Abstract
Neuronal gene therapy potentially offers an effective therapeutic intervention to cure or slow the progression of neurological diseases. However, neuronal cells are difficult to transfect with nonviral vectors, and in vivo their transport across the blood-brain barrier (BBB) is inefficient. We synthesized a series of arginine-rich oligopeptides, grafted with polyethyleneimine (PEI) and modified with a short-chain polyethylene glycol (PEG). We hypothesized that the arginine would enhance cellular uptake and transport of these polyplexes across the BBB, with PEG imparting biocompatibility and “stealth” properties and PEI facilitating DNA condensation and gene transfection. The optimized composition of the polyplexes demonstrated hemocompatibility with red blood cells, causing no lysis or aggregation, and showed significantly better cytocompatibility than PEI in vitro. Polyplexes formulated with luciferase-expressing plasmid DNA could transfect rat primary astrocytes and neurons in vitro. Confocal imaging data showed efficient cellular uptake of DNA and its sustained intracellular retention and nuclear localization with polyplexes. Intravenous administration of the optimized polyplexes in mice led to gene expression in the brain, which upon further immunohistochemical analysis demonstrated gene expression in neurons. In conclusion, we have successfully designed a nonviral vector for in vitro and in vivo neuronal gene delivery.
Keywords: Gene Therapy, Cell Membrane, Nanocomposite, Brain Cells, Blood-Brain Barrier
1 Introduction
Neuronal gene therapy could potentially treat many neurological disorders and neurodegenerative diseases, which remain a major cause of disability and mortality (1, 2). However, neuronal cells are difficult to transfect with nonviral vectors, primarily because of their special polarized and elongated morphology, which affects the internalization of nonviral vectors. DNA translocation into the nucleus presents another hurdle, since neuronal cells are post-mitotic cells (3, 4). Gene delivery to the brain is challenging because the blood-brain barrier (BBB) limits the transport of nonviral vectors to the brain parenchyma (3).
Cell-penetrating peptides, such as transactivating transcriptional activator (TAT) peptides, can effectively cross the plasma membrane to deliver a wide variety of compounds, either intracellularly (3) or across the BBB to the brain (5). Similar to typical cell-penetrating peptides, arginine-rich oligopeptides also possess membrane-permeable characteristics but are less likely to be cytotoxic (6). The internalization mechanism of arginine-rich peptides is hypothesized to be due to the arginine residues (7) which contain guanidine groups that are highly basic in nature (pKa ~12.5). These guanidine groups can form hydrogen bonds with polyhydroxy compounds in cell membranes that facilitate the translocation of arginine peptides across cell membranes (8–10). In addition, the guanidine groups of arginine also have a strong affinity to heparan sulfate, which is ubiquitously expressed on the surface of mammalian cells. It is suggested that these strongly negatively charged heparan sulfate assist arginine-rich peptides to adhere to the cell membrane (11).
In this study, we explored these characteristics of arginine-rich oligopeptides for gene delivery to neuronal cells. The oligopeptides were grafted with polyethyleneimine (PEI) to facilitate DNA condensation and modified with polyethylene glycol (PEG) to afford hemo- and biocompatibility and a “stealth” property to polyplexes for in vivo application. We hypothesized that arginine-rich oligopeptides with a balanced combination of oligopeptide plus PEI, in combination with PEG, would retain their cell-membrane-penetrating property and hence could act as efficient and biocompatible nonviral gene-transfecting vectors. Our data show that the optimized composition of these polyplexes is effective in transfecting neuronal cells both in vitro and in vivo. The polyplexes also transfected glioma cells, demonstrating a broader use as gene expression vectors.
2. Materials and Methods
2.1. Materials
3-(2-Aminoethylamino) propyl-methyl-dimethoxysilane was purchased from Fluka (Sigma-Aldrich, St. Louis, MO). 25-kDa Branched PEI, N-hydroxysuccinimide (NHS), 2-nitrophenylhydrazine and PEG bis(carboxymethyl)ether-600Da, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), fluorescamine, 2-(N-morpholino) ethanesulfonic acid (MES), glycine, pyridine and L-arginine were purchased from Sigma-Aldrich. pGL3-luc vector, Luciferase Assay system with Reporter Lysis Buffer, and VivoGlo luciferin in vivo grade, Anti-Luciferase pAb, and QuantiLum Recombinant Luciferase were purchased from Promega (Madison, WI). Dulbecco’s Modified Eagle’s Medium (DMEM), 0.05% trypsin/0.53 mM, ethylenediaminetetraacetic acid, penicillin and streptomycin were purchased from the Central Cell Services Media Laboratory of our institution. All animal procedures were approved by Cleveland Clinic’s Institutional Animal Care and Use Committee.
2.2. Synthesis of Arginine-modified Siloxane-grafted PEI (PSAr5) Polymers
PEI-based siloxane polymers having arginine groups (PSAr5) were prepared according to the previously described method (12). To 1.8 mM (1 equivalent) of 3-(2-aminoethylamino) propyl-methyldimethoxysilane, 1 equivalent of 1 N NaOH solution was added and mixed (13) (see ref 12 and 13 for schematic). The mixture was stirred using a magnetic stirrer for 20 h at room temperature (RT). The byproducts of the resultant oligomers were evaporated at a reduced pressure of 220 mbar and temperature of 60 °C (BUCHI Rotavapor, R-210/215, New Castle, DE). The residue of oligomer left was diluted to 5 ml with water and neutralized to pH 7 using 1 N HCl. The oligomer was then dried by lyophilization for 2 days at −48 °C, 0.035 mbar (FreeZone 4.5 Liter Benchtop Freeze Dry Systems, Labconco Corp., Kansas City, MO). The oligomer was conjugated to arginine using EDC/NHS chemistry.
The -COOH group of arginine (9 mM) was activated with EDC/NHS (11 mM) in 5 ml of MES buffer (pH 6) for 1 h at RT. To the activated carboxylic acid group of arginine, oligo (alkylaminosiloxane) was added in 3 ml of PBS (pH 7.5), and the reaction was continued for 16 h at RT. The arginine-conjugated oligo (alkylaminosiloxane) [SAr] thus obtained was dialyzed (MWCO 1000, Spectrum Laboratories, Rancho Dominquez, CA) in 2 L of water and then lyophilized for 2 days, as noted above. The resultant SAr (0.05 mM) was then coupled to 0.01 mM PEI; the coupling reaction was carried out using EDC/NHS (0.07 mM) chemistry, as described previously (12). One of the -COOH groups of aspartic acid was activated using half the equivalents of EDC/NHS for 2 h at 4 °C in 2 ml of MES buffer (pH 6). After the acid activation, SAr was dissolved in 5 ml PBS (pH 7.5) and added into the above reaction mixture. The reaction was continued for 18 h at RT; the reaction mixture was then dialyzed (MWCO 1000, Spectrum Laboratories) against 2 L of water to remove unreacted substrates. The remaining -COOH groups of aspartic acid were then activated using EDC/NHS chemistry at 4 °C for 4 h. After acid activation, 1 equivalent of PEI was added into the reaction mixture, and the reaction was continued for 18 h at RT. The resultant mixture was dialyzed (MWCO 12000, Sigma-Aldrich) against 2 L water and then lyophilized as above.
2.3. Synthesis and characterization of PEG-conjugated PSAr5
An arginine-modified oligo (alkylaminosiloxane) conjugated PEI (PSAr5) polymer, where n = 5 represents the molar number of arginine molecules conjugated to oligo (alkylaminosiloxane), was subsequently conjugated to varying equivalents of PEG bis(carboxymethyl) ether (Table 1). Briefly, the -COOH group of the PEG polymer was first activated with half the equivalents of EDC/NHS for 1 h at RT in 2 ml of MES buffer (pH 6). The activated carboxylate group of PEG was then reacted with the amino group of the PSAr5 polymer for 16 h at RT with stirring. The derivatives were named A5Pn, where n represents the equivalents of PEG added for the conjugation reaction and A stands for the arginine-conjugated oligo (alkylaminosiloxane) grafted with PEI (PSAr5). The amount of PEG oligomer conjugated to the PSAr5 polymer was determined by estimating the free acid group of PEG using a 2-nitrophenylhydrazine assay (14). Briefly, 50 μl of PEG conjugates and glycine standards in Milli-Q water (Millipore Corp., Billerica, MA; 0–2 mg/ml) were added successively to 100 μl of 2-nitrophenylhydrazine solutions and 100 μl of EDC solution in 4% pyridine (pH 8.4). Following incubation for 30 min at 40 °C, 100 μl of 1.5 N NaOH was added to the reaction mixture, and the whole mixture was further incubated for 15 min at 60 °C. The reaction mixture was then cooled to RT, and the absorbance was measured at 540 nm against the reagent blank (SpectraMax M2, Molecular Devices, Inc., Sunnyvale, CA). Synthesis of the polymer was confirmed by proton NMR (1H NMR) spectra in D2O using a 300 MHz spectrometer (Varian Mercury-300 NMR Spectrometer, Varian Medical Systems, Palo Alto, CA).
Table 1.
Chemical composition and characterization of PSAr5 grafted with various equivalents of PEG
| Name of polymer | Equivalents of PEI | Equivalents of SAr | Equivalents of PEG | Acid groups μM/mg polymer (glycine)* | Primary amine μM/mg polymer (glycine)* |
|---|---|---|---|---|---|
| PSAr5 | 1 | 5 | 0 | -- | 0.172 ± 0.002 |
| A5P10 | 1 | 5 | 10 | 2.54 ± 0.03 | 0.068 ± 0.005 |
| A5P20 | 1 | 5 | 20 | 4.05 ± 0.04 | 0.065 ± 0.001 |
| A5P30 | 1 | 5 | 30 | 4.57 ± 0.03 | 0.062± 0.0002 |
| A5P40 | 1 | 5 | 40 | 6.60 ± 0.03 | 0.047 ± 0.0004 |
| A5P50 | 1 | 5 | 50 | 8.68 ± 0.02 | 0.043 ± 0.001 |
Data are shown as mean ± SEM; n = 3.
Glycine standard was used for both carboxylic acid and primary amino group detection.
2.4. Amplification and purification of pDNA
The pCMV-luc vector was transformed and propagated in DH5-alpha in Escherichia coli (Invitrogen, Carlsbad, CA), and plasmid DNA (pDNA) was isolated using EndoFree Plasmid Giga Kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol.
2.5. Preparation and characterization of polymer/pDNA polyplexes
Different polymers dissolved in Milli-Q water at a concentration of 1 mg/ml were filtered through a 0.22 μm syringe filter (MILLEX®GP, Millipore Ireland Ltd., Cork, Ireland). The pDNA solution was also prepared at the same concentration of 1 mg/ml in Milli-Q water. Polyplexes were prepared in 5 mM PBS (pH 7.4) by mixing 10 μg/ml of the pDNA solution with different concentrations of polymer in solution to determine the optimal weight:weight ratio of pDNA to polymer in the polyplexes. The pDNA-polymer solutions were mixed well by pipetting up and down and then incubating the formed polyplexes for 15–20 min at RT. Polyplex size and zeta potential were determined using quasi-elastic light scattering (PSS/NICOMP 380/ZLS Particle Sizing Systems, Santa Barbara, CA).
2.6. Evaluation of hemocompatibility of polyplexes
The hemocompatibility of different polyplexes was determined by measuring the lysis of red blood cells (RBCs) and their aggregation in the presence of polyplexes (15). For this step, blood collected from healthy rats in tubes containing 3.8% sodium citrate (Sigma-Aldrich) at a ratio 9:1 v/v (blood: anticoagulant) was used to isolate RBCs. For this, the whole blood collected above was centrifuging at 1,500 g for 10 min at RT (Sorvall Legend RT; Thermo Scientific, Waltham, MA) and RBCs were washed three times in saline. For the aggregation study, polyplexes were incubated with RBCs for 30 min at 37 °C in a 1:4.6 (v/v) ratio of polyplexes to RBCs. Typically 6 μl of polyplexes is mixed with 28 μl of RBC isolated as above. Following incubation, the above polyplex-RBC mixture was diluted in 200 μl saline and 10 μl of the aliquot was mounted on a glass slide and imaged using a microscope (Eclipse TS100 Inverted Microscope, Nikon, Huntley, IL). For the hemolysis study, RBCs collected as above were diluted six times with saline and incubated at RT with polyplexes (pDNA to polymer ratio 1:2 w/w) for 2 h. The RBC-polyplex mixture was centrifuged as above for 10 min, and the supernatant (50 μl) was dissolved in 150 μl of 200:5 (v/v) ethanol: HCl mixture in a Nunc™ 96-well polypropylene MicroWell™ plate (Thermo Scientific). The absorbance of each sample was measured at 399 nm by the SpectraMax M2. RBCs incubated with deionized water (complete hemolysis) were used as the positive control, and RBCs incubated with saline were the negative control (no hemolysis).
2.7. Cell Culture
Rat primary astrocytes (RA) purchased from Invitrogen were maintained in DMEM supplemented with 15% FBS and 1% penicillin-streptomycin. Rat primary cortex neurons purchased from Gibco were cultured in Neurobasal™ medium supplemented with 2.5 ml/L of GlutaMAX™ and 2% B 27 (Gibco, Invitrogen). Rat glioma cells (C-6) and human glioblastoma astrocytoma cells (U-87 MG) purchased from American Type Culture Collection (ATCC, Mannasas, VA) were maintained in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin under conditions of 5% CO2 and 95% humidity at 37 °C. All cells except rat primary cortex neurons were grown in 75 cm2 culture flasks. C-6 and U-87 MG cells were trypsinized with 0.05% trypsin/0.53 mM ethylenediaminetetraacetic acid from the culture flask and RA were detached from the culture flask with StemPro® Accutase® cell dissociation reagent (Invitrogen) and seeded into plates 1 day before the experiments. Rat neurons were directly seeded into poly-D-lysine-coated 48-well plates for transfection as described below.
2.8. Evaluation of cytotoxicity of polyplexes
Cytotoxicity of polyplexes was determined by MTT assay. For this, C-6 and U-87 cells (1 × 104 cells/well) and RA (5 × 104 cells/well) were seeded in a 96-well tissue culture plate in 100 μl DMEM containing 10% FBS. Cells at 70–80% confluency were transfected with polyplexes (0.5 μg pDNA; pDNA to polymer ratio 1:2 w/w) in 100 μL of DMEM containing 10% FBS. Following incubation with polyplexes for 24 h, the medium from the wells was removed, and the cells were washed two times in cold PBS, and 26 μl of MTT (2 mg/ml in DMEM) reagent was added. Cells with reagent were incubated for 4 h, following which 150 μl of Dimethyl sulfoxide (DMSO) was added into each well to dissolve the substrate. The plates were incubated for 30 min at 37 °C prior to measuring absorbance of the samples at 570 nm using the plate reader SpectraMax M2. Cells without polyplexes served as controls. Percent cell viability following incubation with polyplexes was calculated with respect to the above control.
2.9. In vitro transfection
For neuronal cell transfection, 1 × 105 neurons per well were plated in poly-D-lysine (4.5 μg/cm2) coated 48-well plates in Neurobasal™ medium supplemented with GlutaMAX™ and B-27. For coating plates, 200 μl of poly-D-lysine (Santa Cruz Biotechnology, Inc. Paso Robles, CA) solution (100 μg/ml in PBS) was added to each well, plates were incubated for 1 h at RT, then washed three times with PBS prior to cell seeding. RAs were seeded at a density of 2 × 105, whereas C-6 and U-87 MG cells were seeded at a density of 105 cells/well in 24-well plates in DMEM containing 15% FBS. At 24 h post cell seeding, the culture media were removed, and cells were treated with polyplexes containing 2.5 μg of pDNA (pDNA to polymer ratio 1:2 w/w) in 250 μl of DMEM containing 15% FBS for transfection. For comparison, polyplexes of PEI having a molecular weight of 25 kDa were used as transfecting agents, following the identical protocol. For transfecting neurons cultured in 48-well plates, 1 μg of pDNA was used (pDNA to polymer ratio 1:2 w/w/) in 100 μl of DMEM containing 15% FBS. Neuronal cell transfection was compared with Lipofectamine®LTX with PLUS™ (Invitrogen). For this step, DNA/Lipofectamine®LTX with PLUS™ complex was prepared by mixing 1 μg of pDNA with 1 μl of PLUS™ and 5 μl of Lipofectamine®LTX transfecting reagent as per the manufacturer’s instruction. Following incubation of the complex with cells for 24 h, medium in the wells was exchanged with culture medium, and the cells were incubated for another 24 h prior to measure luciferase activity. For transfection with the polyplexes, cells were incubated with the pDNA-polyplex complexes for 3.5 h at 37 °C, medium in the wells was replaced with fresh medium, and the cells were further incubated for 48 h at 37 °C prior to measuring luciferase activity. To do this, the medium from each well was removed, and cells were rinsed with D-PBS two times and then lysed with 100 μl of Reporter Lysis Buffer (Promega). To measure luciferase activity, 25 μl of the lysate was dispensed in a Nunc F96-MicroWell White Polystyrene plate (Nunc, Thermoscientific) with the addition of 50 μl of Luciferase Assay Reagent (Promega). After 10 min, luciferase activity was measured in terms of relative luminescence units (RLUs) using the FLUOstar OPTIMA microplate reader BMG Labtech, Cary, NC). Cell protein content was determined using a Pierce BCA Protein Assay Reagent Kit (Thermo Scientific, Rockford, IL). The results were reported in terms of RLU per milligrams of tissue protein.
2.10. Cellular uptake and nuclear localization of polyplexes
Polyplexes were prepared with tetramethylrhodamine isothiocyanate (TRITC; Invitrogen) tagged polymers and YOYO-1-Iodide (Invitrogen) tagged DNA. pDNA (1 μg/μl) was labeled with YOYO by adding 30 μl of 10 μM YOYO per 1 μg of pDNA for 30 min at RT. TRITC was tagged to the primary amino groups of the polymer A5P50 by stirring 2 mg of it in 2 ml Milli-Q water and 100 μl of 0.5 mg/ml TRITC in bicarbonate buffer at pH 9 for 2 h at RT in the dark. The reaction was stopped by adding 100 μl of 1.5 M hydroxylamine hydrochloride (Aldrich) solution at pH 8.5. The TRITC-tagged polymer was dialyzed overnight to remove unreacted TRITC and any reaction byproducts. YOYO-labeled pDNA that had not been complexed with the polymer was used as the control. The polyplexes with 2 μg pDNA were added to 5 × 103 rat astrocytes in DMEM containing 15% FBS after overnight incubation of cells in culture and incubated for 2, 4, 6 and 24 h in an 8 well chamber slide (Lab-Tek, Rochester, NY). For cellular uptake study in U-87 cell, cells were seeded (2.5 × 103) in DMEM containing 10% FBS in 8 well chamber slides as above. After overnight incubation, cells were incubated with polyplex as mentioned above for 4, 24 and 48 h. After each incubation time, cells were washed three times with PBS, stained for nucleus using VECTASHIELD mounting medium with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories Inc., Burlingame, CA). Cells were then viewed under a laser scanning confocal microscope at excitation/emission wavelengths of 555/580 nm (TRITC, red), 491/509 nm (YOYO-1-Iodide, green), 360/460 nm (DAPI, blue) (Leica TCS-SP2 Laser Scanning Spectral Confocal Microscope, Leica Microsystems, GmbH, Wetzlar, Germany).
2.11. In vivo transfection and immunohistochemistry
Male, athymic nude mice (nu/nu, 5–6 weeks old) were purchased from Charles River Laboratories (Wilmington, MA). Mice were anesthetized with isoflurane (2.5%) in oxygen. Polyplexes containing 50 μg of pDNA in 300 μl saline were injected via tail vein on 3 consecutive days. Animals not injected with polyplexes or DNA were used as controls to check for the background signal. At 24 h following the last injection, animals were euthanized by an intraperitoneal injection of 150 mg/kg sodium pentobarbital. Animals were perfused with normal saline following cardiac puncture to wash off blood. Brain, heart, lungs, kidney, liver, and spleen were collected to determine the extent of gene transfection. All organs following dissection were rinsed with saline, cut into small pieces, incubated with VivoGlo Luciferin (Promega) (1.5 μg per 5 mg tissue weight) for 10 min prior to imaging by IVIS® Lumina II (PerkinElmer, Hopkinton, MA) (15, 16). Each tissue were then homogenized in ice-cold Reporter Lysis Buffer (Promega), vortexed for 15 min and then centrifuged for 5 min at 14,000 rpm at 4 °C in a microcentrifuge (model 5417R, Eppendorf, Inc., Westbury, NY), and the supernatant was analyzed for the protein content using the Pierce BCA Protein Assay Reagent Kit. The data were represented as RLU per milligram of tissue protein.
For immunohistochemical analysis, brains were harvested after reperfusion of animals first with saline and then with 10 ml of 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) diluted in 1X PBS. The harvested brains were cryopreserved in a 20% sucrose (Sigma-Aldrich) solution in 1X PBS at 4 °C overnight. The whole brains were then frozen in OCT embedding medium (Tissue-Tek, Sakura Finetek USA, Inc., Torrance, CA) at −80 °C. Frozen horizontal sections of the brain of 5 μm thickness were prepared with a cryotome cryostat (model CM 1900, Leica, Buffalo Grove, IL). Sections were incubated at 4 °C overnight with anti-luciferase antibody at a 1:500 dilution (Promega) in blocking buffer (2% horse serum, 0.3% Triton X-100 in PBS) and anti-MAP2 antibody at 1:500 dilution (EMD Millipore, Temecula, CA) in blocking buffer. Tissue sections were counterstained with Alexa Fluor-488 donkey anti-goat IgG (Invitrogen) at a 1:400 dilution and Alexa Fluor 568 donkey anti-mouse IgG (1:200) secondary antibodies (Invitrogen). Finally, nuclear staining was done with VECTASHIELD mounting medium with DAPI (Vector Laboratories). The stained sections were scanned using Leica-Scanner Slide SCN400 (Leica, Bannockburn, lL) at 578/603 nm (Alexa Fluor 568, red), 495/519 nm (Alexa Fluor 488, green), 360/400 nm (DAPI, blue).
2.12. Statistical analysis
All numerical data were expressed as mean ± SEM. Statistical analysis was performed with Student’s t test. Differences were considered statistically significant with p ≤ 0.05.
3. Results
3.1. Synthesis and characterization of polymers and polyplexes
To obtain polyplexes that would be hemo- and cytocompatible and would retain transfection ability, the parent polymer PSAr5 was conjugated to different amounts of PEG. For this step, the molar amount of PSAr5 was kept constant and that of PEG was varied (Table 1). Based on the free carboxyl groups of PEG, increasing equivalents of PEG bis (carboxymethyl) ether that were added during the conjugation reaction also increased the amount of PEG conjugated to PSAr5.
This result is also evident from the proportional decrease in primary amine functional groups of PSAr5 (Table 1). For example, based on the molar ratio, the number of micromoles of PEG conjugated per milligrams of A5P50 was 8.68 ± 0.02. The 1H NMR spectra of the PEG conjugate of the parent polymer showed a peak at 3.2 ppm, which indicates the presence of PEG (-CH2CH2O-). Similarly, the peak corresponding to -CH2- protons of PEI was observed at 2.74 ppm (Fig. 1).
Fig. 1.
Spectral characterization of PEG conjugate (A5P40) of the parent polymer PSAr5. 1H NMR spectra show the PEG (-CH2-CH2-O-) groups as well PEI’s ethylene protons (-CH2-).
The particle size of the polyplexes of the parent polymer before and after PEG conjugation ranged from ~190 nm to ~260 nm but did not follow any discernible trend with increasing amounts of PEG conjugated polymers (Table 2). Both the polymers and the corresponding polyplexes followed a similar pattern of change in the positive and negative zeta potential with increasing amounts of PEG used. The surface charge of the PEG conjugates first decreased from a positive zeta potential (PSAr5) to a slightly negative one (A5P20) and then increased again to a positive zeta potential with the A5P50 polymer showing a zeta potential about +10 mV. A similar trend was seen with the polyplexes, but in general, these showed higher positive or negative zeta potentials than the corresponding polymers (Table 2).
Table 2.
Particle size and zeta potential of PEG conjugates and polyplexes
| Name of polymer | Particles size (nm) of polyplex* | Zeta potential (mV) of polymer alone | Zeta potential (mV) of polyplexes* |
|---|---|---|---|
| PSAr5 | 244 ± 4.5 | 4.39 ± 4.925 | 17.33 ±4.925 |
| A5P10 | 192 ± 11.9 | 7.2 ± 6.06 | 24.19 ± 6.06 |
| A5P20 | 239 ± 9.7 | −0.34 ± 0.295 | −1.11 ± 0.295 |
| A5P30 | 264 ± 43.9 | 0.68 ± 0.69 | 2.47 ± 0.69 |
| A5P40 | 241 ± 13.8 | 2.09 ± 0.805 | 8.66 ± 0.805 |
| A5P50 | 253 ± 15.7 | 10.19 ± 4.295 | 10.86 ± 4.295 |
DNA: Polymer ratio = 1:2 (w/w). Data are shown as mean ± SEM; n = 3.
3.2. Biocompatibility of polymers and polyplexes
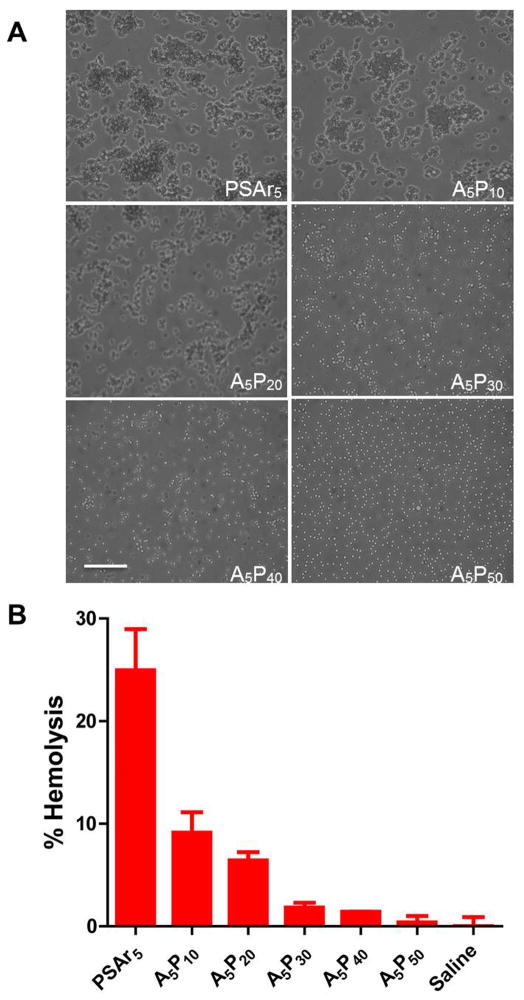
With increasing PEGylation of the parent polymer PSAr5, we found reduced RBC aggregation (Fig. 2A) and hemolysis (Fig. 2B), with A5P50 showing similar results to those of saline controls. Compared with the polyplex of PEI, all the other polyplexes formulated with our polymers showed significantly better cytocompatibility when tested in C6 and U-87 cells (Fig. 3A). The polyplex formulated with A5P50, however, showed some toxicity in rat astrocytes, but that level was lower than the toxicity seen with the polyplex formulated with the parent polymer PSAr5 (Fig. 3B).
Fig. 2.
Hemocompatibility of polyplexes formulated with parent polymer PSAr5 and different PEG conjugates. (A) RBC aggregation following incubation with polyplexes for 30 min at 37 °C (Bar = 20 μm) (B) RBC hemolysis following incubation with polyplexes for 2 h at room temperature. Data are shown as mean ± S.E.M; n = 3.
Fig. 3.

Cytocompatibility of polyplexes formulated with parent polymer PSAr5 and different PEG conjugates. Cells were incubated with polyplexes for 24 h and cell viability was determined using MTT assay. (A) Cytocompatibility of different polyplexes in C6 and U-87 Cell lines. p < 0.01 compared to PEI; (B) Cytocompatibility polyplex formulated with polymer, A5P50 compared with that prepared with parent polymer PSAr5 in rat astrocytes. p < 0.005 compared with PSAr5 or control. Data are shown as mean ± SEM; n = 3.
3.3 In vitro transfection of polyplexes
The in vitro transfection was cell-line and polyplex composition dependent. As shown in Fig. 4A, transfection levels with polymer composition did not change significantly, and the levels were comparable to that achieved with the polyplex formulated with PEI. Although A5P50 showed slightly lower gene transfection than other polymers (Fig. 4A), it demonstrated better cyto- and haemocompatibility than other polymers (Fig. 2 and 3); hence was used for further studies. Transfection levels with polyplexes formulated with polymer A5P50 were higher in tumor cell lines and in astrocytes than in neurons (Fig. 4). pDNA alone did not show any significant transfection (Fig. 4B). When tested in neurons, there was no significant difference in the transfection levels with Lipofectamine®LTX with PLUS™ and polyplex formulated with A5P50 (Fig. 4C). Although there was some difference in the transfection protocol (incubation time), the amount of pDNA used was the same for transfection in neurons with Lipofectamine®LTX with PLUS™ and our polyplex formulation.
Fig. 4.
Transfection with polyplexes in vitro. Cells were transfected with pDNA expressing luciferase gene, luciferase activity was measured at 48 h post-transfection, and data were normalized to cell protein. (A) Luciferase expression with polyplexes formulated with different PEG-modified polymer, parent polymer PSAr5, and PEI in U-87 cells. (B) Luciferase expression with polyplexes formulated with parent polymer PSAr5 and PEG conjugate, A5P50 as compared to plasmid DNA in C-6 and U-87 cells (B) Luciferase expression with polyplex formulated with A5P50 as compared that with Lipofectamine® LTX with Plus™ in neurons. Data are shown as mean ± SEM; n = 3.
3.4 Cellular uptake of DNA with polyplexes
The confocal images of rat astrocytes incubated with polyplexes of A5P50 where DNA is labeled with YOYO (green) and polymer with TRITC (red) showed rapid uptake of the pDNA (Fig. 5A). Although the polymer is labeled with TRITC, it was not visible in the micrographs, possibly because the pDNA labeled with YOYO may have masked the signal of the polymer due to complexation. However, pDNA, when used for transfection with polyplex A5P50, was clearly seen to localize inside the nucleus by as early as 4 h post transfection (green signal in the nucleus), whereas cells incubated with pDNA alone were not observed inside the nucleus until 6 h post transfection. Furthermore, the pDNA was seen retained inside the cells when transfected with polyplex A5P50 even 24 h post transfection, but no significant amount was seen when cells were transfected with pDNA alone (Fig. 5A vs. B). However, cellular uptake of the polyplex when studied in U-87 cells and for a longer incubation time, red color of the polymer was clearly visible and was more prominent at 48 h post-incubation (Fig. 5C).
Fig. 5.
Cellular uptake of pDNA following transfection with (A) polyplex and (B) DNA alone (without polymer) in rat astrocytes and with polyplex in U-87 cells. Confocal images show greater uptake and sustained retention of DNA with polyplex as compared with DNA alone. DNA was labeled with YOYO (green) and polymer with TRITC (red) and nucleus with DAPI (blue). Bar = 10 μm. Red color of the polymer is not clearly visible, but that of DNA is seen to localize in the nucleus with the polyplex. Red color indicates that the dissociation of polymer from DNA was greater and more rapid in U-87 cells than in astrocytes.
3.5. In vivo gene expression and immunohistochemistry of brain tissue
Following intravenous injection of the polyplexes formulated using the polymer A5P50, gene expression was seen in various organs, including the brain (Fig. 6). Of all the tissues, gene expression was higher in the liver. Immunohistochemical analysis of brain sections showed luciferase expression in neurons, as evident from the colocalized images (yellow) of neuron-specific MAP2 antibody-stained neurons (red) and anti-luciferase antibody-stained luciferase protein expressed by luciferase (green) (Fig. 7).
Fig. 6.
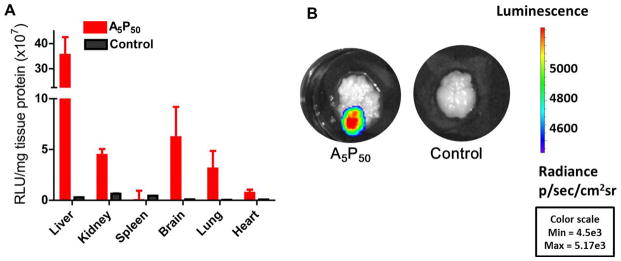
Transfection levels with polyplex formulated with A5P50 in different tissue in vivo. Three doses of the polyplex formulated with the pDNA expressing the luciferase gene were injected through the tail vein in mice on 3 consecutive days, and the organs were harvested at 24 h post last injection. Control is without injection of DNA or polyplexes. (A) Quantification of luciferase expression in different tissues. Data are shown as mean ± SEM; Polyplex A5P50, n = 6; Control, n = 3. (B) Representative images of the brain showing luciferase expression in animals that received polyplex vs. no treatment.
Fig. 7.
Confocal images of the representative horizontal brain section approximately from interaural 4.4 mm and Bregma −5.6 showing luciferase expressed in animals that had received three doses of the polyplex formulated with luciferase gene and A5P50 (1) hippocampus (scale in 500 μm) (2) cerebral cortex (scale in 100 μm) and cerebellum (scale in 100 μm). Colors indicate the following: red, anti-MAP 2; green, anti-luciferase; blue, DAPI. Merged image (yellow) indicates gene expression in neuronal cells. Images were taken at 20X magnification. Representative images from one of the two animals that showed similar results.
4. Discussion
Critical issues for developing effective, nonviral neuronal gene expression vectors are their hemocompatibility, nontoxic nature, and ability to pass through the BBB and transfect neuronal cells. In this study, we demonstrated that PEGylated arginine-rich oligo (alkylaminosiloxane) grafted with PEI at an optimized ratio is hemo- and cytocompatible, and can effectively transfect neuronal cells both in vitro and in vivo.
The 1H NMR spectra confirmed the presence of PEG and PEI in the polymer (Fig 1). The hydrodynamic diameters of polyplexes formulated with different polymers (Table 1) were in the size range 190 nm to 260 nm, which are suitable for intravenous injection (Table 2). We found that the zeta potential of the polymers and polyplexes with increasing PEG amounts first decreased from a positive zeta potential (PSAr5) to a slightly negative one (A5P20) and then increased again to a positive zeta potential (A5P50). Thus it appears that the PEG first masks the cationic charge of the parent polymer but then possibly rearranges the polymer structure with further increases in PEG, exposing the cationic charge of arginine and/or PEI. Also, we found that polyplexes have relatively higher negative or positive zeta potentials than the respective polymers (Table 2). Conventionally, interaction of cationic polymers with anionic DNA should result in the formation of polyplexes with reduced cationic surface charge than the corresponding polymers. However, it is possible that the polymers are in the solution state, whereas polyplexes are in the form of particles, which may change the arrangements of the polymer structure following interaction with DNA. In addition, polyplex formation is a dynamic process, and hence it is difficult to determine the exact structural arrangement of different molecules following interaction with DNA. Nonetheless, as described below, the mild cationic surface charge of the optimized polyplexes seems to balance the transfection without undue toxicity.
PEI is an effective in vitro transfecting agent, but the risk of toxicity from it is a major issue, and PEI is not effective in vivo because of its interaction with proteins (15, 17). This study shows that PEGylation balances the toxicity of PEI while maintaining its ability to condense DNA. In our previous studies in breast cancer cells, we found significantly greater dose-dependent toxicity of PEI than our polymer. For example, at 50 μg/ml concentration, PEI showed only ~20% cell survival whereas our polymer showed over 90% cell survival at that concentration (18). In this study, we used short-chain (600-Da) PEG rather than the more typically used long-chain PEG (2000 to 5000 Da) to balance the steric hindrance to minimize interactions of polyplex with plasma proteins to cause aggregation of polyplex or RBC lysis, but maintain sufficient interaction with cell membranes to internalize and achieve gene transfection (19). As shown in our study, the polyplexes formulated with short-chain PEG conjugates of the parent polymer PSAr5 (Tables 1 and 2) significantly reduce toxicity while improving hemocompatibility and without significantly affecting transfection (Figs. 2–4). Hemolysis and aggregation of RBCs were found to be dependent on the PEG content of polyplexes. Of all the polyplexes formulated, the one formulated with A5P50 seems to possess the right balance of the parent polymer PSAr5 and PEG to impart hemocompatibility (Fig. 2) and cytocompatibility (Fig. 3); hence we used this polyplex for further in vitro and in vivo transfection. For intravenous administration of the vector, it is critical to prevent RBCs from aggregation or hemolysis, a major issue with most highly cationic polymers, including PEI (20).
Transfection data from the various cell lines show that luciferase expression levels were higher in C-6 and U-87 cells, which are fast-dividing cancer cell lines than in neuronal cells (Fig. 4). The polyplexes in rat astrocytes showed relatively higher transfection levels than in rat neurons (Fig. 4C), which could be because neurons are significantly more slowly dividing cells than astrocytes and also their physical structures are different. Astrocytes are more flat whereas neurons are more tubular, thus astrocytes provide larger surface area for contact with the vector than neurons. It has been reported that efficient gene expression involves sequential steps of internalization of the polymer-DNA complexes inside the cells via an endocytosis-like mechanism, escape from endosomes, dissociation of the polyplexes, and finally entry of free DNA into the nucleus (21). Although we have not fully studied the intracellular trafficking of our polyplex formulation, the confocal imaging data in rat astrocytes showed rapid uptake of DNA with polyplexes, where it was retained for a sustained period of time compared when cells were transfected with pDNA alone (Fig. 5A vs. 5B). It is possible that the arginine residue is effective in cellular internalization of the polyplexes and their subsequent localization into the nucleus. Since the polymer signal was not clear, one cannot with certainty conclude that it was the polyplexes that localized into the nucleus, but the signal of the DNA was clearly seen in the nucleus (Fig. 5A). Since the overall DNA signal inside the cells and nucleus was significantly greater with polyplexes than in the cells transfected with DNA alone, one can clearly conclude that the polyplexes facilitated cellular uptake of DNA. It also appears that the dissociation of the polymer from DNA is cell line and incubation time dependent. In U-87 cells, which are fast dividing cells than astrocytes, red color of the polymer was clearly visible at 24 hr post-incubation and significantly greater at 48 hrs (Fig 5C). However, in astrocytes it was mostly the green color (due to DNA complexed with polymer) and scattered yellow color (due to DNA and polymer co-localization) (Fig 5A vs. 5C) were visible. This observation indicates that there is greater dissociation of DNA from the polymer in U-87 cells than in astrocytes, which could also explain higher gene transfection levels seen in U-87 cells than in astrocytes (Fig 4). These results clearly demonstrate that the intracellular processing of polyplexes is cell-line dependent. In our recent study, we found that cellular uptake of DNA is not the only factor that affect gene transfection (18). It may also be the stability of the DNA inside various cellular compartments and how cells process it. Although pDNA uptake is seen in confocal images (Fig 5), the transfection levels with it alone without the polymer complexation was significantly low (Fig 4).
Following intravenous injection of the polyplex, gene expression was significantly greater in the liver than in other tissues (Fig 6). However, gene expression was also seen in the brain tissue, indicating that the polyplexes transported the DNA across the BBB. Also, immunofluorescence studies with the neuronal marker MAP2 and anti-luciferase demonstrate gene expression in neurons in various brain regions, including cerebellum, cerebral cortex and hippocampus (Fig. 7). The hippocampal regions are believed to play an important role in neurological disorders related to aging and also in acute neurological disorders (22–24). Gene delivery to the brain could be of significant importance in treating these neurological conditions. Although we have not used markers for astrocytes for immunohistochemical analysis, it is quite possible that astrocytes are also transfected, considering that our polyplexes transfected astrocytes in vitro (Fig. 4C). Higher gene expression in the liver than in other tissue indicates that PEGylation is not entirely effective in preventing the clearance of the injected polyplexes by the reticuloendothelial system. Others have also reported similar phenomena even when PEG of 2000 molecular weight at different surface densities were evaluated for surface modification of nanoparticles for gene delivery (25). However, since gene expression was seen in the brain in our study, it is likely that our polyplexes remained in the circulation for time sufficient to be transported across the BBB to parenchyma to cause neuronal cell gene transfection (Fig 7).
The swift transport of pDNA inside cells seen with polyplex (Fig. 5) could be due to the arginine moiety of the polymer (12). The receptor-independent translocation of TAT peptides across the biological membrane was also believed to be due to the presence of arginine and lysine residues (5). Our findings concur with earlier reports demonstrating that arginine residues can improve membrane permeability when not in oligopeptide form (26). Recently Aoshima et al. reported a higher gene expression in primary neuronal cultures with arginine-based lipid compared to lysine-based lipid assemblies (27, 28), suggesting that arginine is essential for neuronal gene expression.
Although viral vectors are more effective for gene expression in neurons, in vitro and in vivo, its toxicological and immunological problems are the major issues in their clinical translation (29). Here we show that gene transfection into neural cells in vivo following intravenous injection, suggesting that the A5P50 polyplex maintained the ability of arginine to cross the BBB and ultimately neural cell membranes. It is feasible to develop a cell-specific promoter to target a specific cell population in the brain cells. Recently, we demonstrated in vitro in cancer cells that our polyplex formulation can co-deliver both pDNA and siRNA and was shown to enhance each other’s transfection (i.e., gene expression of DNA by siRNA and the gene-silencing effect of siRNA by DNA) (18). Such a formulation could potentially be explored for simultaneously targeting multiple pathways that may be involved in neurodegenerative diseases to achieve a synergistic effect (30).
5. Conclusions
Our study demonstrates that polyplexes containing arginine-rich oligopeptides (alkylaminosiloxane peptides) grafted with PEI and modified with a short-chain PEG provide an effective, biocompatible, nonviral vector for neuronal gene delivery, both in vitro and in vivo. The vector has potential applications in gene therapy for treating various neurological and other genetic disorders.
Acknowledgments
The study was partly funded by grants 1R01NS048837 and 1R01NS070896 from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health. The NIH had no influence over the study design or any aspect of the data and results reported herein.
The authors thank Judith Drazba, PhD, of Cleveland Clinic’s Imaging Core for guidance on confocal microscopy studies.
Footnotes
Conflict of Interest
The authors declare no conflicts of interest to disclose that could have inappropriately influenced, or be perceived to have influenced, their work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simonato M, Bennett J, Boulis NM, Castro MG, Fink DJ, Goins WF, et al. Progress in gene therapy for neurological disorders. Nat Rev Neurol. 2013;9:277–91. doi: 10.1038/nrneurol.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Honjo K, Black SE, Verhoeff NP. Alzheimer’s disease, cerebrovascular disease, and the beta-amyloid cascade. Can J Neurol Sci. 2012;39:712–28. doi: 10.1017/s0317167100015547. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Martinez FC, Guerra J, Posadas I, Cena V. Barriers to non-viral vector-mediated gene delivery in the nervous system. Pharm Res. 2011;28:1843–58. doi: 10.1007/s11095-010-0364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zabner J, Fasbender AJ, Moninger T, Poellinger KA, Welsh MJ. Cellular and molecular barriers to gene transfer by a cationic lipid. J Biol Chem. 1995;270:18997–9007. doi: 10.1074/jbc.270.32.18997. [DOI] [PubMed] [Google Scholar]
- 5.Malhotra M, Tomaro-Duchesneau C, Prakash S. Synthesis of TAT peptide-tagged PEGylated chitosan nanoparticles for siRNA delivery targeting neurodegenerative diseases. Biomaterials. 2013;34:1270–80. doi: 10.1016/j.biomaterials.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Futaki S. Membrane-permeable arginine-rich peptides and the translocation mechanisms. Adv Drug Deliv Rev. 2005;57:547–58. doi: 10.1016/j.addr.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki T, Futaki S, Niwa M, Tanaka S, Ueda K, Sugiura Y. Possible existence of common internalization mechanisms among arginine-rich peptides. J Biol Chem. 2002;277:2437–43. doi: 10.1074/jbc.M110017200. [DOI] [PubMed] [Google Scholar]
- 8.Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, et al. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J Biol Chem. 2003;278:585–90. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- 9.Calnan BJ, Tidor B, Biancalana S, Hudson D, Frankel AD. Arginine-mediated RNA recognition: the arginine fork. Science. 1991;252:1167–71. doi: 10.1126/science.252.5009.1167. [DOI] [PubMed] [Google Scholar]
- 10.Wang S, Amornwittawat N, Juwita V, Kao Y, Duman JG, Pascal TA, et al. Arginine, a key residue for the enhancing ability of an antifreeze protein of the beetle Dendroides canadensis. Biochemistry. 2009;48:9696–703. doi: 10.1021/bi901283p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tyagi M, Rusnati M, Presta M, Giacca M. Internalization of HIV-1 tat requires cell surface heparan sulfate proteoglycans. J Biol Chem. 2001;276:3254–61. doi: 10.1074/jbc.M006701200. [DOI] [PubMed] [Google Scholar]
- 12.Morris VB, Sharma CP. Enhanced in-vitro transfection and biocompatibility of l-arginine modified oligo (-alkylaminosiloxanes)-graft-polyethylenimine. Biomaterials. 2010;31:8759–69. doi: 10.1016/j.biomaterials.2010.07.073. [DOI] [PubMed] [Google Scholar]
- 13.Kichler A, Sabourault N, Decor R, Leborgne C, Schmutz M, Valleix A, et al. Preparation and evaluation of a new class of gene transfer reagents: poly(-alkylaminosiloxanes) J Control Release. 2003;93:403–14. doi: 10.1016/j.jconrel.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Horikawa R, Tanimura T. Spectrophotometric Determination of Carboxylic acids with 2-Mitrophenylhydrazine in Aqueous Solution. Analytical Letters. 1982;15:1629–42. [Google Scholar]
- 15.Morris VB, Sharma CP. Folate mediated in vitro targeting of depolymerised trimethylated chitosan having arginine functionality. J Colloid Interface Sci. 2010;348:360–8. doi: 10.1016/j.jcis.2010.04.090. [DOI] [PubMed] [Google Scholar]
- 16.McLatchie AP, Burrell-Saward H, Myburgh E, Lewis MD, Ward TH, Mottram JC, et al. Highly sensitive in vivo imaging of Trypanosoma brucei expressing “red-shifted” luciferase. PLoS Negl Trop Dis. 2013;7:e2571. doi: 10.1371/journal.pntd.0002571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin L, Zeng X, Liu M, Deng Y, He N. Current progress in gene delivery technology based on chemical methods and nano-carriers. Theranostics. 2014;4:240–55. doi: 10.7150/thno.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu S, Morris VB, Labhasetwar V. Codelivery of DNA and siRNA via arginine-rich PEI-based polyplexes. Mol Pharmaceutics. 2015;12:621–9. doi: 10.1021/mp5006883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mao S, Neu M, Germershaus O, Merkel O, Sitterberg J, Bakowsky U, et al. Influence of polyethylene glycol chain length on the physicochemical and biological properties of poly(ethylene imine)-graft-poly(ethylene glycol) block copolymer/SiRNA polyplexes. Bioconjug Chem. 2006;17:1209–18. doi: 10.1021/bc060129j. [DOI] [PubMed] [Google Scholar]
- 20.Dai L, Wang L, Deng L, Liu J, Lei J, Li D, et al. Novel multiarm polyethylene glycol-dihydroartemisinin conjugates enhancing therapeutic efficacy in non-small-cell lung cancer. Sci Rep. 2014;4:5871. doi: 10.1038/srep05871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cornelis S, Vandenbranden M, Ruysschaert JM, Elouahabi A. Role of intracellular cationic liposome-DNA complex dissociation in transfection mediated by cationic lipids. DNA Cell Biol. 2002;21:91–7. doi: 10.1089/104454902753604961. [DOI] [PubMed] [Google Scholar]
- 22.Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci. 2011;12:585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sicotte NL, Kern KC, Giesser BS, Arshanapalli A, Schultz A, Montag M, et al. Regional hippocampal atrophy in multiple sclerosis. Brain. 2008;131:1134–41. doi: 10.1093/brain/awn030. [DOI] [PubMed] [Google Scholar]
- 24.Pereira JB, Junque C, Bartres-Faz D, Ramirez-Ruiz B, Marti MJ, Tolosa E. Regional vulnerability of hippocampal subfields and memory deficits in Parkinson’s disease. Hippocampus. 2013;23:720–8. doi: 10.1002/hipo.22131. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Hu Y, Huang L. Influence of polyethylene glycol density and surface lipid on pharmacokinetics and biodistribution of lipid-calcium-phosphate nanoparticles. Biomaterials. 2014;35:3027–34. doi: 10.1016/j.biomaterials.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim TI, Ou M, Lee M, Kim SW. Arginine-grafted bioreducible poly(disulfide amine) for gene delivery systems. Biomaterials. 2009;30:658–64. doi: 10.1016/j.biomaterials.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aoshima Y, Hokama R, Sou K, Sarker SR, Iida K, Nakamura H, et al. Cationic amino acid based lipids as effective nonviral gene delivery vectors for primary cultured neurons. ACS Chem Neurosci. 2013;4:1514–9. doi: 10.1021/cn400036j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarker SR, Aoshima Y, Hokama R, Inoue T, Sou K, Takeoka S. Arginine-based cationic liposomes for efficient in vitro plasmid DNA delivery with low cytotoxicity. Int J Nanomedicine. 2013;8:1361–75. doi: 10.2147/IJN.S38903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346–58. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 30.Blackburn D, Sargsyan S, Monk PN, Shaw PJ. Astrocyte function and role in motor neuron disease: a future therapeutic target? Glia. 2009;57:1251–64. doi: 10.1002/glia.20848. [DOI] [PubMed] [Google Scholar]