Abstract
Objectives
We conducted a randomized, controlled trial comparing the efficacy of an Integrated Risk Reduction Intervention (IRRI) to a control condition with the objective of improving mood stability and psychosocial functioning by reducing cardiometabolic risk factors in overweight/obese patients with bipolar I disorder.
Methods
A total of 122 patients were recruited from our outpatient services and randomly allocated to IRRI (n = 61) or psychiatric care with medical monitoring (n = 61). Individuals allocated to IRRI received psychiatric treatment and assessment, medical monitoring by a nurse, and a healthy lifestyle program from a lifestyle coach. Those allocated to the control condition received psychiatric treatment and assessment and referral, if indicated, for medical problems. A mixed-effects model was used to examine the impact of the interventions on body mass index (BMI). Exploratory moderator analyses were used to characterize those individuals likely to benefit from each treatment approach.
Results
Analyses were conducted on the IRRI (n = 58) and control (n = 56) participants with ≥ 1 study visit. IRRI was associated with significantly greater rate of decrease in BMI (d = −0.51, 95% confidence interval: −0.91 to −0.14). Three variables (C-reactive protein, total cholesterol, and instability of total sleep time) contributed to a combined moderator of faster decrease in BMI with IRRI treatment.
Conclusions
Overweight/obese patients with bipolar disorder can make modest improvements in BMI, even when taking medications with known potential for weight gain. Our finding that a combination of three baseline variables provides a profile of patients likely to benefit from IRRI will need to be tested further to evaluate its utility in clinical practice.
Keywords: bipolar disorder, health risks, weight management
Bipolar disorder is a chronic disease that is associated with a potentially devastating impact on patients’ well-being and social, occupational, and general functioning (1–3). It the sixth leading cause of disability in the world, with an economic burden that in the United States alone has been estimated at $30.7 billion in direct medical costs and $120.3 billion in indirect costs [2009 values (4)]. However, the morbidity, mortality, and costs associated with bipolar disorder are not simply a result of psychiatric symptoms and their attendant dysfunction (5, 6). Medical diseases and risk factors for cardiovascular disease, obesity, and diabetes are exceptionally common in those who suffer from bipolar disorder (7, 8). These medical problems affect the course, severity, and treatment of the bipolar disorder itself (9–14), and lead to even greater morbidity, mortality, and disability (15, 16). Furthermore, because patients with bipolar disorder spend the majority of their lives in the depressive phase of the illness (17–19), the self-discipline and motivation required to reduce cardiometabolic risk factors is frequently difficult to achieve. Indeed, Katon (20) has established a clear relationship between depression and a host of negative health behaviors including poor diet, overeating, smoking, substance use, and a sedentary lifestyle.
In a series of reports over the last decade (12, 13, 21–26), we have articulated a conceptual model in which medical and psychiatric symptoms accumulate over time and converge to cause the marked functional impairment associated with bipolar disorder. In our conceptualization, the same lifestyle parameters have the capacity to represent either risk or protective factors for individuals with bipolar disorder. While having regular sleep/wake rhythms is likely protective in terms of both functioning and weight gain, sleep/wake dysregulation is clearly a risk factor for functional impairment as well as for obesity and cardiovascular disease (26–31).
Based on the view that modifiable health risks adversely affect mood and functioning we sought to examine the benefits of an Integrated Risk Reduction Intervention (IRRI) for patients with bipolar I disorder. The targets of IRRI were: (i) enhanced regularity of sleep/wake cycles; (ii) increased regularity of social rhythms including the timing of social interaction, activities, and meals; (iii) modest increases in physical activity; (iv) improved eating habits including better nutritional balance and modest caloric reduction; and (v) active liaison by a nurse practitioner with patients’ primary care and specialist physicians to improve management of cardiometabolic risk factors and/or disease. IRRI was provided as an adjunct to psychopharmacologic management based on guidelines established for the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) study (32) and further refined in the Bipolar Disorder Center for Pennsylvanians study conducted at our site (13).
Among the aims of our study (NCT00746343) was to determine whether IRRI could stabilize or reduce risk factors for cardiometabolic disease while improving sleep/wake parameters, social rhythm regularity, mood symptoms, and functioning. Further, we expected to demonstrate that baseline cardiometabolic risk factor status moderates the effects of the interventions.
The present report focuses on the effects of IRRI relative to a control condition consisting of guideline-based psychopharmacologic intervention with medical monitoring on body mass index (BMI) over the six months that constituted Phase I of the study. We employ newly developed methods for examining the effect sizes of moderating variables (33, 34) to examine the moderators of those effects.
Methods
Study design
Study participants were randomly allocated to one of two treatment interventions [IRRI or psychiatric care with medical monitoring (PCMM)] at study entry using a stratified permuted block design. The first six months of the study constituted Phase 1; the subsequent 18 months constituted Phase 2. Participants remained in their randomly assigned treatment condition for a total of 24 months, irrespective of the occurrence of depression, mania, or mixed episodes, or psychiatric or medical hospitalization. All participants received guideline-based psychopharmacological intervention and psychiatric management provided by a psychiatrist expert in the treatment of bipolar disorder, that focused on symptom relief, management of side effects, and restoration of functioning. The study was approved by the University of Pittsburgh Institutional Review Board. Following an extensive oral explanation of the study, all participants provided written informed consent.
Participants
Study participants were male and female treatment-seeking outpatients primarily recruited from within the Western Psychiatric Institute and Clinic mood disorder programs who were between the ages of 18 and 55 years-of-age and had a lifetime diagnosis of bipolar I disorder, currently in remission. Remission was defined as a Hamilton Rating Scale for Depression 17-item (HRSD-17) (35) score of ≤ 7, a Young Mania Rating Scale (YMRS) (36) score of ≤ 7, and a Clinical Global Impressions for Bipolar Disorder Scale (CGI-BP-S) (37) score of < 3 for four consecutive weeks prior to randomization and assessed at two time points during those four weeks. In order to reduce potential sources of variation in outcomes not attributable to the randomized interventions, the goal of the pharmacotherapy was to focus as much as possible on a limited set of medications (lithium, divalproex, aripiprazole, and quetiapine); however, in a number of cases the study pharmacotherapists elected to keep patients who had been stable for many months or years on their current pharmacotherapy.
In order to maximize our ability to test our specific hypotheses, we limited study inclusion to those individuals with a definitive diagnosis of bipolar I and a BMI ≥ 25 because, at the time the study was initially designed, those were the patients thought to be at highest cardiometabolic risk. Those with ultra-rapid cycling (> 4 episodes in six months) bipolar disorder, pervasive developmental disorder, antisocial personality disorder, current substance dependence or abuse, organic mental disorder, or unstable and severe medical illness that required immediate and intensive medical attention were excluded, as were individuals who were unwilling or unable to comply with study requirements (i.e., complete forms, attend scheduled evaluations), those who, in the opinion of the investigator were not competent to provide informed consent and women who were planning to become pregnant, currently pregnant, or breast-feeding.
Study interventions
The IRRI consisted of three components: (i) psychiatric treatment by a study psychiatrist; (ii) assessment, referral, monitoring, and coordination by a certified registered nurse practitioner (CRNP) or experienced psychiatric research nurse for medical treatment provided by the patient’s own primary care physician; and (iii) a healthy lifestyle behaviors program delivered by a lifestyle coach. The PCMM condition was intended to mirror high quality team management of individuals with bipolar disorder without special efforts to manage patients’ medical problems or risk factors, although the level of medical monitoring probably exceeded that in most psychiatric settings. PCMM consisted of two components: (i) psychiatric treatment by a study psychiatrist; and (ii) assessment and referral by a psychiatric research nurse for medical treatment provided by the patient’s own primary care physician.
Components of the IRRI
Psychiatric treatment
Study psychiatrists, expert in the treatment of bipolar disorder, guided patients’ psychopharmacologic management according to protocol treatment algorithms for the remitted state (in which all participants entered the study) or for mania, depression, and mixed states when they occurred. Treatment was guided by the Bipolar Disorder Visit Form (BDVF), an adaptation of a similar instrument used in the STEP-BD study (32). Throughout the study, every effort was made to balance optimal symptom control with reduction in cardiometabolic risk factors by discontinuing or reducing the dosage of medications that appeared to be inducing weight gain or changes in other components of the metabolic syndrome unless such changes were associated with clear cut increases in symptomatology.
IRRI nursing management
The IRRI nurses assessed and monitored the patient’s psychiatric symptoms, conducted safety evaluations, monitored study medications, and managed side effects. The IRRI nurse recommended, monitored, and worked to ensure adherence to the medical and psychiatric treatment received by the patient and their adherence to the behavior change program provided by the lifestyle coach. The IRRI nurses attended weekly patient review meetings at which each patient’s status was reviewed and recommendations for adjustments to treatment (e.g., medications, medical issues) were made by the study psychiatrists and medical team. IRRI participants were seen once a month by the IRRI nurse unless they became symptomatic, in which case they were seen every two weeks. Visits with their study psychiatrist were scheduled every two months when participants were stable and monthly when they became symptomatic. Between visits, the IRRI nurses took calls from patients and acted as intermediaries to coordinate and facilitate efficient communication among the patient, psychiatrist, lifestyle coach (see below), and the patient’s primary care physician (PCP). The IRRI nurses helped patients to adhere to their PCP’s recommendations for management of their medical illnesses by providing educational pamphlets, coordinating medical care with their PCP, continually following up with patients on their adherence to prescribed medical treatments, keeping the psychiatrist and lifestyle coach informed about patients’ prescribed treatment and adherence to it, and consulting with the study’s consulting endocrinologist or cardiologist when necessary. A typical session with the psychiatrist and IRRI nurse each lasted approximately one-half hour.
Medical monitoring
Laboratory evaluations were conducted at study entry, every six months, and whenever a patient presented with a medical complaint that necessitated an interim evaluation. Laboratory results were reviewed at the weekly patient review meeting. Patients with abnormal values were referred to their PCP by their study nurse. An independent nurse monitor, blind to the patient’s treatment assignment, conducted weekly chart reviews to ensure that treatment algorithms were being applied accurately and consistently in both treatment conditions.
Healthy Lifestyle Behaviors Program
The third component of IRRI focused on a series of structured modules aimed at educating patients about health risks and teaching them skills to achieve a balanced lifestyle aimed at minimizing risk for medical morbidity and maximizing likelihood of optimal mental and physical health. The overall rationale for the healthy lifestyle program is that more regular, stable sleep/wake and social rhythms as well as modest weight loss can attenuate health risks and improve mood symptoms and functioning. As such, IRRI is a comprehensive, integrative approach that targets health risks on a daily basis across the 24-hour sleep/wake period. See Table 1 for a more detailed description of the Healthy Lifestyle Behaviors Program components. The specific behavioral targets of each of the program components are detailed in the Healthy Lifestyle Behaviors Manual which, along with related patient measures, is available at http://www.wpic.pitt.edu/research/dmdpp/reading.htm. The healthy lifestyle behaviors program was provided by a lifestyle coach who aided patients in the development and maintenance of an individualized lifestyle plan, taught evidence-based strategies for health-related behavior change, and provided support and encouragement to the patient for making progress toward goals. These goals included efforts to: (i) minimize medical risks and promote quality of life through psychoeducation and careful adherence to all prescribed treatments; (ii) improve sleep and social rhythm regularity; (iii) promote weight loss by improving healthy eating habits or, where indicated, preventing weight gain; (iv) increase physical activity; and (v) promote smoking cessation where appropriate. Because alcohol and drug use adversely affect sleep and social rhythms, eating habits, and physical activity, the healthy lifestyle program included psychoeducation about substance use in the relevant modules.
Table 1.
Components of the Healthy Lifestyle Behaviors Program
| Component | Topics addressed | Therapeutic goals |
|---|---|---|
|
Bipolar disorder psychoeducation (3 sessions) |
|
|
|
Healthy sleep/wake and social rhythm practices (4 sessions) |
|
|
|
Weight loss: nutrition (4 sessions) |
|
|
|
Weight loss: physical activity (4 sessions) |
|
|
|
Smoking cessation (2 optional sessions) |
|
|
Each participant’s individualized lifestyle plan was reviewed by his or her study clinicians for consistency with the patient’s medical status to ensure that there were no contraindications to beginning weight loss and exercise programs. The program incorporated psychoeducational material for each component and evidence-based behavior change strategies that were tailored to individual participants’ needs. The program was specifically focused on the circumstances of individuals with bipolar disorder and incorporated principles of social rhythm stabilization (38–40), as well as what is known about self-management in bipolar disorder [see Janney (41) for a detailed review of the current state of knowledge in this area]. Throughout the intervention, the lifestyle coach used the Social Rhythm Metric-5 (SRM-5) (42) as one tool to aid in accomplishing these objectives. Other tools specifically focused on weight management included daily diet records, a calorie counter book, and pedometers (Omron Pocket Pedometer, Walking Style: Model HJ-720ITC) that used the participant’s weight and stride to calculate calories burned, distance walked, fat burned, and aerobic steps. Patients were weighed at each clinic visit using a Tanita stadiometer (model BWB-800). Working with the lifestyle coach, participants set weekly goals to improve their lifestyle and were encouraged to monitor their progress toward these behavioral changes.
During study Phase 1, the lifestyle coach met with the patient for 15 individual, one-hour sessions over the first five to six months of study participation. In study Phase 2, which lasted an additional 18 months, these weekly sessions were followed by five to seven monthly sessions during Study Months 7–12 and then four to five quarterly sessions during Study Months 13–24, depending on the timing of the last of the 15 initial sessions. Participants who smoked could elect to add two sessions to address smoking cessation during the first five months of the study.
Timing and delivery of sessions
These target areas were integrated under the umbrella of the social rhythms framework (38, 39) that explicitly involves emphasis on the interdependence of these areas to achieve healthy life balance. Each session included a review of information from previous visits and of self-monitoring logs for targets being addressed throughout the intervention. Although Table 1 presents a specific order of sessions, at the first IRRI session, participants indicated which components of the lRRI intervention they were most interested in working on—generally it was weight loss and increasing physical activity. The lifestyle coach then attempted to incorporate something the patient was interested in working on as early as possible to achieve an early success and develop a strong therapeutic/coaching relationship. Lifestyle coaches focused on identifying new healthier behaviors that the patients were willing to try and encouraged small changes that were evaluated by the patient and the coach at the next session and modified (if the first was not successful) or advanced (more time exercising or adding an additional change depending on the topic) if it was.
As noted above, following these initial sessions, the study provided five to seven monthly sessions, followed by quarterly sessions to: (i) assess patients’ mood, sleep/wake and social rhythms, nutrition, and exercise; (ii) evaluate progress towards the achievement and maintenance of individual patient goals regarding regular social and sleep/wake rhythms, eating habits, weight management, and physical activity; (iii) reinforce adherence and progress; (iv) provide supportive directions to address difficulties and relapses; and (v) problem-solve barriers the patient has toward achieving his/her goals.
Each one-hour session with the lifestyle coach included the assessment of symptoms, a review of homework, identification of barriers to implementation of earlier recommendations, and troubleshooting to identify strategies to overcome or minimize barriers. Feedback on the regularity of sleep/wake and social rhythms and previous assignments was also provided.
Components of PCMM
Each participant allocated to the PCMM condition was assigned to a research psychiatrist and a psychiatric nurse for the entire 24-month study period.
Psychiatric treatment
The protocol for psychiatric treatment in the PCMM condition was identical to that described above for the IRRI condition.
PCMM nurses
The two PCMM nurses assessed and monitored the patient’s psychiatric symptoms, conducted safety evaluations, monitored study medications, and managed side effects. As in the IRRI condition, the nurses attended weekly patient review meetings at which each patient’s status was reviewed and recommendations for adjustments to treatment (e.g., medications, medical issues) are made. As in the IRRI condition, participants met at least once every month with their assigned PCMM nurse and at least once every two months with their assigned psychiatrist as long as their clinical status remained stable. Visit frequency was increased, as in the IRRI condition, when they became symptomatic. Between visits, the PCMM nurse took calls from patients and communicated psychiatric issues with the patient’s psychiatrist when necessary. A typical session with the psychiatrist and nurse lasted approximately one-half hour, as in the IRRI condition. The PCMM contrast condition did not include the healthy lifestyles behavior program.
Medical monitoring
Likewise, medical monitoring was identical to that in the IRRI condition; however, when an abnormal value was observed the only intervention by the PCMM nurse was to refer patients to their own primary care PCP for follow-up. No further intervention was made on the PCMM nurse’s part, other than to help patients to understand their PCP’s diagnosis of their medical illnesses by providing educational pamphlets. As noted above, an independent nurse monitor, blind to patients’ treatment assignment, conducted weekly chart reviews to ensure that the algorithms were being applied accurately and consistently in both treatment conditions.
Measures
In order to examine all study hypotheses, a wide array of measures was obtained; however, the primary outcome variable for the present report was change in BMI. In order to calculate BMI, height was obtained at the screening visit in our primary medical care department using a Physician Medical Health scale with height rod and weight was measured at screening and each study visit on the same digital medical weight scale.
Study clinicians
The five study physicians were academic psychiatrists expert in the treatment of bipolar disorder. Study nurses were bachelors or masters-prepared psychiatric nurses, also expert in the treatment of bipolar disorders. IRRI nurses were blind to the progress of participants in the PCMM condition and vice versa. The majority of lifestyle coaching sessions were conducted by a health education specialist with advanced degrees in nutrition and exercise physiology (CAJ) who was supervised on a bi-weekly basis for adherence to the healthy lifestyles program by the first author (EF). Three experienced psychiatric social workers, trained in interpersonal and social rhythm therapy (IPSRT) (38, 39) and other behavior modification techniques, filled in when the health educator was on medical leave. All four clinicians conducting the lifestyle coaching sessions received initial training of two days’ duration from experts in sleep medicine (MH) and behavioral weight control and nutrition (Marsha Marcus, Ph.D.) and followed the Healthy Lifestyle Behaviors Program manual. In addition, all four healthy lifestyle clinicians had been trained in motivational interviewing techniques and regularly incorporated motivational enhancement techniques in their work with participants allocated to IRRI.
Data analysis
To examine the impact of IRRI and PCMM conditions on BMI over Phase 1 of the trial (initial six months), we used a mixed-effects model to regress the repeatedly measured BMI observations on log(week+1), treatment condition (coded +0.5 for IRRI and −0.5 for PCMM), and the interaction of log(week+1) with treatment condition. The log(week+1) transformation was selected because it allowed participants to have a more rapid decrease in BMI during the first few weeks of the intervention and it produced the best model fit based on both AIC and BIC. Random intercept and log(week+1) terms were included to allow each participant to vary randomly in their trajectory over time and to account for the correlation induced by each individual’s repeated measures. To estimate the effect size of the treatment by time interaction, we first estimated each individual’s BMI slope using a fixed-effects regression model of BMI on log(week+1). Then we regressed BMI slope on treatment assignment and extracted the estimates required for the Cohen’s d effect size calculation.
To determine whether any differences in change in BMI between the two groups might be a function of their pharmacotherapy regimens, we compared the weight gain potential of the medications participants were taking throughout active treatment phase in the IRRI versus PCMM groups. To accomplish this, four experienced psychiatrists independently rated each medication taken during the active treatment phase on a scale from 0 (no weight gain potential) to 3 (maximum weight gain potential) based on a combination of observation during the study and a review of the relevant literature. Since the consistency of the four raters was high (intra-class correlation coefficient = 0.83), we averaged the raters’ weight gain potential scores to develop a single weight gain potential score for each medication. Using these ratings, we assigned each individual a weight gain potential score for each day he or she was in the active treatment phase, considering only the drug with the largest weight gain potential if multiple drugs were taken in one day. These daily ratings were then averaged to calculate the average daily Phase 1 weight gain potential score for each individual.
Exploratory moderation analyses
We performed an exploratory moderator analysis to identify and characterize individuals who may have a faster decrease in BMI over the first six months using IRRI over PCMM or vice versa. When multiple moderators are identified and used individually, it is possible that they may each suggest a different treatment decision for a given patient. Therefore, we used a newly established method for creating a single, combined moderator out of multiple individual moderators (33). In addition to providing a clear and consistent algorithm for treatment determination, this method has also been shown to produce a combined moderator with a stronger effect size than any of the individual moderators of which it was composed (34). The outcome measure for these moderator analyses was the BMI slope, calculated for each individual by regressing their BMI measurements over six months on log(week+1).
In the exploratory moderator analysis, the first step was to identify a relatively small and independent subset of individual moderators to be used in the calculation of the combined moderator. We began this search by using clinical and scientific rationale to select 59 baseline variables that may moderate the effect of treatment on BMI slope. We then calculated the moderator effect size (33) for each of these individual baseline variables. Twenty-four had at least a small effect size (> 0.10; see Table 2), and thus were entered into an exploratory factor analysis with the aim of selecting one variable to represent each clinically meaningful factor. The factor analysis revealed six factors with eigenvalues > 1, and the first three of these factors had clinically meaningful interpretations with respect to our aim of developing a combined moderator. We then selected one individual moderator to represent each of the first three factors. The selections were made based on clinical and research significance, the factor loading, the available sample size, and the individual moderator effect size.
Table 2.
Baseline variables with a moderator effect size > 0.10
| Moderator | Effect size |
95% CI | N | Baseline profile suggesting faster BMI decrease with IRRI |
|---|---|---|---|---|
| Log CRP (ug/ml) | −0.30 | −0.46 to −0.13 | 100 | Higher CRP |
| Instability in TSTa | 0.24 | 0.05–0.40 | 103 | Less instability in TST |
| Instability in sleep durationa | 0.23 | 0.04–0.40 | 103 | Less instability in sleep duration |
| Instability in wake timea | 0.22 | 0.03–0.40 | 103 | Less instability in TST |
| Age of onset | −0.21 | −0.37 to −0.05 | 111 | Older age of onset |
| Instability in sleep midpointa | 0.21 | −0.01 to 0.40 | 103 | Less instability in sleep midpoint |
| ≥ College or technical degree | −0.20 | −0.37 to −0.04 | 112 | College education or greater |
| Pulse | −0.20 | −0.38 to 0.00 | 111 | Higher pulse |
| Any alcohol use | 0.17 | −0.02 to. 036 | 104 | No alcohol use |
| Log IL6 (pg/ml) | −0.17 | −0.35 to 0.01 | 100 | Higher IL6 |
| Instability in sleep onset timea | 0.16 | −0.04 to 0.33 | 103 | Less instability in sleep onset |
| Young Mania Rating Scale scorea | 0.15 | −0.04 to 0.32 | 112 | Lower mania score |
| Cholesterol | −0.14 | −0.34 to 0.05 | 110 | Higher cholesterol |
| Physical activity (CAPA)b | −0.13 | −0.34 to 0.10 | 111 | More physical activity |
| Taking sedatives or hypnotics | −0.13 | −0.28 to 0.03 | 112 | Taking any hypnotics |
| Work and Social Adjustment (WSAS) score (48) | 0.12 | −0.07 to 0.30 | 112 | Higher functioning (i.e., lower WSAS) |
| Mean sleep onset time | 0.12 | −0.07 to 0.30 | 103 | Earlier mean sleep onset time |
| Low-density lipoprotein (LDL) | −0.12 | −0.31 to 0.07 | 109 | Higher LDL |
| Any anxiety disorder | 0.11 | −0.07 to 0.29 | 112 | No anxiety disorder |
| Systolic blood pressure | 0.11 | −0.07 to 0.29 | 112 | Lower systolic blood pressure |
| Hamilton 17-Item Rating Scale for Depression (HRSD-17)a | 0.11 | −0.12 to 0.32 | 112 | Lower HRSD-17 |
| Age at time of screen | −0.10 | −0.28 to 0.09 | 112 | Older age at screen |
| Mean total sleep time | −0.10 | −0.30 to 0.11 | 103 | More total sleep time |
| Instability in wake after sleep onset (WASO)a | 0.10 | −0.09 to 0.28 | 103 | Less instability in WASO |
CI = confidence interval; BMI = body mass index; IRRI = Integrated Risk Reduction Intervention; TST = total sleep time; CAPA = Clinical Assessment of Physical Activity.
Square root transformation.
CAPA survey score based on the Physical Activity Survey for the Elderly (PASE) algorithm (47).
The three selected individual moderators were: (i) log c-reactive protein (CRP), (ii) cholesterol, and (iii) square root of the instability of total sleep time (TST). Instability of TST was calculated using data from seven nights of wrist actigraphy. The instability metric combines both within-subject variation and temporal dependence (i.e., autocorrelation) from night to night (40). Instability in TST and raw CRP values were transformed to ensure that model assumptions for the moderator analyses were met. Henceforth, we refer to the variables ‘log CRP’ and ‘square root of instability in TST’ as simply ‘CRP’ and ‘TST instability’.
We used the combined moderator method developed by Kraemer (33) to estimate optimal weights for CRP, cholesterol, and TST instability. These three moderators and their optimally derived weights were then used to calculate a single, combined moderator value for each individual. This combined moderator is denoted by M*. The moderator effect size and 95% bootstrap confidence interval (CI) of M* were calculated.
Upon performing this analysis, we found that M* indicated different treatment decisions for individuals with different M* values (i.e., M* is a qualitative moderator). Therefore, we used M* to divide the original sample into two subsamples, each with a different indicated treatment. Within each of these subsamples, we calculated the treatment effect size and 95% bootstrap confidence interval. Finally, we used descriptive statistics to create moderator profiles of these subsamples.
Results
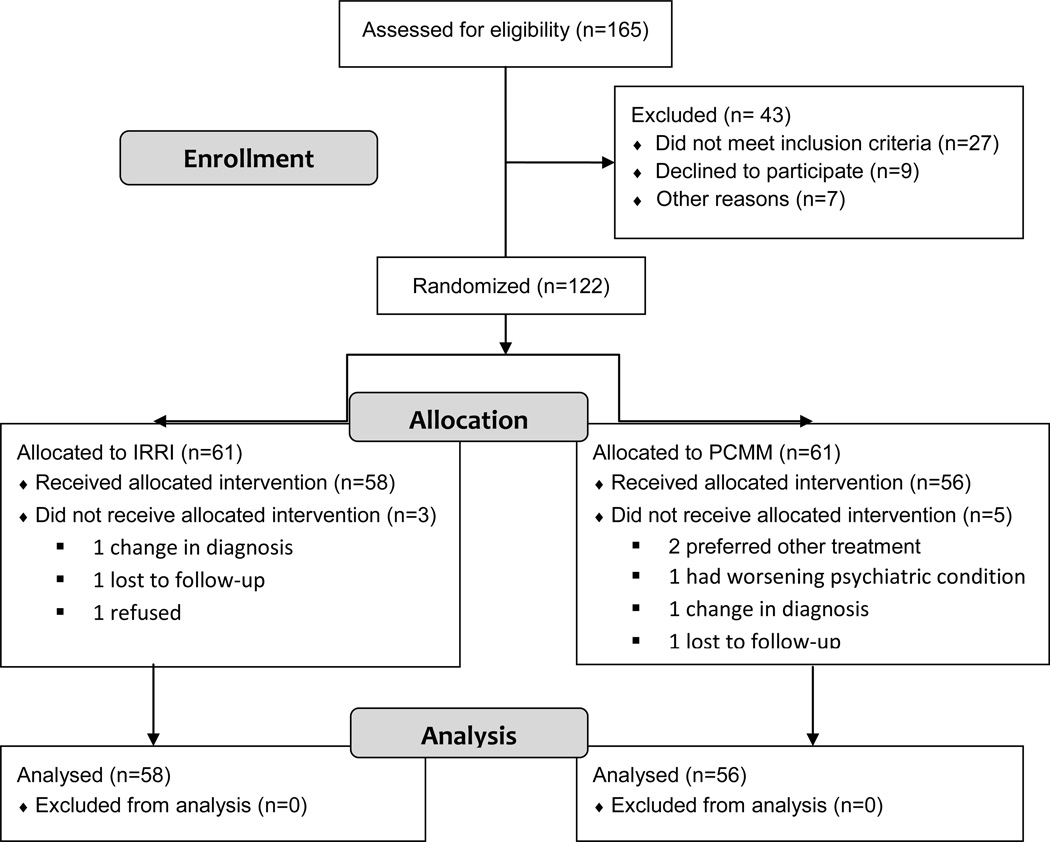
A total of 122 individuals were entered into the trial between 11/12/2008 and 7/14/2011 and were randomly allocated to receive the IRRI (n = 61) or PCMM intervention (n = 61), each in addition to protocol-driven pharmacotherapy. The last participant completed the two-year study on 9/4/2013. Of these 122 individuals, 114 had at least one study visit (IRRI: n = 58; PCMM: n = 56). This group of 114 forms the study population for the present report (see Fig. 1). The demographic and clinical and health risk characteristics of this group are presented in Table 3. The randomization yielded well-matched groups: the IRRI and PCMM participants did not differ significantly on any baseline variable, with the exception of the coefficient of variation (CV) of sleep offset time and the CV of sleep midpoint (t = 3.0, df = 81.9, p < 0.01; t = 2.59, df = 102, p = 0.01). There was no difference in the average daily weight gain potential score between IRRI and PCMM (t = −1.5, df = 95.6, p = 0.14, effect size = −0.28). Because not all of these 114 individuals had data available for the three moderator variables examined, a subset of 90 participants forms the basis for the analysis of treatment moderation. There were no significant differences between these 90 individuals and the remaining 24 on other study variables.
Fig. 1.
Flowchart of study population. IRRI = Integrated Risk Reduction Intervention; PCMM = psychiatric care with medical monitoring.
Table 3.
Participant characteristics
| All participants (N = 114) |
PCMM (n = 56) |
IRRI (n = 58) |
|
|---|---|---|---|
| Demographic characteristics | |||
| Age at screen, mean (SD) | 41.6 (9.5) | 41.4 (9.7) | 41.8 (9.5) |
| Education: ≥ college or technical degree, n (%) | 72 (63.2) | 35 (62.5) | 37 (63.8) |
| Clinical characteristics, mean (SD) | |||
| Age of onset (n = 113) | 18.9 (6.4) | 19.1 (6.4) | 18.6 (6.4) |
| Hamilton 17-item Rating Scale for Depression score | 5.2 (3.5) | 5.3 (4.0) | 5.1 (2.0) |
| Young Mania Rating Scale score | 1.8 (2.1) | 1.7 (2.2) | 1.9 (2.0) |
| Work and Social Adjustment Scale score | 13.4 (9.4) | 12.5 (9.2) | 14.2 (9.6) |
| Medications, n (%) | |||
| Taking sedatives or hypnotics | 13 (11.4) | 7 (12.5) | 6 (10.3) |
| Inflammatory characteristics (n = 102) | |||
| CRP (mg/L), mean (SD) | 4.1 (5.7) | 3.7 (4.7) | 4.5 (6.5) |
| IL6 (pg/mL), mean (SD) | 1.7 (1.4) | 1.6 (1.4) | 1.8 (1.4) |
| CRP > 3 mg/L, n (%) | 35 (34.3) | 19 (38.8) | 16 (30.2) |
| Mean of actigraphy sleep variables, (n = 104), mean (SD) | |||
| Total sleep time (min) | 420.9 (82.0) | 406.3 (76.8) | 435.0 (84.9) |
| Sleep onset time | 00:06 (01:36) | 00:01 (01:43) | 00:11 (01:30) |
| Coefficient of variation of actigraphy sleep variablesa (n = 104), mean (SD) | |||
| Total sleep time (min) | 6.2 (3.3) | 6.8 (3.9) | 5.7 (2.7) |
| Sleep duration (min) | 6.5 (3.2) | 7.1 (3.7) | 6.0 (2.7) |
| Sleep offset timeb | 0.4 (0.2) | 0.4 (0.2) | 0.3 (0.2) |
| Sleep midpointc | 0.3 (0.2) | 0.3 (0.2) | 0.3 (0.1) |
| Sleep onset time | 0.4 (0.3) | 0.4 (0.3) | 0.3 (0.2) |
| Wake after sleep onset (min) | 4.1 (2.0) | 4.4 (2.3) | 3.8 (1.7) |
| Daytime activity, mean (SD) | |||
| Physical activity (CAPA) (n = 96) | 121.7 (68.5) | 138.1 (67.0) | 111.0 (67.9) |
PCMM = psychiatric care with medical monitoring; IRRI = Integrated Risk Reduction Intervention; SD = standard deviation; CRP = c-reactive protein; CAPA = Clinical Assessment of Physical Activity.
We first calculated the instability and mean for each individual over seven days of actigraphy. Instability is a measure of variation that incorporates both the overall within-subject variance and the temporal dependence (autocorrelation) from night to night. To calculate the coefficient of variation, we then divided each individual’s instability by their mean. Because the coefficient of variation was highly skewed, this table shows the results after taking a square root transformation. Larger values represent more instability relative to the mean level.
t = 3.00, df = 81.9, p < 0.01
t = 2.59, df = 102, p = 0.01.
Difference in six-month BMI trajectory
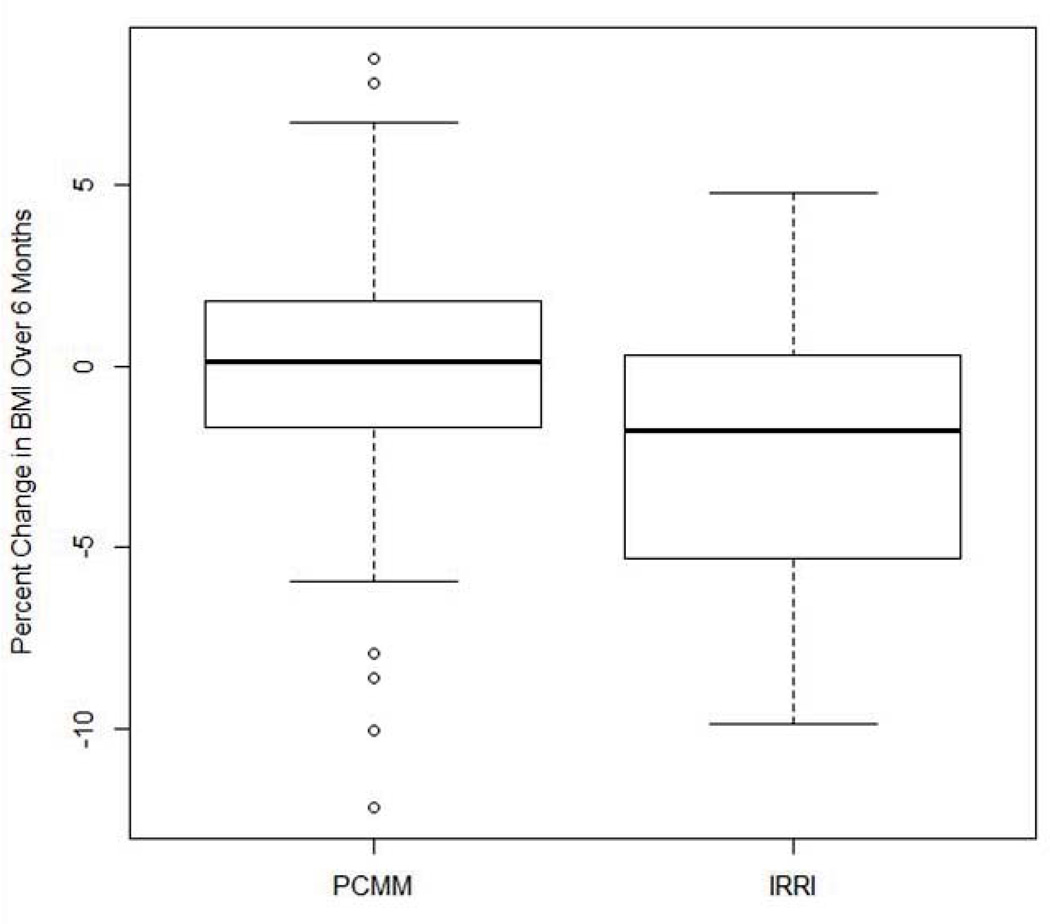
IRRI was associated with a significantly greater rate of decrease in BMI over log time than PCMM (β = −0.26, SE = 0.09, t = −2.81, df = 111, p = 0.006). The estimated Cohen’s d effect size of treatment on the BMI trajectory was −0.51 with a 95% bootstrap CI of (−0.91 to −0.14). Because change in BMI over log time may be difficult to conceptualize clinically, we also tested whether IRRI and PCMM groups differed in the percentage of change in BMI from baseline to their last BMI measurement of the six-month active treatment period. On average, the BMI of individuals in IRRI decreased by 2.3% [standard deviation (SD) = 3.8], while that of individuals in PCMM decreased only 0.2% (SD = 3.9). This difference was significant (t = 2.8, p = 0.006). Figure 2 illustrates the difference in percent change in BMI between the IRRI and PCMM groups. Finally, we note that there were no differences between the IRRI and PCMM groups with respect to the length of time that passed between the baseline BMI measurement and the last BMI measurement of the six-month period.
Fig. 2.
Difference in percent change in body mass index (BMI) between the Integrated Risk Reduction Intervention (IRRI) and psychiatric care with medical monitoring (PCMM) groups.
Moderator analysis
The individual moderator effect sizes with 95% bootstrap CIs of the three variables selected for the combined moderator analysis are shown in Table 4. When considered individually, these three moderators have small effect sizes. The confidence interval for CRP effect size does not span zero, indicating that it is statistically significant. The confidence intervals for cholesterol and instability in TST span zero, indicating that either these individual moderators are not helpful or the sample size is too small to detect their effects. The weights that they contributed to the combined moderator are also shown. Negative effect sizes and weights (cholesterol and CRP) indicate that individuals with larger values of the moderator may have a preferable outcome with IRRI (IRRI > PCMM). Likewise, positive effect sizes and weights (instability of TST) indicate that individuals with larger values of the moderator may have a preferable outcome with PCMM (PCMM > IRRI). The effect size (95% CI) of the combined moderator M* was 0.80 (0.50, 0.94) indicating that it strongly moderates the effect of treatment on the rate of decrease in BMI.
Table 4.
Individual moderator effects sizes (ES) and weights in M* (N = 90)
| Moderator ES (95% CI) | Weight in combined moderator M* |
|
|---|---|---|
| Instability of total sleep time | 0.19 (−0.01 to 0.36) | 0.07 |
| Cholesterol | −0.15 (−0.37 to 0.07) | −0.08 |
| Log c-reactive protein | −0.28 (−0.45 to −0.10) | −0.16 |
CI = confidence interval.
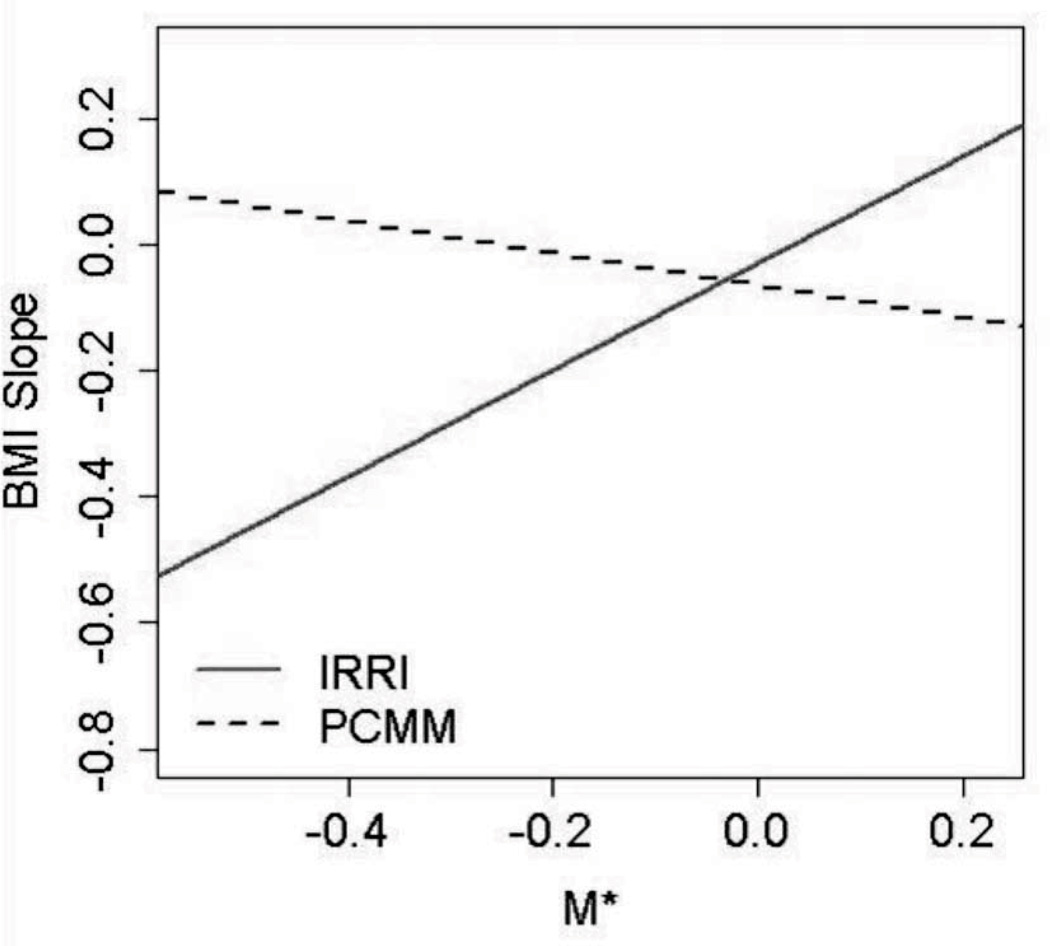
Figure 3 illustrates how the effect of the treatment assignment on BMI slope changes depending on an individual’s value of M*. For individuals with M* > −0.03 (n = 22), the treatment effect size (95% bootstrap CI) is 0.67 (−0.21 to 1.62). Because this effect size CI crosses zero, either PCMM is equivalent to IRRI in this group, or the sample size is too small to detect the difference. Individuals with M* < −0.03 (n = 68) have faster decreases in BMI when they are in the IRRI treatment. For this subgroup of individuals, the treatment effect size (95% bootstrap CI) is −0.94 (−1.52 to −0.46).
Fig. 3.
Illustration of how the effect of the treatment assignment on body mass index (BMI) slope changes depending on an individual’s value of M*. IRRI = Integrated Risk Reduction Intervention; PCMM = psychiatric care with medical monitoring.
Table 5 characterizes the two subgroups. The 68 individuals for whom IRRI was associated with a more negative slope of BMI over time than PCMM (M*< −0.03) had higher CRP values, more stability in their TST, and higher total cholesterol. Conversely, the 22 individuals for whom IRRI was equivalent to PCMM (M*> −0.03) had lower CRP values, less stability in their TST, and lower total cholesterol.
Table 5.
Moderator profiles
| Moderator | IRRI > PCMMa | PCMM > IRRIb | Test | ||
|---|---|---|---|---|---|
| t-value | df | p-value | |||
| Log CRP, mean (SD) | 1.13 (1.07)c | −1.24 (0.90)d | 9.38 | 88 | < 0.001 |
| Square root of instability of TST, mean (SD) | 119.88 (57.32) | 144.17 (76.25) | −1.58 | 88 | 0.116 |
| Cholesterol, mean (SD) | 201.15 (36.45) | 175.41 (30.00) | 3.00 | 88 | 0.004 |
IRRI = Integrated Risk Reduction Intervention; PCMM = psychiatric care with medical monitoring; CRP = c-reactive protein; SD = standard deviation; TST = total sleep time.
n = 68 below cut-point.
n = 22 above cut-point.
Untransformed mean (SD); CRP = 5.30 (6.26).
Untransformed mean (SD); CRP = 0.371 (0.60).
Finally, because values of CRP > 10 may indicate acute rather than chronic inflammation, we performed a sensitivity analysis to determine whether excluding these observations would impact the results of our moderation analyses. There were 13 individuals (13%) with CRP > 10 at baseline. After removing these observations, the inference of the final moderator analysis results did not change.
Discussion
In this report, we demonstrate that it is possible for overweight and obese patients with bipolar I disorder to make modest improvements in BMI over a six-month period, even when regularly taking medications with known potential for weight gain. Providing patients with a clear rationale for why it is important to reduce their health risks, setting reasonable goals for such improvements, providing specific strategies for achieving those goals and the support and encouragement needed to do so—as occurred in the Healthy Lifestyles Program of IRRI—appeared to be a successful strategy for this subset of individuals with bipolar I disorder, at least in the short term.
In this report, we employed a novel, exploratory approach to the analysis of treatment moderation with the goal of providing practicing clinicians with relatively simple profiles of those patients likely to benefit from the sort of integrated approach to treatment provided in the IRRI condition. Using this approach, we found that a combination of three baseline variables (CRP, instability in TST, and total cholesterol) offered a relatively easily obtained profile of such patients. Obtaining CRP and total cholesterol values should represent little challenge for most practitioners. While instability in TST was measured via actigraph in the present study, simple inquiry as to how much a patient’s TST varies from night to night, would likely represent a reasonable proxy in clinical practice. Alternatively, clinicians could ask patients to keep a simple sleep diary. Among participants in the present study, instability in TST as recorded in a sleep diary was a highly significant predictor of instability in TST as recorded by actigraphy (p < 0.001).
What is a little more difficult to understand is why these three variables in these particular directions should emerge as such predictors. Our overall interpretation, looking at all the significant moderators we found (not simply those chosen for analysis in M*) is that factors that represented poorer physical health such as higher CRP, higher cholesterol, lower high-density lipoprotein values, higher low-density lipoprotein (LDL) values, etc. were associated with greater benefit from IRRI/Healthy Lifestyles in terms of weight loss. In contrast, those factors that might be considered correlates of greater functional impairment such as irregular sleep timing and lower educational attainment were associated with less benefit from the IRRI/Healthy Lifestyles approach. It may be that those participants with more physical risk factors were more motivated and more able to engage in the activity and dietary changes recommended in IRRI, while those with greater functional impairment and more disorganized lives were less able to do so.
Our results raise the key question of how we should think about obesity and other features of the cardiometabolic syndrome, in relation to bipolar disorder. Obesity was associated with manic-depressive illness long before the advent of the psychopharmacologic drugs era. Indeed, Kretschmer (43) described a specific manic-depressive body habitus as early as 1925. Obesity with accompanying changes in blood glucose levels, may represent a unique ’bath’ for brain circuitry associated with changes in both physiology and behavior, suggesting a more important relationship between so-called medical and so-called psychiatric factors than previously recognized. We now know that bipolar disorder has a specific impact on the long-term course of hypertension, cardiovascular disease, diabetes, and cerebrovascular disease and vice versa (16, 44–46).
One way to think about the challenges faced by patients with bipolar I disorder is in terms of the concept of allostatic load put forward by McEwen and Stellar (47). They argue that there is a cost to chronic exposure to fluctuating or heightened neural or neuroendocrine activity as a result of the attempt to deal with repeated or chronic environmental challenge. Indeed, in the case of bipolar disorder some of the heightened neural or neuroendocrine activity may also represent an endogenous characteristic of the mood states of mania and/or depression. Thus, the construct of allostatic load may help us to understand the relationship among hypothalamic-pituitary-adrenal (HPA) axis and monoamine system (over)activation, bipolar disorder and with a range of chronic medical conditions, especially those that make up the metabolic syndrome.
It bears noting that the present study has a number of limitations. First, we focused exclusively on patients who were already overweight or obese. We do not know what the effects of the two intervention approaches studied might have been on patients with bipolar disorder not selected for this important risk factor. Second, the population we studied was relatively well-educated, which may have increased their capacity for understanding the need to alter their risk profiles. Their current social class, however, was not reflective of their educational attainment, with more than half of those studied being currently disabled or unemployed. Third, the relative heterogeneity of the medication regimens of these initially stable patients means that we cannot definitively rule out medication effects despite our efforts to determine whether there were any systematic differences in the weight gain potential of the participants in the two study conditions. Fourth, the decision to study an initially euthymic population reduces the variability of episodes and the effects of larger shifts in patients’ clinical state. A study of a more symptomatically diverse population could have shown more potent effects, and possibly provided additional information. On the other hand, adherence to the protocol could have been adversely affected with a more symptomatic population. Fifth, our sample size may have been insufficient to adequately assess the large number of variables analyzed in this study. Finally, the moderator analyses that we employed are clearly exploratory. The M* cutoff we employed and the weights we applied to each individual moderator will require validation in a separate randomized clinical trial in order to evaluate their utility in clinical practice. Nonetheless, our results do provide a modest level of optimism regarding the possibility of improving the health status of individuals with bipolar disorder.
When we are able to examine the longer-term follow-up data beyond six months on these individuals, it will be important to determine whether such weight loss is maintained once patients are no longer receiving IRRI on a more or less weekly basis. As Janney and colleagues point out (41), “ … personally tailored interventions of longer duration and greater frequency may be necessary to achieve the maximal benefit among individuals with [bipolar disorder.]” What we also do not know at this point is whether the modest level of weight reduction achieved by the IRRI participants leads to later reduction in other risk factors such as LDL levels. Nor do we know yet which components of the Healthy Lifestyles Program were associated with the BMI changes we observed, i.e., how much is attributable to increased physical activity, how much to dietary change, and how much to improved sleep/wake regulation.
Acknowledgements
This research supported by National Institute of Mental Health grant MH081003 (DJK) and the Jan Mueller Trust Fund.
EF has received royalties from the American Psychological Association and Guilford Press; is a member of the Valdoxan Advisory Board of Servier International; is an Editorial Consultant for the American Psychiatric Press; has received honoraria from Lundbeck; and is a stockholder in Psychiatric Assessments, Inc. JCL receives royalties from the American Psychological Association books, as well as grant support from the American Psychological Foundation. DJK is a consultant to the American Psychiatric Association (as Chair of the DSM-5 Task Force); holds joint ownership of copyright for the Pittsburgh Sleep Quality Index (PSQI); has received honorarium for manuscript submission to Medicographia (Servier); is a member of the Valdoxan Advisory Board of Servier International; and is a stockholder in AliphCom and Psychiatric Assessments, Inc.
Footnotes
Disclosures
MLW, MH, BH, CAJ, IS, MCF, JB, and FCR do not have any conflicts of interest to report.
References
- 1.Kessler RC, Akiskal HS, Ames M, et al. Prevalence and effects of mood disorders on work performance in a nationally representative sample of U.S. workers. Am J Psychiatry. 2006;163:1561–1568. doi: 10.1176/appi.ajp.163.9.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Revicki DA, Matza LS, Flood E, Lloyd A. Bipolar disorder and health-related quality of life: Review of burden of disease and clinical trials. Pharmacoeconomics. 2005;23:583–594. doi: 10.2165/00019053-200523060-00005. [DOI] [PubMed] [Google Scholar]
- 3.Fagiolini A, Forgione R, Maccari M, et al. Prevalence, chronicity, burden and borders of bipolar disorder. J Affect Disord. 2013;148:161–169. doi: 10.1016/j.jad.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Dilsaver SC. An estimate of the minimum economic burden of bipolar I and II disorders in the United States, 2009. J Affect Disord. 2011;129:79–83. doi: 10.1016/j.jad.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 5.Kupfer DJ. The increasing medical burden in bipolar disorder. JAMA. 2005;293:2528–2530. doi: 10.1001/jama.293.20.2528. [DOI] [PubMed] [Google Scholar]
- 6.Perron BE, Howard MO, Nienhuis JK, Bauer MS, Woodward AT, Kilbourne AM. Prevalence and burden of general medical conditions among adults with bipolar I disorder: Results from the national epidemiologic survey on alcohol and related conditions. J Clin Psychiatry. 2009;70:1407–1415. doi: 10.4088/JCP.08m04586yel. [DOI] [PubMed] [Google Scholar]
- 7.Grande I, Kunz M, Potter W, Balanza-Martinez V, Vieta E, Kapczinski F. Should bipolar disorder be considered a systemic illness? Neuropsychiatry. 2011;1:45–54. [Google Scholar]
- 8.Roshanaei-Moghaddam B, Katon W. Premature mortality from general medical illnesses among persons with bipolar disorder: A review. Psychiatr Serv. 2009;60:147–156. doi: 10.1176/ps.2009.60.2.147. [DOI] [PubMed] [Google Scholar]
- 9.Black DW, Winokur G, Hulbert J, Nasrallah A. Predictors of immediate response in the treatment of mania: the importance of comorbidity. Biol Psychiatry. 1988;24:191–198. doi: 10.1016/0006-3223(88)90274-0. [DOI] [PubMed] [Google Scholar]
- 10.Black DW, Winokur G, Nasrallah A. Effect of psychosis on suicide risk in 1,593 patients with unipolar and bipolar affective disorders. Am J Psychiatry. 1988;145:849–852. doi: 10.1176/ajp.145.7.849. [DOI] [PubMed] [Google Scholar]
- 11.Black DW, Winokur G, Mohandoss E, Woolson RF, Nasrallah A. Does treatment influence mortality in depressives? A follow-up of 1076 patients with major affective disorders. Ann Clin Psychiatry. 1989;1:165–173. [Google Scholar]
- 12.Fagiolini A, Kupfer DJ, Houck PR, Novick DM, Frank E. Obesity as a correlate of outcome in patients with bipolar I disorder. Am J Psychiatry. 2003;160:112–117. doi: 10.1176/appi.ajp.160.1.112. [DOI] [PubMed] [Google Scholar]
- 13.Fagiolini A, Frank E, Scott JA, Turkin S, Kupfer DJ. Metabolic syndrome in bipolar disorder: findings from the Bipolar Disorder Center for Pennsylvanians. Bipolar Disord. 2005;7:424–430. doi: 10.1111/j.1399-5618.2005.00234.x. [DOI] [PubMed] [Google Scholar]
- 14.Swartz HA, Fagiolini A. Cardiovascular disease and bipolar disorder: risk and clinical implications. J Clin Psychiatry. 2012;73:1563–1565. doi: 10.4088/JCP.12ac08227. [DOI] [PubMed] [Google Scholar]
- 15.Hajek T, Slaney C, Garnham J, Ruzickova M, Passmore M, Alda M. Clinical correlates of current level of functioning in primary care-treated bipolar patients. Bipolar Disord. 2005;7:286–291. doi: 10.1111/j.1399-5618.2005.00182.x. [DOI] [PubMed] [Google Scholar]
- 16.Crump C, Sundquist K, Winkleby MA, Sundquist J. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70:931–939. doi: 10.1001/jamapsychiatry.2013.1394. [DOI] [PubMed] [Google Scholar]
- 17.Judd LL, Akiskal HS, Schetteler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530–537. doi: 10.1001/archpsyc.59.6.530. [DOI] [PubMed] [Google Scholar]
- 18.Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60:261–269. doi: 10.1001/archpsyc.60.3.261. [DOI] [PubMed] [Google Scholar]
- 19.Judd LL, Akiskal HS, Schettler PJ, et al. Psychosocial disability in the course of bipolar I and II disorders. Arch Gen Psychiatry. 2005;62:1322–1330. doi: 10.1001/archpsyc.62.12.1322. [DOI] [PubMed] [Google Scholar]
- 20.Katon WJ. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biol Psychiatry. 2003;54:216–226. doi: 10.1016/s0006-3223(03)00273-7. [DOI] [PubMed] [Google Scholar]
- 21.Fagiolini A, Frank E, Houck PR, et al. Prevalence of obesity and weight change during treatment in patients with bipolar I disorder. J Clin Psychiatry. 2002;63:528–533. doi: 10.4088/jcp.v63n0611. [DOI] [PubMed] [Google Scholar]
- 22.Fagiolini A, Kupfer DJ, Scott J, et al. Hypothyroidism in patients with bipolar I disorder treated primarily with lithium. Epidemiol Psichiatr S. 2006;15:123–127. doi: 10.1017/s1121189x00004322. [DOI] [PubMed] [Google Scholar]
- 23.Fagiolini A, Frank E, Soreca I, Houck PR, Kupfer DJ. Integrating medical and psychiatric care in patients with bipolar disorder. J Clin Psychopharmacol. 2008;28:257–258. doi: 10.1097/JCP.0b013e318166f544. [DOI] [PubMed] [Google Scholar]
- 24.Soreca I, Fagiolini A, Frank E, Houck PR, Thompson WK, Kupfer DJ. Relationship of general medical burden, duration of illness and age in patients with bipolar I disorder. J Psychiatry Res. 2008;42:956–961. doi: 10.1016/j.jpsychires.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Soreca I, Frank E, Kupfer DJ. The phenomenology of bipolar disorder: What drives the high rate of medical burden and determines long-term prognosis? Depress Anxiety. 2009;26:73–82. doi: 10.1002/da.20521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soreca I, Fagiolini A, Frank E, Goodpaster BH, Kupfer DJ. Chronotype and body composition in bipolar disorder. Chronobiol Int. 2009;26:780–788. doi: 10.1080/07420520902929060. [DOI] [PubMed] [Google Scholar]
- 27.Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Malden: Blackwell Publishing; 2008. pp. 287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenwasser AM. Circadian clock genes: Non-circadian roles in sleep, addiction, and psychiatric disorders? Neurosci Biobehav R. 2010;34:1249–1255. doi: 10.1016/j.neubiorev.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Mezick EJ, Hall M, Matthews KA. Are sleep and depression independent or overlapping risk factors for cardiometabolic disease? Sleep Med Rev. 2011;15:51–63. doi: 10.1016/j.smrv.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soreca I, Wallace ML, Frank E, Hasler BP, Levenson JC, Kupfer DJ. Sleep duration is associated with dyslipidemia in patients with bipolar disorder in clinical remission. J Affect Disorders. 2012;141:484–487. doi: 10.1016/j.jad.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Depner CM, Stothard ER, Wright KP., Jr Metabolic consequences of sleep and circadian disorders. Curr Diab Rep. 2014;14:507. doi: 10.1007/s11892-014-0507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sachs GS, Thase ME, Otto MW, et al. Rationale, design, and methods of the systematic treatment enhancement program for bipolar disorder. Biol Psychiatry. 2003;53:1028–1042. doi: 10.1016/s0006-3223(03)00165-3. [DOI] [PubMed] [Google Scholar]
- 33.Kraemer HC. Discovering, comparing, and combining moderators of treatment on outcome after randomized clinical trials: a parametric approach. Stat Med. 2013;32:1964–1973. doi: 10.1002/sim.5734. [DOI] [PubMed] [Google Scholar]
- 34.Wallace ML, Frank E, Kraemer HC. A novel approach for developing and interpreting treatment moderator profiles in randomized clinical trials. JAMA Psychiatry. 2013;70:1241–1247. doi: 10.1001/jamapsychiatry.2013.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–61. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 37.Spearing MK, Post RM, Leverich GS, Brandt D, Nolen W. Modification of the clinical global impressions (CGI) scale for use in bipolar illness (BP): The CGI-BP. Psychiatry Res. 1997;73:159–171. doi: 10.1016/s0165-1781(97)00123-6. [DOI] [PubMed] [Google Scholar]
- 38.Frank E, Kupfer DJ, Thase ME, et al. Two-year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Arch Gen Psychiatry. 2005;62:996–1004. doi: 10.1001/archpsyc.62.9.996. [DOI] [PubMed] [Google Scholar]
- 39.Miklowitz DJ, Otto MW, Frank E, et al. Psychosocial treatments for bipolar depression: a 1-year randomized trial from the systematic treatment enhancement program. Arch Gen Psychiatry. 2007;64:419–427. doi: 10.1001/archpsyc.64.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jahng S, Wood PK, Trull TJ. Analysis of affective instability in ecological momentary assessment: Indices using successive difference and group comparison via multilevel modeling. Psychol Methods. 2008;13:354–375. doi: 10.1037/a0014173. [DOI] [PubMed] [Google Scholar]
- 41.Janney CA, Bauer MS, Kilbourne AM. Self-management and bipolar disorder—a clinician’s guide to the literature 2011–2014. Curr Psychiatry Reports. 2014;16:485. doi: 10.1007/s11920-014-0485-5. [DOI] [PubMed] [Google Scholar]
- 42.Frank E. Treating bipolar disorder: a clinician's guide to interpersonal and social rhythm therapy. New York: Guilford Press; 2005. [Google Scholar]
- 43.Kretschmer E. Physique and character. Oxford: Harcourt Brace; 1925. [Google Scholar]
- 44.Fiedorowicz JG, Palagummi NM, Forman-Hoffman V, Miller DD, Haynes WG. Elevated prevalence of obesity, metabolic syndrome, and cardiovascular risk factors in bipolar disorder. Ann Clin Psychiatry. 2008;20:131–137. doi: 10.1080/10401230802177722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fiedorowicz JG, Solomon DA, Endicott J, et al. Manic/hypomanic symptom burden and cardiovascular mortality in bipolar disorder. Psychosom Med. 2009;71:598–606. doi: 10.1097/PSY.0b013e3181acee26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hickie IB, Scott EM, Hermens DF, et al. Applying clinical staging to young people who present for mental health care. Early Interv Psychiatry. 2013;7:31–43. doi: 10.1111/j.1751-7893.2012.00366.x. [DOI] [PubMed] [Google Scholar]
- 47.McEwen BS, Stellar E. Stress and the individual: Mechanisms leading to disease. Arch Intern Med. 1993;153:2093–2101. [PubMed] [Google Scholar]