SUMMARY
Maintaining genomic integrity is of paramount importance to embryonic stem cells (ESCs), as mutations are readily propagated to daughter cells. ESCs display hypersensitivity to DNA damage-induced apoptosis (DIA) to prevent such propagation, although the molecular mechanisms underlying this apoptotic response are unclear. Here, we report the regulatory RNA Apela positively regulates p53-mediated DIA. Apela is highly expressed in mouse ESCs and is repressed by p53 activation, and Apela depletion compromises p53-dependent DIA. Although Apela contains a coding region, this coding ability is dispensable for Apela’s role in p53-mediated DIA. Instead, Apela functions as a regulatory RNA and interacts with hnRNPL, which prevents the mitochondrial localization and activation of p53. Together, these results describe a tri-element negative feedback loop composed of p53, Apela, and hnRNPL that regulates p53-mediated DIA, and further demonstrate that regulatory RNAs add a layer of complexity to the apoptotic response of ESCs following DNA damage.
Keywords: embryonic stem cells, apoptosis, p53, regulatory RNA, hnRNPL, epigenetics
INTRODUCTION
Embryonic stem cells (ESCs) have a unique surveillance system for maintaining genome stability. Compared to differentiated cells, ESCs are easy to undergo apoptosis and differentiation upon DNA damage (Hong and Stambrook, 2004; Liu et al., 2013), probably minimizing the risk of genome instability by removing cells with damaged DNA from the population and maintaining a population with low DNA mutation burden (Cervantes et al., 2002). Indeed, the mutation frequency of ESC population is about 100 times lower than that of differentiated cells (Cervantes et al., 2002). We and others have previously shown that p53 plays important roles in promoting the differentiation of mouse ES cells (mESCs) by repressing Nanog and other master regulators (Li et al., 2012; Lin et al., 2005). However, our understanding of the roles of p53 in DNA damage-induced apoptosis (DIA) of ESCs is incomplete, mainly because the mechanisms and the in vivo support are lacking. On the one hand, p53 is not required for the self-renewal of mESCs since mouse embryos with p53 knockout bypass the ESC stage (Donehower et al., 1992). On the other hand, p53 may have a pro-apoptotic function in mESCs based on a recent in vivo study showing that activated p53 is capable of regulating apoptotic genes in blastocysts (Goh et al., 2012). Given that mESCs have their unique transcriptional program and epigenome (Young, 2011), it is possible that the p53 signaling pathway regulates DIA of ESCs by using part of the ESC-specific transcriptome. Therefore, identifying ESC-specific part of the p53 transcriptional program will provide insights into how p53 regulates DIA of mESCs.
The observation that p53 activates Puma, a pro-apoptotic gene, in blastocysts leads us to investigate whether p53 regulates DIA of mESCs (Goh et al., 2012). After establishing that p53 is required for DIA of mESCs, we employ an integrative genome-wide approach to identify p53 targets that may mediate p53 function in DIA of mESCs. We focus on Apela (Apelin receptor early endogenous ligand) that encodes a putative peptide and find that Apela is involved in p53-mediated DIA of mESCs. Unexpectedly, the coding ability of Apela is dispensable for its pro-apoptotic function in mESCs. We further show that Apela acts as a regulatory RNA that modulates p53 activity by interacting with heterogeneous nuclear ribonucleoprotein L (hnRNPL), an inhibitory regulator of p53. Our results reveal a regulatory RNA-mediated negative feedback loop that regulates p53-mediated DIA of mESCs, and demonstrate that regulatory RNAs are part of p53 signaling in mESCs.
RESULTS
p53 Regulates DIA of mESCs
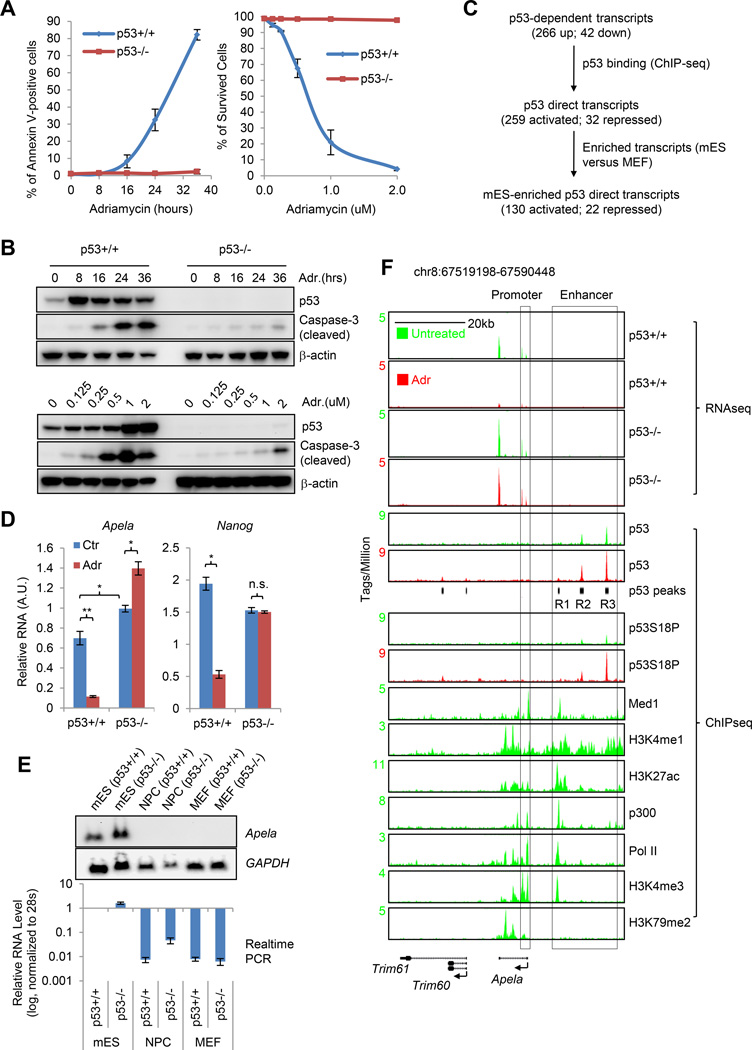
To test whether DIA of mESCs is p53-dependent, we first measured the kinetics of apoptosis in p53+/+ and p53−/− mESCs in response to DNA damage using Annexin V staining (Figure 1A, left panel and Figure S1A). We found that p53 plays a prominent role in regulating DIA of ESCs: around 80% of p53+/+ mESCs became apoptotic 36 hours after the treatment of 0.5 µM Adriamycin (ADR) while less than 5% of p53−/− mESCs were apoptotic (Figure 1A, left panel and Figure S1A). To rule out the dose-specific effect, we treated both p53+/+ and p53−/− mESCs with different doses of ADR for 24 hours and observed p53-dependent DIA at all the tested doses (Figure 1A, right panel). However, the difference of the percentage of apoptotic cells between p53+/+ and p53−/− mESCs is dependent on the dose of ADR (Figure 1A). Using cleaved caspase-3 as another apoptosis marker, we confirmed that p53 regulates DIA of mESCs and apoptosis increased as early as 8 hours after DNA damage (Figure 1B). Our previous study showed that p53+/+ and p53−/− mESCs have similar cell cycle profiles before and after ADR treatment (Li et al., 2012), excluding the contribution of cell cycle arrest to p53-mediated DIA of mESCs.
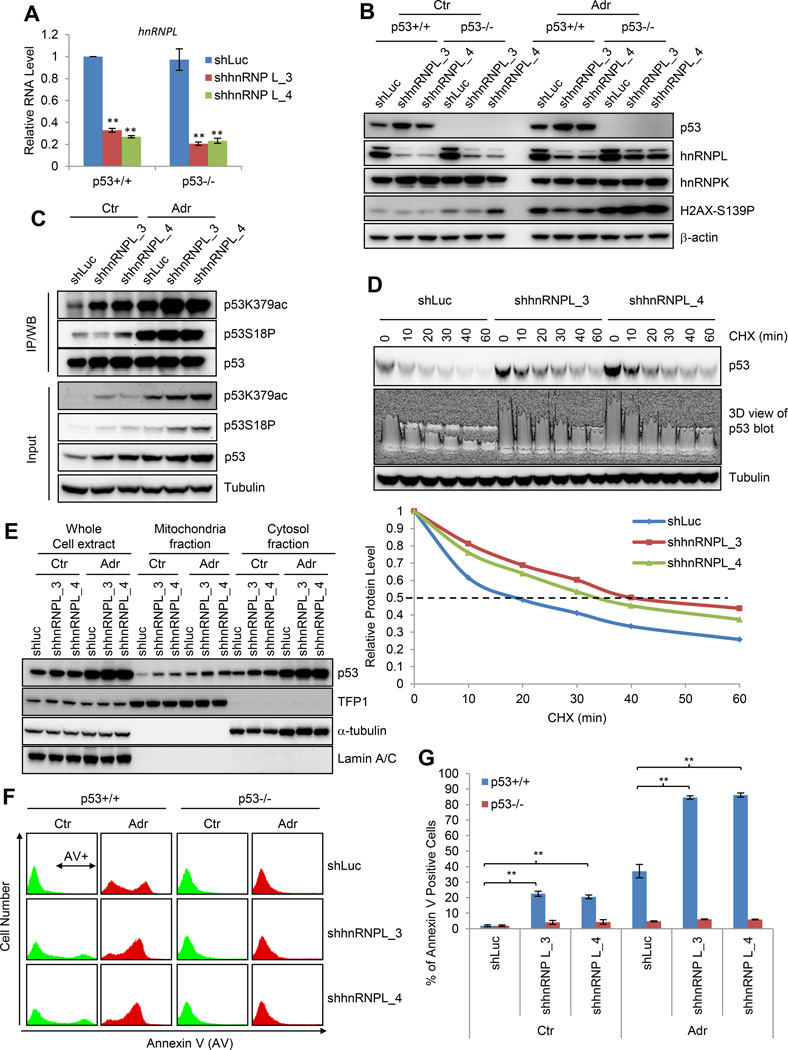
Figure 1. Apela Is Repressed by p53 and Enriched in mESCs.
(A) Left, Annexin V staining to measure the percentage of apoptotic cells. Right, the percentage of survived cells treated with different concentrations of ADR for 24 hours. Error bars are SEM; n=3. (B) Western blot (W.B.) of mESCs treated with 0.5 µM ADR for various time (upper panels) and with various concentrations of ADR for 24 hours (lower panels). (C) Identifying mESC-enriched p53 direct targets upon DNA damage. (D) Real-time PCR to measure the levels of Apela and Nanog (control). Ctr, untreated; Adr, treated with 0.5 µM ADR for 8 hours. A.U., arbitrary unit. Error bars are SEM; n=3. T-test; *, p<0.05; **, p<0.01. (E) Upper, Northern blot with total RNA; lower, real-time PCR. (F) RNA-seq and ChIP-seq on the Apela locus. Black bars underneath the p53 (Adr) view are identified p53 peaks; R1-3, Region 1-3. Promoter and enhancer are annotated using histone modifications, Med1 and Pol II.
Apela Is Repressed by p53 and Highly Expressed in mESCs
We attempted to identify p53 downstream targets that may be involved in p53-mediated DIA of mESCs. For this, we used a genome-wide approach by integrating RNA-seq and ChIP-seq (Li et al., 2012) (Figure 1C). Using RNA-seq data from p53+/+ and p53−/− mESCs that were either untreated or treated with ADR, we identified 266 up-regulated and 42 down-regulated p53-dependent transcripts in response to DNA damage (Figure 1C and Table S1). After integrating with p53 ChIP-seq data, we cataloged 259 up-regulated and 32 down-regulated p53 direct targets (Figure 1C), which were defined as transcripts with expression change in a p53-dependent manner and being associated with at least one p53 binding site (Table S1). Among the enriched pathways in these targets, regulation of cell death (p=0.0031), apoptosis (p=0.0088) and programmed cell death (p=0.0092) pathways are relevant to the apoptotic function of p53 in mESCs (Table S1). Targets enriched in mESCs are more likely to modulate p53’s mESC-specific function. Therefore, we excluded cell type-nonspecific p53 targets by comparing the expression levels of all transcripts in mESCs to those in mouse embryonic fibroblasts (MEFs) (Figure 1C, also see Supplemental Experimental Procedures). This analysis resulted in 130 p53-activated and 22 p53-repressed transcripts that are enriched in mESCs (Table S1 and Table S2). We were interested in p53-repressed transcripts because p53-repressed transcripts are more likely to play ESC-specific functions than that of p53-activated transcripts and their regulation is relatively less well understood (Li et al., 2012; Zhang et al., 2013). Among the 22 p53-repressed, ESC-enriched transcripts, several transcripts, such as Snora15, Dnmt3b, Prdm14, Lef1, Foxd3, Notch4, Cdk6 and Hmga1, have been shown to play critical roles in the regulation of self-renewal and differentiation of ESCs (Table S2). We decided to focus on Apela (also called Gm10664), which had been annotated as a long non-coding RNA in the NCBI database. However, its zebrafish homologue has recently been shown to encode a secretory peptide called Toddler (Pauli et al., 2014) or ELABELA (Chng et al., 2013), which regulates the cell movement during gastrulation. Hereinafter, we used the latest official name in NCBI, Apela, to refer to this RNA species. Because our previous study had shown that p53 has a non-cell autonomous function in mESCs (Lee et al., 2010), our initial excitement was that we had discovered a secretory signaling peptide, Apela, which is part of the cell-non-autonomous function of p53 in mESCs.
Using real-time PCR, we validated that Apela was repressed by DNA damage in a p53-dependent manner (Figure 1D and Figure S1B). In addition, in the absence of extrinsic stress, Apela was significantly higher in p53−/− mESCs than that in p53+/+ mESCs (Figure 1D, compare blue bars in the left panel), suggesting that p53 actively represses Apela even without extrinsic DNA damage. Both Northern blot and real-time PCR verified that Apela was highly expressed in mESCs but not in neural progenitor cells (NPCs) and MEFs (Figure 1E). Specifically, the levels of Apela were about 100 times higher in mESCs than in NPCs and in MEFs (Figure 1E). The expression levels of Apela were less than those of Pou5f1 (also called Oct4) and Nanog mRNAs but were comparable to those of Tfcp2l1 and Klf4 mRNAs, which also encode ESC transcription factors (Figure S1C) (Chen et al., 2008). Northern blot analysis and shRNA-mediated knockdown demonstrated that Apela had only one splicing variant in mESCs and the size of Apela in mESCs was the same as in vitro transcribed Apela (Figure S1D–E).
p53 Binds to the Enhancer Region of the Apela Locus
Next, we inspected our mRNA-seq and public RNA-seq data and found that Apela is spliced in mESCs as annotated in NCBI (Figure 1F and Figure S1F). Using ChIP-seq datasets of RNA polymerase II (Pol II), mediator (Med1), and epigenetic marks, we depicted a precise transcriptional unit, including the enhancer domain, the promoter, and the gene body, for Apela gene (Figure 1F). H3K4me3 marks the promoter while H3K79me2 the gene body (Berger, 2007; Heintzman et al., 2007; Rahl et al., 2010). H3K4me1, H3K27ac, Med1, and p300 together indicated that Apela gene has a distal enhancer domain in mESCs (Figure 1F) (Berger, 2007; Heintzman et al., 2007; Kagey et al., 2010). p53 did not bind to the promoter of Apela gene. Instead, it had three binding regions (R1–3) within the enhancer domain of Apela gene (Figure 1F), which is consistent with our previous finding that enhancer interference is one of the mechanisms of p53-mediated transcriptional repression in mESCs upon DNA damage (Li et al., 2012). The weak binding region (R1) overlapped with strong signal of Med1, H3K4me1, H3K27ac, p300, Pol II and H3K4me3 while the medium binding region (R2) overlapped with weak signal of Med1, H3K4me1, H3K27ac, p300 and Pol II, suggesting a reverse correlation of the signals of p53 and the enhancer markers (also see Supplemental Information). This annotated enhancer domain was also bound by many master transcription factors of mESCs, such as Nanog, Sox2, Oct4, etc. (Figure S1G).
Apela Acts as A Regulatory RNA for p53-Mediated DIA of mESCs Independent of Its Coding Ability
Because Apela is ESC-enriched, we first examined whether Apela affects the self-renewal of mESCs. Knockdown of Apela had no detectable effect on the self-renewal of mESCs based on immunofluorescence staining and immunoblotting of key transcription factors, mESC proliferation and colony formation (Figure 2A, Figure S2A–D). Microarray analysis did not identify any master regulators of mESCs or lineage markers that were regulated by Apela knockdown (Table S3). However, during embryoid body (EB) formation, in which the three germ layers will form, Apela knockdown reduced the mesoderm markers (Flk1 and alpha-actin) and endoderm markers (Foxa2 and Gata4) but not ectoderm markers (Figure S2E). This result is consistent with the studies in zebrafish showing that Apela regulates mesoderm and endoderm development (Chng et al., 2013; Pauli et al., 2014). Therefore, Apela does not affect mESCs under undifferentiated condition.
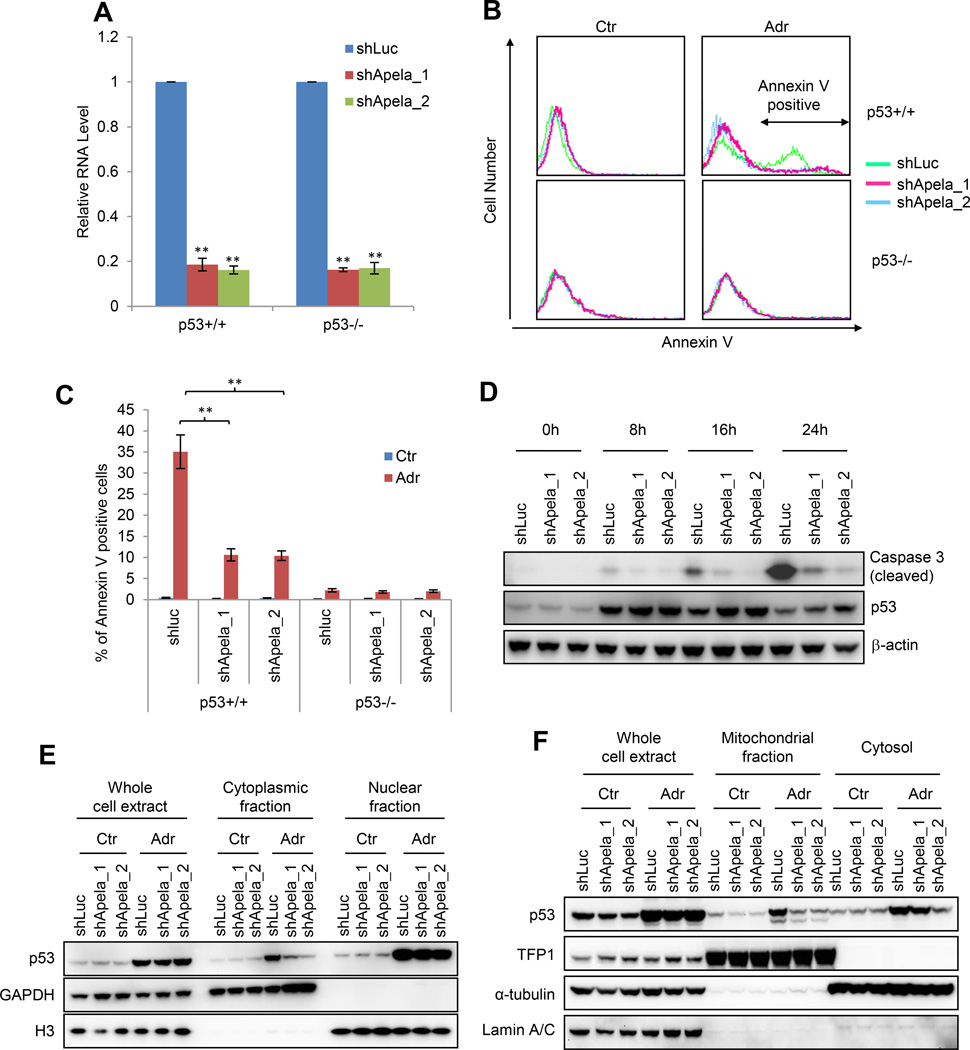
Figure 2. Apela Is Involved in p53-mediated DIA of mESCs.
(A) Real-time PCR showing Apela knockdown. Error bars are SEM; n=3. T-test; *, p<0.05; **, p<0.01. (B) Histograms of the Annexin V staining of mESCs in the presence (shApela_1 and shApela_2) or absence (shLuc) of Apela knockdown. Ctr, untreated; Adr, treated with 0.5 uM ADR for 24 hours. (C) Quantification of (B). (D) W.B. of cleaved caspase-3, p53, and β-actin. Error bars are SEM; n=3. T-test; *, p<0.05; **, p<0.01. (E) W.B. showing the effect of Apela knockdown on the sub-cellular localization of p53. Each lane of cytoplasmic and nuclear fractions was loaded with proteins from same number of cells. GAPDH, a cytoplasmic marker; H3, a nuclear marker. (F) W.B. showing the effect of Apela knockdown on the sub-cellular localization of p53. TFP1, a mitochondrial marker; α-tubulin, a cytosolic marker; Lamin A/C, a nuclear marker. Each lane of mitochondrial and cytosolic fractions contains same amount of protein.
We then reasoned that Apela may be involved in p53-mediated stress response of mESCs since Apela is repressed by p53. To test this, we examined the response of mESCs with Apela knockdown to DNA damage. Both Annexin V staining and immunoblotting of cleaved caspase-3 showed that the reduction of Apela decreased DIA of mESCs in a p53-dependent manner (Figures 2B, 2C, and 2D). Thus, Apela is involved in p53-mediated DIA of mESCs. We then tested whether Apela regulates the transcription of p53-regulated apoptotic genes. We did not observe any consistent effect of Apela knockdown on the expression of Cdkn1a (p21), Mdm2, Bcc3 (Puma), Btg2 and Bax in both untreated and ADR-treated mESCs (Figure S3A). Recently, p53 has been shown to regulate DIA in ESCs through its non-transcriptional role in mitochondria (Han et al., 2008; Liu et al., 2013). We therefore tested whether Apela regulates the non-transcriptional function of p53. For this, we first isolated cytoplasmic and nuclear fractions from ESCs and found that Apela knockdown decreased the cytoplasmic fraction but not the total amount of p53 under DNA damage (Figure 2E). Further, we isolated mitochondrial fraction and observed that Apela knockdown decreased the amount of p53 in the mitochondria of mESCs (Figure 2F), suggesting that Apela regulates p53-mediated apoptosis in mESCs by altering its mitochondrial localization.
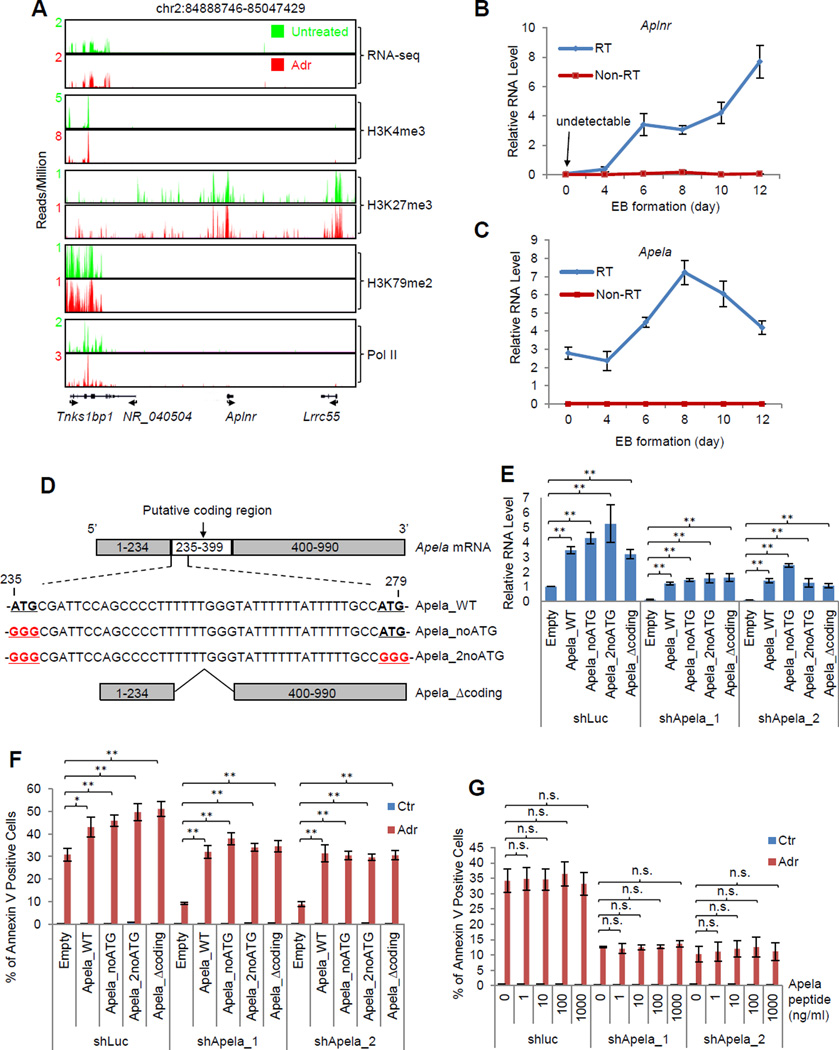
After establishing the role of Apela in p53-dependent DIA of mESCs, we wanted to gain insights into the underlying mechanism. In zebrafish, Apela is a short, secretory peptide and acts as a mitogen through the G protein-coupled Apelin receptor (Aplnr) during gastrulation (Chng et al., 2013; Pauli et al., 2014). Thus, one possibility is that Apela regulates DIA of mESCs through the Apela/Aplnr axis, a mechanism similar to our previously discovered non-cell autonomous axis of p53/Wnt/Wnt receptor in mESCs (Lee et al., 2010). For this, we planned to knock down Aplnr to examine whether Aplnr knockdown phenocopies Apela knockdown. Surprisingly, we found that Aplnr is not expressed in mESCs based on RNA-seq analysis in the absence or presence of DNA damage (Figure 3A). As a control, its neighboring gene, Tnks1bp1, is expressed (Figure 3A). ChIP-seq data showed that the Aplnr locus was blanketed with a repressive histone modification H3K27me3 and essentially had no active marks, H3K4me3 and H3K79me2 (Figure 3A). RNA polymerase II was absent from the locus, firmly demonstrating that Aplnr is silenced in mESCs. To investigate the expression pattern of Aplnr during early developmental events, we measured the levels of Aplnr mRNA during EB formation (Figure 3B). At the ESC stage (0 day EB), there was no expression of Aplnr mRNA (Figure 3B), confirming the result from RNA-seq (Figure 3A). Notably, the expression of Aplnr mRNA gradually increased during differentiation (Figure 3B). In contrast, the levels of Apela increased about twofold from day 0 to day 8 (Figure 3C). These results show that Aplnr, the gene encoding the receptor for Apela, is completely silenced in mESCs but is expressed upon the differentiation of mESCs, explaining the observation that Apela knockdown regulated the expression of mesoderm and endoderm markers during EB formation (Figure S2E). Therefore, in mESCs, Apela has an Aplnr-independent function.
Figure 3. The Coding Ability of Apela Is Dispensable for its Function in p53-Mediated DIA of mESCs.
(A) RNA-seq and ChIP-seq of histone modifications and RNA Pol II on the Aplnr locus. Real-time PCR measuring the expression of Aplnr (B) and Apela (C) during EB formation. 0 day, the mESC stage; non-RT, no reverse transcriptase. Error bars are SEM; n=3. (D) Schematics showing modifications of the coding region of Apela: Apela_WT, wild type Apela; Apela_nonATG, Apela with the first start codon ATG changed to GGG; Apela_2noATG, Apela with both start codons changed to GGG; Apela_Δcoding, Apela without the coding region. (E) Real-time PCR measuring the relative RNA level of Apela in the rescue experiments: empty vector and vectors expressing modified Apela were stably transduced into mESCs containing shLuc, shApela_1, and shApela_2 using a PiggyBac system. Error bars are SEM; n=3. T-test; *, p<0.05; **, p<0.01. (F) Rescue experiment: Annexin V staining of mESCs (shLuc, shApela_1, and shApela_2) transduced with the empty vector or a vector expressing an Apela variant (Apela_noATG, Apela_2noATG, or Apela_Δcoding). Error bars are SEM; n=3. T-test; *, p<0.05; **, p<0.01. (G) Effect of Apela peptide (1–1000 ng/ml) on DIA of mESCs. Error bars are SEM; n=3. T-test; *, p<0.05; **, p<0.01; n.s., not significant.
See also Figure S3.
There are two possible explanations for the Aplnr-independent role of Apela. One is that the Apela peptide has another un-identified receptor that mediates Apela’s function in regulating DIA of mESCs. We carried out BLASTp (Basic Local Alignment Search Tool-protein) search and did not find any protein that has a similar amino acid sequence as Aplnr, therefore arguing against the existence of another receptor of Apela peptide with a similar amino acid sequence to Aplnr. The other possibility is that the Apela RNA acts as a regulatory RNA to regulate p53-mediated DIA in mESCs which is independent of its coding ability and receptor Aplnr. Apela has a putative coding region (235–399 nucleotide, nt). Within this putative coding region, there are two start codons (Figure 3D). To test the second possibility, we disrupted the putative translation of Apela RNA by introducing different modifications to Apela: (1) changing the first start codon of Apela from ATG to GGG (Apela_noATG); (2) changing both start codons from ATG to GGG (Apela_2noATG); (3) deleting the whole putative coding region of Apela (Apela_Δcoding) (Figure 3D). We then performed rescue experiments using these different versions of modified Apela to test whether Apela without the putative coding ability can rescue the decreased apoptosis caused by Apela knockdown (Figure 3E, 3F and Figure S3B). Importantly, both wild type Apela (Apela_WT) and modified Apela rescued the phenotype of Apela knockdown (Figure 3F and Figure S3B), demonstrating that the coding ability of Apela is dispensable for its function in regulating DIA of mESCs. These results together demonstrate that Apela acts as a regulatory RNA to regulate p53-mediated DIA of mESCs even though it has a coding capacity.
To test whether Apela peptide, if produced, has a role in DIA of mESCs, we performed the rescue experiments using synthesized Apela peptide (Figure 3G). We did not observe any rescue effect of the Apela peptide at a wide range (1–1000 ng/ml), suggesting that the mature Apela peptide, if produced, has no function in p53-mediated DIA of mESCs.
Apela Interacts with hnRNPL
Since some regulatory RNAs function through their binding partners (Huarte et al., 2010; Klattenhoff et al., 2013; Rinn et al., 2007), we chose to gain further insights into the role of Apela in p53-mediated DIA of ESCs by identifying the protein(s) that bind to Apela using an RNA pull-down assay (RPA).
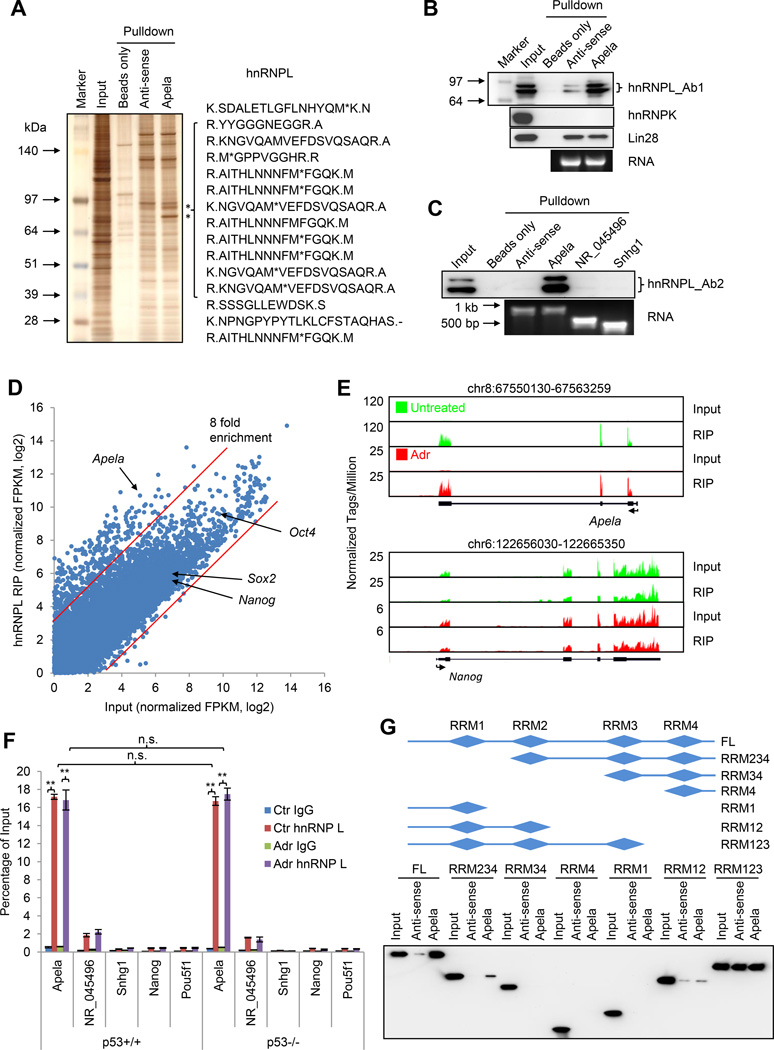
We first used a candidate approach to examine whether Apela binds to several ESC master regulators, such as Oct4, Nanog, Sox2, Klf4, and Utf1 (Figure S4A), and chromatin binding proteins, such as Suz12, Gcnf, G9a, and Dnmt3b (Figure S4B). We neither observed any enrichment of these proteins in Apela RPA versus anti-sense Apela RPA (control) (Figure S4A and S4B), nor did we find any evidence that Apela binds to the p53 protein (Figure S4B). Thus, this candidate approach did not identify any protein that binds Apela. Next, we employed an unbiased approach, which combines RPA with silver staining followed by mass spectrometry (Figure 4A) (Rinn et al., 2007; Tsai et al., 2010). This approach revealed that Apela pulled down two bands, which represented the same protein, hnRNPL (heterogeneous nuclear ribonucleoprotein L) (Figure 4A and S4C). Both hnRNPL splicing variants were enriched at a similar degree (Figure 4B and 4C).
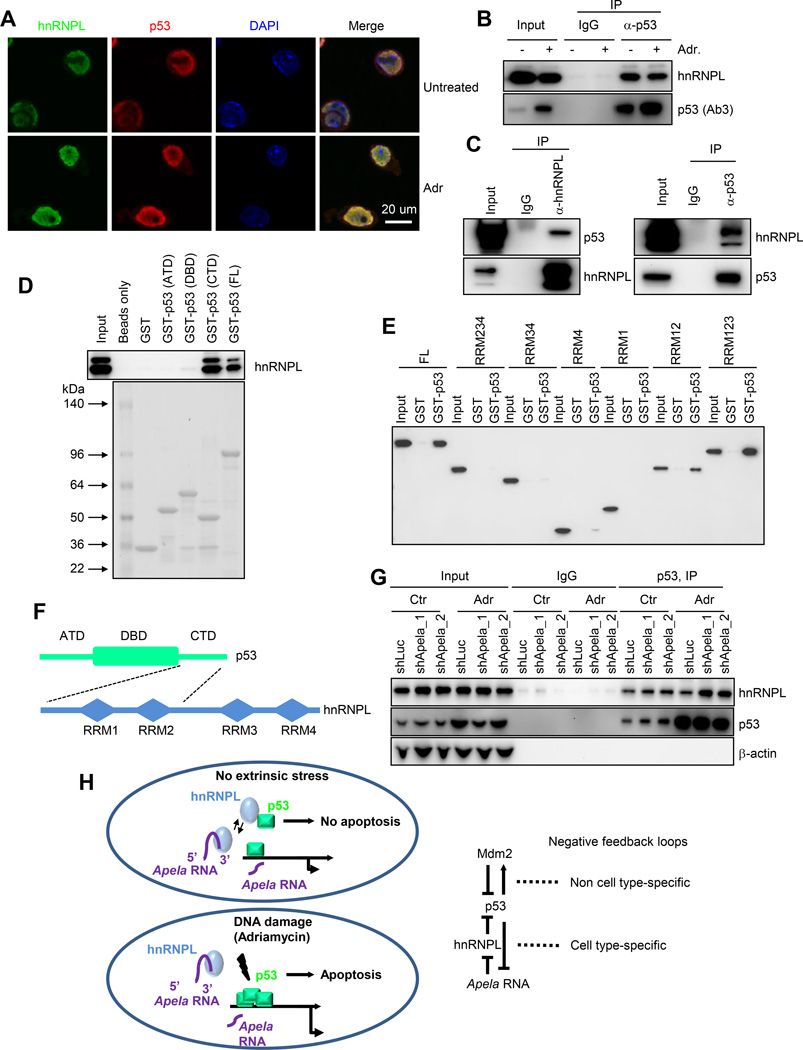
Figure 4. Apela Binds to hnRNPL Independent of p53 Status and DNA Damage.
(A) Silver staining of RPA. Right, representative peptides identified by mass spectrometry. (B) W.B. showing RPA from (A) with hnRNPL antibody (Ab), hnRNPK Ab (control), and Lin28 Ab (control). Ab1, 1st Ab for hnRNPL. (C) W.B. showing RPA using Apela and two other RNA controls, NR_045496 and Snhg1. Ab2, another Ab for hnRNPL. (D) RIP-seq showing average-normalized, log2-transformed FPKM of hnRNPL-RIP and Input. The two red lines are the arbitrary cutoffs of 8-fold enrichment. (E) RIP-seq showing the enrichment of Apela in RIP with hnRNPL Ab. Nanog mRNA, a negative control; green, untreated; red, 0.5 µM ADR for 8 hours. Input and hnRNPL-RIP were scaled to the same level. (F) RIP with hnRNPL Ab followed by real-time PCR. Percentage of input was calculated to measure the relative interaction between hnRNPL and RNAs. Error bars are SEM; n=3. T-test; *, p<0.05; **, p<0.01; n.s., not significant. (G) Upper, schematics of hnRNPL variants; lower, RPA using Apela (antisense and sense) and Flag-tagged hnRNPL fragments.
We confirmed the interaction between Apela and hnRNPL using RPA followed by immunoblotting with two different antibodies (Ab1 and Ab2) recognizing hnRNPL (Figure 4B and 4C). Two lines of evidence suggest that the interaction between Apela and hnRNPL is specific. First, two other RNA binding proteins, hnRNP K and Lin28 did not show enrichment in Apela pull-down (Figure 4B). Second, two other RNAs, NR_045496 and Snhg1, did not bind to hnRNPL (Figure 4C).
To verify the interaction reciprocally, we carried out RNA immunoprecipitation (RIP) and sequencing (RIP-seq) in p53+/+ mESCs (Figure 4D and 4E). 405 transcripts were identified as hnRNPL-bound when using 8 fold as a cutoff (Figure 4E and Table S4). Apela was enriched about 48 times in the immunoprecipitated complex while controls, such as Nanog, Sox2, and Oct4 mRNA, were not enriched (Figure 4D and 4E). RIP-seq result was confirmed by RIP followed by real-time PCR (Figure 4F). hnRNPL Ab enriched Apela around 100-fold versus IgG control (Figure 4F). Moreover, the relative enrichment (percentage of input) was not affected by DNA damage (Figure S4C and Figure 4F, compare Ctr to Adr) and independent of the p53 status (Figure S4C and Figure 4F, compare p53+/+ to p53−/−). In contrast, the interaction between hnRNPL and a control RNA, NR_045496, was weak. The interaction between hnRNPL and Snhg1, Nanog or Oct4 mRNAs was similar to background (IgG). Using recombinant hnRNPL and in vitro transcribed Apela, we detected a direct interaction between hnRNPL and Apela (Figure S4D). In summary, hnRNPL is a bona fide binding partner of Apela.
hnRNPL contains four RNA recognition motifs (RRM1-4) (Figure 4G, upper panel). To map the region(s) within hnRNPL that contributes to Apela binding, we tested the binding of Apela and antisense Apela (as a negative control) to a variety of hnRNPL truncation variants. The first three RRMs of hnRNPL were required for Apela binding (Figure 4G). Interestingly, the fourth RRM was not required for Apela binding. Instead, it determined the binding specificity since RRM123 of hnRNPL bound to antisense Apela as well.
To further test whether Apela and p53 interact, we performed p53 RIP assay (Figure S4E). p53 IP did not pull down Apela, consistent with the result of RPA (Figure S4B). Because alternative RNA splicing is one of the major functions of hnRNPL (Motta-Mena et al., 2010), we investigated the effect of hnRNPL knockdown on Apela splicing and sub-cellular localization. hnRNPL did not affect Apela splicing but slightly altered the sub-cellular localization of Apela (Figure S4F–J).
The 3’UTR of Apela Interacts with hnRNPL and Is Required for Apela ’s Role in p53-mediated DIA of mESCs
To map the region(s) within Apela that interacts with hnRNPL, we performed RPA using different fragments of Apela (Figure 5A). A region (732-974 nt) within the 3’ UTR (400–974 nt) of Apela bound to hnRNPL as efficiently as the full length of Apela (Figure 5A). Further mapping this region (732–974 nt) indicated that a domain within 793-859 nt was required for the interaction (Figure 5B). Since the region binding to hnRNPL falls into the 3’ UTR, we then asked whether the 3’ UTR of Apela has similar function as the wild type in DIA of mESCs. For this, we performed RPA (Figure 5C) and the rescue experiment (Figure 5D and 5E) using the 3’ UTR and full length of Apela. The 3’ UTR of Apela bound to hnRNPL and rescued the reduction of DIA as efficiently as wild type Apela (Apela_WT) (Figure 5D–E), further corroborating the conclusion that the coding ability of Apela is dispensable for its function in DIA of mESCs (Figure 3). RPA showed that the 3’ UTR of Apela does not interact with ribosomal proteins, Rpl26 and Rps3 (Figure S5A–B), ruling out the possibility that Apela regulates DIA of mESCs by interfering with translation in general.
Figure 5. hnRNPL Binds to the CA Tracts within the 3’ UTR of Apela.
(A) RPA using different fragments of Apela to map the interacting domain in Apela with hnRNPL. Numbers are the nucleotide positions from 5’ and 3’ end. Error bars are SEM; n=3. (B) Mapping the region within Apela that binds hnRNPL. (C) RPA using wild type Apela (Apela_WT) and the 3’UTR of Apela (Apela_3UTR). (D) Representative histograms of Annexin V staining of mESCs in the rescue experiment using vector only, Apela_WT and Apela_3UTR in the absence (Ctr) or presence of ADR treatment (Adr, 24 hours). (E) Quantification of (D). Error bars are SEM; n=3. T-test; *, p<0.05; **, p<0.01. (F) Sequences of wild type (WT) Apela and mutants (M1, 1st CA tract disrupted; M2, 2nd CA tract disrupted; M12, both CA tracts disrupted). (G) RPA using WT and mutant Apela. (H) Representative histograms of Annexin V staining of mESCs in the rescue experiment in the absence (Ctr) or presence of ADR treatment (Adr, 24hours). (I) Quantification of (H). Error bars are SEM; n≥3. T-test; *, p<0.05; **, p<0.01; n.s., not significant.
See also Figure S5.
The Interaction between Apela and hnRNPL Is Required for Apela ’s Role in p53-mediated DIA
Because hnRNPL has been shown to bind to the CA tracts in the intronic regions of other genes (Hui et al., 2005), we searched the 732–974 nt domain and found two CA tracts are around this domain (Figure S5C). RNA secondary structure prediction revealed that these two CA tracts were brought to proximity by a loop (Figure S5C). These results suggest that these two CA tracts are the sites interacting with hnRNPL. To test this, we generated Apela mutants containing GU substitutions at the 1st (Apela_M1), 2nd (Apela_M2) or both CA (Apela_M12) tracts and performed RPA (Figure 5F and 5G). We found that both CA tracts were involved in the interaction with hnRNPL, and the 2nd CA tract was dominant (Figure 5G). Substitution of both CA tracts with GU tracts completely disrupted the binding between hnRNPL and Apela (Figure 5G, compare Apela_M12 to anti-sense). Therefore, hnRNPL binds to Apela only at these two CA tracts.
To investigate the functional relevance of the interaction between Apela and hnRNPL to p53-mediated apoptosis in mESCs, we performed rescue experiment using wild type Apela (Apela_WT) and the Apela mutant containing two CA-to-GU substitutions (Apela_M12), which disrupted the interaction between Apela and hnRNPL. Apela_M12 did not rescue the decreased apoptosis caused by Apela knockdown (Figure 5H, 5I and S5D), demonstrating that the binding between hnRNPL and Apela is required for the function of Apela in DIA of mESCs.
hnRNPL Inhibits p53 Activation and Mitochondrial Localization in mESCs
To investigate whether hnRNPL is involved in p53-mediated DIA of mESCs, we used shRNAs to reduce the levels of hnRNPL in both p53+/+ and p53−/− mESCs (Figure 6A). hnRNPL knockdown activated p53 in the absence or presence of ADR treatment (Figure 6B), indicating that hnRNPL inhibits p53 activation. hnRNPL knockdown did not increase DNA damage in mESCs as judged by H2AX-S139P (Figure 6B), suggesting that the increased p53 is not caused by DNA damage. The activation of p53 coincided with the increase of p53K379ac but not p53S18P (Figure 6C). Since acetylation blocks the degradation of p53 (Kruse and Gu, 2009), we tested whether hnRNPL knockdown affects the degradation of p53. Cycloheximide (CHX) was used to inhibit the translation of p53 in untreated control and hnRNPL knockdown mESCs and p53 half-life was measured (Figure 6D). We found that hnRNPL knockdown prolonged the half-life of p53 from about 18 to 33–40 minutes, demonstrating that hnRNPL knockdown inhibits the degradation of p53 in mESCs. We were unable to precisely quantify the effect of hnRNPL knockdown on p53 half-life in ADR-treated mESCs due to rapid cell death induced by the combinatory treatment of ADR and CHX (Figure S6A). We found that hnRNPL knockdown increased the mitochondrial localization (Figure 6E) in addition to the stability of p53 (Figure 6B). Therefore, hnRNPL has a wider role than Apela in regulating p53 in mESCs: hnRNPL regulates both stability and mitochondrial localization of p53 while Apela only affects mitochondrial localization.
Figure 6. hnRNPL Inhibits p53 Activation and p53-dependent Apoptosis of mESCs.
(A) Real-time PCR measuring hnRNPL mRNA levels. Error bars are SEM; n=3. T-test; *, p<0.05; **, p<0.01. (B) W.B. showing the effect of hnRNPL knockdown on p53. (C) Immunoprecipitation (IP) followed by W.B. showing the effect of hnRNPL knockdown on p53 K379ac and S18P. Total p53 amount in each lane was adjusted to the same level for comparing K379ac and S18P levels. (D) W.B. showing the effect of hnRNPL knockdown on p53 degradation; 3D view showing the band intensity of p53; lower panel, quantification of p53 levels. (E) W.B. showing the effect of hnRNPL knockdown on the mitochondrial localization of p53. (F) Representative histograms of Annexin V staining of mESCs without (shLuc) and with hnRNPL knockdown in the absence (Ctr) or presence of ADR treatment (Adr, 24 hours). (G) Quantification of (F). Error bars are SEM; n=3. T-test; *, p<0.05; **, p<0.01; n.s., not significant.
See also Figure S6.
To examine the functional consequence of the increased p53 in response to hnRNPL knockdown, we performed Annexin V staining and found that hnRNPL knockdown significantly increased apoptosis in p53+/+ but not in p53−/− mESCs (Figure 6F and 6G). Thus, hnRNPL prevents mESCs from apoptosis by inhibiting p53 activation. Since both Apela and hnRNPL are involved in p53-mediated apoptosis in mESCs and they bind to each other, we aimed to dissect the hierarchy of these two binding partners in p53-mediated apoptosis. To achieve this, we performed the Annexin V staining of mESCs with the combinatory knockdown of Apela and hnRNPL. We reasoned that if hnRNPL is upstream of Apela, the combinatory knockdown of Apela and hnRNPL would produce similar results as Apela knockdown; if Apela is upstream of hnRNPL, the combinatory knockdown will have similar outcome as hnRNPL knockdown. Our results supported the latter possibility that Apela affects p53-mediated DIA in mESCs upstream of hnRNPL (Figure S6B–C).
Apela Negatively Regulates the Interaction between hnRNPL and p53
Previous studies showed that hnRNPK, another member in the hnRNP family, binds to p53 (Moumen et al., 2005). Thus, we hypothesized that hnRNPL binds to p53. To test this hypothesis, we performed double immunostaining followed by confocal analysis and observed a strong co-localization between p53 and hnRNPL in mESCs in the absence or in the presence of ADR treatment (Figure 7A and Figure S7A–B). The co-localization of hnRNPL and p53 suggests that they bind to each other in mESCs. To test this, we carried out co-immunoprecipitation (co-IP) in mESCs using p53 Ab followed by immunoblotting with hnRNPL Ab and detected an interaction between p53 and hnRNPL (Figure 7B). DNA damage did not obviously alter hnRNPL protein levels but increased p53 protein levels in mESCs (Figure 7B). Despite that more p53 existed in mESCs after DNA damage, the absolute amount of hnRNPL in the p53 immuno-complex did not change, suggesting that the relative portion of p53 interacting with hnRNPL decreases after DNA damage. We also performed co-IP with recombinant hnRNPL and recombinant p53 and observed a direct interaction between hnRNPL and p53 (Figure 7C). To identify the region(s) within p53 that is responsible for the interaction with hnRNPL, we generated recombinant p53 containing the amino terminal domain (ATD), DNA binding domain (DBD) or carboxyl terminal domain (CTD) and performed pulldown followed by hnRNPL immunoblotting (Figure 7D). We found that hnRNPL binds to the CTD of p53 (Figure 7D). In vitro pulldown assay using Flag-tagged hnRNPL mutants containing various RRMs and GST-tagged full length p53 demonstrated that the first two RRMs of hnRNPL contribute to the binding with p53 (Figure 7E). In summary, the CTD of p53 interacts with the first two RRMs within hnRNPL (Figure 7F).
Figure 7. Apela Inhibits hnRNPL/p53 Interaction.
(A) Immunostaining showing the co-localization of hnRNPL and p53 in mESCs untreated or treated with 0.5 µM ADR for 8 hours. (B) Co-IP using p53 Ab followed by W.B. with hnRNPL Ab. (C) Co-IP using recombinant hnRNPL and recombinant p53. (D) Pulldown using purified GST-tagged p53 fragments and mESC lysate. (E) Co-IP using purified GST-tagged FL p53 and Flag-tagged hnRNPL fragments followed by W.B. with Flag Ab. (F) Domain structures of p53 and hnRNPL. The dashed black lines indicate the interacting domains within p53 and hnRNPL. (G) Co-IP showing the effect of Apela knockdown on the interaction between p53 and hnRNPL: co-IP in mESCs (shLuc, shApela_1, and shApela_2) using p53 Ab followed by W.B. with hnRNPL, p53 and β-actin Abs. (H) Left, a model of a tri-element negative feedback loop formed by p53, Apela, and hnRNPL; Right, the well-established negative feedback loop involving p53 and Mdm2 was shown as a comparison.
See also Figure S7.
To explore how Apela affects the regulation of p53 by hnRNPL, we performed co-IP experiments in mESCs untreated or treated with ADR in the absence (shLuc) or in the presence (shApela_1 and shApela_2) of Apela knockdown (Figure 7G). We found that Apela knockdown increased the interaction between hnRNPL and p53 after ADR treatment (Figure 7G), indicating that Apela negatively regulates the interaction between hnRNPL and p53. This result is consistent with the observation that Apela and hnRNPL have opposite roles in regulating p53-mediated DIA of mESCs.
DISCUSSION
A Tri-element Negative Feedback Loop that Regulates p53-mediated DIA in mESCs
The mechanisms underlying DIA of mESCs are not fully appreciated, and the apoptotic role of p53 in mESCs has been still elusive, which can partially be attributed to the lack of understanding of the mechanisms. The discovery of a tri-element negative feedback loop involving p53, Apela, and hnRNPL contributes to the understanding of DIA of mESCs (Figure 7H). This tri-element negative feedback loop differs from the well-established di-element p53/Mdm2 loop in that it is mediated by a regulatory RNA, Apela. In addition, this tri-element loop appears to be cell type specific, consistent with the concept that the regulation of p53 signaling in mESCs is influenced by ESC-specific transcriptome.
In mESCs, p53 activity needs to be kept in check because un-wanted activation of p53 will cause the differentiation and/or apoptosis of mESCs. Nucleolin and aurora kinase A (Aurka) inhibit the pro-differentiation activity of p53 in mESCs (Cinghu et al., 2014; Lee et al., 2012; Montes de Oca Luna et al., 1995). However, it is unknown which factor(s) suppresses the pro-apoptotic function of p53 in mESCs. The fact that Mdm2 and Mdm4 knockout embryos survive beyond the blastocyst stage suggests that other factors prevent p53 activation in mESCs (Jones et al., 1995; Montes de Oca Luna et al., 1995; Parant et al., 2001). Here we find hnRNPL is a factor that antagonizes p53-mediated apoptosis in mESCs (Figure 6 and 7). Thus, multiple proteins are probably needed to subdue p53 activation in mESCs to ensure the normal development under unstressed condition. It is worth noting that hnRNPs are multifunctional proteins (Chaudhury et al., 2010; Ray et al., 2009). Therefore, the inhibition of p53 activation may only be one of the functions of hnRNPL. In the same vein, not every transcript bound by hnRNPL regulates p53 in mESCs (Figure S7C).
Apela binds to hnRNPL and antagonizes its inhibitory effect on p53 during DNA damage response. However, the precise mechanism of this antagonism is currently unknown. It is possible that Apela antagonizes the activity of hnRNPL by modulating its conformation or post-translational modifications or competing with hnRNPL-p53 dimers. Under unstressed condition, the copy number of Apela and mitochondrial p53 in a single mESCs is about 110 and 40, respectively. After DNA damage, the copy number of Apela and mitochondrial p53 is about 25 and 160, respectively (Figure S7D–F and Supplemental Information). These results suggest that the copy number of Apela is enough to influence the mitochondrial localization of p53, although as an activator, Apela does not need to release all the p53 molecules to affect apoptosis. In addition, Apela may regulate many copies of hnRNPL by a dynamic interaction.
Since Apela positively regulates p53, the repression of Apela by p53 probably serves as a means to avoid un-controlled “firing” of p53. Therefore, our results suggest that mESCs employ this p53/Apela/hnRNPL negative feedback loop to balance the needs of suppressing p53 in mESCs without extrinsic DNA damage stress and achieving rapid apoptosis upon DNA damage. We note that Apela is a modulator not a determinant of p53-mediated DIA of mESCs and other mechanisms have been reported. For example, others have shown that the lack of G1/S arrest in mESCs, mitochondrial priming in human ES (hES) cells, and the Golgi localization of Bax also contribute to the sensitivity of ESCs to DIA (Dumitru et al., 2012; Hong and Stambrook, 2004; Liu et al., 2013). It is possible that other un-identified mechanisms exist. Thus, further elucidation of these mechanisms will help us fully understand the DNA damage stress response of ESCs.
The Function of Apela in p53-mediated DIA Does not Require its Coding Ability
A striking finding in this study is that the coding ability is dispensable for the regulatory function of Apela in p53-mediated DIA of mESCs. Four lines of evidence support this conclusion. First, the Aplnr gene that encodes the cognate receptor for the Apela peptide is not expressed in mESCs (Figure 3A and 3B). Second, Apela without the putative coding region rescues the phenotype of Apela knockdown (Figure 3D–F and Figure 5D–E), while the Apela peptide does not (Figure 3G). Third, the region within Apela that binds hnRNPL is at the 3’ UTR (Figure 5). Fourth, Apela peptide has no effect in DIA of ESCs. It is possible that Apela has multiple modes of action depending on the developmental stages: in mESCs, it regulates p53-mediated apoptosis independent of its coding ability while during early development, it controls cell movement through encoded Apela peptide and the Apela/Aplnr axis. Indeed, Apela and Aplnr mRNAs are dynamically expressed during early development using the EB formation model (Figure 3B and 3C). Both Apela and Apelin are ligands for Aplnr in zebrafish; Apela is expressed in the early development of zebrafish while Apelin in the late development (Chng et al., 2013; Pauli et al., 2014).
Although we detected no activity of Apela peptide in p53-mediated DIA of mESCs, it is worth noting that our results do not exclude the possibility that in mESCs Apela is translated into the Apela peptide nor do they contradict to recent studies showing that in zebrafish Apela encodes a secretory peptide (Chng et al., 2013; Pauli et al., 2014). Instead, we identify another mode of action of Apela: it functions as a regulatory RNA in the context of p53-mediated DIA of mESCs. A ribosomal profiling in mESCs suggests that Apela is translated in mESCs (Ingolia et al., 2011). However, we did not have success of detecting the putative endogenous Apela peptide in mESCs so far using mass spectrometry. In zebrafish, Apela (also called Toddler or ELABELA) is expressed only at the gastrulation stage while Aplnr is ubiquitously expressed (Chng et al., 2013; Pauli et al., 2014). In mESCs (this study) and human ESCs (Chng et al., 2013), Apela is highly expressed while the receptor gene, Aplnr, is silenced in mESCs. We speculate that this noncoding mechanism of Apela facilitates a fast DNA damage response of ESCs because it bypasses the step of protein translation. It remains unclear whether this mode of action of Apela in mESCs is conserved across all mammals.
In the last several years, many studies have investigated how regulatory RNAs, especially noncoding RNAs, affect various cellular processes, such as apoptosis (Hung et al., 2011) and differentiation (Klattenhoff et al., 2013). In this study, we serendipitously discovered that a RNA species with a coding ability, Apela, has a noncoding function in mESCs, raising the possibility that other coding RNAs may also have functions beyond their coding ability. Therefore, our results could potentially expand the concept of regulatory RNAs and fuel another line of investigation.
EXPERIMENTAL PROCEDURES
ChIP-Seq, RNA-Seq, and Data Analyses
ChIP-seq and data analyses were performed as previously described (Li et al., 2012). Deep sequencing was done in the Next Generation Sequencing Facility (NGSF) at the Center for Cancer Research (CCR) in NCI. Briefly, 10 ng of IP genomic DNA was used in library construction, cluster generation, and sequenced in the Genome Analyzer IIx (GAIIx) or HiSeq 2000 system. For RNA-seq, 1 µg of total RNA was sent to the NGSF. All the procedures were carried out according to Illumina’s protocols. Data analyses and Z-score algorithm were described in the Supplemental Information.
RNA Pull-down Assay (RPA), RNA Immunoprecipitation (RIP), and RIP-Seq
For RPA, we used a protocol described in a previous publication (Huarte et al., 2010). Briefly, 20 µl M-280 Dynabeads (Life Technologies) were allowed to bind to 300 ng biotin-labeled sense or anti-sense Apela overnight. The Dynabeads/RNA complexes were incubated with 400 µl of 2 µg/µl whole cell lysates at 4°C for 1 hour. Dynabeads were washed three times (5 minutes each) with RPA buffer (50 mM Tris, pH 7.5, 250 mM KCl, 5 mM EDTA, 0.5 mM DTT, 1% NP40 plus protease inhibitors and RNase inhibitors). After washes, beads were boiled and supernatant were run on a 4–12% NuPAGE gel and silver staining was performed using the SilverQuest™ Silver Staining Kit (Life Technologies). Bands were cut out from the gel and sent for protein identification using mass spectrometry.
For RIP, 1 µg hnRNPL Ab was incubated with Protein A-Dynabeads overnight, and then incubated with 500 µg total clear lysate in RPA buffer at 4°C for 4 hours. We washed the beads four times with RPA buffer and extracted the total RNA with Trizol. Concentration was measured and 100 ng RNA was sent to the NGSF at CCR, NCI. For data processing, we applied a normalization approach because input and RIP had different averages of all the FPKMs. We made an assumption that majority of the RNAs were not bound by hnRNPL. Therefore, we normalized the FPKM of every transcript to the average of all the transcripts.
Genomic Datasets
Genomic datasets from this study were submitted to the GEO database (GSE65491). Reanalyzed public datasets were described in Table S5.
Supplementary Material
Highlights.
Apela is enriched in mouse ESCs and repressed by p53
Apela positively regulates DNA damage-induced apoptosis in mouse ESCs
The protein-coding capacity of Apela is not required for DNA damage-induced apoptosis
Apela binds to hnRNPL, an inhibitory binding partner of p53 in mouse ESCs
ACKNOWLEDGEMENTS
We thank Douglas Lowy for reading the manuscript. This study was funded by the intramural research program and partially by the Office of Science and Technology Partnerships at the Center for Cancer Research (CCR), the National Cancer Institute (NCI) at the National Institutes of Health (NIH). The computational analyses utilized the high-performance computational capabilities of the Helix Systems at NIH (http://helix.nih.gov).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental Information includes supplemental figures, tables, experimental procedures and references.
AUTHOR CONTRIBUTIONS
ML and HG conducted the experiments except for confocal microscopy by BT, RNA 2nd structure prediction by JH (HHMI) and ML, mouse breeding and ESC derivation by WD, cloning by SJ, protein id by TW and MZ, computation and statistical analyses by JS and JH (NCI). JH (NCI) designed the project and wrote the manuscript. All authors read the manuscript and approved the submission.
REFERENCES
- Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- Cervantes RB, Stringer JR, Shao C, Tischfield JA, Stambrook PJ. Embryonic stem cells and somatic cells differ in mutation frequency and type. Proc Natl Acad Sci U S A. 2002;99:3586–3590. doi: 10.1073/pnas.062527199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury A, Chander P, Howe PH. Heterogeneous nuclear ribonucleoproteins (hnRNPs) in cellular processes: Focus on hnRNP E1's multifunctional regulatory roles. Rna. 2010;16:1449–1462. doi: 10.1261/rna.2254110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- Chng SC, Ho L, Tian J, Reversade B. ELABELA: a hormone essential for heart development signals via the apelin receptor. Dev Cell. 2013;27:672–680. doi: 10.1016/j.devcel.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Cinghu S, Yellaboina S, Freudenberg JM, Ghosh S, Zheng X, Oldfield AJ, Lackford BL, Zaykin DV, Hu G, Jothi R. Integrative framework for identification of key cell identity genes uncovers determinants of ES cell identity and homeostasis. Proc Natl Acad Sci U S A. 2014;111:E1581–E1590. doi: 10.1073/pnas.1318598111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Dumitru R, Gama V, Fagan BM, Bower JJ, Swahari V, Pevny LH, Deshmukh M. Human embryonic stem cells have constitutively active Bax at the Golgi and are primed to undergo rapid apoptosis. Mol Cell. 2012;46:573–583. doi: 10.1016/j.molcel.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh AM, Lim CY, Chiam PC, Li L, Mann MB, Mann KM, Menendez S, Lane DP. Using targeted transgenic reporter mice to study promoter-specific p53 transcriptional activity. Proc Natl Acad Sci U S A. 2012;109:1685–1690. doi: 10.1073/pnas.1114173109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MK, Song EK, Guo Y, Ou X, Mantel C, Broxmeyer HE. SIRT1 regulates apoptosis and Nanog expression in mouse embryonic stem cells by controlling p53 subcellular localization. Cell Stem Cell. 2008;2:241–251. doi: 10.1016/j.stem.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, Barrera LO, Van Calcar S, Qu C, Ching KA, et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet. 2007;39:311–318. doi: 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- Hong Y, Stambrook PJ. Restoration of an absent G1 arrest and protection from apoptosis in embryonic stem cells after ionizing radiation. Proc Natl Acad Sci U S A. 2004;101:14443–14448. doi: 10.1073/pnas.0401346101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M, et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409–419. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui J, Hung LH, Heiner M, Schreiner S, Neumuller N, Reither G, Haas SA, Bindereif A. Intronic CA-repeat and CA-rich elements: a new class of regulators of mammalian alternative splicing. EMBO J. 2005;24:1988–1998. doi: 10.1038/sj.emboj.7600677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung T, Wang Y, Lin MF, Koegel AK, Kotake Y, Grant GD, Horlings HM, Shah N, Umbricht C, Wang P, et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat Genet. 2011;43:621–629. doi: 10.1038/ng.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia NT, Lareau LF, Weissman JS. Ribosome profiling of mouse embryonic stem cells reveals the complexity and dynamics of mammalian proteomes. Cell. 2011;147:789–802. doi: 10.1016/j.cell.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SN, Roe AE, Donehower LA, Bradley A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature. 1995;378:206–208. doi: 10.1038/378206a0. [DOI] [PubMed] [Google Scholar]
- Kagey MH, Newman JJ, Bilodeau S, Zhan Y, Orlando DA, van Berkum NL, Ebmeier CC, Goossens J, Rahl PB, Levine SS, et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467:430–435. doi: 10.1038/nature09380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klattenhoff CA, Scheuermann JC, Surface LE, Bradley RK, Fields PA, Steinhauser ML, Ding H, Butty VL, Torrey L, Haas S, et al. Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell. 2013;152:570–583. doi: 10.1016/j.cell.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse JP, Gu W. Modes of p53 regulation. Cell. 2009;137:609–622. doi: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DF, Su J, Ang YS, Carvajal-Vergara X, Mulero-Navarro S, Pereira CF, Gingold J, Wang HL, Zhao R, Sevilla A, et al. Regulation of embryonic and induced pluripotency by aurora kinase-p53 signaling. Cell Stem Cell. 2012;11:179–194. doi: 10.1016/j.stem.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Li M, Michalowski AM, Zhang X, Liao H, Chen L, Xu Y, Wu X, Huang J. A genomewide study identifies the Wnt signaling pathway as a major target of p53 in murine embryonic stem cells. Proc Natl Acad Sci U S A. 2010;107:69–74. doi: 10.1073/pnas.0909734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, He Y, Dubois W, Wu X, Shi J, Huang J. Distinct Regulatory Mechanisms and Functions for p53-Activated and p53-Repressed DNA Damage Response Genes in Embryonic Stem Cells. Mol Cell. 2012;46:30–42. doi: 10.1016/j.molcel.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Chao C, Saito S, Mazur SJ, Murphy ME, Appella E, Xu Y. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat Cell Biol. 2005;7:165–171. doi: 10.1038/ncb1211. [DOI] [PubMed] [Google Scholar]
- Liu JC, Guan X, Ryan JA, Rivera AG, Mock C, Agarwal V, Letai A, Lerou PH, Lahav G. High Mitochondrial Priming Sensitizes hESCs to DNA-Damage-Induced Apoptosis. Cell Stem Cell. 2013 doi: 10.1016/j.stem.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes de Oca Luna R, Wagner DS, Lozano G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature. 1995;378:203–206. doi: 10.1038/378203a0. [DOI] [PubMed] [Google Scholar]
- Motta-Mena LB, Heyd F, Lynch KW. Context-dependent regulatory mechanism of the splicing factor hnRNP L. Mol Cell. 2010;37:223–234. doi: 10.1016/j.molcel.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moumen A, Masterson P, O’Connor MJ, Jackson SP. hnRNP K: An HDM2 Target and Transcriptional Coactivator of p53 in Response to DNA Damage. Cell. 2005;123:1065. doi: 10.1016/j.cell.2005.09.032. [DOI] [PubMed] [Google Scholar]
- Parant J, Chavez-Reyes A, Little NA, Yan W, Reinke V, Jochemsen AG, Lozano G. Rescue of embryonic lethality in Mdm4-null mice by loss of Trp53 suggests a nonoverlapping pathway with MDM2 to regulate p53. Nat Genet. 2001;29:92–95. doi: 10.1038/ng714. [DOI] [PubMed] [Google Scholar]
- Pauli A, Norris ML, Valen E, Chew GL, Gagnon JA, Zimmerman S, Mitchell A, Ma J, Dubrulle J, Reyon D, et al. Toddler: an embryonic signal that promotes cell movement via Apelin receptors. Science. 2014;343:1248636. doi: 10.1126/science.1248636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahl PB, Lin CY, Seila AC, Flynn RA, McCuine S, Burge CB, Sharp PA, Young RA. c-Myc regulates transcriptional pause release. Cell. 2010;141:432–445. doi: 10.1016/j.cell.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray PS, Jia J, Yao P, Majumder M, Hatzoglou M, Fox PL. A stress-responsive RNA switch regulates VEGFA expression. Nature. 2009;457:915–919. doi: 10.1038/nature07598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, Shi Y, Segal E, Chang HY. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–693. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RA. Control of the embryonic stem cell state. Cell. 2011;144:940–954. doi: 10.1016/j.cell.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, He Y, Lee KH, Dubois W, Li Z, Wu X, Kovalchuk A, Zhang W, Huang J. Rap2b, a novel p53 target, regulates p53-mediated pro-survival function. Cell Cycle. 2013;12:1279–1291. doi: 10.4161/cc.24364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.