Abstract
Background
The Iowa Gambling Task (IGT; Bechara, Damasio, Damasio, & Anderson, 1994) has frequently been used to assess risky decision making in clinical populations, including patients with schizophrenia (SZ). Poor performance on the IGT is often attributed to reduced sensitivity to punishment, which contrasts with recent findings from reinforcement learning studies in schizophrenia.
Methods
In order to investigate possible sources of IGT performance deficits in SZ patients, we combined data from the IGT from 59 SZ patients and 43 demographically-matched controls with data from the Balloon Analog Risk Task (BART) in the same participants. Our analyses sought to specifically uncover the role of punishment sensitivity and delineate the capacity to integrate frequency and magnitude information in decision-making under risk.
Results
Although SZ patients, on average, made more choices from disadvantageous decks than controls did on the IGT, they avoided decks with frequent punishments at a rate similar to controls. Patients also exhibited excessive loss-avoidance behavior on the BART.
Conclusions
We argue that, rather than stemming from reduced sensitivity to negative consequences, performance deficits on the IGT in SZ patients are more likely the result of a reinforcement learning deficit, specifically involving the integration of frequencies and magnitudes of rewards and punishments in the trial-by-trial estimation of expected value.
Keywords: schizophrenia, reward, risk, reinforcement, decision-making, Iowa Gambling Task (IGT)
INTRODUCTION
Optimal decision-making often requires the ability to learn from the outcomes of previous choices, both rewards and punishments, and to adjust future choices accordingly. The study of the neural substrates of these types of decision processes was pioneered by Bechara and Damasio (Bechara et al. 1994, 1997), who developed the Iowa Gambling Task (IGT). In this task, subjects can choose gambles from four different decks. Two of the decks offer $100 rewards, on average, and two offer $50 rewards, on average. However, the decks offering the higher rewards also involve large punishments and choosing from these higher paying decks is ultimately disadvantageous, and choices from the decks with smaller rewards turn out to be more advantageous. Learning these contingencies typically requires an extended period of sampling across decks, as the frequencies and magnitudes of the punishments vary across decks. Rather remarkably, the initial sample of patients, with lesions encompassing orbitofrontal cortex (OFC), showed a robust preference for the higher-paying but ultimately disadvantageous decks, and appeared to be almost totally indifferent to punishment (Bechara, et al., 1994). Thus, it appeared that their behavior was driven by reward seeking alone, as if the punishments simply failed to occur.
There are multiple lines of evidence suggesting OFC dysfunction in patients with schizophrenia (SZ), including evidence of reduced volumes (Davatzikos et al. 2005), task-evoked hypoactivity (Quintana et al. 2003), and impairments in reversal learning (Waltz & Gold, 2007; Waltz et al., 2013), considered a key cognitive process mediated by OFC. In light of these findings, it would be reasonable to expect that patients with SZ would show robust impairments on the IGT. Surprisingly, the literature is somewhat mixed; most, but not all studies suggest a reduced preference for the advantageous decks relative to the disadvantageous decks, perhaps suggesting a reduced sensitivity to punishments in SZ patients. In an effort to better understand the accumulated literature, we performed a small meta-analysis (weighted by sample size) of the eight studies (Wilder et al. 1998; Ritter et al. 2004; Shurman et al. 2005; Kester et al. 2006; Lee et al. 2007; Sevy et al. 2007; Kim et al. 2009, 2012), in SZ patients, that reported the number of choices from each of the four IGT decks (see Supplementary Table s1 for a list of the studies used in the meta-analysis).
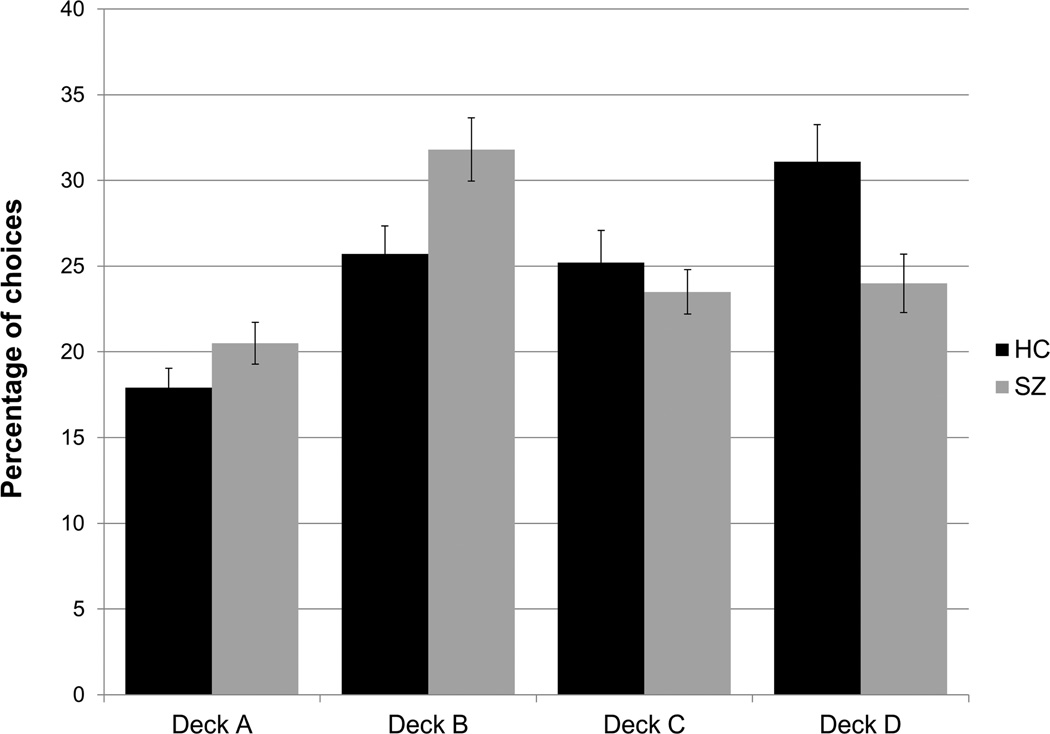
As shown in Figure 1, patients, relative to controls, show an increase in the number of selections from the disadvantageous decks (A and B), and a reduction in the number of selections from the advantageous decks (C and D). While the results from individual published studies vary, the summary figure suggests a relatively robust pattern of group differences. Also seen in Figure 1 is the fact that controls show a clear preference for Deck B relative to A (among the disadvantageous decks) and for Deck D relative to C (among the advantageous decks). Interestingly, Deck A delivers more frequent punishments whereas Deck B delivers larger, but less frequent, punishments. Similarly, Deck C delivers smaller, but more frequent punishments, relative to Deck D. Thus the choice or avoidance of Decks A and C can be primarily attributed to the frequency of punishments. In contrast, Decks B and D require a more complex calculation of expected value over an extended number of selections and experienced outcomes. Patients maximally deviate from controls on Decks B and D (the mean effect-sizes for those differences are the farthest from zero), and show near normal sensitivity to the frequency of punishments. This raises the possibility that the IGT deficit in schizophrenia arises from a problem in calculating expected value rather than a reduced sensitivity to punishment.
Figure 1.
Weighted averages of individual deck choices on the Iowa Gambling Task (IGT) by patients and controls across 8 previous studies reporting individual deck choices. Error bars reflect one standard error. Relative to controls, patients show an increase in the number of selections from both disadvantageous decks (for Deck A, mean Cohen’s d = 0.421; 95% CI of Cohen’s d: [0.23, 0.61]; for Deck B (mean d = 0.670; 95% CI of d: [0.48, 0.86]). Relative to controls, patients show a decrease in the number of selections from both advantageous decks (for Deck C, d = −0.202; 95% CI of d: [−0.39, −0.01]; for Deck D, d = −0.703; 95% CI of d: [−0.90, −0.51]).
To examine this issue, we also administered the Balloon Analog Risk Task (BART; Lejuez et al. 2003), another experiment paradigm designed to examine decision making under risk. In this task, subjects “inflate” a balloon using the space bar on the computer. As the balloon gets bigger, the potential reward gets bigger. However, every trial will end with the balloon popping if the subject continues to press, resulting in a loss of earnings. The only sure way to retain earnings is to decide to stop pressing. Note: the balloon pops randomly somewhere between the first and the 128th potential press, such that the optimal strategy to maximize gains would be to press 64 times each time, thereby ensuring the fewest pops coupled with the maximal retained gains. Thus, unlike the IGT, eventual loss is certain in the BART, and the question is how much risk subjects are willing to take to increase the magnitude of their reward. Prior studies with the BART have found that several clinical populations with impulse control deficits show abnormal risk seeking, whereby individuals make larger than optimal numbers of pumps, on average, seemingly less deterred by impending punishment. By contrast: three previous studies have used the BART in studies of SZ patients, and in all three studies, SZs showed risk aversion (fewer pumps) relative to controls (Cheng et al. 2012; Reddy et al. 2014; Fischer et al. 2015). That is, they appeared to be abnormally sensitive to the prospect of a punishment and settled for lesser gains.
This is not the pattern of results that would be expected based on findings from IGT studies in SZ patients, which appear to show reduced sensitivity to punishments. The results, however, can be reconciled as follows: if patients with SZ have relatively intact sensitivity to the frequency of punishments in guiding choice, and impaired ability to simultaneously consider magnitude and frequency of aversive outcomes, one would expect to find risk aversion on the BART (where punishment will occur on every trial – a pure case of learning based on punishment frequency), and risk seeking on the IGT (resulting in a preference for the disadvantageous decks, coupled with a reduced preference for Deck D). That is, one would expect SZ patients to prefer the advantageous deck with smaller, more frequent punishments (Deck C) to the advantageous deck with larger, but less frequent punishments (Deck D), as estimating the expected value of Deck D requires a more subtle calculation of expected value than Deck C (where more frequent punishments occur).
METHODS
Participants
Fifty-nine patients between the ages of 18–64 with schizophrenia (SZ) or schizoaffective disorder (N=10) by best estimate approach (utilizing the Structured Clinical Interview for DSM-IV Axis I Disorders (First 1997) direct assessment, family informants, and past medical records) were included in this study. Patients were recruited from Maryland Psychiatric Research Center (MPRC) research clinics and from community mental health centers. Exclusion criteria included: acute psychiatric instability (operationalized as change in medication/dose in the last four weeks), mental retardation, co-morbid medical issues, and meeting criteria for substance abuse (in the past three months) or dependence (in the past six months; other than for nicotine). All patients were medicated. Forty-eight were taking atypical antipsychotics (22 clozapine, 10 risperidone, 9 olanzapine, 4 quetiapine, 2 ziprasidone and 1 aripiprazole), 7 were taking typical antipsychotics (2 haloperidol, 3 fluphenazine, 1 chlorpromazine and 1 thiothixene), and 3 patients were taking a combination of first- and second-generation antipsychotics. Medication information was missing for one patient.
Forty-three healthy control (HC) participants, matched for important demographic variables, were recruited from the community via newspaper advertisements. Control volunteers were between the ages of 18–64 and had no history of psychosis or neurological disease/condition that would interfere with test performance. Written documentation of informed consent was obtained from all participants. The institutional review boards of the University of Maryland and the Maryland State Department of Health and Mental Hygiene approved the study.
General Procedures
In SZ patients, overall psychiatric symptom severity was assessed with the Brief Psychiatric Rating Scale (BPRS: Overall and Gorman 1962), and negative symptom severity were measured with the Scale for the Assessment of Negative Symptoms scale (SANS: Andreasen 1984). Patients in the study exhibited relatively mild degrees of negative and overall symptoms (SANS global sum score = 5.8; SD = 4.0; mean BPRS score = 1.9; SD = 0.5). A battery of cognitive and neuropsychological measures was administered to all patients and healthy controls, including the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS: Randolph et al. 1998), the Wechsler Abbreviated Scale of Intelligence (WASI: Wechsler 1999), the Letter Number Sequencing Test (LNS: Gold et al. 1997) and the Digit Symbol Substitution Test (Wechsler 1997). Subject characterizing information is shown in Table 1.
Table 1.
Group demographics, neurocognitive test scores, and results of statistical group comparisons. Values shown represent group means and standard deviations in parentheses.
| Schizophrenia Patients (N=59) |
Healthy Controls (N=43) |
Test Statistic |
|
|---|---|---|---|
| Female/Male | 17/42 | 17/26 | X2=1.28 |
| Age | 42.2 (11.8) | 41.1 (11.8) | t=0.50 |
| Education | 12.6 (2.7) | 14.8 (2.2) | t=4.32*** |
| BPRS Anxiety | 2.1 (0.9) | - | - |
| BPRS Negative | 1.9 (0.8) | - | - |
| BPRS Psychosis | 2.4 (1.1) | - | - |
| BPRS Disorganization | 1.3 (0.5) | - | - |
| SANS Global Sum | 5.8 (4.0) | - | - |
| RBANS Total Score | 78.5 (13.5) | 98.3 (13.6) | t=7.28*** |
| LNS | 8.8 (3.4) | 11.1 (3.4) | t=5.83*** |
| WAIS III Digit Symbol | 7.7 (3.4) | 10.3 (3.5) | t=5.93*** |
| WASI Verbal IQ* | 94.1 (14.3) | 110.7 (13.1) | t=5.94*** |
| WASI Performance IQ* | 92.6 (15.2) | 110.0 (12.5) | t=6.09*** |
= One control did not receive the WAIS
= p < 0.001
Abbreviations: BPRS, Brief Psychiatric Rating Scale; SANS, Scale for the Assessment of Negative Symptoms; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; LNS, University of Maryland Letter-Number Sequencing Test; WAIS, Wechsler Adult Intelligence Scale; WASI, Wechsler Abbreviated Scale of Intelligence.
The experimental tasks were presented in a randomly-determined order for each subject. In order to make the rewards and punishments more concrete, participants earned real money that was relative to the choices they made and points earned on each task; and all subjects were informed of this.
Experimental Tasks
The Iowa Gambling Task (Bechara et al. 1994) uses four simulated card decks arranged in a row. Players draw 100 total cards, with each card specifying a certain amount of money lost or won. Decks A and B always offer a higher reward of $100 and are accompanied by frequent moderate (Deck A, $100–350, 50% chance of loss, expected value of −25) or rare large (deck B, $1,250, 10% chance of loss, expected value of −25) penalties. The other decks, C and D, always present a lower reward of $50, but rewards are accompanied by either frequent small (deck C, $25–75, 50% chance of loss, expected value of +25) or rare moderate penalties (Deck D, $250, 10% chance of loss, expected value of +25). Subjects are not aware of the decks' different reward/penalty schedules but are told to pick freely from any decks. The main dependent measures for this task were the numbers of cards chosen from each deck. We summed choices from multiple decks to produce two additional dependent measures: 1) the proportion of cards drawn from the advantageous (low-risk) decks (C and D, for which the expected value of choices was positive), and 2) the proportion of cards drawn from decks with infrequent losses (Decks B and D, which were accompanied by losses 10% of the time; choices from Decks A and C were accompanied by losses 50% of the time).
In the Balloon Analog Risk Task (Lejuez et al. 2003), subjects view a computer screen on which a simulated balloon and balloon pump is displayed. The participant is instructed that each press of a button inflates the balloon slightly and earns the participant 2 cents. This money is deposited into a temporary cache, and if a balloon pops, the money in the temporary cache is lost. At any time before the balloon pops the participant can choose to stop inflating the balloon and deposit the money from the temporary cache into a permanent bank, the contents of which are displayed on the computer screen at all times. Each participant is presented with 90 total balloons (trials), over 3 blocks of 30 trials each, with the chance of explosion determined by a probability along a normal distribution between 1 and 128, with the average number of pumps to explosion being 64. Dependent measures from this task included the average number of pumps per balloon (adjusted to include only those pumps that did not result in the balloon popping), total number of explosions, and total money earned.
Statistical Analysis
For analyses of data from the IGT, three separate ANOVAs were performed. First, an ANOVA using the rates of selecting from disadvantageous decks (A/B), with factors block (5 levels: 20 trials per block) and group (HC and SZ), were performed to assess learning performance in the two groups. Secondly, an ANOVA was performed using deck (4 levels: Deck A, Deck B, Deck C, Deck D) and group as factors to demonstrate deck effects. Third, a further ANOVA was then done to check for effects of loss frequency, using loss frequency [2 levels: rare (B/D), frequent (A/C) loss decks] as one factor, and group as another. Independent-samples t-tests were used in post-hoc tests to ascertain where differences in cell-means were present. For data from the BART, we performed an ANOVA on numbers of button-presses, with factors of block (3 levels: 30 trials per block) and group. Additionally, we assessed group-differences in the numbers of balloons exploded, and the amount of money earned, using t-tests. Finally, Pearson correlation analyses were performed to explore possible relationships between measures from the two experimental decision-making tasks, between experimental measures and measures of symptom severity in the SZ group, and between experimental measures and measures of cognitive performance within both groups. All statistical analyses were conducted using SPSS 19.0 software (IBM Corp, Armonk, NY).
RESULTS
IGT Results
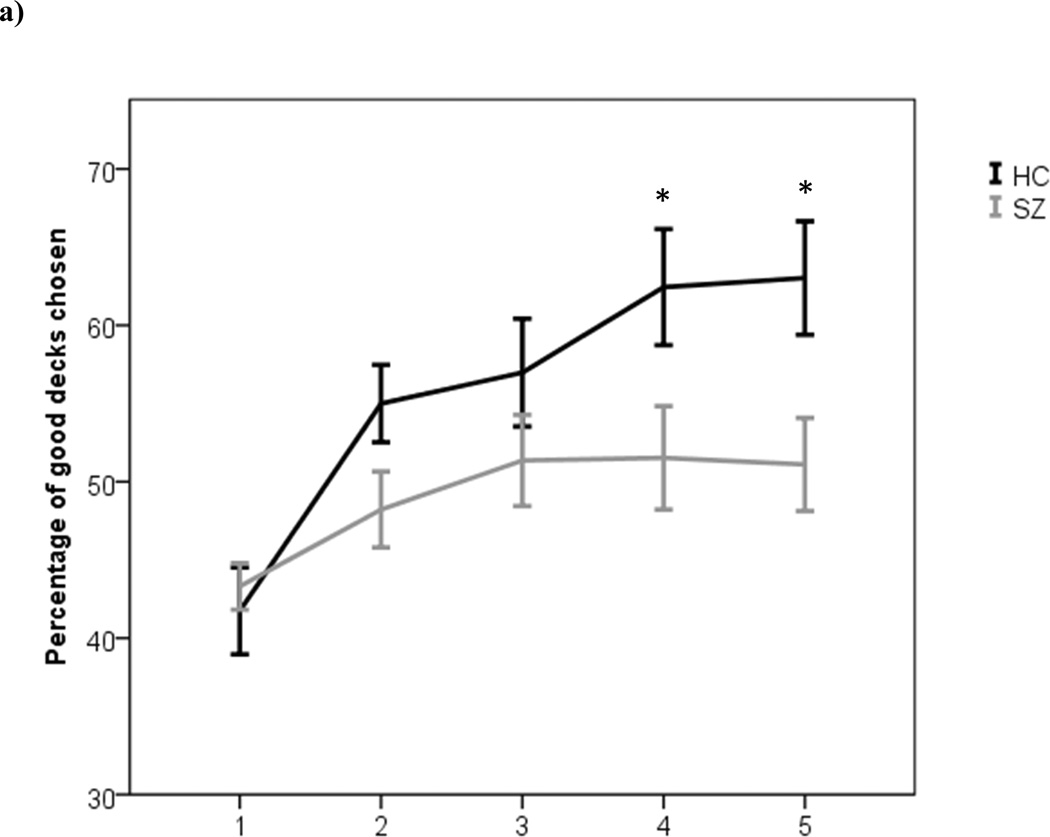
The ANOVA using the rates of selecting from disadvantageous decks (A/B) revealed main effects of both group [F(1,100)=5.02, p=0.03] and block [F(4,400)=13.36, p<0.01], with controls showing higher overall rates of selecting from advantageous decks than patients, and the entire sample showing better performance over time. These main effects were modulated, however, by a significant block×group interaction [F(4,400)=2.66, p=0.03], such that controls gradually learned to avoid the disadvantageous decks whereas the patient group showed impaired learning, relative to controls (Figure 2A). Indeed: patients showed minimal evidence of any performance improvement at all, across the course of the task. From post-hoc analyses of block-by-block learning performance, we observed a group difference on the 2nd block of trials that was approaching significance [t(100)=1.92, p=0.058], and clear significant group differences for the 4th [t(100)=2.18, p=0.03] and 5th blocks of trials [t(100)=2.55, p=0.01]. In essence, controls sampled the disadvantageous decks frequently enough to learn that the punishments exceed the gains. The ANOVA with group and deck as factors showed a main effect of deck [F(3,300)=24.02, p<0.001], but no main effect of group [F(1,100)=0.73, p=0.396], with the deck×group interaction effect approaching significance [F(1,100)=0.27, p=0.081].
Figure 2.
Performance measures from the IGT: current study participants. (a) Percentages of choices from advantageous decks (C and D) across blocks of trials by healthy controls and schizophrenia patients. As participants performed 100 trials of the task, each block represents 20 trials. (b) Percentages of choices from Decks with infrequent punishments (B and D). (c) Percentages of choices from each individual deck of the IGT. Error bars reflect one standard error. (*p<0.05)
The data shown in Figure 2A might be seen as evidence that patients simply responded randomly. However that is not the case, as we found that patients were sensitive to the frequency of losses. The ANOVA used to test for possible group differences in responses to loss frequency revealed a main effect of loss-frequency [F(1, 100)=74.61, p<0.001], and no main effect of group [F(1,100)=0.73, p=0.396], and no significant interaction effect [F(1, 100)=p=0.377].
Post-hoc analysis of the percentage of choices for each individual deck revealed significant group differences on Deck A [t(100)=2.84, p<0.01; 95% CIs [15.79, 18.45], d=0.569] and Deck D [t(100)=1.79, p=0.04, 95% CIs [26.43, 31.39], d=0.359], whereas no significant group differences were found on Decks B [t(100)=1.00, p=0.16, 95% CIs [28.22, 33.28], d=0.201] or C [t(100)=0.79, p=0.22, 95% CIs [21.24, 25.4], d=0.158], as depicted in Figure 2C. We observed a trend towards a group-difference in the amount of money won overall on the IGT [t(100)=1.77, p=0.08]. The effect size of this group difference was small-to-moderate 95% CIs [−0.04, 0.75], d=0.355).
BART Results
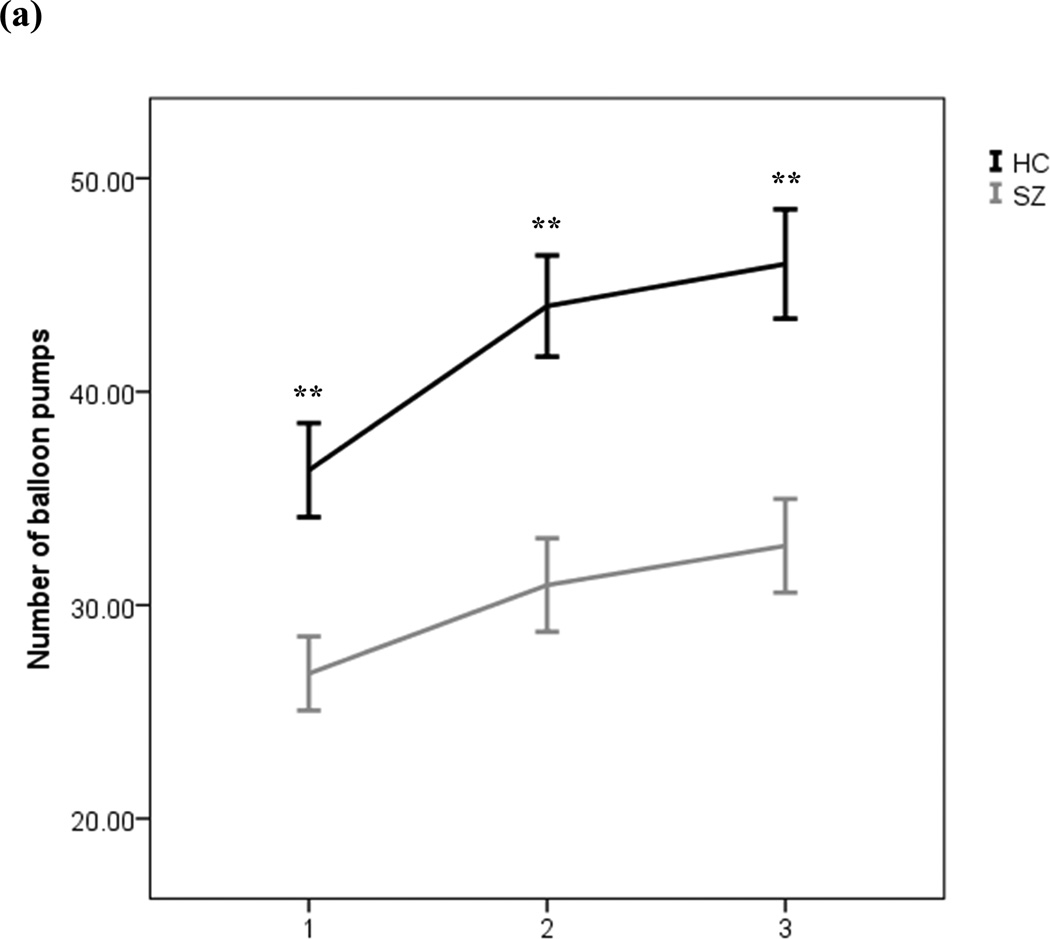
As seen in Figure 3A, healthy controls chose to pump much more per balloon than did patients, with both groups increasing their average numbers of pumps per balloon as the task went on. This was confirmed statistically where an ANOVA on the total number of pumps produced a significant main effect of group [F(1,101)=16.82, p<0.01], such that SZ patients made, on average, fewer pumps per balloon (mean=30.17, SD=14.52), when compared to HCs (mean=42.11, SD=14.52). We also observed a main effect of block on number of pumps [F(1,101)=30.47, p<0.01], with a greater number of pumps in the last block as compared to the first (see Figure 3A), suggesting that both groups learned that they could earn more points by pressing more than they initially did. The interaction between group and block was not significant [F(2,100)=1.99, p=0.14], however, indicating that the groups did not show differential rates of change in their pressing behavior across blocks of trials. It is noteworthy that even though the controls pumped much more often than patients did, the controls chose to pump much less than was optimal in terms of maximizing potential rewards.
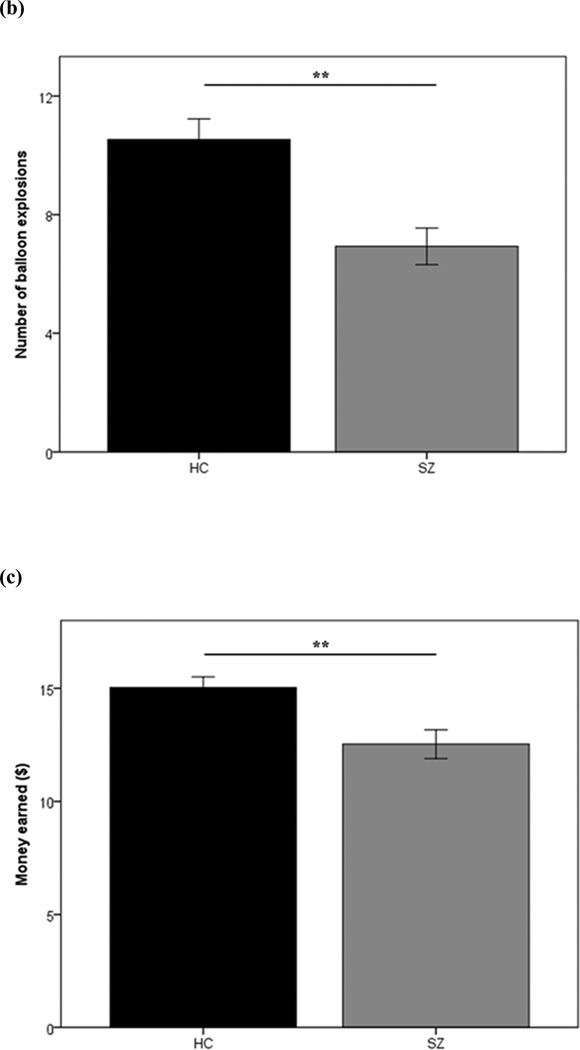
Figure 3.
Performance measures from the Balloon Analogue Risk Task (BART): current study participants. (a) Average numbers of balloon pumps by patients with schizophrenia and healthy controls, across the 3 experimental blocks of trials. (b) Number of explosions experienced and (c) total money earned on the BART by patients with schizophrenia and healthy controls. Error bars reflect one standard error. (**p<0.01)
As a consequence of making fewer average overall pumps, relative to controls, SZ patients also experienced a significantly lower number of explosions [t(100)=3.85, p<0.01; Figure 3B]. However, patients’ greater aversion to risk (or greater preference for certain, but smaller rewards) did not work to their advantage in the context of the BART; although it resulted in fewer unrewarded trials (fewer balloons popped), it also led to less money being won overall [t(100)=2.95, p<0.01; Figure 3C].
Correlation Analyses
Results from analyses of correlations between IGT and BART performance metrics and measures of symptom severity and neurocognitive capacity are presented in Table 2. Significant correlations were observed between disorganized symptoms on the BPRS and several measures from the BART, such that patients with more severe disorganized symptoms tended to pump less, earn less money and have less balloon explosions. We also observed a significant positive correlation between SANS affective blunting/alogia and average number of pumps on the BART, such that patients with greater negative symptoms actually demonstrated more balloon pumps. However, on closer inspection of the distribution of the data, it appears that an outlier is present in the SZ group (pumps more than 3SDs higher than the mean number of pumps for the SZ group), This individual seems to be driving the correlation between pumps and negative symptom scores, as this relationship is no longer statistically significant when this participant is removed from the sample. Figures s1 and s2 in the Supplementary Material illustrate the identification of the outlier, and the distribution of the data in relation to the correlation between BART pumps and negative symptoms.
Table 2.
Results of correlation analyses between performance measures from experimental tasks and symptoms and scores on measures of neurocognition in patients (SZ) and healthy controls (HC).
| IGT | BART | |||||
|---|---|---|---|---|---|---|
| High Risk Deck (AB) Choices |
Frequent Loss Deck (AC) Choices |
Money Earned |
Average Pumps Overall |
Money Earned |
Explosions | |
| Symptoms | ||||||
| BPRS Anxiety | 0.247 | 0.070 | −0.225 | −0.104 | 0.000 | −0.237 |
| BPRS Negative | −0.053 | −0.050 | −0.030 | 0.031 | 0.024 | 0.089 |
| BPRS Psychosis | −0.048 | −0.071 | 0.189 | −0.108 | −0.013 | −0.152 |
| BPRS Disorganization | 0.125 | −0.069 | −0.399* | −0.368* | −0.391* | −0.263* |
| SANS AFB/AL | 0.128 | 0.076 | −0.225 | 0.288* | 0.226 | 0.232 |
| SANS AA/RF | 0.182 | 0.044 | −0.157 | 0.247 | 0.175 | 0.265 |
| Neuropsych (SZ) | ||||||
| RBANS Total | −0.243 | 0.167 | 0.160* | 0.062 | 0.093 | −0.082 |
| LNS scaled | −0.160 | 0.204 | 0.075 | 0.089 | 0.090 | −0.003 |
| WAIS III Digit-Symbol scaled | −0.286* | 0.103 | 0.330* | 0.151 | 0.180 | 0.182 |
| WASI Verbal IQ | −0.358* | 0.100 | 0.374* | −0.053 | 0.021 | −0.164 |
| WASI Performance IQ | −0.157 | 0.211 | 0.093* | 0.156 | 0.173 | −0.006 |
| Neuropsych (HC) | ||||||
| RBANS Total | −0.329* | 0.221 | 0.217 | 0.121 | 0.048 | 0.135 |
| LNS scaled | −0.551* | 0.287 | 0.513* | 0.244 | 0.052 | 0.297 |
| WAIS III Digit-Symbol scaled | −0.220 | 0.284 | 0.409* | −0.130 | −0.004 | −0.066 |
| WASI Verbal IQ | −0.649* | 0.363* | 0.517* | 0.119 | 0.069 | 0.172 |
| WASI Performance IQ | −0.313 | 0.251 | 0.256 | 0.045 | 0.087 | 0.050 |
p < 0.05
Abbreviations: BPRS, Brief Psychiatric Rating Scale; SANS, Scale for the Assessment of Negative Symptoms; AA/RF, Anhedonia/Asociality/Role Functioning; AFB/AL, Affective Blunting/Alogia; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; LNS, University of Maryland Letter-Number Sequencing Test; WAIS, Wechsler Adult Intelligence Scale; WASI, Wechsler Abbreviated Scale of Intelligence.
Finally, there were a number of significant correlations between IGT variables and neuropsychological measures, whereby poorer neurocognition was associated with a greater tendency to choose from disadvantageous decks, a greater tendency to choose from decks with frequent punishments, and less money earned. Significant correlations between the IGT and BART performance measures were only found in the HC group, and not in the SZ group, whereby HCs who chose less from frequent loss decks (A and C) also earned more money on the BART (r=−0.35, p=0.02). Scatterplots illustrating the main significant correlations can be found in the Supplementary Materials in Figures s2–5.
DISCUSSION
This is the first study to investigate the role of punishment sensitivity in risky decision-making in SZ patients using results from both the IGT and the BART to probe the capacity to integrate information about outcome frequency and magnitude in decision-making under risk. We found that SZ patients showed deficits in the acquisition of the contingencies in both tasks. While patients and controls did not differ in their rates of choosing from decks with frequent punishments, patients still showed significantly higher rates of choices from disadvantageous decks, because they chose more often than controls from the disadvantageous deck with infrequent, large punishments (B). Thus, the idea that individuals with SZ choose the disadvantageous decks at higher rates than HCs due to reduced punishment sensitivity is too simple.
Our observation of a preference, in SZ patients, for the deck with rare large losses (Deck B), over the rare moderate loss deck (Deck C), is consistent with a number of previously reported findings in SZ (Ritter et al. 2004; Shurman et al. 2005) and appears to reflect a tendency to utilize outcome-frequency information at the expense of outcome-magnitude information. This represents a sub-optimal decisionmaking strategy, as the rare large loss deck (Deck B) has a negative expected value. The greater preference for deck B seen in patients in our results, as well as the reduced preference for deck D compared to HCs, is well in line with the general pattern of responses of SZ patients in the meta-analysis of previous studies presented in Figure 1. It should be noted, however, that the apparent deficit in utilizing information about outcome magnitude in SZ patients does not necessarily lead to overall reduced earning on the IGT, as we and others (Wilder et al. 1998; Cavallaro et al. 2003; Evans et al. 2005; Rodriguez-Sanchez et al. 2005; Turnbull et al. 2006) have found. In our study, the lack of a significant difference in money earned (p=0.08) is likely attributable to limited power. That is, patients did not experience big losses frequently enough, following choices from Deck B, to lead to an overall difference in money earned.
Nonetheless, a profound deficit in reinforcement learning is evident from the learning curve of patients across blocks of the IGT. Although SZ patients and HCs started off at similar performance levels, SZ patients did not go on to learn to choose good decks more frequently, and group differences emerged in the later trials of the task, after HCs had the chance to learn. The analysis of performance over time offers a more nuanced understanding of the factors influencing decisions than overall summary scores, which have been the typical focus of analysis beginning with the seminal work of Bechara et al. (1994). This finding of an impairment in the ability to use feedback to adaptively update estimations of expected value fits with the findings and formulations of Brambilla et al. (2013), who used expectancy-valence modeling (Busemeyer and Stout 2002) of IGT data in reaching the conclusion that "associative learning underlying the representation of expectancies was disrupted in SZ". As these and other researchers (Maia and McClelland 2004; Brambilla et al. 2013) have noted, there are multiple possible paths to impaired performance on the IGT, and genuine insensitivity to punishments appears to be characteristic of patient groups, such as those with bipolar affective disorder (Brambilla et al. 2013; Burdick et al. 2014) or orbitofrontal lesions (Bechara et al. 1994, 1999).
On the BART, by contrast, patients did demonstrate some degree of learning from losses and gains, as they pumped more as the task progressed. However, SZ patients started off at a lower level of performance than controls and never caught up, resulting in worse performance overall, consistent with the findings of previously-reported studies using the BART in SZ patients (Cheng et al. 2012; Reddy et al. 2014; Fischer et al. 2015). Importantly, SZ patients showed sensitivity to negative outcomes (balloon pops) to such an extent that they earned significantly fewer overall points, relative to controls. Thus, as we hypothesized, simple insensitivity to punishment does not appear to have been the cause of performance deficits in SZ, in the context of either task. A more plausible explanation is that SZ patients effectively utilized information about punishment frequency, while neglecting information about the magnitude of punishments and rewards.
This is not to say that reduced sensitivity to losses plays no role in RL deficits in schizophrenia at all; numerous results suggest the opposite. For example, studies of reversal learning in SZ reveal that patients consistently perform worse than controls (Elliott et al. 1995; Pantelis et al. 1999; Waltz and Gold 2007; Murray et al. 2008b; Leeson et al. 2009). Reversal learning is thought to depend largely on the ability to modify stimulus-responses associations by integrating error signals from ventral striatum and OFC (but see Waltz et al. 2013). Furthermore, negative symptoms in SZ have been shown to be associated with impairments in rapid, trial-to-trial behavioral adjustments during the early phases of learning, thought to reflect prefrontal cortical dysfunction (Waltz et al. 2007, 2011).
An important question that arises from our findings is whether the deficit on the IGT in patients is a result of poor working memory (WM), rather than a problem of reinforcement learning (RL), per se. A recent study from our group (Collins et al. 2014) used a paradigm in which set size and delay between stimulus repetitions were manipulated experimentally in order to assess the specific contribution of working memory. Computational modeling of these data indicated that RL deficits in SZ could be largely accounted for by deficits in WM capacity. That does not appear to be the case with the IGT performance. As seen in Table 2, the correlation between working memory (Letter-Number Sequencing) and the number of disadvantageous choices is more robust in controls than it is in the patient group. That is, working memory capacity appears to be more rate-limiting for decision making in the control group than in the patient group. Similarly, the correlation coefficients with Verbal Intelligence are also substantially higher in controls than in patients. Thus, it appears unlikely that between-group differences on measures of cognitive ability account for the observed differences on the IGT.
Importantly, we did not observe meaningful correlations between performance on either task and the severity of positive and negative symptoms in SZ patients, which may have been due to the generally low symptom severity of the patients in our sample. However, in one case there was a positive correlation between affective flattening/blunting on the SANS and number of patients' average number of pumps on the BART. This was a very counter-intuitive result, as our group recently found that negative symptoms were associated with a reduced willingness to expend effort to obtain higher levels of reward (Gold et al. 2013), a finding replicated by others (Fervaha et al. 2013; Barch et al. 2014). The positive correlation, observed in patients, between affective flattening/blunting on the SANS and number of pumps on the BART seems to have been driven by a single outlier in the patient group. By contrast, we observed more robust associations in the opposite direction between performance measures from both the IGT and the BART and disorganization symptoms in patients, and we observed significant correlations between IGT performance measures and measures of intellectual functioning in both patients and controls. These relationships support a role for DLPFC-dependent "cold" cognitive abilities in successful IGT performance, consistent with previous work (Manes et al. 2002; Fellows and Farah 2005).
Interestingly, correlations between performance on the IGT and the BART were found in HCs, but not in SZs. This finding could be indicative of the different decision-making strategies, and underlying neural substrates, which are utilized across the two tasks. The BART has been associated with impulse control and risk aversion in a context in which loss is inevitable after a certain number of pumps, whereas losses on the IGT are always probabilistic, regardless of the response made. Thus, it may be the case that risk-aversion may have been a factor in the performance of controls on both the BART and the IGT, whereas patients only behaved in a risk-averse manner on the BART, when losses were certain. It appears that the uncertainty and infrequency of losses on the IGT did not evoke the same risk-averse behavior in patients that it did in controls. These discrepant findings on the two tasks could give further insight into subtler, context-dependent abnormalities in risk-aversion in SZ. Therefore, future studies in SZ would benefit from the use of multiple decision-making tasks that assess risk under different circumstances.
One main limitation of this study is the potential confounding effect of antipsychotic medication on reinforcement learning performance. There is some evidence to suggest that SZ patients using second-generation antipsychotics (SGAs) relatively-worse performance on the IGT, when compared with those taking first-generation antipsychotics (FGAs; Beninger et al. 2003). The study from Reddy and colleagues (2014) also found that bipolar patients who were taking antipsychotic medication were more risk averse on the BART, compared to those not taking antipsychotics. However, other evidence points to the presence of reinforcement learning deficits in unmedicated patients with schizophrenia (Murray et al. 2008a; Schlagenhauf et al. 2009), and some data suggest that SGAs do not negatively impact feedback signals in the human brain, perhaps enhancing them (Bates et al. 2004; Schneider et al. 2013). An adequate assessment of this issue is, in any case, only possible in the context of a controlled clinical trial, with random assignment to drug.
We also acknowledge, as a limitation of the study, that socioeconomic status (SES) may have exerted an influence on tasks subject to monetary performance bonuses, such as the ones we used. In the case of our particular study, however, we believe the influence to be negligible, in that patients and controls did not differ in mean parental educational attainment, which served as a proxy for SES. Finally, our study lacked an explicit debriefing, where participants were asked to specifically state their relative preferences for decks; thus, we were unable to ascertain associations between explicit and implicit preferences.
In conclusion, we demonstrate that SZ patients are able to effectively use information about outcome frequency, but simultaneously neglect information about the magnitude of outcomes, and that this is not specific to gains or losses. These findings suggest that a deficit in integrating information about outcome magnitude and frequency, can lead to problems in accurately representing and updating the expected value on a trial-by-trial basis, and consequently lead to overall impairments in reinforcement learning. These findings suggest an alternative explanation for the performance deficits seen in people with SZ on risky decision-making tasks, particularly the IGT, and may also account for some inconsistencies seen in the literature. Future neuroimaging studies using the IGT in conjunction with other decision making tasks, such as the BART, would help to clarify the neural substrates underlying deficits in risky decision making seen in SZ.
Supplementary Material
Highlights.
Patients with schizophrenia show overall impaired learning on the IGT and BART.
These impairments were not attributable to reduced sensitivity to punishments.
Poor integration of reward magnitude and frequency may cause learning deficits.
Acknowledgements
Sharon August, Lindsey Phebus, and Rebecca Wilbur assisted with the collection of the data.
Role of Funding Source This work was supported by Grants R01MH058898 (PI: Carpenter) and R01MH094460 (PI: Waltz) from the National Institute of Mental Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City, IA: University of Iowa; 1984. [PubMed] [Google Scholar]
- Barch DM, Treadway MT, Schoen N. Effort, anhedonia, and function in schizophrenia: reduced effort allocation predicts amotivation and functional impairment. J Abnorm Psychol. 2014 May;123(2):387–397. doi: 10.1037/a0036299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates AT, Liddle PF, Kiehl KA, Ngan ET. State dependent changes in error monitoring in schizophrenia. J Psychiatr Res. 2004 May;38(3):347–356. doi: 10.1016/j.jpsychires.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994 Jun;50(1–3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999 Jul 1;19(13):5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997 Feb 28;275(5304):1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Beninger RJ, Wasserman J, Zanibbi K, Charbonneau D, Mangels J, Beninger BV. Typical and atypical antipsychotic medications differentially affect two nondeclarative memory tasks in schizophrenic patients: a double dissociation. Schizophr Res. 2003 Jun 1;61(2–3):281–292. doi: 10.1016/s0920-9964(02)00315-8. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Perlini C, Bellani M, Tomelleri L, Ferro A, Cerruti S, et al. Increased salience of gains versus decreased associative learning differentiate bipolar disorder from schizophrenia during incentive decision making. Psychol Med. 2013 Mar;43(3):571–580. doi: 10.1017/S0033291712001304. [DOI] [PubMed] [Google Scholar]
- Burdick KE, Braga RJ, Gopin CB, Malhotra AK. Dopaminergic influences on emotional decision making in euthymic bipolar patients. Neuropsychopharmacology. 2014 Jan;39(2):274–282. doi: 10.1038/npp.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busemeyer JR, Stout JC. A contribution of cognitive decision models to clinical assessment: decomposing performance on the Bechara gambling task. Psychol Assess. 2002 Sep;14(3):253–262. doi: 10.1037//1040-3590.14.3.253. [DOI] [PubMed] [Google Scholar]
- Cavallaro R, Cavedini P, Mistretta P, Bassi T, Angelone SM, Ubbiali A, et al. Basal-corticofrontal circuits in schizophrenia and obsessive-compulsive disorder: a controlled, double dissociation study. Biol Psychiatry. 2003 Aug 15;54(4):437–443. doi: 10.1016/s0006-3223(02)01814-0. [DOI] [PubMed] [Google Scholar]
- Cheng GLF, Tang JCY, Li FWS, Lau EYY, Lee TMC. Schizophrenia and risk-taking: impaired reward but preserved punishment processing. Schizophr Res. 2012 Apr;136(1–3):122–127. doi: 10.1016/j.schres.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Collins AGE, Brown JK, Gold JM, Waltz JA, Frank MJ. Working memory contributions to reinforcement learning impairments in schizophrenia. J Neurosci Off J Soc Neurosci. 2014 Oct 8;34(41):13747–13756. doi: 10.1523/JNEUROSCI.0989-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davatzikos C, Shen D, Gur RC, Wu X, Liu D, Fan Y, et al. Whole-brain morphometric study of schizophrenia revealing a spatially complex set of focal abnormalities. Arch Gen Psychiatry. 2005 Nov;62(11):1218–1227. doi: 10.1001/archpsyc.62.11.1218. [DOI] [PubMed] [Google Scholar]
- Elliott R, McKenna PJ, Robbins TW, Sahakian BJ. Neuropsychological evidence for frontostriatal dysfunction in schizophrenia. Psychol Med. 1995 May;25(3):619–630. doi: 10.1017/s0033291700033523. [DOI] [PubMed] [Google Scholar]
- Evans CEY, Bowman CH, Turnbull OH. Subjective awareness on the Iowa Gambling Task: the key role of emotional experience in schizophrenia. J Clin Exp Neuropsychol. 2005 Aug;27(6):656–664. doi: 10.1081/13803390490918354. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cereb Cortex. 2005 Jan;15(1):58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- Fervaha G, Graff-Guerrero A, Zakzanis KK, Foussias G, Agid O, Remington G. Incentive motivation deficits in schizophrenia reflect effort computation impairments during cost-benefit decision-making. J Psychiatr Res. 2013 Nov;47(11):1590–1596. doi: 10.1016/j.jpsychires.2013.08.003. [DOI] [PubMed] [Google Scholar]
- First MB. User’s Guide for the Structured Clinical Interview for DSM-IV Axis II Personality Disorders: SCID-II. American Psychiatric Pub. 1997 [Google Scholar]
- Fischer BA, McMahon RP, Kelly DL, Wehring HJ, Meyer WA, Feldman S, et al. Risk-taking in schizophrenia and controls with and without cannabis dependence. Schizophr Res. 2015 Feb;161(2–3):471–477. doi: 10.1016/j.schres.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Carpenter C, Randolph C, Goldberg TE, Weinberger DR. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry. 1997 Feb;54(2):159–165. doi: 10.1001/archpsyc.1997.01830140071013. [DOI] [PubMed] [Google Scholar]
- Gold JM, Goldberg RW, McNary SW, Dixon LB, Lehman AF. Cognitive correlates of job tenure among patients with severe mental illness. Am J Psychiatry. 2002 Aug;159(8):1395–1402. doi: 10.1176/appi.ajp.159.8.1395. [DOI] [PubMed] [Google Scholar]
- Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, Frank MJ. Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biol Psychiatry. 2013 Jul 15;74(2):130–136. doi: 10.1016/j.biopsych.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kester HM, Sevy S, Yechiam E, Burdick KE, Cervellione KL, Kumra S. Decision-making impairments in adolescents with early-onset schizophrenia. Schizophr Res. 2006 Jul;85(1–3):113–123. doi: 10.1016/j.schres.2006.02.028. [DOI] [PubMed] [Google Scholar]
- Kim YT, Lee K-U, Lee SJ. Deficit in Decision-Making in Chronic, Stable Schizophrenia: From a Reward and Punishment Perspective. Psychiatry Investig. 2009 Mar;6(1):26–33. doi: 10.4306/pi.2009.6.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-T, Sohn H, Kim S, Oh J, Peterson BS, Jeong J. Disturbances of motivational balance in chronic schizophrenia during decision-making tasks. Psychiatry Clin Neurosci. 2012 Dec;66(7):573–581. doi: 10.1111/j.1440-1819.2012.02403.x. [DOI] [PubMed] [Google Scholar]
- Leeson VC, Robbins TW, Matheson E, Hutton SB, Ron MA, Barnes TRE, et al. Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biol Psychiatry. 2009 Sep 15;66(6):586–593. doi: 10.1016/j.biopsych.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Kim Y-T, Seo E, Park O, Jeong S-H, Kim SH, et al. Dissociation of emotional decision-making from cognitive decision-making in chronic schizophrenia. Psychiatry Res. 2007 Aug 30;152(2–3):113–120. doi: 10.1016/j.psychres.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Zvolensky MJ, Pedulla CM. Evaluation of the Balloon Analogue Risk Task (BART) as a predictor of adolescent real-world risk-taking behaviours. J Adolesc. 2003 Aug;26(4):475–479. doi: 10.1016/s0140-1971(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Maia TV, McClelland JL. A reexamination of the evidence for the somatic marker hypothesis: what participants really know in the Iowa gambling task. Proc Natl Acad Sci U S A. 2004 Nov 9;101(45):16075–16080. doi: 10.1073/pnas.0406666101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, et al. Decision-making processes following damage to the prefrontal cortex. Brain. 2002 Mar;125(Pt 3):624–639. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- Murray GK, Cheng F, Clark L, Barnett JH, Blackwell AD, Fletcher PC, et al. Reinforcement and reversal learning in first-episode psychosis. Schizophr Bull. 2008a Sep;34(5):848–855. doi: 10.1093/schbul/sbn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray GK, Clark L, Corlett PR, Blackwell AD, Cools R, Jones PB, et al. Incentive motivation in first-episode psychosis: a behavioural study. BMC Psychiatry. 2008b;8:34. doi: 10.1186/1471-244X-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JE, Gorman DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- Pantelis C, Barber FZ, Barnes TR, Nelson HE, Owen AM, Robbins TW. Comparison of set-shifting ability in patients with chronic schizophrenia and frontal lobe damage. Schizophr Res. 1999 Jun 22;37(3):251–270. doi: 10.1016/s0920-9964(98)00156-x. [DOI] [PubMed] [Google Scholar]
- Quintana J, Wong T, Ortiz-Portillo E, Kovalik E, Davidson T, Marder SR, et al. Prefrontal-posterior parietal networks in schizophrenia: primary dysfunctions and secondary compensations. Biol Psychiatry. 2003 Jan 1;53(1):12–24. doi: 10.1016/s0006-3223(02)01435-x. [DOI] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998 Jun;20(3):310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- Reddy LF, Lee J, Davis MC, Altshuler L, Glahn DC, Miklowitz DJ, et al. Impulsivity and Risk Taking in Bipolar Disorder and Schizophrenia. Neuropsychopharmacology. 2014 Jan;39(2):456–463. doi: 10.1038/npp.2013.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter LM, Meador-Woodruff JH, Dalack GW. Neurocognitive measures of prefrontal cortical dysfunction in schizophrenia. Schizophr Res. 2004 May 1;68(1):65–73. doi: 10.1016/S0920-9964(03)00086-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sanchez JM, Crespo-Facorro B, Perez-Iglesias R, Gonzalez-Blanch C, Alvarez-Jimenez M, Llorca J, et al. Prefrontal cognitive functions in stabilized first-episode patients with schizophrenia spectrum disorders: a dissociation between dorsolateral and orbitofrontal functioning. Schizophr Res. 2005 Sep 15;77(2–3):279–288. doi: 10.1016/j.schres.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Schlagenhauf F, Sterzer P, Schmack K, Ballmaier M, Rapp M, Wrase J, et al. Reward feedback alterations in unmedicated schizophrenia patients: relevance for delusions. Biol Psychiatry. 2009 Jun 15;65(12):1032–1039. doi: 10.1016/j.biopsych.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Schneider S, Bahmer TJ, Metzger FG, Reif A, Polak T, Pfuhlmann B, et al. Quetiapine and flupentixol differentially improve anterior cingulate cortex function in schizophrenia patients: an event-related potential study. Int J Neuropsychopharmacol. 2013 Oct;16(9):1911–1925. doi: 10.1017/S1461145713000540. [DOI] [PubMed] [Google Scholar]
- Sevy S, Burdick KE, Visweswaraiah H, Abdelmessih S, Lukin M, Yechiam E, et al. Iowa gambling task in schizophrenia: a review and new data in patients with schizophrenia and co-occurring cannabis use disorders. Schizophr Res. 2007 May;92(1–3):74–84. doi: 10.1016/j.schres.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurman B, Horan WP, Nuechterlein KH. Schizophrenia patients demonstrate a distinctive pattern of decision-making impairment on the Iowa Gambling Task. Schizophr Res. 2005 Jan 1;72(2–3):215–224. doi: 10.1016/j.schres.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Turnbull OH, Evans CEY, Kemish K, Park S, Bowman CH. A novel set-shifting modification of the iowa gambling task: flexible emotion-based learning in schizophrenia. Neuropsychology. 2006 May;20(3):290–298. doi: 10.1037/0894-4105.20.3.290. [DOI] [PubMed] [Google Scholar]
- Waltz JA, Frank MJ, Robinson BM, Gold JM. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biol Psychiatry. 2007 Oct 1;62(7):756–764. doi: 10.1016/j.biopsych.2006.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz JA, Frank MJ, Wiecki TV, Gold JM. Altered probabilistic learning and response biases in schizophrenia: Behavioral evidence and neurocomputational modeling. Neuropsychology. 2011;25(1):86–97. doi: 10.1037/a0020882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz JA, Gold JM. Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr Res. 2007 Jul;93(1–3):296–303. doi: 10.1016/j.schres.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz JA, Kasanova Z, Ross TJ, Salmeron BJ, McMahon RP, Gold JM, et al. The roles of reward, default, and executive control networks in set-shifting impairments in schizophrenia. PloS One. 2013;8(2):e57257. doi: 10.1371/journal.pone.0057257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wais-III. Psychological Corporation. 1997 [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio TX: The Psychological Corporation; 1999. [Google Scholar]
- Wilder KE, Weinberger DR, Goldberg TE. Operant conditioning and the orbitofrontal cortex in schizophrenic patients: unexpected evidence for intact functioning. Schizophr Res. 1998 Mar 10;30(2):169–174. doi: 10.1016/s0920-9964(97)00135-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.