 10.1016/s0031-9384(99)00257-7. [DOI] [PubMed] [Google Scholar]
- Ma F, Liu D. 17beta-trenbolone, an anabolic-androgenic steroid as well as an environmental hormone, contributes to neurodegeneration. Toxicol Appl Pharmacol. 2015;282:68–76. doi: 10.1016/j.taap.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Magnusson K, Hanell A, Bazov I, Clausen F, Zhou Q, Nyberg F. Nandrolone decanoate administration elevates hippocampal prodynorphin mRNA expression and impairs Morris water maze performance in male rats. Neurosci Lett. 2009;467:189–193. doi: 10.1016/j.neulet.2009.09.041. [DOI] [PubMed] [Google Scholar]
- Markianos M, Panas M, Kalfakis N, Vassilopoulos D. Plasma testosterone in male patients with Huntington’s disease: relations to severity of illness and dementia. Ann Neurol. 2005;57:520–525. doi: 10.1002/ana.20428. [DOI] [PubMed] [Google Scholar]
- McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34:41–54. [PMC free article] [PubMed] [Google Scholar]
- McLaurin J, Golomb R, Jurewicz A, Antel JP, Fraser PE. Inositol stereoisomers stabilize an oligomeric aggregate of Alzheimer amyloid beta peptide and inhibit abeta -induced toxicity. J Biol Chem. 2000;275:18495–18502. doi: 10.1074/jbc.M906994199. [DOI] [PubMed] [Google Scholar]
- Melloni RH, Jr, Ferris CF. Adolescent anabolic steroid use and aggressive behavior in golden hamsters. Ann N Y Acad Sci. 1996;794:372–375. doi: 10.1111/j.1749-6632.1996.tb32546.x. [DOI] [PubMed] [Google Scholar]
- Menard CS, Harlan RE. Up-regulation of androgen receptor immunoreactivity in the rat brain by androgenic-anabolic steroids. Brain Res. 1993;622:226–236. doi: 10.1016/0006-8993(93)90823-6. [DOI] [PubMed] [Google Scholar]
- Michael RP, Clancy AN, Zumpe D. Distribution of androgen receptor-like immunoreactivity in the brains of cynomolgus monkeys. J Neuroendocrinol. 1995;7:713–719. doi: 10.1111/j.1365-2826.1995.tb00813.x. [DOI] [PubMed] [Google Scholar]
- Middleman AB, Faulkner AH, Woods ER, Emans SJ, DuRant RH. High-risk behaviors among high school students in Massachusetts who use anabolic steroids. Pediatrics. 1995;96:268–272. [PubMed] [Google Scholar]
- Midgley SJ, Heather N, Best D, Henderson D, McCarthy S, Davies JB. Risk behaviours for HIV and hepatitis infection among anabolic-androgenic steroid users. AIDS Care. 2000;12:163–170. doi: 10.1080/09540120050001832. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual dimorphism in neuronal number of the posterodorsal medial amygdala is independent of circulating androgens and regional volume in adult rats. J Compar Neurol. 2008;506:851–859. doi: 10.1002/cne.21536. [DOI] [PubMed] [Google Scholar]
- Novaes Gomes FG, Fernandes J, Vannucci Campos D, Cassilhas RC, Viana GM, D’Almeida V, de Moraes Rego MK, Buainain PI, Cavalheiro EA, Arida RM. The beneficial effects of strength exercise on hippocampal cell proliferation and apoptotic signaling is impaired by anabolic androgenic steroids. Psychoneuroendocrinol. 2014;50C:106–117. doi: 10.1016/j.psyneuen.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Okun MS, McDonald WM, DeLong MR. Refractory nonmotor symptoms in male patients with Parkinson disease due to testosterone deficiency: a common unrecognized comorbidity. Arch Neurol. 2002;59:807–811. doi: 10.1001/archneur.59.5.807. [DOI] [PubMed] [Google Scholar]
- Orlando R, Caruso A, Molinaro G, Motolese M, Matrisciano F, Togna G, Melchiorri D, Nicoletti F, Bruno V. Nanomolar concentrations of anabolic-androgenic steroids amplify excitotoxic neuronal death in mixed mouse cortical cultures. Brain Res. 2007;1165:21–29. doi: 10.1016/j.brainres.2007.06.047. [DOI] [PubMed] [Google Scholar]
- Pegna AJ, Caldara-Schnetzer AS, Perrig SH, Lazeyras F, Khateb A, Mayer E, Landis T, Seeck M. Is the right amygdala involved in visuospatial memory? Evidence from MRI volumetric measures. Eur Neurol. 2002;47:148–155. doi: 10.1159/000047973. [DOI] [PubMed] [Google Scholar]
- Pereira FC, Rolo MR, Marques E, Mendes VM, Ribeiro CF, Ali SF, Morgadinho T, Macedo TR. Acute increase of the glutamate-glutamine cycling in discrete brain areas after administration of a single dose of amphetamine. Ann N Y Acad Sci. 2008;1139:212–221. doi: 10.1196/annals.1432.040. [DOI] [PubMed] [Google Scholar]
- Perry PJ, Kutscher EC, Lund BC, Yates WR, Holman TL, Demers L. Measures of aggression and mood changes in male weightlifters with and without androgenic anabolic steroid use. J Forensic Sci. 2003;48:646–651. [PubMed] [Google Scholar]
- Pieretti S, Mastriota M, Tucci P, Battaglia G, Trabace L, Nicoletti F, Scaccianoce S. Brain nerve growth factor unbalance induced by anabolic androgenic steroids in rat. Med Sci Sports Exerc. 2013;45:29–35. doi: 10.1249/MSS.0b013e31826c60ea. [DOI] [PubMed] [Google Scholar]
- Pope H, Kanayama G, Hudson J. Risk factors for illicit anabolic-androgenic steroid use in male weightlifters: a cross-sectional cohort study. Biol Psychiatry. 2012;71:254–261. doi: 10.1016/j.biopsych.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Katz DL. Homicide and near-homicide by anabolic steroid users. J Clin Psychiatry. 1990;51:28–31. [PubMed] [Google Scholar]
- Pope HG, Katz DL. Psychiatric and medical effects of anabolic-androgenic steroid use. A controlled study of 160 athletes. Arch Gen Psychiatry. 1994;51:375–382. doi: 10.1001/archpsyc.1994.03950050035004. [DOI] [PubMed] [Google Scholar]
- Pope HG, Kouri EM, Hudson JI. Effects of supraphysiologic doses of testosterone on mood and aggression in normal men: a randomized controlled trial. Arch Gen Psychiatry. 2000;57:133–140. doi: 10.1001/archpsyc.57.2.133. [DOI] [PubMed] [Google Scholar]
- Pope HG, Wood R, Rogol A, Nyberg F, Bowers L, Bhasin S. Adverse health consequences of performance-enhancing drugs: an Endocrine Society scientific statement. Endo Rev. 2014;35:341–375. doi: 10.1210/er.2013-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Pursani A, Wai B, Hoffman U, Weiner R, Isaacs S, Kanayama G, Hudson JI, Pope HG, Jr, Baggish A. Anabolic-Androgenic Steroid Use Is Associated With Premature Coronary Artery Disease. American Heart Association Annual Meeting; Dallas, Texas. 2013. [Google Scholar]
- Resko JA, Pereyra-Martinez AC, Stadelman HL, Roselli CE. Cellular observations and hormonal correlates of feedback control of luteinizing hormone secretion by testosterone in long-term castrated male rhesus monkeys. Biol Reprod. 2000;63:872–878. doi: 10.1095/biolreprod63.3.872. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Semple J, Kumar R, Truman MI, Shorter J, Ferraro A, Fox B, McKay G, Matthews K. Effects of scopolamine on delayed-matching-to-sample and paired associates tests of visual memory and learning in human subjects: comparison with diazepam and implications for dementia. Psychopharmacol. 1997;134:95–106. doi: 10.1007/s002130050430. [DOI] [PubMed] [Google Scholar]
- Rowland LM, Bustillo JR, Mullins PG, Jung RE, Lenroot R, Landgraf E, Barrow R, Yeo R, Lauriello J, Brooks WM. Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. Am J Psychiatry. 2005;162:394–396. doi: 10.1176/appi.ajp.162.2.394. [DOI] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahakian BJ, Morris RG, Evenden JL, Heald A, Levy R, Philpot M, Robbins TW. A comparative study of visuospatial memory and learning in Alzheimer-type dementia and Parkinson’s disease. Brain. 1988;111:695–718. doi: 10.1093/brain/111.3.695. [DOI] [PubMed] [Google Scholar]
- Salloway S, Sperling R, Keren R, Porsteinsson AP, van Dyck CH, Tariot PN, Gilman S, Arnold D, Abushakra S, Hernandez C, Crans G, Liang E, Quinn G, Bairu M, Pastrak A, Cedarbaum JM. A phase 2 randomized trial of ELND005, scyllo-inositol, in mild to moderate Alzheimer disease. Neurol. 2011;77:1253–1262. doi: 10.1212/WNL.0b013e3182309fa5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer B, Muller BW, Scherbaum N, Hodgins S, Forsting M, Wiltfang J, Gizewski ER, Leygraf N. Disentangling structural brain alterations associated with violent behavior from those associated with substance use disorders. Arch Gen Psychiatry. 2011;68:1039–1049. doi: 10.1001/archgenpsychiatry.2011.61. [DOI] [PubMed] [Google Scholar]
- Shimada Y, Yoritaka A, Tanaka Y, Miyamoto N, Ueno Y, Hattori N, Takao U. Cerebral infarction in a young man using high-dose anabolic steroids. J Stroke Cerebrovasc Dis. 2012;21:906 e909–e911. doi: 10.1016/j.jstrokecerebrovasdis.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Siever LJ. Neurobiology of aggression and violence. Am J Psychiatry. 2008;165:429–442. doi: 10.1176/appi.ajp.2008.07111774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarberg K, Nyberg F, Engstrom I. Is there an association between the use of anabolic-androgenic steroids and criminality? Eur Addict Res. 2010;16:213–219. doi: 10.1159/000320286. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Su TP, Pagliaro M, Schmidt PJ, Pickar D, Wolkowitz O, Rubinow DR. Neuropsychiatric effects of anabolic steroids in male normal volunteers. JAMA. 1993;269:2760–2764. [PubMed] [Google Scholar]
- Swainson R, Hodges JR, Galton CJ, Semple J, Michael A, Dunn BD, Iddon JL, Robbins TW, Sahakian BJ. Early detection and differential diagnosis of Alzheimer’s disease and depression with neuropsychological tasks. Dementia Geriat Cognit Disord. 2001;12:265–280. doi: 10.1159/000051269. [DOI] [PubMed] [Google Scholar]
- Tanehkar F, Rashidy-Pour A, Vafaei AA, Sameni HR, Haghighi S, Miladi-Gorji H, Motamedi F, Akhavan MM, Bavarsad K. Voluntary exercise does not ameliorate spatial learning and memory deficits induced by chronic administration of nandrolone decanoate in rats. Horm Behav. 2013;63:158–165. doi: 10.1016/j.yhbeh.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Thiblin I, Parlklo T. Anabolic androgenic steroids and violence. Acta Psychiatr Scand Suppl. 2002;412:125–128. doi: 10.1034/j.1600-0447.106.s412.27.x. [DOI] [PubMed] [Google Scholar]
- Tugyan K, Ozbal S, Cilaker S, Kiray M, Pekcetin C, Ergur BU, Kumral A. Neuroprotective effect of erythropoietin on nandrolone decanoate-induced brain injury in rats. Neurosci Lett. 2013;533:28–33. doi: 10.1016/j.neulet.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, Wallin D, Pendse G, McDonald L, Griffin M, Anderson J, Nutile L, Renshaw P, Weiss R, Becerra L, Borsook D. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133:2098–2114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttl B. North American Adult Reading Test: age norms, reliability, and validity. J Clin Exper Neuropsychol. 2002;24:1123–1137. doi: 10.1076/jcen.24.8.1123.8375. [DOI] [PubMed] [Google Scholar]
- Veiga S, Azcoitia I, Garcia-Segura LM. Extragonadal synthesis of estradiol is protective against kainic acid excitotoxic damage to the hippocampus. Neuroreport. 2005;16:1599–1603. doi: 10.1097/01.wnr.0000179081.39659.7d. [DOI] [PubMed] [Google Scholar]
- Vekrellis K, Xilouri M, Emmanouilidou E, Stefanis L. Inducible over-expression of wild type alpha-synuclein in human neuronal cells leads to caspase-dependent non-apoptotic death. J Neurochem. 2009;109:1348–1362. doi: 10.1111/j.1471-4159.2009.06054.x. [DOI] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Hermann D, Rabinstein J, Wichert S, Klein O, Ende G, Mann K. Increased activation of the ACC during a spatial working memory task in alcohol-dependence versus heavy social drinking. Alcohol Clin Exp Res. 2010;34:771–776. doi: 10.1111/j.1530-0277.2010.01149.x. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Johnson KM. Differential effects of acute and subchronic administration on phencyclidine-induced neurodegeneration in the perinatal rat. J Neurosci Res. 2005;81:284–292. doi: 10.1002/jnr.20559. [DOI] [PubMed] [Google Scholar]
- Weiner R, Isaacs S, Hutter AM, Kanayama G, Hudson JI, Picard MH, Pope HG, Baggish A. Anabolic Steroid Use Is Associated With Systolic And Diastolic Left Ventricular Dysfunction; American Heart Association Annual Meeting; Dallas, Texas. 2013. [Google Scholar]
- Yates WR, Perry PJ, MacIndoe J, Holman T, Ellingrod V. Psychosexual effects of three doses of testosterone cycling in normal men. Biol Psychiatry. 1999;45:254–260. doi: 10.1016/s0006-3223(98)00028-6. [DOI] [PubMed] [Google Scholar]
- Younger JW, Chu LF, D’Arcy NT, Trott KE, Jastrzab LE, Mackey SC. Prescription opioid analgesics rapidly change the human brain. Pain. 2011;152:1803–1810. doi: 10.1016/j.pain.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]