Abstract
Background
Lipid profiles in the blood are altered in human cocaine users, suggesting that cocaine-exposure can induce lipid remodeling.
Methods
Cocaine-induced locomotor sensitization in rats was followed by shotgun lipidomics using electrospray ionization-mass spectrometry (ESI-MS) and determined changes in brain tissues. To determine if any lipidomic changes were also reflected in the blood, we performed principal component analysis (PCA) of lipidomic spectra isolated from cocaine-treated animals. Alterations in the abundance of phospholipid species were correlated with behavioral changes in the magnitude of either the initial response to drug or locomotor sensitization.
Results
Behavioral sensitization altered the relative abundance of several phospholipid species in the hippocampus and cerebellum, measured one week following the final exposure to cocaine. In contrast, relatively few effects on phospholipids in either the dorsal or the ventral striatum were observed. PCA analysis demonstrated that cocaine altered the relative abundance of several glycerophospholipid species as compared to saline-injected controls. Subsequent MS/MS analysis identified some of these lipids as phosphatidylethanolamines, phosphatidylserines and phosphatidylcholines. The relative abundance of some of these phospholipid species were well correlated (R2 of 0.7 or higher) with either the initial response to cocaine or locomotor sensitization.
Conclusion
Taken together, these data demonstrate that a cocaine-conditioning experience results in the remodeling of specific phospholipids in rat brain tissue in a region-specific manner and also alters the intensities and types of phospholipid species in rat blood. These results further suggest that such changes may serve as biomarkers to assess the neuroadaptations occurring following repeated exposure to cocaine.
Keywords: Phospholipids, Brain Lipids, Lipidomics, Behavioral Sensitization, Cocaine
1. INTRODUCTION
The National Survey on Drug Use and Health (2012) estimates 1.4 million people over the age 12 used cocaine in 2011, and that over 820,000 of these individuals met the Diagnostic and Statistical Manual of Mental Disorders criteria for dependence or abuse of cocaine (2012). Cocaine is responsible for more emergency room visits in the United States than any other illegal drug (http://www.oas.samhsa.gov/2k10/DAWN034/EDHighlights.htm) and there are currently no FDA approved treatments for cocaine substance use disorder.
Although an extensive effort has been made to characterize and evaluate various mechanisms involved in cocaine-induced alterations of brain function, few studies have assessed the effect of cocaine use on brain lipid metabolism in humans (Ross et al., 1996, 2002) and none have identified the specific lipid species in the brain that are altered following drug exposure. It is known that a history of cocaine use alters blood levels of cholesterol (Buydens-Branchey and Branchey, 2003) and fatty acids (Buydens-Branchey et al., 2003). These findings are consistent with the premise that altered cell membrane remodeling activity is occurring in individuals exposed to cocaine. Given that lipid remodeling would be inherent to the changes in neuronal morphology and synaptic plasticity thought to underlie the neuroadaptations associated with substance use disorder, it is somewhat surprising that so few studies have assessed changes in lipid profiles after cocaine exposure, and that none have been described using animal models of addiction.
In addition to data demonstrating that cocaine can induce changes in the lipid profiles, one study reported that a link exists between the brain dopaminergic and phospholipid catabolic systems (Ross and Turenne, 2002), suggesting that changes in phospholipid profiles in cocaine treated subjects may mirror changes in dopaminergic signaling. Additionally, changes in fatty acid levels in the blood have been correlated with relapse (Buydens-Branchey et al., 2003), supporting the hypothesis that blood lipids can serve as biomarkers of cocaine-induced neurological dysfunction. The hypothesis that blood lipids can be related to neurological dysfunction and behavior is further bolstered by a recent finding in Alzheimer’s patients demonstrating that select serum lipids predicted cognitive impairment over 2–3 years with 90% accuracy (Mapstone et al., 2014). Finally, another study has demonstrated that cocaine-induced hepatotoxicity in mice resulted in concurrent changes in serum lipids (Shi et al., 2012).
While the aforementioned studies support the rationale for investigating cocaine-induced alterations in lipidomic profiles, a practical consideration impeding such studies is that thousands of lipids exist in biological tissues, making it difficult to identify specific changes in select lipid species. Furthermore, until recently, the methods used to isolate and characterize such lipids were somewhat laborious and time consuming. One productive approach has been to use matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF MS) imaging in rat brain tissue sections to identify lipid species (Jackson et al., 2005a, 2005b). Subsequent studies have described discrete localization of lipids throughout both the rat (Delvolve et al., 2011; Mikawa et al., 2009) and the human brain (Veloso et al., 2011a, 2011b). These observations suggest that heterogeneity in the relative abundance of lipid species both within and between brain regions may have functional relevance.
We performed shotgun lipidomics using electrospray ionization-mass spectrometry (ESI-MS) to assess the persisting effects of cocaine on the relative abundance of phospholipid species in both brain tissues and blood of rats that had been repeatedly exposed to the drug. The resulting data significantly informs our understanding of how cocaine alters lipid remodeling within specific brain regions and further suggests that changes in the blood lipidome may be useful as prognostic and/or diagnostic indicators of behavioral responses to cocaine and of drug exposure history.
2. METHODS
2.1 Behavior Assays
2.1.1 Cocaine conditioning and the Induction of locomotor sensitization
Male Sprague-Dawley rats, 8 weeks of age (Harlan, Indianapolis, IN, USA) were housed in pairs in clear plastic cages and maintained on a 12 hr light/dark cycle (0700/1900 hr). Food and water was available ad libitum except during the behavioral sessions. Animals were allowed to adapt to the lab conditions for a week before behavioral testing began. Behavioral sessions were conducted daily between 0900 and 1600 hr.
The apparatus and measurement of activity have been described in detail elsewhere (Gosnell, 2005; Seymour and Wagner, 2008). Briefly, locomotor (LM) activity was measured in four 43.2 × 43.2 cm chambers with clear plastic walls and a solid smooth floor (Med Associates, St. Albans, VT, USA). The chambers were individually housed in sound-attenuating cubicles equipped with a house light and a ventilation fan. Two banks of 16 infrared photobeams and detectors, mounted at right angles 3.5 cm above the floor, detected horizontal activity. Activity Monitor software (Med Associates) was used to count photobeam breaks.
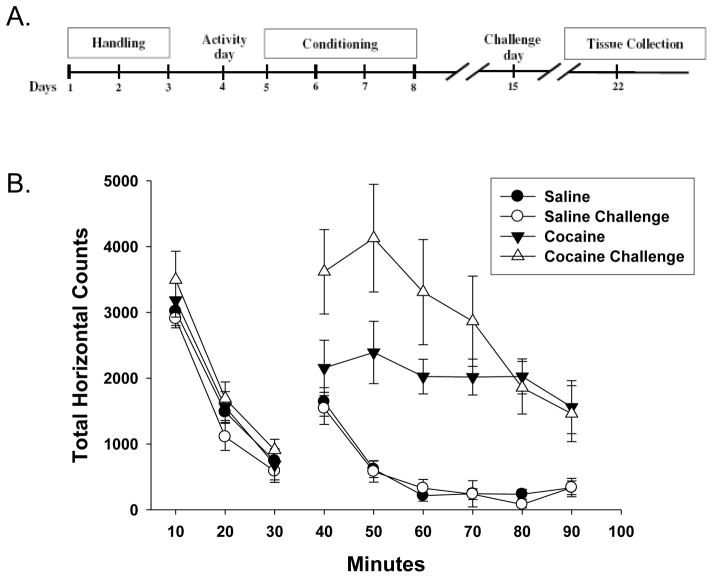
Animals were subjected to a locomotor sensitization protocol over a period of twelve days (Figure 1A). Following 3 days of habituation to experimenter handling, on day 4 animals were placed in the center of the open field activity chamber for 30 minutes to establish a baseline LM activity for each animal. After that time, they were given an i.p. injection of either 10 mg/kg cocaine or 0.9% saline and returned to the chamber for an additional 60 minutes of monitoring for this Activity day session (Figure 1B, cocaine/saline). During conditioning sessions (days 5–8), animals were injected i.p. with either cocaine (15 mg/kg) or 0.9% saline using the same open field procedure. Seven days after the last conditioning session on day 15, animals were again injected during the Challenge day session with either cocaine (10mg/kg) or saline in the open field activity chamber as described above for protocol day 4. For correlation analysis, the initial response to cocaine was determined by the increase in total horizontal counts over the first 30 minutes after exposure to cocaine on day 4 (e.g. Cocaine 30–60 min of Figure 1B). Locomotor sensitization to cocaine was determined by subtracting the total horizontal counts over the first 30 minutes after exposure to cocaine on day 4 from the initial response measured on day 15.
Figure 1. Effect of cocaine conditioning on open-field locomotor activity.
A. Rats were treated according to a standard locomotor sensitization protocol, as indicated. Activity and Challenge day test dose of cocaine was 10mg/kg i.p., conditioning dose on days 5–8 was 15mg/kg. B. Time course quantification of total horizontal counts during the open-field Activity day (filled symbols) and Challenge day (open symbols) sessions prior (10–30 minutes) and post (40–90 minutes) i.p. injection with either saline (circles) or cocaine (triangles). Data represent the mean ± the SEM from groups of n=6 rats.
2.1.2 Isolation of specific brain areas and blood
All experimental protocols were performed following approval by the University of Georgia Animal Care and Use Committee. Rats were anesthetized with halothane prior to decapitation. Brains were removed and cooled in ice-cold oxygenated (95% O2/5% CO2) dissection artificial cerebrospinal fluid containing 120 mM NaCl, 3 mM KCl, 4 mM MgCl2, 1 mM NaH2PO4, 26 mM NaHCO3 and 10 mM glucose. Brain regions were dissected and the tissue was either processed immediately with the Bligh-Dyer lipid extraction (section 2.2.2) or flash frozen in liquid N2. Blood was isolated via cardiac puncture prior to decapitation and immediately mixed, by vortexing, with 1 mL of methanol:water (2.0:0.8 v/v) and then placed at −20°C until lipid extraction.
2.2 Lipidomic Analysis
2.2.1 Lipid nomenclature
Phospholipids differ in terms of the numbers of carbons and double bonds and polar head groups. Typically, the nomenclature used to identify these traits is X:Y, where X = the number of carbons and Y = the number of double bonds; hence, 34:2 would indicate a lipid with 34 carbons and 2 double bonds. Polar head groups are referred to by their abbreviations.
2.2.2 Bligh-Dyer lipid extraction
Phospholipids were extracted using chloroform and methanol according to the method of Bligh and Dyer (1959). After treatment, tissue and blood were isolated as explained above, and tissue was washed with PBS and homogenized in 3 mL of methanol: water (2.0:0.8 v/v). Blood was suspended in 1.25 mL of methanol and 1.25 mL of chloroform. Tubes were vortexed for 30 seconds and allowed to sit for 10 minutes on ice. Tubes were centrifuged at 213 × g for 5 minutes and the bottom chloroform layer was transferred to a new test tube. The extraction steps were repeated a second time and the chloroform layers combined. The collected chloroform layers were dried under nitrogen, reconstituted with 50 μL of methanol: chloroform (2:1 v/v) and stored at −20°C.
2.2.3 Lipid phosphorus assay
Lipid phosphorus was quantified using malachite green (Zhou and Arthur, 1992). Lipids extracts (10 μL) were dried down under nitrogen in a glass test tube, 200 μL of perchloric acid was added to the tube, and heated at 130°C for 2–3 hr. After this time, 1 mL of dH2O was added to the tube while vortexing, then 1.5 mL of reagent C (4.2 grams ammonium molybdate tetrahydrate in 100 mL 5 N HCl and 0.15 grams malachite green oxalate in 300 mL dd H20) was added and vortexed, followed by 200μL of 1.5% v/v Tween 20 and vortexing. After sitting at room temperature for 25 minutes, a 200 μL aliquot was used to measure the absorbance at 590 nm.
2.2.4 Characterization and quantitation of phospholipids using electrospray ionization-mass spectrometry (ESI-MS)
Lipid extract samples (500 pmol/μl) were prepared by reconstitution in chloroform: methanol (2:1, v/v). ESI-MS was performed as described previously (Kinsey et al., 2008; Peterson et al., 2008; Zhang et al., 2005) using a Trap XCT ion-trap mass spectrometer (Agilent Technologies, Santa Clara, CA) with a nitrogen drying gas flow-rate of 8 L/min at 350°C and a nebulizer pressure of 30 psi. The scanning range was from 100 to 2200 m/z on 5 μL of the sample scanned in negative ion mode for 2.5 min with a mobile phase of acetonitrile: methanol: water (2:3:1) in 0.1% ammonium formate. As previously described (Taguchi et al., 2000), qualitative identification of individual phospholipid molecular species was based on their calculated theoretical monoisotopic mass values, subsequent MS/MS analysis, and their level normalized to either the total ion count (TIC) or the most abundant phospholipid.
MSnth fragmentation was performed on an Agilent Trap XCT ion-trap mass spectrometer equipped with an ESI source. The analyte was introduced by direct injection from the HPLC system. The nitrogen drying gas flow-rate was 8.0 L/min at 350°C. The ion source and ion optic parameters were optimized with respect to the positive molecular ion of interest. Identification was based on the loss of the parent head group as assessed by neutral loss scanning. In the event that neutral loss scanning was unable to identify the phospholipid head group, the tentative ID was assigned based on the m/z value and the lipids maps database (http://www.lipidmaps.org). In such cases the lipid species is referred to by it m/z value to indicate the uncertainty in its identification.
2.2.5 Multivariate Statistical Analysis of Blood Lipids
Multivariate techniques, such as principal component analysis (PCA), were performed using MetaboAnalyst 2.0 (http://www.metaboanalyst.ca/MetaboAnalyst/faces/Home.jsp) using default algorithms. Automatic peak detection and spectrum deconvolution was performed using a peak width set to 0.5. Data were normalized to the total ion count, and scaled to the z-value. No outliers were removed from the analysis.
Select lipids identified using a volcano plot analysis (Supplemental Table 11) were further studied and identified using MS/MS analysis (see section 2.2.4). Following identification, the level of each parent lipid was normalized to the total ion count, and the change in the relative abundance of that phospholipid species as compared to its control was determined. This method is standard for lipidomic analysis as reported in our previous studies (Kinsey et al., 2008; Peterson et al., 2008).
2.2.6 Statistical Analysis
All statistical analysis for locomotor data were compiled using SigmaStat for windows version 3.11 (SPSS Science, Chicago, IL). Behavioral sensitization data was analyzed by combining the ambulatory counts and stereotypic counts to get the total horizontal counts (i.e., the total number of horizontal beam breaks) for each animal. The data were binned in 10 min blocks and planned comparisons made at each time point between the treatment activity vs. treatment challenge results using an unpaired Student’s t-test.
All statistical analyses for lipid data were also compiled using SigmaStat for windows version 3.11 (SPSS Science, Chicago, IL). Lipids isolated from one animal source (blood or tissue) equaled an n of 1 and samples from a total of six animals were assessed.
3. Results
3.1 Induction of Locomotor Sensitization (LMS)
We assessed locomotor movement as an indicator of behavioral sensitization to cocaine in rats using the protocol outlined in Figure 1A. We assessed locomotor movement as an indicator of behavioral sensitization to cocaine in rats by comparing locomotion after the initial “Activity” administration of the drug (10mg/kg, i.p.) on day 4 was compared with the movement observed after the “Challenge” administration on day 15. Following habituation to the open field environment during the first 30 minutes, cocaine caused an increase in total horizontal movement in rats compared to those treated with saline (Figure 1B). One week following conditioning with four daily cocaine injections (15mg/kg, i.p.), a cocaine challenge on day 15 again increased movement compared to saline-treated controls. In addition, cocaine-induced movement at the 40, 50, & 60 minute time points was increased (p<0.05, n=6) on the Challenge day as compared to the initial drug-induced movement observed on the Activity day (Figure 1B). This increase in cocaine-induced locomotion following conditioning exposures is indicative of behavioral sensitization (see Steketee and Kalivas, 2011 for review).
Seven days after the final cocaine or saline exposure (protocol day 22), rats were euthanized and specific brain regions and blood were isolated and prepared for lipidomic analysis as explained in the Methods (section 2). Brain tissues isolated included the hippocampus, ventral striatum and dorsal striatum. These structures were chosen as they are known to be involved with reward and reinstatement of drug-seeking. The cerebellum was also isolated as a brain region not directly involved with these behaviors (Koob and Volkow, 2010).
3.2 Differential abundance of phospholipids in the hippocampus, frontal cortex and cerebellum of control (drug naïve) rats
We used ESI-MS to analyze over 1,000 m/z values representing multiple phospholipid classes. Prior to assessing the effect of repeated cocaine exposure on the level of phospholipids, we first determined the relative abundance of phospholipid species between brain structures. These data are shown in Supplemental Figures 1 and 22. Phosphatidylcholines (PCs) were generally the highest expressed lipids in these tissues and their relative abundance did not appear to vary greatly between the different brain regions studied. Relatively low levels of 16:0 lysophosphatidylcholine (LPC) were also detected in all regions. The pattern of intensities of these lipids is in general agreement with several studies assessing regional distribution of phospholipids in the brain (Delvolve et al., 2011; Jackson et al., 2005a, 2005b). Two lipid species with m/z values of 815–817 and 824.5 had relative abundance levels 2-fold higher than baseline; however, the identity of these lipids could not be confirmed by subsequent MS/MS analysis.
The relative abundance of select sphingomyelins (SM) was also determined in the hippocampus, frontal cortex and cerebellum (Supplemental Figure 23). The cerebellum had higher levels of 20:0, 22:1, 24:1 and 26:4 SM, as compared to the hippocampus and frontal cortex regions. The levels of all SM species studied were similar in the hippocampus and frontal cortex tissues. For the subsequent cocaine studies, we chose to add assessments of the dorsal and ventral striatum regions.
3.3 Effect of repeated cocaine exposure on the relative abundance of phospholipid species in the hippocampus
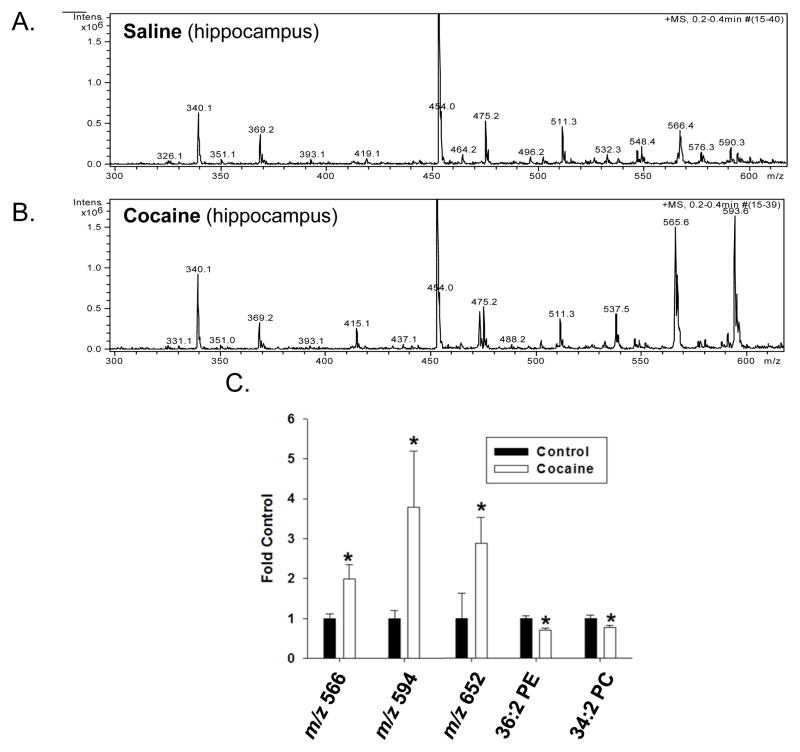
Analysis of spectra derived from the hippocampus of control and cocaine-treated rats showed significant differences in the intensities of m/z values ranging from 300 to 600, which can indicate lysophospholipids or low molecular weight phospholipids (Figure 2). Changes were also detected in intensities for m/z values correlating to higher molecular weight phospholipids (spectra not shown).
Figure 2. Effect of cocaine exposure on the relative abundance of phospholipids in the hippocampus.
Rats were treated with either saline or cocaine as described in Figure 1A. Seven days after the final treatment (Day 22) hippocampal tissue was isolated, subjected to Bligh-Dyer extraction and analyzed by ESI-MS. A. represents positive ion ESI-MS spectra from control rats while B. represents positive ion ESI-MS spectra from cocaine exposed rats. C. represents changes in the relative abundance of select phospholipids in cocaine treated rats compared to saline controls and is presented as the mean ± the SD of at least 6 different rats. *Indicates a significant difference (p < 0.05) as compared to saline control rats. Lipid species indicated by only their m/z value were unable to be fully identified by subsequent MS/MS and neutral loss scanning.
Cocaine treatment specifically increased the intensities of peaks with m/z values of 566 and 594 (Figure 2A and B). The relative abundance of these lipids increased ~2–4-fold in cocaine treated rats compared to saline treated animals. Significant increases in the levels of a lipid species with the m/z value of 652 were also detected. The identity of these lipids could not be fully confirmed by subsequent MS/MS analysis (see Supplemental Data); however, subsequent MS/MS analysis did demonstrate modest decreases in the levels of 36:2 PE and 34:2 PC.
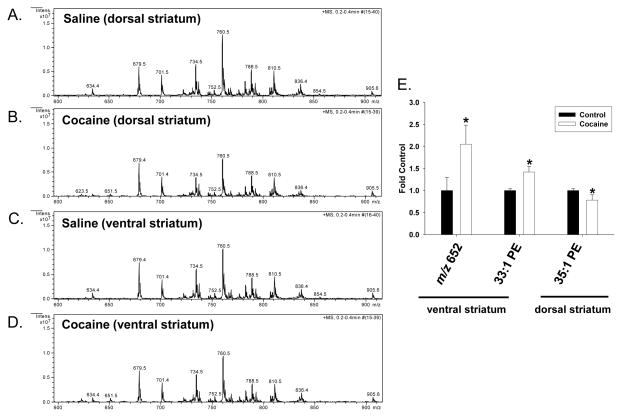
3.4 Effect of repeated cocaine exposure on the relative abundance of phospholipid species in the dorsal and ventral striatum
Analysis of spectra derived from the ventral and dorsal striatum of cocaine- and saline saline-treated rats showed few drug-induced differences with regards to phospholipid levels (Figure 3). The intensity of only three m/z values differed significantly among these two regions, corresponding to a lipid with the m/z value of 652 and 33:1 PE in the ventral striatum and 35:1 PE in the dorsal striatum (Figure 3E). In the ventral striatum, the level of a lipid species with the m/z value of 652 was increased about 2-fold after cocaine treatment, as compared to saline control. The identity of this lipid could not be confirmed by subsequent MS/MS analysis and it may represent in-source fragment of a larger precursor. The level of 33:1 PE increased about 1.5-fold, compared to controls. In contrast, in the dorsal striatum the level of 35:1 PE was decreased in cocaine-treated rats about 20%, as compared to saline-treated rats.
Figure 3. Effect of cocaine exposure on the relative abundance of phospholipids in the dorsal and ventral striatum.
Rats were treated with either saline or cocaine as described in Figure 1A. Seven days after the final treatment (Day 22) ventral and dorsal striatal tissue was isolated, subjected to Bligh-Dyer extraction and analyzed by ESI-MS. A. and C. represent positive ion ESI-MS spectra from control rats for the indicated tissues, while B. and D. represent spectra from cocaine exposed rats. E. represents changes in the relative abundance of select phospholipids in cocaine treated rats as compared to saline controls, and is presented as the mean ± the SD of at least 6 different rats. *Indicates a significant difference (p < 0.05) as compared to saline control rats. Lipid species indicated by only their m/z value were unable to be fully identified by subsequent MS/MS and neutral loss scanning.
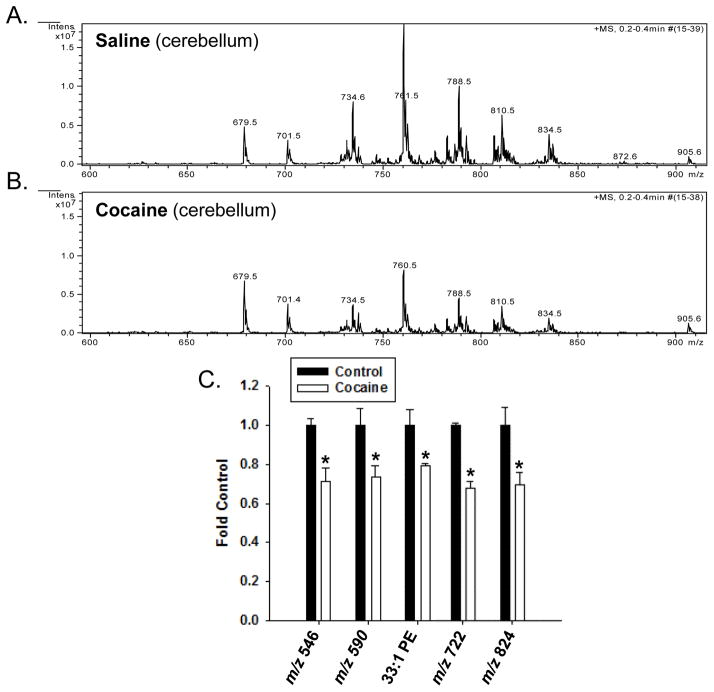
3.5 Effect of repeated cocaine exposure on the relative abundance of phospholipid species in the cerebellum
Analysis of spectra derived from the cerebellum of rats exposed to cocaine showed several m/z values whose intensities significantly decreased compared to control rats (Figure 4). These m/z values corresponded to several phospholipids, many of which could not be fully identified using MS/MS and neutral loss scanning. These include those at 546, 590, 722 and 824 (Figure 4C), and it’s possible that some of these lipids, such as the smaller molecular weight lipids, may be in-source fragments. One lipid that we were able to identify was 33:1 PE, which decreased approximately 20% in cocaine-treated rats, as compared to saline-treated rats.
Figure 4. Effect of cocaine exposure on the relative abundance of phospholipids in the cerebellum.
Rats were treated with either saline or cocaine as described in Figure 1A. Seven days after the final treatment (Day 22) cerebellar tissue was isolated, subjected to Bligh-Dyer extraction and analyzed by ESI-MS. A. represents positive ion ESI-MS spectra from control rats for the indicated tissues, while B. represents spectra from cocaine exposed rats. C. represents changes in the relative abundance of select phospholipids in cocaine treated rats as compared to saline controls, and is presented as the mean ± the SD of at least 6 different rats. Lipid species indicated by only their m/z value were unable to be fully identified by subsequent MS/MS and neutral loss scanning. *Indicates a significant difference (p < 0.05) as compared to saline control rats.
3.6 Effect of repeated cocaine exposure on the blood lipidome
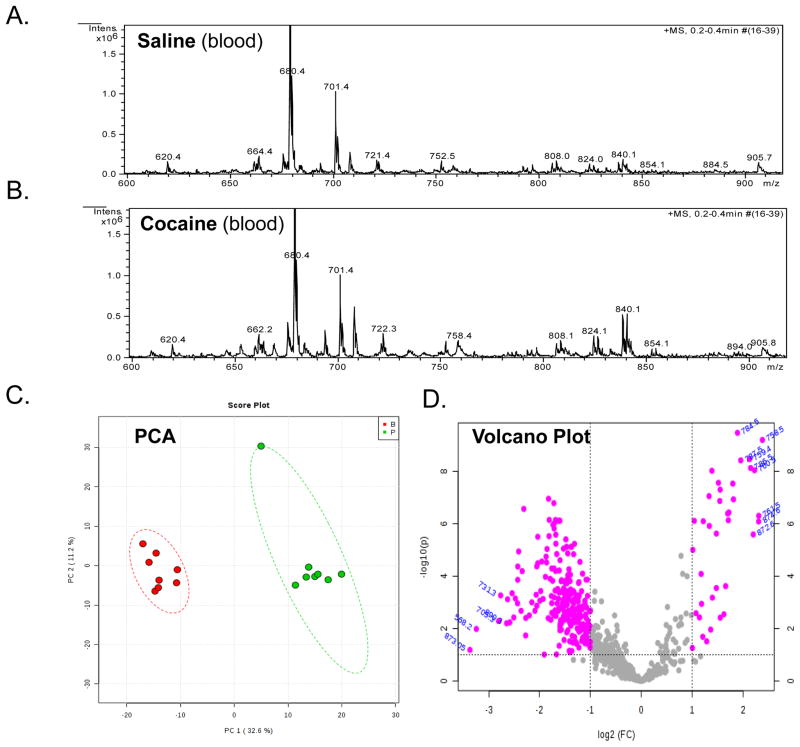
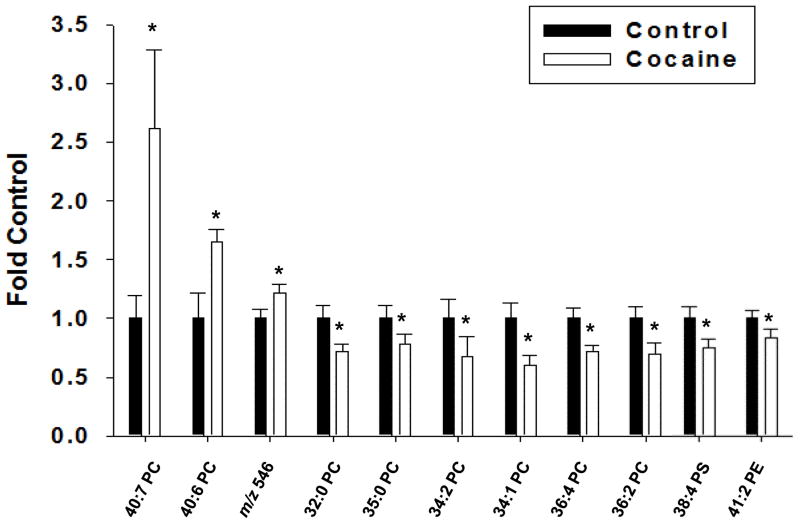
The above data demonstrate the novel finding that cocaine exposure induces region-specific changes in the relative abundance of phospholipid species in the rat brain. We also analyzed spectra to determine if the relative abundance of phospholipids were changed in the blood of rats after cocaine exposure (Figure 5A and B). Subsequent PCA analysis demonstrated that cocaine significantly altered the variability of the blood lipidome in rats compared to saline treatment (Figure 5C). A volcano plot analysis demonstrated several m/z values whose relative abundance was significantly (p < 0.05) altered compared to saline-treated animals (Figure 5D and Supplemental Table 14). In general, cocaine treatment decreased the relative abundance of most lipid species, although increases were seen in some species (Figure 6). We validated some of these data by performing MS/MS analysis (Supplemental Figure 6). This analysis demonstrated increases in 40:7 PC, 40:6 PC and an unidentifiable lipid with an m/z of 546. The level of 40:7 PC in cocaine treated rats was almost 3-fold higher than controls, while 40:6 PC and the lipid with an m/z value of 546 were only increased about 60 and 20%, respectively. In contrast, and in agreement with the volcano plot, the majority of lipids decreased in cocaine treated animals were primarily PC, compared to saline treated animals.
Figure 5. Effect of cocaine exposure on the relative abundance of phospholipids in the blood.
Rats were treated with either saline or cocaine as described in Figure 1A. Seven days after the final treatment (Day 22) blood was collected, subjected to Bligh-Dyer extraction and analyzed by ESI-MS. A. represents positive ion ESI-MS spectra from saline treated rats for the indicated tissue, while B. represents spectra from cocaine exposed rats. C. represents PCA analysis of lipids extracted from saline treated rats (green) and cocaine treated rats (red). The circles represent the 95% confidence intervals. D. Represents a volcano plot analysis comparing the log change in each m/z value (X-axis) to the significance value (Y-axis). The identity of select m/z values are indicated next to each data point.
Figure 6. Identification of blood phospholipids altered after cocaine exposure.
Rats were treated with either saline or cocaine as described in Figure 1A. Seven days after the final treatment (Day 22) blood was collected, subjected to Bligh-Dyer extraction and analyzed by ESI-MS and subsequent PCA analysis using a volcano plot. Select m/z values that demonstrated significant changes in the volcano plot were identified using MS/MS analysis and the fold-change in relative abundance between the saline treated controls and the cocaine treated rats determined. The lipid present at the m/z value of 546.6 was not identified by subsequent MS/MS. The data are presented as the mean ± the SD of at least 6 different rats. *Indicates a significant difference (p < 0.05) as compared to saline control rats.
3.7 Relevance of lipidomic changes in the blood to cocaine-induced behaviors
The above data clearly show that cocaine treatment, under the conditions tested, leads to significant changes in the lipidome of both brain tissues and blood. However, the data do not indicate the relevance of these changes to cocaine-induced neurological dysfunction or behavior. To begin to address this limitation we correlated changes in the relative abundance of the blood phospholipids demonstrated to be significantly altered by cocaine to either the initial response or sensitization to cocaine (both as assessed by changes in locomotion). Interestingly, the two lipid species whose abundance changed the greatest in response to cocaine (40:7 and 40:6 PC), showed very poor correlations to either the initial response or sensitization to cocaine (Table 1). This was also the case with the lipids species at m/z 546 (possibly 20:3 LPC), 38:4 PS and 36:4 PC. Alterations in some phospholipids correlated to either initial response or sensitization, but not both. For example, 36:2 PC had an R2 value of 0.89 to initial response, suggesting that increases in this phospholipid positively correlated to increased locomotor activity during the first exposure to cocaine. In contrast, levels of this same lipid did not correlate to sensitization to cocaine, displaying an R2 value of 0.23. A similar trend was seen for 35:0 PC. There were only a few phospholipids whose abundance correlated to both the initial response to cocaine and sensitization, based on those that had R2 values greater than 0.6. These included 32:0 PC, 34:1 PC and 41:2 PE. Collectively these data show the novel finding that the relative abundance of certain phospholipids in the blood correlate to behavioral responses induced by cocaine.
Table 1.
Correlation between Initial Response and Sensitization to Cocaine and Changes in the Expression of Select Glycerophospholipids in Rat Blooda
| m/z | Tentative Lipidb | R2 Initial Response | R2 Sensitization |
|---|---|---|---|
| 546.6 | 20:3 LPCb | 0.44 | −0.75 |
| 734.5 | 32:0 PCc | 0.75 | 0.66 |
| 735.5 | 35:0 PCc | 0.83 | 0.23 |
| 758.8 | 34:2 PCc | 0.76 | 0.57 |
| 760.6 | 34:1 PCc | 0.64 | 0.63 |
| 782.3 | 36:4 PCc | 0.52 | 0.13 |
| 786.6 | 36:2 PCc | 0.89 | 0.23 |
| 813.6 | 38:4 PSc | 0.59 | 0.53 |
| 815.3 | 41:2 PE | 0.77 | 0.63 |
| 832.4 | 40:7 PCc | −0.36 | −0.48 |
| 834.6 | 40:6 PCc | 0.08 | −0.39 |
As determined by comparing the percent change in lipid to initial response to cocaine
Based on theoretical m/z reported in Lipids Maps (www.lipidmaps.org)
Based on theoretical m/z reported in Lipids Maps (www.lipidmaps.org) and MS/MS analysis with neutral loss scanning
4. DISCUSSION
This study demonstrates that experiencing a cocaine conditioning protocol that induces locomotor sensitization resulted in region-specific changes in select lipid species in the rat brain. The repeated cocaine conditioning protocol also changed the lipidomic profile of the blood. Importantly, the changes in the lipidome of these tissues were present one week following the final administration of cocaine, suggesting that these effects are not merely acute responses following drug exposure. Rather, these lipidomic alterations may reflect persisting consequences of drug use and may therefore serve as markers of drug history and of the resulting behavioral adaptations to drug exposure.
The relative abundance of phospholipid species were significantly changed in the hippocampus, a prominent brain region involved with various aspects of learning and memory (Buzsaki and Moser, 2013). Changes in phospholipids in this brain region may coincide with the role of the hippocampus in drug-seeking behavior that is primed by either conditioned cues (Sun and Rebec, 2003) or environmental context (Fuchs et al., 2005), as such stimuli are activating memories associated with prior drug experience. Given that the hippocampus is directly involved in reinstatement of drug-seeking behavior in extinguished rats with a history of cocaine self-administration (Vorel et al., 2001), this brain region is a likely site for persisting changes related to the memories of addiction, some of which may include membrane remodeling and related lipidomic changes. By comparison, the relatively few changes in phospholipids occurring in either the dorsal or ventral striatum may seem unexpected given the involvement of these areas in dopaminergic signaling and reward. However, it is important to consider the timing of our tissue collections for these assessments. By sampling one week following the final drug exposure, lipidomic changes in brain regions involved with persisting memories associated with drug exposure would be favored as opposed to those involved in mediating acute drug effects.
The observed changes in phospholipids in the cerebellum were unexpected. Originally, the cerebellum was chosen because of its presumed lack of relevance to the mechanisms underlying addictive behavior. However, several sub regions within the cerebellum have bidirectional connections with brain areas mediating drug reward, including the striatum (Bostan et al., 2010) and the ventral tegmental area (Snider et al., 1976). The ventral tegmental area sends dopaminergic projections to the cerebellum (Ikai et al., 1992), forming a midbrain-cerebellar circuit (Carbo-Gas et al., 2014). These and other structural findings have challenged the traditional view of the cerebellum as being independent from the effects of drugs of abuse (Carbo-Gas et al., 2014; Miquel et al., 2009). In light of these considerations, it is not surprising that we observed cocaine-induced changes in phospholipid intensities in the cerebellum, albeit modest in magnitude (less than 30%).
The relative abundance of numerous phospholipids in the blood of cocaine-treated rats was altered. Interestingly, an early magnetic resonance spectroscopy study has reported decreases in phospholipid metabolites in the brains of cocaine-dependent patients (Christensen et al., 1996; MacKay et al., 1993). Decreases in post-mortem brain phospholipid-metabolizing enzymes, such as phospholipase A2 (PLA2) have also been noted in cocaine users (Ross et al., 1996, 2002). PLA2 enzymes metabolize phospholipids, releasing fatty acid and lysophospholipid moieties, and are important mediators of phospholipid remodeling (Akiba and Sato, 2004). Thus, alterations in these enzymes may mediate some changes in lipid profiles in both blood and brain tissues, and such decreases could result in corresponding decreases in blood fatty acid levels, as has been observed in relapsing cocaine addicts (Buydens-Branchey et al., 2003). Decreases in blood cholesterol levels have also been reported in patients that had relapsed to cocaine (Buydens-Branchey and Branchey, 2003), a finding that is also consistent with a decrease in lipid membrane remodeling activity. It remains to be seen if the specific phospholipids identified in our current study using this behavioral sensitization model for cocaine will similarly translate to the human condition, but it is notable that i.p. cocaine administration has previously been shown to reduce PLA2 activity in the rat (Ross and Turenne, 2002), as was mentioned above to occur in human cocaine users. Our results provide further evidence supporting the hypothesis that changes in lipid membrane remodeling and associated levels of these lipids and their metabolites are a consequence of cocaine exposure in both rats and humans.
The lipid isolation procedure used in this study certainly extracts more than just phospholipids; however, we chose to focus our effort on phospholipids due to their important role in the function of plasma membrane, and because the few studies that have assessed the effect of cocaine on the lipid profile in humans suggest that this class is altered. In total, over 1,000 different m/z values were evaluated, representing as many lipids, yet we only detected significant changes in approximately 10% of these lipids. This suggests that phospholipid changes induced by cocaine using this model are not systemic. A variety of other lipids could certainly originate from the metabolism of the phospholipids identified in this study, including fatty acids such as arachidonic acid. Future studies will focus on additional characterization of the specific phospholipids and the metabolites being altered, as well as further refinement of the identification of the specific brain regions where these changes are taking place.
A limitation to this study was that the lipids analyzed were compared based on relative abundance, as opposed to actual quantitation. Direct quantitation of lipids is a goal of future work, but would have been impractical in this current study due to the vast numbers of lipids species initially identified using a shotgun approach. Another limitation is the shotgun approach used in this study did not involve any separation of the lipids prior to analysis. As such, it’s possible that other lipid species may also be represented by a single m/z value, and that these species may be altering the ionization of other lipids. Finally, while the use of MS/MS allowed for identification of polar head groups, fatty acyl chain lengths and double bond numbers, it did not allow us to determine the positions of these double bonds. Future studies focusing on the specific lipids identified in this study are needed to address these limitations.
The above data demonstrate that cocaine conditioning can induce changes in the lipid profiles of brain tissues and blood. However, these data do not indicate if such changes are relevant to cocaine-induced neurological dysfunctions. We addressed this question by correlating changes in lipid abundance to cocaine-induced behavioral changes. We found excellent correlations between the abundance of several phospholipids and the initial response to and sensitization to cocaine, with some of these correlations being greater than 0.7. These observations support the hypothesis that these lipids could serve as novel biomarkers of cocaine exposure, as well as indicators of drug-induced neuroadaptations.
It is unclear as to how changes in blood phospholipids may be related to changes in the dopamine signaling pathways or remodeling that can accompany cocaine abuse. A recent study assessing neurological dysfunctions induced by Alzheimer’s Disease suggested that alterations in serum lipids may be result of changes in cell membrane integrity in the brain (Mapstone et al., 2014). Thus, cocaine-induced changes in neuronal plasticity may lead to plasma membrane remodeling and these changes may be subsequently reflected in the blood. As mentioned above, cocaine exposure in humans has been linked to changes in phospholipid metabolism enzymes, such as PLA2 in the brain (Ross et al., 1996). It is also possible that such changes are not limited to the brain itself and that changes in the blood reflect a global change in PLA2 activity. Further studies are needed to distinguish between these possibilities.
The timing with respect to when cocaine-induced changes in the lipidome are occurring was not a focus of the current study. The behavioral sensitization model was chosen because it has been previously demonstrated to induce neuroadaptations that persist beyond the acute effects the drug. The fact that lipids changes are present as long as one week after cocaine treatment suggests that such changes are persistent; however, it does not indicate if these changes occur after the initial dose of cocaine, are dependent on repeated conditioning exposures, or if they occur during or after the one week of abstinence. Future research will focus on further characterization of the lipid species identified in this study.
Although our study is not the first to demonstrate that repeated cocaine exposure alters the relative abundance of specific phospholipids in the blood, it is the first to show drug-induced changes in phospholipid abundance can also occur in specific regions of the rat brain. A study in mice reported by Shi et al. (2012) demonstrated that hepatotoxic doses of cocaine (2- to 3-fold higher than those used in our study) also induced lipid changes in the serum. Interestingly, several of the same lipids shown to be altered by Shi et al (2012) were also identified in our study, including 40:7 PC, 36:4 PC, 34:2 PC, 36:2 PC and 32:0 PC. In addition to species (mice vs. rats), reasons why not all the same lipids were identified include: the differing doses of cocaine used, the differing timelines for sample collection and that the analytical methods employed by Shi et al. are more accurate and sensitive. Regardless of these differences, the data from our current study further indicate that changes in the abundance of these phospholipids in the blood can be correlated to behavioral responses following cocaine treatment. The relevance of such findings are that these lipid profiles may be collectively used to identify past cocaine exposure and as biomarkers of behavioral sensitization. Finally, the data reported in this study represents one of the most comprehensive analyses of changes in rat brain phospholipids after exposure to cocaine that has been described to date. The general hypothesis that repeated exposure to cocaine can induce lipid remodeling and persisting changes in lipid abundance in both brain and blood is well supported.
Supplementary Material
Electrospray ionization-mass spectrometry assessed the lipidome of rat brain/blood
Cocaine conditioning induced brain region-specific changes in the lipidome
Persisting changes were also observed in the blood lipidome following cocaine exposure
Changes in the relative abundance of specific lipids were correlated to the initial locomotor response and the behavioral sensitization to cocaine
Acknowledgments
Role of funding source
This work was supported by the National Institutes of Health NIBIB EB008153 and EB011610 to BSC and NIDA DA16302 to JJW; a Sloan Graduate Minority Fellowship and an Interdisciplinary Toxicology Program Graduate Stipend to NES and by an International Visiting Scholar Award from Cumhuriyet University to SHB. These organizations had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
This research was funded in part by the NIH NIBIB (EB08153 and EB0116100) to BSC, by NIH NIDA (DA016302) to JJW, by a Sloane Graduate Minority Fellowship and an Interdisciplinary Toxicology Program Graduate Stipend to NES, and by an International Visiting Scholar Award from Cumhuriyet University to SHB.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Contributors
The authors of this paper designed the study (BSC & JJW), collected and oversaw collection of the data (NES, PM, JKC), completed the statistical analyses (SP, SS, POT), and wrote the manuscript (BSC & JJW).
Conflict of interest
The authors of this manuscript declare they have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiba S, Sato T. Cellular function of calcium-independent phospholipase A2. Biol Pharm Bull. 2004;27:1174–1178. doi: 10.1248/bpb.27.1174. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci USA. 2010;107:8452–8456. doi: 10.1073/pnas.1000496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buydens-Branchey L, Branchey M. Association between low plasma levels of cholesterol and relapse in cocaine addicts. Psychosom Med. 2003;65:86–91. doi: 10.1097/01.psy.0000039754.23250.ee. [DOI] [PubMed] [Google Scholar]
- Buydens-Branchey L, Branchey M, McMakin DL, Hibbeln JR. Polyunsaturated fatty acid status and relapse vulnerability in cocaine addicts. Psychiatry Res. 2003;120:29–35. doi: 10.1016/s0165-1781(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbo-Gas M, Vazquez-Sanroman D, Aguirre-Manzo L, Coria-Avila GA, Manzo J, Sanchis-Segura C, Miquel M. Involving the cerebellum in cocaine-induced memory: pattern of cFos expression in mice trained to acquire conditioned preference for cocaine. Addict Biol. 2014;19:61–76. doi: 10.1111/adb.12042. [DOI] [PubMed] [Google Scholar]
- Christensen JD, Kaufman MJ, Levin JM, Mendelson JH, Holman BL, Cohen BM, Renshaw PF. Abnormal cerebral metabolism in polydrug abusers during early withdrawal: a 31P MR spectroscopy study. Magn Reson Med. 1996;35:658–663. doi: 10.1002/mrm.1910350506. [DOI] [PubMed] [Google Scholar]
- Delvolve AM, Colsch B, Woods AS. Highlighting anatomical sub-structures in rat brain tissue using lipid imaging. Anal Methods. 2011;3:1729–1736. doi: 10.1039/C1AY05107E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Gosnell BA. Sucrose intake enhances behavioral sensitization produced by cocaine. Brain Res. 2005;1031:194–201. doi: 10.1016/j.brainres.2004.10.037. [DOI] [PubMed] [Google Scholar]
- Ikai Y, Takada M, Shinonaga Y, Mizuno N. Dopaminergic and non-dopaminergic neurons in the ventral tegmental area of the rat project, respectively, to the cerebellar cortex and deep cerebellar nuclei. Neuroscience. 1992;51:719–728. doi: 10.1016/0306-4522(92)90310-x. [DOI] [PubMed] [Google Scholar]
- Jackson SN, Wang HY, Woods AS. In situ structural characterization of phosphatidylcholines in brain tissue using MALDI-MS/MS. J Am Soc Mass Spectrom. 2005a;16:2052–2056. doi: 10.1016/j.jasms.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Jackson SN, Wang HY, Woods AS, Ugarov M, Egan T, Schultz JA. Direct tissue analysis of phospholipids in rat brain using MALDI-TOFMS and MALDI-ion mobility-TOFMS. J Am Soc Mass Spectrom. 2005b;16:133–138. doi: 10.1016/j.jasms.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Kinsey GR, Blum JL, Covington MD, Cummings BS, McHowat J, Schnellmann RG. Decreased iPLA2gamma expression induces lipid peroxidation, cell death, and sensitizes cells to oxidant-induced apoptosis. J Lipid Res. 2008;49:1477–1487. doi: 10.1194/jlr.M800030-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay S, Meyerhoff DJ, Dillon WP, Weiner MW, Fein G. Alteration of brain phospholipid metabolites in cocaine-dependent polysubstance abusers. Biol Psychiatry. 1993;34:261–264. doi: 10.1016/0006-3223(93)90080-w. [DOI] [PubMed] [Google Scholar]
- Mapstone M, Cheema AK, Fiandaca MS, Zhong X, Mhyre TR, MacArthur LH, Hall WJ, Fisher SG, Peterson DR, Haley JM, Nazar MD, Rich SA, Berlau DJ, Peltz CB, Tan MT, Kawas CH, Federoff HJ. Plasma phospholipids identify antecedent memory impairment in older adults. Nat Med. 2014;20:415–418. doi: 10.1038/nm.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikawa S, Suzuki M, Fujimoto C, Sato K. Imaging of phosphatidylcholines in the adult rat brain using MALDI-TOF MS. Neurosci Lett. 2009;451:45–49. doi: 10.1016/j.neulet.2008.12.035. [DOI] [PubMed] [Google Scholar]
- Miquel M, Toledo R, Garcia LI, Coria-Avila GA, Manzo J. Why should we keep the cerebellum in mind when thinking about addiction? Curr Drug Abuse Rev. 2009;2:26–40. doi: 10.2174/1874473710902010026. [DOI] [PubMed] [Google Scholar]
- Peterson B, Stovall K, Monian P, Franklin JL, Cummings BS. Alterations in phospholipid and fatty acid lipid profiles in primary neocortical cells during oxidant-induced cell injury. Chem Biol Interact. 2008;174:163–176. doi: 10.1016/j.cbi.2008.05.028. [DOI] [PubMed] [Google Scholar]
- Ross BM, Moszczynska A, Kalasinsky K, Kish SJ. Phospholipase A2 activity is selectively decreased in the striatum of chronic cocaine users. J Neurochem. 1996;67:2620–2623. doi: 10.1046/j.1471-4159.1996.67062620.x. [DOI] [PubMed] [Google Scholar]
- Ross BM, Moszczynska A, Peretti FJ, Adams V, Schmunk GA, Kalasinsky KS, Ang L, Mamalias N, Turenne SD, Kish SJ. Decreased activity of brain phospholipid metabolic enzymes in human users of cocaine and methamphetamine. Drug Alcohol Depend. 2002;67:73–79. doi: 10.1016/s0376-8716(02)00022-4. [DOI] [PubMed] [Google Scholar]
- Ross BM, Turenne SD. Chronic cocaine administration reduces phospholipase A(2) activity in rat brain striatum. Prostaglandins Leukot Essent Fatty Acids. 2002;66:479–483. doi: 10.1054/plef.2002.0385. [DOI] [PubMed] [Google Scholar]
- Seymour CM, Wagner JJ. Simultaneous expression of cocaine-induced behavioral sensitization and conditioned place preference in individual rats. Brain Res. 2008;1213:57–68. doi: 10.1016/j.brainres.2008.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Yao D, Gosnell BA, Chen C. Lipidomic profiling reveals protective function of fatty acid oxidation in cocaine-induced hepatotoxicity. J Lipid Res. 2012;53:2318–2330. doi: 10.1194/jlr.M027656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider RS, Maiti A, Snider SR. Cerebellar pathways to ventral midbrain and nigra. Exp Neurol. 1976;53:714–728. doi: 10.1016/0014-4886(76)90150-3. [DOI] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev. 2011;63:348–365. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. 2012 Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: [Google Scholar]
- Sun W, Rebec GV. Lidocaine inactivation of ventral subiculum attenuates cocaine-seeking behavior in rats. J Neurosci. 2003;23:10258–10264. doi: 10.1523/JNEUROSCI.23-32-10258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi R, Hayakawa J, Takeuchi Y, Ishida M. Two-dimensional analysis of phospholipids by capillary liquid chromatography/electrospray ionization mass spectrometry. J Mass Spectrom. 2000;35:953–966. doi: 10.1002/1096-9888(200008)35:8<953::AID-JMS23>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Veloso A, Astigarraga E, Barreda-Gomez G, Manuel I, Ferrer I, Giralt MT, Ochoa B, Fresnedo O, Rodriguez-Puertas R, Fernandez JA. Anatomical distribution of lipids in human brain cortex by imaging mass spectrometry. J Am Soc Mass Spectrom. 2011a;22:329–338. doi: 10.1007/s13361-010-0024-5. [DOI] [PubMed] [Google Scholar]
- Veloso A, Fernandez R, Astigarraga E, Barreda-Gomez G, Manuel I, Giralt MT, Ferrer I, Ochoa B, Rodriguez-Puertas R, Fernandez JA. Distribution of lipids in human brain. Anal Bioanal Chem. 2011b;401:89–101. doi: 10.1007/s00216-011-4882-x. [DOI] [PubMed] [Google Scholar]
- Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- Zhang L, Peterson BL, Cummings BS. The effect of inhibition of Ca2+-independent phospholipase A2 on chemotherapeutic-induced death and phospholipid profiles in renal cells. Biochem Pharmacol. 2005;70:1697–1706. doi: 10.1016/j.bcp.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Zhou X, Arthur G. Improved procedures for the determination of lipid phosphorus by malachite green. J Lipid Res. 1992;33:1233–1236. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.