Abstract
Background
Quantification of lactate dehydrogenase (LDH) release is a widely accepted assay for the quantitative determination of cell viability and late-stage apoptosis. Major disadvantages of commercially available LDH assay kits include proprietary formulations, limited options for optimization and high cost, all resulting in limited reproducibility in research applications.
New Method
Here, we describe a novel, custom LDH assay suitable in the context of plate reader-based screening of drug candidates for glioprotection, but with wide applicability to other cell types and experimental paradigms.
Results
We developed a novel and highly reproducible LDH release assay that is more cost-effective than commercially available assays with comparable performance. The assay was validated by assessing 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid antioxidant protection against tert-butylhydroperoxide-induced oxidative stress in C6 astroglioma cells. Assay performance was validated by direct comparison and compatible with other methods of measuring cellular viability, namely 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and 6-carboxy-2′, 7′ dichlorodihydrofluorescein diacetate assays.
Comparison with Existing Method(s)
There was no statistically significant difference between results obtained with the novel custom assay and a commercially available assay CytoTox96® (Promega, Madison, WI).
Conclusions
The novel custom LDH release assay allows the reproducible quantification of cell viability and is highly cost-effective when compared to commercially available assays (approximately 25 times cheaper). In addition and in contrast to commercially available assays, the identification and detailed description of all assay components and procedures provide greater control over experimental conditions and design. We provide a detailed standard operating procedure permitting our novel assay to be readily adapted depending on experimental requirements.
Keywords: Lactate dehydrogenase, glia, glioprotection, C6 glioma cell, high-throughput screening, cell viability, plate reader
Introduction
Cell viability assays have become a standard tool in neuroscience drug discovery to quantify the number of cells beginning to undergo apoptotic and necrotic processes, as well as to determine the neuroprotective or glioprotective potential of drug candidates (Stoddart, 2011; Vega-Avila and Pugsley, 2011). L-Lactate dehydrogenase is a cytoplasmic enzyme that catalyzes the interconversion of pyruvate to L-lactate with the concomitant interconversion of NADH to NAD+ during glycolysis, and catalyzes the reverse reactions during the Cori cycle (Decker and Lohmann-Matthes, 1988; Nachlas et al., 1960). LDH is released with the cytoplasm in response to cell damage or exogenous insults leading to damage of the plasma membrane and, ultimately, cell death. Given its stability in the extracellular environment and in particular in cell culture media, LDH release has been widely used to evaluate the presence of damage and toxicity in tissues and cells (Stoddart, 2011). Traditionally, LDH activity is determined by utilizing a coupled enzymatic reaction, where LDH oxidizes lactate to pyruvate, which subsequently reacts with the iodonitrotetrazolium chloride (INT) to form the colored formazan. Formazan is water-soluble and can be readily detected colorimetrically by measuring absorbance at 490 nm (Decker and Lohmann-Matthes, 1988). The assay relies on the assumption that the increase in the amount of formazan produced in the culture supernatant is directly correlated with cell viability.
Commercially available LDH assays have drawbacks such as proprietary formulation with unknown components and concentrations, thus limiting assay optimization or adaptation to changing experimental needs, and high cost.
The objective of the present study was to develop a reproducible and at the same time cost-effective LDH assay with known formulation. We have validated the assay for plate reader-based screening of drug candidates for glioprotection utilizing 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) as a prototypic glioprotectant against tert-butylhydroperoxide (tBHP)-induced oxidative stress in C6 astroglioma cells. We report here a low variance LDH assay with a reagent cost estimated to be about 25-fold lower than similarly performing commercial assays.
Furthermore, we established a standardized protocol combining multiple assays for cell viability and proliferation as well as for quantification of reactive oxygen species (ROS). To this end, we here report the successful combination of our custom LDH assay with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and the 6-carboxy-2′, 7′ dichlorodihydrofluorescein diacetate (DCFDA) assay, using the same sample.
Material and methods
Cell culture
C6 astroglioma cells were obtained under Material Transfer Agreement from the American Type Culture Collection (ATTC® CCL-107™; Manassas, VA) and cultured in F-12K medium (Cellgro®; Mediatech, Manassas, VA) supplemented with 15% horse serum (heat inactivated, New Zealand origin; Life Technologies, Carlsbad, CA) and 2.5% fetal bovine serum (Gibco® certified, heat inactivated, US origin; Life Technologies, Carlsbad, CA) at 37 °C/5% CO2/95% humidity in standard tissue culture flasks (TPP®; Midwest Scientific, MidSci, St. Louis, MO). For cell viability experiments, cells were seeded into clear, flat bottom 96 well plates (TPP®; Midwest Scientific, MidSci™, St. Louis, MO) at a density of 7,500 cells/well.
Induction of oxidative stress and drug treatment
In order to induce oxidative stress, cells were exposed to various concentrations of tBHP (Sigma Aldrich Corp., St. Louis, MO) 16–20 hr after seeding. For glioprotection, cells were pre-treated (1 hour) with the prototypic antioxidant Trolox (100 μM in 0.1% v/v ethanol; Sigma Aldrich Corp., St. Louis, MO) or ethanol vehicle (0.1%; Sigma Aldrich Corp., St. Louis, MO).
Custom LDH assay
Following 3 hr exposure to tBHP, 50 μL of cell culture supernatant were transferred into a non-sterile, clear 96-well multiwell plate (Nunc, Thermo Fisher Scientific, Waltham, MA). 50 μL Assay Buffer (2 mM iodonitrotetrazolium chloride, 3.2 mM β-nicotinamide adenine dinucleotide sodium salt, 160 mM lithium lactate, 15 μM 1-methoxyphenazine methosulfate in 0.2 M Tris-HCl, pH 8.2) were added. Plates were incubated at room temperature in the dark for 1 hr. The reaction was stopped by addition of 50 μL 1 M acetic acid. LDH release was quantified by measuring absorbance at 490 nm (A490) using a Synergy H1 plate reader (Biotek, Winooski, VT). Data were exported to Microsoft Excel (Microsoft Corp., Redmond, WA) for processing, normalized to the control condition (0 μM tBHP), and analyzed in Prism 5.0 (Graphpad, La Jolla, CA).
Stock solutions for the LDH Assay Buffer were prepared in advance and consisted of Buffer A (2x; 4 mM INT in 0.2 M Tris-HCl, pH 8.2), Buffer B (2×; 6.4 mM NAD, 320 mM lithium lactate in 0.2 M Tris-HCl buffer, pH 8.2), and MPMS supplement (10,000×; 150 mM MPMS in 0.2 M Tris-HCl buffer, pH 8.2). A single 96-well plate required 5 mL Assay Buffer (2.5 mL Buffer A, 2.5 mL Buffer B, 0.5 μL MPMS supplement). Solutions were aliquoted and stored frozen at −20 °C for up to one month. Assay Buffer was prepared and 50 μl was immediately applied to 50 μl samples. A detailed, step-by-step protocol and standard operating procedure is provided in Appendix A.
CytoTox96® assay
We benchmarked our new custom LDH assay against a commercially available LDH assay (CytoTox96®; Promega, Madison, WI). The assay was performed according to the manufacturer’s instructions.
Carboxy DCFDA assay
In order to quantify the level of oxidative stress we used the fluorescent ROS indicator 6-carboxy-2′, 7′ dichlorodihydrofluorescein diacetate (DCFDA) as described by us previously (Burroughs et al., 2012). Briefly, cells were loaded with 10 μM DCFDA in complete media for 30 min. Subsequently, cells were treated and oxidative stress was induced chemically as described above. At the end of the incubation period and after collection of supernatant samples for LDH release assays, cells were washed twice with 300 μL Hank’s Balanced Salt Solution (HBSS) supplemented with 2 mM CaCl2. Plates were read in a fluorimetric plate reader (Synergy H1 plate reader; Biotek) at 485/530 nm excitation/emission and data were acquired in Gen5 software (Biotek). Data were exported to Microsoft Excel (Microsoft Corp.) for processing, normalized to the control condition (0 μM tBHP), and analyzed in Prism 5.0 (Graphpad).
MTT assay
The MTT assay was performed essentially as described by us previously for HT-22 cells and primary cortical neuron culture (Burroughs et al., 2012; Kaja et al., 2011). Briefly, media was aspirated from the cells and replaced with 100 μl of 1.2 mM MTT in HBSS with calcium and magnesium (Lonza, Walkersville, MD) supplemented with 10 mM 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) at pH 7.3. Plates were incubated at 37° C for 2 hr. Media was aspirated and cells were lysed with 100 μl dimethylsulfoxide (DMSO) and gentle shaking. The conversion of MTT was quantified by measuring absorbance at 570 nm (A570) using a Synergy H1 plate reader (Biotek). Absorbance values were corrected for background, normalized to the control condition (0 μM tBHP / no chemically induced oxidative stress) and analyzed in Prism 5.0 (Graphpad).
Data analysis
Data for all assays were acquired in Gen5 software (Biotek) and exported to Microsoft Excel (Microsoft Corp.) for processing. Data was normalized to the untreated, control condition (0 μM tBHP). Outlier exclusion was applied to data points (single wells) for each insult (tBHP concentration; column of 8 wells). We assumed a Gaussian distribution and excluded any data point 2 standard deviations from the columnar mean. Normalized data were grouped by treatment condition and exported to Prism software (version 5.0; Graphpad) for statistical analysis and plotting. Non-linear regression using a four-parameter logistic equation with variable Hill slope was performed separately for each biological replicate to determine the mean LD50 values for tBHP under each pre-treatment condition (control, vehicle, and Trolox).
Data was analyzed statistically using 2-Way analysis of variance (ANOVA) and the Bonferroni post-hoc test, with pre-treatment condition (control, vehicle, or Trolox) and insult (tBHP concentration) as variables. Statistical significance was defined as P < 0.05.
Results
Optimization of a custom LDH assay to measure cellular viability, effects of chemically induced oxidative stress, and glioprotection in C6 astroglioma cells
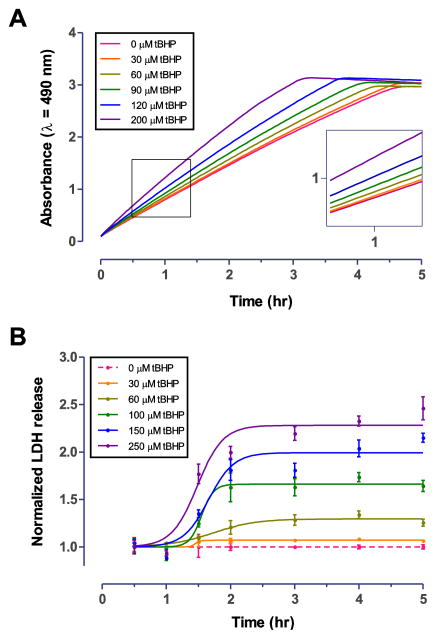
We first determined the optimal LDH assay incubation time using a time course measurement. C6 cells were treated with varying concentrations of tBHP for 3 hr, at which time death of all cells in a given well was observed visually for the group treated with 200 μM tBHP. Following the addition of LDH Assay Buffer to the cell supernatant, the reaction was allowed to proceed for 5 hr while the A490 absorbance was recorded every 2 minutes (Fig. 1A). The absorbance was linear for the first 3 hr of the reaction (Table 1) and the signal plateaued at approximately 3 hr (Fig. 1A) in the group treated with the highest tested concentration of tBHP. Based on this result, we selected the 1 hr time point for the further validation of our custom LDH assay because it provided an adequate dynamic range well below the absorbance maximum.
Figure 1. Time course of the custom LDH assay and optimization of tBHP insult in C6 astroglioma cells.
A. C6 cells were incubated with tBHP for 3 hr, prior to LDH assay. After addition of the substrate, the reaction was allowed to proceed for 5 hr while monitoring A490 absorbance. Increased absorbance is indicative of elevated LDH release in response to oxidative damage by tBHP. The LDH assay showed a linear response for the first 3 hr (Table 1) and started to saturate at approximately 3 hr at higher concentrations of tBHP. We selected the 1 hour time point for further validation of our custom LDH assay. B. We next determined the optimal incubation time of tBHP for C6 astroglioma cells. Separate 96-well plates of C6 cells (n=3 per time point) were incubated with tBHP for 30 min to 5 hr and LDH assay was performed. Maximum LDH release was observed at approximately 2.5 hr. For subsequent experiments, 3 hr incubation with tBHP was chosen.
Table 1.
Linearity of the LDH assay
| tBHP (μM) | Slope | R2 | y-intercept at x = 0 | n (x), n (y) |
|---|---|---|---|---|
| 0 | 0.6634 ± 0.0013 | 0.9996 | 0.1275 ± 0.0023 | 91, 8 |
| 30 | 0.6769 ± 0.0014 | 0.9996 | 0.1317 ± 0.0025 | 91, 8 |
| 60 | 0.7129 ± 0.0017 | 0.9995 | 0.1369 ± 0.0030 | 91, 8 |
| 90 | 0.7634 ± 0.0022 | 0.9993 | 0.1464 ± 0.0038 | 91, 8 |
| 120 | 0.8417 ± 0.0028 | 0.9990 | 0.1604 ± 0.0049 | 91, 8 |
| 200 | 0.9848 ± 0.0058 | 0.9969 | 0.2064 ± 0.0100 | 91, 8 |
The linearity of the LDH assay was determined by linear regression analysis of the A490 absorbance values obtained during the first three hours of the assay at measurement intervals of 2 min. The assay showed a highly linear response (R2 > 0.99) throughout all conditions.
Different cell types respond differently to tBHP-induced oxidative stress. Therefore, it is necessary to determine the optimal treatment duration for each cell type and desired level of oxidative stress. While brief exposure may result in only small detectable differences in LDH release, extended exposure may result in data confounded by LDH degradation (Kendig and Tarloff, 2007). We determined the time course of tBHP-induced oxidative stress in C6 cells with a series of plates similarly treated with tBHP at t = 0. Groups of treated plates were stopped every 30 minutes for the first two hr and hourly thereafter and assayed for LDH release. In C6 cells, maximum LDH release was observed at approximately 2.5 hr, with no additional release detectable for up to 5 hrs (Fig. 1B). For subsequent experiments, 3 hr of incubation with tBHP was chosen.
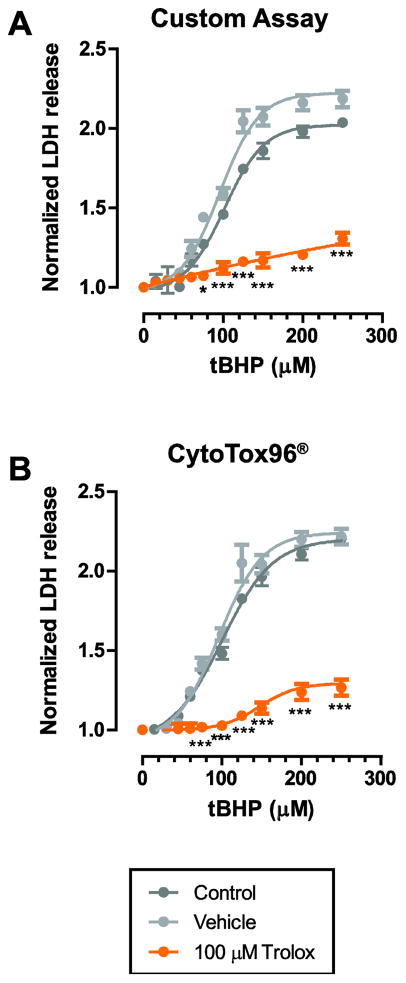
The custom LDH assay performs similar to commercially available assays
Performance of our optimized custom LDH assay was tested against the commercially available CytoTox96® assay (Promega, Madison, WI). C6 cells were treated with either 100 μM Trolox, vehicle (0.1% v/v ethanol) or media one hour prior to tBHP insult.
Trolox was highly glioprotective and significantly reduced LDH release compared to vehicle, as assessed by 2-Way ANOVA with Bonferroni post-hoc test (Fig. 2A). The LD50 for tBHP was shifted from 99 ± 1 μM to 169 ± 18 μM (n=3, P<0.01 compared to vehicle and control) in response to Trolox treatment (Table 2). Furthermore, there was no significant effect of the vehicle (0.1% v/v ethanol) on LDH release (n=3, P=0.44; Fig. 2A; Table 2).
Figure 2. The custom LDH assay performed similar to the commercial CytoTox96® assay by Promega.
A. C6 cells were treated with either 100 μM Trolox, vehicle (0.1% v/v ethanol), or media control one hour prior to tBHP insult. Cells were incubated with tBHP for 3 hr prior to LDH assay. Trolox was highly glioprotective and reduced LDH release significantly compared to vehicle, as assessed by 2-Way ANOVA. There was no significant effect of the vehicle on LDH release. Data was fitted using a 4-Parameter Logistic nonlinear regression model. Trolox shifted the LD50 rightwards by approximately 70 μM tBHP. LD50 values were calculated for each dataset and the summary statistics are provided in Table 2. B. Similar results were obtained using the commercial CytoTox96® assay by Promega. Data is presented as mean ± s.e.m. from 3 separate experiments for each condition. * P<0.05; *** P<0.001 compared to both control and vehicle conditions.
Table 2.
Validation of the custom LDH release assay by comparing the efficacy of Trolox against tBHP-induced oxidative stress.
| LD50 (μM) - Custom Assay | LD50 (μM) - Promega | P (n) | |
|---|---|---|---|
| Control | 99 ± 1 | 97 ± 4 | 0.762 (3) |
| Vehicle | 93 ± 6 | 96 ± 1 | 0.716 (3) |
| 100 μM Trolox | 169 ± 18 **/## | 171 ± 37 */# | 0.971 (3) |
In order to validate the custom LDH assay, we tested the glioprotective effect of Trolox against tBHP-induced oxidative stress in C6 astroglia cells. LD50 values were determined by fitting normalized LDH release. Trolox was protective against oxidative stress-induced damage and increased the LD50 for tBHP significantly compared to both control and vehicle (0.1% v/v ethanol) conditions. There was no significant difference between the LD50 values determined by the custom assay compared with the commercially available Promega kit.
P<0.05,
P<0.01 compared to control condition;
P<0.05,
P<0.01 compared to vehicle condition.
Importantly, we did not identify a statistically significant difference between LDH release values obtained using our custom LDH assay or the CytoTox96® assay (Fig. 2B; Table 2).
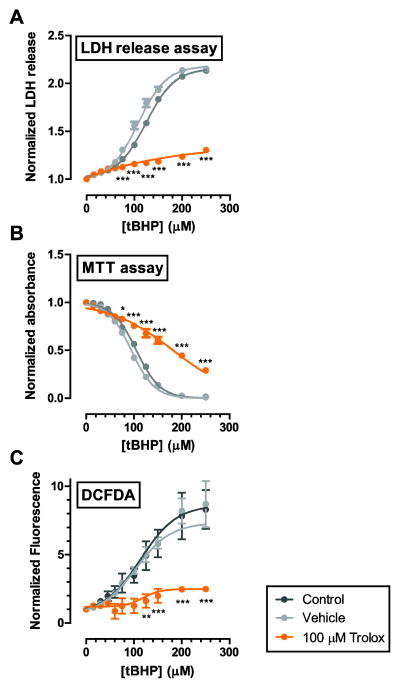
The LDH release assay can be combined with cellular assays quantifying intracellular ROS content and cell viability
In a separate experiment, we aimed at establishing a protocol that allows the quantification of intracellular ROS concentrations and testing of cell viability in a single experiment and on the same cells. For this, we passively loaded C6 cells with the fluorescent ROS indicator, DCFDA, prior to insult as described above. We then performed the custom LDH assay on the cell supernatant and measured fluorescence in the adherent cells prior to exposing the cells to MTT.
In this experimental setup, the custom LDH assay yielded a reliable quantification of LDH release (Fig. 3A). The LD50 values were not significantly different from those determined in our earlier experiments described above (Fig. 2), indicating that DCFDA loading had no significant effect on LDH release. LD50 values obtained from the MTT assay were similar to those obtained from the LDH assay (105 μM, 95 μM and 180 μM for control, vehicle and Trolox, respectively; Fig. 3B). The MTT results were also not affected by prior DCFDA loading (data not shown). DCFDA fluorescence was significantly increaseds at tBHP concentrations equal to or greater than 150 μM indicating elevated ROS concentrations. Statistically significant increases in DCFDA fluorescence were not detected in Trolox-treated cells.
Figure 3. The custom LDH assay was successfully combined with cellular assays quantifying cell viability and ROS generation.
A. In a separate experiment, we tested the functionality of the custom LDH assay in combination with the MTT assay for cell viability and quantification of ROS by the DCFDA assay. The custom assay yielded reliable quantification of LDH release. The LD50 values were not significantly different from those determined in our earlier experiments, presented in Fig. 2A. B. MTT assay confirms the results from the LDH assay. Trolox shifted the LD50 for tBHP rightward by approximately 70 μM. LD50 values obtained from MTT assay were similar to those obtained from the LDH assay (105 μM, 95 μM and 180 μM for control, vehicle and Trolox, respectively). C. Cell were loaded with carboxy-DCFDA-AM to quantify the levels of ROS in C6 cells upon exposure to tBHP. Trolox reduced ROS generation significantly. Data is presented as mean ± s.e.m. from 3 separate experiments for each condition and was fitted to a 4-parameter logistic nonlinear regression model. * P<0.05; ** P<0.01, *** P<0.001 compared to both control and vehicle conditions.
Discussion
We have developed a custom LDH release assay and validated the assay in C6 astroglioma cells exposed to exogenously-applied chemically induced oxidative stress and cell death. Our assay has three major advantages over commercial assays: 1.) cost effectiveness, 2.) optimization potential, and 3.) scalability.
Firstly, the assay presented here is significantly more cost effective than commercially available assays. We estimate that the assay provides an approximately 30–40 fold cost saving in reagent costs over commercially available assays. The labor cost for preparing stock solutions is minimal, ultimately resulting in an approximate 25-fold cost-saving over commercially available assays for LDH release.
Secondly, the components of our LDH assay and their concentrations are known, providing greater potential for optimization as well as an assessment of the potential of certain chemicals to interfere with the assay. It is well established that several chemicals inactivate LDH enzyme activity (Kendig and Tarloff, 2007), calling for careful experimental design and well-designed experimental controls. Our assay provides increased optimization potential due to its known components and their concentrations. For instance, the assay can be performed using either sodium or lithium lactate salts, which does not affect results under standard experimental conditions (data not shown).
Thirdly, our assay is fully scalable. Stock solutions can be prepared for up to thousands of plates eliminating lot to lot variability. Solutions are stable frozen at −20 °C for up to one year without loss of activity (data not shown).
We herein present a detailed protocol for a custom LDH release assay for the quantification of cell viability. Our assay has been validated in C6 astroglioma cells treated with tBHP against a commercially available LDH release assay, CytoTox96® by Promega. In contrast to commercially available assays, our custom LDH is highly cost-effective (approximately 25 times cheaper). More importantly, the assay’s known components and concentrations provide greater control over assay conditions and experimental design.
Highlights.
The novel LDH release assay is more reproducible than commercially available assays.
The novel LDH release assay is more cost-effective than commercial assays.
The assay has the same performance characteristics as commercially available assays.
Its modular design can readily combined with standard assays of cellular viability.
Acknowledgments
Research reported in this publication was supported by grants from the National Eye Institute (EY014227 and EY022774), the Institute on Aging (AG010485, AG022550 and AG027956), the National Center for Research Resources and National Institute of General Medical Sciences (RR022570 and RR027093) of the National Institutes of Health (PK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support by the Felix and Carmen Sabates Missouri Endowed Chair in Vision Research, a Challenge Grant from Research to Prevent Blindness and the Vision Research Foundation of Kansas City is gratefully acknowledged. The authors thank Margaret, Richard and Sara Koulen for generous support and encouragement.
Abbreviations
- ANOVA
analysis of variance
- DCFDA
6-carboxy-2′, 7′ dichlorodihydrofluorescein diacetate
- EtOH
ethanol
- HEPES
4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid
- INT
iodonitrotetrazolium chloride
- LDH
lactate dehydrogenase
- MPMS
1-methoxyphenazine methosulfate
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NAD
beta-nicotinamide adenine dinucleotide sodium salt
- PBS
phosphate-buffered saline, ROS, reactive oxygen species
- tBHP
tert-butylhydroperoxide
- Trolox
6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid
Appendix: Standard Operating Procedure for the custom LDH assay
Reagents
| Compound | CAS number | Catalog number | Supplier |
|---|---|---|---|
| Acetic Acid (glacial) | 64-19-7 | AX0074-6 | Sigma Aldrich Corp., St. Louis, MO |
| 1-Methoxyphenazine methosulfate (MPMS) | 65162-13-2 | M8640 | Sigma Aldrich Corp., St. Louis, MO |
| Iodonitrotetrazolium Chloride (INT) | 146-68-9 | I10406-5G | Sigma Aldrich Corp., St. Louis, MO |
| Beta-Nicotinamide Adenine Dinucleotide Sodium Salt (NAD) | 20111-18-6 | N0632-5G | Sigma Aldrich Corp., St. Louis, MO |
| Lithium L-lactate | 27848-80-2 | L2250-100G | Sigma Aldrich Corp., St. Louis, MO |
| Tris Base (2-Amino-2-(hydroxymethyl)-1,3-propanediol) | 77-86-1 | 252859 | Sigma Aldrich Corp., St. Louis, MO |
| Nunc™ MicroWell F96 Plates | N/A | 12565363 | Fisher Scientific, Waltham, MA |
Solutions and preparation
The volumes of reagents are scalable to users’ needs. Aliquots of 13.5 ml of Buffers A and B are sufficient for 5 plates. The amounts listed below assume preparations scaled for a total of 100 plates. Assay reagents are stable when frozen (−20 °C). In order to prevent a non-specific reaction between the INT dye and MPMS or NAD, store Buffers A and B separately and only prepare the Assay Reagent immediately prior to use.
-
1 M Acetic acid in water
-
57.5ml acetic acid (glacial), water to 1 L.
Filter if necessary. Store at RT or refrigerated.
-
-
0.2 M Tris-HCl, pH 8.2
-
24.2 g Tris Base, 800 ml ultrapure water, adjust to pH 8.2 with HCl, then add ultrapure water to 1L.
Filter to remove particulates. Store refrigerated.
-
-
Buffer A (4mM INT in 0.2 M Tris-HCl, pH 8.2)
Dissolve 0.5057 g INT in 250 ml 0.2 M Tris-HCl, pH 8.2
Mix thoroughly and filter to remove any undissolved particles; some fine, sandy precipitate may not go into solution. Solution should be clear to slightly yellow. (Note: Heating or extensive time spent mixing (>15 minutes) can lead to the formation of a black precipitate. In that case, dispose of the solution and restart.)
Aliquot in 13.5 ml aliquots in 15 ml conical tubes. Store frozen (−20°C).
-
Buffer B (6.4 mM NAD, 320 mM lithium lactate, in 0.2 M Tris-HCl buffer)
Dissolve 1.1 g NAD and 7.7 g lithium lactate in 250 ml 0.2 M Tris-HCl, pH 8.2
Mix thoroughly and filter to remove any undissolved particles. Solution is clear when prepared but will discolor to light yellow after a few days.
Aliquot in 13.5ml in 15 ml conical tubes. Store frozen (−20°C).
-
MPMS Supplement (150 mM MPMS in Tris buffer)
Dissolve 100 mg MPMS in 1.98 ml 0.2 M Tris-HCl, pH 8.2
Do not filter. MPMS Supplement is a dark purple or brown solution. (Note: The solution may occasionally appear grainy, but this does not affect assay quality if the supplement is thoroughly mixed prior to addition to the Assay Reagent.)
Aliquot in 5–6 μl aliquots and store frozen (−20°C).
Procedure: LDH Release (LDHr) assay
-
Seed cells in 96 well plates, the ‘Sample Plate’. Allow sufficient time for cells to adhere and achieve proper confluency (usually 24 hr but cell count and incubation time is cell type dependent).
Cells should be seeded in volumes of 100 μl.
Include at least one control column, we recommend columns 1 and 2 be used for untreated or vehicle controls.
-
Apply drug/insult treatment(s)
Drug and/or insult are most often applied as 50–100 μl/well to ensure even mixing.
Sample plates should have a final volume of at least 150 μl/well.
After treatment time has elapsed, remove 50 μl from each well of the Sample Plate and transfer to a new clear, untreated 96-well multiwell plate (the ‘Assay Plate’).
Prepare the Assay Reagent by combining equal volumes of Buffer A (2.5 ml) and Buffer B (2.5 ml), and 0.5 μl MPMS supplement per plate. Mix thoroughly to a homogenous light pink or crimson color. Proceed quickly to the next step. (Note: The Assay Reagent may change color if left on the bench top.)
Add 50 μl Assay Reagent to each well on the Assay Plate. Mix briefly on an orbital shaker(300–500 rpm for 15 sec).
-
Incubate plate in the dark for 60 min at room temperature.
Note: Assay incubation time is directly related to the degree of color change. The LDH assay is a kinetic assay, users are advised to optimize the incubation time for the cell type to compensate for differing LDH concentrations within the sample. Our results suggest 1 hour is sufficiently below saturation to yield excellent separation between experimental wells. Do not incubate for less than 30 minutes.
-
Add 50 μl 1M acetic acid to each well to stop the reaction and stabilize the product. Mix briefly on an orbital shaker (300–500 rpm for 15 sec).
Note: Excess addition of acid must be avoided. The reduced dye (purple) can spontaneously oxidize back to the initial reactant (light yellow) at pH < 4.0
Record absorbance at 490 nm in a plate reader.
Normalize absorbance data to the control condition.
Footnotes
Disclosure
The authors declare no conflicts of interest. All authors have approved the final version of the manuscript.
Author contributions
Study conception and design: Kaja, Payne, Singh, Ghuman, Sieck, Koulen
Acquisition of data: Kaja, Payne, Singh, Ghuman, Sieck, Koulen
Analysis and interpretation of data: Kaja, Payne, Singh, Ghuman, Sieck, Koulen
Drafting of manuscript: Kaja, Payne, Singh, Ghuman, Sieck, Koulen
Critical revision: Kaja, Payne, Singh, Ghuman, Sieck, Koulen
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Burroughs SL, Duncan RS, Rayudu P, Kandula P, Payne AJ, Clark JL, Koulen P, Kaja S. Plate reader-based assays for measuring cell viability, neuroprotection and calcium in primary neuronal cultures. J Neurosci Methods. 2012;203:141–5. doi: 10.1016/j.jneumeth.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker T, Lohmann-Matthes ML. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J Immunol Methods. 1988;115:61–9. doi: 10.1016/0022-1759(88)90310-9. [DOI] [PubMed] [Google Scholar]
- Kaja S, Duncan RS, Longoria S, Hilgenberg JD, Payne AJ, Desai NM, Parikh RA, Burroughs SL, Gregg EV, Goad DL, Koulen P. Novel mechanism of increased Ca2+ release following oxidative stress in neuronal cells involves type 2 inositol-1,4,5-trisphosphate receptors. Neuroscience. 2011;175:281–91. doi: 10.1016/j.neuroscience.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendig DM, Tarloff JB. Inactivation of lactate dehydrogenase by several chemicals: implications for in vitro toxicology studies. Toxicology in vitro : an international journal published in association with BIBRA. 2007;21:125–32. doi: 10.1016/j.tiv.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachlas MM, Margulies SI, Goldberg JD, Seligman AM. The determination of lactic dehydrogenase with a tetrazolium salt. Anal Biochem. 1960;1:317–26. doi: 10.1016/0003-2697(60)90029-4. [DOI] [PubMed] [Google Scholar]
- Stoddart MJ. Cell Viability Assays: Introduction. In: Stoddart MJ, editor. Mammalian Cell Viability: Methods and Protocols. Humana Press; New York City: 2011. pp. i–xi. [Google Scholar]
- Vega-Avila E, Pugsley MK. An overview of colorimetric assay methods used to assess survival or proliferation of mammalian cells. Proc West Pharmacol Soc. 2011;54:10–4. [PubMed] [Google Scholar]