Abstract
Background and aims
Systematic studies of superficial siderosis(SS) and convexity subarachnoid hemorrhage (cSAH) in patients with suspected cerebral amyloid angiopathy(CAA) without lobar intracerebral hemorrhage(ICH) are lacking. We sought to determine the potential anatomic correlation between SS/cSAH and transient focal neurological episodes (TFNE) and whether SS/SAH is predictor of future cerebral hemorrhagic events in these patients.
Methods
We enrolled 90 consecutive patients with suspected CAA (due to the presence of strictly lobar microbleeds (CMBs) and/or SS/cSAH) but without history of symptomatic lobar ICH who underwent brain MRI including T2*-weighted, diffusion weighted imaging and fluid-attenuated inversion recovery sequences from an ongoing single center CAA cohort from 1998 to 2012. Evaluation of SS, cSAH and CMBs was performed. Medical records and follow-up information were obtained from prospective databases and medical charts. TFNE was defined according to published criteria and electroencephalogram reports were reviewed.
Results
Forty-one patients (46%) presented with SS and/or cSAH. The prevalence of TFNE was significantly higher in those with SS/cSAH (61% versus 10%; p<0.001) and anatomically correlated with the location of cSAH, but not SS. The majority of TFNE in patients with SS/cSAH presented with spreading sensory symptoms. Intermittent focal slowing on electroencephalogram was present in the same area as SS/cSAH in 6 patients, but no epileptiform activity was found in any patients. Among those with available clinical follow-up (76/90 patients, 84%), ten patients with SS/cSAH (29%, median time from the scan for all patients with SS/cSAH: 21 months) had a symptomatic cerebral bleeding event on follow-up (Average time to events: 34 months) compared with only 1 event (2.4%, 25 months from the scan) in patients without SS/cSAH (time to event: 25 months) (p=0.001). The location of hemorrhages on follow-up scan was not in the same location of previously noted SS/cSAH in 9 of 10 patients. Follow-up imaging was obtained in 9 of 17 patients with cSAH and showed evidence of SS in the same location as initial cSAH in all these 9 cases.
Conclusions
SS/cSAH is common in patients with suspected CAA without lobar intracerebral hemorrhage and may have a significantly higher risk of future cerebral bleeding events, regardless of the severity of the baseline CMB burden. The findings further highlight a precise anatomical correlation between TFNE and cSAH, but not SS. Distinct from transient ischemic attack or seizure, the majority of TFNE caused by SS/cSAH appear to present with spreading sensory symptoms.
Keywords: superficial siderosis, convexity subarachnoid hemorrhage, cerebral amyloid angiopathy, transient focal neurological episode, intracerebral hemorrhage
Introduction
Cerebral amyloid angiopathy (CAA) is an age-related small vessel disease characterized by deposition of β-amyloid protein in the walls of cortical and leptomeningeal arteries [1]. Although the most dramatic clinical presentation of CAA is symptomatic lobar intracerebral hemorrhage (ICH), many patients have only cerebral microbleeds (CMBs) [2–4]. In recent years, superficial siderosis (SS) and convexity subarachnoid hemorrhages (cSAH) have been recognized as important MRI markers for CAA [5–8]. These imaging markers, which may be symptomatic or silent, appear to increase sensitivity for diagnosis of CAA [5].
Transient focal neurological episodes (TFNE) in CAA have been previously described [9] and are now increasingly recognized as part of the CAA clinical spectrum [6, 10]. A previous study has correlated TFNE with SS in CAA patients who had suffered lobar ICH [7]. Several case series have suggested that CAA could be common cause of cSAH in the elderly [11,12]. Additionally, two recent studies in CAA with lobar ICH demonstrated that those with SS/cSAH had a higher risk of recurrent ICH compared with those without [13,14]. However, systematic studies regarding the characteristics and the clinical relevance of SS/cSAH in patients without lobar ICH, but still strongly suspected to have CAA, are lacking. Identifying neuroimaging markers that may predict first ever ICH in patients with CAA is of critical importance for early therapeutic intervention in the disease.
We thus performed a retrospective analysis of an ongoing cohort of patients with suspected CAA without lobar ICH seeking to determine 1) the characteristics of SS/cSAH in this population; 2) the prevalence and characteristics of TFNE in patients with SS/cSAH and whether there was a potential anatomic correlation between the two; 3) the future hemorrhagic risk for those patients with SS/cSAH compared with those without; and 4) the potential mechanism of SS/cSAH related TFNE.
Methods
Study participants
Systematic screening and recruitment from both inpatient services and outpatient clinics of all patients with lobar hemorrhage (including microbleeds) at MGH has been previously described [15]. For the present study, only consecutive patients with suspected CAA seen between 1998 and 2012 were deemed eligible. Suspected CAA was defined by the presence of any strictly lobar microbleeds and/or SS/cSAH in patients who did not have a current or previous history of lobar ICH[5,16] and was determined by consensus (J.N., E.A., E.M.G) based on clinical evaluation and imaging characteristics. The term “suspected CAA” was used to indicate that these patients did not strictly fulfill the pathologically-validated Boston criteria for CAA, as this validation study involved only patients with lobar ICH [17]. The patients who had at least one of the following criteria were included in this study: 1) ≥2 Lobar CMBs identified by T2*-weighted GRE without evidence of CMBs in deep brain regions (thalamus, basal ganglia) 2) SS identified by T2*-weighted GRE or cSAH identified by fluid attenuated inversion recovery (FLAIR) sequences. Using these criteria, a total of 90 patients were included. These individuals presented because of self-reported cognitive decline (n=34), TFNE (n=30), or incidental neurological symptoms that prompted MR imaging (including headache, confusion, gait difficulties, dizziness, syncope, transient global amnesia (n=26).
This study was approved by the hospital Institutional Review Board. Participants provided informed consent in accordance with protocols approved by the Partners Health Care Inc. Institutional Review Board.
Data collection
Clinical presentations for all subjects were collected from electronic medical charts. The presence of TFNE was defined by consensus between two raters (J.N. and E.A.) blind to imaging information and according to published criteria [6,10]: a clinical episode of transient focal neurological symptoms including numbness/tingling, weakness, dysarthria or aphasia lasting minutes to 1 hour with subsequent complete resolution. Non-focal symptoms such as dizziness, syncope, or acute confusion were excluded. We collected detailed information of TFNE, including clinical characteristics and precise onset time to further analyze correlations with SS/cSAH. All other variables were collected at time of subject recruitment including patient demographics, vascular risk factors, evidences of prior cerebrovascular events, previous diagnosis of dementia, and use of medications including antiplatelet, anticoagulant agents and statin use. Hypertension, diabetes mellitus, and dyslipidemia were coded as present if subjects were treated with related medications or had a prior medical history of disease. Evidence of atrial fibrillation was based on electrocardiogram or Holter monitoring. EEG reports, interpreted by a neurologist specialized in epilepsy, were also reviewed from medical charts. Routine clinical follow-up or re-hospitalization records were retrospectively analyzed in order to determine the number of patients who subsequently developed symptomatic bleeding events. Complete clinical follow-up information was available in a total of 76 patients (34/41 (83%) patients with SS/cSAH and 42/49 (86%) without; 84% of the whole cohort). Follow-up imaging was available in a subset of patients and was obtained either at the clinician’s discretion in the outpatient clinic, or for the evaluation of new neurologic symptoms in the emergency room (n=37; 19 patients with SS/cSAH and 18 without).
MRI acquisition and analysis
All subjects underwent standard clinical brain MRI on a 1.5T signa scanner. Diffusion weighted imaging (DWI), fluid-attenuated inversion recovery (FLAIR), and gradient-echo (GRE) sequences were obtained using previously reported parameters [3,18].
All images were reviewed for the presence and severity of SS/cSAH by two experienced clinical neurologists (JN and EA) without knowledge of clinical information and based on the following definitions: 1) SS: linear signal changes in the superficial layers of cerebral cortex which appear as low signal on T2*-weighted images [8]; 2) cSAH: localized subarachnoid bleeding identified either as a hyperintensity on computed tomography (CT) and/or a hyperintensity on FLAIR, and restricted to one or several adjacent sulci at the convexity of the brain without recent bleeding in the parenchyma, sylvian fissure, basal cisterns or ventricles [8]. The distribution of SS/SAH was classified as either focal (restricted to ≤3 sulci) or disseminated (≥4 sulci) [5]. After reviewing the images separately, raters met to achieve a final consensus rating for each SS or cSAH lesion. Inter-rater reliability (JN and EA) showed excellent agreement for the presence (Kappa=1.0) and severity (Kappa=0.8193) of SS/cSAH in 10 randomly selected patients.
T2*-weighted images were also evaluated for number of lobar CMBs (JN and EA) according to previously established consensus criteria [19]. We have previously shown excellent inter-rater reliability for CMB rating [4] and current raters exhibited high inter-rater reliability using a standard CAA CMB rating examination established in our group. CMB were defined as small, homogenously hypointense round or ovoid lesions on GRE or SWI sequences, distinct from other potential mimics such as calcification or air-bone susceptibility-related artifacts. The number of lobar CMBs was dichotomized as mild burden (<10) vs. severe burden (≥10) based on the distribution of CMBs number in this cohort.
Sub-group analysis in patients with both SS/cSAH and TFNE
To further determine whether there was a precise correlation between TFNE and SS/cSAH, we studied patients with both SS/cSAH and TFNE at baseline (n=25). We excluded one patient whose severe left ICA stenosis could have potentially explained transient right arm weakness and one patient whose motor status epilepticus could have been potentially caused by CAA related inflammation. This left 23 patients for this sub-analysis.
Statistical analysis
Comparisons between subjects with SS/cSAH vs. without were performed using t-test (for age), chi-square (for categorical variables) for univariable analysis. SPSS/WIN (version 20.0, SPSS, Chicago, IL, USA) software was used to carry out all statistical analyses; a p value of less than 0.05 was considered to be statistically significant.
Results
Baseline characteristics of the cohort are shown in Table 1. In total, 41 patients had SS and/or cSAH based on T2*-weighted GRE or FLAIR. Of them, 24 patients had SS only and 17 patients had cSAH (2 patients had only cSAH and 15 patients had both cSAH and SS). SS/cSAH had a focal distribution in 21 patients and disseminated distribution in 20 patients and occurred in the frontal, parieto-occipital, and the central sulci. There were no differences in the distributions of age, sex, use of medications, vascular risk factors and severe lobar CMB burden between patients with and without SS/cSAH. The prevalence of TFNE was significantly higher (25/41, 61%) in the SS/cSAH group compared with the group without SS/cSAH (5/49, 10%) (p<0.001). Twenty-three of 90 patients with suspected CAA were further diagnosed with definite (n=7) or probable CAA with supporting pathology (n=16) based on Boston criteria [17] (8 patients with SS/cSAH and 15 patients without)
Table 1.
The characteristics of SS/cSAH in patients with suspected CAA without lobar ICH
| Characteristics | SS/cSAH, n=41 | No SS/cSAH, n=49 | P value |
|---|---|---|---|
| Age, y (M±SD) | 76. 2±6.2 | 75.1±8.0 | P=0.477 |
| Male, n (%) | 26 (62) | 27 (55) | P=0.425 |
| Hypertension, n (%) | 24 (59) | 30 (61) | P=0.906 |
| Diabetes mellitus, n (%) | 5 (12) | 8 (16) | P=0.611 |
| Hypercholesterolemia, n (%) | 19 (46) | 22 (45) | P=0.806 |
| Antiplatelet use, n (%) | 16 (39) | 24 (49) | P=0.344 |
| Anticoagulant, n (%) | 1 (2.4) | 5 (10) | P=0.141 |
| Statin use, n (%) | 19 (46) | 21 (43) | P=0.740 |
| Severe lobar CMB burden (≥10), n (%) | 19 (46) | 27(55) | P=0.408 |
| Number of CMBs (Median) [with upper and lower quartiles] | 9 [2, 20.5] | 10 [4, 52.5] | P=0.067 |
| Dementia | 8 (20) | 14(29) | P=0.324 |
| TFNE, n (%) | 25 (61) | 5 (10) | P<0.001 |
| Future cerebral hemorrhage, n (%) | 10 (29)* | 1 (2.4) | P=0.001 |
Illustrations: CAA=cerebral amyloid angiography; ICH=intracerebral hemorrhage; SS=superficial siderosis; cSAH=convexity subarachnoid hemorrhage; CMBs=cerebral microbleeds; TFNE: transient focal neurological episodes.
Characteristics of TFNE and correlation with cSAH/SS
In the 23 individuals with unexplained TFNE (see sub-group analysis), the most common symptom was transient arm/hand/finger numbness/tingling, mostly spreading from the distal to proximal upper extremity (eg fingers to shoulders) in 19 patients. There was accompanied unilateral extremity weakness or slurred speech in 6 of these 19 patients. The remaining 4 patients had no spreading sensory symptoms and exhibited unilateral extremity weakness and word finding difficulty (n=1), unilateral extremity weakness alone (n=1), transient episode of expressive aphasia (n=1) or slurred speech alone (n=1). These transient episodes were mostly recurrent and stereotyped, lasting from less than one minute to a few minutes but not exceeding one hour. By contrast, none of 5 patients with TFNE without SS/cSAH had spreading sensory symptoms (3 presented with acute onset of transient garbled speech, recurrent brief episodes of word finding difficulties and transient limb weakness suggestive of transient ischemic attack (TIA) without spreading symptoms followed by ischemic stroke and 2 with seizure confirmed by EEG).
In those 23 patients with both SS/cSAH and TFNE, MRI including FLAIR was performed within 5 days of onset of the first episode in 17 patients. FLAIR hyperintensity in the corresponding sulci in the contra-lateral hemisphere, suggesting cSAH was present in all 17 patients (see table 2). There was evidence of chronic SS in multiple sulci in 21/23 (91%) patients (table 2 and Figure 1). The most common corresponding sulcus of TFNE was the central sulcus with or without pre or post central sulcus accompaniment (n=19), followed by single or multiple parietal sulci (n=3) and multiple frontal sulci (n=1).
Table 2.
The characteristics of TFNE and anatomical correlation with cSAH/SS in 23 patients
| Patient number |
Sex/age | TFNE characteristics | Time to scan (days) |
Location of cSAH (imaging characteristics) |
SS location (GRE susceptibility) |
|
|---|---|---|---|---|---|---|
| 1 | F/75 | Transient left upper extremity spreading tingling and slurred speech | 1 | Right central sulcus (CT+/FLAIR+) | none | |
| 2 | M/84 | Two brief spells of right hand spreading numbness, weakness and slurred speech for 1 min | 1 | Left central sulcus (CT+/FLAIR+) | Multiple | |
| 3 | M/73 | Transient sensory loss in the right index finger and thumb spreading to the right tongue and shoulder with associated slurring of speech | 1 | Left pre-central sulcus (CT+/FLAI R−+) | Multiple | |
| 4 | M/83 | Recurrent spells with slurred speech | 2 | Left parietal lobe (multiple) (CT+/FLAIR+) | Multiple | |
| 5 | F76 | Transient left arm numbness for several minutes | 1 | Right central sulci (CT+/FLAIR+) | Multiple, right greater than left | |
| 6 | M66 | The first episode: transient episode of expressive aphasia for a few minutes | 1 | left central sulcus (CT+/FLAI R+) | Multiple | |
| 7 | F80 | Transient episode left arm and left leg numbness /tingling with weakness, slurred speech | 0 | Right pre central sulcus (CT+/FLAIR+) | Multiple | |
| 8 | M59 | Spreading right sided numbness from right leg | N/A | Unable to assess. | Multiple, including left central sulcus | |
| 9 | F72 | brief spell of spreading sensory alteration of the right hemi-body with a migrating numbness over 5–10 minutes | 2 | left parietal cortical sulcus (CT[n/a]FLAIR+) | Multiple | |
| 10 | M81 | Spells with tingling in his left thumb spreading to his left shoulder, face and foot for 3 minutes and resolved fully | 3 | Right central sulcus (CT[n/a]FLAIR+) | Multiple | |
| 11 | M67 | Transient intermittent episodes of left face and arm tingling and numbness | N/A | Unable to assess. | Mutiple Including central right sulcus | |
| 12 | M80 | Recurrent episodes of left hand weakness and left lower extremity weakness | N/A | Unable to assess. | Right central sulcus and pre-central sulcus | |
| 13 | F87 | Frequent and transient attacks of right upper extremity numbness/tingling, accompanied by weakness | Left central sulcus (CT+/FLAIR+) | 2 | Left central | Multiple |
| 14 | F73 | Stereotyped episodes of tingling and numbness in the left mouth with some associated dysarthria | N/A | Unable to assess. | Mutiple including central sulcus | |
| 15 | M72 | Spreading sensory loss starting in the left thumb and spreading to the fingers | 5 | Right central sulcus (CT[n/a]/FLAIR+) | Multiple | |
| 16 | M77 | Transient left hand numbness | 1 | Multiple sulci in right frontal lobe (CT[n/a]/FLAIR+) | Multiple | |
| 17 | F81 | Transient left face and hand thumb numbness spreading to fingers | 1 | Right central sulcus (CT+/FLAIR+) | Multiple | |
| 18 | F72 | Stereotyped left arm tingling progressing to left hand weakness | 2 | Right central sulcus (CT[n/a]/FLAIR+) | Multiple | |
| 19 | F77 | Transient left arm numbness | 5 | right central sulcus and post central sulcus (CT+/FLAIR+) | Multiple | |
| 20 | M71 | Transient episodes of language difficulties (spelling) and right hand weakness. | N/A | Unable to assess. | Left parietal | |
| 21 | M65 | Transient right-sided spreading sensory loss | N/A | Unable to assess. | Multiple including left frontal parietal and central sulcus | |
| 22 | M73 | Transient tingling and numbness of right arm | 4 | left central sulcus (CT[n/a]/FLAIR+) | Multiple | |
| 23 | M65 | 3 to 4 episodes of spreading sensory loss from the right hand to the right shoulder (spread over approximately 1 minute, resolving in 5 to 10 minutes) | 2 | left central sulcus (CT[n/a]/FLAIR+) | None |
Illustrations: CAA=cerebral amyloid angiography; cSAH=convexity subarachnoid hemorrhage; FLAIR=Fluid attenuated inversion recovery; ICH=intracerebral hemorrhage; SS=superficial siderosis; TFNE: transient focal neurological episodes; N/A=not available;
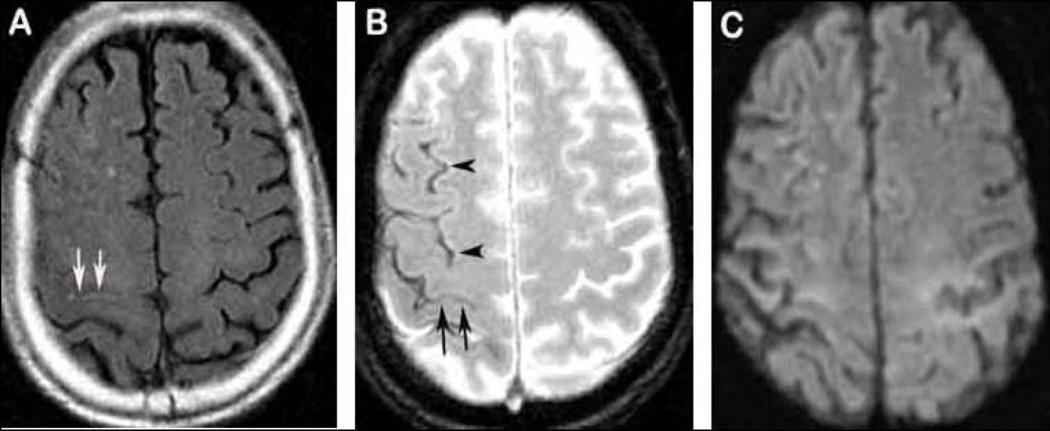
Figure 1. An example of “clinically silent” SS.
A 72-year-old man presents with transient spreading sensory loss starting in the left thumb and spreading to the fingers. Flair shows hyperintensity in right central sulcus (A, arrows). GRE demonstrates multiple hypointensities in the same hemisphere beyond the central sulcus (B, arrow heads for SS and arrows for cSAH). No DWI positive lesion is found (C).
EEG was performed within two days of first episode in 15 patients. No epileptiform activity was found in any of the patients even though one patient did have 3 TFNE during EEG monitoring. Eight patients had a normal EEG. Interestingly, intermittent focal slowing (but no amplitude change) was present in the same hemisphere/location as SS/cSAH in 6 patients. One showed generalized low amplitude.
SS/cSAH and risk of future symptomatic cerebral bleeding
Among those with available follow-up (76/90 patients, 84%), ten patients with SS/cSAH (29%, median time from the scan for all patients with SS/cSAH: 21 months) had a symptomatic cerebral bleeding event (ICH or cSAH) on follow-up (Average time to ICH or cSAH: 34 months) compared with only 1 event (2.4%, 25 months from the scan) in patients without SS/cSAH (time to ICH: 25 months) (p=0.001). Multivariate analyses were not performed due to limited sample size. Of those 10 patients with symptomatic cerebral bleeding events in SS/cSAH group, 2 had new cSAH (Figure 2) and 8 had lobar ICH. The location of hemorrhages on follow-up was not in the same location of previously noted SS/cSAH in 9 patients (Figure 3). Follow-up imaging in all 9 patients with cSAH showed evidence of SS in the same location as the initial cSAH. There were no significant associations between age, sex or lobar CMBs burden and risk of future symptomatic cerebral bleeding events (p>0.1 for all comparisons; data not shown).
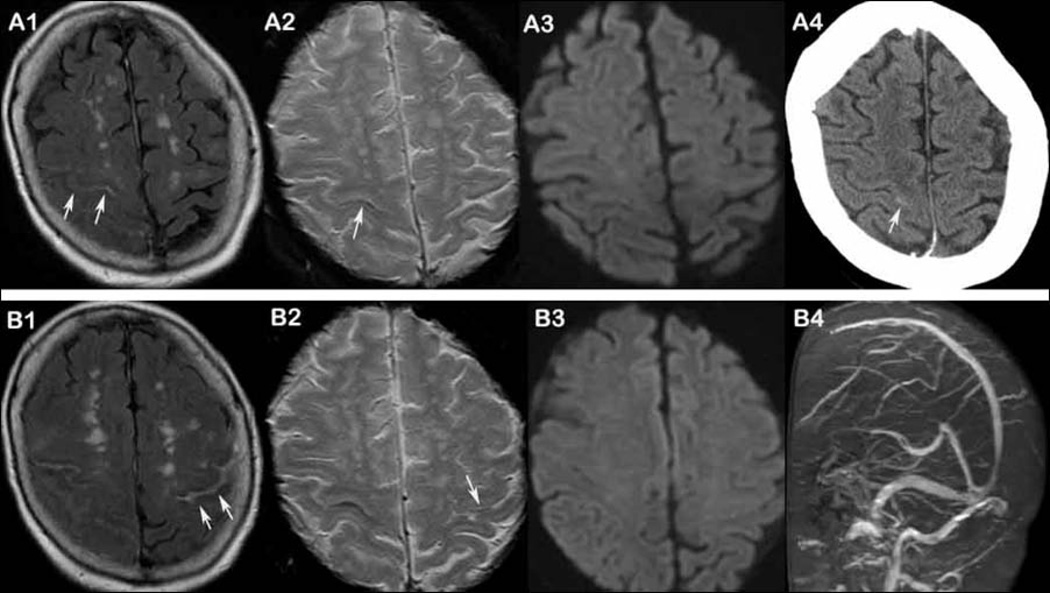
Figure 2. An example of recurrent cSAH.
A 75-year-old woman presented with transient left upper extremity tingling and slurred speech for 20–30 min and right headache before episode. MRI at the same day shows hyperintensity suggesting cSAH within right central sulcus on FLAIR (A1, arrows), single line hypointensity on GRE (A2, arrow) and no positive lesion on DWI (A3). CT shows subtle hyperintensity at same area (A4, arrow). Three weeks later, the patient has a recurrent episode with transient right 4th and fifth finger tingling and slurred speech lasting for 2–3 minutes. MRI within 24 hours shows hyperintensity in left central sulcus on FLAIR (B1, arrows), subtle hypointensity on GRE (B2, arrow) and no DWI positive lesion at the same time (B3). MRV demonstrates no venous sinus thrombosis (B4).
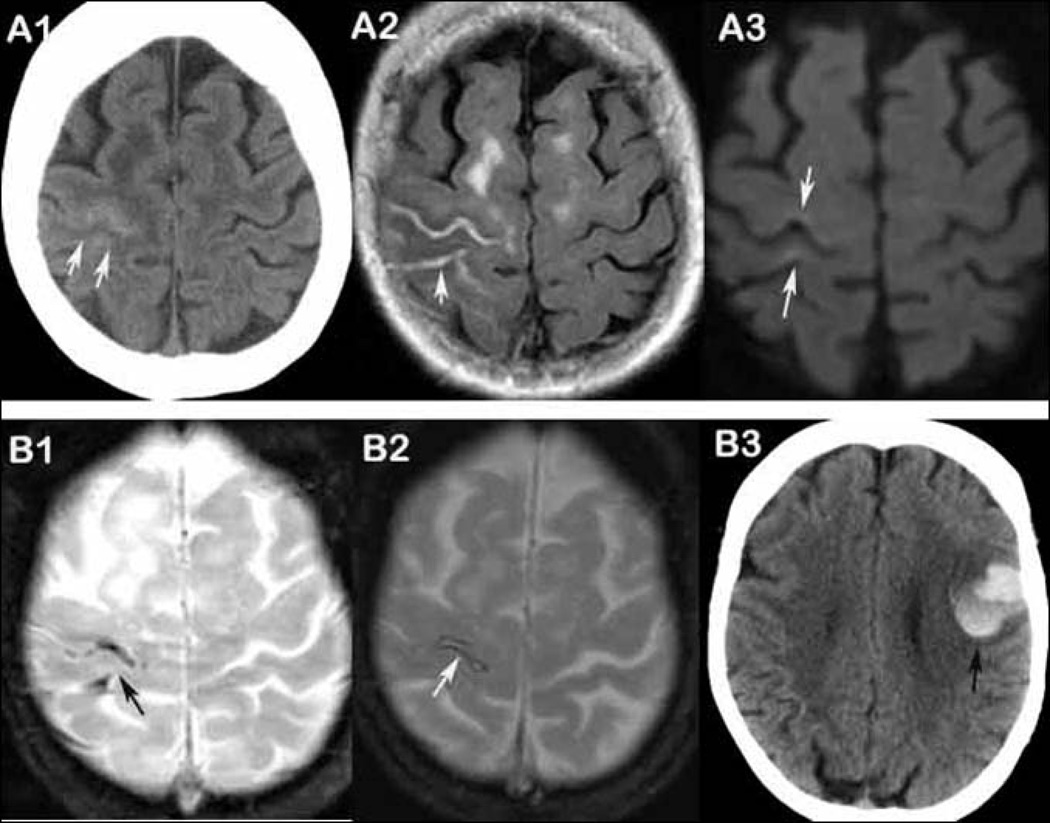
Figure 3. SS is a continuum from acute, focal linear cSAH and SS/cSAH independently predicts symptomatic intracerebral bleeding event.
A 77-year-old woman presented with transient left hand numbness (panel A and B). Both CT (A1, arrows) and FLAIR (A2, arrow) within 2 days demonstrate hyperintensity in right central sulcus and post-central sulcus. Slight elevated DWI signal (A3, arrows) is noted along the right central sulcus associated with areas of cSAH. GRE shows hypointensities at the same areas (B1, arrow). Two-month follow-up GRE shows chronic SS in the same area (B2, arrow). The patient had a left frontal ICH after 40 months (B3, arrow).
Discussion
The major finding from this study highlights a precise anatomical correlation between TFNE and cSAH, in a large majority of patients with suspected CAA without lobar ICH. The common corresponding sulci were either the central sulcus or pre or post central sulci—areas which are close to the primary motor or sensory cortices. This may suggest that acute cSAH located in or near these sulci are primarily responsible for the typical transient focal episodes seen in suspected CAA without lobar ICH. Furthermore, follow-up neuroimaging data from selected patients in this study (Figure 3) may suggest that the superficial siderosis (SS) is likely a continuum from acute, probably repeated, focal convexity linear subarachnoid hemorrhages (cSAH) in these patients. This is in line with hypotheses from previous studies [10,13]. Suspicion of CAA should thus be raised in patients with acute cSAH visualized on FLAIR or CT images and further evaluation with T2*-weighted GRE or SWI may be warranted.
Our study also suggests that the majority of TFNE caused by cSAH appear to present with spreading sensory symptoms. The typical symptoms of TFNE in our patients with cSAH are recurrent, stereotyped spreading numbness and tingling from fingers or toes which extend to the proximal arm, leg or face and resolve completely within a few minutes without associated motor weakness. This is in contrast to a TIA which can often present with acute language or motor symptoms (aphasia, dysarthria, focal weakness) without associated numbness or parathesias. Furthermore, TIA-associated parasthesias, when present, typically occur suddenly and do not spread from the distal to proximal extremities. Thus, these clinical features may distinguish these subpial bleeding events from TIAs in patients with CAA and thus help guide further evaluation and treatment. For example, antithrombotic treatment, which is of benefit in patients with TIA, could potentially have detrimental effects in CAA patients with TFNE with underlying cSAH, by increasing ICH risk [20].
Although the precise mechanism of these TFNEs has not been elucidated previously, focal seizure, TIA and cortical spreading depression have all been hypothesized as potential mechanisms [10]. The results from our study demonstrate that underlying physiological mechanism of CAA-related TFNE might be cortical spreading depression. EEG evaluation performed within 2 days of first onset of symptoms showed intermittent theta slowing but no amplitude change in the corresponding area of cSAH in 6 patients. Cortical spreading depression is becoming increasingly accepted as the basis for migraine aura and the trigger for headache pain [21] and can demonstrate theta slowing without amplitude change on EEG [22]. Similar to cSAH-related TFNE, these migraine auras can also present with transient focal neurologic symptoms and can resolve within a few minutes. Interestingly, no epileptiform activity was found in any of the 15 patients who had EEG monitoring in our cohort. Indeed, one patient had three episodes during EEG monitoring with no electrographic correlate to suggest seizure. It may be that the small amyloid-related hemorrhagic lesions of cSAH act as triggers for cortical spreading depression. Further EEG monitoring studies are warranted in those patients.
Two previous retrospective studies have suggested that SS or cSAH associated with TFNE may be a warning sign for recurrent ICH, which may occur in the same region [13,14]. However, all these previous studies included mostly patients with baseline lobar ICH, which likely influences future hemorrhagic risk. Our study attempts to extend this literature by examining SS/cSAH in suspected CAA without previous lobar ICH. We also found that SS/cSAH was associated with future lobar hemorrhage, although there was no correlation between location of ICH and the previous area of SS/cSAH. These results suggest that the presence SS/cSAH in patients with suspected CAA may reflect increased overall severity of cerebrovascular amyloid deposition in the brain but lack specificity regarding regional differences in degree of vessel pathology in these patients. Further pathological studies to examine this question are ongoing.
Our study has limitations. The single-center retrospective study design may lead to recruitment of patients with SS/cSAH that were clinically distinct from other patients with suspected CAA. However, patients without SS/cSAH in the cohort did not significantly differ in characteristics compared to patients with SS/cSAH. Further large prospective studies with systematic evaluation of TFNE are needed. The inclusion of only patients who underwent MRI with T2*-weighted GRE sequences may have contributed to a selection bias. It is possible that patients with strictly lobar CMBs in the absence of ICH may lack CAA, thus leading to bias. However, recent work suggests that the specificity of lobar CMBs for CAA is high even in the absence of lobar ICH and that lobar CMB alone strongly predict pathologic evidence of advanced CAA [23]. All but one subject with SS/cSAH and TFNE underwent interictal EEG studies, which may have failed to capture the true epileptic nature of the neurologic event. Finally, because our study was retrospective in nature and not designed to systematically examine the risk of incident cerebral bleeding events in patients with suspected CAA without ICH (including the effect of blood pressure and/or antihypertensive agents), our finding that SS/cSAH increases future bleeding risk may have significant bias. Future prospective studies should attempt to investigate this possibility in more detail.
Summary
In conclusion, distinct from TIA or seizure, the majority of TFNE in patients with suspected CAA appears to be caused by cSAH and presents with spreading sensory symptoms. The findings further highlight a precise anatomical correlation between TFNE and acute subpial bleeding in suspected CAA. Patients with SS/cSAH may have a significantly higher risk of future cerebral bleeding events, regardless of the severity of the baseline CMB burden.
Acknowledgments
None
Sources of Funding
The project described was supported by NIH Grant K23AG028726-05, P50AG005134-30, 2R01 AG26484, 5R01AG026484-10 and 5K23AG028726-05.
Footnotes
Completing interests
None
References
- 1.Viswanathan A, Greenberg SM. Cerebral amyloid angiopathy in the elderly. Annals of neurology. 2011;70:871–880. doi: 10.1002/ana.22516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charidimou A, Gang Q, Werring DJ. Sporadic cerebral amyloid angiopathy revisited: recent insights into pathophysiology and clinical spectrum. Journal of neurology, neurosurgery, and psychiatry. 2012;83:124–137. doi: 10.1136/jnnp-2011-301308. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg SM, Eng JA, Ning M, Smith EE, Rosand J. Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke. 2004;35:1415–1420. doi: 10.1161/01.STR.0000126807.69758.0e. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg SM, O'Donnell HC, Schaefer PW, Kraft E. MRI detection of new hemorrhages: potential marker of progression in cerebral amyloid angiopathy. Neurology. 1999;53:1135–1138. doi: 10.1212/wnl.53.5.1135. [DOI] [PubMed] [Google Scholar]
- 5.Linn J, Halpin A, Demaerel P, Ruhland J, Giese AD, Dichgans M, van Buchem MA, Bruckmann H, Greenberg SM. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010;74:1346–1350. doi: 10.1212/WNL.0b013e3181dad605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charidimou A, Peeters A, Fox Z, Gregoire SM, Vandermeeren Y, Laloux P, Jäger HR, Baron JC, Werring DJ. Spectrum of transient focal neurological episodes in cerebral amyloid angiopathy: multicentre magnetic resonance imaging cohort study and meta-analysis. Stroke. 2012;43:2324–2330. doi: 10.1161/STROKEAHA.112.657759. [DOI] [PubMed] [Google Scholar]
- 7.Charidimou A, Jäger RH, Fox Z, Peeters A, Vandermeeren Y, Laloux P, Baron JC, Werring DJ. Prevalence and mechanisms of cortical superficial siderosis in cerebral amyloid angiopathy. Neurology. 2013;81:626–632. doi: 10.1212/WNL.0b013e3182a08f2c. [DOI] [PubMed] [Google Scholar]
- 8.Renou P, Tourdias T, Fleury O, Debruxelles S, Rouanet F, Sibon I. Atraumatic nonaneurysmal sulcal subarachnoid hemorrhages: a diagnostic workup based on a case series. Cerebrovascular diseases. 2012;34:147–152. doi: 10.1159/000339685. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg SM, Vonsattel JP, Stakes JW, Gruber M, Finklestein SP. The clinical spectrum of cerebral amyloid angiopathy: presentations without lobar hemorrhage. Neurology. 1993;43:2073–2079. doi: 10.1212/wnl.43.10.2073. [DOI] [PubMed] [Google Scholar]
- 10.Charidimou A, Law R, Werring DJ. Amyloid “spells” trouble. The Lancet. 2012;380:1620. doi: 10.1016/S0140-6736(12)61333-6. [DOI] [PubMed] [Google Scholar]
- 11.Raposo N, Viguier A, Cuvinciuc V, Calviere L, Cognard C, Bonneville F, Larrue V. Cortical subarachnoid haemorrhage in the elderly: a recurrent event probably related to cerebral amyloid angiopathy. Eur J Neurol. 2011;18:597–603. doi: 10.1111/j.1468-1331.2010.03214.x. [DOI] [PubMed] [Google Scholar]
- 12.Apoil M, Cogez J, Dubuc L, Bataille M, de la Sayette V, Touzé E, Viader F. Focal cortical subarachnoid hemorrhage revealed by recurrent paresthesias: a clinico-radiological syndrome strongly associated with cerebral amyloid angiopathy. Cerebrovasc Dis. 2013;36:139–144. doi: 10.1159/000353676. [DOI] [PubMed] [Google Scholar]
- 13.Linn J, Wollenweber FA, Lummel N, Bochmann K, Pfefferkorn T, Gschwendtner A, Bruckmann H, Dichgans M, Opherk C. Superficial siderosis is a warning sign for future intracranial hemorrhage. Journal of neurology. 2013;260:176–181. doi: 10.1007/s00415-012-6610-7. [DOI] [PubMed] [Google Scholar]
- 14.Charidimou A, Peeters AP, Jäger R, Fox Z, Vandermeeren Y, Laloux P, Baron JC, Werring DJ. Cortical superficial siderosis and intracerebral hemorrhage risk in cerebral amyloid angiopathy. Neurology. 2013;81:1666–1673. doi: 10.1212/01.wnl.0000435298.80023.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Donnell HC, Rosand J, Knudsen KA, Furie KL, Segal AZ, Chiu RI, Ikeda D, Greenberg SM. Apolipoprotein E genotype and the risk of recurrent lobar intracerebral hemorrhage. N Engl J Med. 2000;342(4):240–255. doi: 10.1056/NEJM200001273420403. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Ramirez S, Pontes-Neto OM, Dumas AP, Auriel E, Halpin A, Quimby M, Gurol ME, Greenberg SM, Viswanathan A. Topography of dilated perivascular spaces in subjects from a memory clinic cohort. Neurology. 2013;80:1551–1556. doi: 10.1212/WNL.0b013e31828f1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56:537–539. doi: 10.1212/wnl.56.4.537. [DOI] [PubMed] [Google Scholar]
- 18.Smith EE, Gurol ME, Eng JA, Engel CR, Nguyen TN, Rosand J, Greenberg SM. White matter lesions, cognition, and recurrent hemorrhage in lobar intracerebral hemorrhage. Neurology. 2004;63:1606–1612. doi: 10.1212/01.wnl.0000142966.22886.20. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, Launer LJ, Van Buchem MA, Breteler MM Microbleed Study Group. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biffi A, Halpin A, Towfighi A, Gilson A, Busl K, Rost N, Smith EE, Greenberg MS, Rosand J, Viswanathan A. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology. 2010;75:693–698. doi: 10.1212/WNL.0b013e3181eee40f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogawski MA. Common pathophysiologic mechanisms in migraine and epilepsy. Arch Neurol. 2008;65:709–714. doi: 10.1001/archneur.65.6.709. [DOI] [PubMed] [Google Scholar]
- 22.Drenckhahn C, Winkler MK, Major S, Scheel M, Kang EJ, Pinczolits A, Grozea C, Hartings JA, Woitzik J, Dreier JP COSBID study group. Correlates of spreading depolarization in human scalp electroencephalography. Brain. 2012;135:853–868. doi: 10.1093/brain/aws010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez-Ramirez S, Romero JR, Gurol ME, Ashkan S, McKee AC, van Etten E, Pontes-Neto O, Ayres A, Auriel E, Himali JJ, Beiser AS, DeCarli C, Stein T, Alvarez VE, Frosch M, Rosand J, Greenberg SM, Seshadri S, Viswanathan A. Diagnostic Value of Lobar Hemorrhages for Cerebral Amyloid Angiopathy in Hospital and Community-based Individuals: A Pathological Correlation Study. International Stroke Conference Abstracts, Stroke. 2014;45 AWP 246. [Google Scholar]